Abstract
Many proteins are transported into the endoplasmic reticulum by the universally conserved Sec61 channel. Post-translational transport requires two additional proteins, Sec62 and Sec63, but their functions are poorly defined. Here, we determined cryo-EM structures of several variants of Sec61–Sec62–Sec63 complexes from Saccharomyces cerevisiae and Thermomyces lanuginosus and show that Sec62 and Sec63 induce opening of the Sec61 channel. Without Sec62, the translocation pore of Sec61 remains closed by the plug domain, rendering the channel inactive. We further show that the lateral gate of Sec61 must first be partially opened by interactions between Sec61 and Sec63 in cytosolic and lumenal domains, a simultaneous disruption of which completely closes the channel. The structures and molecular dynamics simulations suggest that Sec62 may also prevent lipids from invading the channel through the open lateral gate. Our study shows how Sec63 and Sec62 work together in a hierarchical manner to activate Sec61 for post-translational protein translocation.
In all organisms, about one third of proteins are transported across or integrated into a membrane upon synthesis by the ribosome. The majority of these translocation processes occur in the endoplasmic reticulum (ER) membrane in eukaryotes or the plasma membrane in prokaryotes, mediated by the conserved heterotrimeric protein-conducting channel called the Sec61 (SecY in prokaryotes) complex1-5. The main α subunit of the channel, comprised of ten transmembrane helices (TMs), forms an hourglass-shaped cavity, through which polypeptides are transported as extended chains. The small β and γ subunits peripherally associate with the α subunit in the membrane6. Previous structures of Sec61 and SecY showed that in the idle state the pore is blocked in the ER lumenal (or extracellular) funnel by the plug domain—a structure formed by a segment immediately following TM1 of the α subunit6-9, whereas in translocating states the plug moves away10-12. The channel can also release polypeptides to the lipid phase through a gap (lateral gate) formed between TM2 and TM7 of the α subunit. The opening of the lateral gate is required for recognition of hydrophobic targeting signals (signal sequences) of soluble secretory proteins and integration of transmembrane proteins. Thus, the channel is gated in two directions: vertically across the membrane by the plug domain and laterally within the membrane by the lateral gate. How these gates are controlled and how they regulate the translocation processes remain incompletely understood.
The Sec61 (or SecY) channel alone is inactive and thus must associate with a partner to enable translocation. In the co-translational mode, common in both prokaryotes and eukaryotes, The channel directly docks with the ribosome-nascent-chain complex11,13,14. Many secretory proteins are targeted to the channel post-translationally after their release from the ribosome15-20. In bacteria, a single cytosolic ATPase called SecA binds to the SecY complex to drive post-translational translocation10,12,19-21. In eukaryotes, post-translational translocation is enabled by association between the Sec61 complex and the two essential integral membrane proteins Sec62 and Sec63, forming a machinery called the Sec complex22-25. In fungal species, the Sec complex also contains the additional nonessential proteins Sec71 and Sec72, which are bound to Sec63 in the cytosol. In the ER lumen, Sec63 recruits the Hsp70 ATPase BiP to the complex to power translocation26,27.
Recently, two cryo-EM studies reported structures of the Sec complex from Saccharomyces cerevisiae at ~4 Å resolution28,29, which suggested a putative role of Sec63 in activating the Sec61 channel for translocation by opening the lateral gate of the channel. However, in both structures, Sec62 was barely visible, and thus its function remains unknown despite its essentiality. Furthermore, the two structures displayed noticeable conformational differences in Sec61, despite essentially identical specimen compositions. Most notably, in one structure the pore is blocked by the plug domain29, whereas in the other structure the plug is displaced leaving the pore open28. Although deemed important given the role of the plug domain in channel gating, the cause of this difference remains a puzzle. Finally, although Sec63 has been suggested to open the lateral gate of Sec6128,29, the mechanism of opening remains speculative without structures of mutants and other conformations. Thus, whether and how Sec62 and Sec63 regulate the function of Sec61 are poorly understood. Addressing these issues is essential for our understanding of eukaryotic post-translational translocation and the mechanism of the Sec61 and SecY channels in general.
Here, using cryo-EM, we analyzed several variants and mutants of the Sec complex from two fungal species, Saccharomyces cerevisiae and Thermomyces lanuginosus. We show that Sec62 and Sec63 cooperate to open both the lateral and vertical gates of the Sec61 channel. The structures and molecular dynamics (MD) simulations also suggest that Sec62 performs an additional function of preventing lipids from invading into the channel through the open lateral gate. Our study provides a detailed mechanistic model for how Sec62 and Sec63 activate the Sec61 channel for post-translational protein translocation in eukaryotes.
Cryo-EM analysis of two fungal Sec complexes
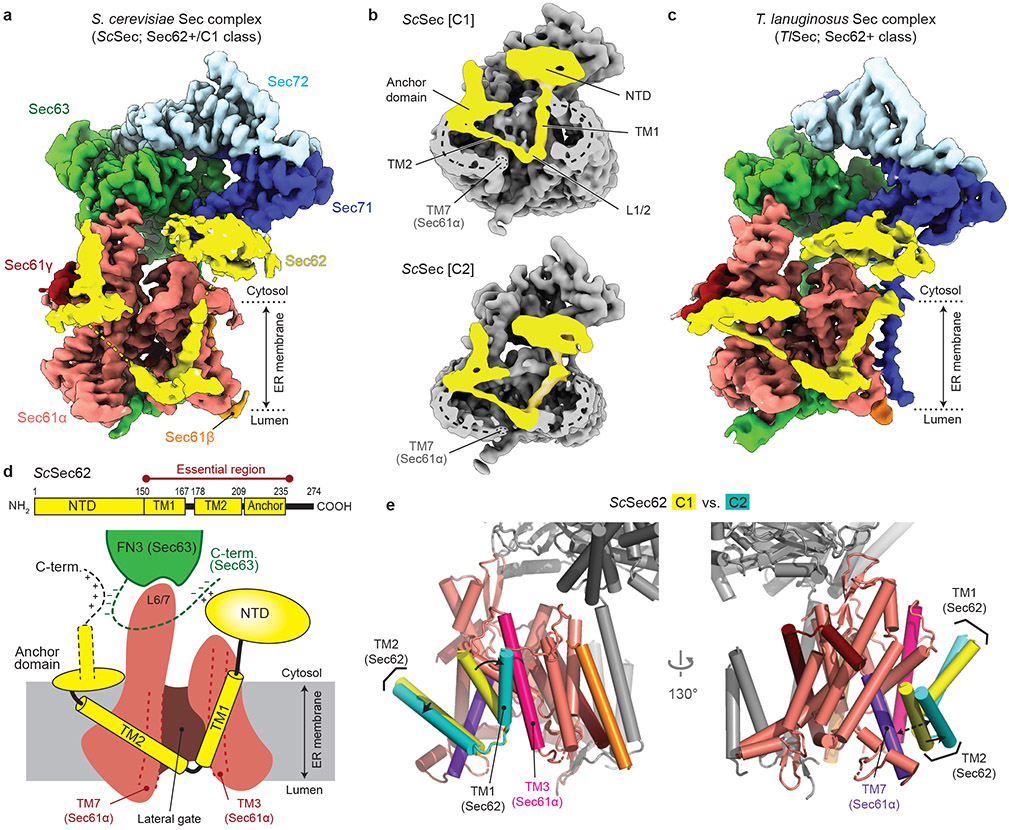
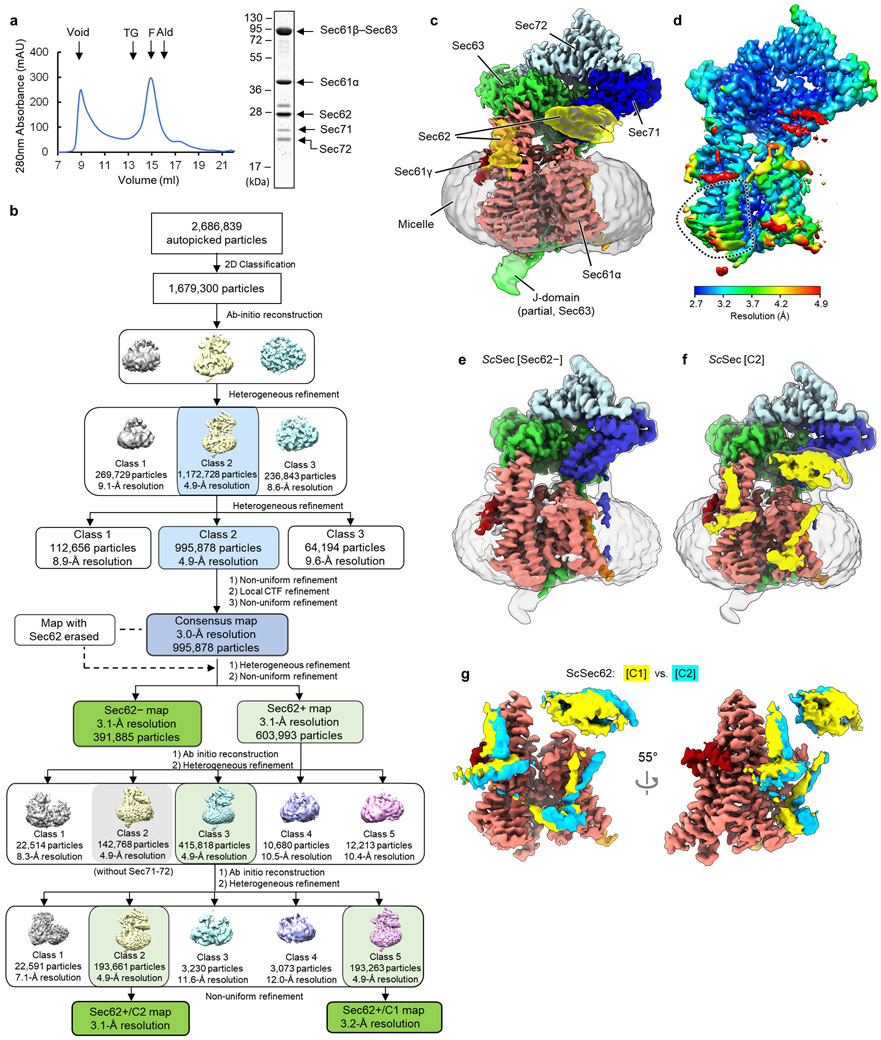
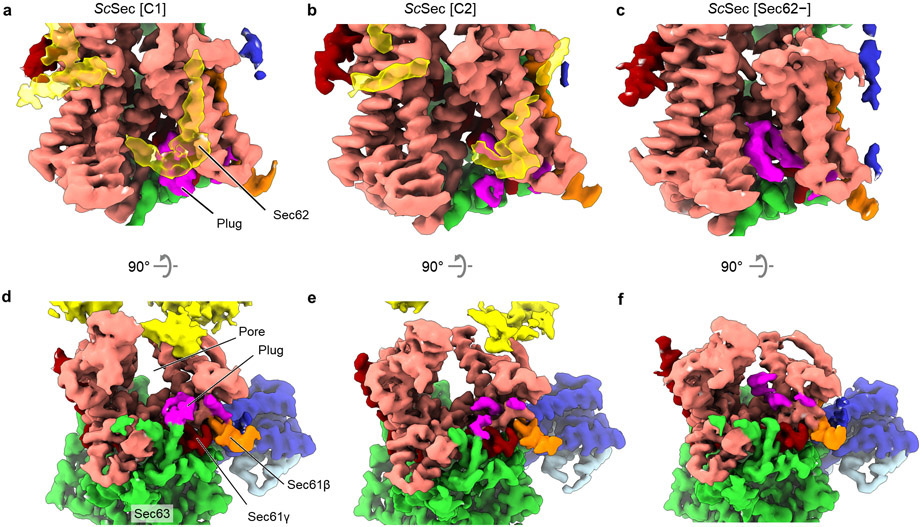
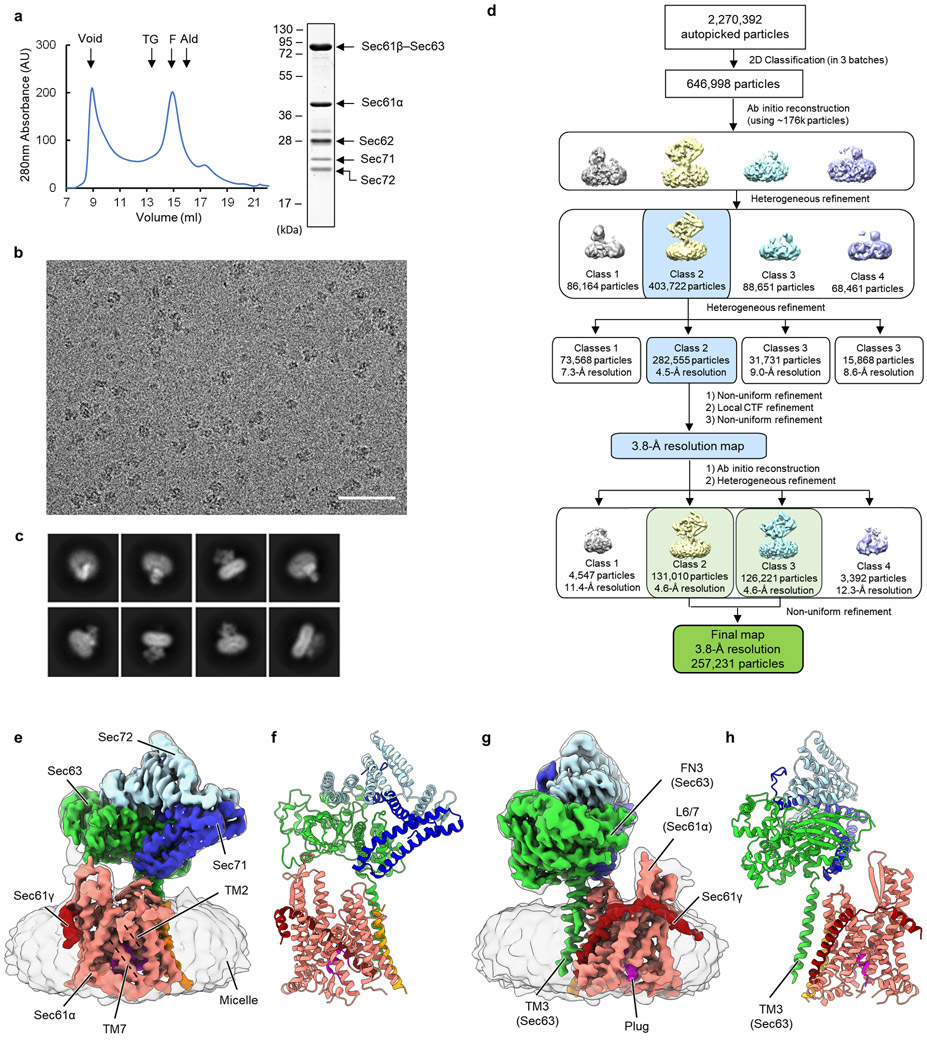
To determine how the gating of the Sec61 channel is regulated in the Sec complex, we first analyzed a large cryo-EM dataset of the wildtype (WT) Sec complex from S. cerevisiae (ScSec) (Fig. 1a,b, Table 1, and Extended Data Fig. 1). While reconstruction from approximately 1 million particles yielded a 3.0-Å-resolution consensus map (Extended Data Fig. 1b-d), we found that the particle set contained subpopulations lacking Sec62 or Sec71–Sec72, despite apparent sample homogeneity (Extended Data Fig. 1a). We therefore performed additional three-dimensional (3D) classifications to separate particles with and without Sec62 (referred to as Sec62+ and Sec62−) (Extended Data Fig. 1b,e). Furthermore, the Sec62+ class could be further separated into two distinct subclasses (referred to as C1 and C2), which show notable conformational differences in Sec62, the lateral gate, and the plug (Fig 1b and Extended Data Fig. 1f,g; see below). The three structures (i.e., Sec62−, C1, and C2) were resolved at overall resolutions of 3.1–3.2 Å. Although an atomic model for Sec62 could not be built due to insufficient local resolution, the classification significantly improved Sec62 features, enabling unambiguous assignment of individual domains (Fig. 1 a,d).
