Abstract
Background
Socio-demographics and comorbidities are involved in determining the severity and fatality in patients with COVID-19 suggested by studies in various countries, but study in Bangladesh is insufficient.
Aims
We designed the study to evaluate the association of sociodemographic and comorbidities with the prognosis of adverse health outcomes in patients with COVID-19 in Bangladesh.
Methods
A multivariate retrospective cohort study was conducted on data from 966 RT-PCR positive patients from eight divisions during December 13, 2020, to February 13, 2021. Variables included sociodemographic, comorbidities, symptoms, Charlson comorbidity index (CCI) and access to health facilities. Major outcome was fatality. Secondary outcomes included hospitalization, duration of hospital stay, requirement of mechanical ventilation and severity.
Results
Male (65.8%, 636 of 966) was predominant and mean age was 39.8 ± 12.6 years. Fever (79%), dry cough (55%), and loss of test/smell (51%) were frequent and 74% patients had >3 symptoms. Fatality was recorded in 10.5% patients. Comorbidities were found in 44% patients. Hypertension (21.5%) diabetes (14.6%), and cardiovascular diseases (11.3%) were most prevalent. Age >60 years (OR: 4.83, 95% CI: 2.45–6.49), and CCI >3 (OR: 5.48, 95% CI: 3.95–7.24) were predictors of hospitalizations. CCI >4 (aOR: 3.41, 95% CI: 2.57–6.09) was predictor of severity. Age >60 years (aOR: 3.77, 95% CI: 1.07–6.34), >3 symptoms (aOR: 2.14, 95% CI: 0.97–4.91) and CCI >3 vs. CCI <3 (aOR: 5.23, 95% CI: 3.77–8.09) were independently associated with fatality.
Conclusions
Increased age, >3 symptoms, increasing comorbidities, higher CCI were associated with increased hospitalization, severity and fatality in patients with COVID-19.
Keywords: Comorbidities, Symptoms, Age, Hospitalization, Fatality, COVID-19
1. Introduction
A global public health emergency situation has been occurred by the pandemic called coronavirus disease-2019 (COVID-19) from the first quarter of 2020 [1]. The COVID-19 pandemic was triggered by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) (family-Coronaviridae) from Wuhan, China during December 2020 [[1], [2], [3], [4]]. As of March 05, 2021, about 116 million cases and 2.5 million fatalities associated with COVID-19 have been documented worldwide [5,6]. Bangladesh, a densely populated least developed country, was first hit by the COVID-19 cases during the first week of March 2020. By March 05, 2021, the number of laboratory-confirmed case number has increased to 548549 and the causality to 8435 [[4], [5], [6]].
Individuals with COVID-19 infection develop various complications of the respiratory system [[7], [8], [9], [10], [11]]. About 80–85% patients are diagnosed with mild symptoms including fever, cough, sore throat, chill, feelings of shaking, loss of taste or smell, headache, rash and muscle pain. In severe cases, symptoms like acute pneumonia, acute respiratory syndrome, kidney failure, difficulty in breathing and failure of multiple organs are commonly reported [[7], [8], [9], [10],[11], [12], [13], [14], [15], [16]]. Patients developing severe health conditions require hospitalization, intensive care unit (ICU) admissions and ventilation support to survive [[7], [8], [9], [10],[11], [12], [13], [14], [15], [16]].
Host demographic factors and preexisting health conditions are involved in determining the outcome of COVID-19 [10]. Presence of comorbid diseases in COVID-19 patients have been involved in increased hospitalizations, prolonged hospitalization and fatality in China, USA, Canada, UK, Spain, Portugal and Hong Kong [10,[17], [18], [19], [20], [21], [22]]. Numerous comorbid diseases have increased the mortality in patients with COVID-19. The most frequent comorbidities associated with hospitalization and fatality of COVID-19 patients are hypertension, cardiovascular diseases (CVD), diabetes mellitus, chronic obstructive pulmonary disease (COPD), kidney diseases, liver diseases, cancer, gastrointestinal diseases, tuberculosis and HIV infection [10,[17], [18], [19], [20], [21], [22],[23], [24], [25], [26], [27],[28], [29], [30], [31], [32]]. A research gap is prominent on the impact of demographic factors and comorbidities in Bangladesh involving larger cohort. Therefore, we conducted a nationwide analysis to investigate the impact of sociodemographic factors and comorbidities in determining the health outcome of COVID-19 patients in Bangladesh. The main objective of this study is to determine the impact of socio-demographic factors and comorbidities on hospitalization and severe outcome of patients with COVID-19 stratified in different age groups in Bangladesh. Other objectives of the study are to investigate the prevalence of various clinical manifestations, age and sex distributions, and nationwide case fatality rate of the study cohort.
2. Materials and methods
2.1. Study data, population and regions
A retrospective cohort study was conducted on the study population. Data were collected from patients admitted on different hospitals and clinics from eight divisions including 95% districts (61 of 64) in Bangladesh. Data were collected from patients by using random sampling. The study was conducted from December 13, 2020, to February 13, 2021, and involved 966 patients with COVID-19 infection who participated willingly. Structured questionnaire and electronic data repositories of hospitals and clinics were used as data collection sources. A total of 889 patients were hospitalized. The presence of comorbidity was confirmed by patient report and evaluated following previously published studies [10,11]. Participants with a COVID-19 positive RT-PCR test on nasopharyngeal swab specimens according to the World Health Organization (WHO) interim guidance were included in this study [10]. Definition of cases, symptoms and comorbidities were determined following WHO guidance and previously published works [1,10,11]. Collected data were sorted, evaluated and stratified according to the quality of the data and responses. Informed consents were taken from the participants before collecting data in this study. Appropriate ethical clearance was taken from the Bio-safety, Bio-security and Ethical Committee at Jahangirnagar University and the reference number is BBEC, JU/M 2021/COVID-19/(1)3.
2.2. Variables and outcomes of the study
The variables included demographics, clinical symptoms and comorbidities. Further, socio-demographic variables included age, sex (male vs. female), occupation (student, housewife, employed, businessman, and unemployed, etc.), monthly family income, and current place of residence (village, district town, and divisional town) and access to health care facilities. The COVID-19 associated variables included duration of symptoms, single symptoms, multiple symptoms, and end point result (hospitalization, death or recovery). The comorbidities were included as categorical variables and classified as patients with single, double, triple or more than three comorbidities. Comorbidities were stratified by the systems of organ namely, respiratory, gastrointestinal, heart disease etc. For evaluating the relation of the comorbidities with the ultimate outcome of COVID-19, Charlson Comorbidity Index (CCI) were computed.
The ultimate outcome found in this study was fatality. Other outcomes included hospitalizations, duration of hospitalization, intensive care unit (ICU) requirement and stay period, ventilation requirement and duration.
2.3. Statistical analysis
The sample size used in this study was sufficient to conduct the statistical analysis. Logistic regression analysis was conducted to determine the association of univariate variables with the outcome of COVID-19. Age adjusted odds ratio were applied in the multivariate analysis. Further, inferential statistics (p-value and 95% confidence interval) were applied for both the univariate and multivariate models. Statistical analysis was performed by using International Business Machines (IBM) Statistical Package for the Social Sciences (SPSS) version 26.0 (Chicago, IL, USA) and Microsoft Excel 2019 [19,20].
