Abstract
Myocardial ischemia continues to be the first cause of morbimortality in the world; the definitive treatment is reperfusion; however, this action causes additional damage to ischemic myocardial tissue; this forces to seek therapies of cardioprotection to reduce this additional damage. There are many cardioprotective agents; within these, cannabinoids have shown to have beneficial effects, mainly cannabidiol (CBD). CBD is a non psychoactive cannabinoid. To evaluate the effect in experimental models of CBD in myocardial ischemia reperfusion in rats, twelve‐week‐old male rats have been used. The animals were divides in 3 groups: control(C), ischemia reperfusion (IR) and CBD pretreatment (1/day/5mg/kg /10days). Langendorff organ isolate studies were performed, and the area of infarction was assessed with triphenyl tetrazolium, in addition to molecular analysis of AT1 and AT2 receptors and Akt and Erk proteins and their phosphorylated forms related to RISK pathways. It was observed that there is an improvement with the use of CBD increasing inotropism and cardiac lusitropism, improving considerably the cardiovascular functionality. These could be related to the reduction of the area of infarction and activation of the AT2 receptor and the RISK pathway with absence of activation of the AT2 receptor (these could relate the reduction of the infarct area and the restoration of cardiovascular function with the activation of the AT2 receptor and the RISK pathway with the absence of activation of the AT2 receptor). The use of cannabinoids was shown to have beneficial effects when used as a treatment for myocardial reperfusion damage.
Keywords: AT1 receptor, AT2 receptor, cannabidiol, MAPK/ERK, myocardial damage by reperfusion, PI3K/AKT, RISK pathways
CBD prior to reperfusion therapy shows a reduction in the necrotic area, improves ventricular function. Reduces the presence of AT1R and increases AT2R in cardiomyocytes. CBD is postulated as a prophylactic treatment prior to reperfusion to improve the survival and function of the heart that will be subjected to reperfusion and reduce the damage caused by it. By molecularly evaluating CBD, it reduces the presence of AT1R and increases the presence of AT2R in cardiomyocytes, which may indirectly explain the decrease in cardiac remodeling.
1. INTRODUCTION
Despite the scientific progress that has been made in the cardiovascular area, myocardial ischemic disease remains as the leading cause of morbidity and mortality worldwide, 1 the worldwide established treatment for this condition is the prompt restoration of coronary blood flow. Paradoxically, these procedures generate additional damage that has been well established as reperfusion damage, causing necrosis and apoptosis of the myocardial cells that are in the area at risk (AAR) of the ischemic zone; in this zone, there is an abundant inflammatory process, oxidative stress, as well as areas of fibrosis patient). 2 , 3 In addition, immediately after myocardial ischemia, the angiotensin renin system is triggered, and AT1 receptor stimulation is responsible for vasoconstriction, sympathetic activation, cell growth (myocardial hypertrophy), and remodeling mediated by fibroblast proliferation and collagen production, 4 while it has been studied that the effects of the AT2 receptor are counter‐regulatory. 5 We are currently in need of continuing to evaluate molecules with potential cardioprotective effects. Cannabinoids have aroused interest in their therapeutic uses and in evaluating their potential cardioprotective effects. Cannabinoids are a group of compounds of endogenous (endocannabinoids), natural (Phyto cannabinoids), or synthetic origin with structure and pharmacology related to the Cannabis sativa plant, all of which have in common the activation of the CB1 and CB2 receptors. 6 , 7
There are more than 70 known cannabinoid derivatives, but the most abundant are two: tetrahydrocannabinol (Δ9‐THC) with important psychoactive effects, activating CB1 receptors which are widely distributed over the central nervous system and (−)‐cannabidiol (CBD) is a non‐psychoactive that has reported that even low doses are an important CB1 receptor antagonist AND CB2 receptor agonist which is distributed mainly on myocardial cells and immune cells. 6 , 8 The CB2 receptor has been attributed important cardioprotective effects in addition to anti‐inflammatory drugs, 9 and therefore, the effects of reducing damage by restoring coronary flow have been evaluated in multiple studies, both during ischemia and prior to reperfusion). 6 , 8
Another interesting finding is that CBD can modulate the activation of other receptors such as the adenosine A1 receptor, generating electrocardiographic and molecular evidence of important myocardial protection. 10
On the other hand, for the last 30 years in the field of cardioprotection, a molecular pathway that has an important effect on reperfusion damage has been extensively studied. This pathway is the reperfusion injury rescue kinases (RISK) pathway, which in turn has been linked to the modulation of G‐protein‐coupled receptors in the heart. 11
Nonetheless, the use of CBD as a cardioprotective agent requires more observation points, and it is of our interest to evaluate if the CBD which is administered to animals during the days prior to an IR event can generate cardioprotection against a global IR event, as well as if this cannabinoid can modulate the activity of the receptors to angiotensin AT1 and AT2, as well as the possible intervention of the RISK pathway in its cardioprotective effects against reperfusion damage. These effects have not been evaluated before.