Figure 1. Cryo-EM analysis of fungal Sec complexes and the structure of Sec62.
a, The 3.1-Å-resolution cryo-EM reconstruction of the S. cerevisiae Sec complex (C1 class, front view into the lateral gate). Yellow dashed lines indicate the connections that are visible at a lower contour level (see panel b). In yeast nomenclature, the α, β, and γ subunits of the Sec61 complex are called Sec61p, Sbh1p, and Sss1p, respectively. b, Cutaway views showing Sec62 (yellow). Shown are 6-Å-lowpass-filtered C1 (upper panel; a tilted view from the ER lumen) and C2 (lower panel; front view) maps. Dashed line, detergent micelle. c, The 3.8-Å-resolution reconstruction of the T. lanuginosus Sec complex (the consensus Sec62+ map). d, Domain organization of Sec62. Previous studies suggest an interaction between the NTD of Sec62 and the C-terminal tail of Sec63 (ref. 30,43). In addition, based on the proximity, the C-terminal tails of Sec62 and Sec63 may also interact with each other through an electrostatic interaction. e, Interactions between the Sec62 TMs and lateral gate. Dashed arrow, a gap between Sec61α TM7 and Sec62 TM2 in the C2 conformation. Sec61α is in salmon with its TM3 and TM7 in magenta and violet, respectively. Sec61β and Sec61γ are in orange and dark red, respectively. Sec62 is in yellow (C1) or cyan (C2). Sec63, Sec71, and Sec72 are in grey.
Table 1.
Cryo-EM data collection, refinement and validation statistics of wildtype ScSec and wildtype and mutant TlSec complexes
|
ScSec [Sec62−] (EMD-22770, PDB 7KAH) |
ScSec[C1] (EMD- 22771, PDB 7KAI) |
ScSec[C2] (EMD- 22772, PDB 7KAJ) |
TlSec [Sec62−] (EMD-22773, PDB 7KAK) |
TlSec [Plug-open] (EMD-22774, PDB 7KAL) |
TlSec [Plug-closed] (EMD-22775, PDB 7KAM) |
ΔSec62 TlSec (EMD-22776, PDB 7KAN) |
Δanchor TlSec (EMD- 22777) |
|
|---|---|---|---|---|---|---|---|---|
| Data collection and processing | ||||||||
| Magnification | 64,000x | 64,000x | 64,000x | 36,000x | 36,000x | 36,000x | 36,000x | 64,000x |
| Voltage (kV) | 300 | 300 | 300 | 200 | 200 | 200 | 200 | 300 |
| Electron exposure | ||||||||
| (e−/Å2) | 49.1 | 49.1 | 49.1 | 50.0 | 50.0 | 50.0 | 50.0 | 49.1 |
| Defocus range (μm) | −0.8 to −2.5 | −0.8 to −2.5 | −0.8 to −2.5 | −0.6 to −2.4 | −0.6 to −2.4 | −0.6 to −2.4 | −0.9 to −2.2 | −0.7 to −2.9 |
| Pixel size (Å) | 1.19 | 1.19 | 1.19 | 1.14 | 1.14 | 1.14 | 1.14 | 1.19 |
| Symmetry imposed | C1 | C1 | C1 | C1 | C1 | C1 | C1 | C1 |
| Initial particle | ||||||||
| images (no.) | 2,686,839 | 2,686,839 | 2,686,839 | 1,632,659 | 1,632,659 | 1,632,659 | 546,712 | 229,825 |
| Final particle images | ||||||||
| (no.) | 391,885 | 193,263 | 193,661 | 155,601 | 114,704 | 143,227 | 222,047 | 76,726 |
| Map resolution (Å) | 3.1 | 3.2 | 3.1 | 3.9 | 4.0 | 3.8 | 3.7 | 4.4 |
| FSC threshold | 0.143 | 0.143 | 0.143 | 0.143 | 0.143 | 0.143 | 0.143 | 0.143 |
| Map resolution range (Å) | 2.6 – 11 | 2.8 – 12 | 2.7 – 12 | 3.4 – 13 | 3.3 – 13 | 3.3 – 12 | 3.3 – 12 | 3.7 – 14 |
| Refinement | ||||||||
| Initial model used | PDB 6N3Q | PDB 7KAH | PDB 7KAH | PDB 7KAN | PDB 7KAN | PDB 7KAN | PDB 6N3Q | - |
| Model resolution (Å) | 3.2 | 3.3 | 3.3 | 4.1 | 4.2 | 4.0 | 4.0 | - |
| FSC threshold | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | - |
| Map sharpening B factor (Å2) | 86.6 | 80.8 | 75.9 | 110.3 | 90.7 | 105.2 | 127.8 | - |
| Model composition | ||||||||
| Non-hydrogen atoms | 10,495 | 10,718 | 10,712 | 10,438 | 10,794 | 10,921 | 10,661 | - |
| Protein residues | 1,349 | 1,399 | 1,399 | 1,371 | 1,429 | 1,445 | 1,371 | - |
| Ligands | - | - | - | - | - | - | 2 | - |
| B factors (Å2) | ||||||||
| Protein | 73 | 61 | 58 | 117 | 126 | 74 | 30 | - |
| Ligand | - | - | - | - | - | - | 34 | - |
| R.m.s. deviations | ||||||||
| Bond lengths (Å) | 0.003 | 0.003 | 0.003 | 0.002 | 0.002 | 0.003 | 0.003 | - |
| Bond angles (°) | 0.522 | 0.508 | 0.513 | 0.524 | 0.489 | 0.521 | 0.623 | - |
| Validation | ||||||||
| MolProbity score | 1.43 | 1.42 | 1.33 | 1.51 | 1.42 | 1.48 | 1.55 | - |
| Clashscore | 4.61 | 4.14 | 3.87 | 6.33 | 5.62 | 5.60 | 6.18 | - |
| Poor rotamers (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - |
| Ramachandran plot | ||||||||
| Favored (%) | 96.83 | 96.58 | 97.01 | 97.09 | 97.42 | 96.96 | 96.72 | - |
| Allowed (%) | 3.17 | 3.42 | 2.99 | 2.91 | 2.58 | 3.04 | 3.28 | - |
| Disallowed (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - |
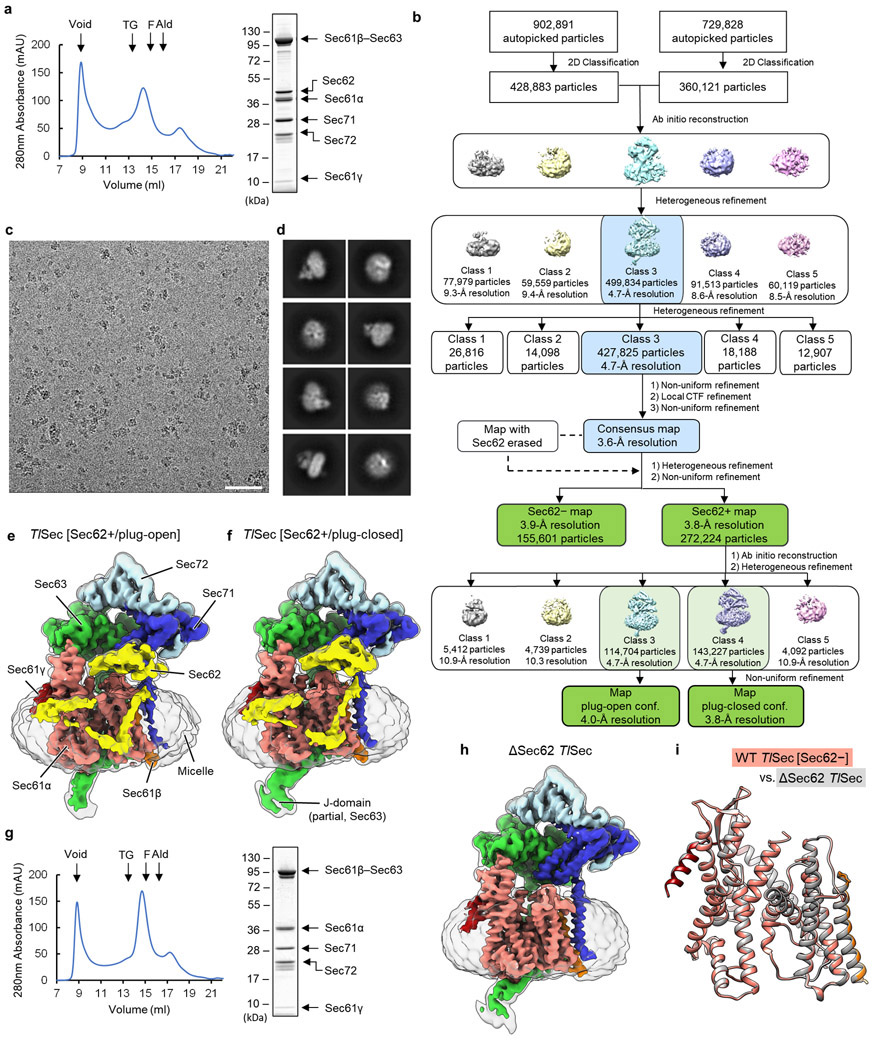
To gain insights into structural and mechanistic conservation across species, we also purified the Sec complex from the thermophilic fungus T. lanuginosus (TlSec) and determined its structures at overall resolutions of 3.6 to 3.9 Å (Fig. 1c, Table 1, and Extended Data Fig. 2). Recombinant expression of the TlSec complex allowed us to analyze complexes completely lacking Sec62 (ΔSec62 TlSec) or containing a mutant Sec62 copy for structural comparisons. Like WT ScSec, the WT TlSec dataset yielded two classes with and without Sec62 (referred to as TlSec[Sec62+] and TlSec[Sec62−]), which closely resemble the ScSec [C2] and [Sec62−] structures, respectively (brackets denote classes). We could not find a C1-equivalent class from the TlSec dataset perhaps because the specimen freezing condition (4°C) might have biased the conformation distribution of this thermophilic complex towards C2. The structure of TlSec[Sec62−] was found to be essentially identical to a separately determined structure of ΔSec62 TlSec, validating our approach to separate distinct subpopulations of the Sec complexes by cryo-EM image analysis (Extended Data Fig. 2g-i). Importantly, the domain arrangement of TlSec62 is the same as that of ScSec62 despite ~30% overall sequence identity (Fig. 1c and Supplementary Fig. 1). This corroborates the conserved architecture of Sec62. Compared to ScSec62, TlSec62 is better resolved such that we could register amino acids to its TM1.
Sec62 forms a V-shaped structure
Sec62 consists of a cytosolic, globular N-terminal domain (NTD), two TMs (TM1 and TM2) connected by a short ER lumenal loop (L1/2), and a cytosolic C-terminal segment (Fig. 1d). Functionally essential regions have previously been mapped to the two TMs and a segment of ~30 amino acids immediately following TM2 (ref. 30). The TMs of Sec62 are arranged as a V shape in front of the lateral gate with L1/2 directed to the lateral gate opening (Fig. 1a-d). The contact with the channel is mainly formed by an interaction of Sec62-TM1 with TM3 and the N-terminal segment of Sec61α.
Following TM2, Sec62 contains an oval-shaped structure lying flat on the membrane interface (Fig. 1a-d, and Extended Data Fig. 3a-b). This amphipathic structure, which we termed the anchor domain, is most likely formed by an ~20-residue-long conserved segment within the abovementioned 30 amino acids, and is rich in hydrophobic amino acids (Supplementary Fig. 1). While single-point mutations of these hydrophobic residues caused no growth defect, alanine substitutions of three consecutive residues in positions 215–220 were lethal (Extended Data Fig. 3d), suggesting that decreased hydrophobicity interrupts its functionally essential interaction with the membrane. The structure of a TlSec mutant (Δanchor TlSec) with the anchor domain replaced with a glycine/serine linker showed virtually no visible Sec62 features (Extended Data Fig. 3e,f), suggesting that Sec62 becomes too mobile without the domain. Taken together, these observations suggest that the function of the anchor domain is to properly position the V-shaped TMs of Sec62 at the lateral gate.
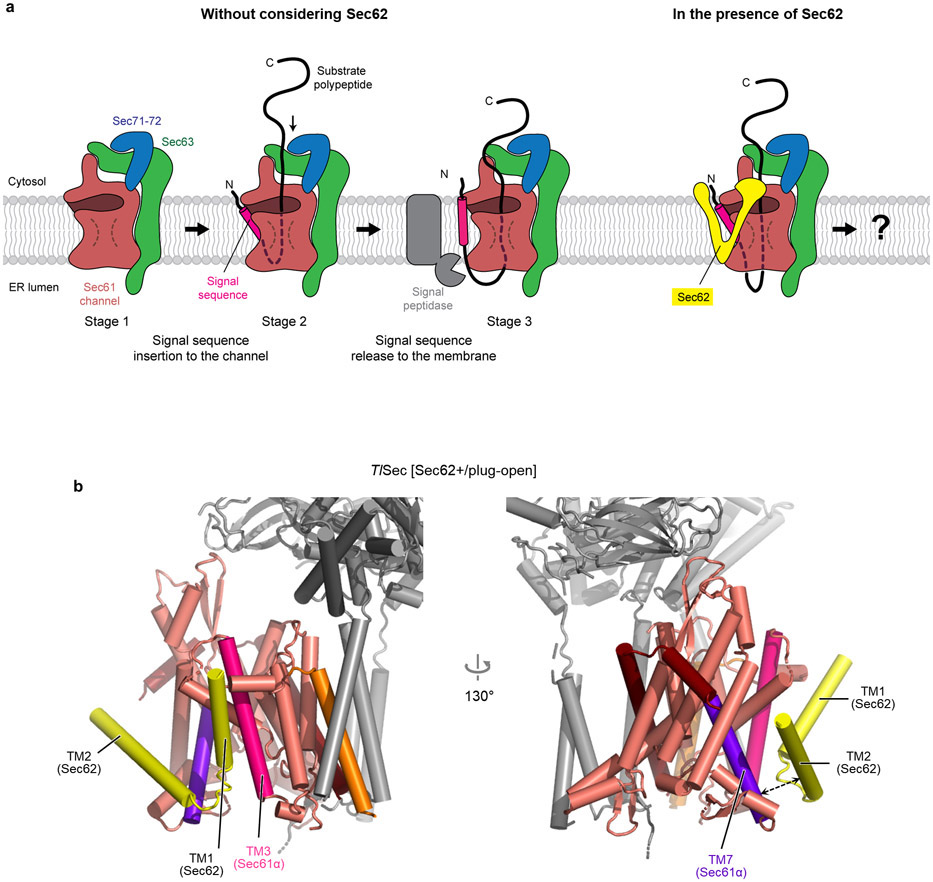
The revealed position and topology of Sec62 raise an important question about how the channel would engage with substrate polypeptides. During the initial stage of post-translational translocation, a substrate polypeptide is expected to insert into the channel as a loop with both its N- and C- termini exposed to the cytosol31 (Extended Data Fig. 4a). While the N-terminal signal sequence may sit initially at the lateral gate as seen in structures of mammalian co-translational and bacterial post-translational complexes10-12, later it must engage with the signal peptidase for cleavage32. Although the exact timing of the signal sequence cleavage remains unknown, the presence of Sec62 might pose a problem in this step because it may block the release of the signal sequence from the lateral gate or prevents the signal peptidase from accessing the cleavage site. The answer may be provided by a conformational transition from C1 to C2 as visualized in the ScSec structure (Fig. 1e). While in both structures the seam between the Sec62-TM1 and Sec61α-TM3 is tight, a sufficient gap is formed on the other side of the lateral gate between the Sec62-TM2 and Sec61α-TM7 in the ScSec[C2] structure. A similar gap also exists in the TlSec structures (Extended Data Fig. 4b). Thus, the signal sequence of the substrate may exit through the gap transiently formed between Sec62-TM2 and Sec61α-TM7 during translocation.
