Abstract
Stathmin, a phosphoprotein that modulates microtubule dynamics, is highly expressed in breast cancer cells. Eribulin, a microtubule‐depolymerizing agent, is used to treat patients with advanced breast cancer. However, the detailed mechanisms underlying the action of eribulin during microtubule catastrophe, and the interaction between eribulin and stathmin dynamics, remain unclear. Here, we investigated the role of stathmin in the antiproliferative activity of eribulin in breast cancer cells. Eribulin induced phosphorylation of stathmin in MCF7 and MDA‐MB‐231 cells; this was attenuated by an inhibitor of protein kinase A (H89) and an inhibitor of Ca2+/calmodulin‐dependent kinase II (KN62). In addition, expression of phosphorylated stathmin was reduced by the protein phosphatase PP2A activator FTY720 but increased by the PP2A inhibitor okadaic acid. Of note, expression of PP2A subunits in eribulin‐treated cells decreased, although eribulin did not affect the phosphatase activity of recombinant PP2A directly. Furthermore, the antiproliferative effect of eribulin was stronger in stathmin‐overexpressing cells. These results suggest that stathmin dynamics are closely associated with the antiproliferative effects of eribulin and stathmin is a possible biomarker for predicting the therapeutic effects of eribulin in breast cancer patients.
Keywords: breast cancer cells, eribulin, microtubule, stathmin
Eribulin, a microtubule targeting agent phosphorylates stathmin by activating the PKA and CaMKII pathways, and by downregulating PP2A level in breast cancer cells. Overexpression of stathmin potentiates the antiproliferative effects of eribulin.
1. INTRODUCTION
Microtubule‐targeting agents are used widely as chemotherapeutic drugs to treat numerous types of cancer. Eribulin, a derivative of halichondon B isolated from the marine sponge Halichondria okadai, is approved in many countries (including the United States, the EU, and Asia) for use as a therapeutic agent for patients with advanced breast cancer and soft tissue sarcoma. Eribulin interacts selectively with the plus ends of microtubules, meaning that it inhibits microtubule polymerization. Its mode of action is distinct from that of other microtubule‐targeting agents such as vinca alkaloids (e.g., vincristine and vinblastine). 1 Eribulin interferes with microtubule growth at much lower concentrations than other microtubule inhibitors. 2 and reversal of epithelial–mesenchymal transition in tumor tissues. 3
Stathmin, also called oncoprotein 18 (Op 18), is a microtubule‐destabilizing protein that promotes microtubule depolymerization by sequestering tubulin heterodimers and increasing microtubule catastrophe. Stathmin has four serine (Ser) phosphorylation sites (Ser‐16, 25, 38, and 63) at its N‐terminus. 4 The de‐polymerization activity of stathmin is turned off by phosphorylation at these serine residues. Various kinases, including protein kinase A (PKA), play roles in regulating stathmin phosphorylation. 5 In addition to kinase‐mediated phosphorylation, phosphorylation status is also modulated by protein phosphatases such as PP2A, which dephosphorylates stathmin. 6
Stathmin is overexpressed in a variety of human malignancies, including breast, ovarian, prostate, and lung cancers. 7 , 8 , 9 , 10 , 11 High stathmin expression by breast cancer cells may be associated with aggressive characteristics and increased mortality. 12 , 13 , 14 Breast cancer cell lines overexpressing stathmin show reduced sensitivity to the microtubule‐targeting drugs paclitaxel and vinca alkaloids,however, the combination of stathmin knockdown and treatment with these drugs exerts stronger antiproliferative effects. Furthermore, Meng et al 15 suggested that low expression of stathmin predicts high sensitivity to docetaxel‐containing chemotherapy regimens. Thus, stathmin may be a key factor and/or potential target for anticancer therapy. 11
Eribulin inhibits binding of stathmin to tubulin in vitro, 4 but no intracellular interaction between eribulin and stathmin has been reported. Therefore, the aim of this study was to examine the association between the antiproliferative activity of eribulin and stathmin dynamics in both hormone receptor‐positive and triple‐negative breast cancer cells.
2. MATERIALS AND METHODS
2.1. Reagents and antibodies
Eribulin was kindly provided by Eisai Co., Ltd. (Tokyo, Japan). H89 was purchased from the D. Western Therapeutics Institute, Inc. (Nagoya, Japan). Paclitaxel (PTX), KN‐62, and FTY720 were purchased from Sigma‐Aldrich (St. Louis, MO, USA). Okadaic acid was obtained from Wako Pure Chemical industries Co., Ltd. (Osaka, Japan).
2.2. Cell culture
MCF7 and MDA‐MB‐231‐Luc cells were obtained from the American Type Culture Collection (Rockville, MD) and the Japanese Collection of Research Bioresources Cell Bank, respectively. MCF7 cells were cultured in DMEM/F‐12 medium supplemented with 10% FBS, 50 µg/ml penicillin, 50 µg/ml streptomycin, and 100 µg/ml neomycin. MDA‐MB‐231 was maintained in Leibovitz's L‐15 medium (FUJIFILM Wako Pure Chemical Corporation) containing 10% FBS, antibiotics, and antimycotics.
