Abstract
Significance: Millions of people worldwide suffer from diabetes mellitus and its complications, including chronic diabetic wounds. To date, there are few widely successful clinical therapies specific to diabetic wounds beyond general wound care, despite the vast number of scientific discoveries in the pathogenesis of defective healing in diabetes.
Recent Advances: In recent years, murine animal models of diabetes have enabled the investigation of many possible therapeutics for diabetic wound care. These include specific cell types, growth factors, cytokines, peptides, small molecules, plant extracts, microRNAs, extracellular vesicles, novel wound dressings, mechanical interventions, bioengineered materials, and more.
Critical Issues: Despite many research discoveries, few have been translated from their success in murine models to clinical use in humans. This massive gap between bench discovery and bedside application begs the simple and critical question: what is still missing? The complexity and multiplicity of the diabetic wound makes it an immensely challenging therapeutic target, and this lopsided progress highlights the need for new methods to overcome the bench-to-bedside barrier. How can laboratory discoveries in animal models be effectively translated to novel clinical therapies for human patients?
Future Directions: As research continues to decipher deficient healing in diabetes, new approaches and considerations are required to ensure that these discoveries can become translational, clinically usable therapies. Clinical progress requires the development of new, more accurate models of the human disease state, multifaceted investigations that address multiple critical components in wound repair, and more innovative research strategies that harness both the existing knowledge and the potential of new advances across disciplines.
Keywords: diabetes, wound healing, murine, diabetic wound, diabetic ulcer, therapeutic

Luisa A. DiPietro, DDS, PhD

Lin Chen, MD, PhD
Scope and Significance
This text reviews the advances in diabetic wound healing research and discusses the obstacles preventing them from successfully progressing to the clinic. The discussion focuses on research using biologically active substances to improve wound healing in murine models of diabetes mellitus (DM).
Approaches relying exclusively on wound care tools, such as inactive dressings, LED lights, bandages, nanoparticles, and gels; as well as preexisting Food and Drug Administration (FDA)-approved ther apies for other conditions; genetic modifications; and physical manipulation of wounds, such as splints, stitches, modified pressure environments, or external structures, are outside the scope of this review and will not be discussed in depth.
Translational Relevance
For the millions of patients affected by DM, impaired wound healing is a life-threatening complication that generates massive social and economic burdens.1 To date, few successful therapies for diabetic wounds have reached the clinic2 despite a vast breadth of research demonstrating improved healing in murine models of the disease. In this work, we summarize these major advances and discuss the remaining challenges that must be addressed to translate these discoveries into successful therapeutics for diabetic ulcers.
Clinical Relevance
Patients with DM face a lifetime of continuous disease management, which is further complicated by sequelae such as the diabetic foot ulcer (DFU). Currently, clinical management of diabetic wounds relies on frequent foot examination, regular care, early detection, wound debridement, appropriate dressings, pressure off-loading, treatment of infection, and, sometimes, skin substitutes.2 Despite these options, diabetic wounds remain a clinical need for which there are few specifically targeted pharmacological or bioactive drugs. As research in murine diabetic models has progressed, promising potential therapeutics have often failed to reach the clinic, leaving high-risk patients with limited options.
Background/Overview
DM is a global health care concern that affects nearly half a billion people and causes over 1 million deaths annually.3 Many can be attributed to the serious complications of DM, which include heart disease, stroke, neuropathy, nephropathy, peripheral artery disease, retinopathy, and poor wound healing.4,5 Of these complications, chronic lower extremity wounds (diabetic foot ulcers, or DFUs) are a leading contributor to the morbidity and mortality of DM, leading to over 100,000 annual amputations in the United States alone and generating a substantial social, economic, and health care burden.1,6
The pathogenesis of deficient wound healing in DM is complex and multifactorial. Contributing factors to the inability of diabetic skin to heal include poor perfusion and angiogenesis; hypoxia; increased inflammation and tissue destruction; increased levels of matrix metalloproteinases (MMPs), free radicals, and advanced glycation end products; and dysregulated cell function.2,5,7,8 Together, these factors predispose the skin to injury and decrease its baseline ability to heal, resulting in chronic wounds that become limb- and life-threatening (Fig. 1).
Figure 1.
Examples of diabetic wounds. Representative images of nonhealing neuropathic, ischemic, or neuroischemic diabetic ulcers affecting the lower extremities. All images generously provided by Dr. Stephanie Wu, DPM, MSc, FACFAS; Dean of Dr. William M. Scholl College of Podiatric Medicine; Professor, Department of Podiatric Surgery and Applied Biomechanics; Professor, Center for Stem Cell and Regenerative Medicine; and Director, Center for Lower Extremity Ambulatory Research (CLEAR) for Dr. William M. Scholl College of Podiatric Medicine at Rosalind Franklin University of Medicine and Science. Color images are available online.
The wound healing process consists of four specifically regulated phases: hemostasis, inflammation, proliferation, and remodeling (Fig. 2).9,10 During hemostasis, blood vessels constrict in response to injury, and the activation and aggregation of platelets forms a blood clot known as the platelet plug, which seals off the injured area to prevent continued bleeding. The inflammatory phase then begins in response to the waves of cytokines and chemokines released during hemostasis. This phase consists of the recruitment of immune cells over the next few hours to days.
Figure 2.
Stages of wound healing. A schematic (not to scale) depicting the four phases of wound healing: hemostasis, inflammation, proliferation, and remodeling. Artwork and renderings in this and subsequent figures are courtesy of Smart Servier Medical Art, freely available at https://smart.servier.com Color images are available online.
First, neutrophils arrive to clear debris and microbes from the injured area. Although they are effective at preventing infection, the enzymes and byproducts generated by neutrophils can often cause additional tissue destruction. Next, monocytes migrate to the wound and differentiate into macrophages, which remove apoptotic cells and debris, and, later, promote fibroblast and keratinocyte proliferation and angiogenesis. As the inflammatory phase resolves over the next few days, the proliferative phase begins to replace and repair the injured tissue. This phase overlaps with the resolution of inflammation and involves the formation and deposition of new epithelial layers, granulation tissue, extracellular matrix, and many new capillaries.
Finally, the healing wound enters the remodeling phase, a long-term and ongoing process responsible for vascular pruning, remodeling of the extracellular matrix, and an overall biological “revising and editing” of the tissue to resemble normal tissue as closely as possible.9,10
In diabetic skin, the wound healing paradigm is disrupted: whole tissue dysfunction contributes to increased injury and impaired healing; the phases of wound healing do not occur or resolve appropriately; individual cells and functions are abnormal; and the increased susceptibility of diabetic patients to external stressors, such as infection, further interferes with wound resolution.7 While these factors are prominent players in the diabetic wound healing problem, they are by no means the exhaustive list.
As scientists understand more about DM itself and about tissue repair and regeneration, their toolkit and the complexity of their questions continue to expand. Scientists have developed animal models to study diabetic wounds, begun deciphering the innumerable factors at play, and described hundreds of targets that improve healing in animal models of DM.
Despite this breadth of progress, however, current research advancements have produced few practical and usable clinical therapeutics for patients with DFUs. This lack of treatment options can be attributed, in part, to the animal model systems of diabetic wound healing, which have yet to sufficiently replicate the human condition—a concern that further underscores this disparity. Still other challenges arise in the complexity of the patient population, which cannot be fully represented in a clinical trial cohort, and the narrow scope of many research studies, which reveal valuable insights into the physiology of the diabetic wound but often do so only a single factor at a time.
The imbalanced ratio between preclinical research and clinically successful therapeutics leaves hundreds of thousands of patients with few options beyond standard wound care. For those with DM, the tenets of ulcer prevention and treatment are good glycemic control, daily foot care, regular examination, early detection of injuries, infection control, and appropriate wound care and dressings. Appropriate care can include wound debridement, systemic antibiotics, revascularization procedures, and pressure off-loading (Fig. 3).2,11
Figure 3.
Current standards of diabetic foot care. A summary of the most important components of diabetic foot care and the treatment and prevention of diabetic foot ulcers. These are good glycemic control, regular foot care and examination, early detection, and appropriate wound care and dressings. There are currently no gold standard pharmaceutical therapies that are specific for and widely usable to treat diabetic wounds, although there are a few FDA-approved options available. FDA, Food and Drug Administration. Color images are available online.
Beyond these standards, wound care clinicians have only a single FDA-approved drug specific to DFUs in their arsenal: Regranex (becaplermin), a topically applied human recombinant platelet-derived growth factor-BB (PDGF-BB) gel12,13 approved for neuropathic diabetic ulcers, which are well-perfused and do not encompass the full complexity of most DFUs. Regranex showed significant efficacy in clinical trials with tightly controlled wound criteria and treatment conditions14,15; however, its clinical use has yet to match up to its suggested potential.16
In addition to Regranex, three biologic medical devices are FDA-approved for the treatment of DFUs. These include Dermagraft17–19 and Apligraf (also known as Graftskin),20–24 both of which are cell-based human skin equivalents or substitutes (HSEs), and Omnigraft, an animal-derived matrix originally used for burns.25–27 While these are the only bioactive medical devices that are approved specifically for use in DFUs, there are many general wound care products and therapies, including other FDA-approved devices; many different types of dressings, scaffolds, bandages, sprays, and closure devices; and drugs with off-label usage; these are all used to varying degrees of success in the management of DFUs.28–32
Throughout this review, various treatment types will be discussed, including pharmacologically active drugs, such as Regranex; medical devices, such as the HSEs; and natural or inactive products, such as honey or inert dressings. The FDA offers clear guidance in the distinction between drugs and devices.33 Put simply, a medical device is a treatment that meets the criteria for drugs but “does not achieve its primary intended purposes through chemical action… and which is not dependent upon being metabolized,” making it exempt from the more rigorous requirements for drug approval.33
It is important to understand these differences between treatments, and, most importantly, the magnitude of difficulty in developing, testing, and launching a new drug, which is exponentially more challenging and can cost hundreds of millions of dollars to achieve.34 These drastically different barriers to entry explain, in part, the relative abundance of non-drug wound care therapies versus novel biologically active drugs.
Although these non-drug products are extremely valuable options, they do not directly target deficits in diabetic skin or the pathophysiology of DFUs, and additional specific and biologically active treatments that work to rescue aberrant healing are necessary. Understanding why barriers to novel drug development exist requires an understanding of the models, methods, and major advances in diabetic wound healing research.
Only then can scientists begin to answer these key questions: What is still missing? How can approaches be updated for novel therapeutic development? Which discoveries can be expanded to surmount the challenges diabetic wound healing presents? Also most importantly, how can sufficient clinical advancement be achieved to protect the growing patient population with life-threatening complications of DM?
Discussion
Common murine models of DM
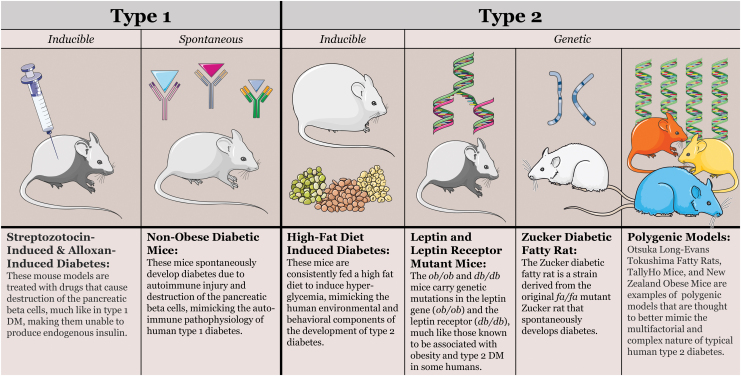
Experimental studies in animal models of DM began centuries ago, with Brunner's early description of polyuria, polydipsia, and polyphagia in pancreatectomized dogs in 1722, Minkowski and von Mering's pancreatectomy-induced canine diabetes model in 1889, and Banting and Best's isolation of insulin in 1921, which led to the life-saving development of exogenous insulin therapy for humans.35,36 Today, many model systems exist to enable investigations into both type 1 (DM1) and type 2 diabetes (DM2), each with their own drawbacks and advantages (Table 1).37–55 The vast majority of these are rodents, categorized by whether they are genetic, inducible, or spontaneous models of DM1 or DM2 (Fig. 4).56
Table 1.
Comparison of common rodent models of diabetes mellitus
| Model+TypeReferences | Description | Onset of Hyperglycemia | Wound Healing Impairment |
||
|---|---|---|---|---|---|
| 2–5 daysa | 6–10 daysa | 11–20 daysa | |||
| Streptozotocin-induced: Inducible, DM138–40 | These mice are dosed with either streptozotocin, a bacterial antibiotic, or alloxan, a pyrimidine derivative similar in structure to glucose. Onset and severity vary based on whether treatments are a single high dose or multiple low doses. Both substances cause destruction of the pancreatic beta cells, mimicking DM1. | Reaches permanent diabetes within days, pattern varies in single vs. multiple dose models | Single dose: 1.53a (95% CI 0.96 to 2.10) Multidose: 1.16a (95% CI 0.84 to 1.47) |
Single dose: 2.09a (95% CI 1.35 to 2.84) Multidose: 1.69a (95% CI 1.27 to 2.11) |
Single dose: 3.58a (95% CI 2.57 to 4.59) Multidose: 1.53a (95% CI 1.06 to 1.99) |
| Alloxan-induced: Inducible, DM139,40 | Single dose: 1.20a (95% CI 0.42 to 1.99) Multidose: 5.35a (95% CI 2.50 to 8.20) |
Single dose: 2.32a (95% CI 0.63 to 4.01) Multidose: 5.89a (95% CI 2.80 to 8.98) |
Single dose: 2.05a (95% CI 0.38 to 3.72) Multidose: 5.84a (95% CI 2.77 to 8.91) |
||
| Nonobese diabetic: Spontaneous, DM139,41,42 | The NOD mouse is a spontaneous autoimmune model of diabetes originating from the Cataract Shionogi mouse. It mimics DM1, and affected mice have some shared autoantibodies with humans. | Develops by 30 weeks, higher incidence in female mice | 1.47a (95% CI 0.68 to 2.27) | 6.09a (95% CI 4.47 to 7.72) | 14.59a (95% CI 4.46 to 24.71) |
| High-fat diet: Inducible, DM239,43–45 | These mice are fed a high-fat diet for a specified time frame to mimic obesity, metabolic syndrome, and DM2. Presentation and onset varies widely based on the diet, duration, and mouse strain. | Severe obesity and hyperglycemia by 16 weeks; varies with diet/strain | 0.17a (95% CI −0.89 to 1.23) | 1.16a (95% CI 0.38 to 1.95) | 1.34a (95% CI 0.65 to 2.03) |
| Ob/Ob: Genetic, DM239,42,47 | These mice are homozygous mutants for the Lep gene, which encodes Leptin, an adipose hormone involved in hunger regulation. These mice mimic human metabolic syndrome and are severely obese. Onset and duration of hyperglycemia varies by background. | Onset at around 4 weeks, transient (up to 14–16 weeks) in C57BL/6J | 3.55a (95% CI −0.07 to 7.18) | 4.57a (95% CI 2.1 to 7.04) | — |
| Db/Db: Genetic, DM239,42,46 | These mice are homozygous mutants for the Lepr gene, the receptor for the Leptin hormone. They mimic human DM2 and exhibit very similar disease onset, progression, presentation, and severity. Db/Db mice are the leading animal model of DM2. | Hyperinsulinemic by 10–14 days, hyperglycemic by 4–8 weeks in C57BLKS/J | 1.18a (95% CI 0.76 to 1.6) | 2.9a (95% CI 2.29 to 3.52) | 3.87a (95% CI 3.11 to 4.63) |
| TallyHo mouse: Genetic, DM249,57 | The TallyHo mouse is polygenic model of DM2, which spontaneously develops diabetes similarly to humans. Its phenotype is less severe than other genetic models like the Db/Db mouse, and it is a more representative model of the complex inheritance of diabetes. | Mild hyperglycemia at 6–8 weeks, severe by 14–16 weeks; usually males only | Known wound healing deficits in incisional, excisional, and ischemic-reperfusion wound models. | ||
| New Zealand Obese: Genetic, DM252,53 | The NZO mouse is a polygenic model of severe obesity that is highly similar to human metabolic syndrome and, in male mice, can progress to mature onset DM2. | Late onset at 20–24 weeks, in males; can be accelerated with high-fat diet | Not commonly used in wound healing studies. | ||
| Otsuka Long-Evans Tokushima Fatty Rat: Genetic, DM251,55 | The OLETF rat is an inbred polygenic model of chronic DM2 with mild obesity and late onset hyperglycemia. Like many of the other genetic models of DM, primarily males develop full-blown disease. | Late onset after 18 weeks; usually males only | Known impairments in corneal wound healing. | ||
| Zucker diabetic fatty rat: Genetic, DM248,50,54 | ZDF rats are derived from select homozygous fa/fa mutants of the original Zucker fatty rat strain. These rats develop early obesity and insulin resistance which mimics the progression to DM2 in humans. Interestingly, most female ZDF rats do not become hyperglycemic. | Mild hyperglycemia at 7–8 weeks, severe by 14–16 weeks; usually males only | Impaired wound closure, abnormalities in wound tissue structure, inflammation, and scarring. | ||
Values represent mean difference in wound size compared to nondiabetic controls, from 2020 meta-analysis of diabetic ulcer models.39
Days, days postwounding; DM, diabetes mellitus; NOD, nonobese diabetic; NZO, New Zealand Obese; OLETF, Otsuka Long-Evans Tokushima Fatty; ZDF, Zucker diabetic fatty.