Sec62 regulates the gates of Sec61
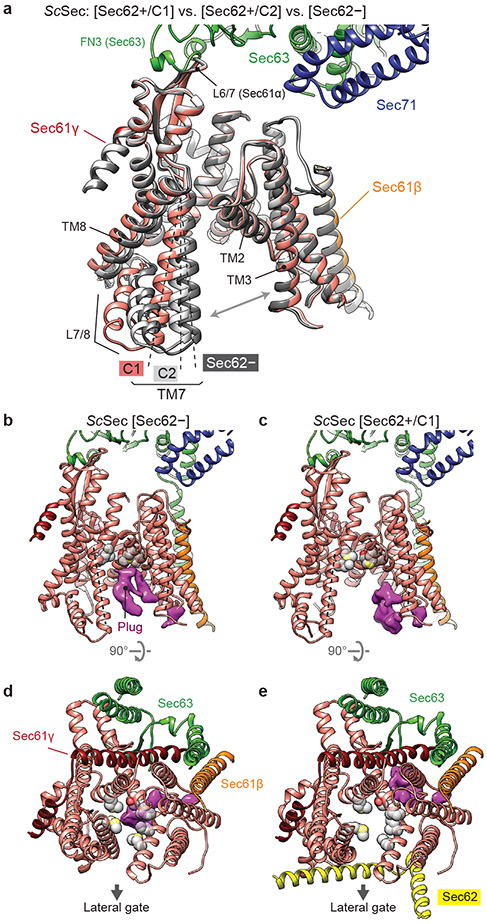
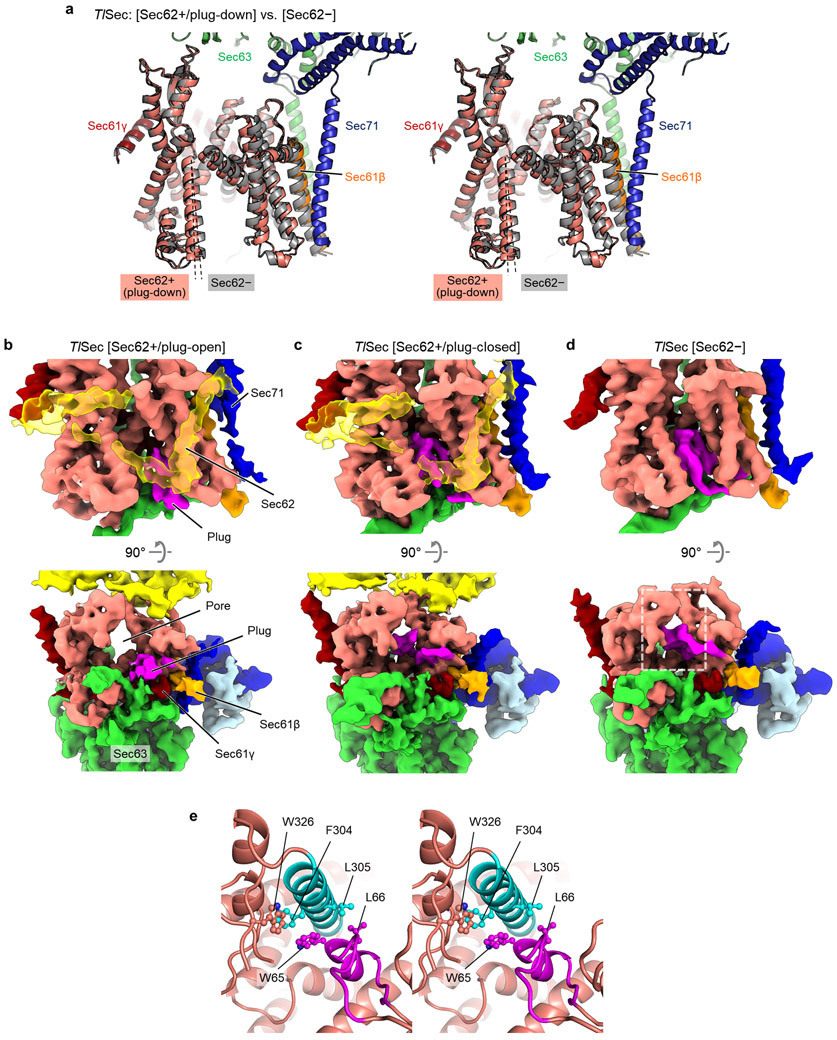
Three distinct classes of ScSec (i.e., C1, C2, and Sec62−) showed notable conformational differences in the lateral gate (Fig. 2a, Supplementary Movie 1). Although open in all three structures, the extent of the lateral gate opening varies on the ER lumenal side, with C1 most open and Sec62− least open. The C2 structure, in which Sec62-TM2 is disengaged, is open to an intermediate degree. The movement is mainly mediated by a rigid-body rotation of the TM7, TM8, and the intervening loop (L7/8) of Sec61α (Fig. 2a), which seems to be induced by the interaction between L1/2 of Sec62 and the lateral gate (Fig. 1a-d). Thus, this movement is distinct from the hinge-like motion between the two halves (TM1–5 and TM6–10) of Sec61α which mediates opening of the channel from the fully closed state6,33-35.
Figure 2. Regulation of the lateral and vertical gates by Sec62.
a, A comparison of the Sec61 channel conformation between the three ScSec classes, C1 (in color), C2 (light grey) and Sec62− (dark grey). Dashed lines, TM7 of Sec61α. Grey arrows, the lateral gate. Sec62 is not shown. b–e, A comparison of the plug domain (purple density) between Sec62-lacking (left) and -containing ScSec (right) classes. Grey spheres, pore ring residues. Dashed lines, lateral gate helices (left to right: TM7, TM2, and TM3 of Sec61α). Shown are front views (b and c) and cytosolic views (d and e).
Importantly, the motion of TM7–8 of Sec61α appears to control the position of the plug (Fig. 2 b-e). In ScSec[Sec62−], the plug is clearly visible immediately below the pore constriction (‘plug-closed’ conformation; Fig. 2 b,d, Extended Data Fig. 5). By contrast, in ScSec[C1], the plug is displaced to a position near the C-terminus of Sec61γ (‘plug-open’ conformation; Fig. 2 c,e), thus opening the pore. The position of the plug in this conformation is consistent with the previous observations that the plug can interact with TM of the SecE (a prokaryotic equivalent of Sec61γ) subunit36,37. In ScSec[C2], the plug seems disordered, probably because it takes intermediate positions between the two conformations. Similar observations were also made with the TlSec structures: compared with the Sec62− and ΔSec62 structures, the Sec62+ structure shows a shifted position of Sec61α TM7–8 as in ScSec[C2] (Extended Data Fig. 6) and concomitant plug mobilization, where 53% and 42% particles classified into the plug- closed and open conformations, respectively (Extended Data Figs. 2 and 6b,c). The plug displacement is likely caused by the Sec62-induced movement of Sec61α TM7 since the plug interacts with TM7 and L7/8 in the plug-closed conformation33,38 (Extended Data Fig. 6e).
Partially open Sec61 is inactive
Despite the observed channel gating by Sec62, physiological importance of this role remained unclear. Without Sec62, the lateral gate can still be opened by Sec63. Even though the pore is blocked by the plug, it has been proposed that insertion of a substrate polypeptide would push the plug away29. To investigate importance of the Sec62-dependent gating, we sought for mutations affecting Sec61 gating as ΔSec62 does, but independently of Sec62. If the gating function of Sec62 is essential, such mutations would be expected to compromise cell viability.
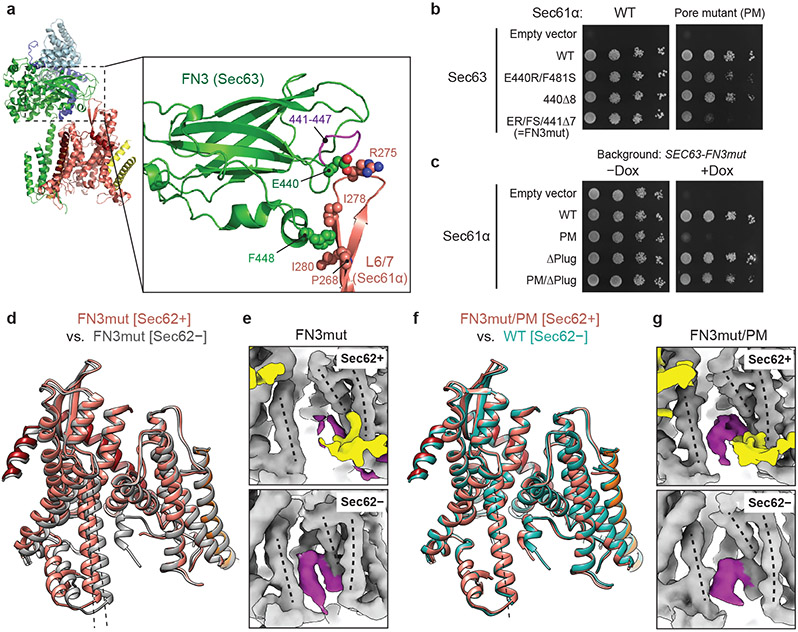
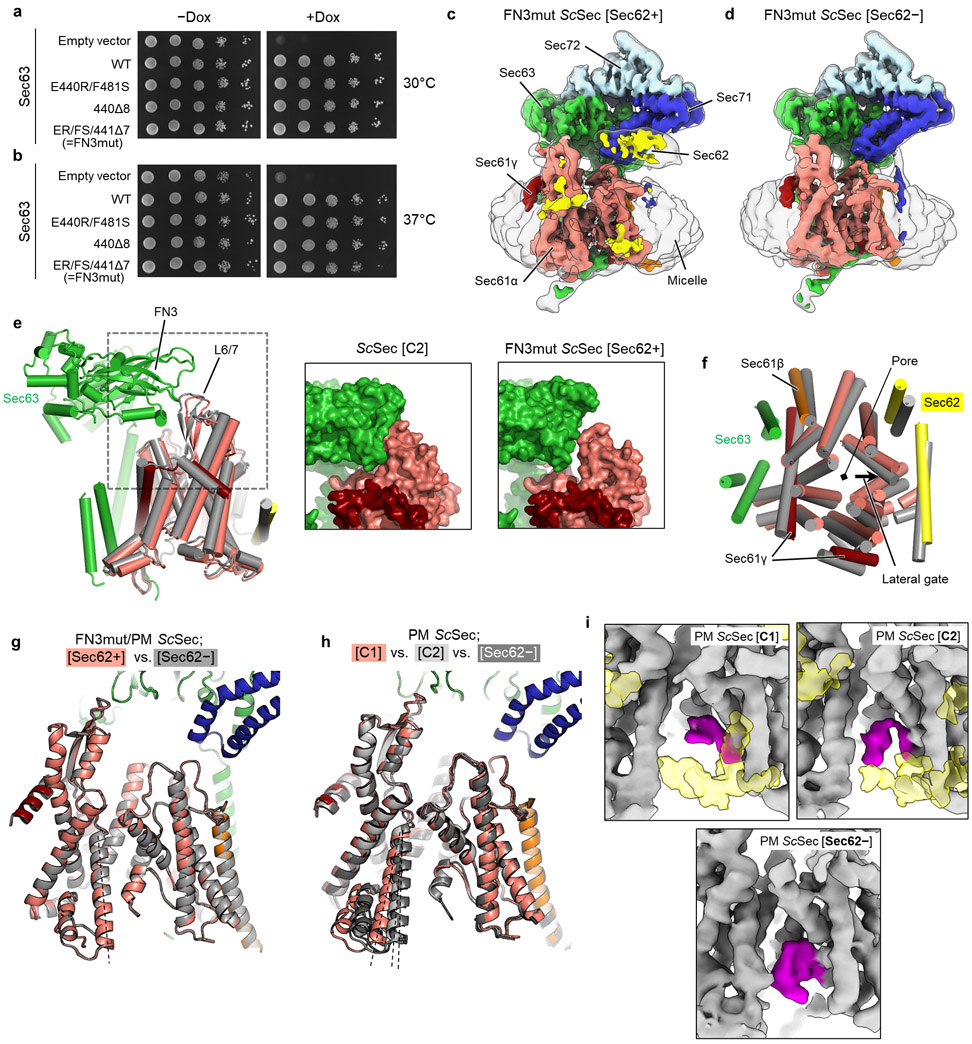
We first chose to mutate the fibronectin III (FN3) domain of Sec63, which interacts with the cytosolic loop 6/7 (L6/7) of Sec61α (Fig. 3a). L6/7 also provides a major interaction site for the ribosome in cotranslational translocation and the SecA ATPase in bacterial post-translational translocation and thus has been universally implicated in priming or activating the channel7,8,21,39,40. We found that none of the FN3 mutants had a growth defect at 30°C (Fig. 3b, left panel). Only a mild defect was seen at 37°C even with the most severe mutant (FN3mut) (Extended Data Fig. 7b). To understand this unexpectedly weak phenotype, we determined the structure of FN3mut ScSec (Fig. 3d,e, Table 2, and Extended Data Fig. 7c,d). The structure showed that the FN3 domain was indeed disengaged from L6/7 by the mutation, causing ~10° rotation of Sec61 along the membrane normal (Extended Data Fig. 7e,f). Nonetheless, the lateral gate was still open (Fig. 3d). Importantly, the FN3mut complex still exhibited Sec62-induced TM7 movement and plug mobilization (Fig. 3 d,e), which may explain the near-WT growth phenotype of the mutant.
Figure 3. Structural and functional analysis of a gating-defective mutant complex.
a, The interaction between the FN3 domain of Sec63 and the L6/7 loop of Sec61α (shown is WT ScSec[C1]). Amino acids involved in the interactions are indicated. b, Yeast growth complementation experiments (at 30°C) testing functionality of indicated FN3 mutants of Sec63 in the background of WT (left) or pore-mutant (PM) Sec61α (right). FN3mut refers to a combination of E440R (ER) and F481S (FS) mutations and a deletion of seven amino acids 441–447 (441Δ7). To repress chromosomal WT Sec63 expression (under a tetracycline promoter), doxycycline (Dox) was added. −Dox control is shown in Extended Data Fig. 7a. c, As in b, but testing for indicated Sec61α mutants in the background of Sec63-FN3mut as a sole Sec63 copy. The addition of Dox represses chromosomal WT Sec61α expression. d, As in Fig. 2a, but with the FN3mut ScSec structures with and without Sec62. e, A comparison of the plug domain (purple density) between the FN3mut ScSec structures with and without Sec62 (yellow). Dashed lines, lateral gate helices (left to right: TM7, TM2, and TM3 of Sec61α). f, As in d, but comparing the Sec62-containing FN3mut/PM structure and the Sec62− class of WT ScSec. g, As in e, but with the FN3mut/PM ScSec structures. The experiments in b and c were repeated at least twice with similar results.
Table 2.