2. MATERIALS AND METHODS
2.1. Animals
Twelve‐week‐old male Wistar rats which were provided by the biotherium of the Escuela Superior de Medicina (Instituto Politécnico Nacional, Mexico City) were kept individually in acrylic cages and randomly distributed in three different groups (n = 6) for functional and morphological and for molecular studies (n = 3): (1) SHAM animals (simulated IR surgery) (C), (2) animals with ischemia‐reperfusion (IR) damage, (3) animals with IR damage treated with CBD (DROPYDIOL) 5 mg/kg every 24 h for 10 days prior to the surgical event injected by intraperitoneal (IP) route. The animals were acclimated to laboratory conditions, including a 12‐h (12:12) light/dark cycle, for at least 7 days. Sterile feed and water were provided ad libitum.
2.2. Ethical approval
The handling of the animals was in accordance with the Mexican Federal Regulations for the Experimentation and Care of Animals (NOM‐062‐ZOO‐1999, Ministry of Agriculture, Mexico City, Mexico) and approved by the Institutional Committee for the Care and Use of Animals (CICUAL‐ESM‐IPN).
2.3. Surgical procedures
The animals were anesthetized by IP injection of ketamine (100 mg/kg; S.A.G.A.R.P.A. Q‐7833‐028) and xylazine (10 mg/kg, S.A.G.A.R.P.A. Q‐7833‐099), intubated and ventilated with positive pressure on a small animal respirator (Harvard). A left thoracotomy was performed to expose the heart. In IR animals, the left anterior descending coronary artery was ligated with a 5‐Fr nylon suture, and a 5‐Fr feeding catheter was placed over it, for 45 min; then, the artery was released by removing the feeding catheter, and the suture was left in place as a reference point. Successful occlusion and reperfusion were verified by visual inspection of left ventricle (LV) color. The thorax was closed in layers, and the animals were extubated, lost body fluids were replaced by subcutaneous administration of 30 ml of saline, and the animals were treated with tramadol analgesia (5 mg/kg ip) for 2 days. The simulated animals underwent the surgical procedure described with the exception of coronary ligation and ischemia.
2.4. Collection of tissues and determination of IM size for morphological analysis (triphenyl tetrazolium staining)
Forty‐eight hours after the surgical procedures, the animals were anesthetized with sodium pentobarbital at a dose of 100 mg/kg and were killed by decapitation. The hearts were then removed and weighed. The AAR was determined by the reocclusion of the anterior descending artery using the suture placed during the surgical procedure and the infusion of 0.5–1 ml of 1%. Evans blue solution in saline was slowly infused in a retrograde fashion through the cannulated aorta into the coronary arteries. The heart was then frozen (−80°C) for 15 min in aluminum foil, and 2‐mm cross sections were made.
Four middle LV rings were taken considering a ratio of the free wall of the LV equivalent to the radius of the ventricular cavity to ensure that all selected rings were taken at the same point and stained with 1% (w/v) triphenyl tetrazolium chloride (TTC) with incubation at 37°C for 15 min in phosphate buffer (pH 7.4) and fixed at 10% (w/v) in formaldehyde solution. The image analysis was performed in Image Pro Plus software. The infarct area (IA) and the AAR were measured. The results are expressed as IA/AAR. In a subgroup of animals from Groups 1, 2, and 3, the hearts were removed and prepared with Krebs solution for ex vivo hemodynamic analysis. In another subgroup of animals from Groups 1, 2, 3, and 4, the hearts were removed and immediately frozen in liquid nitrogen and stored at −80°C for further molecular analysis. Images of unfixed stained rings were also used to measure internal and external chamber diameters and anterior and septal wall thicknesses.