2.3. Immunoblot analysis
Cell lysates were prepared in RIPA buffer (Cell Signaling Technology). Equal amounts of protein (20 µg) were separated in SDS‐PAGE gels and transferred electrophoretically to polyvinylidene difluoride membranes (Millipore). The membranes were probed with primary antibodies specific for phosphorylated (p)‐stathmin, stathmin, PP2Aa, PP2Ab, PP2Ac, and glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH). An antibody specific for p‐stathmin (p‐stathmin Ser16; sc‐12948, 1:2500 dilution) was purchased from Santa Cruz Biotechnology, Inc. Antibodies specific for stathmin (D1Y5A, 1:2500 dilution), PP2Aa (81G5, 1:2500 dilution), PP2Ab (100C1, 1:2500 dilution), and PP2Ac (52F8, 1:2500 dilution) were purchased from Cell Signaling Technology. An antibody specific for GAPDH (1:5000 dilution) was obtained from Sigma‐Aldrich. Immunoreactive bands were detected using an enhanced chemiluminescence system (Western Lightning, PerkinElmer, Inc.) after incubation with the respective horseradish peroxidase‐labeled secondary antibodies (Vector Laboratories). Relative band intensity was assessed by densitometry analysis of digitalized autographic images using ImageJ software (NIH) and normalized to that of GAPDH.
2.4. Immunofluorescent analysis
Cells grown on poly‐L‐lysine‐coated glass cover slips were fixed for 20 min at room temperature in 4% paraformaldehyde and incubated with primary antibodies against p‐stathmin or stathmin. Samples were then washed with PBS and incubated with AlexaFluor 594 goat anti‐rabbit antibody (Molecular Probes). The nuclei were counterstained with 4′,6‐diamino‐2‐phenylindole 2HCl (DAPI).
2.5. PP2A activity
The direct effect of eribulin on PP2A phosphatase activity was measured in a p‐nitrophenyl phosphate (pNPP) assay based on the ability of PP2A to catalyze hydrolysis of pNPP to p‐nitrophenol, a chromogenic product with an absorbance wavelength of 405 nm. 18 Briefly, recombinant PP2A (PP2Aα/PPP2R1A Complex, SignalChem), dissolved in a buffer comprising 50 mM Tris‐HCl (pH 8.4), 10 mM MgCl2, 0.5 M EDTA, 1 mM DTT, and 0.05 mg/ml BSA, was incubated for 5 min with 0.1–10 nM okadaic acid, eribulin, or paclitaxel. Then, pNPP (final concentration, 6.67 mM) was added to the mixture for 1 h at 37°C. Absorbance at 405 nm was measured in a spectrophotometer.
2.6. RNA extraction and real‐time RT‐PCR
Total RNA was extracted using Isogen II (Nippon Gene). RNA (100 ng) was amplified by real‐time RT‐PCR using the SYBR green Luna Universal One‐Step RT‐qPCR kit (New England Biolabs). The sense (S) and antisense (AS) primers were 5′‐CATCTGAATGAGAGTGGTGATCC‐3′ (S) and 5′‐TCTCTGGTTCCTCATGCTGCCT‐3′ (AS) for SET; 5′‐GGAAGTTCCAGATGCAGGTCCT‐3′ (S) and 5′‐AGCAGGTCACTAACAGCCAGGA‐3′ (AS) for PME1; 5′‐TGTGGCTCTACTGCGCTGGTTA‐3′ (S) and 5′‐ TCAGCCGAGGAACAGTTAGCAG‐3′ (AS) for CIP2A; 5′‐ACCGCATGACTACGCTCTTCTG‐3′(S) and 5′‐TTGAAGCGGACATTGGCAACCG‐3′(AS) for PPP2R1A; 5′‐GGTGGTCTCTCGCCATCTATAG‐ 3′(S) and 5′‐CTGGATCTGACCACAGCAAGTC‐3′(AS) for PPP2CA; and 5′‐AGCCACATCGCTCAGACA‐3′ (S) and 5′‐GCCCAATACGACCAAATCC‐3′ (AS) for GAPDH. Expression of each mRNA was normalized to that of GAPDH as a reference transcript and analyzed using the comparative Ct method. 19
2.7. RNA interference
Stathmin‐silencing small interfering (si)RNA and non‐targeting control siRNA were purchased from Sigma‐Aldrich and Qiagen, respectively. The sequences of stathmin siRNAs were as follows: 5’‐AUUGAGAUUCUUCUGCUCCUUGAGG‐3’ and 5’‐CCUCAAGGAGCAGAAGAAUCUCAAU‐3. MCF7 and MDA‐MB‐231, grown to 50% confluency in 24‐well plates, were transfected with control siRNA (50 pmol/well) or stathmin‐targeting siRNA (50 pmol/well) using Lipofectamine RNAiMAX transfection reagent (Invitrogen. After transfection for 24 h, cells were washed with PBS and cultured in fresh medium for 24 h.
2.8. Plasmid construction
The open‐reading frame of the human stathmin gene (STMN1) was amplified by PCR and then subcloned into the pTriEX vector (Novagen). MCF7 cells were transfected for 24 h with pTriEX‐control or pTriEX‐stathmin vectors using Lipofectamine 3000 (Invitrogen). After transfection, cells were given fresh medium and cultured for 24 h. Finally, cells were treated (or not) with 10 nM eribulin for 24 h to examine the effects of stathmin overexpression on cell viability.
2.9. Cell viability assay
The effects of eribulin on viability of stathmin‐knockdown or ‐overexpressing cells were determined in a WST‐8 assay (Cell Counting Kit‐8; Dojindo). Briefly, cells transfected with stathmin‐targeting siRNA or a stathmin expression vector were cultured for 1, 3, or 6 days in the presence/absence of eribulin. Next, the culture medium was replaced with fresh medium containing WST‐8. Absorbance at 460 nm was measured in a spectrophotometer. Data were expressed relative to the control value.