Figure 4.
Common rodent models of DM. An overview of some of the most common murine models of type 1 and type 2 DM. For type 1 DM, these include the streptozotocin and/or alloxan-induced models and the nonobese diabetic mouse.37,56 For type 2 DM, they include the high-fat diet mouse, leptin and leptin receptor mutants, Zucker diabetic fatty rats, and polygenic models such as the New Zealand Obese mouse and the Otsuka Long-Evans Tokushima Fatty Rat.10,11,57 DM, diabetes mellitus. Color images are available online.
The most commonly used models are those that best approximate the human pathogenesis and development of DM. For DM1, these include the streptozotocin- or alloxan-induced mice and nonobese diabetic mice, all of which undergo destruction of the insulin-producing pancreatic beta cell, similar to the autoimmune pathogenesis of DM1 in human patients.37,56 For DM2, which affects the vast majority of diabetic patients, the most prominent models include the genetic leptin mutant ob/ob and leptin receptor mutant db/db mice, Zucker diabetic fatty rats, high-fat-diet-induced mice and rats, and polygenic models such as the Otsuka Long-Evans Tokushima Fatty rats, TallyHo mice, and New Zealand Obese mice.10,11,57
Because murine models remain the most widely studied, they comprise the majority of diabetic wound healing research. However, larger animals, including swine models such as the Ossabaw and Yorkshire pigs, are emerging as model organisms that come increasingly close to mimicking the healing properties of human skin.58–60 When compared to humans, porcine models demonstrate more analogous skin structure, increased concordance (78%) in study results, and more similar responses to treatments than both small mammal (53%) and in vitro (57%) models.58,61
As these more clinically relevant models become increasingly well-characterized, they warrant significant discussion as potential solutions to this problem. Although they present a series of practical considerations, including increased costs and experimental difficulty, the increased concordance between human and porcine models could be key to limiting the number of laboratory discoveries that fall short during clinical trials.
Alongside the animal models of DM, there is a wide array of experimental methods used for the study of wound healing (Fig. 5). Among the in vivo assays, the excisional wound model is the most prominent. In this model, a full-thickness skin segment is removed to enable investigation of wound closure, reepithelialization, cell migration and prolif eration, angiogenesis, formation of granulation tissue, scarring, and more.62 Other commonly used methods include partial thickness wounds, incisional wounds, ischemic cutaneous wounds, surface burn wounds, and abrasion and tape-stripping wounds,62 each of which serves its own utility in investigating the wound healing process and its aberrancy in DM.
Figure 5.
Common experimental methods of wound healing. A summary depicting some of the most commonly used experimental wounding models, including excisional wounds, incisional wounds, ischemic wounds, burn wounds, and abrasion wounds.62,63 Color images are available online.
In addition to these in vivo methods, there are many standard in vitro protocols that are regularly utilized, especially in the early stages of research. These include 3D cultures, co-cultures, single-cell studies, organoids, scratch and migration assays, and more.63 Because the majority of drug development studies progress from these models into animal models before reaching human subjects, in vitro methods are not discussed in depth here. They do, however, play an essential role in wound healing research and are often a key starting point for any potential therapeutic.
Due in no small part to the wide variety of models and methods available, both the quantity and quality of diabetic wound healing research has increased exponentially in recent decades, leading to immense discovery of the individual components that play a role in the development and healing of diabetic ulcers. A PubMed search for “diabetic wound healing” yields only 3 results for the year 1962, but nearly 900 citations appear in the results for the year 2019. Massive growth in this area has brought with it many achievements in the murine models above, suggesting various biological pathways, compounds, cell types, growth factors, small molecules, cytokines, and more that have improved diabetic wound healing experimentally.
Here, we summarize many of these discoveries in murine models and discuss the challenges and constraints that impact their translational success. Of note, the breadth of diabetic wound healing research extends far beyond these direct, biologically derived or bioactive factors and includes other approaches such as novel and supplemented methods of wound care, novel wound dressings and drug delivery methods, gene therapy, adaptations of existing FDA-approved medications, and more, which cannot be discussed in-depth in this review.28,64–67
Growth factors
Among the most prominent and well-studied discoveries for the improvement of diabetic wound healing is the application of growth factors.68–70 Of these, the most successful is PDGF-BB, which was first shown to improve diabetic wound healing in db/db mice in the early 1990s and reached Phase I clinical trials by 2000.14,71–76 To date, Regranex (becaplermin gel), the topical recombinant PDGF-BB therapy, remains the only FDA-approved prescription drug specifically for DFUs, although its usability, practicality, and effectiveness in the clinic are relatively poor despite significant efficacy in clinical trials.12,14,16,77
Additional research continues to investigate the effects of PDGF on diabetic wound healing, including in combination with other growth factors, such as fibroblast growth factor (FGF),71,78 vascular endothelial growth factor (VEGF),79 and transforming growth factor alpha (TGFα).72 Studies in diabetic mice and other healing-impaired models demonstrated that recombinant acidic or basic FGF each improved tissue repair, dermal wound healing, and angiogenesis as early as the 1990s.80–85
Several more recent studies, beginning in 2011, also demonstrated the beneficial effects of FGF on diabetic wound healing using rat models and novel polygenic mouse models; efficacy was also observed in studies using other experimental methods, such as gene transfer, joint applications with other factors, modified applications of FGF, and novel drug delivery vehicles, such as electrospun fibers and fibrin scaffolds.86–91
A similar pattern exists for investigations involving VEGF. Beginning in the early 2000s, Rico et al. and Galiano et al. demonstrated that topical application of VEGF to dermal wounds in db/db mice significantly sped wound closure and improved wound healing.92,93 Additional studies over the next 15 years continued to demonstrate the effectiveness of VEGF therapy in diabetic wound healing models, using methods and tools such as gene therapy, combination therapy with other factors, collagen and fibrin scaffolding, nanospheres, and more.79,86,89,93–96
Others' investigations have also demonstrated the effectiveness of many other growth factors in improving diabetic wound healing experimentally, including keratinocyte growth factor (KGF),97–99 epidermal growth factor (EGF),95,100–105 TGFα and TGFβ,72,106–110 insulin-like growth factor 1,107,111–115 connective tissue growth factor,116 hepatocyte growth factor,117,118 and nerve growth factor.119,120
These discoveries provide an important opportunity to discuss diabetic wound healing research beyond preclinical investigation in model organisms, when a promising experimental treatment reaches human patients and, often unexpectedly, fails during late-stage clinical trials. Growth factors, in particular, represent a large portion of this group, making them an excellent example of the disparity between the laboratory and the clinic and giving a glimpse into where the biggest challenges lie.
Cellular therapies
Another major category yielding experimental success in diabetic murine models is the application of specific cell populations to improve wound healing. These include stem and progenitor cells, stromal cells, immune cells, fibroblasts, and keratinocytes. Among the stem and progenitor cell populations, mesenchymal stem cells (MSCs) are the most extensively studied and have been shown to improve diabetic wound healing in various applications.121–130 The earliest of these studies began in 2007 and used MSCs derived from autologous bone marrow, which improved wound healing both in db/db mice and in a small number of human patients with acute and chronic wounds.121,124
Since then, further preclinical investigations have demonstrated positive effects on wound healing in response to adipose-derived MSCs,125,129,131–142 umbilical cord blood-derived stem cells,123,143–150 embryonic stem cells,151,152 hematopoietic stem cells,153 endothelial progenitor cells (EPCs),147,154–157 and CD34+ cell populations.143,150,158–161 In addition, stromal cells derived from both bone marrow162,163 and adipose tissue,164–166 immune cell populations such as mac rophages167 and mature B cells,167,168 fibroblasts,148,169 keratinocytes,170 and platelets171,172 have all been shown to improve wound healing in murine models of DM.
Each of these cell-based therapies demonstrated preclinical efficacy; however, the majority never reached clinical trials, and even fewer emerged successful. One such example is the macrophage-based therapeutic CureXcell by MacroCure Ltd. The application of macrophages to wounds demonstrated early efficacy in human patients in 2004, leading to the development of MacroCure's namesake product.173 CureXcell, a then cutting-edge biologic, was on track to become one of the few diabetic wound care options on the market, until it failed in Phase III clinical trials,173–177 serving as an example of lost efficacy when study scale increases and practicality becomes a critical metric.
Much like macrophages, platelet therapies for wound healing have also undergone significant investigation in human patients, including multiple clinical trials; several variations, including platelet-rich plasma, platelet-rich fibrin, and autologous platelet gel, are in use clinically for diabetic and other wounds, and further trials are ongoing.178–184
Of the many cell-based advances mentioned here, the most prominent and enduring clinical success is the use of fibroblasts and/or keratinocytes to create HSEs that can be applied with appropriate dressings to improve healing of chronic DFUs. Two such HSEs, Apligraf (Graftskin),21,22,24,185 and Dermagraft,17,186 are FDA-approved for DFUs lasting more than 4–8 weeks, although they cannot be used in with standard topical wound care agents or for any DFU with infection or other common complications. It is of important note that Apligraf, Dermagraft, and their drug counterpart, Regranex, were all approved around the turn of the century: Regranex in 1997, Apligraf in 1998, and Dermagraft in 2001.13,18,23
In the 20 years since, Integra's Omnigraft Dermal Regeneration Matrix, an animal-derived wound dressing containing bovine collagen, shark cartilage, and silicone, originally approved for burns in 1996, gained additional approval for use in diabetic wounds in 2016.27,187 Considering the exponential growth of diabetic wound healing research over the same 20-year period, this statistic underscores a somber scientific reality: although diabetic wound healing research models and methods have been established that produce valuable laboratory results, there clearly remains a knowledge gap impeding the clinical progress they ultimately seek to achieve.
Cytokines, chemokines, and other cellular activation molecules
In addition to harnessing the potential of whole-cell populations, many have investigated the use of individual cytokines, cellular proteins, and signaling molecules to improve diabetic wound healing.155 These include IL2,188,189 IL4,190 IL6,191,192 IL22,193 TNFα,167 and CCL2,194,195 all of which contribute to improved wound repair in diabetic murine models.
Many other endogenous proteins or protein an alogs have also demonstrated contributions to diabetic wound healing, including erythropoietin,105,130,196,197 substance P,198–203 in sulin,103,204,205 C peptide,206,207 nuclear factor erythroid 2-related factor 2 (NRF2),208–212 Sonic Hedgehog,213 homeobox protein HoxD3,214 MMP-9,215–217 oncostatin M,218 nesfatin-1,219 thyroid hormone,220 mineralocorticoid receptor antagonists,221 GnRH antagonists,222 and inositol-requiring enzyme 1.223
One prominent example of a cellular activation-based therapy is the topical application of platelet releasate (PR), also known as platelet-derived wound healing factors/formula, to treat nonhealing wounds. PR is a mixture of platelet-released proteins, chemokines, and cytokines that is usually derived from autologous platelets and first demonstrated improved wound healing in humans in 1986.224 Randomized human trials using PR began in 1990,225–227 and additional research demonstrated the effectiveness of PR for wound healing in several animal models, including diabetic mice and rats.228–231
Over the next two decades, investigation continued into the use of PR for DFUs and other nonhealing wounds, and many clinics today offer autologous PR as a treatment option. While PR and similar substances, such as platelet-rich plasma and autologous platelet gel, are not FDA-approved drugs, their clinical use continues to increase, and further investigations are ongoing.231–234
While the majority of studies demonstrating improvements in diabetic wound healing do so using single factors, PR and its more recent counterparts, such as transcription factors, microRNAs (miRNAs), and exosomes, represent potential therapeutics that may be able to rescue multiple inherent shortcomings in the diabetic wound at once, thereby improving wound healing more significantly than any single factor could alone.
PR serves as a very early example of these broader investigations, a key feature that may explain the longevity of PR as a research target for wound healing and its ongoing clinical use. Expansion into more widely efficacious therapies is rapidly increasing and marks an important step in recognizing the limitations of research discoveries thus far and adapting the investigational approach.
Alongside proteins and cytokines, a multitude of chemical compounds and small molecules have demonstrated experimental efficacy in improving diabetic wound healing. Of the compounds studied, carnosine,235 quadrol,236 sphingosine 1-phosphate,237 deferoxamine,238–240 nonadenine-based purines,241 fullerenes,242 the DPP-4 inhibitor MK0626,243 ND-336,244 hydroxylase inhibitors,245 and hydrogen sulfide246–248 have all been shown to accelerate wound healing in murine models of DM2.
Many studies not discussed here have also investigated the potential repurposing of existing FDA-approved medications for other conditions to be used for DFUs, such as beta-blockers, anesthetics, metformin, and others. Drug repurposing increases the likelihood that clinical trials may be successful since they rely on substances that have already demonstrated safety and efficacy for at least one indication in human patients, making this method an appealing and potentially productive approach for diabetic wound healing and other conditions, including cancers, neurological disorders, and more.249,250
However, no existing DM drugs have been reapproved by the FDA for diabetic wound care, although there are off-label uses. This limitation likely comes on the heels of a few key obstacles: lack of interest from pharmaceutical companies; insufficient funding for the still very costly trials, even for a previously approved compound; and limited access to and strict regulation of existing drugs (especially those for which affordable generics do not yet exist).251,252 These limitations hinder the potential of a vast library of existing and likely underutilized molecules, many of which could have additional therapeutic possibilities.
One such example is Galnobax, a topical DFU treatment containing esmolol hydrochloride, a beta blocker commonly used as an antiarrhythmic, for which recruitment began for a Phase III clinical trial in June of 2019.253 Although the study's outcome is still pending, Galnobax represents an important foray into the repurposing of existing drugs for the treatment of DFUs. Time will tell, however, whether the costly and intricate ventures of a clinical trial, especially during Phase III, can pay off using “old” drugs.
MicroRNAs
As the understanding of cellular functions has deepened, diabetic wound healing research has progressed to previously impossible targets, such as miRNAs. MiRNAs are short noncoding RNA sequences that modulate gene expression by directly targeting matching mRNA sequences to control a wide breadth of cellular functions.254 Diabetic wound healing research has begun to investigate at the miRNA level to understand the effects of DM on miRNA expression during wound healing and the ways in which miRNAs could be modulated to rescue impaired healing. In a single miR profiling study in diabetic rats in 2015, Liu et al. revealed 83 distinct miRNAs that are differentially expressed in DFUs.255
Several individual miRNAs have been studied to further understand their contributions. MiR-129-2-3p,256 miR-132,257 miR-129 and miR-335,258 and miR-27b259 have all been shown to directly improve diabetic wound healing. Others, including miR- 146a,260,261 miR-15b,262–264 miR-200b, miR-126,265,266 and miR-21,267 are known to be involved, although their contributions and therapeutic potential remain less well-understood.