Cryo-EM data collection, refinement and validation statistics of mutant ScSec complexes
| PM ScSec [Sec62−] (EMD-22778, PDB 7KAO) |
PM ScSec [C1] (EMD- 22779, PDB 7KAP) |
PM ScSec [C2] (EMD-22780, PDB 7KAQ) |
FN3mut ScSec [Sec62−] (EMD- 22781, PDB 7KAR) |
FN3mut ScSec [Sec62+] (EMD- 22782, PDB 7KAS) |
PM/FN3mut ScSec [Sec62−] (EMD- 22783, PDB 7KAT) |
PM/FN3mut ScSec [Sec62+] (EMD- 22784, PDB 7KAU) |
FN3mut/ Δ210-216 ScSec (EMD- 22787, PDB 7KB5) |
|
|---|---|---|---|---|---|---|---|---|
| Data collection and processing | ||||||||
| Magnification | 64,000x | 64,000x | 64,000x | 64,000x | 64,000x | 64,000x | 64,000x | 45,000x |
| Voltage (kV) | 300 | 300 | 300 | 300 | 300 | 300 | 300 | 200 |
| Electron exposure | ||||||||
| (e−/Å2) | 48.8 | 48.8 | 48.8 | 49.1 | 49.1 | ~48–49 | ~48–49 | 63 |
| Defocus range (μm) | −1.0 to −2.7 | −1.0 to −2.7 | −1.0 to −2.7 | −0.8 to −2.7 | −0.8 to −2.7 | −1.1 to −2.2 | −1.1 to −2.2 | −0.7 to −2.0 |
| Pixel size (Å) | 1.15 | 1.15 | 1.15 | 1.19 | 1.19 | 1.19 | 1.19 | 0.9 |
| Symmetry imposed | C1 | C1 | C1 | C1 | C1 | C1 | C1 | C1 |
| Initial particle images (no.) | 195,915 | 195,915 | 195,915 | 1,274,219 | 1,274,219 | 267,541 | 267,541 | 2,270,392 |
| Final particle images (no.) | 35,573 | 17,341 | 16,679 | 82,671 | 119,420 | 32,704 | 54,139 | 257,231 |
| Map resolution (Å) | 4.0 | 4.1 | 4.0 | 4.0 | 3.9 | 4.4 | 4.0 | 3.8 |
| FSC threshold | 0.143 | 0.143 | 0.143 | 0.143 | 0.143 | 0.143 | 0.143 | 0.143 |
| Map resolution range (Å) | 3.5 - 14 | 3.5 - 17 | 3.5 - 16 | 3.4 - 14 | 3.3 - 18 | 3.7 - 19 | 3.4 - 16 | 3.2 - 13 |
| Refinement | ||||||||
| Initial model used | PDB 7KAH | PDB 7KAI | PDB 7KAJ | PDB 7KAH | PDB 7KAR | PDB 7KAH | PDB 7KAT | PDB 7KAH |
| Model resolution (Å) | 4.2 | 4.2 | 4.2 | 4.2 | 4.1 | 4.5 | 4.2 | 4.0 |
| FSC threshold | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Map sharpening B factor (Å2) | 50.9 | 39.3 | 32.9 | 74.8 | 73.8 | 59.3 | 58.1 | 119.3 |
| Model composition | ||||||||
| Non-hydrogen atoms | 10,502 | 10,715 | 10,753 | 10,435 | 10,616 | 10,431 | 10,711 | 9,777 |
| Protein residues | 1,349 | 1,398 | 1,402 | 1,341 | 1,385 | 1,340 | 1,396 | 1,252 |
| Ligands | - | - | - | - | - | - | - | - |
| B factors (Å2) | ||||||||
| Protein | 152 | 178 | 187 | 117 | 64 | 255 | 125 | 126 |
| Ligand | - | - | - | - | - | - | - | - |
| R.m.s. deviations | ||||||||
| Bond lengths (Å) | 0.003 | 0.003 | 0.002 | 0.003 | 0.004 | 0.002 | 0.003 | 0.004 |
| Bond angles (°) | 0.520 | 0.519 | 0.494 | 0.581 | 0.596 | 0.501 | 0.533 | 0.626 |
| Validation | ||||||||
| MolProbity score | 1.48 | 1.49 | 1.46 | 1.58 | 1.63 | 1.37 | 1.53 | 1.71 |
| Clashscore | 7.65 | 7.87 | 7.39 | 7.84 | 6.69 | 6.71 | 6.26 | 7.3 |
| Poor rotamers (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ramachandran plot | ||||||||
| Favored (%) | 97.74 | 97.74 | 97.75 | 97.19 | 96.17 | 98.10 | 96.86 | 95.60 |
| Allowed (%) | 2.26 | 2.26 | 2.25 | 2.81 | 3.83 | 1.90 | 3.14 | 4.40 |
| Disallowed (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Next, we mutated the pore of Sec61α. In closed SecY structures6-9, the aliphatic amino acids lining the pore constriction (called the pore ring residues) make a hydrophobic interaction with the plug. Compared to other species, the pore ring of ScSec61α appears significantly less hydrophobic28. Thus, we reasoned that a mutant with a more hydrophobic pore ring (M90L/T185I/M294I/M450L; collectively denoted PM) might bias the plug towards the closed conformation. In growth complementation assays, PM itself did not affect cell growth. However, strong synthetic growth impairment was observed when combined with FN3mut (Fig. 3b, right). Importantly, a plug deletion41 (ΔPlug) could rescue growth of the FN3mut/PM, suggesting that the growth inhibition originates from a gating defect (Fig. 3c). Consistent with this idea, the structures of the combined mutant (FN3mut/PM) showed a strong density of the plug in the closed position and no Sec62-dependent movement of lateral gate helices (Fig. 3f,g, and Extended Data Fig. 7g). This conformation thereby closely resembles the gating state of ScSec[Sec62−] despite the presence of Sec62 in front of the lateral gate. On the other hand, the structure of PM alone still showed Sec62-mediated movements in the lateral gate and plug, similar to WT (Extended Data Fig. 7h,i). Taken together, these results show that the channel conformation seen in the absence of Sec62 is inactive for post-translational translocation.
Sec62 prevents invasion of lipids into the channel
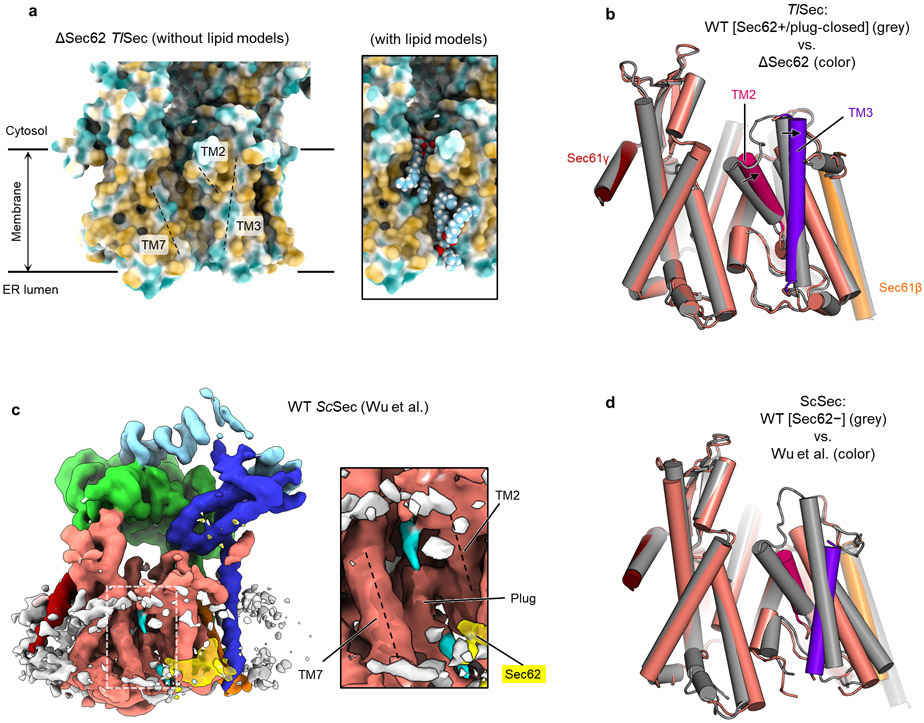
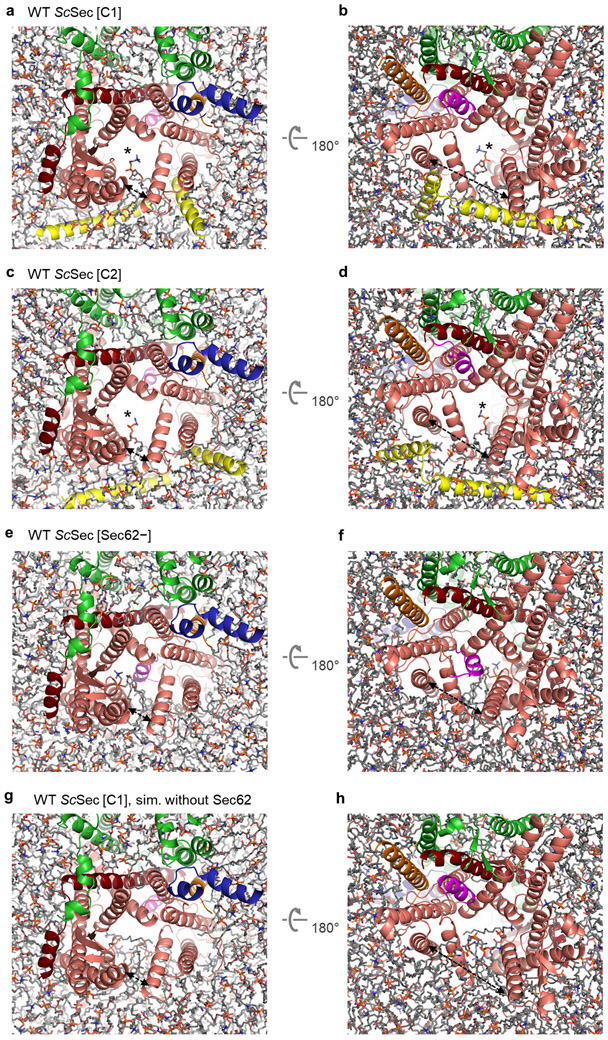
In addition to the role in channel gating, the ΔSec62 TlSec structure suggests another function of Sec62— preventing lipids from moving into the channel. In ΔSec62 TlSec, strong, well-ordered densities of lipid or detergent tails are visible at the lateral gate (Fig. 4a). The densities are vertically aligned along the hydrophobic groove of the open lateral gate (Fig. 4a and Extended Data Fig. 8a). By contrast, in the Sec62+ structures, only weak fragmented densities were observed (Fig. 4b). In the cytosolic leaflet, a lipid/detergent molecule seems to be accommodated with an outward rotation of the TM2–3 of Sec61α (Extended Data Fig. 8b). Sec62 may inhibit lipids from entering the lateral gate by restricting this movement. In the ER lumenal leaflet, the L1/2 of Sec62 seems to sterically block lipids from entering (Fig. 4b). We did not observe a strong lipid/detergent density in the lateral gate of ScSec[Sec62−], perhaps because of a lower affinity to lipid/detergent. However, one of the previous ScSec structures29, the conformation of which resembles the ΔSec62 TlSec structure, has shown a lipid-like density at the lateral gate and the movement of Sec61α TM2–3 similarly to ΔSec62 TlSec (Extended Data Fig. 8c,d). Collectively, these observations suggest that in the absence of Sec62, lipid molecules may penetrate the lateral gate that is opened by Sec63.
Figure 4. Sec62 prevents lipids from invading into the Sec61 channel.
a. Lipid/detergent molecules at the lateral gate in the TlSec structure lacking Sec62 (ΔSec62). The left panel is a front view. Non-protein densities are in grey. Densities in cyan are lipid/detergent molecules intercalated at the lateral gate. The right panel is a zoomed-in view of the lateral gate (area indicated by the white dashed box in the left panel). b, As in a, but with the Sec62+/plug-closed class of WT TlSec. We note that similarly, the Sec62+/plug-open class does not show lipid/detergent densities at the lateral gate. c–h. All-atom MD simulations with indicated TlSec structures in a model membrane. The Sec complex is shown in a ribbon representation in the same colors as in a and b. Lipids are shown in a stick representation. Panels c–e are views from the cytosol, and f–h are views from the ER lumen. In c and f, the translocation pore is marked by an asterisk. The lateral gate openings are indicated by a dashed arrow. The frames are from 200 ns after the initiation of simulations.
To further investigate a role of Sec62 in blocking lipid penetration, we performed 200-ns all-atom MD simulations (Fig. 4 c-h and Extended Data Fig. 9). In simulations of the Sec62-containing structures (i.e., WT TlSec[Sec62+/plug-open] and WT ScSec[C1] and [C2]), the translocation pore largely remained unobstructed and devoid of lipids (Fig. 4c,f, Extended Data Fig. 9 a-d, and Supplementary Movies 2-4). Only one phospholipid molecule partially penetrated the lateral gate of ScSec in the cytosolic leaflet of the membrane with its aliphatic tails remained outside (Extended Data Fig. 9 a-d); further incursion is unlikely as the interior of the cytosolic half of the channel is highly polar42. Notably, no lipids penetrated the channel in the lumenal leaflet during the entire duration of the simulations, despite a larger opening (~20 Å in TlSec and ~30 Å in ScSec) between TM3 and TM7 of Sec61α (Fig. 4f, and Extended Data Fig. 9b,d). Because the plug is displaced in these structures, the lumenal funnel of Sec61α remained completely unoccupied. By contrast, simulations of the Sec62-lacking structures (ΔSec62 TlSec and ScSec[Sec62−]) showed substantially deeper penetration of lipid molecules into the lateral gate (Fig. 4 d,g, Extended Data Fig. 9e,f, and Supplementary Movies 5 and 6). In both the cytosolic and lumenal leaflets of the membrane, the lateral gate became occupied with lipids within ~80 ns. These results are consistent with the lipid/detergent densities seen in the cryo-EM structure of ΔSec62 TlSec.
Our cryo-EM structures and MD simulations suggested that the V-shaped transmembrane domain of Sec62 effectively blocks lipids from entering the open lateral gate, particularly on the ER lumenal leaflet. We thus hypothesized that without Sec62, the pore may be invaded by lipids if both lateral gate and plug remain open. We tested this idea by running another set of MD simulations on TlSec[Sec62+] and ScSec[C1] but excluding the Sec62 subunit (Fig. 4e,h, Extended Data Fig. 9g,h, Supplementary Movies 7 and 8). The results indeed show that in both TlSec and ScSec, lipids invaded into the pore mainly from the lumenal leaflet, substantially obstructing the translocation pathway. It is likely that lipid molecules occupying the pore would inhibit insertion of substrate polypeptides. Thus, Sec62 seems to play an important role in maintaining the functionality of Sec61 by keeping lipids away from the open channel.
Mechanism of Sec61 gating by Sec63
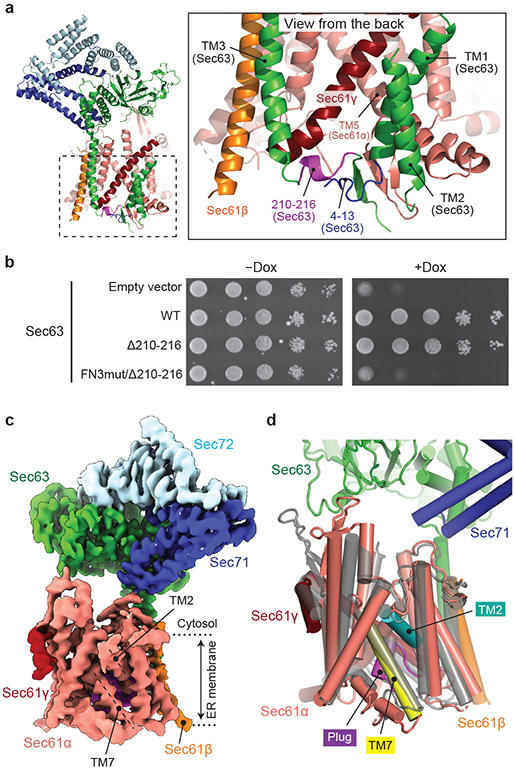
One unexpected finding was that the FN3–L6/7 interaction was dispensable for the protein translocation function of the Sec complex. This indicates that there must be another mechanism for Sec63 to open the lateral gate. Besides the FN3 domain, Sec63 forms major contacts with Sec61 through two other parts: TM3, which anchors Sec63 to the Sec61 complex, and a short ER lumenal segment (residues 210–216) preceding the TM3, which together with the N-terminal segment of Sec63, interacts with a crevice on the back of the channel (opposite from the lateral gate) (Fig. 5a). We reasoned that the latter interaction might control lateral gating through a lever-like mechanism. In the WT background, replacement of this segment with a glycine/serine linker (Δ210-216) alone did not cause growth inhibition (Fig. 5b). However, when combined with FN3mut, cells did not grow (Fig. 5b).
Figure 5. The structure of a fully closed Sec complex.
a, The interaction between Sec61 and Sec63 in the ER lumen (view from the back). The N-terminal segment (positions 4–13) and the segment preceding TM3 (positions 210–216) of Sec63 are in blue and purple, respectively. Shown is the ScSec[C1] structure. b, Yeast growth complementation (at 30°C) testing functionality of the indicated Sec63 mutants. The addition of doxycycline (Dox) represses chromosomal WT Sec63 expression. The experiments were repeated twice with similar results. c, The 3.8-Å-resolution cryo-EM structure of the ScSec complex containing FN3mut/Δ210-216 double-mutant Sec63. The lateral gate helices TM2 and TM7 are indicated. d, As in c, but showing the atomic model of the Sec61 complex. For comparison, the closed M. jannaschii SecY structure (PDB 1RH5; semitransparent grey) is superimposed.
To understand the structural basis of this synthetic defect, we determined the structure of the FN3mut/Δ210-216 ScSec complex (Fig. 5c and Extended Data Fig. 10) The structure showed that indeed both the lateral and vertical gates of the Sec61 channel are completely closed, resembling the idle archaeal SecY channel structure6 (Fig. 5c and Extended Data Fig. 10). This demonstrates that Sec63 uses both its cytosolic and lumenal domains to open the lateral gate in a two-pronged mechanism. The C-terminal cytosolic domain of Sec63 (following TM3) and Sec71–Sec72 are still attached to Sec61 through TM3. However, most of the parts preceding TM3 were invisible due to increased flexibility. Importantly, Sec62 was no longer visible either despite copurification with the complex (Fig. 5c, and Extended Data Fig. 10a). Sec62 is likely associated with Sec63 through an electrostatic interaction with the C-terminal tail of Sec63 (ref. 30,43) (Fig. 1d), but it seems to no longer bind to the lateral gate due to structural incompatibility with the closed gate. Therefore, the lateral gate must be first opened by Sec63 before Sec62 can activate the channel for protein translocation.
