Abstract
Significance: Electric factors such as electric charges, electrodynamic field, skin battery, and interstitial exclusion permeate wound healing physiology and physiopathology from injury to re-epithelialization. The understanding of how electric factors contribute to wound healing and how treatments may interfere with them is fundamental for the development of better strategies for the management of pathological scarring and chronic wounds.
Recent Advances: Angiogenesis, cell migration, macrophage activation hemorheology, and microcirculation can interfere and be interfered with electric factors. New treatments with various types of electric currents, laser, light emitting diode, acupuncture, and weak electric fields applied directly on the wound have been developed to improve wound healing.
Critical Issues: Despite the basic and clinical development, pathological scars such as keloids and chronic wounds are still a challenge.
Future Directions: New treatments can be developed to improve skin wound healing taking into account the influence of electrical charges. Monitoring electrical activity during skin healing and the influence of treatments on hemorheology and microcirculation are examples of how to use knowledge of electrical factors to increase their effectiveness.
Keywords: skin physiological phenomena, galvanic skin response, electrical field, skin electrical conductance, hemorheology, wound healing

Paulo Luiz Farber, MD, PhD
Scope And Significance
After an injury, a movement of electric charges begins to repair the wound. The movement of electric charges leads to the generation of an electric current, which, although measured as direct current, is the result of an electrodynamic field, which after a few days changes its polarity.1–3 These electrodynamic fields guide the orientation and migration of fibroblasts, keratinocytes, macrophages, and epithelial cells.4–7
Electric charges also may interfere with angiogenesis, hemorheology, and blood flow in microcirculation.8,9 The understanding of electric factors in wound healing can lead to the development of new tools and treatments.
Translational Relevance
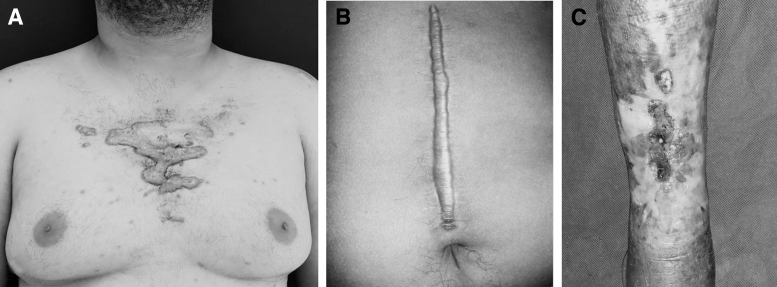
Abnormal wound healing such as keloid, hypertrophic scar, and chronic wounds remains a challenge (Fig. 1).10,11 Depending on how an electric field is applied to the wound, wound healing can be accelerated or delayed.12,13 Many mechanisms such as the movement of electric charges, the orientation and migration of cells, the interference of the electric charge of the molecules of collagen, albumin, glycosaminoglycans, the viscoelasticity of blood in microcirculation, the release of nitric oxide by erythrocytes, and the stimulation of angiogenesis should be taken into consideration and will be presented in this review.
Figure 1.
Abnormal wound healing can occur with excess scar tissue, as in keloids (A) and hypertrophic scars (B) or in healing deficiency as in chronic wounds (C).
Clinical Relevance
Many treatments interfere with electric factors in wound healing, such as various types of electric current, laser, light emitting diode (LED), and acupuncture, while weak electric fields over wounds can improve wound healing.14–17 Probably, many other current treatments also interfere with electric factors. Therefore, physical processes related to blood and oxygen supply, cell migration and interstitial can be relevant for treatments for aberrant wound repair.
Background
Electric factors are involved in the physiology and physiopathology of wound healing. The movements of ions create an endogenous electrodynamic field, which guides many steps of wound healing.
After an injury, an endogenous electric field is created.18,19 Electric fields, both endogenous and exogenous, can direct cell migration and modulate wound healing.4–6,14,20–22 The electricity finds its way through the biologically closed electric circuits (BCECs), the ionic current path through microcirculation.3,23,24 During the following days, alterations in the electric field direct cells, molecules, and drive the wound healing course. The electrodynamic field attracts and repels ions and cells, creating a fluctuating battery whose poles reverse after some days.2,3,25
There are other processes influenced by electric charges. Angiogenesis is enhanced by electric field through release of vascular endothelial growth factor (VEGF), and pathological angiogenesis is linked to abnormal high-voltage electric field.8,26–30 Albumin is excluded from interstitial tissue by negatively charged glycosaminoglycans.31–37
Inside the microcirculation, both blood vessel caliber and blood flow are affected by electric interactions. Sialic acid on the surface of the red blood cell interacts with electric charges that regulate the zeta potential (ZP), thereby influencing the colloidal stability of the blood, preventing erythrocyte aggregation, and ultimately enhancing blood flow in the microcirculation.38–41 Erythrocytes also release nitric oxide when they aggregate, inducing a compensatory vasodilatation.42
Many treatments may enhance wound healing by influencing electric factors. Exogenous electric fields such as pulsed electromagnetic field (PEMF), pulsed high-voltage stimulation (PHVS), and monophasic high-voltage electric stimulation enhance wound healing.43–47 Other treatments that enhance electric factors are low-level laser therapy (LLLT),17,48–52 LED,15,53 and acupuncture.16,54–58 Weak electric fields applied to the wound or in the wound dressing can also improve wound healing.59–63
Finally, electric charge and biological electrodynamic field permeate several phases of wound healing, driving cells and molecules and maintaining the flow of oxygen and nutrients, necessary to heal the wound. The knowledge of the electric physiology of wound healing can also help develop new and more efficient treatments.
Discussion of Findings and Relevant Literature
The electric field in wound healing
Biological electric fields: electrodynamic field
The influence of electric charges (positive or negative charges carried by protons and electrons) in wound healing has been extensively studied since the 1930s.2,64–66 Biological electric fields (fields surrounding electric charges, repelling or attracting other charges) are not chaotic, instead they are organized in well-defined patterns, in vertebrates, invertebrates, and plants.65,67 Local injuries, menstrual cycle, ovulation, and diseases such as cancer have been related to disturbances in the biological electric field.65,68–70
The early experiments used a very sensitive vacuum-tube microvoltmeter, capable of measuring the actual electric field in vivo. The conclusion of the results of these measures was, although the bioelectrical phenomenon had always been measured as direct current, a result from an electrodynamic field coming from different ions with different movements, generating electric fields, which change the distribution and motion of all passive ions in the system.65 Therefore, what is measured as direct current is a result of a living, constantly changing electrodynamic field, resulting from the electrical activity of life.65,71
To understand the role of the electric factors, it is important to mention that beyond wound healing, the electrodynamic field was measured in various physiological and pathological situations in animal and human studies. The development of salamanders was accompanied by switching the electric field measured in the cephalic-caudal orientation of the embryo, and similar alterations could be measured in the development of frog eggs.72,73 These electrodynamic fields were shown before the growth and development of the eggs, and they seem to dictate the orientation of the axis of the embryo. The embryo's development may be guided by the electrodynamic field created by the electric charge distribution.72
These findings mean that electrodynamic field orientates the formation of the embryo, and we have been immersed in that field even before birth.72–74 Disturbances of the electric fields in both humans and animals have been found in cancer development. The electrodynamic field patterns are less variable in cancer-susceptible mice than in cancer-resistant mice.69 There is a switch in the axis of the electric field in women with cervical cancer compared with women without cancer. In women without cancer, the cervix is electropositive towards the abdominal wall while in women with cancer the cervix is electronegative.68,70 Therefore in diseases such as cancer, the changing in the electrodynamic field pattern follows the development of the disease.68,70
As stated, there is a physiological behavior of the electrodynamic field, present since the embryogenesis,72,73 which is corrupted in diseases like cancer.68–70 Also, there are indications that injuries lead to alterations in the electrodynamic field. For example, when the rabbit's sciatic nerve is crushed, the paw innervated by the nerve turns electropositive.66 Block anesthesia also leads to an electropositive shift in the area supplied by the nerve, and there is a return of the original pattern of the electrodynamic field after the end of the anesthetic effect, while the electrical characteristics in the other paw remain unchanged.66,75
Therefore, electrodynamic field follows both nerve injury and anesthesia.66,76,77 These findings mean that since the embryo, living animals are developed under an electrodynamic field, and the development of physiological processes in the body is accompanied and probably guided by these fields.65,72,73
The skin battery
A wound in human skin develops a transcutaneous voltage known as skin battery. The skin of amphibious and mammalians also develope skin battery. In skin wounded of guinea pigs, there is an electric current of 140 mV/mm at the wound edge.19
In humans, the average voltage gradient between the stratum corneum (the outermost layer of skin) related to dermis has been measured in −23 mV, varying according to the anatomical site. Hands and feet have higher skin battery than back and upper arm.18 In children, 1 week after they have fingertip amputation, there is a peak current density of 22 μA/cm2, similar to the electric potential found in amphibian limb regeneration.78
The skin battery and the electric fields are essential to the wound healing. The wound healing process is delayed when an external electric field is applied to reverse the polarity of the physiological electric field, demonstrating that the electrodynamic field must be preserved for skin wound healing.12 Therefore after a skin injury, the electrodynamic field, measured as direct current (resulting from a complex movement of ions), is created to guide the wound healing process.2,12,18,19,65,78
The alternations of electric field in wound healing
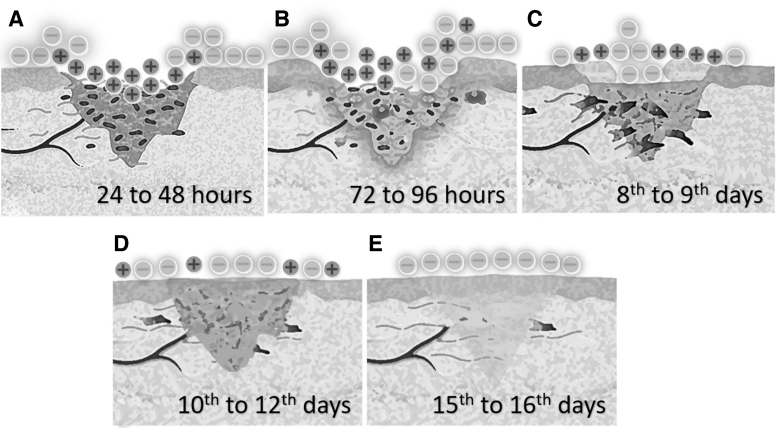
On the days after an injury, shifts in the electric field have been recognized.2,3,79 In a sutured surgical wound in the skin of guinea pigs, the center of the wound turns positive in relation to the surrounding noninjured skin, for the first 24–48 h.2 There is an increase of the electrical potential difference after 24–48 h after a decrease of the electric current, and after 72–96 h of the injury there is a shift of the electrical orientation, when the center of the wound becomes electronegative in relation to the surrounding skin with a gradual increase of the electrical potential difference until the 8th to 9th day. Then, the current reaches its maximum values.2
On the 10th to 12th day after the injury, there is a decrease in the current values, which turns into the original preintervention values after 15th to 16th days.2 Therefore, the healing of the wound is followed by shifts of the orientation of the electrodynamic field. The electric pole behavior changing happens similarly in other tissues; it might drive the process of injuries healing and can explain why application of current and current generation treatments accelerates wound healing.25
The origin of the fluctuation of the electric field might be the results from the changes in the pH secondary of the degradation process inside the wound. Cell death, hypoxic blood, and local enzymes lead to creation of an acid medium, which has an electrical potential different from the surrounding tissue, which leads to attract and repel anions and cations, causing the shifts in the electric field.3 Therefore, the electric charge distribution in tissue during the wound healing creates an electrodynamic field changeable during all the process (Fig. 2).2,3,25
Figure 2.
