Abstract
Aim
Open resection of small bowel neuroendocrine neoplasms (SB-NEN) is still considered standard-of-care, mainly because of frequently encountered multifocality and central mesenteric masses. The aim of this study was to evaluate surgical approach for SB-NEN at a national level and determine predictors for overall survival.
Methods
Patients with SB-NEN who underwent resection between 2005 and 2015 were included from the Netherlands Cancer Registry. Patient and tumor characteristics were compared between laparoscopic and open approach. Overall survival was assessed by Kaplan–Meier and compared with the Log-rank test. Independent predictors were determined by Cox proportional hazards model.
Results
In total, 482 patients were included, of whom 342 (71%) underwent open and 140 (29%) laparoscopic resection. The open resection group had significantly more multifocal tumors resected (24% vs. 14%), pN2 lymph nodes (18% vs. 7%) and stage IV disease (36% vs. 22%). Overall survival after open resection was significantly shorter compared to laparoscopic resection (3-year: 81% vs. 89%, 5-year: 71% vs. 84%, p = 0.004). In multivariable analysis, age above 60-years (60–75, HR 3.38 (95% CI 1.84–6.23); > 75 years, HR 7.63 (95% CI 3.86–15.07)), stage IV disease (HR 1.86 (95% CI 1.18–2.94)) and a laparoscopic approach (HR 0.51 (95% CI 0.28–0.94)) were independently associated with overall survival, whereas multifocal primary tumor, grade and resection margin status were not.
Conclusion
Laparoscopic resection was the approach in 29% of SB-NEN at a national level with selection of the more favorable patients. Laparoscopic resection remained independently associated with better overall survival besides age and stage, but residual confounding cannot be excluded.
Introduction
Small bowel neuroendocrine neoplasms (SB-NEN) are a rare type of gastrointestinal cancer and constitute 15% of all neoplasms of the jejunum and approximately 60% of the ileum, making it the most common gastroenteropancreatic NEN [1, 2]. Patients with stage I-III disease are amenable for curative resection, as well as selected stage IV patients with liver metastases [3, 4]. Resection remains the main treatment modality for these patients, resulting in relatively high 5-year overall survival rates of 70–80% for stage I-III and 35–80% for stage IV disease [3].
The majority of patients with SB-NEN already present with mesenteric lymph node metastases, and multifocal primary tumors can be found in up to 25–44% [5]. These disease characteristics make SB-NEN resection challenging. Although minimally invasive surgery is increasingly gaining acceptance as a standard approach for other gastrointestinal malignancies, minimally invasive surgery is still thought to potentially compromise oncological safety in SB-NEN, thereby potentially worsening survival outcomes [5]. Because of this, guidelines advise laparoscopic resection only in patients in which an appropriate intraoperative assessment of the bowel with proper segmental resection and adequate lymphadenectomy can be performed [3, 5].
Considering the evolution in the application of advanced laparoscopic resection for more complex oncological disease, and more specifically the experience with D3 mesenteric lymphadenectomy [6], application of minimally invasive surgery in SB-NEN might have increased as well. However, there are no population based data or prospective studies on this topic.
The primary aim of this study was to evaluate surgical approach for SB-NEN at a national level considering selection based on patient and tumor characteristics. Secondarily, the aim was to identify independent predictors of overall survival.
Methods
Study design
Data from all patients with SB-NEN diagnosed between 2010 and 2015 were extracted from the Netherlands Cancer Registry (NCR). The NCR contains all cases of cancer in The Netherlands (i.e., total population of 17.4 million), mainly based on notification by the digital pathology archive and the national registry of hospital discharge diagnoses. Independent data-managers collect data on baseline and tumor characteristics as well as treatment and survival data in each Dutch hospital based on hospital records. Full histopathology reports were requested from The Nationwide Network and Registry of Histo- and Cytopathology in The Netherlands (PALGA) [7]. This registry contains histopathology reports from all Dutch pathology laboratories, including all histopathological examined tissues. All histopathology laboratories are connected to PALGA via a special network that enables collection of the histopathology reports. Both NCR and PALGA are independent organizations, funded by the Dutch government. This study is reported in accordance with the STROBE guidelines [8].
