Abstract
Background
Hepatocellular carcinoma (HCC) associated with macroscopic vascular invasion and distant metastasis is an advanced-stage disease with an extremely poor prognosis and low survival rate. Therefore, there is an urgent need to develop novel therapeutic strategies to extend the lives of patients with advanced HCC.
Case Presentation
We represent a case of HCC with macroscopic vascular invasion and pulmonary metastasis responding dramatically to the combination treatment with drug-eluting beads transarterial chemoembolization (DEB-TACE) and Huaier granule. A 64-year-old man with hepatitis B virus (HBV)-induced liver cirrhosis was diagnosed with advanced HCC involved renal vein and inferior vena cava accompanied by pulmonary metastasis. The patient received three cycles of on-demand DEB-TACE from 9th September 2016 to 22nd August 2017 and combined with Huaier granule 20 g three times a day orally. Eight months following the treatment, complete response occurred with regression of HCC and vascular thrombus and disappearance of pulmonary metastasis. The levels of AFP had decreased from 8165.8ng/mL to within the normal range (1.7 ng/mL). This is the first case report of complete response of HCC to the combination treatment with DEB-TACE and Huaier granule. At the most recent follow-up, he remained in remission 36 months after cessation of treatment without clinical or imaging evidence of disease recurrence. The current overall survival is 54 months since the initial treatment.
Conclusion
Data from this clinical case report suggest that the combination treatment with DEB-TACE and Huaier granule is a promising therapeutic option for advanced HCC with macroscopic vascular invasion and distant metastasis.
Keywords: hepatocellular carcinoma, vascular invasion, drug-eluting beads, Huaier, traditional Chinese medicine
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignant tumor and the fourth leading cause of cancer-related death worldwide.1 HCC has a high tendency to invade the vascular system resulting in vascular tumor thrombus and distant metastasis.2 The portal vein invasion and pulmonary metastasis are highly prevalent in advanced HCC. Though the inferior vena cava (IVC) and renal vein (RV) are less frequently involved by HCC, the prognosis of HCC patients with RV and IVC tumor thrombosis (RV-IVCTT) is extremely poor as the tumor thrombosis may flow into the heart and lung, leading to life-threatening or fatal complications.
HCC with RV-IVCTT and pulmonary metastasis is difficult to treat and a standard therapy has not been established. The Barcelona Clinic Liver Cancer (BCLC) staging classification recommends sorafenib as the only treatment for these patients.3 However, survival probabilities differ significantly according to the extent of vascular invasion and the site of distant metastasis. In China, Surgery, ablation therapies, transarterial chemoembolization (TACE), radiotherapy, systemic treatment and traditional Chinese medicine (TCM) were adopted in the management of these cases.4 Herein, we report a case of HCC with RV-IVCTT and pulmonary metastasis responding dramatically to the combination treatment of drug-eluting beads transarterial chemoembolization (DEB-TACE) with CalliSpheres® microsphere and Huaier granule as initial treatment and we hope to explore further study for DEB-TACE and TCM therapy combination for advanced HCC treatment in the future.