3. Results
3.1. Socio-demographics and nationwide distribution of the cohort
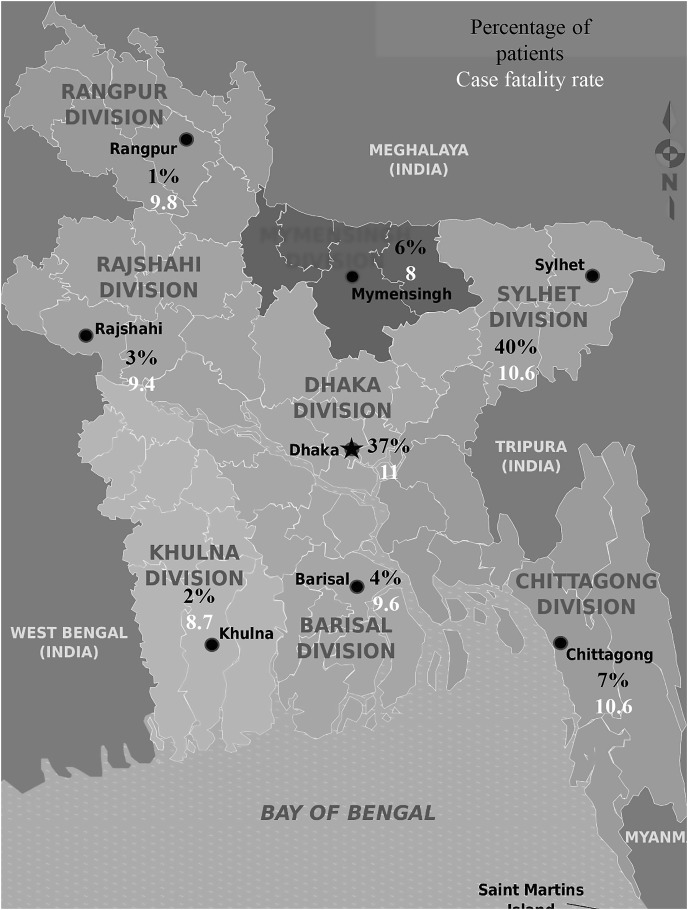
Socio-demographic characteristics of the COVID-19 positive cohort consisting of 966 individuals were analyzed. Male (65.8%, 636 of 966) was predominant sex group followed by female (34.2%, 330 of 966) in the cohort. The study population was distributed in eight age groups including 0–9 years, 10–19 years, 20–29 years, 30–39 years, 40–49 years, 50–59 years, 60–69 years and above 70 years. Most of the patients were confined in age group 20–29 years (33%, mean ± SD: 27.7 ± 11.9 years). The mean ± SD age of the total cohort was 39.8 ± 12.6 years. Most of the cases (84.8%) were detected in urban areas. About 4.6% of the study population was doctors and health workers (Supplementary Table 1). The highest frequency of cases was detected from Sylhet division (40%) followed by Dhaka (37%) and Chittagong (7%) (Fig. 1 ). Case fatality rate was the highest in Dhaka (11 persons per hundred cases) followed by Sylhet (10.6), Chittagong (10.6) and Rangpur (9.8), respectively (Fig. 1).
Fig. 1.
Nationwide distribution of COVID-19 cases and case fatality rates in Bangladesh.
3.2. Clinical manifestations and comorbidities analysis
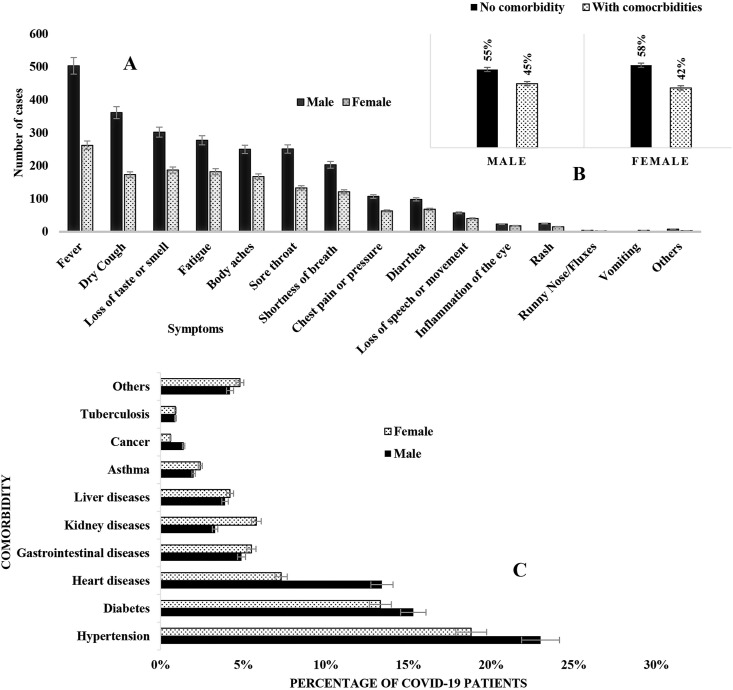
About 100% of the patients developed one or multiple symptoms of COVID-19. Among symptoms of COVID-19, fever (79%) was the most predominant followed by dry cough (55%), loss of test or smell (51%), fatigue (47%), and body aches (43%), respectively (Table 1 ). In cross symptoms analysis, fever + dry cough (479 cases) was the most frequent followed by fever + loss of smell/test (445 cases), fever + fatigue (414 cases), fever + body aches (386 cases) and fever + sore throat (361 cases), respectively. Rare symptoms included pain in ear in 1 case, excessive sweating in 1 case and constipation in 1 case. About 17% of the patients had 3 symptoms, followed by 4 symptoms (16%), 5 symptoms (13%), 6 symptoms (12%), 7 symptoms (6%), 8 symptoms (5%) and more than 8 symptoms (5%) related to COVID-19, respectively.
Table 1.
Age stratified clinical manifestations of patients with COVID-19 in Bangladesh.
