Abstract
Background
This systematic review and meta-analysis aimed to compare the levels of von Willebrand Factor (vWF) antigen in patients with coronavirus disease 2019 (COVID-19) with a poor outcome compared with those with a good outcome, and explored factors that may affect the difference in terms of vWF antigen between the two groups.
Methods
A comprehensive literature search of PubMed, Embase and Scopus databases was undertaken from inception until 7 April 2021. The primary outcome was poor outcome, which is a composite of mortality and severity of COVID-19.
Results
Ten studies including a total of 996 patients were included in this systematic review and meta-analysis. vWF antigen was higher in patients with poor outcomes [standardized mean difference (SMD) 0.84 [0.45–1.23], P<0.001; I2=87.3, P<0.001). For subgroup analysis on studies that reported the vWF antigen level as a percentage, the mean difference was 121.6 [(53.7–189.4), P<0.001; I2=92.0, P<0.001]. Meta-regression showed that the SMD between poor outcome and good outcome was affected by the platelet count (coefficient 0.0061, P=0.001), d-dimer level (coefficient 0.0007, P=0.026) and factor VIII level (coefficient 0.0057, P=0.031), but not by age (coefficient -0.0610, P=0.440), gender (coefficient 0.0135, P=0.698), obesity (coefficient 0.0282, P=0.666), hypertension (coefficient 0.0273, P=0.423), diabetes (coefficient 0.0317, P=0.398) or malignancy (coefficient 0.0487, P=0.608).
Conclusion
This meta-analysis showed that the level of vWF antigen was significantly higher in patients with COVID-19 with a poor outcome, signalling marked endotheliopathy. Meta-regression showed that the differences became larger as the platelet count, d-dimer level and factor VIII level increased.
Keywords: Coronavirus, Coagulopathy, Endothelial dysfunction, Thrombosis, Mortality
Background
Coronavirus disease 2019 (COVID-19) is currently one of the most common diseases in the world, and has a considerable death toll (WHO, 2021). Although most patients have mild–moderate clinical manifestations, a significant proportion of patients develop life-threatening complications (Lim et al., 2020; Pranata et al., 2020a, 2021c). Complications caused by coagulopathy are among the most important. Activation of the coagulation pathway and endothelial cells (ECs) is a hallmark of severe COVID-19, which is consistent with high rates of venous thromboembolism (VTE), pulmonary embolism (PE) and disseminated intravascular coagulation (DIC) (Mancini et al., 2021a; Ward et al., 2021). von Willebrand factor (vWF) is a platelet adhesive and aggregator protein which carries coagulation factor VIII, produced exclusively by ECs and megakaryocytes. Thus, vWF acts as a marker of EC activation, and is released after inflammation-mediated vascular damage (Mancini et al., 2021a). The present systematic review and meta-analysis aimed to compare vWF antigen levels in patients with COVID-19 with a poor outcome compared with those with a good outcome, and explored factors that may affect the difference in terms of vWF antigen between the two groups.
Methods
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines, and is registered in PROSPERO (CRD42021247507).
Eligibility criteria
The inclusion criteria were: (1) observational prospective and retrospective studies reporting patients with COVID-19; (2) studies reporting vWF antigen levels in patients with a poor outcome and patients with a good outcome; and (3) studies reporting mortality/severity/acute respiratory distress syndrome/need for intensive care unit (ICU) or high dependency unit (HDU) admission or mechanical ventilation.
Articles published as pre-prints, reviews, non-research letters or commentaries/viewpoints/editorials, and articles published in any language other than English were excluded
The primary outcome of this study was poor outcome, which is a composite of mortality and severity. COVID-19 was defined as severe if it met the criteria for severe pneumonia (Metlay et al., 2019) or required ICU/HDU care or mechanical ventilation.
Search strategy and study selection
A comprehensive literature search of PubMed, Embase and Scopus databases was undertaken from inception until 7 April 2021 using the following keywords: ‘2019-nCoV’ OR ‘SARS-CoV-2’ OR ‘COVID-19’ AND ‘von Willebrand’ OR ‘vWF’ OR ‘endothelium’. The PubMed search strategy was ((2019-nCoV) OR (SARS-CoV-2) OR (COVID-19)) AND ((von Willebrand) OR (vWF) OR (endothelium)). Titles and abstracts were screened by two independent authors after duplicates had been removed. Article eligibility was assessed using the inclusion and exclusion criteria above.