Some miRNAs have been shown to contribute to impaired wound healing in DM, making them good targets for inhibitory therapy. MiR-155 inhibition,268,269 miR-15b and miR-200b inhibition,264 the modified miR-92a inhibitor MRG-110,270 and the miR-26a inhibitor LNA-anti-miR-26a271 improve cutaneous wound healing in murine models of DM.
Importantly, investigation into the roles of miRNAs in diabetic wound healing also requires consideration of epigenetic regulation, which both alters gene expression levels and affects protein translation. Differences in epigenetic patterns have been observed in human patients with DM and other conditions,272 and both miR-200b and miR-19a have been implicated in the regulation of vascular and tissue factors in DM.273,274 These and other epigenetic targets are a young area of diabetic wound healing research with important advantages.275
MiRNAs and epigenetic regulation can readily influence gene expression within a cell, drastically expanding their potential for efficacy over a single soluble factor. Because of this increased breadth, they represent an important level of innovation that could potentially overcome the deficits that single factors have so far been unable to conquer.
Microvesicles and exosomes
Similarly, recent diabetic wound healing studies beginning in 2015 have turned to extracellular vesicles as potential therapeutics. Both microvesicles and exosomes are specific types of extracellular membrane vesicles that carry packaged proteins, RNAs, signaling molecules, and other substances from cell to cell, playing a crucial role in communication and regulation.276 Because these biological packages are capable of carrying a heterogenous combination of factors and information from their parent cells, they are more broad-spectrum research targets that could inform the understanding of diabetic wound healing with greater resolution.
Exosomes from cell populations, including fibrocytes,277 EPCs,156,157 platelet-rich plasma,278,279 MSCs,280,281 and adipose stem cells,211 have all demonstrated efficacy in diabetic mouse and rat models. Like their miRNA counterparts, microvesicles offer the potential promise of much more widespread targeting within the diabetic wound, with their contents representing an amalgam of factors that are, together, more potent and diverse in their effects.
Bio- and bioengineered materials
Another state-of-the-art research approach has become increasingly more common over the last 15 years: the use of bio- and bioengineered materials to modulate wound repair.66 These efforts aim to modernize and expand the boundaries of diabetic wound healing research by taking advantage of rapid technological advancement. Many of these studies have used biomaterials to elaborate on previously studied factors, making unique modifications to improve the target's potential therapeutic effects.
Such examples include biomaterial-grown EPCs,282 electrospun biomaterial seeded with MSCs,283 drug-eluting bioengineered scaffolds containing macrophage chemokines,284 adipose stem cells within an artificial dermis,134 EGF-releasing hydrogel,285 and more. Still others of these more tech-savvy approaches have produced a rapidly growing body of preclinical research demonstrating promising potential for diabetic wound care, including a variety of hydrogels,141,286–302 nanoparticles,89,261,303–309 and scaffolds.89,96,290,300,305,310–316
Here, it is critical to note the many other mechanical, physical, and external wound-modulating treatments, such as LED light therapy,317–321 vacuum-assisted wound closure,322–326 a wide variety of dressings and bandages, and many more. Although they are generally not bioactive, these options are also rapidly evolving, due to the same technological advances that drive the new wave of bioengineered and biomaterial solutions. Together, these studies represent an important but still-young shift in the approach to diabetic wound healing research, pushing beyond the traditional low-tech approaches that comprise the majority of the existing body of work.
As bioengineered and more tech-driven methods continue to expand, they may be able to clear the hurdles that have delayed clinical progress thus far and achieve previously impossible efficacy in driving wound repair.
Plant- and animal-derived substances
By far the largest and most diverse group of factors that have been suggested to improve diabetic wound healing are plant- and animal-derived substances, including readily accessible natural products, herbal treatments, ingredients commonly included in skincare products, and substances found in our everyday diet. The most common and well-studied of these are curcumin199,308,327–330 and curcumol,331 bee venom,210,332,333 honey,334–337 propolis,338,339 resveratrol,340,341 aloe vera,342,343 and whey protein.344–346
Other common, although less thoroughly investigated, substances that have been shown to improve diabetic wound healing include nicotine,347 vitamin D,348 l-arginine,349 yeast extract,350 citrus peel extract,351 Annona squamosa (sugar apple),352,353 Rosmarinus (rosemary),354 and spinach extract.355 There are several animal-derived substances that have also demonstrated some therapeutic potential, including frog skin peptides,356 chum salmon skin gelatin,357 acellular fish skin,358,359 powdered shells of the snail Megalobulimus lopesi,360 and camel milk peptides,361 as well two funguses, Sparassis crispa (cauliflower mushroom),362,363 and proteins from Phellinus gilvus (mustard yellow polypore mushroom).364
The majority of the other substances studied are derived from plants, including aescin (from horse chestnut),365 Martynia annua (cat's claw),366 Lithospermum,367 Catharanthus roseus (periwinkle),368 Momordica charantia (bitter gourd/melon),369,370 pi per betel,371 the herbal medicine “Yi Bu A Jie”,372 Mallotus philippinensis (kamala tree),373 Cotinus coggygria (smoke tree),374 Stachys hissarica,375 Angelica dahurica,376 Moringa oleifera,377 Rehmanniae radix,378,379 Lycium depressum,380 Morinda citrifolia (noni) juice,381 Carica papaya,382 and Strychnos pseudoquina extract.383 It is of note that some of these substances, most especially honey, have long been incorporated into standard wound care products, such as honey dressings, bandages, and topical gels and creams, which have proven clinically successful for general wound care.384
Clinical trials
Although more and more potential therapies are being developed in small mammal models of DM, only a few go on to large animal testing, and even fewer still make it through clinical trials to achieve FDA approval. This creates a costly and disheartening stream of failed trials, marking the most critical bottleneck in the development of effective, practical treatments for DFUs.
Among the most notable downfalls are CureXcell, the macrophage-based topical therapy discussed previously, which failed its Phase III clinical trial in 2015174,177; Trafermin, also known as Fiblast, a recombinant human basic FGF spray for DFUs, which failed its Phase III clinical trial in 2014385,386; DermaPro, a topical diperoxochloric acid, which failed a phase III clinical trial in 2015387,388; Aclerastide, a gel containing the compound DSC127, for which the Phase III trial was terminated during recruitment due to demonstrated futility of the drug389–391; Locilex (pexiganan), a topical cream containing a 22-amino acid polypeptide to treat infected DFUs, which failed two Phase III clinical trials in 2016388,392,393; and Repifermin, a topical treatment containing KGF2, which failed a Phase II clinical trial in 2003.394,395
These highly publicized attempts represent only a small fraction of the total number of clinical trials for DFUs. As of early 2020, a search for “diabetic foot ulcer” trials in the ClinicalTrials.gov database reveals 63 Phase I trials, 105 Phase II trials, and 73 Phase III trials—a total of 196, of which only 22 have listed results.396 Of the 196 trials, 96 are listed as completed, 26 are listed as terminated, suspended, or withdrawn, 29 are in the prerecruitment, recruitment, or invitation phases, and only 7 are currently active (leaving the remaining 38 studies with status unknown).396 While the ongoing recruitment and active trials offer great promise for new treatments, these numbers also hint at the bigger picture: a massive amount of progress that often yields little to no clinically usable result by Phase III.
The factors driving this high rate of late stage attrition are not limited to the shortcomings of the animal models and the limited scope of research targets. Arguably one of the most critical hurdles is inherent to the clinical trials themselves: the simplicity of the trial cohort compared to the highly complex and diverse patient population. To avoid confounding variables and ensure reliable results, clinical trials must be designed with strict and specific inclusion and exclusion criteria. This aspect of study design, while essential for high-quality data, introduces its own dilemma: if the patient population does not match the study cohort, how generalizable are the results?
In the case of patients with DFUs, this is a common problem. Take Regranex, for example: in clinical trials, it was tested only in diabetic patients with neuropathic ulcers of a limited size that lacked any other complicating factors, such as vasculopathy or infection. The general population of patients with DFUs, however, includes large numbers of patients with peripheral vasculopathies, wound complications, and many additional comorbidities that could all reduce the effectiveness of any treatment. This added complexity likely explains the difference between Regranex's impressive success in clinical trials compared to its more modest Phase IV results.397
For therapies deemed ineffective in Phase III, this issue may simply be coming to light earlier on, when the increase in cohort size introduces enough variability to eliminate significant results. When considered alongside the limitations of preclinical research, the complexity of the patient population is of great consequence. While it would be entirely unfeasible to conduct clinical trials large and varied enough to fully account for patient diversity, it must be considered during trial design to maximize the impact of the study results.
Bench-to-bedside barrier
The individual research targets discussed here (summarized in Table 2) are only a small subset of the many hundreds of studies that have unveiled potential therapeutic targets for diabetic wound healing. Countless other types of advances exist in murine models of DM, including discoveries in wound care, drug repurposing, light-based therapies, novel application methods, genetic editing, retroviral studies, and much more. In total, there are significantly more potential advances than any one review can encompass. Yet, the clinical achievements derived from all of these discoveries can be easily summarized, highlighting the incredible bottleneck between the bench and the bedside.
Table 2.
Murine diabetic wound healing research targets
| Growth Factors | Cellular Therapies | Microvesicles and Exosomes From | Bio- and Bioengineered Materials | ||
|---|---|---|---|---|---|
| PDGF-BB | EGF | Stem/Progenitor cells (MSCs, adipose-derived cells, umbilical/cord blood stem cells, HSCs, CD34+ cells, EPCs) | Fibrocytes | Biogrown EPCs | LED light |
| FGF | IGF-1 | Stromal cells | EPCs | Electrospun fibers | Vacuum-assisted closure |
| VEGF | CTGF | Immune cells (macrophages, monocytes, B cells) | Platelet-rich plasma | Drug-eluting scaffolds | Negative pressure therapy |
| TGFα, TGFβ | HGF | Fibroblasts | MSCs | Hydrogels | DermaPACE |
| Keratinocytes | Adipose stem cells | Nanoparticles | Engineered dressings | ||
| Platelet-rich plasma, platelet gel | |||||
| Plant- and Animal-Derived Substances | Cytokines, Chemokines, and Signaling Molecules | MicroRNAs | ||||
|---|---|---|---|---|---|---|
| Curcumin |
Powdered snail shell (Megalobulimus lopesi) |
Carica papaya |
IL2 |
MMP-9 |
Nonadenine purines |
miR-129-2-3p |
| Curcumol |
Camel milk peptides |
Martynia annua (cat's claw) |
IL4 |
Oncostatin |
Fullerenes |
miR-132 |
| Bee venom |
Stachys hissarica |
Lithospermum |
IL6 |
Nesfatin-1 |
Hydrogen sulfide |
miR-129 |
| Honey |
Phellinus gilvus (mustard yellow polypore mushroom) |
Catharanthus roseus (periwinkle) |
IL22 |
Thyroid hormone |
β-Blockers |
miR-335 |
| Propolis |
Chum salmon skin gelatin |
Momordica charantia (bitter gourd/melon) |
TNFα, TNFβ |
Mineralocorticoid receptor agonists |
Anesthetics |
miR-27b |
| Piper betel |
Yeast extract |
“Yi Bu A Jie” (herbal medicine) |
CCL2 |
GnRH antagonists |
Metformin |
miR-146a |
| Resveratrol |
Citrus peel extract |
Mallotus philippinensis (kamala tree) |
EPO |
Inositol-requiring enzyme |
Sonic hedgehog |
miR-15b |
| Aloe vera |
Spinach extract |
Angelica dahurica |
Substance P |
Carnosine |
HoxD3 |
miR-21 |
| Whey protein |
Rosmarinus (rosemary) |
Sparassis crispa (caulifolower mushroom) |
Quadrol |
Insulin, C peptide |
DPP-4 inhibitors |
miR-155 inhibitors |
| Nicotine |
Annona squamosa (sugar apple) |
Cotinus coggygria (smoke tree) |
NRF2 |
Deferoxamine |
ND-336 |
miR-92a inhibitor (MRG-110) |
| Vitamin D |
Frog skin peptides |
Moringa oleifera |
Platelet releasate |
Sphingosine 1-phosphate |
Hydroxylase inhibitors |
miR-26a inhibitor (LNA-anti-miR-26a) |
|
l-Arginine |
Lycium depressum |
Rehmanniae radix |
||||
| Aescin | Morinda citrifolia (noni) juice | Strychnos pseudoquina | ||||
CTGF, connective tissue growth factor; EGF, epidermal growth factor; EPC, endothelial progenitor cell; EPO, erythropoietin; FGF, fibroblast growth factor; HGF, hepatocyte growth factor; HSC, hematopoietic stem cell; IGF-1, insulin-like growth factor 1; MMP-9, matrix metalloproteinase 9; MSC, mesenchymal stem cell; NRF2, nuclear factor erythroid 2-related factor 2; PDGF, platelet-derived growth factor; TGF, transforming growth factor; VEGF, vascular endothelial growth factor.
While the factors contributing to this limited progress are not fully understood, three noteworthy contributors stand out. The first is the relative insufficiency of the murine models of diabetic wound healing. Despite their variety, they all fall short of one of the most critical features of the diabetic wound: a chronic inability to heal. In the commonly studied murine models of DM, experimentally induced wounds do heal independently, although more slowly than their wild-type counterparts, and they do not develop chronic, nonhealing wounds without significant additional interventions such as induced infection or repetitive injury.
Furthermore, animal models of DM do not spontaneously develop ulcers. In every case, wounds must be created or induced to investigate their healing, meaning that (a) the wounds are acute, rather than chronic, and (b) they do not represent the insidious, involuntary, and often initially unnoticed development of ulcers seen in the diabetic patient. These caveats suggest that, fundamentally, the prominent animal models of DM do not sufficiently recapitulate the human condition for DFUs.61 Therefore, the many scientific discoveries that show immense promise in these models can easily fall short when translated to humans, likely because their contribution is enough to speed the healing of an acute, slow-healing wound but not enough to rescue one that remains chronically unable to heal.
The second notable contributor is the repeated pattern of investigating single factors in the diabetic wound. Of course, this is not to minimize the value of specific, mechanistic investigations. These studies have been invaluable in their contribution to the understanding of the wound environment and have uncovered critical details about the aberrant processes and inherent deficits in the diabetic wound. Furthermore, they have proven critical in enabling mechanistic insights and a more functional understanding of the pathogenesis of chronically nonhealing diabetic ulcers. However, single-factor studies have a major shortcoming in terms of clinical translation: insufficient power to correct the level of aberrancy in the human diabetic wound.
Despite their value at the basic science level, each individual small molecule, extract, or compound has a very narrow scope of effect. This level of specificity renders each factor highly informative relative to its own targets, but leaves much to be desired in the grand scheme of the wound environment. As a consequence, the effect size of each factor becomes relatively small and is almost always insufficient to rescue the severe healing impairment observed in human DFUs.
Finally, and relatedly, the third major contributor is the clinical diversity of the patient population. Because of the large of number of complications and comorbidities among patients with DM, clinical trial cohorts cannot perfectly represent the population and must rely on more specific subgroups to obtain scientifically valuable results. When therapies go on to reach larger patient populations, where those complicating factors are reintroduced, the efficacy of the new treatment is often greatly reduced.
When considered in combination, these major factors reveal an important paradigm regarding diabetic wound healing research: because the prominent models do not fully recapitulate the human condition, the majority of investigations center on single factors affecting healing, and study cohorts cannot replicate the diversity of the patient population, an insufficient research approach for the development of therapeutics for chronic diabetic wounds has been established.