| Variables | Age group in years (%) |
P value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–9 | 10–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | >70 | ||
| Total number, N | 5 | 51 | 319 | 205 | 161 | 139 | 63 | 23 | .027 |
| Symptoms | 4 (80%) | 39 (76.5%) | 239 (74.9%) | 164 (80%) | 127 (78.9%) | 119 (85.6%) | 52 (82.5%) | 21 (91.3%) | .014 |
| Fever | |||||||||
| Dry Cough | 4 (80%) | 23 (45.1%) | 153 (48%) | 126 (61.5%) | 89 (55.3%) | 88 (63.3%) | 39 (61.9%) | 12 (52.2%) | .043 |
| Loss of taste or smell | 1 (20%) | 28 (54.9%) | 162 (50.8%) | 106 (51.7%) | 85 (52.8%) | 72 (51.8%) | 26 (41.3%) | 9 (39.1%) | .541 |
| Fatigue | 2 (40%) | 24 (47.1%) | 155 (48.6%) | 99 (48.3%) | 69 (42.9%) | 68 (48.9%) | 32 (50.8%) | 10 (43.5%) | .674 |
| Body aches | 0 (0%) | 26 (51%) | 133 (41.7%) | 80 (39%) | 73 (45.3%) | 64 (46%) | 27 (42.9%) | 14 (60.9%) | .034 |
| Sore throat | 1 (20%) | 20 (39.2%) | 116 (36.4%) | 83 (40.5%) | 65 (40.4%) | 68 (48.9%) | 25 (39.7%) | 6 (26.1%) | .061 |
| Shortness of breath | 0 (0%) | 14 (27.5%) | 85 (26.6%) | 58 (28.3%) | 55 (34.2%) | 59 (42.4%) | 37 (58.7%) | 16 (69.6%) | .011 |
| Chest pain or pressure | 0 (0%) | 10 (19.6%) | 43 (13.5%) | 29 (14.1%) | 27 (16.8%) | 36 (25.9%) | 16 (25.4%) | 9 (39.1%) | .042 |
| Diarrhea | 0 (0%) | 6 (11.8%) | 52 (16.3%) | 34 (16.6%) | 31 (19.3%) | 25 (18%) | 12 (19%) | 6 (26.1%) | .013 |
| Loss of speech or movement | 1 (20%) | 5 (9.8%) | 31 (9.7%) | 12 (5.9%) | 13 (8.1%) | 18 (12.9%) | 11 (17.5%) | 6 (26.1%) | .027 |
| Inflammation of the eye | 0 (0%) | 4 (7.8%) | 16 (5%) | 5 (2.4%) | 3 (1.9%) | 6 (4.3%) | 5 (7.9%) | 2 (8.7%) | .062 |
| Rash | 0 (0%) | 5 (9.8%) | 8 (2.5%) | 6 (2.9%) | 5 (3.1%) | 7 (5%) | 3 (4.8%) | 6 (26.1%) | .041 |
| Runny nose/Fluxes | 0 (0%) | 1 (2%) | 1 (0.3%) | 2 (1%) | 0 (0%) | 1 (0.7%) | 0 (0%) | 1 (4.3%) | .083 |
| Vomiting | 0 (0%) | 0 (0%) | 1 (0.3%) | 3 (1.5%) | 0 (0%) | 1 (0.7%) | 0 (0%) | 0 (0%) | .493 |
| Others | 0 (0%) | 1 (2%) | 2 (0.6%) | 2 (1%) | 3 (1.9%) | 1 (0.7%) | 2 (3.2%) | 0 (0%) | .792 |
| No Symptoms | 0 (0%) | 1 (2%) | 4 (1.3%) | 3 (1.5%) | 0 (0%) | 6 (4.3%) | 1 (1.6%) | 0 (0%) | .137 |
In the cohort, 44% (425 of 966) had one or multiple comorbidities. Among the comorbidities, hypertension (208 cases) was the most prevalent followed diabetes (141 cases), cardiovascular diseases (CVDs) (109 cases), gastrointestinal diseases (49 cases), kidney diseases (40 cases), liver diseases (39 cases), asthma (21 cases) and cancer (11 cases), respectively (Table 2 ). About 22% of the COVID-19 patients had more than one comorbidities. In multiple comorbidity analysis, hypertension + diabetes (76 cases), hypertension + CVDs (76 cases) were predominant, followed by diabetes + CVDs (57 cases), hypertension + kidney diseases (27 cases) and CVDs + kidney diseases (20 cases), respectively.
Table 2.
Distribution of comorbidities in various age group of patients with COVID-19 positive.
| Variables | Age group in years (%) |
P value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–9 | 10–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | Above 70 | ||
| Total, N | 5 | 51 | 319 | 205 | 161 | 139 | 63 | 23 | .011 |
| Comorbidities | 0 (0%) | 3 (5.9%) | 21 (6.6%) | 29 (14.1%) | 50 (31.1%) | 58 (41.7%) | 36 (57.1%) | 11 (47.8%) | .05 |
| Hypertension | |||||||||
| Diabetes | 0 (0%) | 0 (0%) | 4 (1.3%) | 10 (4.9%) | 34 (21.1%) | 48 (34.5%) | 32 (50.8%) | 13 (56.5%) | .001 |
| Heart diseases | 0 (0%) | 0 (0%) | 7 (2.2%) | 6 (2.9%) | 25 (15.5%) | 37 (26.6%) | 23 (36.5%) | 11 (47.8%) | .024 |
| Gastrointestinal diseases | 0 (0%) | 3 (5.9%) | 17 (5.3%) | 5 (2.4%) | 8 (5%) | 9 (6.5%) | 4 (6.3%) | 3 (13%) | .041 |
| Kidney diseases | 0 (0%) | 2 (3.9%) | 2 (0.6%) | 4 (2%) | 6 (3.7%) | 8 (5.8%) | 10 (15.9%) | 8 (34.8%) | .033 |
| Liver diseases | 0 (0%) | 0 (0%) | 6 (1.9%) | 6 (2.9%) | 13 (8.1%) | 6 (4.3%) | 5 (7.9%) | 3 (13%) | .001 |
| Asthma | 0 (0%) | 1 (2%) | 6 (1.9%) | 8 (3.9%) | 3 (1.9%) | 2 (1.4%) | 1 (1.6%) | 0 (0%) | .496 |
| Cancer | 0 (0%) | 0 (0%) | 2 (0.6%) | 0 (0%) | 1 (0.6%) | 1 (0.7%) | 3 (4.8%) | 4 (17.4%) | .731 |
| Tuberculosis | 0 (0%) | 2 (3.9%) | 7 (2.2%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | .435 |
| Others | 0 (0%) | 2 (3.9%) | 14 (4.4%) | 6 (2.9%) | 7 (4.3%) | 6 (4.3%) | 3 (4.8%) | 5 (21.7%) | .142 |
| No comorbidity | 5 (100%) | 40 (78.4%) | 252 (79%) | 135 (65.9%) | 65 (40.4%) | 39 (28.1%) | 4 (6.3%) | 1 (4.3%) | .019 |
| ∗With Comorbidity | 0 (0%) | 11 (21.6%) | 67 (21%) | 70 (34.1%) | 96 (59.6%) | 100 (71.9%) | 59 (93.7%) | 22 (95.7%) | .004 |
3.3. Distribution of symptoms and comorbidities
The frequency of fever was highest in age group above 70 years (91%), dry cough in 0–9 years (80%), loss of smell or taste in 10–19 years (55%) fatigue in 60–69 years (51%), shortness of breath, chest pain and loss of movement in above 70 years (70%, 39% and 26%) were highest in patients (Table 1). Fever was the most predominant symptoms in female patients (79.4%) followed by loss of taste/smell (57%) (Fig. 2 ). However, in male group, fever (79%) was followed by dry cough (57%) (Fig. 2). The frequency of comorbidities was highest in male (58%) followed by female (42%). Patients aged above 70 years had the highest frequency of comorbidities (96%), followed by age group 60–69 years (94%), 50–59 years (72%) and 40–49 years (60%), respectively (Table 2). Hypertension (55%, 47 of 86), diabetes (52%, 45 of 86) and heart diseases (39%, 34 of 86) were most prevalent in COVID-19 patients aged above 60 years, while gastrointestinal diseases was most frequent in patients aged 20–29 years. The frequency of gastrointestinal diseases (5.5%), liver diseases (4.2%), kidney diseases (5.8%) and asthma (2.4%) were higher in female than male (Fig. 2).
Fig. 2.
Sex-wise prevalence distribution of A. Clinical manifestations and B–C. Comorbidities in COVID-19 positive cohort.