Case Presentation
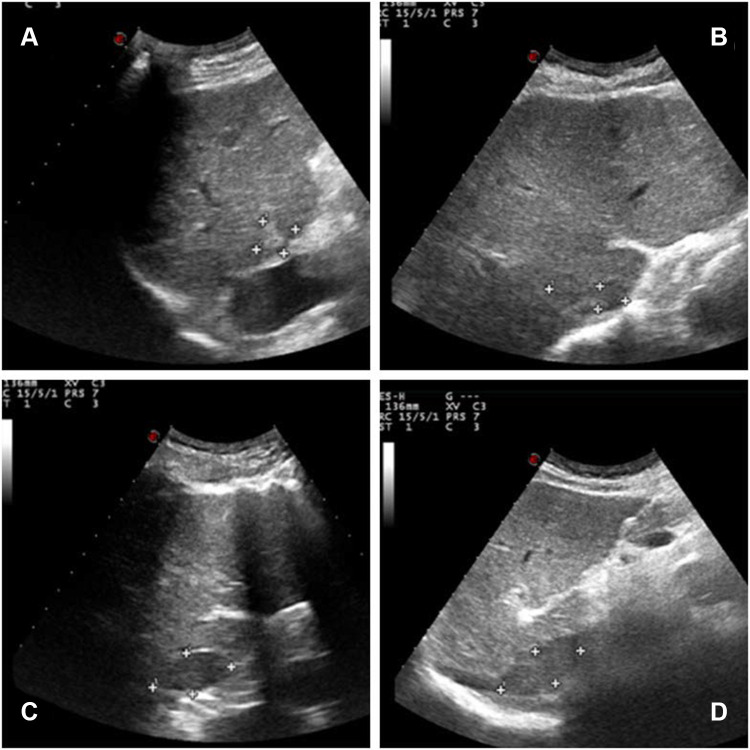
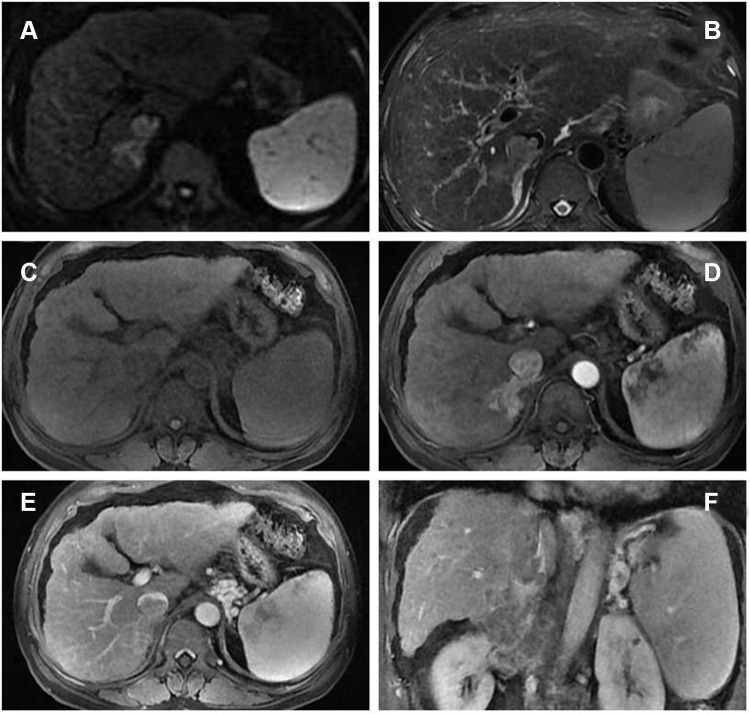
A 64-year-old man with hepatitis B virus (HBV)-related cirrhosis for ten years, and positive for a hepatitis B surface antigen (HBsAg) with high-level hepatitis B virus replication (HBV-DNA was 8.11×104 IU/mL), and he had no remarkable chief complaints. He was followed up regularly by a local hospital as he was an asymptomatic hepatitis B virus carrier, and the abdominal ultrasonography at that clinic revealed a mass in the right lobe of the liver with right renal vein and inferior vena cava thrombosis. He was referred to our department for further examination and treatment. The abdomen was soft and flat. Any digestive symptoms such as abdominal pain or weight loss were not observed (Eastern Cooperative Oncology Group: 0). Laboratory investigation results revealed an aspartate aminotransferase level of 69 IU/L (normal range, 8 to 40 U/L), alanine aminotransferase level of 53 IU/L (normal range, 5 to 40 U/L), alkaline phosphatase level of 99 IU/L (normal range, 40 to 150 U/L), a gamma-glutamyl-transpeptidase level of 21 IU/L (normal range, 11 to 50 U/L), albumin level of 41.8 g/L (normal range, 35 to 55 g/L), and the total bilirubin level of 29 μmol/L (normal range, 0 to 21 μmol/L). The parameters of the blood routine and coagulation function were within the normal range. Among tumor markers, the serum alpha-fetoprotein (AFP) was markedly elevated at 8165.8 ng/mL (normal range, 0 to 20 ng/mL). carcinoembryonic antigen (CEA) level was 1.2 ng/mL (normal range, 0 to 5 ng/mL), CA125 level was 40.5 U/mL (normal range, 0 to 35 U/mL) and CA19-9 level was 11.9 U/mL (normal range, 0 to 37 U/mL). The abdominal ultrasonography revealed an isoechoic mass (3.5cm×1.0 cm in size) in the posterior right lobe (Figure 1A–B), and a hypoechoic strip-like area measuring 3.7 cm × 1.9 cm was noted in the retrohepatic segment of the inferior vena cava (tumor thrombosis suspected) (Figure 1C–D). The contrast enhanced-computed tomography (CECT) scan showed that an irregular tumor mass located in segment V of the right lobe of the liver with renal vein and inferior vena cava involved. The tumor had enhancement in the arterial phase and was washed out in the venous phase (Figure 2). Dynamic contrast‐enhanced magnetic resonance imaging (DCE‐MRI) displayed enhancement in the arterial phase and a defect in the venous phase (Figure 3). The chest CT scan showed multiple nodular lesions in both lungs (Figure 6A–B) and pulmonary metastasis was considered.