Alternation of electrical field: after the injury, an electric current is formed between the healthy and injured tissues, whose poles alternate with the passage of days. Initially the center of the wound is positive in relation to intact skin (A), there is a shift of charges after 72 h (B), an increase of electric current until about the 8th day (C), a decrease of the current from about the 10th day (D), and after 15–16 days the electric current turns to the original values (E). This effect may be related to changes in pH caused by tissue destruction, attracting or repelling anions and cations.
Effects of endogenous electric field on cells
Fibroblasts from human and animals alike have their axes oriented perpendicular to the electric field.20 When fibroblasts are placed under exogenous electric field, there is an increase in 20% of DNA syntheses and ∼100% increase of collagen synthesis, leading to a faster wound healing in pig's skin.14
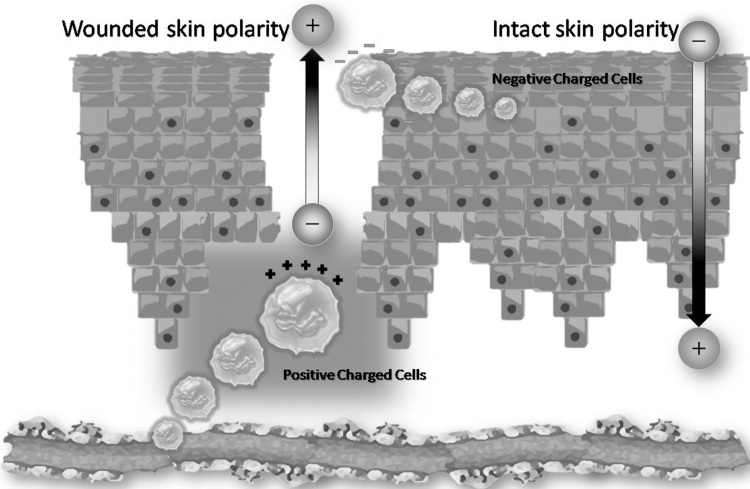
Cells respond to the exogenous electric field migrating to the negative (cathode) or positive (anode) pole. This phenomenon is known as electrotaxis or galvanotaxis (Fig. 3). Corneal epithelial cells and osteoblasts are attracted to the cathode, while corneal stroma fibroblasts and osteoclasts migrate to the anode. When the polarity of the exogenous electric field is reversed, the migration is also reversed, demonstrating the importance of the polarity and strength of the biological electric field.5 The migration of fibroblast is voltage dependent and needs an electric field of ∼100 mV/mm.6
Figure 3.
Electrotaxis or galvanotaxis: cells are attracted to or repelled from electrical poles depending on their polarity, a phenomenon known as galvanotaxis or electrotaxis. Positively charged cells such as corneal epithelial cells, osteoblasts, and monocytes are attracted to the negative pole (cathode), while negatively charged cells such as corneal stroma fibroblasts, osteoclasts, and macrophages are attracted to the positive pole (anode).
In this sense, human keratinocytes also migrate to the cathode at a rate of ∼1 μm/min in fields of physiological strength (100 mV/mm).80 Keratinocytes are also driven by electric fields, but the results of the cells' polarity are conflicting. The application of a direct current of 200 mV/mm, corresponding to the physiological current found in skin wounds, directs keratinocytes to the negative pole and induces autophagy in vitro.21 A pulsed electric field of ∼150 mV/mm also drives keratinocytes toward the cathode dependent on the electric current but regardless of the frequency of the pulses.22 Another in vitro study that uses an electric field of 50 mV/mm reports a keratinocyte migration toward the anode.4 This apparent contradiction of the migration of keratinocytes may be due to different cell lines used in the studies. One possibility is the use of keratinocytes with different integrin expression. Integrins may adjust keratinocyte functions during wound healing. The same type of cells can be attracted to anode or cathode depending on the kind of integrin expressed.81
The migration of keratinocytes by galvanotaxis can be stimulated in the presence of tissue hypoxia. Hypoxia usually occurs immediately after an injury to the skin, representing an initial stimulus to trigger the wound healing process. Conceptually, the oxygen tension rate changes during the healing phases and also in the different regions of the wound, ranging from 0 to 10 mmHg (0–1.3% O2) in the central region, and ∼60 mmHg (7.9% O2) in the periphery of the wound.82 In summary, but still under intense investigation, keratinocytes must have positive charge because they show a migration directed to the cathode region during the skin wound healing (the wound becomes more negative) in equivalent electric fields.79
Defense cells also can be driven by endogenous electrodynamic field. Macrophages, one of the main immune cells for wound repair, are attracted to the positive pole from an electric field comparable with the endogenous electrodynamic field and have their phagocytic capacity also increased, while their precursor, the monocyte, is attracted to the negative pole.83 The immune system can also be activated by electric field, despite the interference with cytokines expression such as chemokines and interleukins.84,85
The influences in cell migration and immune system, described previously, suggest that endogenous electric fields and exogenous electric fields have an important role not only in wound healing but probably in all physiological processes. Nowadays, we are immersed in an electrical and electromagnetic pollution generated by a number of electric appliances, power transmission lines, home wiring, radio, television, smartphones and smart watches, computers, tablets, microwave ovens, radar transmitters, medical diagnostic image equipment, and others. The influence of the electric and electromagnetic pollution on health is still not completely elucidated.86
Biologically closed electric circuits
The best way to mimic the influence of the endogenous electric fields in the cell migration and wound healing is a model of microchannels, known as microfluidic devices.87 By using this kind of device, it is possible to monitor the electrotaxis upon different currents and polarity as well the electric action on wound healing promoting drugs such as β-lapachone.88
The “microchannels” that modulate both electrotaxis and electricity generation have been studied in vivo, in humans and animals. The biological system has been described as BCECs, a series of various closed electric systems formed by vessels, arteries, and nerve-conducting channels. The BCECs work in the same way as electric circuits, whereas the blood vessels operate as insulating electric cables due to the fact that their walls are 200–300 times more resistant than the electrical conductance of plasma.23
The existence of BCECs has been demonstrated in various types of lesions of various tissues. In humans with pulmonary lesions such as cancer, benign tumors, and inflammatory diseases, there is an electric field between the core of the lesion and the surrounding tissue. In cancer cases, the electric field was higher; therefore endogenous electric fields occur in lesions of internal organs in the same way as they occur in skin wounds.3
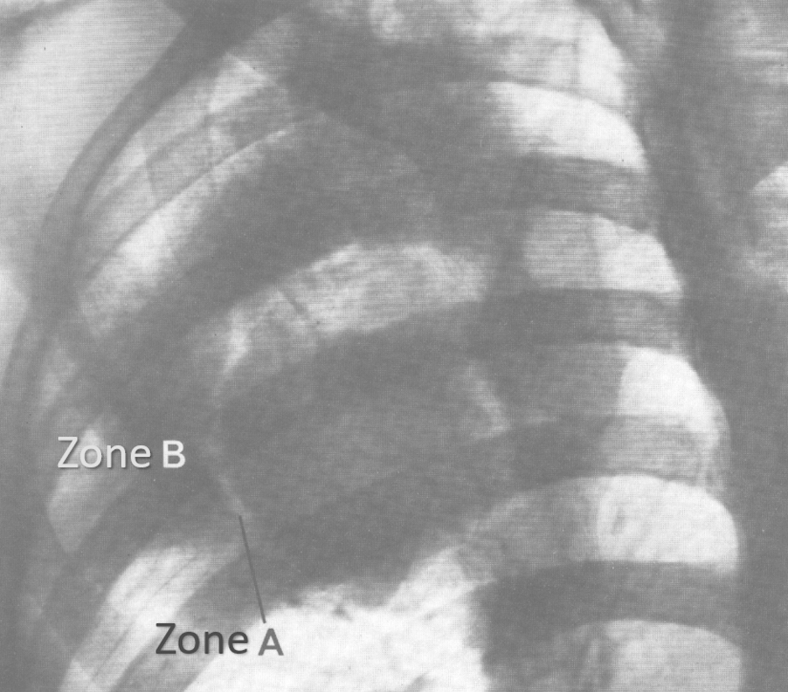
BCECs have been described initially in lung cancer observing the X-ray images that appear in the form of corona complex, consisting of 12 radiographic constituents. Zone “A” is a radiolucency, which appears around the electrically polarized lesion. Surrounding the “A” zone appears a radiopaque region, zone “B.” The A and B zones are predominantly the result of electro-osmotic outflow of water. Small arches form an interface between A and B regions (Fig. 4). As a result of the electrical flow, various elements of interstitial tissue turn into a radiating fibrous structure. As a result of the collapse of the tumor, irregular elongated opacities appear, named Lamella. Microthrombosis and perifocal emphysema lead to circumferential structures. Electro-osmotic inflow of water causes perifocal edema, which contributes to the circular displacement of the structures. Retraction of fibrous tissues appears as an effect of local dehydration due to electro-osmosis, leading to a retraction pocket of pleura, similar to the retraction of the skin in the wound healing process. Interlobular edema is developed as a result of the changes of the polarity of the tissue. The fluctuation of the electric field leads to a calcification of the tissue, which is a part of the healing process.