3.4. Outcomes of patients with COVID-19
Overall case fatality rate was 10.5 in the study cohort. The frequency of mortality was highest in patients aged above 70 years (mean ± SD: 78.4 ± 4.3 years) (61%, 14 of 23), followed by 60–69 years (66.1 ± 5.9) (36.5%, 23 of 63) and 50–59 years (58.4 ± 8.9) (18%, 25 of 139), respectively (Table 3 ). Fatality was most frequent in patients with symptoms prevailing for mean ± SD of 11.6 ± 2.3 days. Hospitalization required for about 92% (889 of 966) patients. The mean length of hospitalization was 8.9 ± 2.7 days. Admission to intensive care unit was required in 284 (29.4%) patients. Supplemental oxygen was provided to 496 (51%) patients, and 109 (11.3%) patients was given mechanical ventilation support for a mean of 6 ± 2.4 days. About 193 (20%) of the patients faced problems to receive treatment for preexisting comorbidities during the pandemic (Table 3).
Table 3.
Age stratified analysis on gender distribution, symptoms and fatalities of nationwide patients with COVID-19 in Bangladesh.
| Variables |
Age groups in years (%) |
P value |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0–9 |
10–19 |
20–29 |
30–39 |
40–49 |
50–59 |
60–69 |
>70 |
|||
| Total number | 5 | 51 | 319 | 205 | 161 | 139 | 63 | 23 | .041 | |
| Sex | Male | 2 (40%) | 24 (47.1%) | 214 (67.1%) | 143 (69.8%) | 99 (61.5%) | 89 (64%) | 47 (74.6%) | 18 (78.3%) | .003 |
| Female | 3 (60%) | 27 (52.9%) | 105 (32.9%) | 62 (30.2%) | 62 (38.5%) | 50 (36%) | 16 (25.4%) | 5 (21.7%) | .014 | |
| Duration of symptoms | 7–14 days | 5 (100%) | 36 (70.6%) | 219 (68.7%) | 98 (47.8%) | 82 (50.9%) | 68 (48.9%) | 30 (47.6%) | 10 (43.5%) | .001 |
| 15–28 days | 0 (0%) | 10 (19.6%) | 92 (28.8%) | 96 (46.8%) | 69 (42.9%) | 55 (39.6%) | 25 (39.7%) | 10 (43.5%) | .005 | |
| 1–2 months | 0 (0%) | 4 (7.8%) | 8 (2.5%) | 8 (3.9%) | 10 (6.2%) | 14 (10.1%) | 8 (12.7%) | 3 (13%) | .012 | |
| > 2 months | 0 (0%) | 1 (2%) | 0 (0%) | 3 (1.5%) | 0 (0%) | 2 (1.4%) | 0 (0%) | 0 (0%) | .034 | |
| Comorbidities (before COVID-19) | Yes | 0 (0%) | 11 (21.6%) | 50 (15.7%) | 31 (15.1%) | 65 (40.4%) | 61 (43.9%) | 37 (58.7%) | 16 (69.6%) | .001 |
| No | 5 (100%) | 40 (78.4%) | 269 (84.3%) | 174 (84.9%) | 96 (59.6%) | 78 (56.1%) | 26 (41.3%) | 7 (30.4%) | .017 | |
| Comorbidities (after COVID-19) | Yes | 0 (0%) | 6 (11.8%) | 41 (12.9%) | 20 (9.8%) | 23 (14.3%) | 33 (23.7%) | 16 (25.4%) | 7 (30.4%) | .043 |
| No | 5 (100%) | 45 (88.2%) | 278 (87.1%) | 185 (90.2%) | 138 (85.7%) | 106 (76.3%) | 47 (74.6%) | 16 (69.6%) | .022 | |
| Symptoms of other disease increased after COVID-19 infection | Yes | 0 (0%) | 8 (15.7%) | 64 (20.1%) | 56 (27.3%) | 59 (36.6%) | 58 (41.7%) | 23 (36.5%) | 10 (43.5%) | .005 |
| No | 5 (100%) | 43 (84.3%) | 255 (79.9%) | 149 (72.7%) | 102 (63.4%) | 81 (58.3%) | 40 (63.5%) | 13 (56.5%) | .049 | |
| Treatment problems during COVID-19 | Yes | 0 (0%) | 11 (21.6%) | 60 (18.8%) | 33 (16.1%) | 27 (16.8%) | 45 (32.4%) | 14 (22.2%) | 8 (34.8%) | .019 |
| No | 5 (100%) | 40 (78.4%) | 259 (81.2%) | 172 (83.9%) | 134 (83.2%) | 94 (67.6%) | 49 (77.8%) | 15 (65.2%) | .348 | |
| Death due to COVID-19 | Yes | 0 (0%) | 2 (3.9%) | 22 (6.9%) | 4 (2%) | 11 (6.8%) | 25 (18%) | 23 (36.5%) | 14 (60.9%) | .002 |
| No | 5 (100%) | 49 (96.1%) | 297 (93.1%) | 201 (98%) | 150 (93.2%) | 114 (82%) | 40 (63.5%) | 9 (39.1%) | .043 | |
In hospitalization tendency, higher odds were detected in patients aged >60 years (OR: 4.83, 95% CI: 2.45–6.49), symptoms prevailing >14 days (OR: 4.12, 95% CI: 2.16–6.34), with higher income (OR: 1.45, 95% CI: 0.81–2.44) and residing in urban areas (OR: 1.78, 95% CI: 0.85–3.15) with better medical facilities (OR: 1.92, 95% CI: 0.48–1.93) (Table 4 a). Patients with comorbidities had greater odds (OR: 5.86, 95% CI: 3.47–7.93) for hospitalization. Higher odds of hospitalization were present in patients with higher Charlson Comorbidity Index (CCI), CCI >3 (OR: 5.48, 95% CI: 3.95–7.24) with diabetes (OR: 2.83, 95% CI: 1.54–3.96), heart disease (OR: 2.44, 95% CI: 1.82–3.13) and hypertension (OR: 2.16, 95% CI: 1.41–3.53) (Table 4a).
Table 4a.
Logistic regression analyses of different variables to determine their role in hospitalizations of patients with COVID-19.
| Univariate analysis | OR (95% CI) | P value |
|---|---|---|
| Gender | 1.21 (0.72–1.64) | .001 |
| Age: >60 years | 4.83 (2.45–6.49) | .004 |
| Residence: Urban areas | 1.78 (0.85–3.15) | .023 |
| Better access to health facilities | 1.92 (0.48–1.93) | .041 |
| High income | 1.45 (0.81–2.44) | .976 |
| Symptoms prevailing >14 days | 4.12 (2.16–6.34) | .001 |
| Comorbidity | ||
| With comorbidities | 5.86 (3.47–7.93) | .003 |
| CCI >3 vs. CCI <3 | 5.48 (3.95–7.24) | .001 |
| Hypertension | 2.16 (1.41–3.53) | .024 |
| Diabetes | 2.83 (1.54–3.96) | .004 |
| Heart diseases | 2.44 (1.82–3.13) | .016 |
| Gastrointestinal diseases | 1.12 (0.76–1.97) | .037 |
| Kidney diseases | 1.67 (0.93–2.11) | .001 |
| Liver diseases | 1.27 (0.87–2.43) | .049 |
| Asthma | 1.94 (0.97–2.77) | .034 |
| Cancer | 1.19 (0.78–2.46) | .041 |
| Tuberculosis | 1.35 (0.93–2.27) | .097 |
| COPD | 1.79 (0.62–3.76) | .004 |
| Lung disease | 1.94 (1.02–3.83) | .016 |
| Paralysis | 1.21 (0.82–2.17) | .011 |
| Typhoid | 1.01 (0.43–1.73) | .294 |
| Dengue | 1.18 (0.62–2.01) | .068 |
| Arthritis | 1.09 (0.72–1.81) | .043 |
| Anemia | 1.28 (0.68–2.31) | .438 |
| Hypothyroidism | 1.15 (0.76–2.28) | .001 |
| Surgical infection | 1.19 (0.79–2.0) | .000 |
P value < .05 was considered statistically significant. OR-odds ratio, COPD-chronic obstructive pulmonary disease, CCI- Charlson comorbidity index.