Figure 1.
The abdominal ultrasonography revealed an isoechoic mass (3.5cm×1.0 cm in size) in the posterior right lobe (A and B), and a hypoechoic strip-like area measuring 3.7 cm × 1.9 cm was noted in the retrohepatic segment of the inferior vena cava (tumor thrombosis suspected, (C and D).
Figure 2.
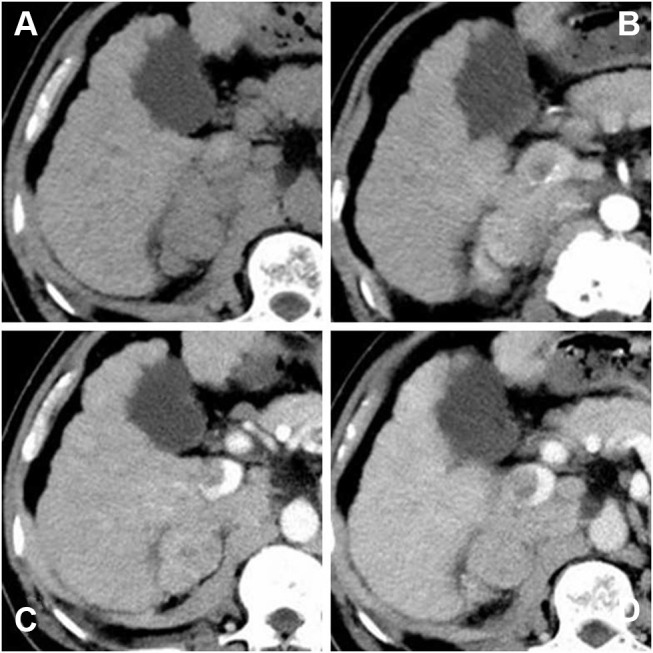
Contrast-enhanced computed tomography (CECT) scan showed that an irregular tumor mass located in segment V of the right lobe of the liver with renal vein and inferior vena cava involved. The tumor had enhancement in the arterial phase and was washed out in the venous phase. ((A) precontrast scan, (B) arterial phase, (C) portal phase, (D) delayed phase).
Figure 3.
Dynamic contrast‐enhanced magnetic resonance imaging (DCE‐MRI) displayed enhancement in the arterial phase and a defect in the venous phase ((A) DWI, (B) T2WI, (C) T1WI, (D) arterial phase, (E) portal phase, (F) coronal portal phase T1WI).
Figure 6.