2.10. Statistical analysis
Data are expressed as the mean ± SEM for at least three independent experiments in duplicate. Statistical significance was determined using the Tukey–Kramer multiple comparisons test. A p‐value <.05 was considered statistically significant.
3. RESULTS
3.1. Eribulin induces phosphorylation of stathmin in breast cancer cell lines
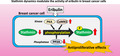
The effect of eribulin on the phosphorylation status of stathmin was examined in breast cancer cell lines MCF7 and MDA‐MB‐231 (Figure 1). Cells were treated with different concentrations of eribulin (1, 3, and 10 nM) or PTX (10 nM) for 24 h, and phosphorylation of stathmin was assessed by immunoblotting and immunofluorescence analysis (Figure 1). Treatment with eribulin increased phosphorylation of stathmin in both MCF7 and MDA‐MB‐231 cells in a dose‐dependent manner (Figure 1A, B). The total amount of stathmin, including the phosphorylated form, was not changed by eribulin treatment. PTX had no effect on stathmin phosphorylation in either cell line. Immunofluorescence analysis also revealed an intense signal corresponding to phosphorylated stathmin in eribulin‐treated cells (Figure 1C, D).
FIGURE 1.
Effects of eribulin on phosphorylation of stathmin in breast cancer cell lines. MCF7 and MDA‐MB‐231 cells were treated for 24 h with various concentrations of eribulin (1, 3, or 10 nM) or paclitaxel (PTX, 10 nM). (A, B) Cell lysates were subjected to immunoblot analysis to detect phosphorylated (p)‐stathmin and stathmin. GAPDH served as a loading control. (C, D) Immunofluorescence analysis of p‐stathmin and stathmin in eribulin‐treated MCF7 (C) and MDA‐MB‐231 cells (D). Representative data from three independent experiments in duplicate are shown
3.2. Eribulin induces phosphorylation of stathmin through the PKA and CaMKII signaling pathways
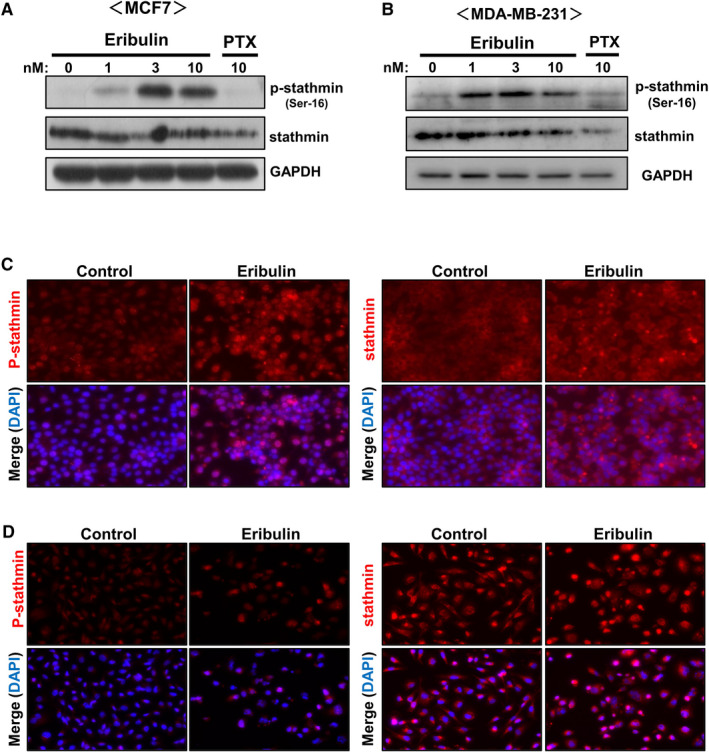
To determine the mechanism underlying eribulin‐induced phosphorylation of stathmin, we examined the effects of protein kinase inhibitors. Protein kinase A (PKA) and calcium/calmodulin‐dependent protein kinase II (CaMKII) phosphorylate stathmin. 5 , 20 As shown in Figure 2, pretreatment of MCF7 cells with a PKA inhibitor (H89, 10 µM) or a CaMKII inhibitor (KN62, 10 µM) partially suppressed eribulin‐stimulated phosphorylation of stathmin, whereas both inhibitors markedly reduced stathmin phosphorylation in MDA‐MB‐231 cells. These results indicate that eribulin induces phosphorylation of stathmin in breast cancer cells via the PKA and CaMKII signaling pathways.
FIGURE 2.