Data extraction
The following data were extracted from eligible studies using a standardized extraction form: author, study design, year of publication, age, gender, obesity, hypertension, diabetes, malignancy, platelet count, d-dimer level, factor VIII level, and primary outcome. Data extraction was performed by two independent authors, and discrepancies were resolved through discussion.
The primary outcome was poor outcome; the pooled effect estimate was standardized mean difference (SMD) in terms of vWF antigen level between patients with a poor outcome and patients with a good outcome. Effect estimates were reported along with standard deviation (SD).
Risk-of-bias assessment
Two independent authors assessed the risk of bias using the Newcastle–Ottawa Scale. Discrepancies that arose were resolved by discussion.
Statistical analysis
This meta-analysis was performed using Stata Version 16 (StataCorp, College Station, TX, USA). Continuous variables were pooled using Hedges’ random-effects method to populate the pooled SMD in terms of Hedges’ g and SDs. Restricted maximum likelihood (REML) random-effects models were used for the meta-analysis regardless of heterogeneity. P-values <0.05 were taken to indicate statistical significance. All P-values were two-tailed, and statistical significance was set at ≤0.05. Heterogeneity was assessed using the Cochran Q test and I 2 statistic, with P<0.10 or I 2>50% taken to indicate significant heterogeneity. Subgroup analysis to calculate the mean difference instead of SMD was performed for studies that reported vWF antigen as a percentage. Funnel plot analysis was used for qualitative measurement of publication bias, followed by non-parametric trim-and-fill analysis using Run 0 estimator. Egger's test was used for quantitative assessment of the potential for small study effects. REML random-effects meta-regression was performed for age, gender, obesity, hypertension, diabetes, malignancy, platelet count, d-dimer level and factor VIII level.
Results
Baseline characteristics
Ten studies including a total of 996 patients were included in this systematic review and meta-analysis (Figure 1 ) (Goshua et al., 2020; Rauch et al., 2020;Cugno et al., 2021; De Jongh et al., 2021; Mancini et al., 2021b; Philippe et al., 2021; Rodríguez Rodríguez et al., 2021; Sweeney et al., 2021; Vassiliou et al., 2021; von Meijenfeldt et al., 2021). The baseline characteristics of the included studies are presented in Table 1 .
Figure 1.
PRISMA flowchart. COVID-19, coronavirus disease 2019.
Table 1.
Baseline characteristics of the included studies.
| Authors | Design | Samples | Severe (%) | Age (years) | Male (%) | Obesity (%) | Hypertension (%) | Diabetes (%) | Malignancy (%) | Platelets (x109) | D-dimer (ng/mL) | FVIII (%) | Outcome | Percentage of outcome (%) | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cugno 2020 | Cohort | 148 | 31.1 | 63 | 58.8 | NA | NA | NA | NA | 245 | 1071 | NA | Severity | 31.1 | 6 |
| De Jongh 2021 | CS | 16 | 100 | 67 | NA | NA | NA | NA | NA | 286 | 1600 | 332 | Mortality | 31.3 | 5 |
| Goshua 2020 | CS | 68 | 70.6 (ICU) | 62 | 60 | 37 | 56 | 29 | 4 | NA | 1585 | 354 | ICU | 70.6 | 7 |
| Mancini 2020 | CS | 50 | 38.0 (HDU) | 59 | 64 | 22 | 28 | 8 | 4 | 328 | NA | NA | HDU | 38.0 | 6 |
| Philippe 2021 | CS | 50 | 48.1 (critical) | 63 | 65.9 | 27.6 | 55.7 | 29.2 | 15.7 | NA | 2638 | NA | Critical | 48.1 | 7 |
| Rauch 2020 | PC | 243 | NA | 64 | 63.8 | NA | 48.6 | 23 | 9.5 | 228 | 1000 | 241 | 30-day agg | 29.2 | 8 |
| Rodriguez 2021 | RC | 100 | 50 | 60.5 | 70 | NA | NA | NA | NA | 326 | 1089 | NA | Severity | 50.0 | 6 |
| Sweeney 2021 | CC | 181 | NA | 67 | 59 | NA | NA | NA | NA | 213 | 1800 | 175 | Mortality | 49.7 | 6 |
| Vassiliou 2021 | PC | 38 | 100 | 64 | 81.6 | NA | 44.7 | 13.2 | NA | 227 | 390 | NA | Mortality | 26.3 | 6 |
| von Meijinfeldt 2021 | PC | 102 | 11.8 (HDU) | 59.5 | 64 | NA | NA | 25.5 | NA | 231 | 1110 | 219 | HDU | 11.8 | 7 |
CS, cross-sectional; FVIII, factor VIII; ICU, intensive care unit; HDU, high-dependency unit; PC, prospective cohort; RC, retrospective cohort; NA, not available; NOS, Newcastle–Ottawa Scale.