2.5. Hemodynamic studies (Langendorff heart isolated ex vivo)
The rats were given heparin (100 U/kg, s.c.); after 15 min, they were anesthetized with sodium pentobarbital (50 mg/kg, ip). The animals were slaughtered; their hearts were immediately removed and immersed in Krebs–Henseleit solution (KHS). The hearts were perfused in a retrograde fashion, using an aortic cannula, with a constant flow rate (10 ml/min). Perfusion was performed using KHS containing (mM): NaCl (118), KCl (4.7), CaCl2 (1.5), MgSO4 (1.2), NaHCO3 (25), KH2PO4 (1.2), dextrose (11), and double‐distilled water, balanced with continuous gassing (95% O2 and 5% CO2 at 37°C) to maintain a pH of 7.4. After a 30‐min stabilization period, cardiac function was determined using an isovolumetric technique by Langendorff. 12 Left ventricular pressure was quantified by means of a balloon constructed of plastic film (3–5 mm in diameter) inserted into the LV via the left atrium and connected to a pressure transducer (TSD104A, Biopac Systems Inc.) coupled to a software (Acknowledge program; MP 100WSW, Biopac Systems Inc.) for data acquisition. The balloon volume was adjusted to produce a pressure at the end of the diastole of 8–10 mmHg in all groups. 13
Measurements of left ventricular pressure development (LVDP), maximum value of the first derivative of positive ventricular pressure (+dP/dt max), minimum negative (−dP/dt min), and heart rate (HR) was made. Coronary perfusion pressure (CPP) was measured through a lateral connection on the perfusion cannula, connected to a perfusion transducer (TSD104A, Biopac Systems Inc.). Once the baseline values of the cardiac mechanics were obtained, the isolated hearts obtained from animals in each experimental group were subjected to a period of 0 flow global ischemia, completely obstructing the retrograde perfusion for 30 min. Subsequently, the isolated hearts were re‐infused with KHS for 90 min; measurements were taken at 15, 30, 45, 60, and 90 min.14, 15
2.6. Molecular analysis (western blot)
One hundred milligrams of previously collected heart tissue were taken and kept frozen at −80°C. They were homogenized in polytron in Tris solution pH 7.4 with protease and phosphatase inhibitors (miniComplete Cocktail). They were centrifuged at 4°C at 10,000 g for 15 min, and protein quantification was performed by Bradford technique. Western Blot was used to determine the variations in AT1R, AT2R, phosphorylated ERK AKT, and their relationship with their total forms. Subsequently, 100 µg of total protein were taken from each sample and separated by 10% SDS‐PAGE gels under reducing conditions. They were then transferred to a polyvinylidene dichloride membrane (Immobilon PVDF, 0.45 µm; Millipore). The membranes were then blocked with 5% bovine serum albumin in TBS −0.1% Tween 20 (TBS‐T, pH 7.4) for 2 h, washed three times with TBS‐T, and incubated with primary antibodies listed in Table 1 at 4°C overnight on continuous agitation. After proper washing with TBS‐T, the membranes were incubated with secondary antibodies, at room temperature for 2 h on continuous agitation. Detection was carried out using the enhanced chemiluminescence method (Western transfer luminol reagent; Santa Cruz Cat., 2048). The membranes were photographed, and the image digitized for densitometric analysis using the Image Studio Lite software (LI‐COR Biosciences). The relative presence of each protein was normalized with β‐actin as the housekeeping protein.
2.6.1. Primary antibodies
AT1 anti‐rabbit (Pro‐sci 5391) concentration 1:2000, AT2 anti‐rabbit (Pro‐sci 5393) concentration 1:2000, Akt Anti‐mouse (sc‐81434) concentration 1:2500, pAkt anti‐mouse (sc‐377556) concentration 1:2500, Erk 1/2 anti‐rabbit (sc‐153) concentration 1:3000, pERK1/2 anti‐rabbit (sc‐7383) concentration 1:3000 Β actina anti‐goat (sc‐1615) concentration 1:3000.
2.6.2. Secondary antibodies: (HRP‐conjugated)
Anti‐rabbit (invitrogen 656120) concentration 1:6000, anti‐goat (invitrogen 81–1620) concentration 1:12,500 anti‐mouse (Santa Cruz sc‐2005) concentration 1:8000.
2.7. Statistical analysis
The data are presented as the mean ± SEM. A comparison between three groups in the respective data was analyzed, using the one‐way ANOVA test with its subsequent post hoc Tukey test considering significant data with a p < .05. All analyses and graphs were performed with Prisma software version 8.0.