Effects of PKA and CaMKII inhibitors on eribulin‐induced phosphorylation of stathmin. MCF7 and MDA‐MB‐231 cells were treated for 1 h with inhibitors of PKA (H89, 10 µM) and CaMKII (KN62, 10 µM), and then stimulated for 24 h with 10 nM eribulin. Cell lysates were subjected to immunoblot analysis to detect p‐stathmin and stathmin. GAPDH served as a loading control. Representative data from three independent experiments in duplicate are shown
3.3. Eribulin induces phosphorylation of stathmin by downregulating expression of protein phosphatase PP2A
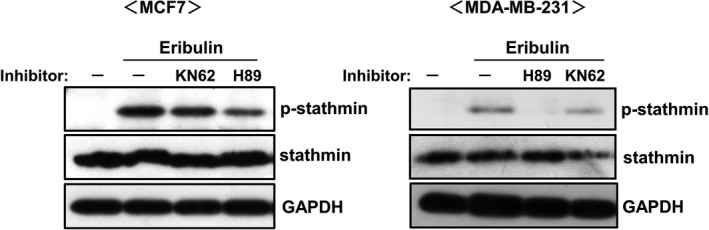
Phosphorylation of stathmin may be regulated by protein phosphatase 2A (PP2A) 21 in addition to PKA and CaMKII. Therefore, we used PP2A modulators to examine the role of PP2A in eribulin‐mediated stathmin phosphorylation (Figure 3). The PP2A inhibitor okadaic acid induced phosphorylation of stathmin in both MCF7 and MDA‐MB‐231 cells, even in the absence of eribulin. By contrast, the PP2A activator FTY720 is capable of binding the intrinsic PP2A inhibitor SET, 22 and reduced the basal level of phosphorylated stathmin. The level of eribulin‐stimulated stathmin phosphorylation was further increased by okadaic acid but decreased by FTY720. Based upon the above result showing possible involvement of PP2A in eribulin‐induced stathmin phosphorylation, we next investigated the direct effect of eribulin on PP2A activity in a pNPP assay using a recombinant PP2A protein (Figure 4A). Okadaic acid attenuated PP2A activity in a dose‐dependent manner, but neither eribulin nor PTX had a direct effect. We examined whether eribulin affects endogenous inhibitory factors SET nuclear proto‐oncogene (SET), 23 protein phosphatase methylesterase 1 (PME‐1), 24 and cancerous inhibitor of protein phosphatase 2A (CIP2A), 25 all of which modulate PP2A activity in MCF7 and MDA‐MB‐231 cells (Figure 4B). Although eribulin treatment had no effect on expression of SET and PME‐1 in MCF7 cells, it downregulated expression of CIP2A. In MDA‐MB‐231 cell, the expression of the PP2A modulators was not affected by eribulin treatment. Next, we examined the effect of eribulin on PP2A protein levels (Figure 4C). PP2A is a heterotrimeric holoenzyme comprising a core dimer composed of scaffolding A subunit (PP2Aa) and a catalytic C subunit (PP2Ac) associated with a regulatory B subunit (PP2Ab). 26 Eribulin decreased protein levels of the PP2Aa and PP2Ac subunits in both MCF7 and MDA‐MB‐231 cells.
FIGURE 3.
Effects of a PP2A inhibitor and activator on eribulin‐induced phosphorylation of stathmin. MCF7 and MDA‐MB‐231 cells were treated for 1 h with a PP2A inhibitor (okadaic acid: OA) or activator (FTY‐720: FTY), and then stimulated for 24 h with 10 nM eribulin. Cell lysates were subjected to immunoblot analysis to detect p‐stathmin and stathmin. GAPDH served as a loading control. Representative data from three independent experiments in duplicate are shown
FIGURE 4.
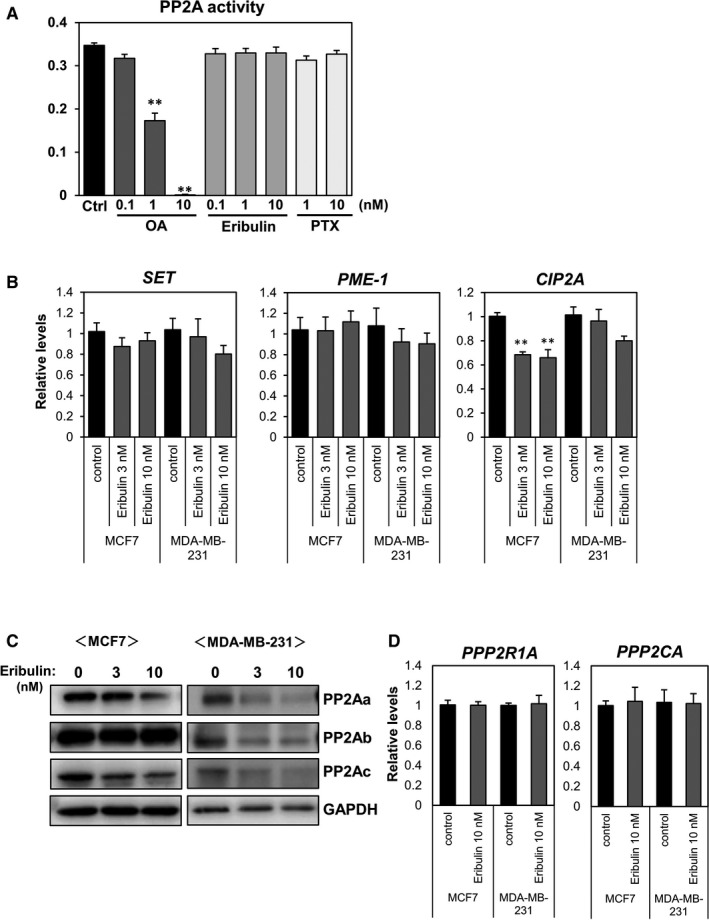
Effects of eribulin on PP2A activity and expression. (A) Recombinant PP2A was incubated for 5 min with various concentrations of okadaic acid (OA), eribulin, or paclitaxel (PTX), followed by p‐nitrophenyl phosphate (pNPP) for 1 h. Absorbance at 405 nm was measured in a microplate reader. (B) MCF7 and MDA‐MB‐231 cells were treated with eribulin for 24 h. Total RNA was analyzed by real‐time RT‐PCR to determine expression of SET, PME‐1, and CIP2A. (C) Cells were stimulated with eribulin (3 or 10 nM) for 24 h. Cell lysates were subjected to immunoblot analysis to detect PP2A subunits PP2Aa, PP2Ab, and PP2Ac. GAPDH served as a loading control. (D) Expression of PPP2R1A and PPP2CA was analyzed by real‐time RT‐PCR
In contrast, the expression of PP2Aa (PPP2R1A) and PP2Ac (PPP2CA) mRNA was not changed by eribulin treatment (Figure 4D). These results suggest that eribulin also induces phosphorylation of stathmin by downregulating PP2A level, but not its activity, in breast cancer cells.