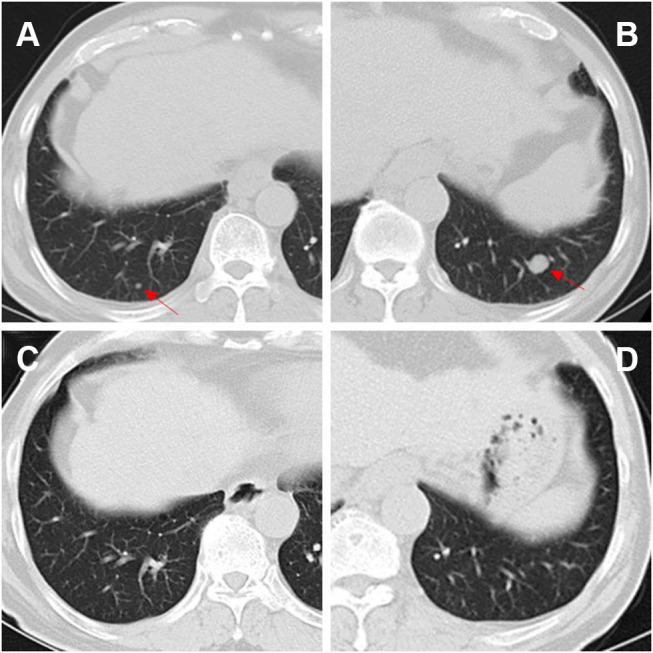
CT images before and after treatment. On admission before treatment, pulmonary metastasis (red arrow) were seen in the posterior segment of lower lobes of the lungs (A and B). After three cycles of treatments, the pulmonary metastasis had disappeared completely (C and D).
Using both his medical history and imaging findings, he was diagnosed as having advanced HCC with RV-IVCTT and pulmonary metastasis according to the American Association for the Study of Liver Diseases (AASLD) criteria, and the clinical stage was BCLC stage C. The multi-kinase inhibitor sorafenib was recommended as the first-line therapy for him. But he refused sorafenib because it was not on the list of drugs covered by National Health Insurance at that time though it had become available in China. Therefore, we had to seek alternative therapies for him.
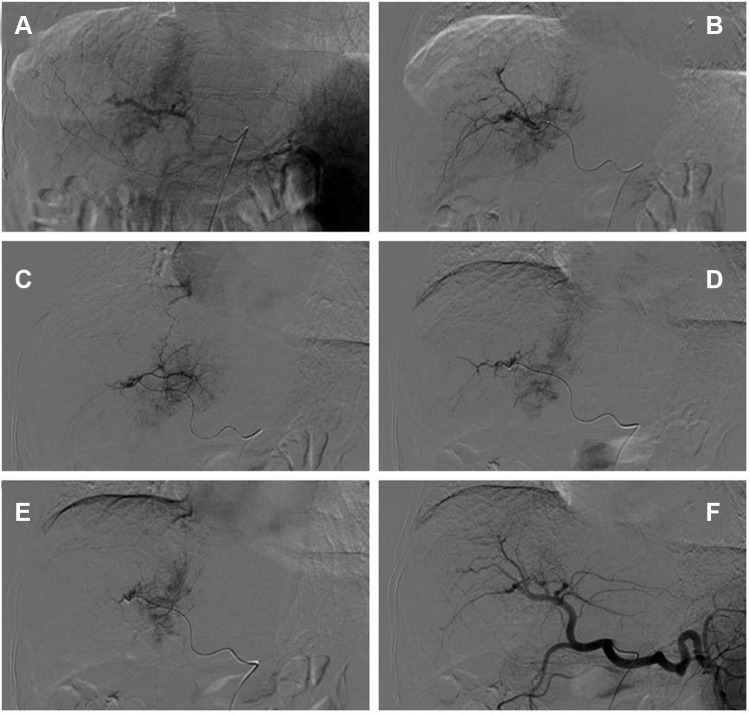
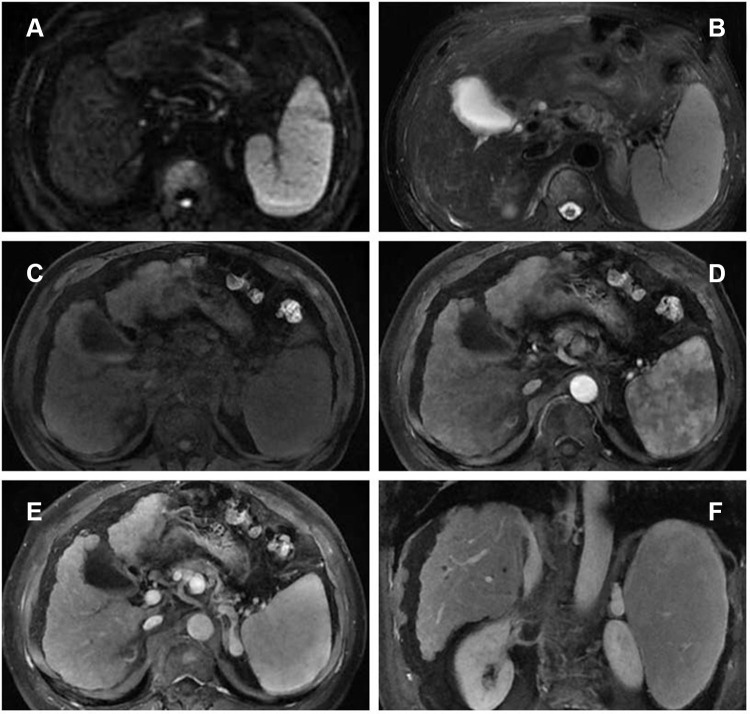
TACE is widely used when curative therapies cannot be performed, and it is recommended for intermediate‐stage HCC and as a palliative treatment in advanced‐stage HCC. Additionally, traditional Chinese medicine (TCM), such as Huaier granule, functions as chemotherapy or adjuvant chemotherapy attracted much attention for easy to obtain and exhibited significant antitumor effects accompanied with less toxic and side effects.5 Many studies suggested that the combination of TCM with TACE may synergistically affect the advanced HCC.6 Therefore, as the patient had normal hepatic function with good performance status, DEB-TACE loaded with doxorubicin combined with Huaier granule was planned for him. After three cycles of DEB-TACE (Figure 4) from 9th September 2016 to 22nd August 2017 and combined with Huaier granule 20 g three times a day orally, the level of AFP had decreased from 8165.8ng/mL to within the normal range (1.7 ng/mL). In April 2018, the level of AFP was still normal and the follow-up chest CT demonstrated that the lung metastases disappeared (Figure 6C–D) and the DCE‐MRI displayed that the liver tumor and RV-IVCTT showed complete response (Figure 5) according to the modified Response Evaluation Criteria in Solid Tumors criteria. Huaier granule was discontinued in July 2019 after he maintains his excellent serological and radiological complete response for 14 months.