Figure 4.
X-ray image of a lung cancer surrounded by a radiolucent zone A and a radiopaque zone B, resulting in electro-osmotic outflow of water (reproduced with authorization from Jorgen Nordenström).3
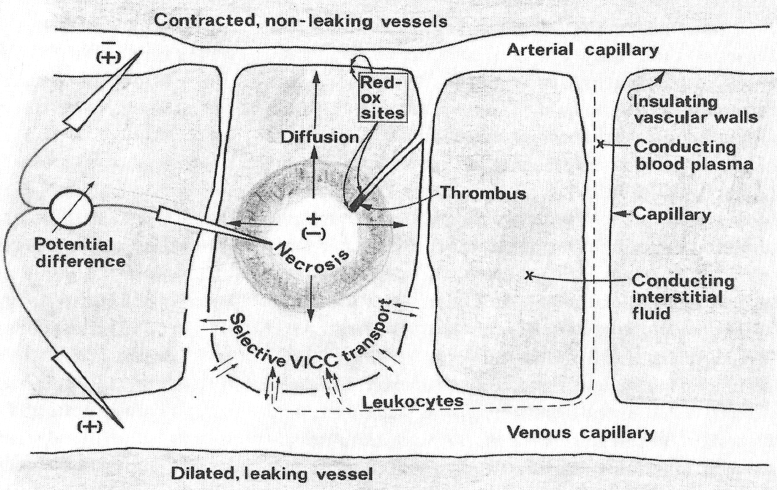
These subsets of BCECs have been named VICCs, vascular interstitial closed circuits (Fig. 5). The system is activated by the presence of necrosis or degrading blood, forming the electric field. Ions, cells, and water are transported in VICCs, leading to the image of corona complex. Inside capillaries and interstitial fluids, the electricity flows between injured and noninjured tissues, forming an electric circuit. These 12 components have been reproduced in animals and humans during the electrochemical treatment of cancer.24 Therefore, the corona complex is the radiographic image of the battery, which generates the electricity to the physiological process of tissue repair. The flow of energy, ions, and cells form a myriad of images when crossed by X-rays.3,23,24 The flow of energy through BCEC can be activated by external electric or electromagnetic fields to enhance wound healing.23
Figure 5.
VICCs: the injured tissues generate differences in electrical potential, causing water, cells, and ions to circulate through biological electrical circuits known as VICC. VICC, vascular interstitial closed circuits (reproduced with authorization from Jorgen Nordenström).3
Consequently, the electrodynamic field measured as direct current is, at least, part of it, consequence of the electric charges moving on biologically closed electric circuits. The movement of electric charge is both causes and consequences of the healing process.
Interstitial exclusion
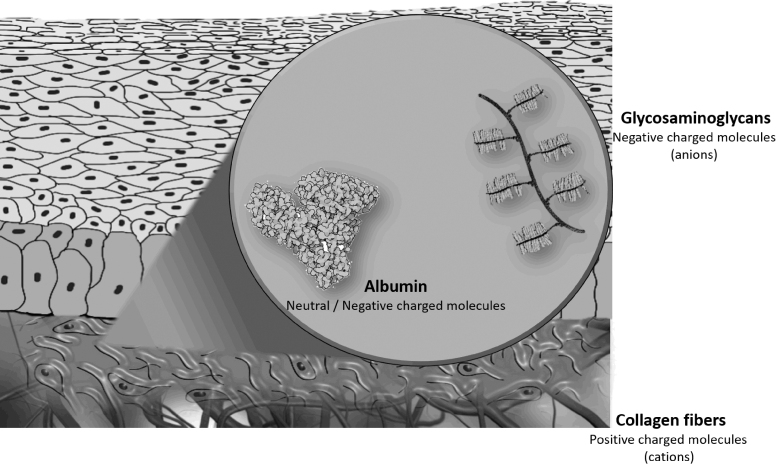
Interstitium or interstitial space is a place outside blood and lymphatic vessels and outside parenchyma cells. In the interstitium, the space is occupied by charged molecules as collagen and glycosaminoglycans. Due to their negative charge, glycosaminoglycans have a repulsive effect over other negatively charged molecules in the interstitium.89
Glycosaminoglycans are linear polysaccharides important in the inflammatory process, the recognition of injury, and the initiation of wound healing.32 The effect of the glycosaminoglycans on the albumin has been described as interstitial exclusion, because these two structures cannot be in the same confined place at the same time.33 In the human dermis, collagen fibers exclude albumin.34 Glycosaminoglycans are negatively charged molecules (anions) at physiological pH, which can explain their effects of albumin. Using cationized labeled albumin, it was possible to verify the exclusion of albumin by glycosaminoglycans (hyaluronan and uronic acid) in swollen dermis.35 Albumin plays an important role in the hydration of the intercellular matrix. The interstitial exclusion of the albumin by negatively charged glycosaminoglycans is partially blocked by tissue dehydration.31
Therefore, the balances of the charges in the interstitial tissue play a role in the distribution of the molecules during the wound healing (Fig. 6).64 There are cations as collagen, which has a slight positive charge as physiological pH, and anions as glycosaminoglycans interacting electrically with albumin and other charged substances of the interstitial matrix. The distribution of the electric charges is also determined by the hydration of the tissue. Other example of the charge interaction is cationic neutrophil-derived proteinase, which impairs the wound healing and can be inhibited by negatively charged glycosaminoglycans.36,37
Figure 6.
Interstitial exclusion: during inflammation, negatively charged glycosaminoglycans interact with albumin and positively charged collagen in interstitium.
Hemorheology, ZP, and microcirculation
Wound healing is dependent on the tissue perfusion and the tissue oxygenation. Tensile strength and collagen deposition can be increased or decreased according to the oxygen tension of the environment. Collagen deposition in healing wounds is directly related to tissue oxygenation, independent of blood hemoglobin.90
Wound healing is dependent on microcirculation. After a burn injury, the recovery of microcirculation is related to the interruption of the wound extension and wound healing.91 Impaired wound healing in chronic wounds such as venous leg ulcer and diabetic foot ulcer is related to poor microcirculation.92,93 Therefore, microcirculation and tissue oxygenation are essential factors for wound healing process.90–93
Hemorheology is a science of the deformation and flow of blood.94 Blood is a non-Newtonian suspension, and its viscosity varies according to the shear stress. The viscoelasticity of flowing blood is related to the behavior of red blood cells suspended in plasma and dependent on the shear rate. At lower shear rates, blood viscosity is extremely sensitive. Deformability and aggregation of red blood cells interfere with the rheology of blood. The decrease of erythrocyte deformability and the increase of erythrocyte aggregation lead to the increase of blood viscosity. Plasma is a Newtonian liquid, and its viscosity is independent of shear rate. When red blood cells, platelets, and white blood cells are added, shear stress and shear rate become important for blood viscosity. At low shear rate, as in microcirculation, erythrocytes tend to aggregate, increasing the viscosity.9
Therefore, in situations of low shear stress as in microcirculation around wound healing, factors that interfere with the hemorheology are fundamental to the wound healing process. Among these factors, electric charge has an important rule, mainly over red blood cell aggregation.95,96 To understand how electric charge interferes with erythrocyte aggregation, first we should understand colloidal stability.
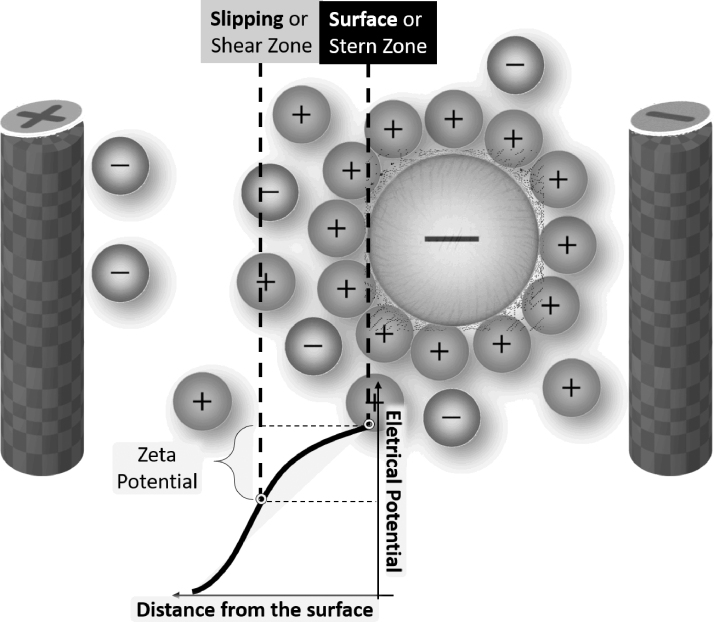
Colloids are suspensions in which the dispersed molecules or particles are larger in size than the solvent molecules, with the diameter of the dispersed material ranging from ∼1 nm to 1 μm.97 One of the most important factors determining colloidal stability is the small electric potential around every dispersed particle, known as ZP (Fig. 7). If the ZP of the blood becomes less negative than the threshold value, the blood loses its colloidal stability, leading to the aggregation of erythrocytes and other colloidal particles.38
Figure 7.
Zeta potential: zeta potential, the small electric potential around negatively charged RBC in blood, prevents RBC aggregation. RBC, red blood cell.
Therefore, ZP prevents erythrocyte aggregation, caused by attraction forces (hydrophobic bonds, van der Waals forces, and hydrogen bonds). The main origin of negative charges that contribute to negative ZP and consequently to repulsive forces are the sialic acids located on red blood cell membrane.39,95,96
Erythrocytes have a large negative ZP, which acts preventing erythrocyte aggregation.40 Diseases such as diabetes commonly show impaired wound healing, microcirculation abnormality, and increased red blood cell aggregation.41 Infectious diseases, such as malaria, also modify erythrocytes' ZP.96 Therefore, the electric charge may play a role in the impairment of wound healing in conditions such as diabetes, leading to erythrocyte aggregation, higher blood viscosity, and impairment of microcirculation. Therefore, electric charges contribute to the colloidal stability and the repulsive forces between the erythrocytes.