To address this growing and unmet need, scientists must work to overcome these obstacles by expanding and adapting the research methodology. Novel approaches must be considered, including the development and validation of more specific and relevant model systems, expanded clinical trial populations, multifaceted research strategies that view the diabetic wound through a wider lens, and layered innovations into the management of diabetic wounds, such as combination therapeutics and preventative measures.
Of course, many of these efforts are not without their own challenges. Combination therapies in particular pose a unique set of problems: even with solid scientific foundations behind them, these potential treatments face hurdles in regulation and testing, higher costs, licensing and patent difficulties, and complex battles in garnering the interest and cooperation of the parties involved.398 In some cases, these challenges may prove insurmountable, but existing combination therapies for conditions such as HIV infection and cancer offer hope that they are not entirely impossible.
Cutting-edge advances across disciplines, including medicine, technology, and mathematics, must also be used to empower forward progress, such as the use of complex computational models, identification of new biomarkers, and investigation into the possibilities of personalized medicine for wound care. Promisingly, many innovations are already beginning to tackle these obstacles head-on, and the rapid expansion of these new research areas suggests a bright future for diabetic wound healing therapies.
Evidence of this progress is already beginning to improve diabetic wound care. Several recent multicenter randomized controlled trials investigating the effectiveness of Grafix, LeucoPatch, and sucrose octasulfate in improving diabetic wound healing have demonstrated positive results, prompting the inclusion of new recommendations in the International Working Group in Diabetic Foot's recent guidelines, published in 2020.399–402 These included several new interventions to improve healing of chronic DFUs, including the use of autologous leukocyte therapies, platelet and fibrin-based products, placental products, and sucrose octasulfate dressings.402
As these new guidelines are implemented, patients who previously had few options for care may finally receive new treatments that could prevent amputation, underscoring both the need for a variety of effective therapies and the significant benefits of the latest advances in diabetic wound care.
Summary
Chronic, nonhealing diabetic wounds are a common, high-risk complication of DM that can lead to serious impairments, life-threatening infections, and amputation. To date, there are few treatment options available for patients DFUs, and the majority of their management relies on treatments for DM and standard wound care. To address this clinical need, a wide array of research investigations have sought to identify, understand, and rescue the deficits in diabetic wound healing.
These studies have revealed hundreds of potential therapeutic targets, including cell types, growth factors, cytokines, small molecules, miRNAs, extracellular vesicles, plant- and animal-based substances, and more. Despite this breadth of research success, however, nearly all of these discoveries have failed to reach human patients.
Three major factors likely contribute to this scientific bottleneck. The first is the comparative insufficiency of the animal models of DM, which do not develop or heal wounds in the same manner as human patients; the second is the relatively narrow scope of diabetic wound healing research, which tends to focus on individual components in healing; and the third is the sheer complexity of the patient population. Together, these factors create an environment in which a large body of valuable scientific work yields a more detailed understanding of diabetic wound healing, but produces limited therapies to manage it.
To surmount these challenges, scientists must work to develop more accurate model systems, to further expand into multifactorial investigations, and to consider more multifaceted and compound approaches to diabetic wound care. Important examples of this much-needed progress are already rising to the forefront, including broader research targets, such as miRNAs, transcription factors, novel biomarkers, and bioengineered materials; improved experimental model organisms, such as pigs and other large animal models; and multidisciplinary advances, such as personalized medicine and complex computational modeling.
These efforts are testament to the vast amount of progress ahead and demonstrate invaluable steps toward novel therapeutic development. Although ongoing obstacles are inevitable, scientists continue to evolve in pursuit of innovative therapies to address the critical needs of patients with chronic wounds. As the new frontiers of wound healing research continue to expand, so too will their resulting successes and, of course, the countless patients they will serve.
Take-Home Messages
Chronic, nonhealing wounds are a serious complication of DM and lead to thousands of deaths and amputations annually.
To date, there are four biologically active FDA-approved treatments for diabetic wounds: one drug, Regranex; two human cell devices, Dermagraft and Apligraf; and one animal-derived device, Omnigraft.
The significant disadvantages and caveats to the available therapies and a lack of novel therapeutic development leave the vast majority of diabetic wound patients with limited treatment options.
Significant research demonstrates the experimental effectiveness of a wide variety of factors in improving diabetic wound healing in murine models. These prominent models elucidate many viable, informative research targets, but do not sufficiently replicate the human condition.
The majority of experimental discoveries rely on single factors, highlighting a potential shortcoming in the most common investigational approach.
Despite the vast number of research advances, little progress has been made in successfully translating potential therapeutics into the clinic. Those that make it to clinical trials often fail in Phase III, likely due to the complexity and diversity of the patient population.
The most commonly used models and approaches for the investigation of diabetic wound healing are largely insufficient for the development of novel therapeutics. To overcome these barriers, scientists must consider more innovative tools and approaches for diabetic wound healing research.
Abbreviations and Acronyms
- CTGF
connective tissue growth factor
- DFU
diabetic foot ulcer
- DM
diabetes mellitus
- EGF
epidermal growth factor
- EPC
endothelial progenitor cell
- EPO
erythropoietin
- FDA
Food and Drug Administration
- FGF
fibroblast growth factor
- HFD
high-fat diet
- HGF
hepatocyte growth factor
- HSC
hematopoietic stem cell
- HSE
human skin equivalent/substitute
- IGF-1
insulin-like growth factor 1
- KGF
keratinocyte growth factor
- miR
microRNA (specific)
- miRNA
microRNA
- MMP
matrix metalloproteinase
- MSC
mesenchymal stem cell
- NGF
nerve growth factor
- NIH
National Institutes of Health
- NOD
nonobese diabetic
- NRF2
nuclear factor erythroid 2-related factor 2, often written as NFE2L2
- NZO
New Zealand Obese
- OLETF
Otsuka Long-Evans Tokushima Fatty
- PAD
peripheral arterial disease
- PDGF
platelet-derived growth factor
- PR
platelet releasate
- TGF
transforming growth factor
- VEGF
vascular endothelial growth factor
- ZDF
Zucker diabetic fatty
Acknowledgments and Funding Sources
The authors thank the other members of the DiPietro laboratory: Junhe Shi, Anna Salapatas, Trevor Leonardo, Dr. Bruna Romano De Souza, and Dr. Wendy Cerny for their assistance during the revision of this work. They also extend special thanks to Dr. Stephanie Wu, for generously providing the clinical photographs for Fig. 1, and Dr. Veronica Haywood, for her valuable feedback. Funding is provided by NIH grants R01-GM-50875 and R01-AR069541-01A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used.
About the Authors
May Barakat is an MD/PhD candidate in the laboratory of Dr. Luisa A. DiPietro. Her research interests are in diabetic wound healing and tissue repair and regeneration. Dr. Luisa A. DiPietro is a professor of Periodontics and the Director of the Center for Wound Repair and Regeneration at the University of Illinois at Chicago College of Dentistry. Her laboratory investigates the process of wound repair, including inflammation, angiogenesis, scar formation, mucosal and cutaneous tissue repair, and wound healing outcomes in abnormal states such as DM or keloid scars. Dr. Lin Chen is a Research Associate Professor in the Center for Wound Repair and Regeneration at the University of Illinois at Chicago College of Dentistry, whose research focuses on mucosal and cutaneous wound healing, angiogenesis, and immune response in wound repair.
References
- 1. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med 2017;376:2367–2375 [DOI] [PubMed] [Google Scholar]
- 2. Baltzis D, Eleftheriadou I, Veves A. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: new insights. Adv Ther 2014;31:817–836 [DOI] [PubMed] [Google Scholar]
- 3. WHO. Global Report on Diabetes. Geneva, Switzerland: World Health Organization, 2016 [Google Scholar]
- 4. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services, 2017 [Google Scholar]
- 5. McCulloch DK, Robertson P. Pathogenesis of Type 2 Diabetes Mellitus. Waltham, MA: UpToDate, 2019 [Google Scholar]
- 6. Amin N, Doupis J. Diabetic foot disease: from the evaluation of the “foot at risk” to the novel diabetic ulcer treatment modalities. World J Diabetes 2016;7:153–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 2005;366:1736–1743 [DOI] [PubMed] [Google Scholar]
- 8. Sen CK. Human wounds and its burden: an updated compendium of estimates. Adv Wound Care 2019;8:39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res 2010;89:219–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Okonkwo UA, DiPietro LA. Diabetes and wound angiogenesis. Int J Mol Sci 2017;18:1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Armstrong DG, McCulloch DK, de Asla RJ. Management of Diabetic Foot Ulcers. Waltham, MA: UpToDate, 2019 [Google Scholar]
- 12. Smith & Nephew, Inc. REGRANEX (becaplermin) gel. 2019. https://regranex.com (last accessed August2020)
- 13. FDA. Regranex (Drugs@FDA: FDA-Approved Drugs). Drugs@FDA. 1997. 2020. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=103691 (last accessed September2020)
- 14. Balfour JA, Noble S. Becaplermin. BioDrugs 1999;11:359–364 [DOI] [PubMed] [Google Scholar]
- 15. FDA. Regranex Label. FDA.gov Silver Spring, MD: U.S. Food and Drug Administration, 2008
- 16. Papanas N, Maltezos E. Becaplermin gel in the treatment of diabetic neuropathic foot ulcers. Clin Interv Aging 2008;3:233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marston WA, Hanft J, Norwood P, Pollak R; Dermagraft Diabetic Foot Ulcer Study Group. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 2003;26:1701–1705 [DOI] [PubMed] [Google Scholar]
- 18. FDA. PMA: Dermagraft. 2001. 2020. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=p000036 (last accessed February2020)
- 19. Organogenesis. Dermagraft: The Difference is Dermagraft. 2019. Dermagraft Website. http://dermagraft.com (last accessed October2019)
- 20. Brem H, Balledux J, Bloom T, Kerstein MD, Hollier L. Healing of diabetic foot ulcers and pressure ulcers with human skin equivalent: a new paradigm in wound healing. Arch Surg 2000;135:627–634 [DOI] [PubMed] [Google Scholar]
- 21. Novartis. Apligraf: Add Life to Healing. 2019; Apligraf website. www.apligraf.com/professional (last accessed November2019)
- 22. Falanga V, Sabolinski M. A bilayered living skin construct (APLIGRAF®) accelerates complete closure of hard-to-heal venous ulcers. Wound Repair Regen 1999;7:201–207 [DOI] [PubMed] [Google Scholar]
- 23. FDA. PMA: Apligraf (Graftskin). 1998; Original PMA for Apligraf. 2020. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P950032 (last accessed February2020)
- 24. Streit M, Braathen L. Apligraf–a living human skin equivalent for the treatment of chronic wounds. Int J Artif Organs 2000;23:831–833 [PubMed] [Google Scholar]
- 25. Integra. Omnigraft: Dermal Regeneration Matrix. 2016; 2020. Omnigraft Website. http://omnigraft.com/about-omnigraft.php (last accessed February2020)
- 26. FDA. PMA Supplement: Integra Dermal Regeneration Template, Omnigraft. 2016. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P900033S042 (last accessed January2020)
- 27. FDA. FDA Approves Integra Omnigraft Dermal Regeneration Matrix to Treat Diabetic Foot Ulcers. FDA.gov: Silver Spring, MD: U.S. Food and Drug Administration, 2016 [Google Scholar]
- 28. Powers JG, Higham C, Broussard K, Phillips TJ. Wound healing and treating wounds: chronic wound care and management. J Am Acad Dermatol 2016;74:607–625; quiz 625-606. [DOI] [PubMed] [Google Scholar]
- 29. Greer N, Foman NA, MacDonald R, et al. Advanced wound care therapies for nonhealing diabetic, venous, and arterial ulcers: a systematic review. Ann Intern Med 2013;159:532–542 [DOI] [PubMed] [Google Scholar]
- 30. Boateng JS, Matthews KH, Stevens HN, Eccleston GM. Wound healing dressings and drug delivery systems: a review. J Pharm Sci 2008;97:2892–2923 [DOI] [PubMed] [Google Scholar]
- 31. Dhivya S, Padma VV, Santhini E. Wound dressings—a review. Biomedicine (Taipei) 2015;5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murphy PS, Evans GR. Advances in wound healing: a review of current wound healing products. Plast Surg Int 2012;2012:190436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. FDA. Classification of Products as Drugs and Devices and Additional Issues. Silver Spring, MD: U.S. Food and Drug Administration, 2018 [Google Scholar]
- 34. Morgan S, Grootendorst P, Lexchin J, Cunningham C, Greyson D. The cost of drug development: a systematic review. Health Policy 2011;100:4–17 [DOI] [PubMed] [Google Scholar]
- 35. Karamanou M, Protogerou A, Tsoucalas G, Androutsos G, Poulakou-Rebelakou E. Milestones in the history of diabetes mellitus: the main contributors. World J Diabetes 2016;7:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Keck FS, Duntas LH. Brunner's missing ‘Aha experience’ delayed progress in diabetes research by 200 years. Hormones (Athens) 2007;6:251–254 [PubMed] [Google Scholar]
- 37. King A, Bowe J. Animal models for diabetes: understanding the pathogenesis and finding new treatments. Biochem Pharmacol 2016;99:1–10 [DOI] [PubMed] [Google Scholar]
- 38. Wu KK, Huan Y. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol 2008;Chapter 5:Unit 5.47. [DOI] [PubMed] [Google Scholar]
- 39. Huynh P, Phie J, Krishna SM, Golledge J. Systematic review and meta-analysis of mouse models of diabetes-associated ulcers. BMJ Open Diabetes Res Care 2020;8:e000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Radenkovic M, Stojanovic M, Prostran M. Experimental diabetes induced by alloxan and streptozotocin: the current state of the art. J Pharmacol Toxicol Methods 2016;78:13–31 [DOI] [PubMed] [Google Scholar]