Study population
Patients with histopathologically proven SB-NEN of any stage and differentiation grade were included. The diagnosis was based on the International Classification of Disease-Oncology (ICD-O-3) topography and morphology codes [9]. Surgical approach (open/laparoscopic) is registered in the NCR since 2010, hence only patients with a diagnosis between 2010 and 2015 were included for the present study. Exclusion criteria were: grade 3 NEN, mixed neuroendocrine-non-neuroendocrine tumors (MiNEN), duodenal NENs, double tumors (e.g., concomitant SB-NEN and adenocarcinoma of the colon), autopsy and cytology data, benign neoplasms and non-neuroendocrine neoplasms. Grade 3 NEN were excluded because of the essentially different prognosis and rarity for small bowel localization, therefore it should be considered a separate disease entity.
Data collection
Primary tumor location was classified as jejunum (C17.1), ileum (C17.2) or small bowel not otherwise specified (C17.9), according to the ICD-O-3 codes. Missing TNM stage was assessed using supplementary data on “extend of disease” present in the NCR database.
Data in both NCR and PALGA databases correspond based on unique NCR-codes. This feature was used to couple both datasets. Data regarding topography, differentiation grade, resection margins, TNM staging and tumor positive lymph nodes were extracted from the full histopathology reports provided by PALGA. Morphology codes were used in case of a mismatch in differentiation grade [10]. Data from PALGA prevailed, in case of disagreement between both datasets. Finally, all tumors were restaged according to the 8th edition of the TNM classification [11].
In case of multiple histopathology reports (e.g., two biopsies followed by a resection), the first date was used as ‘date of diagnosis’. Time to treatment analyses could not be performed because the diagnosis was based on pathology data, which was often the date of surgery. Overall survival was defined as the time between date of diagnosis and date of death or censored at the end of follow-up.
Statistical analysis
Categorical data are presented as number of cases and percentages, whilst continuous data are presented as either mean with standard deviation (SD) or median with interquartile range (IQR), depending on the data distribution. Overall survival analyses were performed using the Kaplan–Meier method and compared with the Log-Rank test. Univariable and multivariable Cox proportional hazards regression models were used to estimate hazard ratios (HR) with 95% confidence intervals (CI) to identify factors associated with overall survival. Factors with a p value < 0.2 in univariable analyses were added to multivariable analyses in a forward stepwise fashion. The study period was divided into two time periods (2010–2012 and 2013–2015), and added to the Cox proportional hazards regression model to correct for historical improvements in outcomes. A two sided p value ≤ 0.05 was considered statistically significant. Data were analyzed using the Statistical Package for Social Sciences (SPSS) version 26 (IBM Corp. Armonk, NY, USA).
Results
In total, 482 patients were included over a period of six years (2010–2015), of whom 342 (71%) underwent open and 140 (29%) laparoscopic resection (Table 1). There was a minor increase in the proportion of laparoscopic resections during the study period: 46% (2010–2012) versus 54% (2013–2015). Academic centers performed less often laparoscopic resections than regional hospitals (24/121 (20%) vs. 111/339 (33%), p = 0.012). Patients undergoing open resection were more often male (58% vs. 43%, p = 0.003) and older (64 vs. 60 years, p = 0.009) compared to patients undergoing laparoscopic resection. Emergency procedures constituted a minority of patients, with a slightly skewed distribution toward more emergencies in the open group: 5% versus 3% obstruction (p = 0.36) and 2% vs. 0% perforation (p = 0.07), respectively.
Table 1.