vWF antigen and outcome
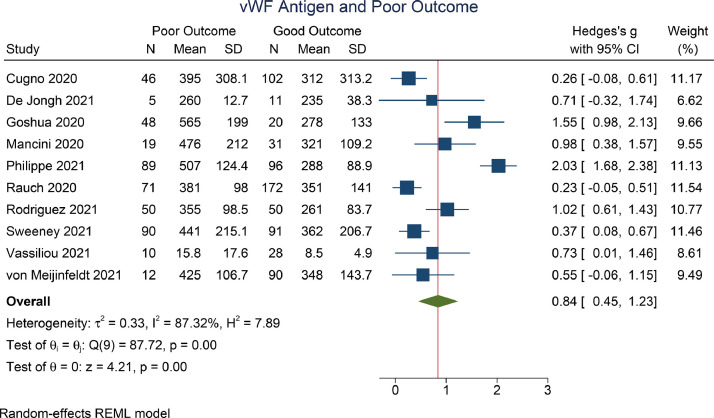
The vWF antigen level was higher in patients with a poor outcome [SMD 0.84 (0.45–1.23), P<0.001; I 2=87.3, P<0.001] (Figure 2 ). For subgroup analysis on studies that reported the vWF antigen level as a percentage, the mean difference was 121.6 [(53.7–189.4), P<0.001; I 2=92.0, P<0.001)] (Figure 3 ).
Figure 2.
von Willebrand factor (vWF) antigen and poor outcome. REML, restricted maximum likelihood; SD, standard deviation; CI, confidence interval.
Figure 3.
von Willebrand factor antigen and poor outcome (percentage subgroup). REML, restricted maximum likelihood; SD, standard deviation; CI, confidence interval.
Meta-regression
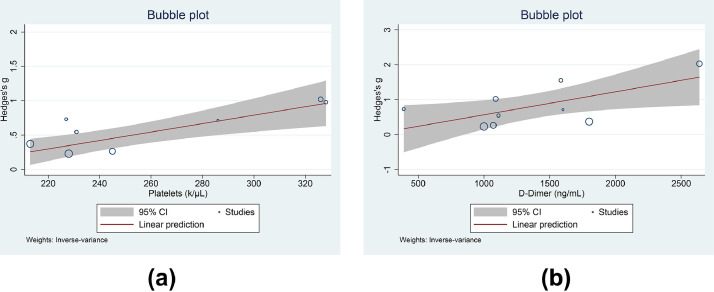
Meta-regression showed that the SMD between poor outcome and good outcome was affected by the platelet count (coefficient 0.0061, P=0.001) (Figure 4 A), d-dimer level (coefficient 0.0007, P=0.026) (Figure 4B) and factor VIII level (coefficient 0.0057, P=0.031), but not by age (coefficient -0.0610, P=0.440), gender (coefficient 0.0135, P=0.698), obesity (coefficient 0.0282, P=0.666), hypertension (coefficient 0.0273, P=0.423), diabetes (coefficient 0.0317, P=0.398) or malignancy (coefficient 0.0487, P=0.608).