- 41. Mullen Y. Development of the nonobese diabetic mouse and contribution of animal models for understanding type 1 diabetes. Pancreas 2017;46:455–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yeadon J. Choosing Among Type II Diabetes Mouse Models. Bar Harbor, Maine: The Jackson Laboratory, 2015 [Google Scholar]
- 43. Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes 1988;37:1163–1167 [DOI] [PubMed] [Google Scholar]
- 44. Wang C-Y, Liao JK. A mouse model of diet-induced obesity and insulin resistance. Methods Mol Biol 2012;821:421–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Heydemann A. An Overview of Murine High Fat Diet as a Model for Type 2 Diabetes Mellitus. J Diabetes Res 2016;2016:2902351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Db/Db Mouse. Strain 000642—BKS.Cg-Dock7m +/+ Leprdb/J. Bar Harbor, Maine: The Jackson Lab, 2020 [Google Scholar]
- 47. Ob/Ob Mouse. Strain 000632—B6.Cg-Lepob/J. Bar Harbor, Maine: The Jackson Lab, 2020 [Google Scholar]
- 48. Shiota M, Printz RL. Diabetes in Zucker diabetic fatty rat. Methods Mol Biol 2012;933:103–123 [DOI] [PubMed] [Google Scholar]
- 49. Kim JH, Saxton AM. The TALLYHO mouse as a model of human type 2 diabetes. In: Joost H-G, Al-Hasani H, Schürmann A, eds. Animal Models in Diabetes Research. Totowa, NJ: Humana Press, 2012:75–87 [DOI] [PubMed] [Google Scholar]
- 50. Shiota M, Printz RL. Diabetes in Zucker diabetic fatty rat. In: Joost H-G, Al-Hasani H, Schürmann A, eds. Animal Models in Diabetes Research. Totowa, NJ: Humana Press, 2012:103–123 [DOI] [PubMed] [Google Scholar]
- 51. Kawano K, Hirashima T, Mori S, Natori T. OLETF (Otsuka Long-Evans Tokushima Fatty) rat: a new NIDDM rat strain. Diabetes Res Clin Pract 1994;24 Suppl:S317–S320 [DOI] [PubMed] [Google Scholar]
- 52. Joost H-G. The genetic basis of obesity and type 2 diabetes: lessons from the New Zealand obese mouse, a polygenic model of the metabolic syndrome. In: Meyerhof W, Beisiegel U, Joost H-G, eds. Sensory and Metabolic Control of Energy Balance. Berlin, Heidelberg: Springer Berlin Heidelberg, 2010:1–11 [DOI] [PubMed] [Google Scholar]
- 53. NZO Mice. Strain 002105—NZO/HlLtJ. Bar Harbor, Maine: The Jackson Lab, 2020 [Google Scholar]
- 54. Slavkovsky R, Kohlerova R, Tkacova V, et al. Zucker diabetic fatty rat: a new model of impaired cutaneous wound repair with type II diabetes mellitus and obesity. Wound Repair Regen 2011;19:515–525 [DOI] [PubMed] [Google Scholar]
- 55. Nagai N, Murao T, Ito Y, Okamoto N, Sasaki M. Enhancing effects of sericin on corneal wound healing in Otsuka Long-Evans Tokushima fatty rats as a model of human type 2 diabetes. Biol Pharm Bull 2009;32:1594–1599 [DOI] [PubMed] [Google Scholar]
- 56. King AJ. The use of animal models in diabetes research. Br J Pharmacol 2012;166:877–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Buck DW, 2nd, Jin DP, Geringer M, Hong SJ, Galiano RD, Mustoe TA. The TallyHo polygenic mouse model of diabetes: implications in wound healing. Plast Reconstr Surg 2011;128:427e–437e [DOI] [PubMed] [Google Scholar]
- 58. Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen 2001;9:66–76 [DOI] [PubMed] [Google Scholar]
- 59. Renner S, Dobenecker B, Blutke A, et al. Comparative aspects of rodent and nonrodent animal models for mechanistic and translational diabetes research. Theriogenology 2016;86:406–421 [DOI] [PubMed] [Google Scholar]
- 60. Kleinert M, Clemmensen C, Hofmann SM, et al. Animal models of obesity and diabetes mellitus. Nat Rev Endocrinol 2018;14:140–162 [DOI] [PubMed] [Google Scholar]
- 61. Gordillo GM, Bernatchez SF, Diegelmann R, et al. Preclinical models of wound healing: is man the model? Proceedings of the Wound Healing Society Symposium. Adv Wound Care 2013;2:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. DiPietro LA, Burns AL. Wound Healing: Methods and Protocols, vol. 78. Totowa, NJ: Springer Humana Press, 2003 [Google Scholar]
- 63. Ud-Din S, Bayat A. Non-animal models of wound healing in cutaneous repair: in silico, in vitro, ex vivo, and in vivo models of wounds and scars in human skin. Wound Repair Regen 2017;25:164–176 [DOI] [PubMed] [Google Scholar]
- 64. Branski LK, Gauglitz GG, Herndon DN, Jeschke MG. A review of gene and stem cell therapy in cutaneous wound healing. Burns 2009;35:171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Moura LI, Dias AM, Carvalho E, de Sousa HC. Recent advances on the development of wound dressings for diabetic foot ulcer treatment—a review. Acta Biomater 2013;9:7093–7114 [DOI] [PubMed] [Google Scholar]
- 66. Kasiewicz LN, Whitehead KA. Recent advances in biomaterials for the treatment of diabetic foot ulcers. Biomater Sci 2017;5:1962–1975 [DOI] [PubMed] [Google Scholar]
- 67. Andrews KL, Houdek MT, Kiemele LJ. Wound management of chronic diabetic foot ulcers: from the basics to regenerative medicine. Prosthet Orthot Int 2015;39:29–39 [DOI] [PubMed] [Google Scholar]
- 68. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen 2008;16:585–601 [DOI] [PubMed] [Google Scholar]
- 69. Steed DL. The role of growth factors in wound healing. Surg Clin North Am 1997;77:575–586 [DOI] [PubMed] [Google Scholar]
- 70. Barrientos S, Brem H, Stojadinovic O, Tomic-Canic M. Clinical application of growth factors and cytokines in wound healing. Wound Rep Regen 2014;22:569–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Greenhalgh DG, Sprugel KH, Murray MJ, Ross R. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol 1990;136:1235–1246 [PMC free article] [PubMed] [Google Scholar]
- 72. Brown RL, Breeden MP, Greenhalgh DG. PDGF and TGF-alpha act synergistically to improve wound healing in the genetically diabetic mouse. J Surg Res 1994;56:562–570 [DOI] [PubMed] [Google Scholar]
- 73. Margolis DJ, Crombleholme T, Herlyn M. Clinical protocol: phase I trial to evaluate the safety of H5.020CMV.PDGF-B for the treatment of a diabetic insensate foot ulcer. Wound Rep Rege 2000;8:480–493 [DOI] [PubMed] [Google Scholar]
- 74. Steed DL. Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity diabetic ulcers. Diabetic Ulcer Study Group. J Vasc Surg 1995;21:71–78; discussion 79–81. [DOI] [PubMed] [Google Scholar]
- 75. Steed DL. Becaplermin: a viewpoint by David L. Steed. BioDrugs 1999;11:366. [DOI] [PubMed] [Google Scholar]
- 76. Steed DL. Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity ulcers. Plast Reconstr Surg 2006;117:143S–149S; discussion 150S–151S. [DOI] [PubMed] [Google Scholar]
- 77. Chan RK, Liu PH, Pietramaggiori G, Ibrahim SI, Hechtman HB, Orgill DP. Effect of recombinant platelet-derived growth factor (Regranex) on wound closure in genetically diabetic mice. J Burn Care Res 2006;27:202–205 [DOI] [PubMed] [Google Scholar]
- 78. Albertson S, Hummel RP, 3rd, Breeden M, Greenhalgh DG. PDGF and FGF reverse the healing impairment in protein-malnourished diabetic mice. Surgery 1993;114:368–372; discussion 372–363. [PubMed] [Google Scholar]
- 79. Shi R, Lian W, Han S, et al. Nanosphere-mediated co-delivery of VEGF-A and PDGF-B genes for accelerating diabetic foot ulcers healing in rats. Gene Ther 2018;25:425–438 [DOI] [PubMed] [Google Scholar]
- 80. Tsuboi R, Rifkin DB. Recombinant basic fibroblast growth factor stimulates wound healing in healing-impaired db/db mice. J Exp Med 1990;172:245–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Klingbeil CK, Cesar LB, Fiddes JC. Basic fibroblast growth factor accelerates tissue repair in models of impaired wound healing. Progr Clin Biol Res 1991;365:443–458 [PubMed] [Google Scholar]
- 82. Mellin TN, Cashen DE, Ronan JJ, Murphy BS, DiSalvo J, Thomas KA. Acidic fibroblast growth factor accelerates dermal wound healing in diabetic mice. J Invest Dermatol 1995;104:850–855 [DOI] [PubMed] [Google Scholar]
- 83. Okumura M, Okuda T, Nakamura T, Yajima M. Effect of basic fibroblast growth factor on wound healing in healing-impaired animal models. Arzneimittelforschung 1996;46:547–551 [PubMed] [Google Scholar]
- 84. Okumura M, Okuda T, Nakamura T, Yajima M. Acceleration of wound healing in diabetic mice by basic fibroblast growth factor. Biol Pharm Bull 1996;19:530–535 [DOI] [PubMed] [Google Scholar]
- 85. Okumura M, Okuda T, Okamoto T, Nakamura T, Yajima M. Enhanced angiogenesis and granulation tissue formation by basic fibroblast growth factor in healing-impaired animals. Arzneimittelforschung 1996;46:1021–1026 [PubMed] [Google Scholar]
- 86. Jazwa A, Kucharzewska P, Leja J, et al. Combined vascular endothelial growth factor-A and fibroblast growth factor 4 gene transfer improves wound healing in diabetic mice. Genet Vaccines Ther 2010;8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Huang Z, Lu M, Zhu G, et al. Acceleration of diabetic-wound healing with PEGylated rhaFGF in healing-impaired streptozocin diabetic rats. Wound Repair Regen 2011;19:633–644 [DOI] [PubMed] [Google Scholar]
- 88. Yang Y, Xia T, Chen F, et al. Electrospun fibers with plasmid bFGF polyplex loadings promote skin wound healing in diabetic rats. Mol Pharm 2012;9:48–58 [DOI] [PubMed] [Google Scholar]
- 89. Losi P, Briganti E, Errico C, et al. Fibrin-based scaffold incorporating VEGF- and bFGF-loaded nanoparticles stimulates wound healing in diabetic mice. Acta Biomater 2013;9:7814–7821 [DOI] [PubMed] [Google Scholar]
- 90. Blaber SI, Diaz J, Blaber M. Accelerated healing in NONcNZO10/LtJ type 2 diabetic mice by FGF-1. Wound Repair Regen 2015;23:538–549 [DOI] [PubMed] [Google Scholar]
- 91. Choi SM, Lee KM, Kim HJ, et al. Effects of structurally stabilized EGF and bFGF on wound healing in type I and type II diabetic mice. Acta Biomater 2018;66:325–334 [DOI] [PubMed] [Google Scholar]
- 92. Galiano RD, Tepper OM, Pelo CR, et al. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol 2004;164:1935–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rico T, Green J, Kirsner RS. Vascular endothelial growth factor delivery via gene therapy for diabetic wounds: first steps. J Invest Dermatol 2009;129:2084. [DOI] [PubMed] [Google Scholar]
- 94. Yan X, Chen B, Lin Y, et al. Acceleration of diabetic wound healing by collagen-binding vascular endothelial growth factor in diabetic rat model. Diabetes Res Clin Pract 2010;90:66–72 [DOI] [PubMed] [Google Scholar]
- 95. Ko J, Jun H, Chung H, et al. Comparison of EGF with VEGF non-viral gene therapy for cutaneous wound healing of streptozotocin diabetic mice. Diabetes Metab J 2011;35:226–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tan Q, Chen B, Yan X, et al. Promotion of diabetic wound healing by collagen scaffold with collagen-binding vascular endothelial growth factor in a diabetic rat model. J Tissue Eng Regen Med 2014;8:195–201 [DOI] [PubMed] [Google Scholar]
- 97. Marti G, Ferguson M, Wang J, et al. Electroporative transfection with KGF-1 DNA improves wound healing in a diabetic mouse model. Gene Ther 2004;11:1780–1785 [DOI] [PubMed] [Google Scholar]
- 98. Marti GP, Mohebi P, Liu L, Wang J, Miyashita T, Harmon JW. KGF-1 for wound healing in animal models. Methods Mol Biol 2008;423:383–391 [DOI] [PubMed] [Google Scholar]
- 99. Peng Y, Wu S, Tang Q, Li S, Peng C. KGF-1 accelerates wound contraction through the TGF-beta1/Smad signaling pathway in a double-paracrine manner. J Biol Chem 2019;294:8361–8370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Choi JK, Jang JH, Jang WH, et al. The effect of epidermal growth factor (EGF) conjugated with low-molecular-weight protamine (LMWP) on wound healing of the skin. Biomaterials 2012;33:8579–8590 [DOI] [PubMed] [Google Scholar]
- 101. Choi JS, Leong KW, Yoo HS. In vivo wound healing of diabetic ulcers using electrospun nanofibers immobilized with human epidermal growth factor (EGF). Biomaterials 2008;29:587–596 [DOI] [PubMed] [Google Scholar]
- 102. Dogan S, Demirer S, Kepenekci I, et al. Epidermal growth factor-containing wound closure enhances wound healing in non-diabetic and diabetic rats. Int Wound J 2009;6:107–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hennessey PJ, Black CT, Andrassy RJ. Growth factors and diabetic wound healing: epidermal growth factor and insulin. Curr Surg 1989;46:285–286 [PubMed] [Google Scholar]
- 104. Tsang MW, Wong WK, Hung CS, et al. Human epidermal growth factor enhances healing of diabetic foot ulcers. Diabetes Care 2003;26:1856–1861 [DOI] [PubMed] [Google Scholar]
- 105. Hong JP, Park SW. The combined effect of recombinant human epidermal growth factor and erythropoietin on full-thickness wound healing in diabetic rat model. Int Wound J 2014;11:373–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Benn SI, Whitsitt JS, Broadley KN, et al. Particle-mediated gene transfer with transforming growth factor-beta1 cDNAs enhances wound repair in rat skin. J Clin Invest 1996;98:2894–2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bitar MS, Labbad ZN. Transforming growth factor-beta and insulin-like growth factor-I in relation to diabetes-induced impairment of wound healing. J Surg Res 1996;61:113–119 [DOI] [PubMed] [Google Scholar]
- 108. Broadley KN, Aquino AM, Hicks B, et al. The diabetic rat as an impaired wound healing model: stimulatory effects of transforming growth factor-beta and basic fibroblast growth factor. Biotechnol Ther 1989;1:55–68 [PubMed] [Google Scholar]
- 109. Chesnoy S, Lee PY, Huang L. Intradermal injection of transforming growth factor-beta1 gene enhances wound healing in genetically diabetic mice. Pharm Res 2003;20:345–350 [DOI] [PubMed] [Google Scholar]
- 110. Lee PY, Chesnoy S, Huang L. Electroporatic delivery of TGF-beta1 gene works synergistically with electric therapy to enhance diabetic wound healing in db/db mice. J Invest Dermatol 2004;123:791–798 [DOI] [PubMed] [Google Scholar]
- 111. Tsuboi R, Shi CM, Sato C, Cox GN, Ogawa H. Co-administration of insulin-like growth factor (IGF)-I and IGF-binding protein-1 stimulates wound healing in animal models. J Invest Dermatol 1995;104:199–203 [DOI] [PubMed] [Google Scholar]
- 112. Aghdam SY, Eming SA, Willenborg S, et al. Vascular endothelial insulin/IGF-1 signaling controls skin wound vascularization. Biochem Biophys Res Commun 2012;421:197–202 [DOI] [PubMed] [Google Scholar]
- 113. Bitar MS. Insulin-like growth factor-1 reverses diabetes-induced wound healing impairment in rats. Horm Metab Res 1997;29:383–386 [DOI] [PubMed] [Google Scholar]
- 114. Achar RA, Silva TC, Achar E, Martines RB, Machado JL. Use of insulin-like growth factor in the healing of open wounds in diabetic and non-diabetic rats. Acta Cir Bras 2014;29:125–131 [DOI] [PubMed] [Google Scholar]
- 115. Thaller SR, Lee TJ, Armstrong M, Tesluk H, Stern JS. Effect of insulin-like growth factor type 1 on critical-size defects in diabetic rats. J Craniofac Surg 1995;6:218–223 [DOI] [PubMed] [Google Scholar]