Patient and pathology characteristics
| Surgery, no. (%)a | |||
|---|---|---|---|
| Characteristics, no. (%) | Open (n = 342) | Laparoscopic (n = 140) | p value |
| Diagnosis year | |||
| 2010–2012 | 169 (49) | 65 (46) | 0.55 |
| 2013–2015 | 173 (51) | 75 (54) | |
| Treatment centerb | 335 | 135 | |
| Regional hospital | 238 (71) | 111 (82) | 0.012 |
| Academic center | 97 (29) | 24 (18) | |
| Sex | |||
| Male | 197 (58) | 60 (43) | 0.003 |
| Age, years, mean (SD) | 64 (12) | 60 (12) | 0.009 |
| Tumor grade: no evaluable | 341 | 138 | |
| Grade 1 | 270 (79) | 110 (80) | 0.90 |
| Grade 2 | 71 (21) | 28 (20) | |
| Clinical TNM classificationb | |||
| registered cT stage | 88 | 33 | < 0.001 |
| cT4 | 26 (30) | 4 (12) | |
| registered cN stage | 259 | 109 | < 0.001 |
| cN1-2 | 165 (64) | 37 (34) | |
| registered cM stage | 341 | 139 | < 0.001 |
| cM1 | 140 (41) | 28 (20) | |
| Pathological T classificationb | 320 | 133 | |
| pT1 | 8 (2) | 20 (15) | < 0.001 |
| pT2 | 31 (10) | 20 (15) | |
| pT3 | 169 (53) | 56 (42) | |
| pT4 | 112 (35) | 37 (28) | |
| Pathological N classificationb | 296 | 123 | |
| pN0 | 45 (15) | 24 (20) | 0.018 |
| pN1 | 198 (67) | 90 (73) | |
| pN2 | 53 (18) | 9 (7) | |
| Pathological M classificationb | |||
| pM1 | 112 (33) | 28 (20) | 0.005 |
| Multifocal tumors | 81 (24) | 19 (14) | 0.014 |
| Size of (largest) primary tumor, mm, mean (SD) | 21 (10) | 18 (10) | 0.007 |
| Lymph nodes | |||
| Number of examined LNs, mean (SD) | 12 (10) | 11 (7) | 0.81 |
| Number of tumor positive LNs, mean (SD) | 3 (4) | 3 (3) | 0.43 |
| Disease stageb | 312 | 124 | |
| Stage I-II | 25 (8) | 17 (14) | 0.012 |
| Stage III | 175 (56) | 79 (64) | |
| Stage IV | 112 (36) | 28 (22) | |
| Resection margin | 300 | 118 | |
| R0 | 244 (81) | 105 (89) | 0.06 |
| R1/2 | 56 (19) | 13 (11) | |
| Conversion ratec | – | 8/22 (36) | – |
| 30-day mortality | 16 (5) | 3 (2) | 0.19 |
aUnless stated otherwise
bData are reported for evaluable cases
cConversion rates were only reported in 2015, during which 22 laparoscopic resections were performed
SD Standard deviation, mm millimeter, LN Lymph node
Patients in the open resection group had a significantly higher clinical stage of NEN with higher proportions of cN1-2 and cM1 stage. Also pathological outcomes were significantly different between the two surgical approaches, with higher pT, pN and pM stages in the open group, as well as a higher percentage of multifocal tumors and larger size of the (largest) primary tumor. A trend toward more positive resection margins in the open resection group was observed (19% vs. 11%, p = 0.06).
Conversion rate was only available for the year 2015, in which 8 of 30 (27%) laparoscopic procedures were converted. Although no strict reasons for conversion were documented, the following outcomes were observed: pT4 tumors were present in 5/8 (63%) patients, multifocal tumors in 3/8 (38%), pN2 lymph node metastases in 2/8 (25%) and R1/2 resection margin in 1/8 (13%) patients. Mean (SD) tumor size was 27 (9) in the converted cases and 21 (9) mm in the non-converted cases (p = 0.10).