Figure 4.
Celiac arteriogram demonstrates the hypervascular mass but does not provide much assistance in determining the number and location of feeding vessels to the target (A). Subselective arteriogram with a microcatheter clearly demonstrates the suitable target vessels to treat this tumor (B–E). Celiac arteriogram shows no tumor staining after DEB-TACE treatment with CalliSpheres microsphere (F).
Figure 5.
DCE‐MRI displayed the intrahepatic tumor and RV-IVCTT were complete responses after three cycles of treatments. ((A) DWI, (B) T2WI, (C) T1WI, (D) arterial phase, (E) portal phase, (F) coronal portal phase T1WI).
At his last follow-up, nearly 5 years have elapsed since the diagnosis of HCC, and it is up to 20 months since treatment discontinued. He was in very good condition, without evidence of disease progression till now. Currently, 54 months have passed, and he is still alive without recurrence.
Discussion
Hepatocellular carcinoma (HCC) with RV-IVCTT is rare and regarded as an advanced disease stage with a poor prognosis. Survival of those patients is extremely shortened and it is reported to be merely 2 to 4 months with the best supportive care.7,8 Conventionally, the treatment algorithm proposed by the BCLC system considers the presence of vascular invasion and/or distant metastasis (BCLC stage C) as a contraindication to surgery. Sorafenib is recommended as the only standard treatment for these patients; however, the effects of treatment with sorafenib alone on overall survival (OS) have not been satisfactory.9
TACE is performed worldwide for patients with intermediate-stage HCC, and as a palliative treatment in advanced‐stage HCC. Recently, TACE has been reported to have similar or even better safety and efficacy in some advanced HCC cases compared with sorafenib, including HCC with vascular invasion.10 However, the efficacy of conventional lipiodol-TACE (cTACE) is still limited, with a 1-year overall survival rate of 22.0% to 30.9%.11 Therefore, treatment of advanced HCC has always been challenging.
