Abstract
Background
Transarterial chemoembolization (TACE) is an important therapy for hepatocellular carcinoma (HCC) in cirrhosis. In particular in advanced cirrhosis, post-TACE hepatic failure liver (PTHF) failure may develop. Currently, there is no standardization for the periinterventional risk assessment. The liver maximum capacity (LiMAx) test assesses the functional liver capacity, but has not been investigated in this setting.
Aims
The aim of this study was to prospectively evaluate periinterventional LiMAx and CT volumetry measurements in patients with cirrhosis and HCC undergoing repetitive TACE.
Methods
From 06/2016 to 11/2017, eleven patients with HCC and cirrhosis undergoing TACE were included. LiMAx measurements (n = 42) were conducted before and after each TACE. Laboratory parameters were correlated with the volume–function data.
Results
The median LiMAx levels before (276 ± 166 µg/kg/h) were slightly reduced after TACE (251 ± 122 µg/kg/h; p = 0.08). This corresponded to a median drop of 7.1%. Notably, there was a significant correlation between LiMAx levels before TACE and bilirubin (but not albumin nor albumin–bilirubin [ALBI] score) increase after TACE (p = 0.02, k = 0.56). Furthermore, a significantly higher increase in bilirubin in patients with LiMAx ≤ 150 µg/kg/h was observed (p = 0.011). LiMAx levels at different time points in single patients were similar (p = 0.2).
Conclusion
In our prospective pilot study in patients with HCC and cirrhosis undergoing multiple TACE, robust and reliable LiMAx measurements were demonstrated. Lower LiMAx levels before TACE were associated with surrogate markers (bilirubin) of liver failure after TACE. Specific subgroups at high risk of PTHF should be investigated. This might facilitate the future development of strategies to prevent occurrence of PTHF.
Keywords: Cirrhosis, Hepatocellular carcinoma, Liver function, Liver maximum capacity, Transarterial chemoembolization, Volume–function analysis
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third most common cancer-dependent death cause worldwide [1]. Transarterial chemoembolization (TACE) is the gold standard palliative therapy in non-resectable Barcelona Clinic Liver Cancer Classification (BCLC) stage B patients and also used for bridging patients to liver transplantation [2]. It is well known that the prognosis depends on tumor burden and liver function capacity. TACE is less effective in patients with extrahepatic spread or macrovascular invasion, and/or decompensated liver disease [2]. Post-TACE hepatic failure (PTHF) is severe complication after TACE. Several scoring systems and algorithms for HCC patients have been evaluated in the past [3]. Child–Pugh stage B or C and serum bilirubin level > 2 mg/dl are considered as risk factors for liver failure after TACE [4, 5]. Currently, the ALBI-[6] and other scores are applied. However, there is still no widely accepted standard risk assessment procedure in patients undergoing TACE.
The liver maximum capacity (LiMAx) test was introduced for the evaluation of the liver function capacity. LiMAx was initially developed and proved beneficial to predict postoperative liver function in patients after hepatectomy [7]. In the further course, it was used to assess the severity of liver cirrhosis and the short-term mortality of cirrhosis patients and HCC patients undergoing hepatectomy [8, 9]. Previous studies found a strong correlation between LiMAx levels and histological severity of liver disease [10] and different clinical stages of cirrhosis [11].
Moreover, we previously evaluated the ability to predict the functional reserve in patients with cirrhosis and transhepatic intrahepatic portosystemic shunt (TIPS) implantation [8].
The aim of this study was to prospectively assess LiMAx in HCC patients with cirrhosis and HCC undergoing repetitive TACE.
Patients and Methods
Study Population
The study was reviewed and approved by an institutional research review board (Approval 266/16). Each patient signed a written informed consent for the study and TACE procedure, as well as informed consent to use the clinical data for this research project. All patients evaluated for TACE in our departments between June 2016 and November 2017 were screened for inclusion. Only patients with HCC and cirrhosis were included in the analysis. HCC was diagnosed according to EASL criteria [2]. All patients were discussed in the local tumor board, and TACE was recommended. Since a total bilirubin level > 3.0 mg/dl is considered as a contraindication for TACE in our center, no such patients were enrolled. Age < 18 years, paracetamol allergy, and the inability to perform all the necessary study examinations were exclusion criteria. Before TACE, all patients received a clinical examination including laboratory liver function tests and magnetic resonance as well as computed tomography (CT) imaging of the liver. The following clinical data were analyzed: age, sex, etiology of cirrhosis, Child–Pugh score (CPS), and model for end-stage liver disease (MELD) score. Moreover, the following laboratory values were collected: serum levels of hemoglobin, creatinine, bilirubin, albumin, sodium, international normalized ratio (INR), aminotransferases, white and red blood cell and platelet counts as well as ammonium serum concentrations and alpha-fetoprotein (AFP) levels.