Figure 4.
Meta-regression analysis for von Willebrand factor antigen and poor outcome with platelet count (A) and d-dimer level (B) as covariates. CI, confidence interval.
Funnel plot
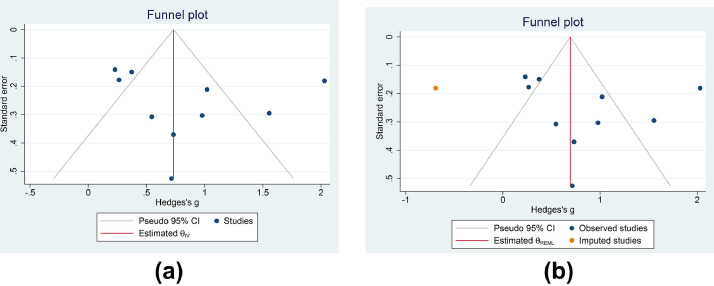
Qualitative assessment of the funnel plot indicates an asymmetrical shape (Figure 5 A), and subsequent non-parametric trim-and-fill analysis (Run 0) showed that imputation of one study on the left side of the plot resulted in an SMD of 0.693 (0.244–1.142) (Figure 5B). Quantitative Eggers's test indicated no indication of small study effects (P=0.767).
Figure 5.
Publication bias. Funnel plot analysis (A) and trim-and-fill analysis (B). CI, confidence interval.
Discussion
This meta-analysis showed that the level of vWF antigen was significantly higher in patients with a poor outcome. Meta-regression showed that the differences became larger as the platelet count, d-dimer level and factor VIII level increased.
Activation of the coagulation pathway and ECs is found in patients with severe COVID-19, which may result in VTE, PE and DIC (Mancini et al., 2021a; Ward et al., 2021). These biological mechanisms play a role in the pathophysiology of serious complications of COVID-19, including cardiorespiratory collapse, thrombotic and bleeding events, sepsis, multiple organ dysfunction, and death (Lim et al., 2020). Marked hypercoagulability is characterized by changes in various inflammatory coagulation biomarkers, including D-dimer which is a biomarker for thrombosis and an independent predictor of poor clinical outcome; fibrinogen and fibrin degradation product which indicate blood viscosity and fibrinolysis; P-selectin which modulates the interaction between ECs and blood cells; and vWF which is a marker of EC damage and bleeding (when low) and thrombotic (when high) (Grobler et al., 2020; Ladikou et al., 2020; Mancini et al., 2021a). The D-dimer level often rises at a relatively early stage, while the fibrinogen level and platelet count change over the course of the disease (Grobler et al., 2020; Huang et al., 2020; Pranata et al., 2021e). vWF is a platelet adhesive and aggregator protein which carries coagulation factor VIII, produced exclusively by ECs and megakaryocytes. The levels of vWF and factor VIII are often massively elevated (more than four times the upper limit of normal) in patients with COVID-19, which is comparable to patients admitted to the ICU with severe sepsis (Escher et al., 2020; Grobler et al., 2020; Ladikou et al., 2020; Zachariah et al., 2020). Apart from its role in primary haemostasis, vWF is also a marker of EC activation, released after inflammation-mediated vascular damage (Mancini et al., 2021a). An increased level of vWF antigen is consistent with EC activation and reflects disease severity, but plasma vWF propeptide has been shown to be a more sensitive and specific indicator of acute EC activation due to a shorter plasma half-life (∼2 versus 12 h), and its levels are not consumed by platelet aggregation or influenced by ABO blood group (Mancini et al., 2021a; Philippe et al., 2021; Ward et al., 2021). However, the plasma vWF propeptide/vWF antigen ratio was decreased, suggesting that a reduced VWF clearance rate plays a role in increasing the vWF antigen level in patients with severe COVID-19 (Ward et al., 2021). vWF activity, vWF antigen level, D-dimer level and factor VIII clotting activity were persistently and massively elevated, while ADAMTS13 (A Disintegrin And Metalloprotease with ThromboSpondin 1 repeats, number 13) activity and platelet count were relatively normal in the majority of patients with severe COVID-19. This suggests that coagulopathy may be a different form of highly prothrombotic alteration, most likely an endothelial disease (Escher et al., 2020). Due to the ultra-large size of vWF multimers (5000–10,000 kDa), their high levels can only be reduced by means of plasma exchange (haemodialysis only removes molecules <60 kDA in size). As vWF molecules are cleared by macrophages, the activation of EC (depicted by increased levels of vWF) may contribute to the activation of macrophages in patients with COVID-19 (Zachariah et al., 2020).