3. RESULTS
Basal heart function was evaluated by measuring LVDP (mmHg), HR (bpm) and LVDP +dP/dt max and −dP/dt min (mmHg/s) and CPP (mmHg). During the stabilization period, they represent an average of the last 5 min of a total of 30 min of stabilization prior to the total ischemia event, by cutting the continuous flow of solution and carbogen administered by the Langendorff system. The hearts of rats treated with CBD showed a better and faster recovery being significantly higher from the first 15 min and persisting until 60 min where the total reperfusion period was completed (Figure 1).
FIGURE 1.
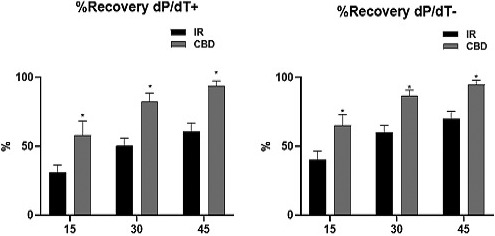
Recovery percentages at 15, 30, and 45 min after restoring flow and carbogen (reperfusion) in untreated hearts (IR) and in hearts treated 10 days before with CBD in doses of 5 mg/kg, c/24 h IP. Means ± SEM are shown considering n = 6 rats per group, *p ≤ .005 CBD versus IR
Notwithstanding, the group treated with CBD showed significantly increased recovery with increased cardiac inotropism: with elevated LVDP and +dP/dt máx (%) compared to the IR group as well and increased lusitropy with increased LVDP and increased −dP/dt máx (%). Also, the CPP was significantly increased from the first 15 min in the CBD group and remained so until the end of the experiment (Figure 2). This establishes an increase in the force of contraction and the speed of relaxation as well as an increase in the available coronary flow.
FIGURE 2.
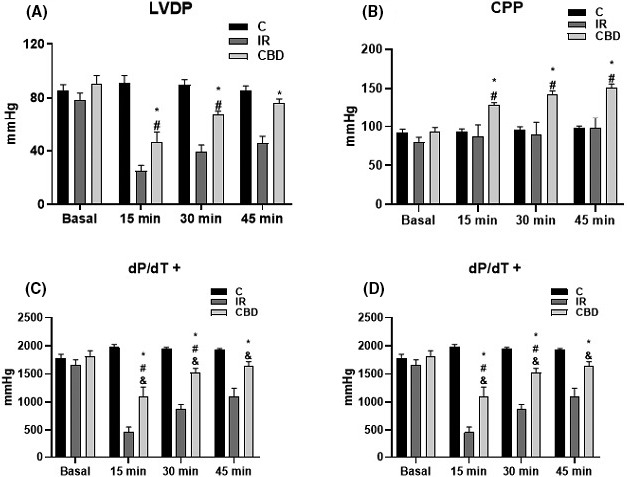
The graphs show (A) left ventricular pressure development (LVDP), (B) coronary perfusion pressure (CPP), (C) maximum value of the first derivative of positive ventricular pressure (+dP/dt max), and (D) first derivative of minimum negative ventricular pressure (−dP/dt min). From isolated hearts in Langendorff system from untreated (IR) rats and rats treated with CBD dose of 5 mg/kg, c/24 h, for 10 days prior to the experiment. Baseline measurements were made before reperfusion and at 15, 30, and 45 min after reperfusion, concluding the experiment at 60 min after reperfusion (n = 6) *p < .05 CBD versus IR
However, the heart rate showed no change in both IR and CBD groups, either during the basal period of stabilization or after reperfusion and continued unchanged until the end of the experiment, suggesting that no harm was done to cardiac automatism (Figure 3).
FIGURE 3.
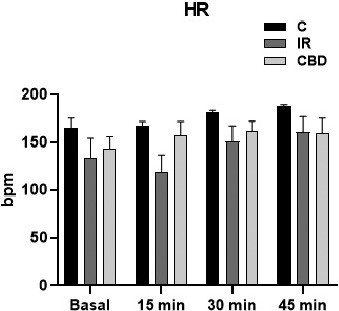
The heart rate (bpm) of the isolated hearts of untreated rats and rats treated with CBD doses of 5 mg/kg, c/24 h IP, was observed for 10 days prior to the experiment, placed in the Langendorff system. Basal responses were recorded prior to reperfusion and at 15, 30, and 45 min after reperfusion, concluding the experiment at 60 min. No significant differences in this variable were found in any temporality
After the first functional evaluation, confirming that there were effects with the use of CBD, we performed the surgical procedures of anterior descending artery ligation with 45‐min regional ischemia and subsequent reperfusion and were stained with triphenyl tetrazolium stain to assess the AAR (%) and IA/LV (%).