Patients were screened for the occurrence of complications until dismissal and at presentation at the following treatments. The LiMAx measurements were performed 1 ± 1 day prior and 1 ± 1 day post-TACE treatment to study early changes after TACE. According to the pre-TACE LiMAx value, patients were divided into two groups: higher and lower than 150 µg/kg/h. This cutoff was based on a large study by Stockman et al. [7] where significantly worse outcome for patients after hepatectomy with LiMAx under 150 µg/kg/h was found. The ALBI score was calculated as follows and as previously described [6]: log10 bilirubin [mmol/L] × 0.66) + (albumin [g/L] × (− 0.0852)). ALBI classes were assessed as follows: ALBI score ≤ 2.60 (ALBI grade 1), ≥ 2.60 to ≤ 1.39 (ALBI grade 2), and ≥ 1.39 (ALBI grade 3) according to Johnson et al. [12].
TACE Procedure
TACE procedures were routinely prepared using analgetic and antiemetic oral medication including dexamethasone, ondansetron, and pethidine. Angiography was performed via a transfemoral route using an appropriate 4 French guiding catheter for catheterization of the celiac trunk or mesenteric artery to evaluate anatomical feasibility. A coaxial 2.7 French microcatheter was used for selective angiography of the hepatic vasculature and to identify segmental hepatic tumor feeding arteries. For chemoembolization, a mixture of 10 ml Lipiodol® Ultra Fluid (Guerbet, France) and 50 mg of doxorubicin was slowly injected to avoid non-target embolization. A CT scan was conducted in all patients post-TACE to allow for evaluation of embolized liver volume.
LiMAx Test
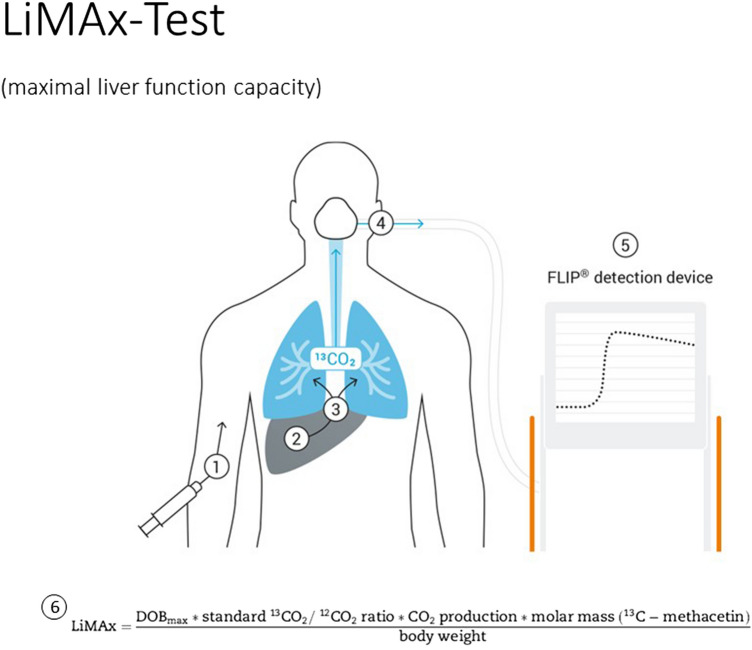
Liver tests were performed at the time of inclusion after fasting for a minimum of 3 h. The procedure is based on body weight-adjusted (2 mg/kg) intravenous 13C-labeled methacetin bolus injection and subsequent injection of 20 ml 0.9% sodium chloride as previously described [13]. Exhaled breath is collected by a distinct two-way face mask and analyzed by means of a special device (Humedics, Berlin, Germany). Herein, we are able to achieve a continuous real-time sampling rate and optimal analysis of delta-over-baseline (DOB) curves of 13CO2/12CO2 ratios. 13C-methacetin is a substrate of the hepatic CYP1A2 enzyme, which exclusively metabolizes it into paracetamol and 13CO2 [14]. Prior to substrate injection, the baseline 13CO2/12CO2 ratio is recorded in the native expired air to calculate the individual baseline. Using the individual 13CO2/12CO2 baseline, the results are not influenced by obstructive pulmonary disease or ventilation. Maximum delta-over-baseline (DOBmax) of the 13CO2/12CO2 ratio was determined by analyzing the continuous DOB curve over a maximum of 60 min, and the LiMAx value is calculated as previously described [13]. The principle of the LiMAx test is illustrated in Fig. 1 (according to Stockmann et al. [13]).
Fig. 1.