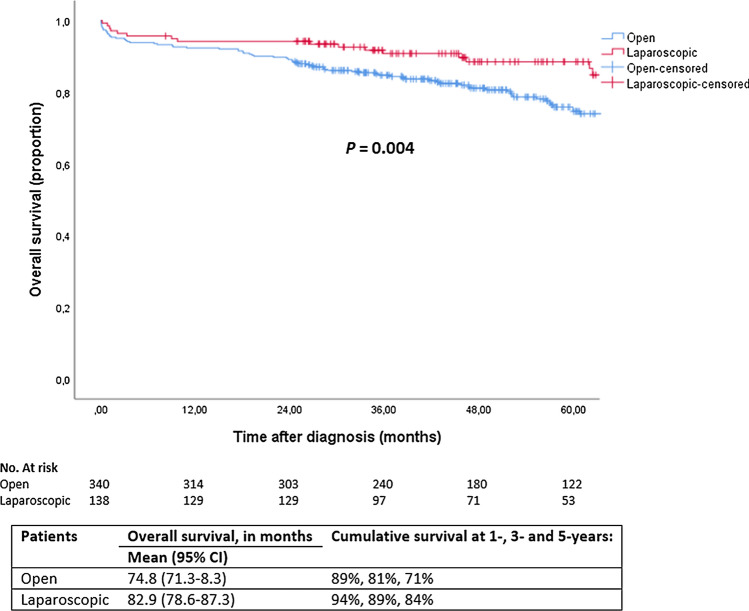
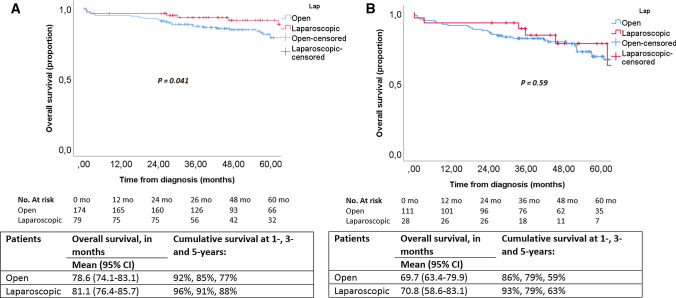
Within 30 days postoperatively, 16 patients (5%) died in the open group and 3 patients (2%) after laparoscopic resection (p = 0.19). Estimated 5-year overall survival of the entire cohort (i.e., patients amenable for resection) was 74%. Without correction for confounders, patients undergoing laparoscopic resection had significantly higher 5-year overall survival rates compared to open resection: 84% versus 71% (p = 0.004), respectively (Fig. 1). Survival rates were also separately analyzed for stage III and stage IV disease (Fig. 2). A statistically significant higher 5-year overall survival was found after laparoscopic surgery in stage III patients (88% vs. 77%; p = 0.041), while there was no significant difference between the two surgical approaches for stage IV (59% vs. 63%; p = 0.59).
Fig. 1.
Overall survival, stratified by surgical approach
Fig. 2.
Overall survival for a stage III, and b stage IV disease, stratified by surgical approach
In univariable analysis, age above 60 years, multifocal tumors, stage IV disease and laparoscopic resection showed an association with overall survival (Table 2). In multivariable analyses, age between 60 and 75 years (HR 3.39, 95% CI [1.85–6.25], p < 0.001) and ≥ 75 years (HR 7.69, 95% CI [3.89–15.18], p < 0.001), stage IV disease (HR 1.89, 95% CI [1.20–2.99], p = 0.006), and laparoscopic resection (HR 0.52, 95% CI [0.28–0.95], p = 0.032) remained significantly associated with overall survival. The results of univariable and multivariable analyses for overall survival are shown in Table 2.
Table 2.
Uni- and multivariable survival analyses of patients with SB-NEN in The Netherlands
| Risk factors | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Diagnosis year | ||||
| 2010–2012 | 1.47 (0.93 − 2.32) | 0.10 | 1.33 (0.82 − 2.15) | 0.25 |
| 2013–2015 | 1 [Reference] | 1 [Reference] | ||
| Sex | ||||
| Male | 1 [Reference] | – | ||
| Female | 0.84 (0.57 − 1.25) | 0.40 | – | |
| Age | ||||
| < 60 | 1 [Reference] | |||
| 60–75 | 3.20 (1.81 − 5.66) | < 0.001 | 3.38 (1.84 − 6.23) | < 0.001 |
| ≥ 75 | 6.86 (3.68 − 12.82) | < 0.001 | 7.63 (3.86 − 15.07) | < 0.001 |
| Multifocal tumors | ||||
| No | 1 [Reference] | 1 [Reference] | ||
| Yes | 1.40 (0.90 − 2.19) | 0.14 | 1.25 (0.78 − 2.00) | 0.35 |
| Disease stage | ||||
| Stage I-II | 1.60 (0.73 − 2.50) | 0.24 | – | |
| Stage III | 1 [Reference] | 1 [Reference] | ||
| Stage IV | 1.96 (1.25 − 3.06) | 0.003 | 1.86 (1.18 − 2.94) | 0.043 |
| Tumor grade | ||||
| Grade 1 | 1 [Reference] | – | ||
| Grade 2 | 1.33 (0.83 − 2.14) | 0.24 | – | |
| Resection margin | ||||
| R0 | 1 [Reference] | – | ||
| R1/2 | 1.16 (0.67 − 1.99) | 0.60 | – | |
| Surgery | ||||
| Open | 1 [Reference] | 1 [Reference] | ||
| Laparoscopic | 0.47 (0.28 − 0.80) | 0.005 | 0.51 (0.28 − 0.94) | 0.032 |
Discussion
The main finding of this nationwide study was that 29% of the patients with SB-NEN were planned for a laparoscopic approach. There was a slight but non-significant increase in laparoscopic resection rate over time. Case selection was clearly seen, with less favorable tumors in patients who underwent open resection, as reflected by significantly higher stage, larger size, and more multifocal tumors. Academic centers performed less laparoscopic resections as compared to regional hospitals, likely reflecting tertiary referral of more advanced cases. With the available variables in the dataset, the association between surgical approach and overall survival was corrected for confounding as much as possible. The multivariable model revealed better overall survival after a laparoscopic approach, with age and stage as the other independent predictors. Potential prognostic factors such as margin status, grade and multifocal tumor location were not found to be associated with overall survival in this patient cohort.