ECs that line blood vessels normally function to prevent pathological thrombosis. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) gains cellular entry to human cells using angiotensin-converting enzyme 2 receptors, which are found in the ECs of various organs and tissues (Lim et al., 2020; Ward et al., 2021). EC activation in response to high shear stress and other inflammatory mediators results in the substantial release of vWF multimers into the circulation; these are cleaved and can be activated by metalloprotease ADAMTS13 (Grobler et al., 2020; Yang et al., 2020; Mancini et al., 2021a). A decrease in ADAMTS1 was seen in some patients with COVID-19, which suggests the loss of vWF cleaving protease and subsequently its activity, as well as increased risk of thrombosis in patients with myocardial infarction and ischaemic stroke (Ladikou et al., 2020). Severe ADAMTS13 deficiency (activity <10 IU/dL) indicates thrombotic thrombocytopenic purpura, a life-threatening diffuse thrombotic microangiopathy caused by the accumulation of hyperactive vWF multimers (Escher et al., 2020; Mancini et al., 2021a; Philippe et al., 2021). Imbalance between vWF and ADAMTS13 could lead to a prothrombotic state in inflammatory-driven conditions, as seen in sepsis and DIC (Yang et al., 2020; Mancini et al., 2021a).
Once activated, platelets can bind to vWF via an exposed binding site for GPIbα (part of the GPIb-IX-V receptor complex), initiating the thrombogenic process and leading to integrin αIIbβ3 activation and the pivotal role of the FcRγ chain and FcγRIIa immunoreceptor tyrosine-based activation motif pathway. vWF binding to the upregulated αIIbβ3 integrin promotes platelet adhesion and aggregation, and vWF binding to fibrinogen augments thrombus formation (Grobler et al., 2020; Mancini et al., 2021a). αvβ3 integrin is the best-characterized EC receptor for vWF, which relates to EC (and smooth muscle cell) adhesion, migration proliferation, differentiation and survival. Complex responses that depend on αvβ3 function include angiogenesis, vasculogenesis and vascular cell survival, and it also plays a crucial role in inflammatory endothelial responses (Grobler et al., 2020). vWF can bind to red blood cells under conditions such as decreased shear rates. After inflammatory damage and increased generation of reactive oxygen species (Grobler et al., 2020), vWF is released from Weibel-Palade bodies of ECs. Some enters the circulation, and some remains bound to the EC surface (Philippe et al., 2021). During inflammation (and oxidative stress), red blood cells may undergo eryptosis, characterized by cell shrinkage, membrane blebbing and cell membrane scrambling. vWF mediates erythrocyte–erythrocyte linking as well as platelet-independent erythrocyte adhesion to ECs, causing microvascular occlusion and interfering with dynamic blood flow (Nicolay et al., 2018; Grobler et al., 2020).
Bleeding and thrombotic pathologies often occur in patients with multiple risk factors that are likely to create severe symptoms and complications. Old age, excessive body mass index, debilitating and frail conditions, and various chronic, non-communicable diseases are comorbidities associated with worse outcomes in patients with COVID-19 (Tuty Kuswardhani et al., 2020; Martha et al., 2021; Pranata et al., 2021, 2021a, 2021b, 2021c). These unfavourable conditions drive chronic and systemic inflammation, even in individuals without COVID-19. Pro-inflammatory cytokine release, complement activation and severe hypoxia can aggravate EC damage in patients with severe COVID-19. Due to the hyperinflammatory reaction in the COVID-19-induced cytokine storm, various inflammatory biomarkers, including interleukins, creatine kinase, erythrocyte sedimentation rate, C-reactive protein, ferritin and procalcitonin are often found to be elevated (Huang et al., 2020; Yonas et al., 2020; Akbar et al., 2021). Interestingly, meta-regression analysis showed that obesity, hypertension, diabetes and malignancy did not affect the difference in vWF antigen between the two groups. These factors are usually associated with endotheliopathy; nevertheless, the medications used in the patients in the studies included in this review were obscure. Several long-term medications used may be beneficial in patients with COVID-19 with comorbidities, and have an effect on the endothelium (Lukito et al., 2020; Pranata et al., 2020b, 2021d); this may have affected the analysis.