It has been reported that erythrocytes that have different shapes from biconcave such as echinocytes, stomatocytes, or spherocytes show decreased deformability and increased aggregability.98–100 Also, it has been recently proposed that erythrocyte shape and erythrocyte ZP are correlated.101 It was suggested that the healthy erythrocytes, which display biconcave, torus-like shape with a proportion known by Golden Ratio can be considered a type of capacitor that can store electric and magnetic energy generated by the interaction of iron with a dielectric medium (chloride anion), enhancing ZP and delivering oxygen to the tissues efficiently. While changes in their shape can reduce the ability for gas exchange.102–104
Chloride also modulates Band 3/AE1 anion exchange channel. The positively charged ion H+ separated from H2CO3 by toroidal flow goes to the membrane surface, and negatively charged HCO3− goes to the plasma through Band 3.103 Band 3 has also been linked to erythrocyte aggregation, and both phosphorylation and dephosphorylation of Band 3 decrease erythrocyte aggregation.105 Therefore, a healthy erythrocyte can work as a capacitor, generating an electric field that flows at its toroidal shape to increase ZP. It is important to note that during the passage through the microcirculation, the erythrocyte changes its shape because it is squeezed by the capillaries, but the healthy erythrocytes return to their biconcave shape when they return to the larger vessels.106
Red blood cells, blood vessels, and microcirculation
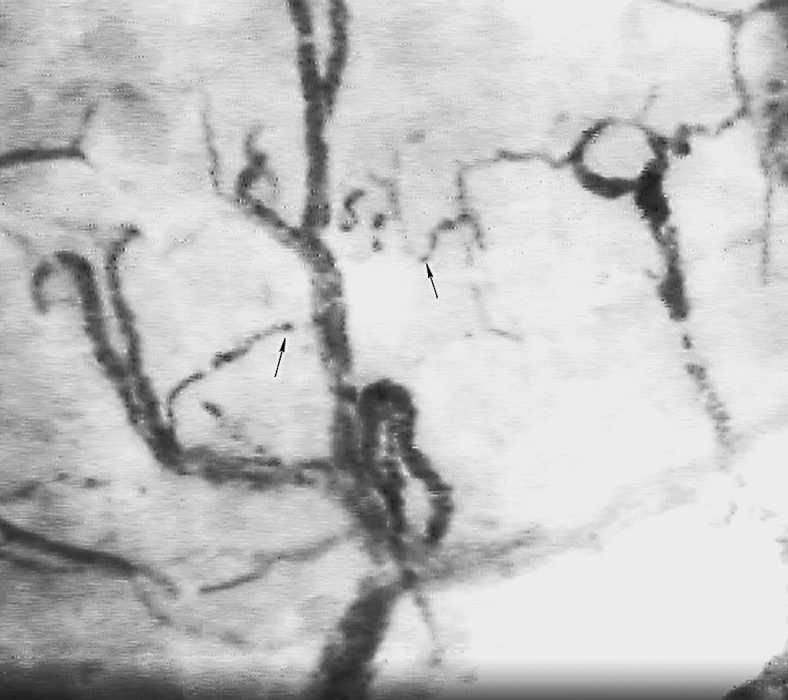
The influence of erythrocytes on blood flow is more dramatic when the diameter of blood vessels reduces to 0.3 mm, because of the relationships between red blood cells and blood vessels, altering the viscoelasticity of the blood, and they are responsible for the pressure gradient inside the microcirculation (Fig. 8).107 The proportion of red blood cells in relation to total plasma (hematocrit) also changes depending on the vase caliber. When the diameters of the vessels become <0.3 mm, hematocrit decreases. This phenomenon is known as the Fåhraeus effect. There is also a decrease of the viscosity as the vessel diameter decreases, phenomenon known as Fåhraeus and Lindqvist effect, reducing the microvascular resistance. Also, the erythrocytes tend to stay in the core of the vessels away for the surrounding.108
Figure 8.
RBCs and microcirculation: sublingual microscopy showing RBCs in the microcirculation. Negatively charged RBCs influence blood viscosity, especially when the diameter of the blood vessel is <0.3 mm (arrows).
Hemorheology, microcirculation, fibrinogen, acetylcholine, and nitric oxide
Blood vessels are not rigid conducts but flexible and contractile ones. Endothelium cells can release vasoactive molecules, among them nitric oxide, a vasodilator molecule.109 After injury, large amounts of nitric oxide are produced by inducible nitric oxide synthase in response to bacterial products and inflammation.109
Erythrocytes can rescue the nitric oxide released by endothelium cells and liberate it according to partial pressure of oxygen in the tissues. When oxygen in the tissues is decreased, nitric oxide is released by erythrocyte. According to endothelium integrity and amount of nitric oxide, acetylcholine released by endothelium cells and lymphocytes can induce vasodilatation or vasoconstriction.110
When acetylcholine is added to blood from some patients (with arterial hypertension, renal transplantation, or hypercholesterolemia), erythrocytes release nitric oxide. These patients have some hemorheological disorders, such as an increase of erythrocyte aggregation, an increase of plasma viscosity, and a decrease of red blood cells deformability.42 These findings may indicate compensation from increase of blood viscoelasticity by vasodilator molecules such as nitric oxide. Acetylcholine levels increase in plasma secondary from inflammation, same behavior of fibrinogen. Fibrinogen is associated with erythrocyte aggregation and impaired microcirculation by increased red blood cell aggregation. On the contrary, fibrinogen helps erythrocytes scavenge nitric oxide by binding membrane protein CD47.111,112 At physiological pH, fibrinogen has negative ZP but low ionic strength.113
Electric factors and angiogenesis
The new vascular formation occurs from the injury to the end of wound healing. Disruption of new capillary pattern leads to impaired wound healing and chronic ulcers.26 VEGF is thought to be the main factor for angiogenesis. Electrical pulses in some frequencies ∼50 Hz elicited the expression of VEGF-messenger RNA (mRNA) in muscle cells in vitro, while other frequencies like 24 Hz increase VEGF-mRNA in artery smooth muscles, and increase VGF protein synthesis. In a rat's model of ischemic hindlimb, continuous electric stimulation increases blood flow and increases VEGF only between the electrode and the stimulated muscle.27
The production of VEGF elicited by electric stimulation of endothelial cells decreases when VEGF receptor is inhibited.28,114 The synthesis of VEGF by VEGF receptor also increases angiogenesis in a three-dimensional environment of vascular endothelial cells.8 Therefore, the changes of electric field might also stimulate wound healing through augmented angiogenesis. In addition, experimental studies have shown that photobiomodulation is an important tool for better conditioning of skin grafts and flaps, favoring wound healing.115–117
Pathological angiogenesis was studied in retinas from rat's models of retina neovascularization and from humans with diabetic retinopathy. The results showed that aberrant neovascularization generates extraordinary high-voltage measures associated with abnormal response to hypoxia, leading to vasoconstriction instead of the compensatory vasodilatation seen in normal retina.29
Treatments to improve wound healing based on electric factors
The literature presents further investigations about the attributions of the modalities of electric current for the treatment of chronic wounds. However, there are applications in acute wounds and skin incisions in an attempt to improve the healing process from the beginning. In general, there are improvements in skin vascularization and blood flow, hemoglobin level and local cells organization.118,119
As discussed above, various stages of wound healing are related to electrical factors. Several wound treatments can affect wound healing through electric factors and some will be discussed below.
PEMF has been used for improving bone fracture repair.30 More recently, PEMF has also been tested for wound healing. PEMF improves wound tissue repair after plastic surgery.43 PEMF can also accelerate wound closure and improve microcirculation in diabetic ulcers.120 In diabetic rats, PEMF improves wound healing, increases myofibroblast proliferation, and improves the tensile biomechanical strength.44,45 Although PEMF seems to enhance microcirculation,120 there is no improvement in hemorheological parameters after PEMF in postmenopausal women.46
PHVS has been used to accelerate wound healing instead of unilateral direct current because direct current has been reported to produce chemical and thermal burns.121 PHVS improves wound healing in pressure ulcers, and has recently been reported as improving wound healing in donor sites of skin grafts used for treating burns.47,122
Monophasic high-voltage electric stimulation can also enhance wound healing.47 When monophasic high voltage is applied to the donor site of skin grafts, there are decrease of pain and decrease of time for complete epithelization.47
LLLT has also been used to improve wound healing.17 LLLT improves skin flaps survival in rats, pressure ulcers, and diabetic foot ulcers.48–50 Similar to PHVS, LLLT also improves the healing process of donor sites of burnt patients subjected to skin grafting.51 When a blood sample is irradiated with LLLT, hemorheological parameters such as blood viscosity and red blood cells deformability are improved.123 LLLT also decreases erythrocyte sedimentation rate in vitro, probably by enhanced colloidal stability and ZP.124
LLLT also has important interactions in the physiology of skin healing. The initial effects of the interaction between the laser and the wound promote the release of histamine, serotonin, and bradykinin, accelerating or delaying the mechanisms and reactions of the various metabolic pathways involved.50,51 It also generates an increase in the production of ATP, improving the efficiency of the sodium–potassium pump. This is one of the points in which there is electrical interaction through the use of the laser; the difference in electrical potential between the inside and outside of the cell is guaranteed.50 LLLT also decreases the electrical resistance of the skin while accelerating wound healing.52
Recently, there has been a discussion about the fact that LLLT uses laser (a coherent source of light), but the fact that coherence is not essential for the treatment has been discussed. LED, a noncoherent source of light, has been used in the same way to enhance wound healing. The photons emitted by the LED have a narrow emission band (±20 nm), output power close to that emitted by low-intensity lasers (20–100 mW). The electromagnetic radiation emitted by an LED is absorbed by cytochrome C oxidase, present in mitochondria, increasing the metabolic activity and cell proliferation of fibroblasts, in addition to the synthesis of collagen fibers.15,53,125,126 Both LLLT and LED have comparable power of angiogenesis stimulation.53 LED and LLLT promote similar biological effects such as decrease of inflammation, increase of angiogenesis, and increase of collagen synthesis.15
Acupuncture needles can generate an electric field due to difference of temperature from various metals present in the needle.54 Acupuncture needles also can be stimulated by electric current (electroacupuncture).55 Acupuncture has been reported to be beneficial for burn-related wound healing.56,57 In humans, acupuncture seems to improve wound healing and the quality of healing, with no scars in almost all treated patients.56 Acupuncture also improves wound healing in burn-injured mice, decreases the wound size, decreases inflammation, and induces epidermal regeneration.57 In a rabbit's model of soft tissue defects, electroacupuncture improves wound healing and is reported to be effective in treatment of chronic nonhealing skin wounds.16,58
Acupuncture improves the microcirculation in healthy humans.127 Electroacupuncture also enhances blood fluidity in rats, and acupuncture improves microcirculation in rabbits along with recovery of microcirculatory changes caused by thermal stimulation.128,129 In humans with multiple cerebral infarction and dementia, acupuncture improves various hemorheological parameters such as whole blood viscosity, erythrocyte aggregation, and erythrocyte deformability, with the improvement of the microcirculation.130 Therefore, the effect of acupuncture in enhancing the wound healing might be explained by the improvement of hemorheology and microcirculation.