Discussion
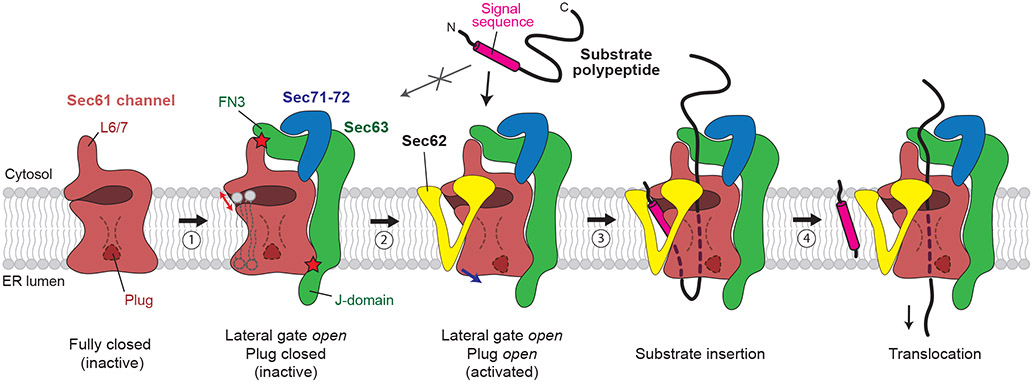
In summary, our study defines the functions of Sec62 and reveals the mechanism by which Sec63 and Sec62 regulate the gates of the Sec61 channel. The function of Sec62 had been elusive for three decades since its discovery as an essential component in eukaryotic post-translational translocation22,23,44. Our study shows that once the lateral gate of the Sec61 channel is opened by Sec63, Sec62 fully activates the channel by further mobilizing the plug domain (Fig. 6). At the same time, Sec62 seems to prevent lipids from penetrating the channel interior through the open lateral gate by forming a barrier in front of the lateral gate. Such lipid penetration into the lateral gate and translocation pore would likely impair the protein translocation activity by competitively inhibiting insertion of polypeptide substrates into the channel. The lipids may also affect movements of polypeptides in later stages of protein translocation. The V-shaped structure formed by the transmembrane domain of Sec62 is rather dynamic with respect to the rest of the complex and loosely associated with the lateral gate, as suggested by its relatively low-resolution densities in our cryo-EM maps. This flexibility may be important for insertion of signal sequences into and its egress from the lateral gate. It is also possible that the movement of Sec62 is modulated by binding of signal sequences and other protein translocation factors (e.g., BiP) to the Sec61 channel.
Figure 6. A model for activation of the Sec61 channel by Sec62 and Sec63.
The Sec61 channel alone assumes a fully closed conformation (the leftmost cartoon). Step 1, association of Sec63 opens the lateral gate (indicated by a red arrow) through interactions with Sec61 in both the cytosol and ER lumen (indicated by red stars). The channel in this conformation is inactive due to the closed state of the plug domain. In addition, without Sec62, lipids may enter the open lateral gate. Step 2, Sec62 interacts with the lateral gate of Sec61 and further opens the lateral gate (blue arrow), which results in opening of the plug. The V-shaped transmembrane domain of Sec62 excludes lipids from the channel. Step 3, a substrate polypeptide inserts into the open pore of the channel as a loop with the signal sequence sitting at the lateral gate. Step 4, The signal sequence is cleaved by the signal peptidase (not shown), and the polypeptide is translocated into the ER lumen. For simplicity, the BiP ATPase, which drives translocation by interactions with the polypeptide and J-domain, is not shown.
The fully open conformation of the WT Sec complexes observed in our cryo-EM structures likely represents a resting state prior to substrate engagement. Although the channel’s conformation and their dynamics in the native membrane environment remains to be determined, we speculate that this open state is likely a predominant form in the native ER membrane, based on the stable association between Sec61, Sec62, and Sec63. A pre-opened Sec61 channel in the post-translational complex contrasts with a relatively closed Sec61 channel seen with resting co-translational complexes, where the lateral gate is only marginally open, and the plug domain remains in the closed position8. It has been generally thought that during initial substrate engagement, the Sec61 channel would be opened by a hydrophobic interaction between the signal sequence (or TM helix) and the lateral gate2,8,11,14,45. Our mutagenesis analysis however indicates that such a partially open state, like the one induced by Sec63 alone, is insufficient for post-translational protein translocation. This is probably because the plug domain in the closed position would impose a too high energy barrier for post-translational polypeptide substrates to insert into the pore.
Many post-translational substrates are known to contain a signal sequence with relatively lower hydrophobicity15. Eukaryotic post-translational substrates are also expected to interact more transiently with Sec61 during initial insertion because they are not tethered to the ribosome as in the co-translational mode or to the SecA ATPase as in the bacterial post-translational mode. These features of substrates for the Sec complex may require both the lateral and vertical gates of the channel to be pre-opened for efficient insertion. A reduced energy barrier for substrate insertion by pre-opening the gates would allow polypeptides to promptly engage with the Sec61 complex, without which polypeptides may lose translocation competency because of premature folding or aggregation. Maintaining a stably open conformation by Sec63 and Sec62 may also be important for subsequent translocation steps as it may reduce friction in polypeptide movements. Our structural analysis shows that Sec63 and Sec62 open the gates of the Sec61 channel in a stepwise fashion to activate the channel, explaining their essentiality in cell viability. Given the high degree of sequence conservation of these components, the gating mechanism we discovered here is likely conserved across all eukaryotic species.
Methods
Yeast strains
A list of yeast strains used in this study is given in Supplementary Table 1.
The yeast strain (ySI7) used for purification of the wildtype (WT) ScSec complex has been described previously28. Briefly, this strain expresses a fusion protein of Sec61β (Sbh1), Sec63, and a green fluorescent protein (GFP) from the genomic SEC63 locus under the endogenous promoter of SEC63 (the endogenous SBH1 copy is deleted). The C-terminus of Sbh1p and the N-terminus of Sec63 are separated by a 15-amino-acid-long glycine-serine linker. There is a flexible linker containing a Tobacco etch virus (TEV) cleavage sequence between the C-terminus of Sec63 and GFP.
To enable purification of the PM (pore mutant) ScSec complex, we generated strain ySI8 by modifying ySI7. We first clone the SEC61 gene (from 1965-bp upstream to 668-bp downstream of the Sec61 coding sequence (CDS) of BY4741) into a pBlueScript-derived cloning vector (comprised of a pUC origin and an ampicillin resistance gene). We then inserted a LEU2 selection marker cassette (amplified by PCR using pYTK075 as a template, a forward primer: 5’- agctaaataagatctTCGAGGAGAACTTCTAGTATATCTACATAC, and a reverse primer: 5’- tatatataggagctcCTGCCTATTTAACGCCAAC; uppercase for LEU2-specific sequences and lowercase for SEC61-specific sequences) was inserted between 125 bp and 126 bp downstream of the Sec61 stop codon by In-Fusion cloning (Takara Bio). Pore mutations (M90L/T185I/M294I/M450L) were introduced to Sec61 by site-specific mutagenesis. The resulting plasmid was then linearized by cutting the plasmid backbone with NotI. The DNA fragment was introduced into ySI7 by a standard lithium acetate/polyethylene glycol transformation protocol. Recombinants were selected on a leucine drop-out synthetic complete [SC(−Leu)] agar medium. Incorporation of the mutations was verified by PCR and Sanger sequencing of single colonies.
To purify the FN3mut ScSec complex, we generated strain ySI73 by modifying strain TH_5187 (Horizon Discovery) from the Hughes collection46, where the expression of chromosomal Sec63 is under the control of a tetracycline repressible promoter. First, the endogenous copy of SBH1 of TH_5187 was replaced with a hygromycin resistance marker (hphMX) cassette. An hphMX cassette fragment was amplified by PCR using pFA6a-hphMX6 (ref. 47) as a template (forward primer: 5’-gggaaaagatttcaaccaccacttcaaaacaccacactctacctcctaccatactccataAGCTTGCCTCGTCCCC; reverse primer: 5’-tagtcttgttttgtcaaatagggtggataaaagctgaatcattactgaagaaaattcttaCAGTATAGCGACCAGCATTCAC; uppercase for vector specific sequences and lowercase for sequences homologous to yeast chromosomal sequences). The DNA fragment was transformed into TH_5187. Single colonies were isolated from YPD (1% yeast extract, 2% peptone, 2% glucose) agar plates containing 400 μg/ml hygromycin (Gold Biotechnology), and integration was verified by PCR. The resulting strain (ySI48) was then further modified by integration of the FN3mut Sec63 construct into the HO locus using the transforming plasmid pSI74 (see below) linearized with HindIII, which cuts at the E. coli kanamycin resistance marker. Transformed cells were selected on SC(−Leu) agar medium, and integration was verified by PCR.
For purification of the FN3mut/PM ScSec complex, we used strain ySI74. To generate ySI74, we first modified TH_5187 to contain the pore mutations in the SEC61 gene, similarly as described above for ySI8, but using a nourseothricin resistance cassette (natMX6) instead of the LEU2 marker. The natMX6 cassette was amplified from pFA6a-natMX6 (ref. 47) and inserted into the pBlueScript-Sec61 plasmid at 125 bp downstream of the Sec61 stop codon. After transformation of the linearized plasmid, recombinants were selected on YPD agar plates containing 100 μg/ml nourseothricin (Gold Biotechnology), resulting in ySI42. Subsequently, SBH1 deletion and Sec63 FN3mut mutation were introduced to the strain as described for ySI73.
For purification of the FN3mut/Δ210-216 ScSec complex, we used strain ySI112. To generate ySI112 we modified strain ySI48 (TH_5187 sbh1Δ::hphMX6) by integration of the FM3mut/Δ210-216 Sec63 construct into the HO locus using the transforming plasmid pSI120 (see below) linearized with HindIII.
The WT and mutant TlSec complexes were expressed using the yeast strains ySI67 (for WT), ySI77 (for ΔSec62), and ySI113 (for Δanchor). These strains were generated from the parental strain yMLT62 (a gift from J. Thorner; ref. 48), which expresses the β-estradiol-responsive chimeric transcription activator Gal4dbd.ER.VP16. All TlSec subunits were co-expressed with an integration vector (pYTK-e101) generated using MoClo Yeast ToolKit (YTK)49 (see below). Expression of each gene is driven by a GAL1 promoter. pYTK-e101 contains a nourseothricin resistance marker and URA3 homology sequences for chromosomal integration. pYTK-e101 encoding WT (pSI65) or mutant TlSec (pSI87 for ΔSec62 and pSI94 for Δanchor) were linearized with NotI and transformed into yMLT62. Recombinants were selected by growth in YPD agar plates containing 100 μg/ml nourseothricin. Integration was verified by PCR as described49.
For yeast growth complementation assays for Sec62, we generated ySI62, whose chromosomal Sec62 expression can be repressed in the presence of doxycycline. The tetracycline response element (TRE) as well as the upstream kanMX cassette were PCR amplified from genomic DNA of TH_5187 with primers containing 60 bps overhangs homologous to the N-terminus of Sec62 (forward primer: 5’-gacggaatagacgtgtcgttttcccaatactggcatacaaatcaagagggagaagagtggGGCGTTAGTATCGAATCG; reverse primer: 5’-tgtagcagatccgccattgacactagcacctgcattgctacctggacctacggctgacatGGATCCCCCGAATTG; uppercase for sequence specific to TRE-kanMX and lowercase for sequences homologous to yeast chromosomal sequences). The amplicon was then transformed into the strain R1158 (the parental strain for TH_5187) which contains the “tet activator” (tTA). Transformed cells were selected on 300 μg/ml G418 (Fisher Chemical) containing YPD-agar plates and integration was verified by PCR.
Strain ySI89 was used for complementation assays testing synthetic growth defects of FN3mut Sec63 and Sec61 mutants (Fig. 3c). To generate this strain, TH_4087 (Hughes strain with the chromosomal SEC61 expressing under a tetracycline-repressible promoter) was modified such that its endogenous Sec63 was mutated to FN3mut. A DNA segment encoding part of FN3mut Sec63 was amplified by PCR from plasmid pSI16 (from 164th amino acid to the stop codon) and inserted to pFA6a-natMX6 immediately before an ADH1 terminator, which precedes the natMX6 cassette. The resulting construct was amplified by PCR to include 773 bp upstream of the first mutated amino acid (E440R) and 50 bp of the 3’ untranslated region of the SEC63 locus (forward primer: 5’- CCCTTACTGACGAATTGGTTAGGC; reverse primer: 5’- atgtatctatttttataaagatgaaatatatacgtctaagagctaaaatgGGCCGCATAGGCCACTAG; uppercase for sequence specific to the plasmid and lowercase for sequences homologous to yeast chromosomal sequence). The amplicon was transformed into TH_4087 and selected on 100 μg/ml nourseothricin containing YPD-agar plates. Incorporation of the mutation was verified using Sanger sequencing.
Plasmids
A list of plasmids used in this study is given in Supplementary Table 1.
The integration vectors pYTK-e101 and pYTK-e106, and CEN/ARS plasmid pYTK-e112 were generated using Golden Gate BsaI assembly of parts from MoClo YTK49 (for pTYK-e101, parts were 8, 47, 73, 78, 86, 90, and 92; for pYTK-e106, part numbers were 8, 47, 73, 75, 88, 90, and 94; for pYTK-e112, part numbers were 8, 47, 73, 75, 81, and 84). pYTK-e101 contains a natMX6 marker and integrates into the URA3 locus. pYTK-e106 contains a LEU2 auxotroph marker and integrates into the HO locus. pYTK-e112 contains a LEU2 auxotroph marker.
The TlSec-expressing pYTK-e101 plasmids were generated using MoClo YTK as follows. First, gene fragments encoding TlSec subunits were chemically synthesized based on protein sequences of T. lanuginosus ATCC 2000065 (https://gb.fungalgenomics.ca) and cloned into the YTK entry vector pYTK001 (ref. 49). Codons were optimized for yeast. In the case of the Sec63 and Sec61β subunits, a fusion construct (TlSec61β– GGSGGSGGSGGSGGS–TlSec63–TEV–GFP) was synthesized similarly to the expression of the ScSec complex. Each synthesized CDS was then cloned into the pYTK095 vector (ref. 49) as an expression cassette together with a GAL1 inducible promoter, an ENO1 terminator, and connector parts by Golden Gate BsaI assembly. Subsequently, the multigene expression construct was generated by Golden Gate BsmBI assembly of the pYTK095 plasmids and pYTK-e101 resulting in pSI65 (the Sec gene placed in tandem in the following order: TlSec61α, TlSec61γ, Sec62, TlSec61β-TlSec63-GFP, TlSec71, and TlSec72). For ΔSec62 TlSec (plasmid pSI87), pYTK095-TlSec62 was replaced by a non-expressing spacer cassette in the BsmBI assembly. For Δanchor TlSec (plasmid pSI94), amino acid residues N319LF…WNE338 of TlSec62 were replaced with a Gly/Ser linker (GGSGGSGGS) before the multigene BsmBI assembly.
For expression of Sec63-mutant ScSec complexes (FN3mut and FN3mut/Δ210-216), WT ScSec63 was first amplified from genomic DNA of BY4741 by PCR to include the endogenous promoter and terminator (187 bp upstream and 97 downstream of the CDS) and cloned into pYTK-e112 between the two BsaI sites (pSI5). This plasmid was then further modified to have Sbh1 and GFP flanking Sec63 as in other Sbh1-Sec63 fusion constructs (i.e., Sbh1–GGSGGSGGSGGSGGS–Sec63–TEV–GFP). Then FN3mut (E440R/F481S/Δ441-447) or FN3mut/Δ210-216 (FN3mut and residues L210PRFLVD216, replaced with SGSGGSG) mutations were introduced by site-specific mutagenesis. For generation of strains ySI74 and ySI112 (chromosomal integration of mutant Sec63 to the HO locus), the expression cassette for Sbh1-Sec63-GFP was transferred to pYTK-e106 by restriction digestion and ligation.