The application of a laparoscopic approach for resection of SB-NEN is mainly determined by the extensiveness of mesenteric lymph node metastases. Ohrvall et al. proposed a classification of these metastases, ranging from resectable stage I (close to the intestine) to irresectable stage IV (retroperitoneal, peri-pancreatic or encasement of the mesenteric artery with involvement of proximal jejunal arteries) [12]. In this study, 38% of lymph node metastases extended along the superior mesenteric artery without encasement, whilst 16% were irresectable. Depending on the laparoscopic experience, one would expect that at least 40% of patients are amenable to a laparoscopic approach based on these data. The 29% laparoscopic resection rate as found in the present study suggests a still restricted application.
The recent European Society of Medical Oncology guideline states that patients with SB-NEN often present in the emergency setting [13]. An emergency resection without prior knowledge of the presence or nature of a small bowel neoplasm might lead to oncologic inferior resections. Interestingly, emergency resections for obstruction or perforation were performed in only a small minority (8%) of this nationwide cohort. This finding suggests that patients might have been offered ‘up-front’ resection to prevent bowel obstruction and/or ischemia [13]. However, the value of ‘up-front’ resection for SB-NEN has been debated in literature, as this is associated with significantly more reoperations, rather than yielding a survival advantage [14].
A common misunderstanding of laparoscopic resection is the inability of palpating the small bowel. However, after completion of the lymph node dissection, almost the entire small bowel can be exteriorized through an umbilical extraction site, enabling meticulous palpation [15]. Identification of multifocal primary disease is regarded a critical step during resection, although recent analyses suggest that the presence of multifocal disease does not affect overall survival [5, 16]. Therefore, we carefully hypothesize that multifocality is not a contraindication for laparoscopic resection. Nevertheless, multifocal primary tumors were more often found in the open resection group, although there might not be a causal relationship, but rather a reflection of case selection and more advanced tumor stage.
The conversion rate (36%) was only reported in the last registration year (2015) and was higher compared to previous studies (25–30%) [15, 17]. In contrast, the conversion rate for laparoscopic D3 lymphadenectomy for colon cancer was 5% in a randomized clinical trial [18]. The substantially higher conversion rate reflects the level of complexity that might be encountered during resections for SB-NEN. The essential difference between central mesenteric lymph node metastases that originate from colon cancer or SB-NEN is related to the infiltrative growth with sometimes extensive mesenteric fibrosis and vascular encasement in the latter tumor type. Likely, D3 lymphadenectomy requires even more skills if performed for SB-NEN than for colon cancer. A handport-assisted laparoscopic procedure can sometimes be an alternative for conversion.
Pedrazzani et al. described a case series of nine patients undergoing laparoscopic right hemicolectomy with complete mesocolic excision for terminal ileum/right colon/appendix NEN [19]. Although it comprises a small cohort, peri-operative and long-term survival outcomes were promising: 1/9 had a Clavien-Dindo grade III complication, no mesenteric locoregional recurrence and all patients with an R0 resection were disease free after a median follow-up of 18 months (range 6–50). One randomized trial of laparoscopic versus open D3 lymphadenectomy has been published in stage II-III colon cancer, which revealed beneficial short-term outcomes for minimally invasive surgery (less blood loss, shorter time to pass first flatus, decreased use of postoperative analgesics and shorter hospital stay), without compromising 5-year overall survival [18, 20]. Interestingly, a recent meta-analyses that pooled the results of this trial with comparative cohort series, suggested that laparoscopic resection was even associated with better oncological outcomes for colon cancer [21], similar to the present study. However, the methodological issues of non-randomized comparisons do not allow for definitive conclusions on this observed association. There is a high risk of bias, and laparoscopic resection might just be a reflection of treatment by more specialized surgeons in dedicated centers with optimized peri-operative care.