- 116. Henshaw FR, Boughton P, Lo L, McLennan SV, Twigg SM. Topically applied connective tissue growth factor/CCN2 improves diabetic preclinical cutaneous wound healing: potential role for CTGF in human diabetic foot ulcer healing. J Diabetes Res 2015;2015:236238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Li JF, Duan HF, Wu CT, et al. HGF accelerates wound healing by promoting the dedifferentiation of epidermal cells through beta1-integrin/ILK pathway. Biomed Res Int 2013;2013:470418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Yoshida S, Matsumoto K, Tomioka D, et al. Recombinant hepatocyte growth factor accelerates cutaneous wound healing in a diabetic mouse model. Growth Factors 2004;22:111–119 [DOI] [PubMed] [Google Scholar]
- 119. Matsuda H, Koyama H, Sato H, et al. Role of nerve growth factor in cutaneous wound healing: accelerating effects in normal and healing-impaired diabetic mice. J Exp Med 1998;187:297–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Muangman P, Muffley LA, Anthony JP, et al. Nerve growth factor accelerates wound healing in diabetic mice. Wound Repair Regen 2004;12:44–52 [DOI] [PubMed] [Google Scholar]
- 121. Falanga V, Iwamoto S, Chartier M, et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng 2007;13:1299–1312 [DOI] [PubMed] [Google Scholar]
- 122. Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007;25:2648–2659 [DOI] [PubMed] [Google Scholar]
- 123. Tark KC, Hong JW, Kim YS, Hahn SB, Lee WJ, Lew DH. Effects of human cord blood mesenchymal stem cells on cutaneous wound healing in leprdb mice. Ann Plast Surg 2010;65:565–572 [DOI] [PubMed] [Google Scholar]
- 124. Kuo YR, Wang CT, Cheng JT, Wang FS, Chiang YC, Wang CJ. Bone marrow-derived mesenchymal stem cells enhanced diabetic wound healing through recruitment of tissue regeneration in a rat model of streptozotocin-induced diabetes. Plast Reconstr Surg 2011;128:872–880 [DOI] [PubMed] [Google Scholar]
- 125. Maharlooei MK, Bagheri M, Solhjou Z, et al. Adipose tissue derived mesenchymal stem cell (AD-MSC) promotes skin wound healing in diabetic rats. Diabetes Res Clin Pract 2011;93:228–234 [DOI] [PubMed] [Google Scholar]
- 126. Argolo Neto NM, Del Carlo RJ, Monteiro BS, et al. Role of autologous mesenchymal stem cells associated with platelet-rich plasma on healing of cutaneous wounds in diabetic mice. Clin Exp Dermatol 2012;37:544–553 [DOI] [PubMed] [Google Scholar]
- 127. Kim SW, Zhang HZ, Guo L, Kim JM, Kim MH. Amniotic mesenchymal stem cells enhance wound healing in diabetic NOD/SCID mice through high angiogenic and engraftment capabilities. PLoS One 2012;7:e41105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kong P, Xie X, Li F, Liu Y, Lu Y. Placenta mesenchymal stem cell accelerates wound healing by enhancing angiogenesis in diabetic Goto-Kakizaki (GK) rats. Biochem Biophys Res Commun 2013;438:410–419 [DOI] [PubMed] [Google Scholar]
- 129. Guo J, Hu H, Gorecka J, et al. Adipose-derived mesenchymal stem cells accelerate diabetic wound healing in a similar fashion as bone marrow-derived cells. Am J Physiol Cell Physiol 2018;315:C885–C896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Lu H, Wu X, Wang Z, et al. Erythropoietin-activated mesenchymal stem cells promote healing ulcers by improving microenvironment. J Surg Res 2016;205:464–473 [DOI] [PubMed] [Google Scholar]
- 131. Kim EK, Li G, Lee TJ, Hong JP. The effect of human adipose-derived stem cells on healing of ischemic wounds in a diabetic nude mouse model. Plast Reconstr Surg 2011;128:387–394 [DOI] [PubMed] [Google Scholar]
- 132. Nie C, Yang D, Xu J, Si Z, Jin X, Zhang J. Locally administered adipose-derived stem cells accelerate wound healing through differentiation and vasculogenesis. Cell Transplant 2011;20:205–216 [DOI] [PubMed] [Google Scholar]
- 133. Gu JH, Lee JS, Kim DW, Yoon ES, Dhong ES. Neovascular potential of adipose-derived stromal cells (ASCs) from diabetic patients. Wound Repair Regen 2012;20:243–252 [DOI] [PubMed] [Google Scholar]
- 134. Shen T, Pan ZG, Zhou X, Hong CY. Accelerated healing of diabetic wound using artificial dermis constructed with adipose stem cells and poly (L-glutamic acid)/chitosan scaffold. Chin Med J (Engl) 2013;126:1498–1503 [PubMed] [Google Scholar]
- 135. Zografou A, Papadopoulos O, Tsigris C, et al. Autologous transplantation of adipose-derived stem cells enhances skin graft survival and wound healing in diabetic rats. Ann Plast Surg 2013;71:225–232 [DOI] [PubMed] [Google Scholar]
- 136. Sumi Y, Ishihara M, Kishimoto S, et al. Effective wound healing in streptozotocin-induced diabetic rats by adipose-derived stromal cell transplantation in plasma-gel containing fragmin/protamine microparticles. Ann Plast Surg 2014;72:113–120 [DOI] [PubMed] [Google Scholar]
- 137. Ammar HI, Sequiera GL, Nashed MB, et al. Comparison of adipose tissue- and bone marrow-derived mesenchymal stem cells for alleviating doxorubicin-induced cardiac dysfunction in diabetic rats. Stem Cell Res Ther 2015;6:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Cronk SM, Kelly-Goss MR, Ray HC, et al. Adipose-derived stem cells from diabetic mice show impaired vascular stabilization in a murine model of diabetic retinopathy. Stem Cells Transl Med 2015;4:459–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kuo YR, Wang CT, Cheng JT, Kao GS, Chiang YC, Wang CJ. Adipose-derived stem cells accelerate diabetic wound healing through the induction of autocrine and paracrine effects. Cell Transplant 2016;25:71–81 [DOI] [PubMed] [Google Scholar]
- 140. Li J, Pincu Y, Marjanovic M, et al. In vivo evaluation of adipose- and muscle-derived stem cells as a treatment for nonhealing diabetic wounds using multimodal microscopy. J Biomed Opt 2016;21:86006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Kaisang L, Siyu W, Lijun F, Daoyan P, Xian CJ, Jie S. Adipose-derived stem cells seeded in Pluronic F-127 hydrogel promotes diabetic wound healing. J Surg Res 2017;217:63–74 [DOI] [PubMed] [Google Scholar]
- 142. Na YK, Ban JJ, Lee M, Im W, Kim M. Wound healing potential of adipose tissue stem cell extract. Biochem Biophys Res Commun 2017;485:30–34 [DOI] [PubMed] [Google Scholar]
- 143. Chotinantakul K, Dechsukhum C, Dejjuy D, Leeanansaksiri W. Enhancement of wound closure in diabetic mice by ex vivo expanded cord blood CD34+ cells. Cell Mol Biol Lett 2013;18:263–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Cil N, Oguz EO, Mete E, Cetinkaya A, Mete GA. Effects of umbilical cord blood stem cells on healing factors for diabetic foot injuries. Biotech Histochem 2017;92:15–28 [DOI] [PubMed] [Google Scholar]
- 145. Han Y, Sun T, Han Y, et al. Human umbilical cord mesenchymal stem cells implantation accelerates cutaneous wound healing in diabetic rats via the Wnt signaling pathway. Eur J Med Res 2019;24:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Harris DT, Rogers I. Umbilical cord blood: a unique source of pluripotent stem cells for regenerative medicine. Curr Stem Cell Res Ther 2007;2:301–309 [DOI] [PubMed] [Google Scholar]
- 147. Kim JY, Song SH, Kim KL, et al. Human cord blood-derived endothelial progenitor cells and their conditioned media exhibit therapeutic equivalence for diabetic wound healing. Cell Transplant 2010;19:1635–1644 [DOI] [PubMed] [Google Scholar]
- 148. Moon KC, Lee JS, Han SK, Lee HW, Dhong ES. Effects of human umbilical cord blood-derived mesenchymal stromal cells and dermal fibroblasts on diabetic wound healing. Cytotherapy 2017;19:821–828 [DOI] [PubMed] [Google Scholar]
- 149. Shrestha C, Zhao L, Chen K, He H, Mo Z. Enhanced healing of diabetic wounds by subcutaneous administration of human umbilical cord derived stem cells and their conditioned media. Int J Endocrinol 2013;2013:592454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Whiteley J, Chow T, Adissu H, Keating A, Rogers IM. Topical application of culture-expanded CD34+ umbilical cord blood cells from frozen units accelerates healing of diabetic skin wounds in mice. Stem Cells Transl Med 2018;7:591–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Lee KB, Choi J, Cho SB, et al. Topical embryonic stem cells enhance wound healing in diabetic rats. J Orthop Res 2011;29:1554–1562 [DOI] [PubMed] [Google Scholar]
- 152. Qian de J, Guo XK, Duan HC, et al. An application of embryonic skin cells to repair diabetic skin wound: a wound reparation trail. Exp Biol Med 2014;239:1630–1637 [DOI] [PubMed] [Google Scholar]
- 153. Chan RK, Garfein E, Gigante PR, et al. Side population hematopoietic stem cells promote wound healing in diabetic mice. Plast Reconstr Surg 2007;120:407–411; discussion 412–403. [DOI] [PubMed] [Google Scholar]
- 154. Asai J, Takenaka H, Ii M, et al. Topical application of ex vivo expanded endothelial progenitor cells promotes vascularisation and wound healing in diabetic mice. Int Wound J 2013;10:527–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Tecilazich F, Dinh T, Pradhan-Nabzdyk L, et al. Role of endothelial progenitor cells and inflammatory cytokines in healing of diabetic foot ulcers. PLoS One 2013;8:e83314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Li X, Jiang C, Zhao J. Human endothelial progenitor cells-derived exosomes accelerate cutaneous wound healing in diabetic rats by promoting endothelial function. J Diabetes Complications 2016;30:986–992 [DOI] [PubMed] [Google Scholar]
- 157. Zhang J, Chen C, Hu B, et al. Exosomes derived from human endothelial progenitor cells accelerate cutaneous wound healing by promoting angiogenesis through Erk1/2 signaling. Int J Biol Sci 2016;12:1472–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Elsharawy MA, Naim M, Greish S. Human CD34+ stem cells promote healing of diabetic foot ulcers in rats. Interact Cardiovasc Thorac Surg 2012;14:288–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Kanji S, Das M, Aggarwal R, et al. Nanofiber-expanded human umbilical cord blood-derived CD34+ cell therapy accelerates murine cutaneous wound closure by attenuating pro-inflammatory factors and secreting IL-10. Stem Cell Res 2014;12:275–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Kanji S, Das M, Joseph M, et al. Nanofiber-expanded human CD34(+) cells heal cutaneous wounds in streptozotocin-induced diabetic mice. Sci Rep 2019;9:8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Sivan-Loukianova E, Awad OA, Stepanovic V, Bickenbach J, Schatteman GC. CD34+ blood cells accelerate vascularization and healing of diabetic mouse skin wounds. J Vasc Res 2003;40:368–377 [DOI] [PubMed] [Google Scholar]
- 162. Javazon EH, Keswani SG, Badillo AT, et al. Enhanced epithelial gap closure and increased angiogenesis in wounds of diabetic mice treated with adult murine bone marrow stromal progenitor cells. Wound Repair Regen 2007;15:350–359 [DOI] [PubMed] [Google Scholar]
- 163. Kwon DS, Gao X, Liu YB, et al. Treatment with bone marrow-derived stromal cells accelerates wound healing in diabetic rats. Int Wound J 2008;5:453–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Nambu M, Kishimoto S, Nakamura S, et al. Accelerated wound healing in healing-impaired db/db mice by autologous adipose tissue-derived stromal cells combined with atelocollagen matrix. Ann Plast Surg 2009;62:317–321 [DOI] [PubMed] [Google Scholar]
- 165. Amos PJ, Kapur SK, Stapor PC, et al. Human adipose-derived stromal cells accelerate diabetic wound healing: impact of cell formulation and delivery. Tissue Eng Part A 2010;16:1595–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Di Rocco G, Gentile A, Antonini A, et al. Enhanced healing of diabetic wounds by topical administration of adipose tissue-derived stromal cells overexpressing stromal-derived factor-1: biodistribution and engraftment analysis by bioluminescent imaging. Stem Cells Int 2010;2011:304562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Gu XY, Shen SE, Huang CF, et al. Effect of activated autologous monocytes/macrophages on wound healing in a rodent model of experimental diabetes. Diabetes Res Clin Pract 2013;102:53–59 [DOI] [PubMed] [Google Scholar]
- 168. Sirbulescu RF, Boehm CK, Soon E, et al. Mature B cells accelerate wound healing after acute and chronic diabetic skin lesions. Wound Repair Regen 2017;25:774–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Lee E, Kim DY, Chung E, Lee EA, Park KS, Son Y. Transplantation of cyclic stretched fibroblasts accelerates the wound-healing process in streptozotocin-induced diabetic mice. Cell Transplant 2014;23:285–301 [DOI] [PubMed] [Google Scholar]
- 170. Kasap S, Barutcu A, Guc H, Yazgan S, Kivanc M, Vatansever HS. Effects of keratinocytes differentiated from embryonic and adipogenic stem cells on wound healing in a diabetic mouse model. Wounds 2017;29:297–305 [PubMed] [Google Scholar]
- 171. Ding Y, Cui L, Zhao Q, Zhang W, Sun H, Zheng L. Platelet-rich fibrin accelerates skin wound healing in diabetic mice. Ann Plast Surg 2017;79:e15–e19 [DOI] [PubMed] [Google Scholar]
- 172. Zhang C, Zhu Y, Lu S, Zhong W, Wang Y, Chai Y. Platelet-rich plasma with endothelial progenitor cells accelerates diabetic wound healing in rats by upregulating the Notch1 signaling pathway. J Diabetes Res 2019;2019:5920676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. MacroCure. MacroCure Products: CureXcell. Products. 2020. MacroCure. http://polusdigital.com/macrocure/curexcell (last accessed January2020)
- 174. BioSpace. Macrocure Sinks as CureXcell Flunks Phase III Study. Urbandale, Lowa: BioSpace, 2015 [Google Scholar]
- 175. Evaluation of CureXcell® in Treating Lower Extremity Chronic Ulcers in Adults With Diabetes—Full Text View—ClinicalTrials.gov National Institutes of Health. 2011. https://clinicaltrials.gov/ct2/show/NCT01421966 (last accessed 2019)
- 176. BioSpace. Macrocure Stock Plunges 63% as Lead Drug Fails Phase III Study. Urbandale, Lowa: BioSpace, 2015 [Google Scholar]
- 177. Carey D. Macrocure Ltd. Announces Results for Phase III Clinical Trial of CureXcell® in Diabetic Foot Ulcers and Provides Corporate Update. Framingham, MA: Fierce Biotech, 2015 [Google Scholar]
- 178. ClinicalTrials.gov. Clinical Trials Search Results for: “platelet” in Condition/Disease: Diabetic Foot Ulcer ClinicalTrials.gov Bethesda, MD: U.S. National Library of Medicine, 2020 [Google Scholar]
- 179. Del Pino-Sedeno T, Trujillo-Martin MM, Andia I, et al. Platelet-rich plasma for the treatment of diabetic foot ulcers: a meta-analysis. Wound Repair Regen 2019;27:170–182 [DOI] [PubMed] [Google Scholar]
- 180. Ding H, Fu XL, Miao WW, Mao XC, Zhan MQ, Chen HL. Efficacy of autologous platelet-rich gel for diabetic foot wound healing: a meta-analysis of 15 randomized controlled trials. Adv Wound Care 2019;8:195–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Driver VR, Hanft J, Fylling CP, Beriou JM, Autologel Diabetic Foot Ulcer Study Group. A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manage 2006;52:68–70, 72, 74 passim. [PubMed] [Google Scholar]
- 182. Hu Z, Qu S, Zhang J, et al. Efficacy and safety of platelet-rich plasma for patients with diabetic ulcers: a systematic review and meta-analysis. Adv Wound Care 2019;8:298–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Piccin A, Di Pierro AM, Canzian L, et al. Platelet gel: a new therapeutic tool with great potential. Blood Transfus 2017;15:333–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Singh SP, Kumar V, Pandey A, Pandey P, Gupta V, Verma R. Role of platelet-rich plasma in healing diabetic foot ulcers: a prospective study. J Wound Care 2018;27:550–556 [DOI] [PubMed] [Google Scholar]