Principle of the LiMAx test. The test starts with a body weight-adjusted (2 mg/kg) intravenous 13C-labeled methacetin bolus injection and a subsequent injection of 20 ml 0.9% sodium chloride (1). 13C-methacetin is a substrate of the hepatic CYP1A2 enzyme, which is metabolized into paracetamol and 13CO2 (2), which is excreted pulmonary (3). Consecutively exhaled air is collected by a facemask (4) and then analyzed in a FLIP ® device according to the following formula (6). Adapted from Stockmann et al. [12]
Liver Volume Reduction and LiMAx Value Drop
Semi-automated liver volumetry was performed using a dedicated medical image viewing and postprocessing software (iNtuition, TeraRecon, NC, USA) pre- and post-TACE CT scans (available in all patients). The total liver volume (TLV) and tumor volume (TuV) were measured before TACE. The embolization volume (EV) was measured directly after TACE. LiMAx was performed 1 day (± 1) before and 1 day (± 1) after TACE treatment. A LiMAx drop was defined as: (LiMAx before TACE – LiMAx after TACE)/LiMAx before TACE *100 [%]. The Liver volume (LV) reduction was defined as: (TLV-TuV)-(TLV-EV)/(TLV-TuV) [%].
Assessment of Liver Function After TACE
LiMAx and liver function tests (thrombin time, bilirubin, transaminases) were measured at the first day after TACE treatment. Moreover, deterioration in clinical outcome, hospital stay, and post-interventional morbidity according to the Clavien–Dindo classification were recorded. A total bilirubin increase (total bilirubin after TACE – total bilirubin before TACE), albumin decrease (total albumin after TACE – total albumin before TACE), and ALBI increase (total ALBI after TACE – total ALBI before TACE) were calculated as surrogate parameters for post-TACE treatment liver function deterioration.
Statistical Analysis
All variables are described as proportions, means with standard deviations (SD), or medians with interquartile ranges (IQR). Univariate analysis was performed with Chi2-squared test, t test or Mann–Whitney U test, according to the distribution of the test variable. The statistical analyses were performed using SPSS 22.0 (SPSS, Munich, Germany). Two-sided p values < 0.05 were regarded as statistically significant. Coefficient of variations as well as Friedmann two-factorial rank analysis for repeated measures was used to compare repetitive LiMAx measurements before and after consecutive TACE in the same patient.
Results
Baseline Data
The patient baseline characteristics are summarized in Table 1. Twenty-one TACE procedures in 11 patients were included in the analysis. The patients were predominantly male (72%); the median age was 67 (± 11 SD) years. All of the patients had cirrhosis. The most common etiology was alcohol (64%), followed by hepatitis B (18%) and primary biliary cholangitis (18%)-induced cirrhosis. The patients were mostly in an early stage of cirrhosis (Child–Pugh stages: A 82%, B 18%, C 0%; median MELD score 9.9 points ± 4 SD). Most patients were ALBI score grade 1 (81.8%); 2 patients (18.2%) were grade 2. Median LiMAx in the ALBI grade 1 patients was 292 ± 168 µg/kg/h; in ALBI grade 2 patients LiMAx levels were lower (117 ± 37 µg/kg/h), even though not reaching statistical significance (p = 0.12). No ALBI grade 3 patients were included.
Table 1.
Baseline patients characteristics
| Age (years) | 67 ± 11 |
| Sex (male) | 8 (72) |
| Child–Pugh stage | |
| A | 9 (82) |
| B | 2 (18) |
| C | 0 |
| ALBI score (mean) | − 3.01 ± 0.4 |
| Total bilirubin before TACE [mg/dl] | 1.22 ± 0.8* |
| Total bilirubin after TACE [mg/dl] | 1.91 ± 1.3* |
| Albumin [mg/dl] | 36.4 ± 4 |
| INR | 1.16 ± 0.2 |
| Creatinine [mg/dl) | 1.05 ± 0.3 |
| Platelets | 102 ± 63 |
| ALT [U/l] | 39.6 ± 20 |
| MELD | 9.9 ± 4 |
| MELD-Na | 11.2 ± 4 |
| Etiology of cirrhosis | |
| Alcoholic | 7 (64) |
| HBV | 2 (18) |
| PBC | 2 (18) |
Unless specified differently, values are given as median and standard deviation (SD), or frequencies and percentages
*p = 0.001 vs serum bilirubin before TACE. Significant p-values are indicated in bold
HCC and TACE Treatments
Characteristics of the HCCs (Table 2) and TACE treatments (Table 3) are provided. The median size of the largest HCC nodule was 58.4 ± 24 mm. Most patients (87%) were BCLC stage B; 3 patients (13%) were in stage A3. The median TLV was 1755 ± 400 ml; median TuV of the HCCs was 64.5 ± 6 ml. The median EV was 693 ± 400 ml. Most TACE was conducted in a selective lobar approach (70%).