3.4. Eribulin downregulates expression of stathmin
As shown in Figure 1, treatment of breast cancer cells with eribulin for 24 h did not affect stathmin expression. However, the effects of long‐term treatment with eribulin on stathmin expression have not been studied. To clarify the effect of long‐term exposure to eribulin on stathmin expression, MCF7 and MD‐MB‐231 cells were treated for 3 or 6 days with eribulin, and stathmin expression was examined by immunoblotting (Figure 5A). Treatment with eribulin for 3 or 6 days reduced stathmin expression by both cell lines (Figure 5A). In addition, cell viability was decreased (Figure 5B). Thus, eribulin not only induces phosphorylation of stathmin but also reduces stathmin protein expression upon long‐term exposure.
FIGURE 5.
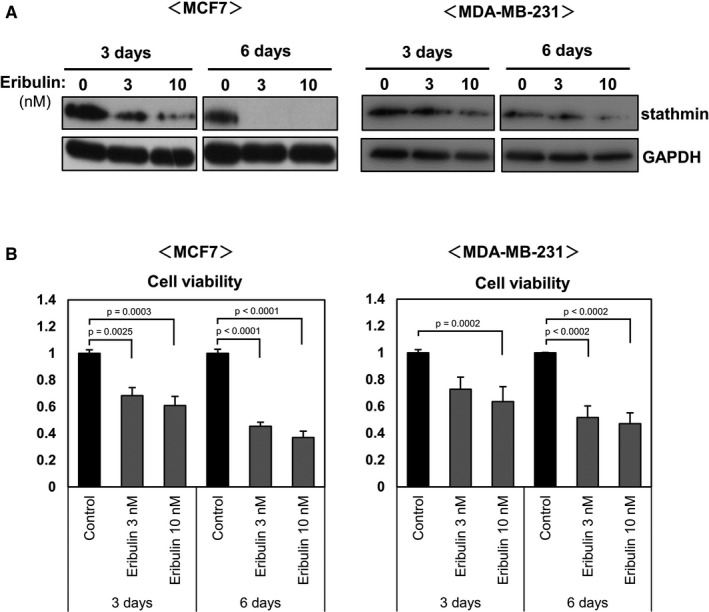
Effects of eribulin on stathmin expression in breast cancer cell lines. MCF7 and MDA‐MB‐231 cells were treated for 3 or 6 days with various concentrations of eribulin (1, 3, or 10 nM). A: Cell lysates were subjected to immunoblot analysis to detect stathmin. GAPDH served as a loading control. B: Cell viability was evaluated in a WST‐8 assay
3.5. Stathmin modulates the antiproliferative effects of eribulin
To investigate the role of stathmin in the antiproliferative activity of eribulin, we examined the effects of stathmin knockdown on eribulin‐induced antiproliferative activity (Figure 6). Transfection of MCF7 or MDA‐MB‐231 cells with stathmin‐targeting siRNA suppressed endogenous expression of stathmin protein (Figure 6A). Next, cells transfected with control or stathmin siRNA were cultured in the presence or absence of eribulin (10 nM), and cell viability was evaluated after 24 h. Cell viability tended to decrease only in stathmin‐knockdown cells. Eribulin treatment decreased cell viability only in MDA‐MB‐231 cells. Stathmin knockdown had no effect on the efficacy of eribulin in either cell line (Figure 6A). Next, we examined the effects of stathmin overexpression on the activity of eribulin in MCF7 cells (Figure 6B, C). Overexpression of stathmin induced by a stathmin vector was confirmed by immunoblotting (Figure 6B). The viability of stathmin‐overexpressing cells was similar to that of control cells. However, the viability of stathmin‐overexpressing cells exposed to eribulin was markedly lower than that of eribulin‐treated control cells (Figure 6C). These results indicate that eribulin has antitumor activity in breast cancer cells expressing high levels of stathmin.
FIGURE 6.