Drug-eluting beads TACE (DEB-TACE), a novel TACE based on the use of microspheres to release chemotherapeutic agents within a target lesion with controlled pharmacokinetics. It has been demonstrated in clinical studies that DEB-TACE had better tolerability and fewer complications compared with cTACE, especially in patients with advanced liver disease.12–14 Our previous study also demonstrates that DEB-TACE with CalliSpheres microsphere was efficient and well-tolerated in Chinese HCC patients.15 In this case, the advanced HCC patient with RV-IVCTT reached complete tumor response after three cycles of DEB-TACE with CalliSpheres microsphere. Therefore, we think that DEB-TACE with CalliSpheres microsphere may be a good choice for HCC patients with RV-IVCTT. However, it is a challenge how to select patients who are more likely to be responsive to DEB-TACE, and further large-scale prospective studies are required to prove the effect of DEB-TACE with CalliSpheres microsphere in HCC with vascular invasion.
Huaier is a sandy beige mushroom found on the trunks of trees and has been widely used in traditional Chinese medicine (TCM) for more than 1,600 years. A large number of clinical studies have shown that it has good inhibitory effect on tumor proliferation, recurrence and metastasis,16,17 and its aqueous extract namely Huaier granule is the most common type used in clinical treatment as an adjuvant anticancer drug. A multicentre randomized clinical trial demonstrated Huaier granule could significantly improve recurrence-free survival and reduce extrahepatic recurrence in HCC patients who received radical surgical resection.5 Moreover, a systematic review and network meta-analysis revealed that Huaier granule might improve clinical therapeutic effects and immune functions without increasing side effects for patients with gastrointestinal cancers and it may best enhance the treatment effectiveness in patients with HCC going through treatment with TACE.18 In our case, it was beyond doubt Huaier granule enhance the DEB-TACE treatment effectiveness resulting in a significant prolonged progression-free survival and overall survival of this advanced HCC patient. What was even more surprising was that the lung metastases achieved complete remission unexpectedly without any other therapy except Huaier granule orally. Huaier granule had promising therapeutic effects against cancer and may serve as an adjuvant treatment for advanced HCC with macroscopic vascular invasion and distant metastasis. However, the mechanisms that underlie the combination therapy anti-tumor effects remain unclear and further investigation in future prospective randomized clinical studies is warranted.
Conclusion
The management of advanced HCC with macroscopic vascular invasion and pulmonary metastasis has always been challenging and DEB-TACE is an accepted palliative treatment for those patients with well-preserved liver function. Huaier granule has promising therapeutic effects against cancer and can enhance the therapeutic effectiveness of DEB-TACE. The present case suggests DEB-TACE with CalliSpheres microsphere combined with Huaier granule is a promising therapeutic option for advanced HCC with macroscopic vascular invasion and distant metastasis.
Acknowledgments
The authors would like to thank the patient for his participation and his consent to the publication of the case details and associated images.
Funding Statement
This work was sponsored by Zhejiang Provincial Natural Science Foundation of China (Grant No.LZ18H180001), National Natural Science Foundation of China (Grant No. 81971713), National S&T Major Project of China (NO.2018ZX10301201), Grant from the Health Commission of Zhejiang Province (JBZX-202004), Research Unit of Collaborative Diagnosis and Treatment For Hepatobiliary and Pancreatic Cancer, Chinese Academy of Medical Sciences(2019RU019) and Research Fund for Interventional Oncology of China Health Promotion Foundation(No.XM_2018_011_0006_01).
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval and Consent to Participate
Review board of the First Affiliated Hospital, Zhejiang University School of Medicine approval.