Table 2.
HCC characteristics
| Largest nodule diameter [mm] | 58.4 ± 24 |
|---|---|
| Number noduli | |
| 1 | 9 (39) |
| 2 | 1 (4) |
| 3 | 5 (22) |
| > 3 | 8 (35) |
| BCLC score | |
| A1 | 0 (0) |
| A2 | 0 (0) |
| A3 | 3 (13) |
| A4 | 0 (0) |
| B | 20 (87) |
| C | 0 (0) |
| D | 0 (0) |
| AFP pre-TACE [µg/l] | 842 ± 2106 |
| TNM T-status prior TACE | |
| T1a | 1 (4) |
| T1b | 2 (9) |
| T2 | 3 (13) |
| T3 | 17 (74) |
| T4 | 0 (0) |
Values are given as median and standard deviation (SD), or frequencies and percentages
Significant p-values are indicated in bold
Table 3.
TACE and liver volume–function analysis parameters
| TLV [ml] | 1755 ± 400 |
| TuV [ml] | 64.5 ± 6 |
| EV [ml] | 693 ± 400 |
| TACE approach (%) | |
| Subsegmental | 0 |
| Segmental | 2 (9) |
| Lobar | 16 (70) |
| Bilobar | 5 (22) |
| LiMAx before TACE [µg/kg/h] | 276 ± 166 |
| LiMAx after TACE [µg/kg/h] | 251 ± 122* |
| LiMAx drop [%] | 7.1 ± 23 |
| LV reduction [%] | 37.5 ± 23 |
Values are given as median and standard deviation (SD), or frequencies and percentages
*p = 0.08 versus LiMAx before TACE. Significant p-values are indicated in bold
LiMAx and Function–Volume Analysis Pre- and Post-TACE
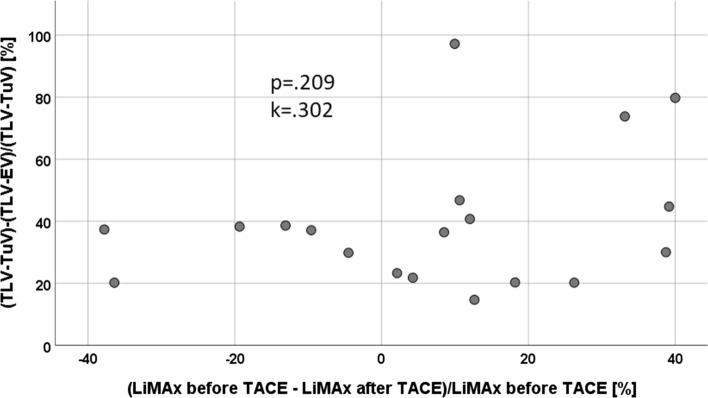
The data are provided in Tables 2 and 3. The LiMAx levels pre-TACE correlated with the pre-TACE ALBI score (p = 0.017, k = − 0.513) and albumin (p = 0.016, k = 0.519) levels, pre-TACE bilirubin (as item of the ALBI score) only (p = 0.065, k = − 0.41) were not significantly correlated with LiMAx levels pre-TACE though. Serum bilirubin levels were significantly higher after (1.91 ± 1.3 SD), than before (median 1.22 mg/dl ± 0.8 SD; p = 0.001) TACE. The median LiMAx levels before TACE were 276 ± 166 µg/kg/h; they were slightly reduced after TACE (251 ± 122 µg/kg/h; p = 0.08). This corresponded to a median drop of 7.1% of LiMAx levels after TACE. The LV was reduced median 37.5 ± 23% SD. The reduction in LV did not correlate with the drop in LiMAx levels as presented in Fig. 2.
Fig. 2.
Poor correlation of FRLF und FLRV in patients after TACE
LV reduction (10.7–100%) and change of LiMAx levels (− 37.8% to +40.0%) after TACE varied substantially among the patients and did not correlate significantly (p = 0.209, k = 0.302). When we compared patients with lower versus those with a higher (LiMAx ≤ 150 µg/kg/h versus > 150 µg/kg/h) LiMAx levels (Table 4), higher bilirubin and INR levels, larger spleen sizes, and lower platelet counts were observed in patients with lower LiMAx levels, which additionally in the setting of TACE confirms the well-known association of reduced LiMAx levels with the increasing stage of cirrhosis.
Table 4.