- 185. Veves A, Falanga V, Armstrong DG, Sabolinski ML. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care 2001;24:290–295 [DOI] [PubMed] [Google Scholar]
- 186. Hart CE, Loewen-Rodriguez A, Lessem J. Dermagraft: use in the treatment of chronic wounds. Adv Wound Care (New Rochelle) 2012:138–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. FDA. PMA: Integra Dermal Regeneration Template 1996; Original PMA for Integra/Omnigraft. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P900033 (last accessed January2020) [Google Scholar]
- 188. Chan SW. Interleukin 2 topical cream for treatment of diabetic foot ulcer: experiment protocol. JMIR Res Protoc 2015;4:e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Doersch KM, DelloStritto DJ, Newell-Rogers MK. The contribution of interleukin-2 to effective wound healing. Exp Biol Med 2017;242:384–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Kucukcelebi A, Harries RH, Hennessey PJ, et al. In vivo characterization of interleukin-4 as a potential wound healing agent. Wound Repair Regen 1995;3:49–58 [DOI] [PubMed] [Google Scholar]
- 191. Lin ZQ, Kondo T, Ishida Y, Takayasu T, Mukaida N. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. J Leukoc Biol 2003;73:713–721 [DOI] [PubMed] [Google Scholar]
- 192. Gallucci RM, Simeonova PP, Matheson JM, et al. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. FASEB J 2000;14:2525–2531 [DOI] [PubMed] [Google Scholar]
- 193. Avitabile S, Odorisio T, Madonna S, et al. Interleukin-22 promotes wound repair in diabetes by improving keratinocyte pro-healing functions. J Invest Dermatol 2015;135:2862–2870 [DOI] [PubMed] [Google Scholar]
- 194. Ishida Y, Kuninaka Y, Nosaka M, et al. CCL2-mediated reversal of impaired skin wound healing in diabetic mice by normalization of neovascularization and collagen accumulation. J Invest Dermatol 2019;139:2517–2527.e2515. [DOI] [PubMed] [Google Scholar]
- 195. Wood S, Jayaraman V, Huelsmann EJ, et al. Pro-inflammatory chemokine CCL2 (MCP-1) promotes healing in diabetic wounds by restoring the macrophage response. PLoS One 2014;9:e91574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Galeano M, Altavilla D, Cucinotta D, et al. Recombinant human erythropoietin stimulates angiogenesis and wound healing in the genetically diabetic mouse. Diabetes 2004;53:2509–2517 [DOI] [PubMed] [Google Scholar]
- 197. Hamed S, Ullmann Y, Masoud M, Hellou E, Khamaysi Z, Teot L. Topical erythropoietin promotes wound repair in diabetic rats. J Invest Dermatol 2010;130:287–294 [DOI] [PubMed] [Google Scholar]
- 198. Kant V, Kumar D, Kumar D, et al. Topical application of substance P promotes wound healing in streptozotocin-induced diabetic rats. Cytokine 2015;73:144–155 [DOI] [PubMed] [Google Scholar]
- 199. Kant V, Kumar D, Prasad R, et al. Combined effect of substance P and curcumin on cutaneous wound healing in diabetic rats. J Surg Res 2017;212:130–145 [DOI] [PubMed] [Google Scholar]
- 200. Leal EC, Carvalho E, Tellechea A, et al. Substance P promotes wound healing in diabetes by modulating inflammation and macrophage phenotype. Am J Pathol 2015;185:1638–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Park JH, Kim S, Hong HS, Son Y. Substance P promotes diabetic wound healing by modulating inflammation and restoring cellular activity of mesenchymal stem cells. Wound Repair Regen 2016;24:337–348 [DOI] [PubMed] [Google Scholar]
- 202. Um J, Yu J, Park KS. Substance P accelerates wound healing in type 2 diabetic mice through endothelial progenitor cell mobilization and Yes-associated protein activation. Mol Med Rep 2017;15:3035–3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Zhu FB, Liu DW, Zhang HY, et al. Effect of substance P combined with epidermal stem cells on wound healing and nerve regeneration in rats with diabetes mellitus [in Chinese]. Zhonghua Shao Shang Za Zhi 2012;28:25–31 [PubMed] [Google Scholar]
- 204. Greenway SE, Filler LE, Greenway FL. Topical insulin in wound healing: a randomised, double-blind, placebo-controlled trial. J Wound Care 1999;8:526–528 [DOI] [PubMed] [Google Scholar]
- 205. Hanam SR, Singleton CE, Rudek W. The effect of topical insulin on infected cutaneous ulcerations in diabetic and nondiabetic mice. J Foot Surg 1983;22:298–301 [PubMed] [Google Scholar]
- 206. Langer S, Born F, Breidenbach A, Schneider A, Uhl E, Messmer K. Effect of C-peptide on wound healing and microcirculation in diabetic mice. Eur J Med Res 2002;7:502–508 [PubMed] [Google Scholar]
- 207. Lim YC, Bhatt MP, Kwon MH, et al. Proinsulin C-peptide prevents impaired wound healing by activating angiogenesis in diabetes. J Invest Dermatol 2015;135:269–278 [DOI] [PubMed] [Google Scholar]
- 208. Long M, Rojo de la Vega M, Wen Q, et al. An essential role of NRF2 in diabetic wound healing. Diabetes 2016;65:780–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Hosur V, Burzenski LM, Stearns TM, et al. Early induction of NRF2 antioxidant pathway by RHBDF2 mediates rapid cutaneous wound healing. Exp Mol Pathol 2017;102:337–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Hozzein WN, Badr G, Badr BM, et al. Bee venom improves diabetic wound healing by protecting functional macrophages from apoptosis and enhancing Nrf2, Ang-1 and Tie-2 signaling. Mol Immunol 2018;103:322–335 [DOI] [PubMed] [Google Scholar]
- 211. Li X, Xie X, Lian W, et al. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med 2018;50:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Rabbani PS, Ellison T, Waqas B, et al. Targeted Nrf2 activation therapy with RTA 408 enhances regenerative capacity of diabetic wounds. Diabetes Res Clin Pract 2018;139:11–23 [DOI] [PubMed] [Google Scholar]
- 213. Luo JD, Hu TP, Wang L, Chen MS, Liu SM, Chen AF. Sonic hedgehog improves delayed wound healing via enhancing cutaneous nitric oxide function in diabetes. Am J Physiol Endocrinol Metab 2009;297:E525–E531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Hansen SL, Myers CA, Charboneau A, Young DM, Boudreau N. HoxD3 accelerates wound healing in diabetic mice. Am J Pathol 2003;163:2421–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Cho H, Balaji S, Hone NL, et al. Diabetic wound healing in a MMP9-/- mouse model. Wound Repair Regen 2016;24:829–840 [DOI] [PubMed] [Google Scholar]
- 216. Jones JI, Nguyen TT, Peng Z, Chang M. Targeting MMP-9 in diabetic foot ulcers. Pharmaceuticals (Basel) 2019;12:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Nguyen TT, Ding D, Wolter WR, et al. Validation of matrix metalloproteinase-9 (MMP-9) as a novel target for treatment of diabetic foot ulcers in humans and discovery of a potent and selective small-molecule MMP-9 inhibitor that accelerates healing. J Med Chem 2018;61:8825–8837 [DOI] [PubMed] [Google Scholar]
- 218. Shin SH, Han SK, Jeong SH, Kim WK. Potential of oncostatin M to accelerate diabetic wound healing. Int Wound J 2014;11:398–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Solmaz A, Bahadir E, Gulcicek OB, et al. Nesfatin-1 improves oxidative skin injury in normoglycemic or hyperglycemic rats. Peptides 2016;78:1–10 [DOI] [PubMed] [Google Scholar]
- 220. Safer JD, Crawford TM, Holick MF. Topical thyroid hormone accelerates wound healing in mice. Endocrinology 2005;146:4425–4430 [DOI] [PubMed] [Google Scholar]
- 221. Nguyen VT, Farman N, Palacios-Ramirez R, et al. Cutaneous wound healing in diabetic mice is improved by topical mineralocorticoid receptor blockade. J Invest Dermatol 2020;140:223–234.e227. [DOI] [PubMed] [Google Scholar]
- 222. Lee YS, Kang SU, Lee MH, et al. GnRH impairs diabetic wound healing through enhanced NETosis. Cell Mol Immunol 2020;17:856–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Wang JM, Qiu Y, Yang ZQ, Li L, Zhang K. Inositol-requiring enzyme 1 facilitates diabetic wound healing through modulating microRNAs. Diabetes 2017;66:177–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Knighton DR, Ciresi KF, Fiegel VD, Austin LL, Butler EL. Classification and treatment of chronic nonhealing wounds. Successful treatment with autologous platelet-derived wound healing factors (PDWHF). Ann Surg 1986;204:322–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Knighton DR, Ciresi K, Fiegel VD, Schumerth S, Butler E, Cerra F. Stimulation of repair in chronic, nonhealing, cutaneous ulcers using platelet-derived wound healing formula. Surg Gynecol Obstet 1990;170:56–60 [PubMed] [Google Scholar]
- 226. Krupski WC, Reilly LM, Perez S, Moss KM, Crombleholme PA, Rapp JH. A prospective randomized trial of autologous platelet-derived wound healing factors for treatment of chronic nonhealing wounds: a preliminary report. J Vasc Surg 1991;14:526–532; discussion 532–536. [PubMed] [Google Scholar]
- 227. Steed D, Goslen B, Hambley R, Abell E, Hebda P, Webster M. Clinical trials with purified platelet releasate. Progr Clin Biol Res 1991;365:103–113 [PubMed] [Google Scholar]
- 228. Ksander GA, Sawamura SJ, Ogawa Y, Sundsmo J, McPherson JM. The effect of platelet releasate on wound healing in animal models. J Am Acad Dermatol 1990;22:781–791 [DOI] [PubMed] [Google Scholar]
- 229. Moulin V, Lawny F, Barritault D, Caruelle JP. Platelet releasate treatment improves skin healing in diabetic rats through endogenous growth factor secretion. Cell Mol Biol (Noisy-le-grand) 1998;44:961–971 [PubMed] [Google Scholar]
- 230. Phillips GD, Stone AM, Whitehead RA, Knighton DR. Platelet derived wound healing factors (PDWHF) accelerate and augment wound healing angiogenesis in the rat. In Vivo 1994;8:167–171 [PubMed] [Google Scholar]
- 231. Tanaka R, Ichioka S, Sekiya N, et al. Elastic plasma protein film blended with platelet releasate accelerates healing of diabetic mouse skin wounds. Vox Sang 2007;93:49–56 [DOI] [PubMed] [Google Scholar]
- 232. Kirsner RS, Warriner R, Michela M, Stasik L, Freeman K. Advanced biological therapies for diabetic foot ulcers. Arch Dermatol 2010;146:857–862 [DOI] [PubMed] [Google Scholar]
- 233. Margolis DJ, Kantor J, Santanna J, Strom BL, Berlin JA. Effectiveness of platelet releasate for the treatment of diabetic neuropathic foot ulcers. Diabetes Care 2001;24:483–488 [DOI] [PubMed] [Google Scholar]
- 234. Zelen CM, Serena TE, Gould L, et al. Treatment of chronic diabetic lower extremity ulcers with advanced therapies: a prospective, randomised, controlled, multi-centre comparative study examining clinical efficacy and cost. Int Wound J 2016;13:272–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Ansurudeen I, Sunkari VG, Grunler J, et al. Carnosine enhances diabetic wound healing in the db/db mouse model of type 2 diabetes. Amino Acids 2012;43:127–134 [DOI] [PubMed] [Google Scholar]
- 236. Bhide MV, Dunphy MJ, Mirkopulos N, Smith DJ. Promotion of wound collagen formation in normal and diabetic mice by quadrol. Immunopharmacol Immunotoxicol 1988;10:513–522 [DOI] [PubMed] [Google Scholar]
- 237. Kawanabe T, Kawakami T, Yatomi Y, Shimada S, Soma Y. Sphingosine 1-phosphate accelerates wound healing in diabetic mice. J Dermatol Sci 2007;48:53–60 [DOI] [PubMed] [Google Scholar]
- 238. Hou Z, Nie C, Si Z, Ma Y. Deferoxamine enhances neovascularization and accelerates wound healing in diabetic rats via the accumulation of hypoxia-inducible factor-1alpha. Diabetes Res Clin Pract 2013;101:62–71 [DOI] [PubMed] [Google Scholar]
- 239. Wang C, Cai Y, Zhang Y, Xiong Z, Li G, Cui L. Local injection of deferoxamine improves neovascularization in ischemic diabetic random flap by increasing HIF-1alpha and VEGF expression. PLoS One 2014;9:e100818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Ram M, Singh V, Kumawat S, et al. Deferoxamine modulates cytokines and growth factors to accelerate cutaneous wound healing in diabetic rats. Eur J Pharmacol 2015;764:9–21 [DOI] [PubMed] [Google Scholar]
- 241. Jiang S, Zavitz CC, Wang J, et al. Non-adenine based purines accelerate wound healing. Purinergic Signal 2006;2:651–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Zhou Z, Joslin S, Dellinger A, et al. A novel class of compounds with cutaneous wound healing properties. J Biomed Nanotechnol 2010;6:605–611 [DOI] [PubMed] [Google Scholar]
- 243. Whittam AJ, Maan ZN, Duscher D, et al. Small molecule inhibition of dipeptidyl peptidase-4 enhances bone marrow progenitor cell function and angiogenesis in diabetic wounds. Transl Res 2019;205:51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Gao M, Nguyen TT, Suckow MA, et al. Acceleration of diabetic wound healing using a novel protease-anti-protease combination therapy. Proc Natl Acad Sci U S A 2015;112:15226–15231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Botusan IR, Sunkari VG, Savu O, et al. Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci U S A 2008;105:19426–19431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Liu F, Chen DD, Sun X, et al. Hydrogen sulfide improves wound healing via restoration of endothelial progenitor cell functions and activation of angiopoietin-1 in type 2 diabetes. Diabetes 2014;63:1763–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Yang CT, Chen L, Chen WL, et al. Hydrogen sulfide primes diabetic wound to close through inhibition of NETosis. Mol Cell Endocrinol 2019;480:74–82 [DOI] [PubMed] [Google Scholar]
- 248. Zhao H, Lu S, Chai J, et al. Hydrogen sulfide improves diabetic wound healing in ob/ob mice via attenuating inflammation. J Diabetes Complications 2017;31:1363–1369 [DOI] [PubMed] [Google Scholar]
- 249. Pushpakom S, Iorio F, Eyers PA, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov 2019;18:41–58 [DOI] [PubMed] [Google Scholar]
- 250. Strittmatter SM. Overcoming drug development bottlenecks with repurposing: old drugs learn new tricks. Nat Med 2014;20:590–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Breckenridge A, Jacob R. Overcoming the legal and regulatory barriers to drug repurposing. Nat Rev Drug Discov 2019;18:1–2 [DOI] [PubMed] [Google Scholar]
- 252. Kato S, Moulder SL, Ueno NT, et al. Challenges and perspective of drug repurposing strategies in early phase clinical trials. Oncoscience 2015;2:576–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. ClinicalTrials.gov. Phase 3 Study to Evaluate the Safety and Efficacy of Galnobax® in Treating Diabetic Foot Ulcers—Full Text View—ClinicalTrials.gov 2020. https://clinicaltrials.gov/ct2/show/NCT03998436 (last accessed February2020) [Google Scholar]
- 254. He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 2004;5:522–531 [DOI] [PubMed] [Google Scholar]
- 255. Liu YF, Ding M, Liu DW, Liu Y, Mao YG, Peng Y. MicroRNA profiling in cutaneous wounds of diabetic rats. Genet Mol Res 2015;14:9614–9625 [DOI] [PubMed] [Google Scholar]
- 256. Umehara T, Mori R, Mace KA, et al. Identification of specific miRNAs in neutrophils of type 2 diabetic mice. Overexpression of miRNA-129-2-3p accelerates diabetic wound healing. Diabetes 2019;68:617–630 [DOI] [PubMed] [Google Scholar]
- 257. Li X, Li D, Wang A, et al. MicroRNA-132 with therapeutic potential in chronic wounds. J Invest Dermatol 2017;137:2630–2638 [DOI] [PubMed] [Google Scholar]