There was a statistically significant reduction in the area of IA/LV ischemia (20.55%) and a recovery of healthy tissue of 47.67%. Comparing hearts treated with CBD at a dose of 5 mg/kg, c/24 h IP, for 10 days prior to the surgical procedure compared to those that received no treatment but underwent ischemic surgery 45 min and reperfusion (IR). There was no significant change in the AAR (Figure 4).
FIGURE 4.
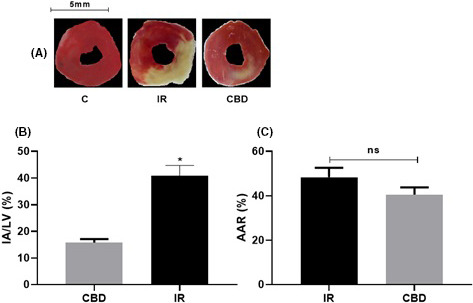
(A) Representative sections of the ring cross section of the left ventricle (LV) animals with ischemia 45 min and reperfusion and animals treated with CBD stained with triphenyltetrazolium chloride. (B) Graph of the area of infarct/left ventricle (IA/LV) in coronary occlusion groups for 45 min with subsequent reperfusion (n = 6). The values are the means ± SEM. *p < .001 CBD versus IR
Finally we evaluated the molecular effect in the LVs of the animals submitted to the surgery simulation (SHAM) that will be the control group (C), animals submitted to regional ischemia for 45 min with subsequent re‐fusion (IR) and the animals with the CBD treatment (CBD) in doses of 5 mg/kg, c/24 hr IP, during 10 days previous to the procedure. Figure 4 shows the differences in the molecular expression of the AT1 and AT2 receptors. It shows a significant increase in the expression of the AT1 receptor in the IR animals without generating elevation of this receptor in the CBD‐treated animals (p = .0306) or the C group (p = .0470) and a significant increase of the AT2 receptor in the CBD‐treated group compared to the IR group (p = .0002) but also exceeding the basal levels of the AT2 receptor in the control group (p = .0005).
Figure 5 shows the differences in the molecular expression of the AT1 and AT2 receptors. It shows a significant increase in the expression of the AT1 receptor in the IR animals without generating elevation of this receptor in the CBD‐treated animals (p = .0306) or the C group (p = .0470) and a significant increase of the AT2 receptor in the CBD‐treated group compared to the IR group (p = .0002) but also exceeding the basal levels of the AT2 receptor in the control group (p = .0005). Figure 4 shows the differences in the molecular expression of the AT1 and AT2 receptors. It shows a significant increase in the expression of the AT1 receptor in the IR animals without generating elevation of this receptor in the CBD‐treated animals (p = .0306) or the C group (p = .0470) and a significant increase in the expression of the AT2 receptor in the CBD‐treated group compared to the IR group (p = .0002) but also exceeding the basal levels of the AT2 receptor in the control group (p = .0005).
FIGURE 5.
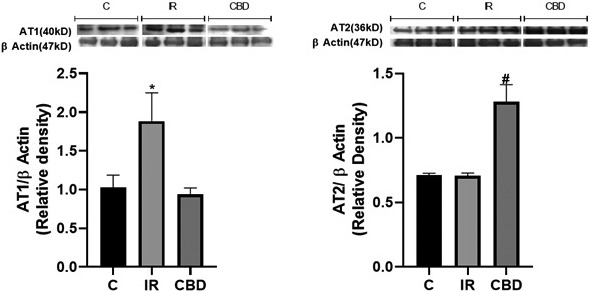
Western blot analysis (A) for AT1 receptor (40 kDa) and (B) for AT2 receptor (36 kDa), in left ventricle of rats submitted to surgery simulation (C), ischemia surgery for 45 min and subsequent reperfusion and those treated with CBD doses of 5 mg/kg c/24 h IP, 10 days prior to the ischemia‐reperfusion procedure. A representative western blot is shown above each graph. The intensity of the bands was quantified by densitometric analysis and normalized with the corresponding β‐Actin (47 kDa). The values are averages ± SEM (n = 3). (A) *p < .05 IR versus C and CBD; (B) *p < .05 CBD versus C and IR
The activation of the RISK pathways characterized by the modulation of the Akt/PKB and ERK proteins and their phosphorylated forms was evaluated. A significant increase in the total forms of AKT was observed in the group treated with CBD compared to group C (p ≤ .0001) and also a significant decrease of this protein in the IR animals (p ≤ .0001). This decrease is also different from the baseline values of group C (p = .0164). As for the phosphorylated forms, we also found a significant increase in the p‐Akt levels of the group treated with CBD compared to IR (p = .0013) and to group C (p = .0003). ERK protein levels were also increased in both total and phosphorylated forms in CBD‐treated animals compared to IR‐treated and control animals. In general terms, we can consider that there is an activation of the RISK pathway at this level (Figure 6).