Parameters stratified by LiMAx levels ≤/> 150 µg/kg/h. Values are given as median and standard deviation (SD), or frequencies and percentages
| LiMAx before TACE ≤ 150 µg/kg/h | LiMAx before TACE > 150 µg/kg/h | p-Value | |
|---|---|---|---|
| Total bilirubin before TACE [mg/dl] | 1.4 ± 0.6 | 0.8 ± 0.6 | 0.01 |
| Total bilirubin after TACE [mg/dl] | 2.2 ± 0.8 | 1.1 ± 0.7 | 0.02 |
| Total bilirubin rise [mg/dl] | 0.81 ± 0.5 | 0.1 ± 0.2 | 0.01 |
| Spleen size [cm] | 18.0 ± 5 | 11.8 ± 3 | 0.004 |
| Platelets count [109/l] | 70.0 ± 55 | 143.4 ± 42 | 0.01 |
| ALT [U/l] | 76.7 ± 150 | 42.3 ± 21 | 0.31 |
| GGT [U/l] | 148 ± 106 | 264 ± 245 | 0.55 |
| AP [U/l] | 179 ± 100 | 145 ± 73 | 0.19 |
| INR | 1.35 ± 0.1 | 1.01 ± 0.1 | 0.001 |
Significant p-values are indicated in bold
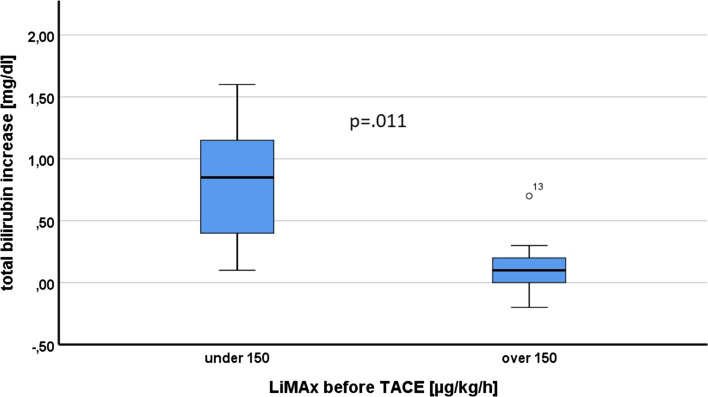
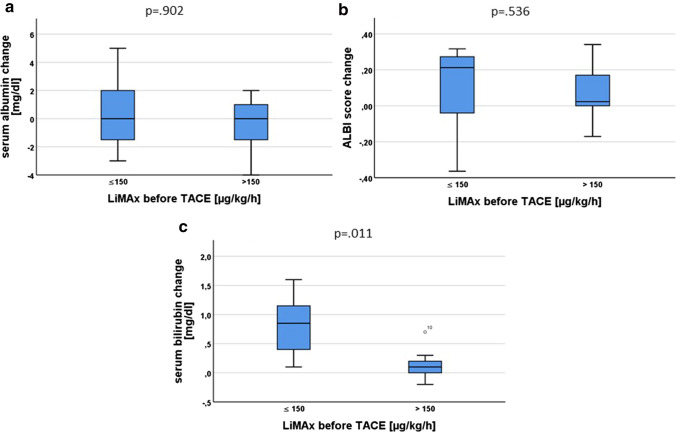
Notably, there was a significant correlation between LiMAx levels pre-TACE and the bilirubin increase after TACE (p = 0.019; Fig. 3). The increase in bilirubin was higher in the group of patients with LiMAx ≤ 150 µg/kg/h (Fig. 4c), whereas no difference could be detected for ALBI score and albumin levels (Fig. 4a and b).
Fig. 3.
Correlation of bilirubin increase after TACE and LiMAx levels before TACE
Fig. 4.
a Post-TACE albumin change stratified by LiMAx levels. b Post-TACE ALBI change stratified by LiMAx levels. c Post-TACE bilirubin change stratified by LiMAx levels
Repetitive TACE
Most of the patients were treated multiple times (n = 18, 78%): one patient was treated 5 times, one 4 times, one 3 times, and 3 patients were treated twice. The longitudinal data of LiMAx measurements in the patient with five treatments are presented in Fig. 5, there was no significant difference between LiMAx levels at different time points (p = 0.2), and this could also not be observed in the other patients. Overall, we noticed only few and minor complications post-TACE; 21 Patients (74%) had a normal post-interventional course, in one patient (4%) Clavian–Dindo Grade I, and in five patients (22%) Clavian–Dindo Grade II (mostly acute cholecystitis) morbidity were observed.
Fig. 5.