Various weak electric fields applied to the wound or in the wound dressing can also improve wound healing.59–62 The use of a wireless electroceutical dressing, which generates continuous microcurrent, in conjunction with a negative pressure wound therapy decreases the frequency of dressing change without compromising the wound closure and might decrease wound infections.60,61 This bioelectric wound dressing made with zinc and silver can accelerate keratinocytes migration and increase H2O2 production.63 Wearable nanogenerator, which produces a small electrical current but sufficient to drive the migration of fibroblasts in vitro, accelerates skin wound healing in rats.62
These weak electric fields can have properties that prevent or treat biofilm in a chronic wound.61,131,132 This was evidenced in the early 1990s. The bacteria also use electrostatic interactions to guarantee their adhesion to the surfaces of the epithelia, a vital property in the formation of biofilm.61,131 Barki et al.61 tested an animal model (porcine) of a type of dressing that uses a weak electric current called a wireless electroceutic dressing (WED), in the treatment of polymicrobial biofilm infection. Scanning electron microscopy showed that WED prevented and interrupted the evolution and biofilm aggregates in chronic wounds. WED also optimized the wound closure, restoring the integrity of the skin. In addition, WED attenuated persistent inflammation induced by the open wound and the presence of biofilm, bypassing the activation of growth factors and inflammatory pathways with their pertinent cellular responses.61
Randomized controlled trials have been identified for electrical stimulation for the treatment of diabetic foot in a systematic review.133 The first one was characterized by a weak methodology and resulted in no benefit of this therapy to patients.134 On the contrary, the second study resulted in a trend, without statistical significance, to improve the treatment of the wound in ∼12 weeks.135 A third cohort study was also presented with a weak methodology, with no difference in reducing the raw area of ulcers after 60 days of therapy.136 The fourth study compared the use of electrical stimulation with local heating of the skin (control group).137
Some biases in the methodology called into question the positive finding of electrotherapy in significantly reducing the wound area in 4 weeks. As for the types of electrodes that could be used in electrotherapies,138–140 it is difficult to list them all. The different devices could be mono, bi, or triphasic, of which the current flows simultaneously from an active electrode (source) to the other reference electrode (receiver).141,142
The electric stimulation can also modify gene expression in healthy humans. A device producing pulsed microcurrent in the skin modified the expression of 105 genes, some of them related to be upregulated in skin wounds.143 Endogenous and exogenous electric field can drive nanoparticles. As nanoparticles are electrically charged, they are attracted by the electric fields. A potential use is electrostimulated drug delivery by electroresponsive hydrogels or conductive polymers.144
Summary
Since embryo, the flow of electric charges generates an electrodynamic field able to drive several steps of physiology, from embryo development to wound healing. The electrodynamic field shown after skin injury is known as skin battery. Its polarity shifts after a few days mainly due to pH changes in the wound site, causing the movement of ions. The electrodynamic field flows through microchannels known as BCEC, and causes cell migration such as fibroblasts, keratinocytes, and macrophages. Other charged molecules that participate in wound healing are negatively charged glycosaminoglycans, which cause interstitial exclusion of albumin.
Wound healing is dependent on tissue perfusion and oxygenation. Hemorheology or viscoelasticity of blood in microcirculation is dependent on ZP, which causes repulsive forces in the blood and red blood cells, preventing them from aggregating. Therefore, the blood flow in the microcirculation is dependent on electric charges. Nitric oxide released by red blood cells can provoke vasodilatation, compensating the impairment of hemorheology. Electric field also increases angiogenesis through the synthesis of VEGF (Fig. 9).
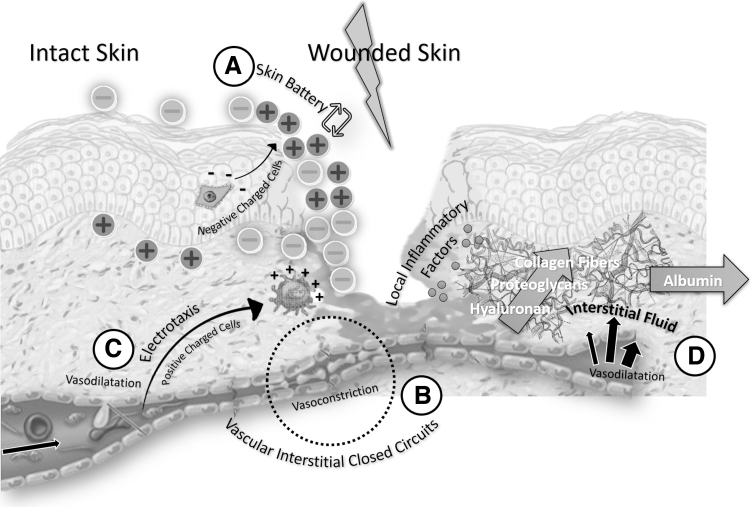
Figure 9.
Electric factors in wound healing. A wound provokes a transcutaneous voltage known as skin battery with alternations in the electric field during wound healing (A). Ion cells and water are transported in the VICC (B). Positively charged cells are attracted to the negative pole and negatively charged cells to the positive pole (electrotaxis) (C). In interstitium, albumin is excluded by glycosaminoglycans as a consequence of inflammatory process (D).
Several treatments can improve wound healing altering electric factors. PEMF, PHVS, monophasic high-voltage electric stimulation, LLLT, acupuncture, and electrical dressing are some examples. In conclusion, electric factors drive wound healing and can be modified for the improvement of scarring and chronic wounds.
Take-Home Messages
After skin injury, a skin battery is generated by electrodynamic field created by the movements of electric charges.
The electrodynamic field orientates and stimulates the migration of electric charged cells such as fibroblasts, keratinocytes, and macrophages and stimulates angiogenesis.
The electric current generated by the electrodynamic field circulates in humans and animals similar to electrical circuits (BCECs).
The interaction of electric charges in blood alters erythrocyte aggregation and consequently the viscosity in microcirculation.
Several treatments can enhance skin wound healing interfering with electric factors. Electric stimulation, laser, LED, acupuncture, and electroceutical dressing are some of them.
Abbreviations and Acronyms
- BCEC
biologically closed electric circuits
- LED
light emitting diode
- LLLT
low-level laser therapy
- mRNA
messenger RNA
- PEMF
pulsed electromagnetic field
- PHVS
pulsed high-voltage stimulation
- RBC
red blood cell
- UNIFESP
Universidade Federal de São Paulo
- VEGF
vascular endothelial growth factor
- VICC
vascular interstitial closed circuits
- WED
wireless electroceutic dressing
- ZP
zeta potential
Acknowledgment and Funding Sources
The main author acknowledges Frank Hartman whose contribution to this work was of great significance. No funding was received for this article.
Author Disclosure and Ghostwriting
All authors have nothing to disclose. All authors contributed to the writing of the article. There was no ghostwriter participation in this article.
About The Authors
Paulo Luiz Farber, MD, PhD obtained his degree in medicine and his PhD from University of Sao Paulo Medical School, and was a professor of postgraduate program of Plastic Surgery of UNIFESP. Currently is a medical doctor of Hospital da Luz de Aveiro, secretary-general of Portuguese Society of Hemorheology and Microcirculation and external researcher of the IMM, Institute of Biochemistry, Faculty of Medicine, University of Lisbon.
Felipe Contoli Isoldi, MD, MHS obtained his degree in medicine, Medical Residence in Plastic Surgery and is currently a PhD student by Universidade Federal de São Paulo – Paulista Medical School (UNIFESP). Master in Health Sciences by the Professional Master Course in Science, Technology and Management Applied to Tissue Regeneration of UNIFESP. He is a collaborator in the Sector of Pathological Scars of the Discipline of Plastic Surgery of UNIFESP.
Lydia Masako Ferreira, MD, PhD is Full Professor, Chair, and Head of the Plastic Surgery Division at the Universidade Federal de Sao Paulo (UNIFESP). Researcher of the Brazilian National Council for Scientific and Technological Development (CNPq). Former Head of the General Surgery Department UNIFESP, Former coordinator of the Post Graduate Program in Plastic Surgery. Doctorate in Plastic Surgery at UNIFESP. Post doctorate Plastic Surgery at the University of California San Francisco.