FIGURE 6.
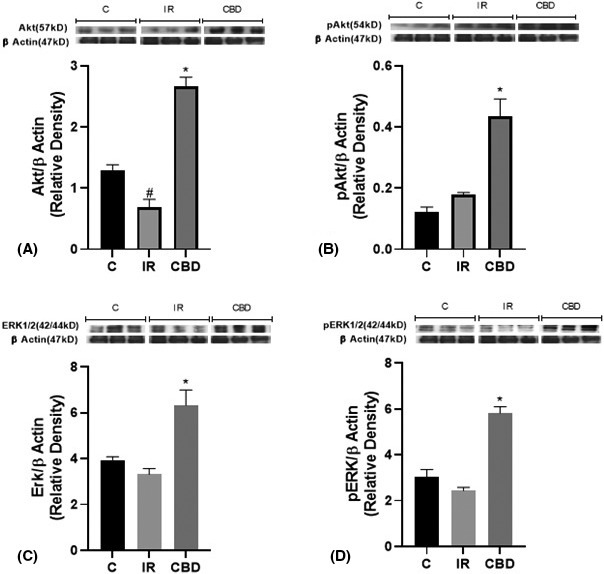
Western blot analysis (A) for the Akt/PKB protein (57 kDa) and (B) its phosphorylated p‐Akt form (54 kDa); (C) densitometry of the MAPK/ERK1/2 protein (42/44 kDa) and (D) its phosphorylated p‐ERK1/2 form (44/42 kDa), in the left ventricle of rats subjected to surgical simulation (C), ischemia surgery for 45 min and subsequent reperfusion (IR) and those treated with CBD doses of 5 mg/kg c/24 h IP, 10 days prior to the ischemia‐reperfusion procedure. A representative western blot is shown above each graph. The intensity of the bands was quantified by densitometric analysis and normalized with the corresponding β‐Actin (47 kDa). The values are averages ± SEM (n = 3). (A) *p < .05 CBD versus IR and C and # p < .05 IR versus C and CBD; (B) *p < .05 CBD versus C and IR: (C, D)* versus C and IR
4. DISCUSSION
Our findings in ex vivo hearts mounted in the Langendorff system allowed us to establish that CBD treatment administered prior to an IR event can produce a cardioprotective effect that is perpetuated in the myocardial tissue and that is importantly dependent on functional changes at the myocardial level. It is important to note that although no functional change occurs at the baseline, myocardial tissue subjected to reperfusion injury has a higher percentage of recovery of function and this occurs more rapidly within 15 min (Figures 1 and 2). This is mentioned by some authors who used CBD during the ischemic event and prior to reperfusion, interestingly previous administration of CBD on an ongoing basis exerts similar effects. 2 , 7
On the other hand, results in the in vivo hearts confirmed that CBD used prior to an IR event has a significant cardioprotective effect against additional damage induced by anterior descending artery ligation, with reduction in infarct size measured as a percentage of the AAR for TTC staining (Figure 3). This was accompanied by an increase in left ventricular function for inotropism and lusitropism (Figure 1). 16 , 17
Reduced stroke size was associated with increased expression of the AT2 receptor but no elevation of the AT1 receptor, which has been described as cardioprotective by several authors. 4 , 18 It has been mentioned that in an acute ischemic event, the AT1 receptor is immediately activated and modulates vasoconstriction, leukocyte infiltration, inflammation, and cardiac remodeling actions, which will end up decreasing the physiological capacities of the heart. However, what we find when CBD is administered is an increase in AT2 that can be related to the increase of ventricular functions in the isolated heart (Figures 1 and 3), by relating these data to the increase in the AT2 receptor which has been linked to the generation of cardioprotection by having counter‐regulatory effects on the AT1 receptor, helping to establish a strong functional relationship of cannabinoids and the modulation of the angiotensin renin system. 19