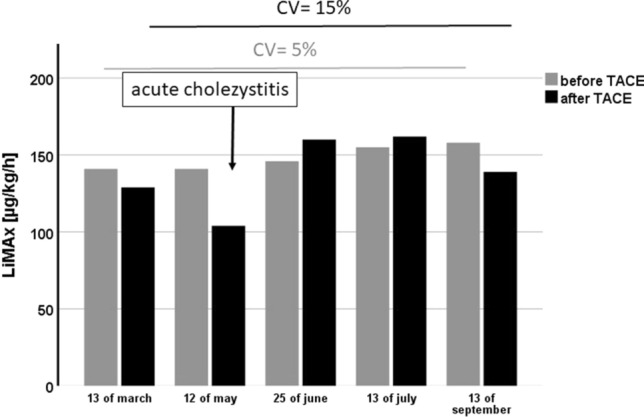
Course of LiMAx before and after TACE in the patient with five treatments. There was no significant difference between the single measurements (p = 0.16). The coefficient of variations (CV) for the LiMAx before TACE was 15% and after TACE 5%. There was a drop of LiMAx value after the second TACE. This was associated with an episode of acute cholecystitis induced by TACE
Discussion
TACE is the gold standard therapy for patients with HCC in intermediate stages as endorsed by both the guidelines from the European Association for the Study of the Liver (EASL) [2] and the American Association for the Study of Liver Diseases (AASLD) [15, 16]. Two randomized controlled trials confirm the benefit of treatment for those patients in comparison with the best supportive care [17–19]. Post-TACE hepatic failure (PTHF) is one of the major complications after TACE. There are several risk factors for PTHF, including the presence of a portal vein thrombosis, reduced serum sodium and serum albumin levels, increased serum total bilirubin and AFP levels and reduced platelets count and large diameter of HCC [20]. Different scores including those parameters were developed. The ALBI score (albumin–bilirubin grade) [3–5, 21] and the BCLC criteria (based on the CPS [22]) are widely used prognostic markers for PTHF, but still are suboptimal. Therefore, other additional tests were developed in order to reduce the PTHF rate. Huang et al. [23] showed the superiority of the monoethylglycinexylidide test over conventional liver function tests and clinical parameters to predict PTHF. Similarly, Khisti et al. [24] showed a specificity of 87.5% and sensitivity of 90% for the liver function test with indocyanine green clearance to predict PTHF. There are also reports though, showing a non-superiority of the indocyanine green plasma disappearance rate in order to predict PTHF compared to MELD, MELD-Na, and CPS [25]. Still, there is no widely accepted gold standard for the evaluation of the liver function reserve in patients prior TACE treatment.
In this prospective pilot study, the feasibility of LiMAx measurements to assess the pre-TACE liver function was demonstrated. The LiMAx test has been intensively evaluated since its introduction in the year 2009 [13]. It was initially evaluated in hepatectomy patients. Using an algorithm to predict posthepatectomy liver failure, including volume–function analysis [7], a significant improvement in the postoperative liver failure and mortality rate can be achieved as shown by Jara et al. [26]. Since there were some considerations regarding an HCC functional status and the feasibility of volume–function analysis in those patients, Blüthner et al. [27] analyzed this algorithm in HCC patients with and without liver cirrhosis, yielding a similar result. As shown by Jara et al. [28], LiMAx can be repeatedly performed even before and after extra-abdominal surgery leading to similar results.
A recent study by Barzakova et al. [29] also investigated LiMAx measurements in a similar approach to our cohort in patients undergoing TACE. Similar reductions to our cohort of LiMAx levels after TACE were found (10.0 versus 7.1%). As the cohort by Barzakova et al. contained > 65% of the patients with no cirrhosis, results can be not compared with our cohort.
In the present study, we found that LiMAx measurements are reliable reproducible in patients with HCC and cirrhosis undergoing multiple TACE treatments. LiMAx levels did not correlate with ALBI levels, likely due to the similar stage of cirrhosis of the included patients. The functional liver volume reduction correlates very well with the drop of liver function measured by LiMAx in hepatectomy patients [13, 30]. LV changes were not correlated with changes of the LiMAx levels in our TACE patients though. We assume that this is due the fact that after TACE, there is no ''real'' liver parenchyma loss, since only portal, but no arterial branches are embolized. In addition, there is typically no necrosis within the embolized liver after TACE. We thus assume that liver volume analysis prior TACE cannot stratify the risk of post-interventional liver failure, as it is the case in hepatectomy patients [7].
In contrast to ALBI and albumin levels, reduced LiMAx levels were associated with an increase in bilirubin after TACE. Even though this might also reflect changes in vascularization after TACE or other confounders and, therefore, further studies are required, this may indicate that LiMAx could be an important marker to predict PTHF.
Our study has limitations that have to be acknowledged: our cohort size was small, and we had no case of major hepatic failure after TACE. This is due to a rigid selection of TACE candidates regarding preserved liver function. However, in this limited cohort of patients, results showed significant results indicating LiMAx as a robust method for assessment of liver function in cirrhotic liver. Occurrence of PTHF in the follow-up could therefore not be evaluated, and we therefore evaluated surrogate markers, but not clinical endpoints. Additionally, further studies investigating the optimal time point of LiMAx measurements after TACE are required.