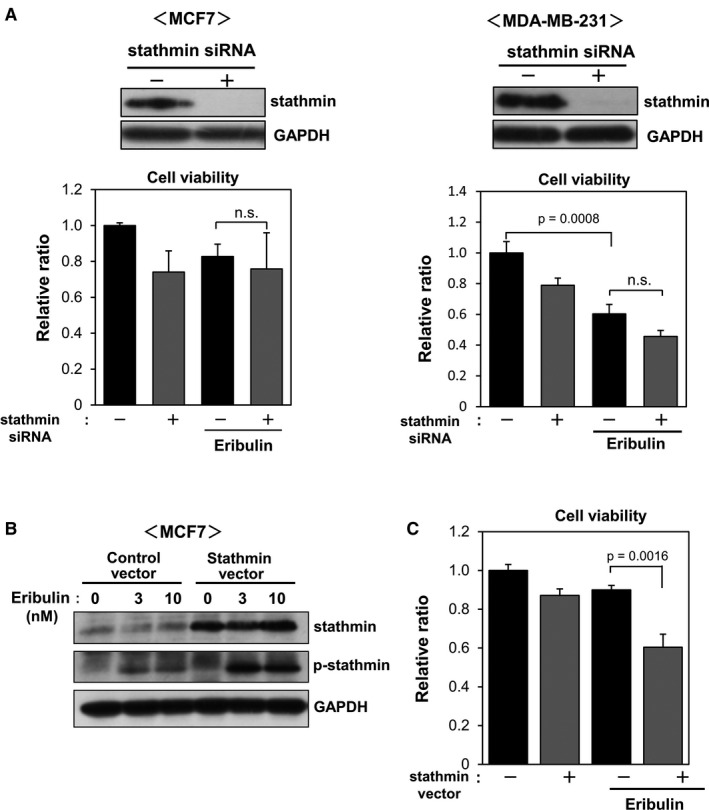
Effects of stathmin knockdown and overexpression on cell viability. (A) MCF7 and MDA‐MB‐231 cells were transfected with stathmin‐targeting or control siRNA for 2 days and then stimulated with eribulin for 24 h. Cell lysates were subjected to immunoblot analysis to detect stathmin and GAPDH. Cell viability was evaluated in a WST‐8 assay. (B, C) MCF7 cells were transfected with a control or stathmin vector and then treated with various concentrations of eribulin (3 and 10 nM) for 24 h. Cell lysates were subjected to immunoblot analysis to detect stathmin and p‐stathmin (B). Cell viability was evaluated in a WST‐8 assay (C)
4. DISCUSSION
Here, we showed that eribulin induces phosphorylation of stathmin in hormone receptor‐positive and triple‐negative breast cancer cell lines (Figure 7). Several protein kinases, including PKA and CaMKII, phosphorylate stathmin on Ser‐16, which is the major site that regulates microtubule‐destabilizing activity. 5 , 20 The reduction in eribulin‐induced stathmin phosphorylation by PKA and CaMKII inhibitors implies the involvement of these kinases in phosphorylation. The phosphorylation status of stathmin is also modulated by phosphatases, including PP2A. 6 The stimulatory action of the PP2A inhibitor okadaic acid on eribulin‐induced phosphorylation of stathmin, along with the inhibitory effect of the PP2A activator FTY720, suggests the inhibitory action of eribulin on PP2A‐mediated stathmin dephosphorylation. Of note, eribulin did not abrogate PP2A activity directly,rather, it downregulated the level of its subunits in breast cancer cells. Our results that eribulin did not affect mRNA expression of PP2A subunits suggest the post‐transcriptional downregulation of PP2A by eribulin. There were no significant changes in expression of endogenous PP2A inhibitors SET, PME‐1, and CIP2A in eribulin‐treated MCF7 and MDA‐MB‐231 cells (except CIP2A in MCF7 cells), supporting the notion that decreased levels of PP2A, but not its activity, is associated with increased phosphorylation of stathmin by eribulin. Thus, eribulin may phosphorylate stathmin by activating the PKA and CaMKII pathways, and by downregulating PP2A level in breast cancer cells (Figure 7). Since phosphorylation of stathmin turns off the microtubule depolymerization activity, eribulin‐induced phosphorylation of stathmin may cause dysfunction of microtubule dynamics and cell cycle arrest. Some studies show that chemotherapeutic agents that target microtubules affect the phosphorylation status of stathmin. Vinblastine, a microtubule destabilizer, increases stathmin phosphorylation in a non‐small cell lung cancer (NSCLC) cell line, 27 and Machado‐Neto et al 28 showed that PTX, a microtubule stabilizer, induces stathmin phosphorylation in acute lymphoblastic leukemia cells. Vinblastine reduces PP2A expression, whereas PTX increases it markedly, in NSCLC cells. 27 Because we found no significant effect of PTX on stathmin phosphorylation in breast cancer cell lines, the action of microtubule‐targeting drugs on phosphorylation may vary according to cancer cell type.
FIGURE 7.
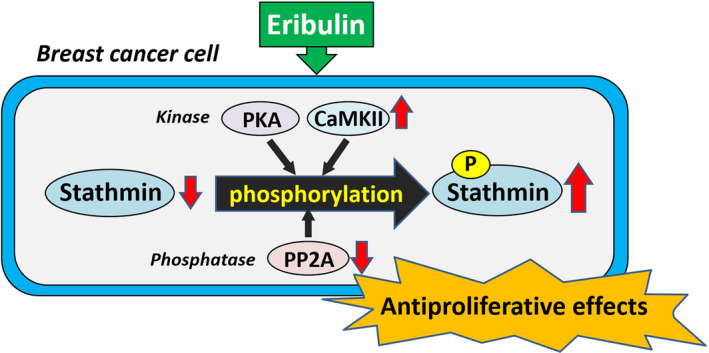
Proposed mechanism by which eribulin exerts antiproliferative effects through modulating stathmin dynamics in breast cancer cell. Eribulin phosphorylates stathmin by activating the PKA and CaMKII pathways, and by downregulating PP2A level in breast cancer cells. Overexpression of stathmin potentiates the antiproliferative effects of eribulin
The molecular mechanisms by which eribulin activates PKA and CaMKII, and downregulates PP2A level, remain unknown; further studies should clarify the mechanism by which eribulin exerts post‐transcriptional regulation of stathmin.
In addition to eribulin‐induced stathmin phosphorylation, we also found that continuous exposure to eribulin reduced stathmin expression by breast cancer cell lines. Regarding the relationship between stathmin expression and microtubule‐targeting agents, previous reports suggest that paclitaxel decreases stathmin expression in NSCLC and nasopharyngeal carcinoma cells. 27 , 29 Because overexpression of stathmin is associated with a poor prognosis and with chemoresistance in a variety of cancer cell types, 30 the inhibitory effects of eribulin on stathmin expression may increase sensitivity to other anticancer drugs. Notably, we showed that overexpression of stathmin, but not its knockdown, augments the antiproliferative effect of eribulin in breast cancer cells. These results suggest that stathmin plays a major role in the antiproliferative action of eribulin (Figure 7), and that it is a potential predictive biomarker for breast cancer responses to eribulin treatment. But the main limitation of the present study is the lack of in vivo studies using animal model and the patients. There is no evidence that similar changes in stathmin dynamics observed in this study are reflected in cancer tissues of the patients. Further studies would be required to evaluate the significance of stathmin as a biomarker for efficacy of eribulin against cancer therapy.