Both multivariate and univariate analysis were conducted to determine the roles of variables on mortality. Greater odds of association of mortality were present in patients aged >60 years (OR: 5.43, 95% CI: 2.85–7.68) with symptoms prevailing >14 days (OR: 2.92, 95% CI: 1.43–4.19), comorbidities (OR: 4.62, 95% CI: 3.09–6.33) and CCI >3 vs. CCI <3 (OR: 5.98, 95% CI: 3.65–7.63) (Table 4 b). In multivariate analysis CCI was used to replace comorbidities. Independent predictors of mortality were patients aged >60 years (aOR: 3.77, 95% CI: 1.07–6.34), >3 symptoms of COVID-19 (aOR: 2.14, 95% CI: 0.97–4.91), symptoms persisting >14 days (aOR: 2.34, 95% CI: 0.81–4.63), and CCI >3 vs. CCI <3 (aOR: 5.23, 95% CI: 3.77–8.09) (Table 4b). However, better access to health facilities (aOR: 0.73, 95% CI: 0.22–1.96) had lower odds of association with mortality.
Table 4b.
Logistic regression analyses (both univariate and multivariate) of different variables to determine their role in fatality of patients with COVID-19 in Bangladesh.
| Univariate analysis | ||
|---|---|---|
| Variables | OR (95% CI) | P value |
| Gender | 1.84 (0.91–2.94) | .003 |
| Age: >60 years | 5.43 (2.85–7.68) | .001 |
| Urban areas vs. village areas | 1.38 (0.65–2.86) | .037 |
| Better access to health facilities | 0.71 (0.39–1.61) | .346 |
| High income | 1.83 (0.76–2.21) | .067 |
| Symptoms prevailing >14 days | 2.92 (1.43–4.19) | .008 |
| Comorbidity | ||
| With comorbidities vs without comorbidities | 4.62 (3.09–6.33) | .005 |
| CCI >3 vs. CCI <3 | 5.98 (3.65–7.63) | .011 |
| Hypertension | 2.04 (1.41–3.53) | .004 |
| Diabetes | 2.57 (1.42–3.83) | .009 |
| Heart diseases | 2.92 (1.71–4.34) | .029 |
| Gastrointestinal diseases | 1.16 (0.67–1.73) | .391 |
| Kidney diseases | 1.39 (0.75–2.30) | .005 |
| Liver diseases | 1.34 (0.92–2.49) | .019 |
| Asthma | 1.51 (0.73–2.82) | .008 |
| Cancer | 1.20 (0.63–2.16) | .001 |
| Tuberculosis | 1.45 (0.81–2.67) | .004 |
| COPD | 2.19 (0.97–3.82) | .342 |
| Lung disease | 2.64 (1.54–4.03) | .005 |
| Paralysis | 1.29 (0.76–2.67) | .014 |
| Typhoid | 1.11 (0.63–2.49) | .037 |
| Dengue | 1.62 (0.82–2.76) | .001 |
| Arthritis | 1.72 (0.64–2.93) | .006 |
| Anemia | 1.39 (0.91–2.86) | .277 |
| Hypothyroidism | 1.05 (0.46–2.18) | .160 |
| Surgical infection | 1.23 (0.53–2.61) | .005 |
| More than three symptoms |
1.84 (0.92–3.84) |
.006 |
| Multivariate analysis | ||
|
Variables |
Adjusted OR |
P value |
| Age: >60 years vs. <60 years | 3.77 (1.07–6.34) | .007 |
| Urban areas vs. village areas | 2.04 (0.83–4.26) | .001 |
| Better access to health facilities vs. worse access to health facilities | 0.73 (0.22–1.96) | .043 |
| High income vs. low income | 1.19 (0.61–3.57) | .009 |
| Symptoms prevailing >14 days vs. <14 days | 2.34 (0.81–4.63) | .004 |
| CCI >3 vs. CCI <3 | 5.23 (3.77–8.09) | .001 |
| Symptoms >3 vs. <3 | 2.16 (0.97–4.91) | .035 |
P value < .05 was considered statistically significant. OR-odds ratio, COPD-chronic obstructive pulmonary disease, CCI- Charlson comorbidity index.
3.5. Prognostic analysis by age, sex and Charlson comorbidity index
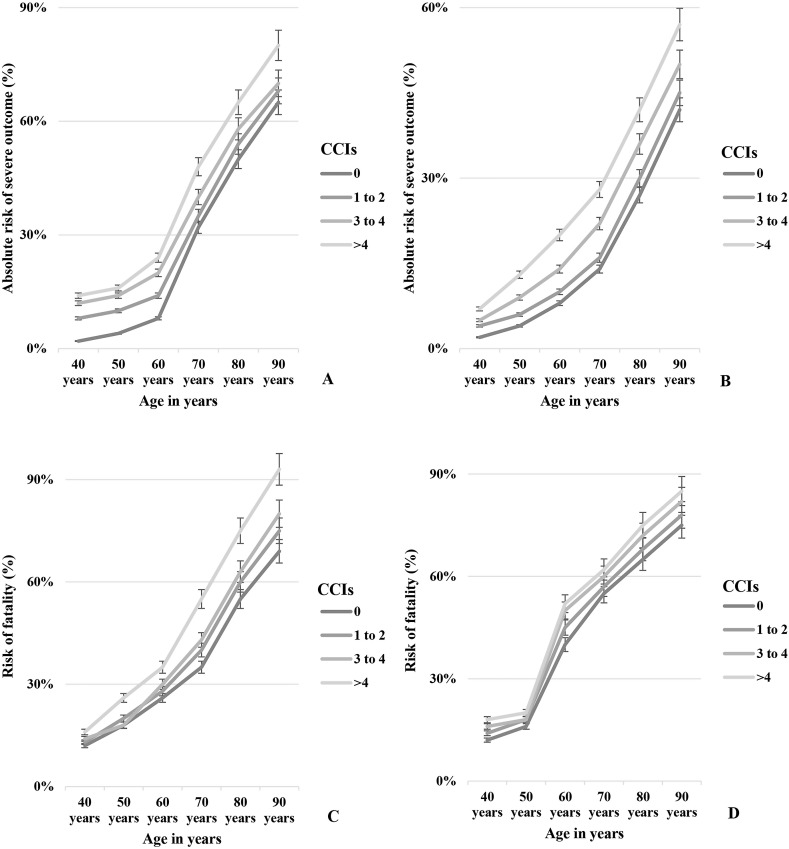
Four groups of CCI including CCI 0, CCI 1–2, 3–4, and >4 were evaluated for the association with severe outcome and mortality in COVID-19 patients. About 41 (4%) patients developed severe outcome and 12 (12%) died in CCI 0 group, 72 (7%), 91 (9%), and 64 (6%) patients had severe outcome in the CCI 1–2, 3–4, and >4 groups, respectively. About 39 (38%), 36 (36%), and 14 (14%) patients died in CCI 1–2, 3–4, and >4 groups, respectively. The highest odds of severe COVID-19 outcome were present in CCI >4 (aOR: 3.41, 95% CI: 2.57–6.09), followed by CCI 3–4 (aOR: 2.96, 95% CI: 2.01–5.39), CCI 1–2 (aOR: 2.39, 95% CI: 2.11–5.59) and CCI 0 (aOR: 1.35, 95% CI: 0.83–2.28), respectively (Fig. 3 ). The odds of fatality were most significant in group CCI >4 (aOR: 4.22, 95% CI: 2.92–6.89), followed by CCI 3–4 (aOR: 3.67, 95% CI: 2.98–5.78), CCI 1–2 (aOR: 2.89, 95% CI: 2.11–5.59) and CCI 0 (aOR: 1.93, 95% CI: 0.98–2.76), respectively. The absolute risks of severity associated with COVID-19 and fatality exacerbated gradually from CCI 0 to CCI 1–2, 3–4, and >4 in both sex with increased age (Fig. 3).