References
- 1. Nuccitelli R. A role for endogenous electric fields in wound healing. Curr Top Dev Biol 2003;58:1–26 [DOI] [PubMed] [Google Scholar]
- 2. Burr HS, Harvey SC, Taffel M. Bio-electric correlates of wound healing. Yale J Biol Med 1938;11:103–107 [PMC free article] [PubMed] [Google Scholar]
- 3. Nordenström B. Biologically Closed Electric Circuits: Clinical, Experimental, and Theoretical Evidence for an Additional Circulatory System, 1st ed. Stockholm, Sweden: Nordic Medical Publications, 1983 [Google Scholar]
- 4. Cho Y, Son M, Jeong H, Shin JH. Electric field-induced migration and intercellular stress alignment in a collective epithelial monolayer. Mol Biol Cell 2018;29:2292–2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tai G, Reid B, Cao L, Zhao M. Electrotaxis and wound healing: experimental methods to study electric fields as a directional signal for cell migration. Methods Mol Biol 2009;571:77–97 [DOI] [PubMed] [Google Scholar]
- 6. Guo A, Song B, Reid B, et al. Effects of physiological electric fields on migration of human dermal fibroblasts. J Invest Dermatol 2010;130:2320–2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhao M. Electrical fields in wound healing—an overriding signal that directs cell migration. Semin Cell Dev Biol 2009;20:674–682 [DOI] [PubMed] [Google Scholar]
- 8. Chen Y, Ye L, Guan L, et al. Physiological electric field works via the VEGF receptor to stimulate neovessel formation of vascular endothelial cells in a 3D environment. Biol Open 2018;7:bio035204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baskurt OK, Meiselman HJ. Blood rheology and hemodynamics. Semin Thromb Hemost 2003;1:435–450 [DOI] [PubMed] [Google Scholar]
- 10. Hochman B, Isoldi FC, Furtado F, Ferreira LM. New approach to the understanding of keloid: psychoneuroimmune-endocrine aspects. Clin Cosmet Investig Dermatol 2015;8:67–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferreira LM, Gragnani A, Furtado F, Hochman B. Control of the skin scarring response. An Acad Bras Cienc 2009;81:623–629 [DOI] [PubMed] [Google Scholar]
- 12. Borgens B. What is the role of naturally produced electric current in vertebrate regeneration and healing? Int Rev Cytol 1982;76:245–298 [DOI] [PubMed] [Google Scholar]
- 13. Weiss DS, Kirsner REW. Electrical stimulation and wound healing. Arch Dermatol 1990;126:222–225 [PubMed] [Google Scholar]
- 14. Alvarez OM, Mertz PM, Smerbeck RV, Eaglstein WH. The healing of superficial skin wounds is stimulated by external electrical current. J Invest Dermatol 1983;81:144–148 [DOI] [PubMed] [Google Scholar]
- 15. Chaves MEA, de Araújo AR, Piancastelli ACC, Pinotti M. Effects of low-power light therapy on wound healing: LASER × LED. An Bras Dermatol 2014;89:616–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parmen V, Taulescu M, Ober C, Pestean C, Oana L. Influence of electroacupuncture on the soft tissue healing process. J Acupunct Meridian Stud 2014;7:243–249 [DOI] [PubMed] [Google Scholar]
- 17. Avci P, Gupta A, Sadasivam M, et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg 2013;32:41–52 [PMC free article] [PubMed] [Google Scholar]
- 18. Foulds IS, Barker AT. Human skin battery potentials and their possible role in wound healing. Br J Dermatol 1983;109:515–522 [DOI] [PubMed] [Google Scholar]
- 19. Barker A, Jaffe L, Vanable J. The glabrous epidermis of cavies contains a powerful battery. Am J Physiol 1982;242:R358–R366 [DOI] [PubMed] [Google Scholar]
- 20. Ross SM, Ferrier JM, Aubin JE. Studies on the alignment of fibroblasts in uniform applied electrical fields. Bioelectromagnetics 1989;10:371–384 [DOI] [PubMed] [Google Scholar]
- 21. Yan T, Jiang X, Lin G, et al. Autophagy is required for the directed motility of keratinocytes driven by electric fields. FASEB J 2019;33:3922–3935 [DOI] [PubMed] [Google Scholar]
- 22. Ren X, Sun H, Liu J, et al. Keratinocyte electrotaxis induced by physiological pulsed direct current electric fields. Bioelectrochemistry 2019;127:113–124 [DOI] [PubMed] [Google Scholar]
- 23. Nordenstrom B, Ipavec S, Alfas S. Interferences of electromagnetic field with biological matter. Int J Environ Stud 1992;42:157–167 [Google Scholar]
- 24. Nordenström B. Fleischner lecture. Biokinetic impacts on structure and imaging of the lung: the concept of biologically closed electric circuits. Am J Roentgenol 1985;145:447–467 [DOI] [PubMed] [Google Scholar]
- 25. Nordenström B. Electric potentials in pulmonary lesions: a preliminary report. Acta Radiol 1971;11:1–16 [DOI] [PubMed] [Google Scholar]
- 26. Sorg H, Tilkorn DJ, Hager S, Hauser J, Mirastschijski U. Skin wound healing: an update on the current knowledge and concepts. Eur Surg Res 2017;58:81–94 [DOI] [PubMed] [Google Scholar]
- 27. Kanno S, Nobuyuki O, Mayumi A, et al. Establishment of a simple and practical procedure applicable to therapeutic angiogenesis. Circulation 1999;99:2682–2687 [DOI] [PubMed] [Google Scholar]
- 28. Bai H, Forrester JV, Zhao M. DC electric stimulation upregulates angiogenic factors in endothelial cells through activation of VEGF receptors. Cytokine 2011;55:110–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Puro DG, Kohmoto R, Fujita Y, Gardner TW, Padovani-Claudio DA. Bioelectric impact of pathological angiogenesis on vascular function. Proc Natl Acad Sci U S A 2016;113:9934–9939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hannemann PFW, Mommers EHH, Schots JPM, Brink PRG, Poeze M. The effects of low-intensity pulsed ultrasound and pulsed electromagnetic fields bone growth stimulation in acute fractures: a systematic review and meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg 2014;134:1093–1106 [DOI] [PubMed] [Google Scholar]
- 31. Wiig H, Tenstad O, Bert JL. Effect of hydration on interstitial distribution of charged albumin in rat dermis in vitro. J Physiol 2005;569:631–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Taylor KR, Gallo RL. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. FASEB J 2006;20:9–22 [DOI] [PubMed] [Google Scholar]
- 33. Ogston A, Phelps C. The partition of solutes between buffer solutions and solutions containing hyaluronic acid. Biochem J 1961;78:827–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bert JL, Mathieson JM, Pearce RH. The exclusion of human serum albumin by human dermal collagenous fibres and within human dermis. Biochem J 1982;201:395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wiig H, Kolmannskog O, Tenstad O, Bert JL. Effect of charge on interstitial distribution of albumin in rat dermis in vitro. J Physiol 2003;550:505–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li ST, Katz EP. An electrostatic model for collagen fibrils. The interaction of reconstituted collagen with Ca++, Na+, and Cl−. Biopolymers 1976;15:1439–1460 [DOI] [PubMed] [Google Scholar]
- 37. Peplow PV. Glycosaminoglycan: a candidate to stimulate the repair of chronic wounds. Thromb Haemost 2005;94:4–16 [DOI] [PubMed] [Google Scholar]
- 38. Riddick T. Control of Colloid Stability Through Zeta Potential. (Livingston Pub. Co., ed.). Wynnewood, PA: Zeta-Meter, 1968 [Google Scholar]
- 39. Fernandes HP. Electrical properties of the red blood cell membrane and immunohematological investigation. Rev Bras Hematol Hemoter 2011;33:297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Silva DCN, Jovino CN, Silva CAL, et al. Optical tweezers as a new biomedical tool to measure zeta potential of stored red blood cells. PLoS One 2012;7:e31778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cicco G, Giorgino F, Cicco S. Wound healing in diabetes: hemorheological and microcirculatory aspects. In: Lambris, Crusio, Rezaei, eds. Advances in Experimental Medicine and Biology. Vol. 701. New York: Springer, LLC, 2011:263–269 [DOI] [PubMed] [Google Scholar]
- 42. Carvalho FA, Maria AV, Nogueira JMB, Guerra J, Martins-Silva J. The relation between the erythrocyte nitric oxide and hemorheological parameters. Clin Hemorheol Microcirc 2006;35:341–347 [PubMed] [Google Scholar]
- 43. Strauch B, Herman C, Dabb R, Ignarro LJ PA. Evidence-based use of pulsed electromagnetic field therapy in clinical plastic surgery. Aesthet Surg J 2009;29:135–143 [DOI] [PubMed] [Google Scholar]
- 44. Cheing GLY, Li X, Huang L, Kwan RLC, Cheung KK. Pulsed electromagnetic fields (PEMF) promote early wound healing and myofibroblast proliferation in diabetic rats. Bioelectromagnetics 2014;35:161–169 [DOI] [PubMed] [Google Scholar]
- 45. Choi HM, Cheing AK, Ng GY, Cheing GLY. Effects of pulsed electromagnetic field (PEMF) on the tensile biomechanical properties of diabetic wounds at different phases of healing. PLoS One 2018;13:e0191074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu H, Yang L, He H, Zhou J, Liu Y, Wang C. The hemorheological safety of pulsed electromagnetic fields in postmenopausal women with osteoporosis in southwest China: a randomized, placebo controlled clinical trial. Clin Hemorheol Microcirc 2013;55:285–295 [DOI] [PubMed] [Google Scholar]
- 47. Gomes RC, Guirro ECO, Gonçalves AC, Farina Junior JA, Murta Junior LO, Guirro RRJ. High-voltage electric stimulation of the donor site of skin grafts accelerates the healing process. A randomized blinded clinical trial. Burns 2018;44:636–645 [DOI] [PubMed] [Google Scholar]
- 48. Pinfildi CE, Liebano RE, Hochman BS, Ferreira LM. Helium-neon laser in viability of random skin flap in rats. Lasers Surg Med 2005;37:74–77 [DOI] [PubMed] [Google Scholar]
- 49. Machado RS, Viana S, Sbruzzi G. Low-level laser therapy in the treatment of pressure ulcers: systematic review. Lasers Med Sci 2017;32:937–944 [DOI] [PubMed] [Google Scholar]