Because stathmin expression is associated with cell proliferation, downregulation of stathmin expression after continuous eribulin treatment is probably caused by eribulin‐induced suppression of proliferation. Based upon the results of stathmin overexpression, it is also conceivable that cells expressing substantial level of stathmin are sensitive to eribulin and preferentially removed, and cells with relatively low levels of stathmin may survive because of their low sensitivity. Our results presented herein highlight the importance of determination of the molecular mechanisms by which eribulin downregulates stathmin expression in breast cancer cells.
Some studies show that the affinity of stathmin for tubulin is increased in the presence of microtubule destabilizers, vinblastine or vinflunine, suggesting that stathmin modulates the activity of microtubule‐targeting agents. 31 , 32 Since abnormally high levels of stathmin are associated with human malignancies and correlate with poor prognosis of breast cancer, 12 , 13 , 14 eribulin may effectively inhibit proliferation of stathmin‐overexpressing cancer cells. Also, a combination of PTX and stathmin knockdown inhibits growth and promotes apoptosis of nasopharyngeal, 33 gastric, 34 and endometrial 35 carcinoma cells. Therefore, combined treatment with eribulin, which downregulates stathmin, may increase the antiproliferative effects of PTX against drug‐resistant cancer cells.
In conclusion, we demonstrated a novel mechanism by which eribulin exerts antiproliferative effects in breast cancer cells; these effects are mediated by modulation of stathmin dynamics (Figure 7). Taken together, the results suggest that stathmin is a possible biomarker for predicting the therapeutic effects of eribulin in breast cancer patients.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
M.Y. and K.T. conceived and designed the study. M.Y., A.I., H.A., N.N., and M.A. performed the experiments. M.Y. and K.T. wrote the manuscript. All authors interpreted the data. All authors reviewed the manuscript.
ACKNOWLEDGMENT
We thank Eisai Co., Ltd., for providing eribulin.
Contributor Information
Mikihiro Yoshie, Email: yoshie@toyaku.ac.jp.
Kazuhiro Tamura, Email: yoshie@toyaku.ac.jp, Email: hiro@toyaku.ac.jp.
DATA AVAILABILITY STATEMENT
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Jordan MA, Kamath K, Manna T, et al. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol Cancer Ther. 2005;4(7):1086‐1095. [DOI] [PubMed] [Google Scholar]
- 2. Funahashi Y, Okamoto K, Adachi Y, et al. Eribulin mesylate reduces tumor microenvironment abnormality by vascular remodeling in preclinical human breast cancer models. Cancer Sci. 2014;105(10):1334‐1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yoshida T, Ozawa Y, Kimura T, et al. Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial‐mesenchymal transition (EMT) to mesenchymal‐epithelial transition (MET) states. Br J Cancer. 2014;110(6):1497‐1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. A. Curmi P, Gavet O, Charbaut E, et al. Stathmin and its phosphoprotein family. General properties, biochemical and functional interaction with tubulin. Cell Struct Funct. 1999;24(5):345‐357. 10.1247/csf.24.345. [DOI] [PubMed] [Google Scholar]
- 5. Gradin HM, Larsson N, Marklund U, Gullberg M. Regulation of microtubule dynamics by extracellular signals: cAMP‐dependent protein kinase switches off the activity of oncoprotein 18 in intact cells. J Cell Biol. 1998;140(1):131‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mistry SJ, Li HC, Atweh GF. Role for protein phosphatases in the cell‐cycle‐regulated phosphorylation of stathmin. Biochem J. 1998;334(1):23‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ghosh R, Gu G, Tillman E, et al. Increased expression and differential phosphorylation of stathmin may promote prostate cancer progression. Prostate. 2007;67(10):1038‐1052. [DOI] [PubMed] [Google Scholar]
- 8. Obayashi S, Horiguchi J, Higuchi T, et al. Stathmin1 expression is associated with aggressive phenotypes and cancer stem cell marker expression in breast cancer patients. Int J Oncol. 2017;51(3):781‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Price DK, Ball JR, Bahrani‐Mostafavi Z, et al. The phosphoprotein Op18/stathmin is differentially expressed in ovarian cancer. Cancer Invest. 2000;18(8):722‐730. [DOI] [PubMed] [Google Scholar]
- 10. Yurong L, Biaoxue R, Wei L, et al. Stathmin overexpression is associated with growth, invasion and metastasis of lung adenocarcinoma. Oncotarget. 2017;8(16):26000‐26012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Friedrich B, Grönberg H, Landström M, Gullberg M, Bergh A. Differentiation‐stage specific expression of oncoprotein 18 in human and rat prostatic adenocarcinoma. Prostate. 1995;27(2):102‐109. 10.1002/pros.2990270207. [DOI] [PubMed] [Google Scholar]