We conclude that in our prospective pilot trial, LiMAx measurements before TACE in order to predict PTHF are feasible and safe and show a strong intra-patient correlation with reliable reproducible measurements in repetitive treatments. Lower LiMAx levels before TACE were associated with surrogate markers indicating the liver function drop after TACE. Specific subgroups at high risk of PTHF should be investigated in dedicated future prospective trials. This may facilitate the future development of strategies to prevent the development of PTHF and identify patients at highest risk.
Acknowledgments
We would like to thank all patients who participated in the study and Sarah Igel, Sabine Bunjes-Schmieger, Oluwatosin Raphael Famuyiwa, and Julian Schröder for their assistance.
Abbreviations
- ALBI
Albumin–bilirubin
- ALT
Alanine aminotransferase
- AFP
Alpha-fetoprotein
- BCLC
Barcelona Clinic Liver Cancer Classification
- CPS
Child–Pugh score
- CT
Computed tomography
- EV
Embolization volume
- FLRV
Functional remnant liver volume
- FRLF
Future remnant liver function
- HBV
Hepatitis B virus
- HCC
Hepatocellular carcinoma
- INR
International normalized ratio
- LiMAx
Liver maximum capacity
- LV
Liver volume
- MELD
Model for end-stage liver disease
- PBC
Primary biliary cholangitis
- PTHF
Post-TACE hepatic failure
- TLV
Total liver volume
- TuV
Tumor volume (TuV)
- SD
Standard deviation
- TACE
Transarterial chemoembolization
- TIPS
Transhepatic intrahepatic portosystemic shunt
- TKI
Tyrosinkinase inhibitor
Author's contribution
Author’s contributions (according to Uniform Requirements for Manuscripts Submitted to Biomedical Journals: Ethical Considerations in the Conduct and Reporting of Research: Authorship and Contributorship, ICMJE): MCR, MM, and FL designed the study; MCR, AS, AM, and MM participated in the acquisition of clinical data, drafted the manuscript, and together with MG, AB and FL analyzed the data and finalized the manuscript, which was then revised by all authors. The final draft of the manuscript has been approved by all authors. The contents of this manuscript are our original work and have not been published, in whole or in part, prior to or simultaneous with our submission of the manuscript.
Funding
This study was supported by the Liver Systems Medicine (LiSyM) network (BMBF 031L0051 to MG and FL) and funds from Saarland University (HOMFOR Grant T201000815). Open access funding provided by Projekt DEAL.
Compliance with Ethical Standards
Conflict of interest
The authors who have taken part in this study declared that they do not have anything to disclose regarding conflicts of interest with respect to this manuscript.
Ethical approval
Informed consent was obtained from all individual participants included in the study. The study protocol has been approved by the research institute’s committee on human research (Approval 266/16). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002 CA Cancer J Clin. 2005;55:74–108. [DOI] [PubMed]
- 2.EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. [DOI] [PubMed]
- 3.Hiraoka A, Kumada T, Michitaka K, Kudo M. Newly proposed ALBI Grade and ALBI-T score as tools for assessment of hepatic function and prognosis in hepatocellular carcinoma patients. Liver Cancer. 2019;8:312–325. doi: 10.1159/000494844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Baere T, Arai Y, Lencioni R, et al. Treatment of liver tumors with lipiodol TACE: Technical recommendations from experts opinion. Cardiovasc Intervent Radiol. 2016;39:334–343. doi: 10.1007/s00270-015-1208-y. [DOI] [PubMed] [Google Scholar]
- 5.Raoul JL, Sangro B, Forner A, et al. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. 2011;37:212–220. doi: 10.1016/j.ctrv.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Gui B, Weiner AA, Nosher Jet al. Assessment of the albumin-bilirubin (ALBI) grade as a prognostic indicator for hepatocellular carcinoma patients treated with radioembolization. Am J Clin Oncol. 2018;41:861–866. [DOI] [PMC free article] [PubMed]
- 7.Stockmann M, Lock JF, Malinowski M, Niehues SM, Seehofer D, Neuhaus P. The LiMAx test: a new liver function test for predicting postoperative outcome in liver surgery HPB (Oxford). 2010;12:139–146. doi: 10.1111/j.1477-2574.2009.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malinowski M, Jara M, Luttgert K, et al. Enzymatic liver function capacity correlates with disease severity of patients with liver cirrhosis: a study with the LiMAx test. Dig Dis Sci. 2014;59:2983–2991. doi: 10.1007/s10620-014-3250-z. [DOI] [PubMed] [Google Scholar]