- 50. Tchanque-Fossuo CN, Ho D, Dahle SE, et al. A systematic review of low-level light therapy for treatment of diabetic foot ulcer. Wound Repair Regen 2016;24:418–426 [DOI] [PubMed] [Google Scholar]
- 51. Vaghardoost R, Momeni M, Kazemikhoo N, et al. Effect of low-level laser therapy on the healing process of donor site in patients with grade 3 burn ulcer after skin graft surgery (a randomized clinical trial). Lasers Med Sci 2018;33:603–607 [DOI] [PubMed] [Google Scholar]
- 52. Solmaz H, Dervisoglu S, Gulsoy M, Ulgen Y. Laser biostimulation of wound healing: bioimpedance measurements support histology. Lasers Med Sci 2016;31:1547–1554 [DOI] [PubMed] [Google Scholar]
- 53. De Sousa APC, Paraguassú GM, Silveira NTT, et al. Laser and LED phototherapies on angiogenesis. Lasers Med Sci 2013;28:981–987 [DOI] [PubMed] [Google Scholar]
- 54. Cohen M, Kwok G, Cosic I. Acupuncture needles and the Seebeck effect: do temperature gradients produce electrostimulation? Acupunct Electrother Res 1997;22:9–15 [DOI] [PubMed] [Google Scholar]
- 55. Napadow V, Makris N, Liu J, Kettner NW, Kwong KK, Hui KKS. Effects of electroacupuncture versus manual acupuncture on the human brain as measured by fMRI. Hum Brain Mapp 2005;24:193–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Loskotova A, Loskotova J. The use of acupuncture in first aid of burns—clinical report. Burns 2017;43:1782–1791 [DOI] [PubMed] [Google Scholar]
- 57. Lee JA, Jeong HJ, Park HJ, Jeon S, Hong SU. Acupuncture accelerates wound healing in burn-injured mice. Burns 2011;37:117–125 [DOI] [PubMed] [Google Scholar]
- 58. Walton EW. Electroacupuncture: applications in the treatment of chronic nonhealing wounds. Adv Skin Wound Care 2013;26:495–497 [DOI] [PubMed] [Google Scholar]
- 59. Maijer A, Gessner A, Trumpatori B, Varhus JD. Bioelectric dressing supports complex wound healing in small animal patients. Top Companion Anim Med 2018;33:21–28 [DOI] [PubMed] [Google Scholar]
- 60. Ghatak P Das, Schlanger R, Ganesh K, et al. A wireless electroceutical dressing lowers cost of negative pressure wound therapy. Adv Wound Care 2015;4:302–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Barki KG, Das A, Dixith S, et al. Electric field based dressing disrupts mixed-species bacterial biofilm infection and restores functional wound healing. Ann Surg 2019;269:756–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Long Y, Wei H, Li J, et al. Effective wound healing enabled by discrete alternative electric fields from wearable nanogenerators. ACS Nano 2018;12:12533–12540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Banerjee J, Das Ghatak P, Roy S, et al. Improvement of human keratinocyte migration by a redox active bioelectric dressing. PLoS One 2014;9:e89239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Farber PL, Hochman B, Furtado F, Ferreira LM. Electricity and colloidal stability: how charge distribution in the tissue can affects wound healing. Med Hypotheses 2014;82:199–204 [DOI] [PubMed] [Google Scholar]
- 65. Burr HS, Northrop FS. Evidence for the existence of an electro-dynamic field in living organisms. Proc Natl Acad Sci U S A 1939;25:284–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Grenell RG, Burr H. Surface potentials and peripheral nerve injury, a clinical test. Yale J Biol Med 1946;18:517–525 [PMC free article] [PubMed] [Google Scholar]
- 67. Lee RC, Canaday DJ, Doong H. A review of the biophysical basis for the clinical application of electric fields in soft-tissue repair. J Burn Care Rehabil 1993;14:319–335 [DOI] [PubMed] [Google Scholar]
- 68. Langman L, Burr H. Electrometric studies in women with malignancy of cervix uteri. Science (80-) 1947;105:209–210 [DOI] [PubMed] [Google Scholar]
- 69. Burr HS, Smith GM, Strong LC. Bio-electric properties of cancer-resistant and cancer-susceptible mice. Am J Cancer 1938;32:240–248 [Google Scholar]
- 70. Langman L, Burr H. A technique to aid in the detection of malignancy of the female genital tract. Am J Obstet Gynecol 1949;57:274–281 [DOI] [PubMed] [Google Scholar]
- 71. Liu Q, Song B. Electric field regulated signaling pathways. Int J Biochem Cell Biol 2014;55:264–268 [DOI] [PubMed] [Google Scholar]
- 72. Burr H, Hovland C. Bio-electric correlates of development in amblystoma. Yale J Biol Med 1937;9:540–549 [PMC free article] [PubMed] [Google Scholar]
- 73. Burr H. Field properties of the developing frog's egg. Nature 1941;147:780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nuccitelli R. Endogenous electric fields in embryos during development, regeneration and wound healing. Radiat Prot Dosimetry 2003;106:375–383 [DOI] [PubMed] [Google Scholar]
- 75. Willand MP, Nguyen MA, Borschel GH, Gordon T. Electrical stimulation to promote peripheral nerve regeneration. Neurorehabil Neural Repair 2016;30:490–496 [DOI] [PubMed] [Google Scholar]
- 76. Modrak M, Talukder MAH, Gurgenashvili K, Noble M, Elfar JC. Peripheral nerve injury and myelination: potential therapeutic strategies. J Neurosci Res 2020;98:780–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Qian Y, Cheng Y, Cai J, et al. Advances in electrical and magnetic stimulation on nerve regeneration. Regen Med 2019;14:969–979 [DOI] [PubMed] [Google Scholar]
- 78. Illingworth CM, Barker AT. Measurement of electrical currents emerging during the regeneration of amputated finger tips in children. Clin Phys Physiol Meas 1980;1:87 [Google Scholar]
- 79. Hunckler J, de Mel A. A current affair: electrotherapy in wound healing. J Multidiscip Healthc 2017;10:179–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fang KS, Ionides E, Oster G, Nuccitelli R, Isseroff R. Epidermal growth factor receptor relocalization and kinase activity are necessary for directional migration of keratinocytes in DC electric fields. J Cell Sci 1999;112:1967–1978 [DOI] [PubMed] [Google Scholar]
- 81. Zhu K, Takada Y, Nakajima K, et al. Expression of integrins to control migration direction of electrotaxis. FASEB J 2019;33:9131–9141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Guo X, Jiang X, Ren X, et al. The galvanotactic migration of keratinocytes is enhanced by hypoxic preconditioning. Sci Rep 2015;5:10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hoare JI, Rajnicek AM, McCaig CD, Barker RN, Wilson HM. Electric fields are novel determinants of human macrophage functions. J Leukoc Biol 2016;99:1141–1151 [DOI] [PubMed] [Google Scholar]
- 84. Jennings JA, Chen D, Feldman DS. Upregulation of chemokine (C-C motif) ligand 20 in adult epidermal keratinocytes in direct current electric fields. Arch Dermatol Res 2010;302:211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Jones AM, Griffiths JL, Sanders AJ, et al. The clinical significance and impact of interleukin 15 on keratinocyte cell growth and migration. Int J Mol Med 2016;38:679–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Redlarski G, Lewczuk B, Żak A, et al. The influence of electromagnetic pollution on living organisms: historical trends and forecasting changes. Biomed Res Int 2015;2015:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sun YS. Studying electrotaxis in microfluidic devices. Sensors (Switzerland) 2017;17;2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sun YS, Peng SW, Cheng JY. In vitro electrical-stimulated wound-healing chip for studying electric field-assisted wound-healing process. Biomicrofluidics 2012;6:34117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Øien AH, Justad SR, Tenstad O, Wiig H. Effects of hydration on steric and electric charge-induced interstitial volume exclusion—a model. Biophys J 2013;105:1276–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Jonsson K, Jensen JA, Goodson WH, et al. Tissue oxygenation, anemia, and perfusion in relation to wound healing in surgical patients. Ann Surg 1991;214:605–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Qin W, Li Y, Wang J, Qi X, Wang RK. In vivo monitoring of microcirculation in burn healing process with optical microangiography. Adv Wound Care 2016;5:332–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Raffetto JD. Pathophysiology of wound healing and alterations in venous leg ulcers-review. Phlebology 2016;31:56–62 [DOI] [PubMed] [Google Scholar]
- 93. Lowry D, Saeed M, Narendran P, Tiwari A. The difference between the healing and the nonhealing diabetic foot ulcer: a review of the role of the microcirculation. J Diabetes Sci Technol 2017;11:914–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Antonova N. Methods in hemorheology and their clinical applications 1. Clin Hemorheol Microcirc 2016;64:509–515 [DOI] [PubMed] [Google Scholar]
- 95. Jan KM, Chien S. Role of surface electric charge in red blood cell interactions. J Gen Physiol 1973;61:638–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tokumasu F, Ostera GR, Amaratunga C, Fairhurst RM. Modifications in erythrocyte membrane zeta potential by Plasmodium falciparum infection. Exp Parasitol 2012;131:245–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hunter RJ, White LR. Foundations of Colloid Science. Oxford, UK: Oxford University Press, 1987 [Google Scholar]
- 98. Geekiyanage NM, Sauret E, Saha SC, Flower RL, Gu YT. Deformation behaviour of stomatocyte, discocyte and echinocyte red blood cell morphologies during optical tweezers stretching. Biomech Model Mechanobiol 2020;19:1827–1843 [DOI] [PubMed] [Google Scholar]
- 99. Berling C, Lacombe C, Lelievre JC, Allary M, Saint-Blancard J. The RBC morphological dependance of the RBC disaggregability. Biorheology 1988;25:791–798 [DOI] [PubMed] [Google Scholar]