For growth complementation assays, SEC61 (710 bp upstream to 264 bp downstream of CDS) and SEC62 (251 bp upstream to 123 bp downstream of CDS) were amplified by PCR using genomic DNA of BY4741 and cloned into pYTK-e112, resulting in pSI123 and pSI39, respectively. Plasmids used for growth complementation assays of Sec63 mutants were derived from pSI5 (see above) by adding a TEV-GFP tag at the C-terminus of Sec63 (the Sbh1 fusion was not introduced, and thus the constructs have the native N-terminus).
Yeast growth complementation assays
The yeast strains were transformed with each pYTK-e112 plasmid encoding the indicated protein under its endogenous promoter. Cells were selected on SC(−Leu) agar medium. Single colonies were picked and grown overnight in SC(−Leu) medium. The cultures were diluted with water to OD600 of 1.0 and further serially diluted by factors of 10 (in Fig 3b,c, starting concentration was OD600 of 0.1). The diluted cultures (10 μL each) were spotted on SC(−Leu) agar plates. In the case of Extended Data Fig. 4 (bottom two panels only), YPD agar medium was used. Where indicated, plates contained 10 μg/ml doxycycline. Plates were incubated at 30°C unless otherwise stated. The following strains were used for the indicated experiment: TH_5187 (Fig. 3b left panel, Fig. 4, and Extended Data Fig. 8a,b), ySI42 (Fig. 3b right panel), ySI89 (Fig. 3c), and ySI62 (Extended Data Fig. 4c)
Protein purification
For purification of ScSec complexes, yeast cells (ySI7 for WT, ySI8 for PM, ySI73 for FN3mut, ySI74 for FN3/Δ210-216) were grown in YPD medium to OD600 of 2–3, before harvest. For purification of TlSec, cells were grown in YPD medium to OD of 1.0. After adding 50 nM β-estradiol, cells were further grown until reaching OD 2–3. All cultures were grown in 30°C, except for the FN3mut/PM and FN3/Δ210-216 variants of ScSec, for which cells were grown at 22°C. Cells were harvested by centrifugation (8 min at 6,400 xg), washed once with ice-cold tris-buffered saline (20mM Tris pH 7.5 and 150 mM NaCl), frozen in liquid nitrogen, and stored at −75°C before use.
All ScSec and TlSec complexes were purification as described previously28. Briefly, cells were lysed by cryo-milling and resuspended in buffer containing 50 mM Tris pH 7.5, 200 mM NaCl, 1 mM EDTA, 10% glycerol, 2mM DTT, 5 μg/ml aprotinin, 5 μg/ml leupeptin, 1 μg/ml pepstatin A, and 1.2 mM PMSF. Membranes were solubilized by adding 1% lauryl maltose neopentyl glycol (LMNG; Anatrace) and 0.2% cholesteryl hemisuccinate (CHS; Anatrace) directly to the whole-cell lysate for 1.5 h at 4°C. The lysate was clarified by ultracentrifugation at 125,000 xg for 1 h. The Sec complex was bound to agarose beads conjugated with anti-GFP nanobody and the buffer was exchanged to 50 mM Tris pH 7.5, 200mM NaCl, 1.0 mM EDTA, 2 mM DTT, 0.02% glycol-diosgenin (GDN; Anatrace), and 10% glycerol. The complex was eluted by incubating the beads with TEV protease (~10 μg/ml) overnight and further purified by size-exclusion chromatography (Superose 6 Increase; GE Lifesciences) in 20 mM Tris pH 7.5, 100 mM NaCl, 1mM EDTA, 2 mM DTT, and 0.02% GDN. Peak fractions were concentrated to ~5 mg/ml and used immediate for cryo-EM. We note that the yields of all mutant ScSec complexes were comparable to that of the WT complex.
Cryo-EM grid preparation and data collection
Purified samples were supplemented with 3 mM fluorinated Fos-Choline-8 (FFC8; Anatrace) before plunge freezing. The samples were applied on holey carbon gold grids (Quantifoil 1.2/1.3, 400 mesh) that were glow discharged for 35 seconds using PELCO easiGlow glow discharge cleaner. Plunge freezing was performed using Vitrobot Mark IV (FEI) set at 4°C and 100% humidity. Whatman No. 1 filter paper was used to blot the samples.
Datasets for TlSec, ΔSec62 TlSec and FN3mut/Δ210-216 ScSec were collected on an FEI Talos Arctica electron microscope operated at an acceleration voltage of 200 kV. Datasets for WT ScSec, PM ScSec, FN3mut ScSec, FN3mut/PM ScSec and Δanchor TlSec were collected on an FEI Titan Krios electron microscope operated at an acceleration voltage of 300 kV and equipped with a Gatan Quantum Image Filter (a slit width of 20 eV). Both microscopes operated using SerialEM software50. Movies were recorded on a Gatan K3 Summit direct electron detector under the super-resolution mode (with a physical pixel size of 1.14 Å for TlSec and ΔSec62 TlSec, 0.9 Å for FN3mut/Δ210-216 ScSec and 1.19 Å for WT ScSec, FN3mut ScSec, FN3mut/PM ScSec and Δanchor TlSec,) with the exception of PM ScSec, which utilized a Gatan K2 Summit direct election detector (with physical pixel size of 1.15 Å). The samples were exposed to a total dose of ~50 e− per Å2 applied over 42 frames. Defocus target was typically set between −0.8 μm and −2.4 μm. For detailed parameters, also see Tables 1 and 2.
Cryo-EM image analysis
Micrographs collected from the microscopes were preprocessed by Warp51. Movie stacks were corrected for gains and subjected to tile-based motion correction and CTF estimation (7 by 5 tiles for datasets from the K3 detector and 5 by 5 for datasets from the K2 detector). Particles were automatically picked using the BoxNet algorithm of Warp. Low quality micrographs and particles, such as those containing crystalline ice or showing excessive motion blur, were removed by manual inspection. Motion corrected movies were exported with 2x-pixel- and 2x-frame-binning. Local particle motion corrections were performed in cryoSPARC v2 (ref. 52) after importing particle metadata and motion-corrected movie stacks. Box sizes of extracted particle images were 256 pixels except for the FN3mut/Δ210-216 ScSec dataset, which was 320 pixels. All subsequent single-particle analyses were performed with cryoSPARC v2 as described below. In the cases of the WT and FN3mut/Δ210-216 ScSec datasets, particle images extracted from Warp were directly used without local motion correction.
(1) WT ScSec: The single-particle analysis procedure for WT ScSec is outlined in Extended Data Fig. 1b. First, 2,686,839 picked particles were subjected to 2D classification, where empty micelles and classes of poor quality were removed. Selected 1,679,300 particles were then subjected to ab-initio reconstruction to yield three initial models, followed by heterogenous refinement using the initial maps (unless state otherwise, particle images were 2x scaled down to 128 by 128 pixels in all heterogenous refinement). Features of the Sec complex appeared in only one class (1,172,728 particles), particles of which were subjected to a second iteration of heterogenous refinement with the three classes from the first heterogenous refinement as references to further remove poor-quality particles. The resulting 995,878 particles were then subjected to a round of non-uniform refinement, local CTF refinement, and another round of non-uniform refinement, yielding a map at 2.98-Å resolution (consensus map). In order to separate the particles into classes containing and lacking Sec62, the N-terminal cytosolic domain (NTD) density of Sec62 in the consensus map was manually erased used UCSF Chimera53 and was used alongside the consensus map as initial references for heterogenous refinement. This yielded two classes: ScSec[Sec62−] with 391,885 particles which is largely devoid of detectable Sec62 and ScSec[Sec62+] with 603,993 particles. After non-uniform refinement, both classes refined to resolution of 3.07 Å. To further separate into subclasses containing different conformations of Sec62, the particles of ScSec[Sec62+] were subjected to a round of ab-initio reconstruction and heterogeneous refinement to yield five new classes. This step produced two major classes: one lacking Sec71-Sec72 (142,768 particles), and one showing the full complex features (415,818 particles). Particles of the latter class were subjected to a second round of ab-initio reconstruction and heterogenous refinement, yielding five new classes. Of these, two major classes showing the prominent features of the Sec complex (the other three classes did not show clear features of ScSec) were further refined using non-uniform refinement to yield the final maps of ScSec[C1] (from 193,263 particles) and ScSec[C2] (from 193,661 particles) at overall resolutions of 3.16 and 3.14 Å, respectively.
(2) PM ScSec: The PM ScSec dataset was analyzed using essentially the same procedure as for WT ScSec but starting with a dataset of 195,915 auto-picked particles (see Supplementary Fig. 2). After a round of 2D classification, ab-initio reconstruction, and heterogeneous refinement, the consensus class (91,813 particles) was obtained, which was subjected to non-uniform refinement to yield a 3.53-Å-resolution map. As with WT ScSec, the particles were further classified to [Sec62−] and [Sec62+] classes by heterogeneous refinement (35,573 and 56,240 particles, respectively), and the structures were refined to maps at resolutions of 4.02 and 3.78 Å, respectively. Particles of the [Sec62+] class was further classified by ab-initio reconstruction and heterogeneous refinement (five classes). One class (13,752 particles) lacked the Sec71-72 subunits, and the main class (36,506 particles) showed features of the full complex. The particles from the latter class were subjected another round of ab-initio reconstruction and heterogenous refinement, yielding two main classes, PM ScSec[C1] (17,341 particles) and PM ScSec[C2] (16,679 particles), which were further refined with non-uniform refinement to overall resolutions of 4.06 and 4.04 Å, respectively.
(3) FN3mut ScSec: The single-particle analysis procedure is outlined in Supplementary Fig. 2). The initial set of 1,274,219 auto-picked particles were subjected to 2D classification. After discarding empty micelle classes and classes showing poor features (resulting in 412,129 particles), we generated five initial models with ab-initio reconstruction. Only one class showed features of the Sec complex. Particles were subjected to two rounds of heterogeneous refinement to further remove particles of poor quality. The resulting 202,091 particles used for non-uniform refinement, which was followed by local CTF refinement and a second round of non-uniform refinement to obtain a consensus map at 3.73-Å resolution. Like with WT ScSec, these particles were further classified into two classes, one with Sec62 (FN3mut ScSec[Sec62+], 119,420 particles) and the other without Sec62 (FN3mut ScSec[Sec62−], 82,671 particles) using the consensus map and Sec62-NTD-erased map as initial references for heterogeneous refinement. FN3mut ScSec [Sec62+] and [Sec62−] particles were separately subjected to local CTF refinement and non-uniform refinement to yield final maps at 3.90- and 4.01-Å resolution, respectively. Further 3D classification of particles from the [Sec62+] class did not result in classes with a significant conformational difference.
(4) FN3mut/PM ScSec: The single-particle analysis procedure is outlined in Supplementary Fig. 2). The analysis was processed similarly as FN3mut ScSec. The initial set of 267,541 auto-picked particles were first cleaned up by 2D classification. The resulting 146,399 particles were subjected to ab-initio reconstruction (three classes). Only one main class showed features of the Sec complex. The 146,399 particles were then subjected to two rounds of heterogeneous refinement to remove non-Sec-complex particles. The resulting 86,843 particles were then subjected to non-uniform refinement, which was followed by local CTF refinement and a second round of non-uniform refinement to obtain a consensus map at 3.73-Å resolution. The particles were further classified to [Sec62+] and [Sec62−] classes (54,139 and 32,704 particles, respectively) with heterogeneous refinement, and final maps of PM/FN3mut ScSec [Sec62+] and [Sec62−] at 3.99- and 4.35- Å resolution respectively were obtained by non-uniform refinement followed by local CTF refinement and a second round of non-uniform refinement. Further 3D classification of particles from the [Sec62+] class did not yield classes with a significant conformational difference.
(5) FN3mut/Δ210-216 ScSec: The single-particle analysis procedure is outlined in Extended Data Fig. 10d. The initial set of 2,270,392 auto-picked particles were cleaned up by 2D classification. The resulting 646,998 particles were used to generate four initial maps with ab-initio reconstruction. Only one main class showed features of the Sec complex. Two rounds of heterogeneous refinement (with particle image 2x scaled down to 160 by 160 pixels) were performed to enrich particles of the Sec complex. The resulting 282,555 particles were subjected to non-uniform refinement, followed by local CTF refinement and a second round of non-uniform refinement to produce a 3.80-Å resolution map. The particles were then subjected to a second round of ab-initio reconstruction and heterogeneous refinement to generate four classes. Out of these classes, two showed features of the Sec complex (131,010 and 126,221 particles), maps of which were nearly identical. Particles of the two classes were combined for non-uniform refinement to yield the final map at 3.75-Å resolution.
(6) WT TlSec: The single-particle analysis procedure is outlined in Extended Data Fig. 2b. The initial set of 1,632,719 auto-picked particles were subjected to 2D classification in two batches to remove empty micelles and poor-quality particles. The resulting 789,004 particles were used to generate five initial 3D maps with ab-initio reconstruction. Only one (main) class showed features of the Sec complex. The 789,004 particles were subjected to heterogeneous refinement using the initial maps as references, which was followed by a second round of heterogeneous refinement. The resulting main class (427,835 particles) was refined using non-uniform refinement, local CTF refinement and second non-uniform refinement yielding a consensus map at 3.61-Å resolution. As with WT ScSec, particles were further classified to [Sec62+] and [Sec62−] classes with heterogeneous refinement using the consensus map and a Sec62-NTD-erased map as references (272,224 and 155,601 particles, respectively). The classes were further refined with non-uniform refinement yielding a 3.88-Å-resolution map of TlSec[Sec62−] and a 3.75-Å-resolution map of TlSec[Sec62+]. Particles of the [Sec62+] class (272,224 particles) were further subjected to ab-initio reconstruction and heterogeneous refinement (five classes). Two major classes (114,704 and 143,227 particles) showed the features of the Sec complex, which were further refined to the final maps of [Sec62+/plug-open] and [Sec62+/plug-closed] at overall resolutions of 4.02 and 3.76 Å, respectively. Unlike WT ScSec, a class lacking Sec71-Sec72 was not identified.
(7) ΔSec62 TlSec: The single-particle analysis procedure is outlined in Supplementary Fig. 2. The initial set of 546,712 auto-picked particles was subjected to two rounds of 2D classification with removal of poor classes in each round, resulting in 258,743 particles. Five initial 3D models were generated from the 258,743 selected particles by ab-initio reconstruction and further refined by heterogenous refinement. This produced two major classes (77,524 and 114,523 particles) which showed features of the Sec complex. Particles from the two classes were combined and refined with non-uniform refinement, local CTF refinement, and second non-uniform refinement, yielding the final map at 3.74-Å overall resolution.
(8) Δanchor TlSec: The initial set of 229,825 auto-picked particles were subjected to 2D classification, resulting in 105,578 particles. Three initial 3D models were generated by ab-initio reconstruction and refined by heterogenous refinement. One major class (76,726 particles) showed features of the Sec complex, and the particles from this class were used to generate the final map at 4.38-Å resolution with non-uniform refinement.
For additional Fourier shell correlation curves between the two half maps of final reconstructions, particle orientation distributions, local resolution distributions, see Supplementary Figs. 3-5.
Atomic model building
Atomic models were built using Coot (ref. 54). We first built models for ScSec[Sec62−] and ΔSec62 TlSec using our previous ScSec model (PDB ID 6N3Q; ref. 28) as a template. For ΔSec62 TlSec, we generated a homology model using SWISS-MODEL55, which was rebuilt into the map using Coot. The ScSec[Sec62−] model was then used to build models for ScSec[C1] and ScSec[C2]. For ScSec62, a poly-alanine model was built into densities. Atomic models for all the mutant ScSec structures lacking Sec62 were also built starting from the ScSec[Sec62−] model. The ScSec[Sec62−] model was first fitted into each map using UCSF chimera and further fitted into the map in groups of domains and subunits using rigid-body refinement in Phenix (ref. 56). The models were then locally adjusted in Coot. Models for PM ScSec[C1] and PM ScSec[C2] were built similarly using the WT ScSec[C1] and ScSec[C2] models as starting models. Models for FN3mut ScSec[Sec62+] and PM/FN3mut ScSec[Sec62+] were built starting with the WT ScSec[C2] and PM/FN3mut ScSec[Sec62−] model, respectively. ΔSec62 TlSec was used as a starting model to build all TlSec structures.
The models were refined with Phenix real-space refinement using combined maps that were sharpened with a B-factor estimated based on the Guinier plot and low-pass-filtered at their overall resolution (produced by cryoSPARC). The refinement resolution was also limited to the overall resolution of the maps in Phenix. Secondary structure restraints were used during the refinement. MolProbity57 was used for structural validation. For refinement and validation statistics, see Tables 1 and 2.