- 9.Jara M, Dziodzio T, Malinowski M, et al. Prospective assessment of liver function by an enzymatic liver function test to estimate short-term survival in patients with liver cirrhosis. Dig Dis Sci. 2019;64:576–584. doi: 10.1007/s10620-018-5360-5. [DOI] [PubMed] [Google Scholar]
- 10.Buechter M, Thimm J, Baba HA, et al. Liver maximum capacity: a novel test to accurately diagnose different stages of liver fibrosis. Digestion. 2019;100:45–54. doi: 10.1159/000493573. [DOI] [PubMed] [Google Scholar]
- 11.Buechter M, Kersting S, Gerken G, Kahraman A. Enzymatic liver function measured by LiMAx-a reliable diagnostic and prognostic tool in chronic liver disease. Sci Rep. 2019;9:13577. doi: 10.1038/s41598-019-49746-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stockmann M, Lock JF, Riecke B, et al. Prediction of postoperative outcome after hepatectomy with a new bedside test for maximal liver function capacity. Ann Surg. 2009;250:119–125. doi: 10.1097/SLA.0b013e3181ad85b5. [DOI] [PubMed] [Google Scholar]
- 14.Pfaffenbach B, Gotze O, Szymanski C, Hagemann D, Adamek RJ. [The 13C-methacetin breath test for quantitative noninvasive liver function analysis with an isotope-specific nondispersive infrared spectrometer in liver cirrhosis] Deutsche medizinische Wochenschrift. 1998;123:1467–1471. [DOI] [PubMed]
- 15.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 16.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 17.Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 18.Min YW, Kim J, Kim S, et al. Risk factors and a predictive model for acute hepatic failure after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. Liver Int. 2013;33:197–202. doi: 10.1111/liv.12023. [DOI] [PubMed] [Google Scholar]
- 19.Siriwardana RC, Niriella MA, Dassanayake AS, et al. Factors affecting post-embolization fever and liver failure after trans-arterial chemo-embolization in a cohort without background infective hepatitis- a prospective analysis. BMC Gastroenterol. 2015;15:96. doi: 10.1186/s12876-015-0329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hickey R, Mouli S, Kulik L, et al. Independent analysis of albumin-bilirubin grade in a 765-patient cohort treated with transarterial locoregional therapy for hepatocellular carcinoma. J Vasc Interv Radiol. 2016;27:795–802. doi: 10.1016/j.jvir.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 22.Huang YS, Chiang JH, Wu JC, Chang FY, Lee SD. Risk of hepatic failure after transcatheter arterial chemoembolization for hepatocellular carcinoma: predictive value of the monoethylglycinexylidide test. Am J Gastroenterol. 2002;97:1223–1227. doi: 10.1111/j.1572-0241.2002.05709.x. [DOI] [PubMed] [Google Scholar]
- 23.Khisti R, Patidar Y, Garg L, Mukund A, Thomas SS, Sarin SK. Correlation of baseline Portal pressure (hepatic venous pressure gradient) and Indocyanine Green Clearance Test With Post-transarterial Chemoembolization Acute Hepatic Failure. J Clin Exp Hepatol. 2019;9:447–452. doi: 10.1016/j.jceh.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shalimar JainS, Gamanagatti SR, et al. Role of indocyanine green in predicting post-transarterial chemoembolization liver failure in hepatocellular carcinoma. J Clin Exp Hepatol. 2018;8:28–34. doi: 10.1016/j.jceh.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jara M, Reese T, Malinowski M, et al. Reductions in post-hepatectomy liver failure and related mortality after implementation of the LiMAx algorithm in preoperative work-up: a single-centre analysis of 1170 hepatectomies of one or more segments. HPB. 2015;17:651–658. doi: 10.1111/hpb.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bluthner E, Jara M, Shrestha R, et al. The predictive value of future liver remnant function after liver resection for HCC in noncirrhotic and cirrhotic patients. HPB. 2019;21:912–922. doi: 10.1016/j.hpb.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 27.Jara M, Bednarsch J, Valle E, et al. Reliable assessment of liver function using LiMAx. J Surg Res. 2015;193:184–189. doi: 10.1016/j.jss.2014.07.041. [DOI] [PubMed] [Google Scholar]
- 28.Barzakova ES, Schulze-Hagen M, Zimmermann M, et al. Monitoring liver function of patients undergoing transarterial chemoembolization (TACE) by a 13C breath test (LiMAx) Cardiovasc Intervent Radiol. 2019;42:1702–1708. doi: 10.1007/s00270-019-02325-3. [DOI] [PubMed] [Google Scholar]
- 29.Malinowski M, Lock JF, Seehofer D, et al. Preliminary study on liver function changes after trisectionectomy with versus without prior portal vein embolization. Surg Today. 2016;46:1053–1061. doi: 10.1007/s00595-015-1293-1. [DOI] [PubMed] [Google Scholar]