Fig. 3.
Estimated absolute risks of sever outcomes of COVID-19 patients in relation with age and Charlson Comorbidity Index (CCI) in A. Male and B. Female. Trends of risks of fatality of COVID-19 patients in relation with age and CCI in C. Male and D. Female.
4. Discussions
The health burden of COVID-19 is still on the rise globally. The preexisting health conditions of the patients infected with COVID-19 are involved in determining the clinical manifestations and outcome of the infection [10,11,19,20]. This is the first nationwide study that comprehensively investigates the association and impact of comorbid diseases and health conditions on the severity, hospitalization and prognosis in patients with COVID-19 in Bangladesh. Hypertension (21.5%), diabetes (14.6%) and cardiovascular diseases (CVDs) (11.3%) were the most common comorbidities among patients infected with COVID-19. The severity of the clinical outcomes in patients exacerbated with the increasing number of comorbidities. Findings in this study including a large sample size from every regions in Bangladesh have rendered equitable proof for considering common comorbidities in the comprehensive risk assessment of patients with COVID-19. The prevalence and impact of comorbidities on the outcome among patients with COVID-19 published in recent studies were reflected by the findings of our study [10,11,19,20,[22], [23], [24], [25], [26], [27], [28], [29], [30],[32], [33], [34], [35], [36], [37], [38],[39], [40], [41], [42], [43], [44]].
In this cohort of COVID-19 patients 65.8% were male and the mean ± SD age of patients was 39.8 ± 12.6 years. Lower mean ± SD age than previously published studies reflected that the frequency of young patients is higher in Bangladesh [10,11,19,20]. About 84.8% cases were reported from urban areas and the highest case-fatality rate was detected in Dhaka with high population density. The tendency of hospitalization increased with the increased availability of health facilities and monthly family income of the cohort. Overall, male aged above 50 years with >3 symptoms, at least one or more comorbidities, with difficulties in getting regular treatment of comorbid diseases during the pandemic were reported with increased hospitalization and severe outcome of COVID-19. These findings are in good agreement with the previous studies conducted in USA, China, UK and Spain [10,11,[19], [20], [21], [22], [23], [24], [25],[26], [27], [28], [29], [30], [31], [32], [33], [34], [35],[36], [37], [38], [39], [40],[41], [42], [43], [44]]. More than twenty clinical manifestations associated with COVID-19 were evaluated in this study. Fever (79%) was the most prevalent followed by dry cough (55%) and loss of test or smell (51%) among the patients in the cohort and 74% patients had >3 symptoms. Regardless to the size of the cohort and duration of the study, the findings on symptoms echoed the previously conducted studies [1,4,10,11,19,20].
About 44% patients with COVID-19 had comorbidities. Among the patients 15% diagnosed with comorbidities after getting COVID-19. The circulatory diseases including hypertension and cardiovascular diseases were the most common comorbidities (33%) in the study population and endocrine diseases like diabetes was also prevalent in the cohort. Regardless of sample size and study regions hypertension, CVDs and diabetes were most common among comorbidities in patients with COVID-19 [[1], [2], [3],10,11,[11], [19], [20], [21], [22], [23], [24], [25],[26], [27], [28], [29], [30], [31]]. At least two and more than two comorbidities were detected in 22% of the patients and gastrointestinal disease, liver and kidney problems were prevalent after hypertension, CVDs and diabetes. These findings on liver and kidney comorbidities were inconsistent from previous studies as the frequency were higher [10,11,[34], [35], [36], [37]]. People in least developed countries like Bangladesh suffer severely with liver and kidney problems due to the unhealthy living conditions and poor nutrients and contaminated foods. However respiratory problems including COPD (3%) were reported in lower frequency in patients with COVID-19 than other comorbidities. This finding is consistent with previous studies and may be due to the lack of consciousness and proper diagnosis of respiratory diseases like COPD in the community [10,32,33]. However about 1% patients with COVID-19 were reported to have tuberculosis. The frequency of patients with comorbid paralysis and cancer was low and the finding is consistent with previous reports [10,[38], [39], [40]]. The frequency of comorbidities was higher in male (58%) aged above 70 years. The frequency of gastrointestinal diseases (5.5%), liver diseases (4.2%), kidney diseases (5.8%) and asthma (2.4%) as comorbidity were higher in female patients with COVID-19. These findings reflected previously published works [[1], [2], [3], [4],10,11,[19], [20], [21], [22], [23], [24], [25], [26],[27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42]]. This is the first study on the impact of comorbidities on outcome of COVID-19 in Bangladesh. Therefore, our findings will append to the available knowledge on the frequency of comorbidities and distribution in age and sex group in patients with COVID-19 established on the analysis of nationwide representative larger sample in Bangladesh. In previous studies, comorbidities including CVDs, diabetes, hypertension, respiratory diseases, cardiac diseases, pregnancy, renal diseases, liver diseases and cancer were detected to increase the risk of severity and fatality in patients with COVID-19, SARS-CoV, avian influenza (bird flu), and MERS-CoV infections [[43], [44], [45], [46], [47], [48], [49]]. In similar with previous studies on outbreaks of respiratory diseases, our findings concluded that, comorbidities including CVDs, hypertension, diabetes, liver diseases, COPD, and cancer persuaded to severe clinical outcomes and fatality in patients with COVID-19 [[19], [20], [21], [22], [23], [24], [25], [26],[27], [28], [29], [30], [31], [32], [33], [34], [35],[36], [37], [38], [39], [40], [41], [42],[43], [44], [45], [46], [47], [48], [49]].