Consent for Publication
Written informed consent was obtained from the case patient/family for publication of this report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Cao F, Shen L, Qi H, et al. Tree-based classification system incorporating the HVTT-PVTT score for personalized management of hepatocellular carcinoma patients with macroscopic vascular invasion. Aging. 2019;11(21):9544–9555. doi: 10.18632/aging.102403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 4.Cheng S, Chen M, Cai J, et al. Chinese expert consensus on multidisciplinary diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus (2018 Edition). Liver Cancer. 2020;9(1):28–40. doi: 10.1159/000503685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Q, Shu C, Laurence AD, et al. Effect of Huaier granule on recurrence after curative resection of HCC: a multicentre, randomised clinical trial. Gut. 2018;67(11):2006–2016. doi: 10.1136/gutjnl-2018-315983 [DOI] [PubMed] [Google Scholar]
- 6.Tang CW, Zhu M, Feng WM, Bao Y, Zheng YY. Chinese herbal medicine, Jianpi Ligan decoction, improves prognosis of unresectable hepatocellular carcinoma after transarterial chemoembolization: a retrospective study. Drug Des Devel Ther. 2016;10:2461–2466. doi: 10.2147/DDDT.S113295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Yuan L, Ge RL, Sun Y, Wei G. Survival benefit of surgical treatment for hepatocellular carcinoma with inferior vena cava/right atrium tumor thrombus: results of a retrospective cohort study. Ann Surg Oncol. 2013;20(3):914–922. doi: 10.1245/s10434-012-2646-2 [DOI] [PubMed] [Google Scholar]
- 8.Le Treut YP, Hardwigsen J, Ananian P, et al. Resection of hepatocellular carcinoma with tumor thrombus in the major vasculature. A European case-control series. J Gastrointest Surg. 2006;10(6):855–862. doi: 10.1016/j.gassur.2005.12.011 [DOI] [PubMed] [Google Scholar]
- 9.Lee S, Kim BK, Kim SU, et al. Clinical outcomes and prognostic factors of patients with advanced hepatocellular carcinoma treated with sorafenib as first-line therapy: a Korean multicenter study. J Gastroenterol Hepatol. 2014;29(7):1463–1469. doi: 10.1111/jgh.12542 [DOI] [PubMed] [Google Scholar]
- 10.Pinter M, Hucke F, Graziadei I, et al. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology. 2012;263(2):590–599. doi: 10.1148/radiol.12111550 [DOI] [PubMed] [Google Scholar]
- 11.Luo J, Guo RP, Lai EC, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011;18(2):413–420. doi: 10.1245/s10434-010-1321-8 [DOI] [PubMed] [Google Scholar]
- 12.Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33(1):41–52. doi: 10.1007/s00270-009-9711-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogl TJ, Lammer J, Lencioni R, et al. Liver, gastrointestinal, and cardiac toxicity in intermediate hepatocellular carcinoma treated with PRECISION TACE with drug-eluting beads: results from the PRECISION V randomized trial. AJR Am J Roentgenol. 2011;197(4):W562–W570. doi: 10.2214/AJR.10.4379 [DOI] [PubMed] [Google Scholar]
- 14.Farid K, Elalfy H, Abo El-khair SM, et al. Prognostic value of vascular endothelial growth factor in both conventional and drug eluting beads transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma in HCV patients. Expert Rev Gastroenterol Hepatol. 2020;14(12):1203–1214. doi: 10.1080/17474124.2020.1823215 [DOI] [PubMed] [Google Scholar]
- 15.Zhou GH, Han J, Sun JH, et al. Efficacy and safety profile of drug-eluting beads transarterial chemoembolization by CalliSpheres(R) beads in Chinese hepatocellular carcinoma patients. BMC Cancer. 2018;18(1):644. doi: 10.1186/s12885-018-4566-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shan L, Li Y, Jiang H, et al. Huaier restrains proliferative and migratory potential of hepatocellular carcinoma cells partially through decreased yes-associated protein 1. J Cancer. 2017;8(19):4087–4097. doi: 10.7150/jca.21018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Wu H, Wang X, et al. Huaier Granule extract inhibit the proliferation and metastasis of lung cancer cells through down-regulation of MTDH, JAK2/STAT3 and MAPK signaling pathways. Biomed Pharmacother. 2018;101:311–321. doi: 10.1016/j.biopha.2018.02.028 [DOI] [PubMed] [Google Scholar]
- 18.Ma Y, Wang C, Zhang Q, Peng X, Feng Y, Meng X. The effects of polysaccharides from Auricularia auricula (Huaier) in adjuvant anti-gastrointestinal cancer therapy: a systematic review and network meta-analysis. Pharmacol Res. 2018;132:80–89. doi: 10.1016/j.phrs.2018.04.010 [DOI] [PubMed] [Google Scholar]