Finally, it was allowed to link the cardioprotective action of CBD to the activation of the RISK pathway, 5 which has long been described to exert intrinsic cellular regulatory effects on the protection of myocardial cells from reperfusion injury of the RISK pathways headed by the activation of the PI3K/Akt pathway and the MAPK/RISK pathway,it has been determined that when elevated as in the results obtained in Figure 6; interestingly, it has been described that a reduction of these pathways can generate heart failure due to pathological myocardial hypertrophy, with the consequent remodeling. 11 So, we could consider that its lack of activation has similar consequences to the activation of the AT1 receptor, so by having the results obtained, we can correlate that the activation of the AT2 receptor is somehow linked to the positive modulation of the RISK pathways and that together they produce a cardioprotection and a fast and better recovery of the ventricular capacities besides reducing the size of the damaged area. 20 , 21
DISCLOSURE
The authors have no conflicts of interest and declare that this article has not been published nor is being considered for publication elsewhere.
AUTHOR CONTRIBUTIONS
Conception and design of study: Castillo‐Hernandez M.C. and Franco‐Vadillo A. Acquisition of data: Franco‐Vadillo A., Lopez‐Sanchez P., Rivera‐Herrera Z., and Toledo‐Blas M. Analysis and/or interpretation of data:Orihuela‐Rodriguez O., Guevara‐Bacazar, Kormanovski‐Kovzova A., and Morales‐Carmona J.A. Drafting the manuscript: Franco‐Vadillo A. and Castillo‐Hernandez M.C. Revising the manuscript critically for important intellectual content: Franco‐Vadillo A. and Castillo‐Hernandez M.C., Ivan Rubio‐Gayosso.
ACKNOWLEDGEMENT
The current study was supported by the SIP project (Escuela Superior de Medicina, IPN) and COFAA.
Contributor Information
Alexandre Kormanovski‐Kovzova, Email: kormanovski@yahoo.com.mx.
Maria del Carmen Castillo‐Hernandez, Email: ccastillohe@yahoo.com.mx, Email: castillohernandezmc@gmail.com.
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article.
REFERENCES
- 1. Huxley R, Mendis S, Zheleznyakov E, Reddy S, Chan J. Body mass index, waist circumference and waist:hip ratio as predictors of cardiovascular risk – a review of the literatura. Eur J Clin Nutr. 2010;64:16‐22. [DOI] [PubMed] [Google Scholar]
- 2. Montecucco F, Lenglet S, Braunersreuther V, et al. CB2 cannabinoid receptor activation is cardioprotective in a mouse model of ischemia/reperfusion. J Mol Cell Cardiol. 2009;46(5):612‐620. 10.1016/j.yjmcc.2008.12.014 [DOI] [PubMed] [Google Scholar]
- 3. Wilkins E, Wilson L, Wickramasinghe K, et al. European Cardiovascular Disease Statistics. Vol 2. 19th ed. Brussels: European Heart Network; 2017. [Google Scholar]
- 4. Adachi Y, Saito Y, Kishimoto I, et al. Angiotensin II type 2 receptor deficiency exacerbates heart failure and reduces survival after acute myocardial infarction in mice. Circulation. 2003;107(19):2406‐2408. 10.1161/01.CIR.0000072763.98069.B4 [DOI] [PubMed] [Google Scholar]
- 5. Zhu YC, Zhu YZ, Lu N, Wang MJ, Wang YX, Yao T. Role of angiotensin AT1 and AT2 receptors in cardiac hypertrophy and cardiac remodelling. Clin Exp Pharmacol Physiol. 2003;30(12):911‐918. 10.1111/j.1440-1681.2003.03942.x [DOI] [PubMed] [Google Scholar]
- 6. Durst R, Lotan C. The potential for clinical use of cannabinoids in treatment of cardiovascular diseases. Cardiovasc Ther. 2011;29:17‐22. 10.1111/j.1755-5922.2010.00233.x [DOI] [PubMed] [Google Scholar]
- 7. Durst R, Danenberg H, Gallily R, et al. Cannabidiol, a nonpsychoactive Cannabis constituent, protects against myocardial ischemic reperfusion injury. Am J Physiol Heart Circ Physiol. 2020;293(6):H3602‐H3607. 10.1152/ajpheart.00098.2007 [DOI] [PubMed] [Google Scholar]