- 100. Ryazantseva NV, Novitskii VV, Stepovaya EA, Bulavina YV, Fokin VA. Typical changes of reversible erythrocyte aggregation in various pathological processes. Bull Exp Biol Med 2003;135:26–28 [DOI] [PubMed] [Google Scholar]
- 101. Purnell MC, Ramsey R. The influence of the golden ratio on the erythrocyte. In: Erythrocyte. IntechOpen, 2019. https://www.intechopen.com/books/erythrocyte/the-influence-of-the-golden-ratio-on-the-erythrocyte (last accessed June24, 2020)
- 102. Papasimakis N, Fedotov VA, Savinov V, Raybould TA, Zheludev NI. Electromagnetic toroidal excitations in matter and free space. Nat Mater 2016;15:263–271 [DOI] [PubMed] [Google Scholar]
- 103. Purnell MC, Butawan MBA, Ramsey RD. Bio-field array: a dielectrophoretic electromagnetic toroidal excitation to restore and maintain the golden ratio in human erythrocytes. Physiol Rep 2018;6:e13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Dao M, Li J, Suresh S. Molecularly based analysis of deformation of spectrin network and human erythrocyte. Mater Sci Eng C 2006;26:1232–1244 [Google Scholar]
- 105. Saldanha C, Silva AS, Gonçalves S, Martins-Silva J. Modulation of erythrocyte hemorheological properties by band 3 phosphorylation and dephosphorylation. Clin Hemorheol Microcirc 2007;36:183–194 [PubMed] [Google Scholar]
- 106. Pretorius E. Erythrocyte deformability and eryptosis during inflammation, and impaired blood rheology. Clin Hemorheol Microcirc 2018;69:545–550 [DOI] [PubMed] [Google Scholar]
- 107. Dimakopoulos Y, Kelesidis G, Tsouka S, Georgiou GC, Tsamopoulos J. Hemodynamics in stenotic vessels of small diameter under steady state conditions: effect of viscoelasticity and migration of red blood cells. Biorheology 2015;52:183–210 [DOI] [PubMed] [Google Scholar]
- 108. Chebbi R. Dynamics of blood flow: modeling of the Fåhraeus–Lindqvist effect. J Biol Phys 2015;41:313–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Korhonen R, Lahti A, Kankaanranta H, Moilanen E. Nitric oxide production and signaling in inflammation. Curr Drug Targets Inflamm Allergy 2005;4:471–479 [DOI] [PubMed] [Google Scholar]
- 110. Saldanha C, Silva-Herdade A. Erythrocyte nitric oxide. In: Atukeren P, ed. Novel Prospects in Oxidative and Nitrosative Stress. InTech, 2018:131 [Google Scholar]
- 111. Saldanha C. Fibrinogen interaction with the red blood cell membrane. 2013;53:39–44 [DOI] [PubMed] [Google Scholar]
- 112. De Oliveira S, De Almeida VV, Calado A, Rosário HS, Saldanha C. Integrin-associated protein (CD47) is a putative mediator for soluble fibrinogen interaction with human red blood cells membrane. Biochim Biophys Acta 2012;1818:481–490 [DOI] [PubMed] [Google Scholar]
- 113. Adamczyk Z, Bratek-Skicki A, Dabrowska P, Nattich-Rak M. Mechanisms of fibrinogen adsorption on latex particles determined by zeta potential and AFM measurements. Langmuir 2012;28:474–485 [DOI] [PubMed] [Google Scholar]
- 114. das Neves LMS, Leite G de PMF, Marcolino AM, et al. Laser photobiomodulation (830 and 660 nm) in mast cells, VEGF, FGF, and CD34 of the musculocutaneous flap in rats submitted to nicotine. Lasers Med Sci 2017;32:335–341 [DOI] [PubMed] [Google Scholar]
- 115. de Souza TR, de Souza AK, Garcia SB, et al. Photobiomodulation increases viability in full-thickness grafts in rats submitted to nicotine. Lasers Surg Med 2020;52:449–455 [DOI] [PubMed] [Google Scholar]
- 116. Leite GPMF, das Neves LMS, Silva CA, et al. Photobiomodulation laser and pulsed electrical field increase the viability of the musculocutaneous flap in diabetic rats. Lasers Med Sci 2017;32:641–648 [DOI] [PubMed] [Google Scholar]
- 117. das Neves LMS, de Oliveira Guirro EC, de Almeida Albuquerque FL, Marcolino AM. Effects of high-voltage electrical stimulation in improving the viability of musculocutaneous flaps in rats. Ann Plast Surg 2016;77:e50–e54 [DOI] [PubMed] [Google Scholar]
- 118. Ud-Din S, Perry D, Giddings P, et al. Electrical stimulation increases blood flow and haemoglobin levels in acute cutaneous wounds without affecting wound closure time: evidenced by non-invasive assessment of temporal biopsy wounds in human volunteers. Exp Dermatol 2012;21:758–764 [DOI] [PubMed] [Google Scholar]
- 119. Machado AFP, Liebano RE, Furtado F, Hochman B, Ferreira LM. Effect of high-and low-frequency transcutaneous electrical nerve stimulation on angiogenesis and myofibroblast proliferation in acute excisional wounds in rat skin. Adv Skin Wound Care 2016;29:357–363 [DOI] [PubMed] [Google Scholar]
- 120. Kwan RLC, Wong WC, Yip SL, Chan KL, Zheng YP, Cheing GLY. Pulsed electromagnetic field therapy promotes healing and microcirculation of chronic diabetic foot ulcers. Adv Skin Wound Care 2015;28:212–219 [DOI] [PubMed] [Google Scholar]
- 121. Kawasaki L, Mushahwar VK, Ho C, Dukelow SP, Chan LLH, Chan KM. The mechanisms and evidence of efficacy of electrical stimulation for healing of pressure ulcer: a systematic review. Wound Repair Regen 2014;22:161–173 [DOI] [PubMed] [Google Scholar]
- 122. Girgis B, Duarte JA. High voltage monophasic pulsed current (HVMPC) for stage II-IV pressure ulcer healing. A systematic review and meta-analysis. J Tissue Viability 2018;27:274–284 [DOI] [PubMed] [Google Scholar]
- 123. Mi XQ, Chen JY, Cen Y. A comparative study of 632. 8 and 532 nm laser irradiation on some rheological factors in human blood in vitro. 2004;74:7–12 [DOI] [PubMed] [Google Scholar]
- 124. Al Musawi MS, Jaafar MS, Al-Gailani B, Ahmed NM, Suhaimi FM. Laser-induced changes of in vitro erythrocyte sedimentation rate. Lasers Med Sci 2017;32:2089–2095 [DOI] [PubMed] [Google Scholar]
- 125. Karu T. Photobiology of low-power laser effects. Health Phys 1989;56:691–704 [DOI] [PubMed] [Google Scholar]
- 126. Karu TI, Pyatibrat LV, Afanasyeva NI. Cellular effects of low power laser therapy can be mediated by nitric oxide. Lasers Surg Med 2005;36:307–314 [DOI] [PubMed] [Google Scholar]
- 127. Hsiu H, Hsu WC, Hsu CL, Bau JG, Chen CT, Liu YS. Complexity analysis of the microcirculatory-blood-flow response following acupuncture stimulation. Microvasc Res 2013;89:34–39 [DOI] [PubMed] [Google Scholar]
- 128. Ishikawa S, Suga H, Fukushima M, et al. Blood fluidity enhancement by electrical acupuncture stimulation is related to an adrenergic mechanism. J Acupunct Meridian Stud 2012;5:21–28 [DOI] [PubMed] [Google Scholar]
- 129. Asano M. Reminiscences of microcirculatory studies on application of acupuncture needles to the rabbit in vivo. Clin Hemorheol Microcirc 2006;34:89–96 [PubMed] [Google Scholar]
- 130. Qingguo L. Effects of acupuncture on hemorheology, blood lipid content and nail fold microcirculation in multiple infarct dementia patients. J Tradit Chin Med 2004;24:219–223 [PubMed] [Google Scholar]
- 131. Blenkinsopp SA, Khoury AE, Costerton JW. Electrical enhancement of biocide efficacy against Pseudomonas aeruginosa biofilms. Appl Environ Microbiol 1992;58:3770–3773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Blackiston DJ, McLaughlin KA, Levin M. Bioelectric controls of cell proliferation: ion channels, membrane voltage and the cell cycle. Cell Cycle 2009;8:3527–3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Game FL, Apelqvist J, Attinger C, et al. Effectiveness of interventions to enhance healing of chronic ulcers of the foot in diabetes: a systematic review. Diabetes Metab Res Rev 2016;32:154–168 [DOI] [PubMed] [Google Scholar]
- 134. Baker LL, Chambers R, Demuth SK, Villar F. Effects of electrical stimulation on wound healing in patients with diabetic ulcers. Diabetes Care 1997;20:405–412 [DOI] [PubMed] [Google Scholar]
- 135. Peters EJ, Lavery LA, Armstrong DG, Fleischli JG. Electric stimulation as an adjunct to heal diabetic foot ulcers: a randomized clinical trial. Arch Phys Med Rehabil 2001;82:721–725 [DOI] [PubMed] [Google Scholar]
- 136. Janković A, Binić I. Frequency rhythmic electrical modulation system in the treatment of chronic painful leg ulcers. Arch Dermatol Res 2008;300:377–383 [DOI] [PubMed] [Google Scholar]
- 137. Petrofsky JS, Lawson D, Berk L, Suh H. Enhanced healing of diabetic foot ulcers using local heat and electrical stimulation for 30 min three times per week. J Diabetes 2010;2:41–46 [DOI] [PubMed] [Google Scholar]
- 138. Lambert H, de Bisschop F, de Mey G, et al. Electric current distribution in tissues upon electrotherapy. Acta Belg Med Phys 1989;12:31–40 [PubMed] [Google Scholar]
- 139. Livshitz LM, Mizrahi J, Einziger PD. Interaction of array of finite electrodes with layered biological tissue: effect of electrode size and configuration. IEEE Trans Neural Syst Rehabil Eng 2001;9:355–361 [DOI] [PubMed] [Google Scholar]
- 140. Petrofsky J, Schwab E, Cúneo M, et al. Current distribution under electrodes in relation to stimulation current and skin blood flow: are modern electrodes really providing the current distribution during stimulation we believe they are? J Med Eng Technol 2006;30:368–381 [DOI] [PubMed] [Google Scholar]
- 141. Kloth LC. Electrical stimulation technologies for wound healing. Adv Wound Care 2014;3:81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Houghton PE. Clinical trials involving biphasic pulsed current, microcurrent, and/or low-intensity direct current. Adv Wound Care 2014;3:166–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Lallyett C, Yeung CYC, Nielson RH, et al. Changes in S100 proteins identified in healthy skin following electrical stimulation: relevance for wound healing. Adv Skin Wound Care 2018;31:322–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kolosnjaj-Tabi J, Gibot L, Fourquaux I, Golzio M, Rols MP. Electric field-responsive nanoparticles and electric fields: physical, chemical, biological mechanisms and therapeutic prospects. Adv Drug Deliv Rev 2019;138:56–67 [DOI] [PubMed] [Google Scholar]