The case fatality rate in the study cohort was 10.5 that is higher than the overall case fatality rate of COVID-19 in Bangladesh. The frequency of fatality was 61% in patients aged >70 years (mean ± SD: 78.4 ± 4.3 years). The mean length of hospital stay was 8.9 ± 2.7 days for the 92% hospitalized patients. Higher odds were present for hospitalization in patients aged >60 years (OR: 4.83, 95% CI: 2.45–6.49) with symptoms prevailing >14 days (OR: 4.12, 95% CI: 2.16–6.34), CCI >3 (OR: 5.48, 95% CI: 3.95–7.24), diabetes (OR: 2.83, 95% CI: 1.54–3.96), and heart disease (OR: 2.44, 95% CI: 1.82–3.13). The highest odds of severity of COVID-19 were present in CCI >4 (aOR: 3.41, 95% CI: 2.57–6.09) and CCI 3–4 (aOR: 2.96, 95% CI: 2.01–5.39). These findings on the association of demographic factors, symptoms and comorbidities with the severity of COVID-19 had reflected the previously published works [10,11,19,20].
Consistent and higher odds of fatality were present in patients aged >60 years (OR: 5.43, 95% CI: 2.85–7.68), with comorbidities (OR: 4.62, 95% CI: 3.09–6.33). The higher odds of fatality were present in group CCI >4 (aOR: 4.22, 95% CI: 2.92–6.89). The absolute risks of severity and fatality in patients with COVID-19 gradually increased from group CCI 0 to CCI 1–2, 3–4, and >4. This study is with good agreement with previous works reporting the impact of comorbidities on the prognosis of the severity of health outcomes in patients with COVID-19 [10,11,19,20]. Further, having easy access to health facilities of comorbid patients with COVID-19 (aOR: 0.73, 95% CI: 0.22–1.96) reduced the odds of association with mortality.
The odds of association among comorbidities and outcomes in this study was significantly consistent with the previous literature reports [10,11,[19], [20], [21], [22], [23], [24], [25],[26], [27], [28], [29], [30], [31], [32],[33], [34], [35], [36], [37], [38]]. In accordance with the available studies we detected poor prognosis of COVID-19 patients with comorbidities [10,11,19]. This study found that COVID-19 patients with comorbidities have greater risk of developing severity and fatality than those without. Further, the severity and fatality increased with increased CCI, number of symptoms and age. The proper screening of patients with COVID-19 and comorbidities should be conducted by examining the previous medical history to identify the risk group immediately. Major concluding remark of this study is the suggestion to implement immediate medical assistance among aged patients with COVID-19 and comorbidities in Bangladesh.
The main limitation of the study was self-reporting of comorbidities which could result in under-reporting of comorbidities. Under-reporting of comorbidities might contribute to the underestimation or overestimation of the true strength of association with the clinical prognosis and severity. We carefully reduced the possibility of under-reporting through history taking of the patients for longer time at four points and our findings were consistent with previous studies [[1], [2], [3],10,11,[19], [20], [21], [22], [23], [24], [25],[26], [27], [28],[29], [30], [31], [32]]. This is the first cohort study to predict the association of demographics, symptoms and comorbidities with the prognosis of adverse health outcomes in patients with COVID-19 in Bangladesh. This study will be a guideline for future studies focusing similar outbreak dynamics. In future, studies including more patients should be conducted to power up the statistical analysis and elucidate the association of comorbidities with the severity and fatality among patients with COVID-19.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contribution
Nadim Sharif: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Investigation (equal); Methodology (lead); Project administration (lead); Software (lead); Validation (lead); Writing-original draft (lead); Writing-review & editing (lead). Rubayet Rayhan Opu: Data curation (equal); Investigation (equal). Shamsun Nahar Ahmed: Data curation (equal); Methodology (equal). Mithun Kumar Sarkar: Data curation (equal); Methodology (supporting). Raisah Jaheen: Data curation (supporting); Investigation (supporting). Muktasid Ud Daullah: Data curation (supporting); Investigation (supporting). Shahriar Khan: Data curation (supporting); Investigation (supporting). Mir Mubin: Data curation (supporting); Investigation (supporting). Habibur Rahman: Data curation (supporting). Faiza Islam: Data curation (equal). Nusaira Haque: Data curation (supporting). Suchana Islam: Data curation (supporting). Fariha Bushra Khan: Data curation (supporting). Nabila Haque: Data curation (equal). Umme Ayman: Data curation (equal). Abdullah Mohammad Shohael: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Investigation (equal); Methodology (lead); Project administration (lead); Software (lead); Validation (lead); Writing-original draft (lead); Writing-review & editing (lead). Shuvra Kanti Dey: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Investigation (equal); Methodology (lead); Project administration (lead); Software (lead); Validation (lead); Writing-original draft (lead); Writing-review & editing (lead). Ali Azam Talukder: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Investigation (equal); Methodology (lead); Project administration (lead); Software (lead); Validation (lead); Writing-original draft (lead); Writing-review & editing (lead).
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dsx.2021.05.021.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Peeri N.C., Shrestha N., Rahman M.S., Zaki R., Tan Z., Bibi S., et al. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? Int J Epidemiol. 2020;49(3):717–726. doi: 10.1093/ije/dyaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burki T. Outbreak of coronavirus disease 2019. Lancet Infect Dis. 2020;20:292–293. doi: 10.1016/S1473-3099(20)30076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matoba Y., Abiko C., Ikeda T., Aoki Y., Suzuki Y., Yahagi K., et al. Detection of the human coronavirus 229E, HKU1, NL63, and OC43 between 2010 and 2013 in Yamagata, Japan. Jpn J Infect Dis. 2015;68(2):138–141. doi: 10.7883/yoken.JJID.2014.266. [DOI] [PubMed] [Google Scholar]
- 4.Sharif N., Dey S.K. Phylogenetic and whole genome analysis of first seven SARS-CoV-2 isolates in Bangladesh. Future Virol. 2020;15(11):735–746. [Google Scholar]
- 5.COVID-19 map - Johns Hopkins coronavirus resource center. https://coronavirus.jhu.edu/map.html
- 6.WHO coronavirus disease (COVID-19) dashboard. https://covid19.who.int/
- 7.Symptoms of coronavirus-CDC. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html
- 8.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharif N., Sarkar M.K., Ahmed S.N., Ferdous R.N., Nobel N.U., Parvez A.K., et al. Environmental correlation and epidemiologic analysis of COVID-19 pandemic in ten regions in five continents. Heliyon. 2021;7(3) doi: 10.1016/j.heliyon.2021.e06576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M., et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5) doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu K., Fang Y.Y., Deng Y., Liu W., Wang M.F., Ma J.P., et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J. 2020;133(9):1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu X.W., Wu X.X., Jiang X.G., Xu K.J., Ying L.J., Ma C.L., et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case studies. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharif N., Dey S.K. Impact of population density and weather on COVID-19 pandemic and SARS-CoV-2 mutation frequency in Bangladesh. Epidemiol Infect. 2021:149. doi: 10.1017/S0950268821000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang B., Li R., Lu Z., Huang Y. Does comorbidity increase the risk of patients with covid-19: evidence from meta-analysis. Aging. 