- 8. Walsh SK, Hepburn CY, Kane KA, Wainwright CL. Acute administration of cannabidiol in vivo suppresses ischaemia‐induced cardiac arrhythmias and reduces infarct size when given at reperfusión. British J Pharmacol. 2010;160(5):1234‐1242. 10.1111/j.1476-5381.2010.00755.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sun H, Lu Y, Wang H, et al. Activation of endocannabinoid receptor 2 as a mechanism of propofol pretreatment‐induced cardioprotection against ischemia‐reperfusion injury in rats. Oxid Med Cell Longev. 2017;2017:1‐18. 10.1155/2017/2186383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gonca E, Darici F. The effect of cannabidiol on ischemia/reperfusion‐induced ventricular arrhythmias: the role of adenosine a1receptors. J Cardiovasc Pharmacol Ther. 2015;20(1):76‐83. 10.1177/1074248414532013 [DOI] [PubMed] [Google Scholar]
- 11. Rossello X, Yellon DM. The RISK pathway and beyond. Basic Res Cardiol. 2018;113(1):1‐5. 10.1007/s00395-017-0662-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Skrzypiec‐Spring M, Grotthus B, Szelag A, Schulz R. Isolated heart perfusion according to Langendorff‐Still viable in the new millennium. J Pharmacol Toxicol Methods. 2007;55(2):113‐126. 10.1016/j.vascn.2006.05.006 [DOI] [PubMed] [Google Scholar]
- 13. Liu J, Saul D, Böker KO, Ernst J, Lehman W, Schilling AF. Current methods for skeletal muscle tissue repair and regeneration. Biomed Res Int. 2018;16:1984879. 10.1155/2018/1984879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Do Carmo H, Arjun S, Petrucci O, Yellon DM, Davidson SM. The caspase 1 inhibitor VX‐765 protects the isolated rat heart via the RISK pathway. Cardiovasc Drugs Ther. 2018;32(2):165‐168. 10.1007/s10557-018-6781-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lindsey ML, Bolli R, Canty JM, et al. Guidelines for experimental models of myocardial ischemia and infarction. Am J Physiol ‐ Heart Circ Physiol. 2018;314(4):H812‐H838. 10.1152/ajpheart.00335.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herr DJ, Aune SE, Menick DR. Induction and assessment of ischemia‐reperfusion injury in Langendorff‐perfused rat hearts. J Vis Exp. 2015;101:e52908. 10.3791/52908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reichelt ME, Willems L, Hack BA, Peart JN, Headrick JP. Cardiac and coronary function in the Langendorff‐perfused mouse heart model. Exp Physiol. 2009;94(1):54‐70. 10.1113/expphysiol.2008.043554 [DOI] [PubMed] [Google Scholar]
- 18. Tavares FM, Bezerra I, Gomes DA, Barreto‐Chaves MLM. Angiotensin II type 2 receptor (AT2R) is associated with increased tolerance of the hyperthyroid heart to ischemia‐reperfusion, (Ang II). Cardiovasc Drugs Ther. 2013;27:393‐402. 10.1007/s10557-013-6473-x [DOI] [PubMed] [Google Scholar]
- 19. Ramalingam A, Budin SB, Fauzi NM, Ritchie RH, Zainalabidin S. Angiotensin II type I receptor antagonism attenuates nicotine‐ induced cardiac remodeling, dysfunction, and aggravation of myocardial ischemia‐reperfusion injury in rats. Front Pharmacol. 2019;12(10):1493. 10.3389/fphar.2019.01493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lépicier P, Bouchard JF, Lagneux C, Lamontagne D. Endocannabinoids protect the rat isolated heart against ischaemia. Br J Pharmacol. 2003;139(4):805‐815. 10.1038/sj.bjp.0705313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Duerr GD, Heinemann JC, Suchan G, et al. The endocannabinoid‐CB2 receptor axis protects the ischemic heart at the early stage of cardiomyopathy. Basic Res Cardiol. 2014;109:425. 10.1007/s00395-014-0425-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.