Long-term nationwide population-based data were used for this study, making it more representative than cohort studies. However, the findings of this study should be seen in light of some limitations. The NCR database is primarily focused on oncologic characteristics, which limits analysis of (peri-) operative characteristics (indication, conversion rate, postoperative morbidity) and imaging data such as postoperative CT or PET scanning to assess the completeness of mesenteric lymphadenectomy. Time to recurrence, which is a relevant marker of “surgical success” in regard to lymphadenectomies, could not be reported, as recurrent disease is often not diagnosed with biopsies. The most important missing data concern the reasons for choosing an open or laparoscopic approach and whether surgery was performed in the emergency setting.
We propose that guidelines should adapt their recommendations regarding selection criteria for laparoscopic resection, for example using the classification as proposed by Ohrvall et al. [12]. It seems that more SB-NENs are eligible for laparoscopic resection, especially in hands of colorectal surgeons with experience in D3 lymphadenectomies. Also, less emphasis on multifocality as a reasons not to perform laparoscopic resection should be given.
Further work is required to establish the role of laparoscopic resection for SB-NEN. Ideally, this would be a multicenter international randomized clinical trial with stratification for extent of lymph node metastases. However, such a trial would be challenging because of the rarity of the disease and potential lack of equipoise. To overcome this issue, a prospective international cohort study with clearly documented inter-hospital variability in surgical practice could also provide relevant data. To really be of added value, such a study should include variables that reflect “surgical success”, which are part of standard follow-up protocols (pathology reports of recurrent lesions, blood tests, PET/CT) [3].
In conclusion, this study showed that laparoscopic resection of SB-NENs was performed in 29% of patients in the Netherlands. Current data do not raise major concern regarding oncologic adequacy of laparoscopic resection in selected cases.
Acknowledgement
The authors thank IKNL, NCR and PALGA for providing data.
Declarations
Conflict of interest
The authors have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Raghav K, Overman MJ. Small bowel adenocarcinomas–existing evidence and evolving paradigms. Nat Rev Clin Oncol. 2013;10:534–544. doi: 10.1038/nrclinonc.2013.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335–1342. doi: 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niederle B, Pape UF, Costa F, Gross D, Kelestimur F, Knigge U, Oberg K, Pavel M, Perren A, Toumpanakis C, O'Connor J, O'Toole D, Krenning E, Reed N, Kianmanesh R. ENETS consensus guidelines update for neuroendocrine neoplasms of the jejunum and ileum. Neuroendocrinology. 2016;103:125–138. doi: 10.1159/000443170. [DOI] [PubMed] [Google Scholar]
- 4.Kacmaz E, Heidsma CM, Besselink MGH, Dreijerink KMA, Klumpen HJ, Nieveen van Dijkum EJM, Engelsman AF. Treatment of liver metastases from midgut neuroendocrine tumours: a systematic review and meta-analysis. J Clin Med. 2019;8:403. doi: 10.3390/jcm8030403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howe JR, Cardona K, Fraker DL, Kebebew E, Untch BR, Wang YZ, Law CH, Liu EH, Kim MK, Menda Y, Morse BG, Bergsland EK, Strosberg JR, Nakakura EK, Pommier RF. The surgical management of small bowel neuroendocrine tumors: consensus guidelines of the north american neuroendocrine tumor society. Pancreas. 2017;46:715–731. doi: 10.1097/MPA.0000000000000846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Negoi I, Hostiuc S, Negoi RI, Beuran M. Laparoscopic vs open complete mesocolic excision with central vascular ligation for colon cancer: a systematic review and meta-analysis. World J Gastrointest Oncol. 2017;9:475–491. doi: 10.4251/wjgo.v9.i12.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casparie M, Tiebosch AT, Burger G, Blauwgeers H, van de Pol A, van Krieken JH, Meijer GA. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol. 2007;29:19–24. doi: 10.1155/2007/971816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin LH, Parkin DM, Whelan SL, World Health O (2000) International classification of diseases for oncology , 3rd ed. World Health Organization, Geneva