UCSF Chimera (ref. 53), ChimeraX (ref. 58), and PyMOL (Schrödinger) were used to prepare figures in the paper. Unless stated otherwise, all shown cryo-EM maps are unsharpened maps that were low-pass-filtered at their overall resolution.
Molecular dynamics (MD) simulations
Protein models of ScSec[C1], ScSec[C2], ScSec[Sec62−], WT TlSec, and ΔSec62 TlSec suitable for molecular dynamics (MD) simulation were built from the cryo-EM-derived atomic models. Missing areas of the overall complexes were modeled in using SWISS-MODEL55, however omitting the unstructured region of TlSec63, residues 482 to 526. The sequence of the TM portion of Sec62 was mapped onto the structure; gaps in the structures of other proteins were modeled except for the J-domain of Sec63. Molecular dynamics flexible fitting59 was used to fit the newly modeled pieces of the structures to optimize their positions within the density maps, including the Sec61 plug. All five systems were placed in a realistic yeast endoplasmic reticulum membrane with 47% POPC, 20% POPE, 10% PLPI, 8% POPS, 3% POPA, 10% ERG, 1% TLCL, and 1% DYGL (ref. 60,61) using CHARMM-GUI (ref. 62,63). The membrane protein systems were placed in a TIP3 (ref. 64) water box and neutralized with 0.15M KCl. The all-atom systems ranged from 250,000-270,000 atoms in size.
All simulations were run using NAMD 2.14 (ref. 65) with the CHARMM36m (protein)66,67 and CHARMM36 (lipid)68 force fields as well as hydrogen mass repartitioning (HMR)69. Positional restraints were initially placed on all atoms in each system and were gradually released in two consecutive runs: 0.5 ns with only the lipid tails unrestrained and 1 ns with the protein restrained. Subsequent 200-ns runs maintained restraints on the protein backbone in order to focus on the behavior of lipids for a given conformation of the Sec complex. All simulations were performed at a constant temperature of 310 K using Langevin dynamics (damping coefficient 1/ps), a constant pressure of 1 atm using Langevin piston, and periodic boundary conditions. Since HMR was used, the time step was set to 4 fs. Short range non-bonded interactions were cut off at 12 Å, with a force-based switching function starting at 11 Å. Long range non-bonded interactions were calculated using particle-mesh Ewald method with grid spacing of at least 1/Å3 (ref. 70). Total simulation time between all systems was 1.4 μs. Setup, analysis, and visualization were carried out using VMD71.
Reporting Summary statement
Further information on experimental design is available in the Nature Research Reporting Summary linked to this article
Data availability
The atomic coordinates and cryo-EM density maps of the Sec complexes were deposited to the Protein Data Bank (wwPDB) and Electron Microscopy Data Bank (EMDB), respectively. Their PDB and EMDB accession codes are as follows: EMD-22785 for ScSec[consensus]; PDB 7KAH and EMD-22770 for ScSec[Sec62−]; PDB 7KAI and EMD-22771 for ScSec[C1]; PDB 7KAJ and EMD22772 for ScSec[C2]; PDB 7KAO and EMD-22778 for PM ScSec[Sec62−]; PDB 7KAP and EMD-22779 for PM ScSec[C1]; PDB 7KAQ and EMD-22780 for PM ScSec[C2]; PDB 7KAR and EMD-22781 for FN3mut ScSec[Sec62−]; PDB 7KAS and EMD-22782 for FN3mut ScSec[Sec62+]; PDB 7KAT and EMD-22783 for PM/FN3mut ScSec[Sec62−]; PDB 7KAU and EMD-22784 for PM/FN3mut ScSec[Sec62+]; PDB 7KB5 and EMD-22787 for FN3mut/Δ210-216 ScSec; EMD-22786 for WT TlSec[Sec62+]; PDB 7KAK and EMD-22773 for TlSec[Sec62−]; PDB 7KAL and EMD-22774 for TlSec[Sec62+/plug-open]; PDB 7KAM and EMD-22775 for TlSec[Sec62+/plug-closed]; PDB 7KAN and EMD-22776 for ΔSec62 TlSec; EMD-22777 for Δanchor TlSec. Source data are available with the paper online.
Extended Data
Extended Data Fig. 1. Cryo-EM analysis of the wild-type (WT) S. cerevisiae Sec complex (ScSec).
a, Purification of the WT ScSec complex. Left, a chromatogram from Superose 6 size-exclusion chromatography of the affinity purified WT ScSec complex (MW standards: Tg, thyroglobulin; F, ferritin; Ald, aldolase). Right, the Coomassie-stained SDS-PAGE gel of the Superose 6 peak fraction. In this gel, Sec61γ (~10 kDa) migrated off the bottom. b, A diagram of the cryo-EM single particle analysis procedure. c, The 3.0-Å-resolution consensus map of ScSec. Salmon, Sec61α; orange, Sec61β; red, Sec61γ; yellow, Sec62; green, Sec63; blue, Sec71; light blue, Sec72; Grey, detergent micelle. Semitransparent surface, lowpass-filtered (5 Å for Sec62 and the J-domain and 7 Å for the micelle) maps shown at a lower contour level. d, As in c, but showing a local resolution map. Note that in addition to Sec62, the TM7-TM8 region of Sec61α (dotted line) displays noticeably lower solution than the overall resolution due to conformational heterogeneity (see Fig. 2a). e, As in c, but with the 3.1-Å-resolution map of the Sec62− class. Semitransparent surface, 6-Å-lowpass-filtered map at a lower contour level. f, As in c, but with the 3.1-Å-resolution map of the Sec62+/C2 class. g, The Sec62 densities of the C1 (yellow) and C2 (cyan) classes were compared after aligning the two cryo-EM maps. For simplicity, only Sec61 (from the C1 class) and Sec62 are shown. An uncropped image for panel a is available as source data.
Extended Data Fig. 2. Cryo-EM analysis of the WT and ΔSec62 T. lanuginosus Sec complexes (TlSec).
a, Purification of the WT TlSec complex. Left, a chromatogram from Superose 6 size-exclusion chromatography of the affinity purified WT TlSec complex. Right, the Coomassie-stained SDS-PAGE gel of the Superose 6 peak fraction. b, A diagram of the cryo-EM single particle analysis procedure. c, A representative cryo-EM micrograph. Scale bar, 50 nm. d, Examples of selected 2D class averages. e, The 4.0-Å-resolution map of the Sec62+/plug-open class of WT TlSec. The color scheme is the same as in Fig. 1. Semitransparent surface, a 7-Å-lowpass-filtered map shown at a lower contour level. f, As in e, but showing the 3.8-Å-resolution map of the Sec62+/plug-closed class. g, As in a, but purification of the ΔSec62 TlSec complex. h, As in e, but with the 3.7-Å-resolution map of ΔSec62 TlSec complex. i, The atomic models of the Sec61 complexes from the Sec62− class of WT TlSec (in color) and the ΔSec62 TlSec structure (in grey) were aligned and compared (RMSD of Cα atoms is 0.24Å). Uncropped images for panels a and g are available as source data.
Extended Data Fig. 3. Structure and mutagenesis analysis of the anchor domain of Sec62.
a, As in Fig. 1a, but a side view additionally showing a 6-Å-lowpass-filtered map at a lower contour level (semitransparent surface). b, As in a, but showing a view from cytosol. The anchor domain of Sec62 is indicated by a dotted oval. c, As in b, but showing the map of the Sec62+/plug-open class of TlSec. Semitransparent surface is a 7-Å-lowpass-filtered map. d, Yeast growth complementation tests for Sec62 anchor mutants. The yeast strain (ySI62) whose endogenous Sec62 is expressed under a tetracycline-repressible promoter was transformed with a CEN/ARS plasmid expressing WT or indicated mutant Sec62 under its native promoter. As a control, empty vector was used. In the right panels, 10 μg/mL doxycycline was included to repress the expression of endogenous Sec62. All growth assays were performed at 30°C. The top two panels (single and double mutants) were grown on synthetic complete (SC) medium lacking leucine, and the bottom two panels (triple and quadruple Ala mutants) were grown on YPD medium. e, As in Extended Data Fig 2a, but with the Δanchor mutant of TlSec. f, As in Extended Data Fig. 2e, but showing the 4.4-Å-resolution map of Δanchor TlSec. Semitransparent surface, 7-Å-lowpass-filtered map at a lower contour level. We note that the conformation of Δanchor TlSec is essentially identical to ΔSec62 TlSec. An uncropped image for panel e is available as source data.
Extended Data Fig. 4. A potential path for signal sequence release from the lateral gate into the membrane.
a, Schematic model for substrate insertion into the Sec complex and translocation. Substrates are expected to insert into the Sec61 channel as a loop with both N- and C-termini exposed in the cytosol. The N-terminal signal sequence may sit at the lateral gate (Stage 2) as seen in the structures of eukaryotic co-translational and bacterial post-translational translocation intermediates10-12. In a later stage, the signal sequence is cleavage by the signal peptidase. Although the exact timing remains unclear, one likely scenario is that the signal sequence needs to be released into the membrane prior to cleavage. Alternatively, the signal sequence may be cleaved by the signal peptidase while it is sitting at the lateral gate. In the former case, the presence of Sec62 may cause the signal sequence to be trapped at the lateral gate if Sec62 forms tight contacts with the lateral gate (right). In the latter scenario, signal sequence cleavage may be inhibited due to reduced accessibility of the cleavage site for the signal peptidase. b, As in Fig. 1e, but with the TlSec [Sec62+/plug-down] structure. Dashed arrow, a gap between TM2 of Sec62 and TM7 of Sec61α.
Extended Data Fig. 5. The presence of Sec62 induces opening of the vertical gate of Sec61 by displacing the plug domain from the closed position.
a–c, Views into the lateral gate (front view) of the three classes of the WT ScSec structure, C1, C2, and Sec62−. Color scheme: salmon, Sec61α; orange, Sec61β; red, Sec61γ; yellow, Sec62; green, Sec63; blue, Sec71; light blue, Sec72; magenta, the plug domain. d–f, as in a–c, but showing views from the ER lumen.
Extended Data Fig. 6. Conformational changes in the lateral gate and plug domain by Sec62 in the TlSec complex.
a, As in Fig. 2a, but with the WT TlSec (stereo view). Two classes, Sec62+/plug-open (in color) and Sec62− (grey) are shown. Dashed line, TM7 of Sec61α. For simplicity, Sec62 and the plug are not shown. We note that in the Sec62+/plug-closed class, TM7 assumes an intermediate position of the plug-open and Sec62− classes. b–d, As in Extended Data Fig. 6 but with the WT TlSec structures. Shows are front views (upper panels) and views from the ER lumen (lower panels). We note that in both the plug-open and plug-closed classes, the conformation of Sec62 (yellow) is similar to that of the ScSec[C2] structure. The area indicated by a white dashed box in g are shown in e. e, A stereo view showing an interaction between the plug domain (magenta) and TM7 (cyan) of Sec61α. Side chains that are involved in the interaction are shown in a ball and stick representation. W326 belongs to the loop 7/8 of Sec61α. Shown is the ΔSec62 TlSec structure. We note that a highly similar interaction is also present in the crystal structure of P. furiosus SecY (ref. 33; PDB ID 3MP7).
Extended Data Fig. 7. Structures of the ScSec complex containing mutations in the FN3 domain of Sec62 and the pore ring of Sec61α.
a, The same yeast growth complementation experiment shown in Fig. 3b (left panel), but additionally showing a control without doxycycline. The experiments were repeated three times with similar results. b, As in a, but the plates were incubated at 37°C. c, The 3.9-Å-resolution cryo-EM map of the Sec62+ class of the FN3mut ScSec complex. d, As in c, but with the 4.0-Å-resolution cryo-EM map of the Sec62− class of the FN3mut ScSec complex. e, The interaction between the FN3 domain of Sec63 and the L6/7 of Sec61α. Left, a side view showing Sec63 (green) and the Sec61 complex (grey, C2 class of WT ScSec; color, Sec62+ class of FN3mut ScSec). The structures were aligned with respect to Sec63. The area indicated by a grey dashed box is shown in the middle and right panels with a solvent-accessible surface representation. f, As in the left panel of e, but showing the cytosolic view into the Sec61 complex. g, As in Fig. 2a, but comparing the Sec62+ and Sec62− classes of FN3mut/PM ScSec. h, As in Fig. 2a, but comparing the three classes of PM ScSec. i, As in Fig. 3e, but with PM ScSec. We note that although the plug domain is partly visible in the C1 and C2 classes of PM ScSec, its density is substantially weaker than that of the Sec62− class.
Extended Data Fig. 8. Lipid/detergent molecules at the lateral gate.
a, A view into the lateral gate of the Sec61 channel in ΔSec62 TlSec. The left panel shows a surface representation of the complex (front view) showing the distribution of hydrophobic (yellow) and hydrophilic (cyan) amino acids. In the right panel, phosphatidylcholine lipid molecules modelled into the cryo-EM densities were additionally shown in a space-filling representation. b, A comparison of the Sec61 atomic models of ΔSec62 TlSec (in color) and WT TlSec [Sec62+/plug-closed] (in grey). Movements of TM2 (purple) and TM3 (violet) are indicated. c. As in Fig. 4a, but the WT ScSec structure by Wu et al. (EMDB-0440; ref. 29). Densities in cyan are detergent/lipid-like features. f, As in b, but comparing the structure by Wu et al. (in color; PDB 6ND1; ref. 29) and the ScSec[Sec62−] structure of the present study (in grey).
Extended Data Fig. 9. All-atom MD simulations of the ScSec complex in a model lipid bilayer.
a–h, As in Fig. 4 c-h, but with indicated ScSec structures. Panels a, c, e, and g are views from the cytosol, and b, d, f, and h are views from the ER lumen. In panels a–d, the translocation pore is marked by an asterisk. The lateral gate openings are indicated by a dashed arrow. The frames are from 200 ns after the initiation of the simulations.
Extended Data Fig. 10. Structure of a fully closed ScSec complex containing FN3mut/Δ210-216 Sec63.
a, Purification of the FN3mut/Δ210-216 ScSec complex. Left, a chromatogram from Superose 6 size-exclusion chromatography of the affinity purified mutant ScSec complex. Right, Coomassie-stained SDS-PAGE gel of the Superose 6 peak fraction. In this gel, Sec61γ (~10 kDa) migrated off the bottom. b, A representative cryo-EM micrograph. Scale bar, 50 nm. c, Examples of selected 2D class averages. d, A diagram of the cryo-EM single particle analysis procedure. e–h, The cryo-EM map (e and g) and atomic model (f and h). The color scheme is the same as in Fig. 1 a,d. The plug domain is shown in magenta. Shown are front (e and f) and side (g and h) views. An uncropped image for panel a is available as source data.
Supplementary Material
Supplementary Video 1 Morph between the three different conformations of WT S. cerevisiae Sec complex (ScSec).
Supplementary Video 2 Molecular dynamics simulation of the T. lanuginosus Sec complex (TlSec) in a lipid bilayer (Sec62+/plug-open conformation).
Supplementary Video 3 Molecular dynamics simulation of ScSec in a lipid bilayer (C1 conformation).
Supplementary Video 4 Molecular dynamics simulation of ScSec in a lipid bilayer (C2 conformation).
Supplementary Video 5 Molecular dynamics simulation of ΔSec62 TlSec in a lipid bilayer.
Supplementary Video 6 Molecular dynamics simulation of ScSec in a lipid bilayer (Class without Sec62).
Supplementary Video 7 Molecular dynamics simulation of TlSec [Sec62+/plug-open] in a lipid bilayer performed without the Sec62 subunit.
Supplementary Video 8 Molecular dynamics simulation of ScSec [C1] in a lipid bilayer performed without the Sec62 subunit.