Clinical implications
This meta-analysis indicates that the vWF antigen level is higher in patients with severe COVID-19, thus establishing the importance of endotheliopathy from the pathophysiological perspective in patients with COVID-19 as the disease progresses. From the clinical perspective, elevated vWF antigen signals poor prognosis. For therapeutic purposes, the result of this review may serve as a basis for further research; for example, several studies indicated that the use of antiplatelet or anticoagulant drugs was associated with improved prognosis, while others did not. Investigating the effect of antiplatelet or anticoagulant drugs on prognosis in patients with high vWF antigen levels compared with patients with low vWF antigen levels may explain the heterogeneity between the studies.
Limitations
The studies did not report optimal cut-off points for prognostication purposes. To be more useful clinically, the cut-off points need to be determined. Additionally, most of the studies included in this review did not report the use of drugs such as aspirin, anticoagulants, nitric-oxide-related drugs, statins and other medications that may affect endothelial function. These medications may affect the dynamics of vWF antigen or outcome in these patients. Some of the included studies were cross-sectional, so some of the events may already have occurred when the blood was drawn. Future studies should address the optimal cut-off point for prognostic purposes, and whether antiplatelet/anticoagulant drugs affect outcome in patients with high vWF antigen levels.
Conclusion
This meta-analysis showed that the level of vWF antigen was significantly higher in patients with COVID-19 with a poor outcome, signalling marked endotheliopathy. Meta-regression showed that the differences became larger as the platelet count, d-dimer level and factor VIII level increased.
Acknowledgments
Author contributions
AW: investigation, and writing – original draft.
RP: conceptualization, methodology, software, data curation, formal analysis meta-analysis), investigation, validation, writing – original draft, and writing – review and editing.
MAL: data curation, investigation, and writing – original draft.
MRA: investigation, and writing – review and editing.
JWM: Investigation, writing – review and editing, and supervision.
Conflict of interest statement
None declared.
Funding
None.
Ethical approval
Not required.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2021.06.051.
Appendix. Supplementary materials
References
- Akbar M.R., Pranata R., Wibowo A., Lim M.A., Sihite T.A., Martha J.W. The prognostic value of elevated creatine kinase to predict poor outcome in patients with COVID-19 – a systematic review and meta-analysis: creatinine kinase in COVID-19. Diabetes Metab Syndr Clin Res Rev. 2021;15:529–534. doi: 10.1016/j.dsx.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cugno M., Meroni P.L., Gualtierotti R., Griffini S., Grovetti E., Torri A., et al. Complement activation and endothelial perturbation parallel COVID-19 severity and activity. J Autoimmun. 2021;116 doi: 10.1016/j.jaut.2020.102560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jongh R., Ninivaggi M., Mesotten D., Bai C., Marcus B., Huskens D., et al. Vascular activation is a strong predictor of mortality in coronavirus disease 2019 patients on the ICU. Blood Coagul Fibrinolysis. 2021:2019–2022. doi: 10.1097/MBC.0000000000001007. [DOI] [PubMed] [Google Scholar]
- Escher R., Breakey N., Lämmle B. ADAMTS13 activity, von Willebrand factor, factor VIII and D-dimers in COVID-19 inpatients. Thromb Res. 2020;192:174–175. doi: 10.1016/j.thromres.