- 10.Korse CM, Taal BG, van Velthuysen ML, Visser O. Incidence and survival of neuroendocrine tumours in the Netherlands according to histological grade: experience of two decades of cancer registry. Eur J Cancer. 2013;49:1975–1983. doi: 10.1016/j.ejca.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 11.Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR. AJCC Cancer Staging Manual. Cham: Springer International Publishing; 2017. [Google Scholar]
- 12.Öhrvall U, Eriksson B, Juhlin C, Karacagil S, Rastad J, Hellman P, Åkerström G. Method for dissection of mesenteric metastases in mid-gut carcinoid tumors. World J Surg. 2000;24:1402–1408. doi: 10.1007/s002680010232. [DOI] [PubMed] [Google Scholar]
- 13.Pavel M, Öberg K, Falconi M, Krenning EP, Sundin A, Perren A, Berruti A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:844–860. doi: 10.1016/j.annonc.2020.03.304. [DOI] [PubMed] [Google Scholar]
- 14.Daskalakis K, Karakatsanis A, Hessman O, Stuart HC, Welin S, Tiensuu Janson E, Öberg K, Hellman P, Norlén O, Stålberg P. Association of a prophylactic surgical approach to stage IV small intestinal neuroendocrine tumors with survival. JAMA Oncol. 2018;4:183–189. doi: 10.1001/jamaoncol.2017.3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaçmaz E, Van Eeden S, Koppes JCC, Klümpen HJ, Bemelman WA, Nieveen van Dijum EJM, Engelsman AF, Tanis PJ (2020) Value of laparoscopy for resection of small bowel neuroendocrine neoplasms including central mesenteric lymphadenectomy. Dis Colon Rectum [DOI] [PubMed]
- 16.Gangi A, Siegel E, Barmparas G, Lo S, Jamil LH, Hendifar A, Nissen NN, Wolin EM, Amersi F. Multifocality in small bowel neuroendocrine tumors. J Gastrointest Surg. 2018;22:303–309. doi: 10.1007/s11605-017-3586-8. [DOI] [PubMed] [Google Scholar]
- 17.Ethun CG, Postlewait LM, Baptiste GG, McInnis MR, Cardona K, Russell MC, Kooby DA, Staley CA, Maithel SK. Small bowel neuroendocrine tumors: a critical analysis of diagnostic work-up and operative approach. J Surg Oncol. 2016;114:671–676. doi: 10.1002/jso.24390. [DOI] [PubMed] [Google Scholar]
- 18.Kitano S, Inomata M, Mizusawa J, Katayama H, Watanabe M, Yamamoto S, Ito M, Saito S, Fujii S, Konishi F, Saida Y, Hasegawa H, Akagi T, Sugihara K, Yamaguchi T, Masaki T, Fukunaga Y, Murata K, Okajima M, Moriya Y, Shimada Y. Survival outcomes following laparoscopic versus open D3 dissection for stage II or III colon cancer (JCOG0404): a phase 3, randomised controlled trial. Lancet Gastroenterol Hepatol. 2017;2:261–268. doi: 10.1016/S2468-1253(16)30207-2. [DOI] [PubMed] [Google Scholar]
- 19.Pedrazzani C, Conti C, Valdegamberi A, Davì MV, Cingarlini S, Scarpa A, Guglielmi A (2020) Is laparoscopic cme right hemicolectomy an optimal indication for net of the right colon and terminal ileum?. J Gastrointest Surg [DOI] [PubMed]
- 20.Yamamoto S, Inomata M, Katayama H, Mizusawa J, Etoh T, Konishi F, Sugihara K, Watanabe M, Moriya Y, Kitano S. Short-term surgical outcomes from a randomized controlled trial to evaluate laparoscopic and open D3 dissection for stage II/III colon cancer: japan clinical oncology group study JCOG 0404. Ann Surg. 2014;260:23–30. doi: 10.1097/SLA.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 21.Chaouch MA, Dougaz MW, Bouasker I, Jerraya H, Ghariani W, Khalfallah M, Nouira R, Dziri C. Laparoscopic versus open complete mesocolon excision in right colon cancer: a systematic review and meta-analysis. World J Surg. 2019;43:3179–3190. doi: 10.1007/s00268-019-05134-4. [DOI] [PubMed] [Google Scholar]