Acknowledgments
We thank D. Toso and J. Remis for support for electron microscope operation, and J. Hurley and E. Nogales for critical reading of manuscript. This work was supported by the Vallee Scholars Program (E.P.) and the National Institutes of Health (J.C.G.; R01-GM123169). Computational resources were provided through the Extreme Science and Engineering Discovery Environment (XSEDE; TG-MCB130173), which is supported by a National Science Foundation Grant (ACI-1548562). Additional resources were provided by the Partnership for an Advanced Computing Environment (PACE) at the Georgia Institute of Technology.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Rapoport TA, Li L & Park E Structural and Mechanistic Insights into Protein Translocation. Annu Rev Cell Dev Biol 33, 369–390, doi: 10.1146/annurev-cellbio-100616-060439 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Voorhees RM & Hegde RS Toward a structural understanding of co-translational protein translocation. Curr Opin Cell Biol 41, 91–99, doi: 10.1016/j.ceb.2016.04.009 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Collinson I, Corey RA & Allen WJ Channel crossing: how are proteins shipped across the bacterial plasma membrane? Philos Trans R Soc Lond B Biol Sci 370, doi: 10.1098/rstb.2015.0025 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsirigotaki A, De Geyter J, Sostaric N, Economou A & Karamanou S Protein export through the bacterial Sec pathway. Nat Rev Microbiol 15, 21–36, doi: 10.1038/nrmicro.2016.161 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Seinen AB & Driessen AJM Single-Molecule Studies on the Protein Translocon. Annu Rev Biophys 48, 185–207, doi: 10.1146/annurev-biophys-052118-115352 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Van den Berg B et al. X-ray structure of a protein-conducting channel. Nature 427, 36–44, doi: 10.1038/nature02218 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Tsukazaki T et al. Conformational transition of Sec machinery inferred from bacterial SecYE structures. Nature 455, 988–991, doi: 10.1038/nature07421 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voorhees RM, Fernandez IS, Scheres SH & Hegde RS Structure of the mammalian ribosome-Sec61 complex to 3.4 A resolution. Cell 157, 1632–1643, doi: 10.1016/j.cell.2014.05.024 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka Y et al. Crystal Structures of SecYEG in Lipidic Cubic Phase Elucidate a Precise Resting and a Peptide-Bound State. Cell Rep 13, 1561–1568, doi: 10.1016/j.celrep.2015.10.025 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Li L et al. Crystal structure of a substrate-engaged SecY protein-translocation channel. Nature 531, 395–399, doi: 10.1038/nature17163 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voorhees RM & Hegde RS Structure of the Sec61 channel opened by a signal sequence. Science 351, 88–91, doi: 10.1126/science.aad4992 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma C et al. Structure of the substrate-engaged SecA-SecY protein translocation machine. Nat Commun 10, 2872, doi: 10.1038/s41467-019-10918-2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braunger K et al. Structural basis for coupling protein transport and N-glycosylation at the mammalian endoplasmic reticulum. Science 360, 215–219, doi: 10.1126/science.aar7899 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gogala M et al. Structures of the Sec61 complex engaged in nascent peptide translocation or membrane insertion. Nature 506, 107–110, doi: 10.1038/nature12950 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Ng DT, Brown JD & Walter P Signal sequences specify the targeting route to the endoplasmic reticulum membrane. J Cell Biol 134, 269–278 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller G & Zimmermann R Import of honeybee prepromelittin into the endoplasmic reticulum: structural basis for independence of SRP and docking protein. EMBO J 6, 2099–2107 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lakkaraju AK et al. Efficient secretion of small proteins in mammalian cells relies on Sec62-dependent posttranslational translocation. Mol Biol Cell 23, 2712–2722, doi: 10.1091/mbc.E12-03-0228 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Rhoads D & Tai PC Alkaline phosphatase and OmpA protein can be translocated posttranslationally into membrane vesicles of Escherichia coli. J Bacteriol 161, 973–980 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cabelli RJ, Chen L, Tai PC & Oliver DB SecA protein is required for secretory protein translocation into E. coli membrane vesicles. Cell 55, 683–692, doi: 10.1016/0092-8674(88)90227-9 (1988). [DOI] [PubMed] [Google Scholar]
- 20.Brundage L, Hendrick JP, Schiebel E, Driessen AJ & Wickner W The purified E. coli integral membrane protein SecY/E is sufficient for reconstitution of SecA-dependent precursor protein translocation. Cell 62, 649–657, doi: 10.1016/0092-8674(90)90111-q (1990). [DOI] [PubMed] [Google Scholar]
- 21.Zimmer J, Nam Y & Rapoport TA Structure of a complex of the ATPase SecA and the protein-translocation channel. Nature 455, 936–943, doi: 10.1038/nature07335 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothblatt JA, Deshaies RJ, Sanders SL, Daum G & Schekman R Multiple genes are required for proper insertion of secretory proteins into the endoplasmic reticulum in yeast. J Cell Biol 109, 2641–2652 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deshaies RJ, Sanders SL, Feldheim DA & Schekman R Assembly of yeast Sec proteins involved in translocation into the endoplasmic reticulum into a membrane-bound multisubunit complex. Nature 349, 806–808, doi: 10.1038/349806a0 (1991). [DOI] [PubMed] [Google Scholar]
- 24.Meyer HA et al. Mammalian Sec61 is associated with Sec62 and Sec63. J Biol Chem 275, 14550–14557 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Tyedmers J et al. Homologs of the yeast Sec complex subunits Sec62p and Sec63p are abundant proteins in dog pancreas microsomes. Proc Natl Acad Sci U S A 97, 7214–7219 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feldheim D, Rothblatt J & Schekman R Topology and functional domains of Sec63p, an endoplasmic reticulum membrane protein required for secretory protein translocation. Mol Cell Biol 12, 3288–3296 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matlack KE, Misselwitz B, Plath K & Rapoport TA BiP acts as a molecular ratchet during posttranslational transport of prepro-alpha factor across the ER membrane. Cell 97, 553–564 (1999). [DOI] [PubMed] [Google Scholar]
- 28.Itskanov S & Park E Structure of the posttranslational Sec protein-translocation channel complex from yeast. Science 363, 84–87, doi: 10.1126/science.aav6740 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu X, Cabanos C & Rapoport TA Structure of the post-translational protein translocation machinery of the ER membrane. Nature 566, 136–139, doi: 10.1038/s41586-018-0856-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wittke S, Dunnwald M & Johnsson N Sec62p, a component of the endoplasmic reticulum protein translocation machinery, contains multiple binding sites for the Sec-complex. Mol Biol Cell 11, 3859–3871, doi: 10.1091/mbc.11.11.3859 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw AS, Rottier PJ & Rose JK Evidence for the loop model of signal-sequence insertion into the endoplasmic reticulum. Proc Natl Acad Sci U S A 85, 7592–7596, doi: 10.1073/pnas.85.20.7592 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paetzel M, Karla A, Strynadka NC & Dalbey RE Signal peptidases. Chem Rev 102, 4549–4580, doi: 10.1021/cr010166y (2002). [DOI] [PubMed] [Google Scholar]
- 33.Egea PF & Stroud RM Lateral opening of a translocon upon entry of protein suggests the mechanism of insertion into membranes. Proc Natl Acad Sci U S A 107, 17182–17187, doi: 10.1073/pnas.1012556107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park E et al. Structure of the SecY channel during initiation of protein translocation. Nature 506, 102–106, doi: 10.1038/nature12720 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfeffer S et al. Structure of the native Sec61 protein-conducting channel. Nat Commun 6, 8403, doi: 10.1038/ncomms9403 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flower AM, Osborne RS & Silhavy TJ The allele-specific synthetic lethality of prlA-prlG double mutants predicts interactive domains of SecY and SecE. EMBO J 14, 884–893 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris CR & Silhavy TJ Mapping an interface of SecY (PrlA) and SecE (PrlG) by using synthetic phenotypes and in vivo cross-linking. J Bacteriol 181, 3438–3444, doi: 10.1128/JB.181.11.3438-3444.1999 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hizlan D et al. Structure of the SecY complex unlocked by a preprotein mimic. Cell Rep 1, 21–28, doi: 10.1016/j.celrep.2011.11.003 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng Z, Jiang Y, Mandon EC & Gilmore R Identification of cytoplasmic residues of Sec61p involved in ribosome binding and cotranslational translocation. J Cell Biol 168, 67–77, doi: 10.1083/jcb.200408188 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Becker T et al. Structure of monomeric yeast and mammalian Sec61 complexes interacting with the translating ribosome. Science 326, 1369–1373, doi: 10.1126/science.1178535 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Junne T, Schwede T, Goder V & Spiess M The plug domain of yeast Sec61p is important for efficient protein translocation, but is not essential for cell viability. Mol Biol Cell 17, 4063–4068, doi: 10.1091/mbc.e06-03-0200 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gumbart J, Chipot C & Schulten K Free energy of nascent-chain folding in the translocon. J Am Chem Soc 133, 7602–7607, doi: 10.1021/ja2019299 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willer M, Jermy AJ, Young BP & Stirling CJ Identification of novel protein-protein interactions at the cytosolic surface of the Sec63 complex in the yeast ER membrane. Yeast 20, 133–148, doi: 10.1002/yea.954 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Deshaies RJ & Schekman R SEC62 encodes a putative membrane protein required for protein translocation into the yeast endoplasmic reticulum. J Cell Biol 109, 2653–2664 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kater L et al. Partially inserted nascent chain unzips the lateral gate of the Sec translocon. EMBO Rep 20, e48191, doi: 10.15252/embr.201948191 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Methods-only references
- 46.Hughes TR et al. Functional discovery via a compendium of expression profiles. Cell 102, 109–126, doi: 10.1016/s0092-8674(00)00015-5 (2000). [DOI] [PubMed] [Google Scholar]
- 47.Hentges P, Van Driessche B, Tafforeau L, Vandenhaute J & Carr AM Three novel antibiotic marker cassettes for gene disruption and marker switching in Schizosaccharomyces pombe. Yeast 22, 1013–1019, doi: 10.1002/yea.1291 (2005). [DOI] [PubMed] [Google Scholar]
- 48.McIsaac RS et al. Fast-acting and nearly gratuitous induction of gene expression and protein depletion in Saccharomyces cerevisiae. Mol Biol Cell 22, 4447–4459, doi: 10.1091/mbc.E11-05-0466 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee ME, DeLoache WC, Cervantes B & Dueber JE A Highly Characterized Yeast Toolkit for Modular, Multipart Assembly. ACS Synth Biol 4, 975–986, doi: 10.1021/sb500366v (2015). [DOI] [PubMed] [Google Scholar]
- 50.Mastronarde DN Automated electron microscope tomography using robust prediction of specimen movements. J Struct Biol 152, 36–51, doi: 10.1016/j.jsb.2005.07.007 (2005). [DOI] [PubMed] [Google Scholar]
- 51.Tegunov D & Cramer P Real-time cryo-electron microscopy data preprocessing with Warp. Nat Methods 16, 1146–1152, doi: 10.1038/s41592-019-0580-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Punjani A, Rubinstein JL, Fleet DJ & Brubaker MA cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat Methods 14, 290–296, doi: 10.1038/nmeth.4169 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Pettersen EF et al. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem 25, 1605–1612, doi: 10.1002/jcc.20084 (2004). [DOI] [PubMed] [Google Scholar]
- 54.Emsley P, Lohkamp B, Scott WG & Cowtan K Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66, 486–501, doi: 10.1107/S0907444910007493 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waterhouse A et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res 46, W296–W303, doi: 10.1093/nar/gky427 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Afonine PV et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr D Struct Biol 74, 531–544, doi: 10.1107/S2059798318006551 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen VB et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr 66, 12–21, doi: 10.1107/S0907444909042073 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goddard TD et al. UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci 27, 14–25, doi: 10.1002/pro.3235 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trabuco LG, Villa E, Mitra K, Frank J & Schulten K Flexible fitting of atomic structures into electron microscopy maps using molecular dynamics. Structure 16, 673–683, doi: 10.1016/j.str.2008.03.005 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sugiura S & Mima J Physiological lipid composition is vital for homotypic ER membrane fusion mediated by the dynamin-related GTPase Sey1p. Sci Rep 6, 20407, doi: 10.1038/srep20407 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ganesan S, Shabits BN & Zaremberg V Tracking Diacylglycerol and Phosphatidic Acid Pools in Budding Yeast. Lipid Insights 8, 75–85, doi: 10.4137/LPI.S31781 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jo S, Kim T, Iyer VG & Im W CHARMM-GUI: a web-based graphical user interface for CHARMM. J Comput Chem 29, 1859–1865, doi: 10.1002/jcc.20945 (2008). [DOI] [PubMed] [Google Scholar]
- 63.Wu EL et al. CHARMM-GUI Membrane Builder toward realistic biological membrane simulations. J Comput Chem 35, 1997–2004, doi: 10.1002/jcc.23702 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW & Klein ML Comparison of Simple Potential Functions for Simulating Liquid Water. J Chem Phys 79, 926–935, doi:Doi 10.1063/1.445869 (1983). [DOI] [Google Scholar]
- 65.Phillips JC et al. Scalable molecular dynamics with NAMD. J Comput Chem 26, 1781–1802, doi: 10.1002/jcc.20289 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Best RB et al. Optimization of the Additive CHARMM All-Atom Protein Force Field Targeting Improved Sampling of the Backbone phi, psi and Side-Chain chi(1) and chi(2) Dihedral Angles. J Chem Theory Comput 8, 3257–3273, doi: 10.1021/ct300400x (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang J et al. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat Methods 14, 71–73, doi: 10.1038/nmeth.4067 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klauda JB et al. Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J Phys Chem B 114, 7830–7843, doi: 10.1021/jp101759q (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Balusek C et al. Accelerating Membrane Simulations with Hydrogen Mass Repartitioning. J Chem Theory Comput 15, 4673–4686, doi: 10.1021/acs.jctc.9b00160 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Darden T, York D & Pedersen L Particle Mesh Ewald - an N.Log(N) Method for Ewald Sums in Large Systems. J Chem Phys 98, 10089–10092, doi:Doi 10.1063/1.464397 (1993). [DOI] [Google Scholar]
- 71.Humphrey W, Dalke A & Schulten K VMD: Visual molecular dynamics. J Mol Graph Model 14, 33–38, doi:Doi 10.1016/0263-7855(96)00018-5 (1996). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Video 1 Morph between the three different conformations of WT S. cerevisiae Sec complex (ScSec).
Supplementary Video 2 Molecular dynamics simulation of the T. lanuginosus Sec complex (TlSec) in a lipid bilayer (Sec62+/plug-open conformation).
Supplementary Video 3 Molecular dynamics simulation of ScSec in a lipid bilayer (C1 conformation).
Supplementary Video 4 Molecular dynamics simulation of ScSec in a lipid bilayer (C2 conformation).
Supplementary Video 5 Molecular dynamics simulation of ΔSec62 TlSec in a lipid bilayer.
Supplementary Video 6 Molecular dynamics simulation of ScSec in a lipid bilayer (Class without Sec62).
Supplementary Video 7 Molecular dynamics simulation of TlSec [Sec62+/plug-open] in a lipid bilayer performed without the Sec62 subunit.
Supplementary Video 8 Molecular dynamics simulation of ScSec [C1] in a lipid bilayer performed without the Sec62 subunit.
Data Availability Statement
The atomic coordinates and cryo-EM density maps of the Sec complexes were deposited to the Protein Data Bank (wwPDB) and Electron Microscopy Data Bank (EMDB), respectively. Their PDB and EMDB accession codes are as follows: EMD-22785 for ScSec[consensus]; PDB 7KAH and EMD-22770 for ScSec[Sec62−]; PDB 7KAI and EMD-22771 for ScSec[C1]; PDB 7KAJ and EMD22772 for ScSec[C2]; PDB 7KAO and EMD-22778 for PM ScSec[Sec62−]; PDB 7KAP and EMD-22779 for PM ScSec[C1]; PDB 7KAQ and EMD-22780 for PM ScSec[C2]; PDB 7KAR and EMD-22781 for FN3mut ScSec[Sec62−]; PDB 7KAS and EMD-22782 for FN3mut ScSec[Sec62+]; PDB 7KAT and EMD-22783 for PM/FN3mut ScSec[Sec62−]; PDB 7KAU and EMD-22784 for PM/FN3mut ScSec[Sec62+]; PDB 7KB5 and EMD-22787 for FN3mut/Δ210-216 ScSec; EMD-22786 for WT TlSec[Sec62+]; PDB 7KAK and EMD-22773 for TlSec[Sec62−]; PDB 7KAL and EMD-22774 for TlSec[Sec62+/plug-open]; PDB 7KAM and EMD-22775 for TlSec[Sec62+/plug-closed]; PDB 7KAN and EMD-22776 for ΔSec62 TlSec; EMD-22777 for Δanchor TlSec. Source data are available with the paper online.