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshua G., Pine A.B., Meizlish M.L., Chang C.H., Zhang H., Bahel P., et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobler C., Maphumulo S.C., Grobbelaar L.M., Bredenkamp J.C., Laubscher G.J., Lourens P.J., et al. COVID-19: the rollercoaster of fibrin(ogen), d-dimer, von Willebrand factor, p-selectin and their interactions with endothelial cells, platelets and erythrocytes. Int J Mol Sci. 2020;21:1–25. doi: 10.3390/ijms21145168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang I., Pranata R., Lim M.A., Oehadian A., Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14 doi: 10.1177/1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladikou E.E., Sivaloganathan H., Milne K.M., Arter W.E., Ramasamy R., Saad R., et al. Von Willebrand factor (vWF): marker of endothelial damage and thrombotic risk in COVID-19? Clin Med J R Coll Physicians London. 2020;20:E178–E182. doi: 10.7861/clinmed.2020-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim M.A., Pranata R., Huang I., Yonas E., Soeroto A.Y., Supriyadi R. Multiorgan failure with emphasis on acute kidney injury and severity of COVID-19: systematic review and meta-analysis. Can J Kidney Health Dis. 2020;7 doi: 10.1177/2054358120938573. 2054358120938573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukito A.A., Pranata R., Henrina J., Lim M.A., Lawrensia S., Suastika K. The effect of metformin consumption on mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. Diabetes Metab Syndr Clin Res Rev. 2020;14:2177–2183. doi: 10.1016/j.dsx.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini I., Baronciani L., Artoni A., Colpani P., Biganzoli M., Cozzi G., et al. The ADAMTS13-von Willebrand factor axis in COVID-19 patients. J Thromb Haemost. 2021;19:513–521. doi: 10.1111/jth.15191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini I., Baronciani L., Artoni A., Colpani P., Biganzoli M., Cozzi G., et al. The ADAMTS13-von Willebrand factor axis in COVID-19 patients. J Thromb Haemost. 2021;19:513–521. doi: 10.1111/jth.15191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martha J.W., Pranata R., Wibowo A., Lim M.A. Tricuspid annular plane systolic excursion (TAPSE) measured by echocardiography and mortality in COVID-19: a systematic review and meta-analysis. Int J Infect Dis. 2021;105:351–356. doi: 10.1016/j.ijid.2021.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metlay J.P., Waterer G.W., Long A.C., Anzueto A., Brozek J., Crothers K., et al. Diagnosis and treatment of adults with community-acquired pneumonia. Am J Respir Crit Care Med. 2019;200:E45–E67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolay J.P., Thorn V., Daniel C., Amann K., Siraskar B., Lang F., et al. Cellular stress induces erythrocyte assembly on intravascular von Willebrand factor strings and promotes microangiopathy. Sci Rep. 2018;8:10945. doi: 10.1038/s41598-018-28961-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe A., Chocron R., Gendron N., Bory O., Beauvais A., Peron N., et al. Circulating von Willebrand factor and high molecular weight multimers as markers of endothelial injury predict COVID-19 in-hospital mortality. Angiogenesis. 2021 doi: 10.1007/s10456-020-09762-6. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata R., Huang I., Raharjo S.B. Incidence and impact of cardiac arrhythmias in coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Indian Pacing Electrophysiol J. 2020;20:193–198. doi: 10.1016/j.ipej.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata R., Permana H., Huang I., Lim M.A., Soetedjo N.N.M., Supriyadi R., et al. The use of renin angiotensin system inhibitor on mortality in patients with coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Diabetes Metab Syndr Clin Res Rev. 2020;14:983–990. doi: 10.1016/j.dsx.2020.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata R.., Lim M.A., Yonas E., Vania R., Lukito A.A., Siswanto B.B., et al. Body mass index and outcome in patients with COVID-19: a dose–response meta-analysis. Diabetes Metab. 2021;47 doi: 10.1016/j.diabet.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata R., Henrina J., Lim M.A., Lawrensia S., Yonas E., Vania R., et al. Clinical frailty scale and mortality in COVID-19: a systematic review and dose–response meta-analysis: Clinical Frailty Scale in COVID-19. Arch Gerontol Geriatr. 2021;93 doi: 10.1016/j.archger.2020.104324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata R., Henrina J., Raffaello W.M., Lawrensia S., Huang I. Diabetes and COVID-19: the past, the present, and the future. Metabolism. 