- 12. Askeland C, Wik E, Finne K, et al. Stathmin expression associates with vascular and immune responses in aggressive breast cancer subgroups. Sci Rep. 2020;10(1):2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bieche I, Lachkar S, Becette V, et al. Overexpression of the stathmin gene in a subset of human breast cancer. Br J Cancer. 1998;78(6):701‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Curmi P, Nogues C, Lachkar S, et al. Overexpression of stathmin in breast carcinomas points out to highly proliferative tumours. Br J Cancer. 2000;82(1):142‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meng XL, Su D, Wang L, et al. Low expression of stathmin in tumor predicts high response to neoadjuvant chemotherapy with docetaxel‐containing regimens in locally advanced breast cancer. Genet Test Mol Biomarkers. 2012;16(7):689‐694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rana S, Maples PB, Senzer N, Nemunaitis J. Stathmin 1: a novel therapeutic target for anticancer activity. Expert Rev Anticanc. 2008;8(9):1461‐1470. 10.1586/14737140.8.9.1461. [DOI] [PubMed] [Google Scholar]
- 17. Alday PH, Correia JJ. Macromolecular interaction of halichondrin B analogues eribulin (E7389) and ER‐076349 with tubulin by analytical ultracentrifugation. Biochemistry. 2009;48(33):7927‐7938. 10.1021/bi900776u. [DOI] [PubMed] [Google Scholar]
- 18. Ikehara T, Imamura S, Yoshino A, Yasumoto T. PP2A inhibition assay using recombinant enzyme for rapid detection of okadaic acid and its analogs in shellfish. Toxins (Basel). 2010;2(1):195‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yoshie M, Kaneyama K, Kusama K, et al. Possible role of the exchange protein directly activated by cyclic AMP (Epac) in the cyclic AMP‐dependent functional differentiation and syncytialization of human placental BeWo cells. Hum Reprod. 2010;25(9):2229‐2238. [DOI] [PubMed] [Google Scholar]
- 20. le Gouvello S, Manceau V, Sobel A. Serine 16 of stathmin as a cytosolic target for Ca2+/calmodulin‐dependent kinase II after CD2 triggering of human T lymphocytes. J Immunol. 1998;161(3):1113‐1122. [PubMed] [Google Scholar]
- 21. Tournebize R, Andersen SS, Verde F, Dorée M, Karsenti E, Hyman AA. Distinct roles of PP1 and PP2A‐like phosphatases in control of microtubule dynamics during mitosis. EMBO J. 1997;16(18):5537‐5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oaks JJ, Santhanam R, Walker CJ, et al. Antagonistic activities of the immunomodulator and PP2A‐activating drug FTY720 (Fingolimod, Gilenya) in Jak2‐driven hematologic malignancies. Blood. 2013;122(11):1923‐1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li M, Makkinje A, Damuni Z. The myeloid leukemia‐associated protein SET is a potent inhibitor of protein phosphatase 2A. J Biol Chem. 1996;271(19):11059‐11062. [DOI] [PubMed] [Google Scholar]
- 24. Ogris E, Du X, Nelson KC, et al. A protein phosphatase methylesterase (PME‐1) is one of several novel proteins stably associating with two inactive mutants of protein phosphatase 2A. J Biol Chem. 1999;274(20):14382‐14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Junttila MR, Puustinen P, Niemelä M, et al. CIP2A inhibits PP2A in human malignancies. Cell. 2007;130(1):51‐62. [DOI] [PubMed] [Google Scholar]
- 26. Baskaran R, Velmurugan BK. Protein phosphatase 2A as therapeutic targets in various disease models. Life Sci. 2018;210:40‐46. [DOI] [PubMed] [Google Scholar]
- 27. Shen F, Long D, Yu T, et al. Vinblastine differs from Taxol as it inhibits the malignant phenotypes of NSCLC cells by increasing the phosphorylation of Op18/stathmin. Oncol Rep. 2017;37(4):2481‐2489. [DOI] [PubMed] [Google Scholar]
- 28. Machado‐Neto JA, Rodrigues Alves APN, Fernandes JC, et al. Paclitaxel induces Stathmin 1 phosphorylation, microtubule stability and apoptosis in acute lymphoblastic leukemia cells. Heliyon. 2017;3(9):e00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu Y, Tang M, Wu Y, et al. A combination of paclitaxel and siRNA‐mediated silencing of Stathmin inhibits growth and promotes apoptosis of nasopharyngeal carcinoma cells. Cell Oncol. 2014;37(1):53‐67. 10.1007/s13402-013-0163-3. [DOI] [PubMed] [Google Scholar]
- 30. Sun R, Liu Z, Wang L, et al. Overexpression of stathmin is resistant to paclitaxel treatment in patients with non‐small cell lung cancer. Tumour Biol. 2015;36(9):7195‐7204. [DOI] [PubMed] [Google Scholar]
- 31. Devred F, Tsvetkov PO, Barbier P, et al. Stathmin/Op18 is a novel mediator of vinblastine activity. FEBS Lett. 2008;582(17):2484‐2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Malesinski S, Tsvetkov PO, Kruczynski A, Peyrot V, Devred F. Stathmin potentiates vinflunine and inhibits Paclitaxel activity. PLoS One. 2015;10(6):e0128704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lin X, Yu T, Zhang L, et al. Silencing Op18/stathmin by RNA interference promotes the sensitivity of nasopharyngeal carcinoma cells to taxol and high‐grade differentiation of xenografted tumours in nude mice. Basic Clin Pharmacol Toxicol. 2016;119(6):611‐620. [DOI] [PubMed] [Google Scholar]
- 34. Bai T, Yokobori T, Altan B, et al. High STMN1 level is associated with chemo‐resistance and poor prognosis in gastric cancer patients. Br J Cancer. 2017;116(9):1177‐1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Werner HM, Trovik J, Halle MK, et al. Stathmin protein level, a potential predictive marker for taxane treatment response in endometrial cancer. PLoS One. 2014;9(2):e90141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.