2020;12(7):6049–6057. doi: 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanyaolu A., Okorie C., Marinkovic A., Patidar R., Younis K., Desai P., et al. Comorbidity and its impact on patients with COVID-19. SN Compr Clin Med. 2020;2(8):1069–1076. doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imam Z., Odish F., Gill I., O'Connor D., Armstrong J., Vanood A., et al. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID-19 patients in Michigan, United States. J Intern Med. 2020;288(4):469–476. doi: 10.1111/joim.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye C., Zhang S., Zhang X., Cai H., Gu J., Lian J., et al. Impact of comorbidities on patients with COVID-19: a large retrospective study in Zhejiang, China. J Med Virol. 2020;92(11):2821–2829. doi: 10.1002/jmv.26183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ejaz H., Alsrhani A., Zafar A., Javed H., Junaid K., Abdalla A.E., et al. COVID-19 and comorbidities: deleterious impact on infected patients. J Infect. Public Health. 2020;13:1833–1839. doi: 10.1016/j.jiph.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nogueira P.J., de Araújo Nobre M., Costa A., Ribeiro R.M., Furtado C., Bacelar Nicolau L., et al. The role of health preconditions on COVID-19 deaths in Portugal: evidence from surveillance data of the first 20293 infection cases. J Clin Med. 2020;9(8):2368. doi: 10.3390/jcm9082368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lippi G., Wong J., Henry B.M. Hypertension in patients with coronavirus disease 2019 (COVID-19): a pooled analysis. Pol Arch Intern Med. 2020;130(4):304–309. doi: 10.20452/pamw.15272. [DOI] [PubMed] [Google Scholar]
- 24.Pranata R., Lim M.A., Huang I., Raharjo S.B., Lukito A.A. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. J Renin-Angiotensin-Aldosterone Syst JRAAS. 2020;21(2) doi: 10.1177/1470320320926899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parveen R., Sehar N., Bajpai R., Agarwal N.B. Association of diabetes and hypertension with disease severity in covid-19 patients: a systematic literature review and exploratory meta-analysis. Diabetes Res Clin Pract. 2020;166:108295. doi: 10.1016/j.diabres.2020.108295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharif N., Ahmed S.N., Opu R.R., Tani M.R., Dewan D., Daullah M.U., et al. Prevalence and impact of diabetes and cardiovascular disease on clinical outcome among patients with COVID-19 in Bangladesh. Diabetes Metab Syndr. 2021;15(3):1009–1016. doi: 10.1016/j.dsx.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li B., Yang J., Zhao F., Zhi L., Wang X., Liu L., et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr. 2020;14(3):247–250. doi: 10.1016/j.dsx.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo W., Li M., Dong Y., Zhou H., Zhang Z., Tian C., et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metabol Res Rev. 2020;36(7) doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia – a systematic review, meta-analysis, and meta-regression: diabetes and COVID-19. Diabetes Metab Syndr. 2020;14(4):395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh A.K., Gupta R., Ghosh A., Misra A. Diabetes in COVID-19: prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab Syndr. 2020;14(4):303–310. doi: 10.1016/j.dsx.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Q., Meng M., Kumar R., Wu Y., Huang J., Lian N., et al. The impact of COPD and smoking history on the severity of COVID-19: a systemic review and meta-analysis. J Med Virol. 2020;92(10):1915–1921. doi: 10.1002/jmv.25889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pranata R., Soeroto A.Y., Huang I., Lim M.A., Santoso P., Permana H., et al. Effect of chronic obstructive pulmonary disease and smoking on the outcome of COVID-19. Int J Tubercul Lung Dis. 2020;24(8):838–843. doi: 10.5588/ijtld.20.0278. [DOI] [PubMed] [Google Scholar]
- 34.Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L., et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang X., Jin Y., Li R., Zhang Z., Sun R., Chen D. Prevalence and impact of acute renal impairment on COVID-19: a systematic review and meta-analysis. Crit Care. 2020;24(1):1–8. doi: 10.1186/s13054-020-03065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yip T.C., Lui G.C., Wong V.W., Chow V.C., Ho T.H., Li T.C., et al. Liver injury is independently associated with adverse clinical outcomes in patients with COVID-19. Gut. 2020:1–10. doi: 10.1136/gutjnl-2020-321726. 0. [DOI] [PubMed] [Google Scholar]
- 37.Ji D., Zhang D., Yang T., Mu J., Zhao P., Xu J., et al. Effect of COVID-19 on patients with compensated chronic liver diseases. Hepatol Int. 2020;14(5):701–710. doi: 10.1007/s12072-020-10058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y., Rubinstein S.M., Rivera D.R., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang K., Sheng Y., Huang C., Jin Y., Xiong N., Jiang K., et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:904–913. doi: 10.1016/S1470-2045(20)30310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saini K.S., Tagliamento M., Lambertini M., McNally R., Romano M., Leone M., et al. Mortality in patients with cancer and coronavirus disease 2019: a systematic review and pooled analysis of 52 studies. Eur J Canc. 2020;139:43–50. doi: 10.1016/j.ejca.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Moheb M., Naar L., Christensen M.A., Kapoen C., Maurer L.R., Farhat M., et al. Gastrointestinal complications in critically Ill patients with and without COVID-19. J Am Med Assoc. 2020;324:1899–1901. doi: 10.1001/jama.2020.19400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhaskaran K., Rentsch C.T., MacKenna B., Schultze A., Mehrkar A., Bates C.J., et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV. 2021;8(1):e24–e32. doi: 10.1016/S2352-3018(20)30305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Booth C.M., Matukas L.M., Tomlinson G.A., Rachlis A.R., Rose D.B., Dwosh H.A., et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. J Am Med Assoc. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 44.Shiley K.T., Nadolski G., Mickus T., Fishman N.O., Lautenbach E. Differences in the epidemiological characteristics and clinical outcomes of pandemic (H1N1) 2009 influenza, compared with seasonal influenza. Infect Control Hosp Epidemiol. 2010;31:676–682. doi: 10.1086/653204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martínez A., Soldevila N., Romero-Tamarit A., Torner N., Godoy P., Rius C., et al. Risk factors associated with severe outcomes in adult hospitalized patients according to influenza type and subtype. PLoS One. 2019;14 doi: 10.1371/journal.pone.0210353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gutiérrez-González E., Cantero-Escribano J.M., Redondo-Bravo L., San Juan-Sanz I., Robustillo-Rodela A., Cendejas-Bueno E. Effect of vaccination, comorbidities and age on mortality and severe disease associated with influenza during the season 2016–2017 in a Spanish tertiary hospital. J Infect Public Health. 2019;12:486–491. doi: 10.1016/j.jiph.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 47.Alqahtani F.Y., Aleanizy F.S., Mohamed R.A., Alanazi M.S., Mohamed N., Alrasheed M.M., et al. Prevalence of comorbidities in cases of Middle East respiratory syndrome coronavirus: a retrospective study. Epidemiol Infect. 2018;5:1–5. doi: 10.1017/S0950268818002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alanazi K.H., Abedi G.R., Midgley C.M., Alkhamis A., Alsaqer T., Almoaddi A., et al. Diabetes mellitus, hypertension, and death among 32 patients with MERS-CoV infection, Saudi Arabia. Emerg Infect Dis. 2020;26:166–168. doi: 10.3201/eid2601.190952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Y.M., Hsu C.Y., Lai C.C., Yen M.F., Wikramaratna P.S., Chen H.H., et al. Impact of comorbidity on fatality rate of patients with Middle East Respiratory Syndrome. Sci Rep. 2017;7:11307. doi: 10.1038/s41598-017-10402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.