2021 doi: 10.1016/j.metabol.2021.154814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata R., Huang I., Lim M.A., Yonas E., Vania R., Kuswardhani R.A.T. Delirium and mortality in coronavirus disease 2019 (COVID-19) – a systematic review and meta-analysis. Arch Gerontol Geriatr. 2021;95 doi: 10.1016/j.archger.2021.104388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata R., Huang I., Lim M.A., Yonas E., Vania R., Kuswardhani R.A.T. Delirium and mortality in coronavirus disease 2019 (COVID-19) – a systematic review and meta-analysis. Arch Gerontol Geriatr. 2021;95 doi: 10.1016/j.archger.2021.104388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata R., Lim M.A., Yonas E., Huang I., Nasution S.A., Setiati S., et al. Thrombocytopenia as a prognostic marker in COVID-19 patients: diagnostic test accuracy meta-analysis. Epidemiol Infect. 2021;149:e40. doi: 10.1017/S0950268821000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch A., Labreuche J., Lassalle F., Goutay J., Caplan M., Charbonnier L., et al. Coagulation biomarkers are independent predictors of increased oxygen requirements in COVID-19. J Thromb Haemost. 2020;18:2942–2953. doi: 10.1111/jth.15067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez Rodríguez M., Castro Quismondo N., Zafra Torres D., Gil Alos D., Ayala R., Martinez-Lopez J. Increased von Willebrand factor antigen and low ADAMTS13 activity are related to poor prognosis in COVID-19 patients. Int J Lab Hematol. 2021 doi: 10.1111/ijlh.13476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney J.M., Barouqa M., Krause G.J., Gonzalez-Lugo J.D., Rahman S., Gil M.R. Low ADAMTS13 activity correlates with increased mortality in COVID-19 patients. TH Open. 2021;05:e89–103. doi: 10.1055/s-0041-1723784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuty Kuswardhani R.A., Henrina J., Pranata R., Anthonius Lim M., Lawrensia S., Suastika K. Charlson comorbidity index and a composite of poor outcomes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Metab Syndr Clin Res Rev. 2020;14:2103–2109. doi: 10.1016/j.dsx.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassiliou A.G., Keskinidou C., Jahaj E., Gallos P., Dimopoulou I., Kotanidou A., et al. ICU Admission levels of endothelial biomarkers as predictors of mortality in critically ill COVID-19 patients. Cells. 2021;10:1–13. doi: 10.3390/cells10010186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Meijenfeldt F.A., Havervall S., Adelmeijer J., Lundström A., Rudberg A.S., Magnusson M., et al. Prothrombotic changes in patients with COVID-19 are associated with disease severity and mortality. Res Pract Thromb Haemost. 2021;5:132–141. doi: 10.1002/rth2.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S.E., Curley G.F., Lavin M., Fogarty H., Karampini E., McEvoy N.L., et al. Von Willebrand factor propeptide in severe coronavirus disease 2019 (COVID-19): evidence of acute and sustained endothelial cell activation. Br J Haematol. 2021;192:714–719. doi: 10.1111/bjh.17273. [DOI] [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva: 2021. Weekly epidemiological update –2 March 2021. [Google Scholar]
- Yang J., Wu Z., Long Q., Huang J., Hong T., Liu W., et al. Insights into immunothrombosis: the interplay among neutrophil extracellular trap, von Willebrand factor, and ADAMTS13. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.610696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonas E., Alwi I., Pranata R., Huang I., Lim M.A., Yamin M., et al. Elevated interleukin levels are associated with higher severity and mortality in COVID 19 – a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr Clin Res Rev. 2020;14:2219–2230. doi: 10.1016/j.dsx.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariah U., Nair S.C., Goel A., Balasubramanian K.A., Mackie I., Elias E., et al. Targeting raised von Willebrand factor levels and macrophage activation in severe COVID-19: consider low volume plasma exchange and low dose steroid. Thromb Res. 2020;192:2. doi: 10.1016/j.thromres.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.