- 258. Wang W, Yang C, Wang XY, et al. MicroRNA-129 and -335 promote diabetic wound healing by inhibiting Sp1-mediated MMP-9 expression. Diabetes 2018;67:1627–1638 [DOI] [PubMed] [Google Scholar]
- 259. Wang JM, Tao J, Chen DD, et al. MicroRNA miR-27b rescues bone marrow-derived angiogenic cell function and accelerates wound healing in type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol 2014;34:99–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Xu J, Wu W, Zhang L, et al. The role of microRNA-146a in the pathogenesis of the diabetic wound-healing impairment: correction with mesenchymal stem cell treatment. Diabetes 2012;61:2906–2912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Zgheib C, Hilton SA, Dewberry LC, et al. Use of cerium oxide nanoparticles conjugated with microRNA-146a to correct the diabetic wound healing impairment. J Am Coll Surg 2019;228:107–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Xu J, Zgheib C, Hu J, Wu W, Zhang L, Liechty KW. The role of microRNA-15b in the impaired angiogenesis in diabetic wounds. Wound Repair Regen 2014;22:671–677 [DOI] [PubMed] [Google Scholar]
- 263. Chan YC, Roy S, Khanna S, Sen CK. Downregulation of endothelial microRNA-200b supports cutaneous wound angiogenesis by desilencing GATA binding protein 2 and vascular endothelial growth factor receptor 2. Arterioscler Thromb Vasc Biol 2012;32:1372–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Pizzino G, Irrera N, Galfo F, et al. Effects of the antagomiRs 15b and 200b on the altered healing pattern of diabetic mice. Br J Pharmacol 2018;175:644–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Pishavar E, Behravan J. miR-126 as a therapeutic agent for diabetes mellitus. Curr Pharm Des 2017;23:3309–3314 [DOI] [PubMed] [Google Scholar]
- 266. Zhang D, Li Z, Wang Z, Zeng F, Xiao W, Yu A. MicroRNA-126: a promising biomarker for angiogenesis of diabetic wounds treated with negative pressure wound therapy. Diabetes Metab Syndr Obes 2019;12:1685–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Madhyastha R, Madhyastha H, Nakajima Y, Omura S, Maruyama M. MicroRNA signature in diabetic wound healing: promotive role of miR-21 in fibroblast migration. Int Wound J 2012;9:355–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Ye J, Kang Y, Sun X, Ni P, Wu M, Lu S. MicroRNA-155 inhibition promoted wound healing in diabetic rats. Int J Low Extrem Wounds 2017;16:74–84 [DOI] [PubMed] [Google Scholar]
- 269. Moura J, Sorensen A, Leal EC, et al. microRNA-155 inhibition restores fibroblast growth factor 7 expression in diabetic skin and decreases wound inflammation. Sci Rep 2019;9:5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Gallant-Behm CL, Piper J, Dickinson BA, Dalby CM, Pestano LA, Jackson AL. A synthetic microRNA-92a inhibitor (MRG-110) accelerates angiogenesis and wound healing in diabetic and nondiabetic wounds. Wound Repair Regen 2018;26:311–323 [DOI] [PubMed] [Google Scholar]
- 271. Icli B, Nabzdyk CS, Lujan-Hernandez J, et al. Regulation of impaired angiogenesis in diabetic dermal wound healing by microRNA-26a. J Mol Cell Cardiol 2016;91:151–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Ling C, Rönn T. Epigenetics in human obesity and type 2 diabetes. Cell Metab 2019;29:1028–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Singh K, Pal D, Sinha M, et al. Epigenetic modification of microRNA-200b contributes to diabetic vasculopathy. Mol Ther 2017;25:2689–2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Witkowski M, Tabaraie T, Steffens D, et al. MicroRNA-19a contributes to the epigenetic regulation of tissue factor in diabetes. Cardiovasc Diabetol 2018;17:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. den Dekker A, Davis FM, Kunkel SL, Gallagher KA. Targeting epigenetic mechanisms in diabetic wound healing. Transl Res 2019;204:39–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 2013;200:373–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Geiger A, Walker A, Nissen E. Human fibrocyte-derived exosomes accelerate wound healing in genetically diabetic mice. Biochem Biophys Res Commun 2015;467:303–309 [DOI] [PubMed] [Google Scholar]
- 278. Guo SC, Tao SC, Yin WJ, Qi X, Yuan T, Zhang CQ. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics 2017;7:81–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Xu N, Wang L, Guan J, et al. Wound healing effects of a Curcuma zedoaria polysaccharide with platelet-rich plasma exosomes assembled on chitosan/silk hydrogel sponge in a diabetic rat model. Int J Biol Macromol 2018;117:102–107 [DOI] [PubMed] [Google Scholar]
- 280. Tao SC, Guo SC, Li M, Ke QF, Guo YP, Zhang CQ. Chitosan wound dressings incorporating exosomes derived from microRNA-126-overexpressing synovium mesenchymal stem cells provide sustained release of exosomes and heal full-thickness skin defects in a diabetic rat model. Stem Cells Transl Med 2017;6:736–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Dalirfardouei R, Jamialahmadi K, Jafarian AH, Mahdipour E. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J Tissue Eng Regen Med 2019;13:555–568 [DOI] [PubMed] [Google Scholar]
- 282. Chen W, Wu Y, Li L, et al. Adenosine accelerates the healing of diabetic ischemic ulcers by improving autophagy of endothelial progenitor cells grown on a biomaterial. Sci Rep 2015;5:11594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. He S, Shen L, Wu Y, et al. Effect of brain-derived neurotrophic factor on mesenchymal stem cell-seeded electrospinning biomaterial for treating ischemic diabetic ulcers via milieu-dependent differentiation mechanism. Tissue Eng Part A 2015;21:928–938 [DOI] [PubMed] [Google Scholar]
- 284. Yin H, Ding G, Shi X, et al. A bioengineered drug-Eluting scaffold accelerated cutaneous wound healing In diabetic mice. Colloids Surf B Biointerfaces 2016;145:226–231 [DOI] [PubMed] [Google Scholar]
- 285. Lao G, Yan L, Yang C, Zhang L, Zhang S, Zhou Y. Controlled release of epidermal growth factor from hydrogels accelerates wound healing in diabetic rats. J Am Podiatr Med Assoc 2012;102:89–98 [DOI] [PubMed] [Google Scholar]
- 286. Lee PY, Li Z, Huang L. Thermosensitive hydrogel as a Tgf-beta1 gene delivery vehicle enhances diabetic wound healing. Pharm Res 2003;20:1995–2000 [DOI] [PubMed] [Google Scholar]
- 287. Lee PY, Cobain E, Huard J, Huang L. Thermosensitive hydrogel PEG-PLGA-PEG enhances engraftment of muscle-derived stem cells and promotes healing in diabetic wound. Mol Ther 2007;15:1189–1194 [DOI] [PubMed] [Google Scholar]
- 288. Chen S, Shi J, Zhang M, et al. Mesenchymal stem cell-laden anti-inflammatory hydrogel enhances diabetic wound healing. Sci Rep 2015;5:18104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Tokatlian T, Cam C, Segura T. Porous hyaluronic acid hydrogels for localized nonviral DNA delivery in a diabetic wound healing model. Adv Healthc Mater 2015;4:1084–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Chen H, Jia P, Kang H, et al. Upregulating Hif-1alpha by hydrogel nanofibrous scaffolds for rapidly recruiting angiogenesis relative cells in diabetic wound. Adv Healthc Mater 2016;5:907–918 [DOI] [PubMed] [Google Scholar]
- 291. Hajimiri M, Shahverdi S, Esfandiari MA, et al. Preparation of hydrogel embedded polymer-growth factor conjugated nanoparticles as a diabetic wound dressing. Drug Dev Ind Pharm 2016;42:707–719 [DOI] [PubMed] [Google Scholar]
- 292. Shukla R, Kashaw SK, Jain AP, Lodhi S. Fabrication of Apigenin loaded gellan gum-chitosan hydrogels (GGCH-HGs) for effective diabetic wound healing. Int J Biol Macromol 2016;91:1110–1119 [DOI] [PubMed] [Google Scholar]
- 293. Xiao Y, Reis LA, Feric N, et al. Diabetic wound regeneration using peptide-modified hydrogels to target re-epithelialization. Proc Natl Acad Sci U S A 2016;113:E5792–E5801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Yoon DS, Lee Y, Ryu HA, et al. Cell recruiting chemokine-loaded sprayable gelatin hydrogel dressings for diabetic wound healing. Acta Biomater 2016;38:59–68 [DOI] [PubMed] [Google Scholar]
- 295. da Silva LP, Santos TC, Rodrigues DB, et al. Stem cell-containing hyaluronic acid-based spongy hydrogels for integrated diabetic wound healing. J Invest Dermatol 2017;137:1541–1551 [DOI] [PubMed] [Google Scholar]
- 296. He H, Xia DL, Chen YP, et al. Evaluation of a two-stage antibacterial hydrogel dressing for healing in an infected diabetic wound. J Biomed Mater Res B Appl Biomater 2017;105:1808–1817 [DOI] [PubMed] [Google Scholar]
- 297. Thangavel P, Ramachandran B, Chakraborty S, Kannan R, Lonchin S, Muthuvijayan V. Accelerated healing of diabetic wounds treated with L-glutamic acid loaded hydrogels through enhanced collagen deposition and angiogenesis: an in vivo study. Sci Rep 2017;7:10701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Zhao L, Niu L, Liang H, Tan H, Liu C, Zhu F. pH and glucose dual-responsive injectable hydrogels with insulin and fibroblasts as bioactive dressings for diabetic wound healing. ACS Appl Mater Interfaces 2017;9:37563–37574 [DOI] [PubMed] [Google Scholar]
- 299. Liu J, Chen Z, Wang J, et al. Encapsulation of Curcumin nanoparticles with MMP9-responsive and thermos-sensitive hydrogel improves diabetic wound healing. ACS Appl Mater Interfaces 2018;10:16315–16326 [DOI] [PubMed] [Google Scholar]
- 300. Veerasubramanian PK, Thangavel P, Kannan R, et al. An investigation of konjac glucomannan-keratin hydrogel scaffold loaded with Avena sativa extracts for diabetic wound healing. Colloids Surf B Biointerfaces 2018;165:92–102 [DOI] [PubMed] [Google Scholar]
- 301. Xu Q, A S, Gao Y, et al. A hybrid injectable hydrogel from hyperbranched PEG macromer as a stem cell delivery and retention platform for diabetic wound healing. Acta Biomater 2018;75:63–74 [DOI] [PubMed] [Google Scholar]
- 302. Wang T, Zheng Y, Shi Y, Zhao L. pH-responsive calcium alginate hydrogel laden with protamine nanoparticles and hyaluronan oligosaccharide promotes diabetic wound healing by enhancing angiogenesis and antibacterial activity. Drug Deliv Transl Res 2019;9:227–239 [DOI] [PubMed] [Google Scholar]
- 303. Blecher K, Martinez LR, Tuckman-Vernon C, et al. Nitric oxide-releasing nanoparticles accelerate wound healing in NOD-SCID mice. Nanomed Nanotechnol Biol Med 2012;8:1364–1371 [DOI] [PubMed] [Google Scholar]
- 304. Chen SA, Chen HM, Yao YD, Hung CF, Tu CS, Liang YJ. Topical treatment with anti-oxidants and Au nanoparticles promote healing of diabetic wound through receptor for advance glycation end-products. Eur J Pharm Sci 2012;47:875–883 [DOI] [PubMed] [Google Scholar]
- 305. Karri VV, Kuppusamy G, Talluri SV, et al. Curcumin loaded chitosan nanoparticles impregnated into collagen-alginate scaffolds for diabetic wound healing. Int J Biol Macromol 2016;93:1519–1529 [DOI] [PubMed] [Google Scholar]
- 306. Turner CT, McInnes SJ, Melville E, Cowin AJ, Voelcker NH. Delivery of flightless I neutralizing antibody from porous silicon nanoparticles improves wound healing in diabetic mice. Adv Healthc Mater 2017;6. DOI: 10.1002/adhm.201600707 [DOI] [PubMed] [Google Scholar]
- 307. Abdelkader DH, Tambuwala MM, Mitchell CA, et al. Enhanced cutaneous wound healing in rats following topical delivery of insulin-loaded nanoparticles embedded in poly(vinyl alcohol)-borate hydrogels. Drug Deliv Transl Res 2018;8:1053–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Kamar SS, Abdel-Kader DH, Rashed LA. Beneficial effect of Curcumin Nanoparticles-Hydrogel on excisional skin wound healing in type-I diabetic rat: histological and immunohistochemical studies. Ann Anat 2019;222:94–102 [DOI] [PubMed] [Google Scholar]
- 309. Kasiewicz LN, Whitehead KA. Lipid nanoparticles silence tumor necrosis factor alpha to improve wound healing in diabetic mice. Bioeng Transl Med 2019;4:75–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Lee CH, Chang SH, Chen WJ, et al. Augmentation of diabetic wound healing and enhancement of collagen content using nanofibrous glucophage-loaded collagen/PLGA scaffold membranes. J Colloid Interface Sci 2015;439:88–97 [DOI] [PubMed] [Google Scholar]
- 311. Gholipour-Kanani A, Bahrami SH, Rabbani S. Effect of novel blend nanofibrous scaffolds on diabetic wounds healing. IET Nanobiotechnol 2016;10:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Martin JR, Nelson CE, Gupta MK, et al. Local delivery of PHD2 siRNA from ROS-degradable scaffolds to promote diabetic wound healing. Adv Healthc Mater 2016;5:2751–2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Dwivedi C, Pandey I, Pandey H, et al. In vivo diabetic wound healing with nanofibrous scaffolds modified with gentamicin and recombinant human epidermal growth factor. J Biomed Mater Res A 2018;106:641–651 [DOI] [PubMed] [Google Scholar]
- 314. Yan W, Liu H, Deng X, Jin Y, Wang N, Chu J. Acellular dermal matrix scaffolds coated with connective tissue growth factor accelerate diabetic wound healing by increasing fibronectin through PKC signalling pathway. J Tissue Eng Regen Med 2018;12:e1461–e1473 [DOI] [PubMed] [Google Scholar]
- 315. Kanda N, Morimoto N, Ayvazyan AA, et al. Evaluation of a novel collagen-gelatin scaffold for achieving the sustained release of basic fibroblast growth factor in a diabetic mouse model. J Tissue Eng Regen Med 2014;8:29–40 [DOI] [PubMed] [Google Scholar]
- 316. Tong C, Hao H, Xia L, et al. Hypoxia pretreatment of bone marrow-derived mesenchymal stem cells seeded in a collagen-chitosan sponge scaffold promotes skin wound healing in diabetic rats with hindlimb ischemia. Wound Repair Regen 2016;24:45–56 [DOI] [PubMed] [Google Scholar]
- 317. Al-Watban FA, Andres BL. Polychromatic LED in oval full-thickness wound healing in non-diabetic and diabetic rats. Photomed Laser Surg 2006;24:10–16 [DOI] [PubMed] [Google Scholar]
- 318. Al-Watban FA, Zhang XY, Andres BL. Low-level laser therapy enhances wound healing in diabetic rats: a comparison of different lasers. Photomed Laser Surg 2007;25:72–77 [DOI] [PubMed] [Google Scholar]
- 319. Akyol U, Gungormus M. The effect of low-level laser therapy on healing of skin incisions made using a diode laser in diabetic rats. Photomed Laser Surg 2010;28:51–55 [DOI] [PubMed] [Google Scholar]
- 320. Mao Z, Wu JH, Dong T, Wu MX. Additive enhancement of wound healing in diabetic mice by low level light and topical CoQ10. Sci Rep 2016;6:20084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Ahmed OM, Mohamed T, Moustafa H, Hamdy H, Ahmed RR, Aboud E. Quercetin and low level laser therapy promote wound healing process in diabetic rats via structural reorganization and modulatory effects on inflammation and oxidative stress. Biomed Pharmacother 2018;101:58–73 [DOI] [PubMed] [Google Scholar]
- 322. Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care 2008;31:631–636 [DOI] [PubMed] [Google Scholar]
- 323. Noble-Bell G, Forbes A. A systematic review of the effectiveness of negative pressure wound therapy in the management of diabetes foot ulcers. Int Wound J 2008;5:233–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Mohseni S, Aalaa M, Atlasi R, Tehrani MRM, Sanjari M, Amini MR. The effectiveness of negative pressure wound therapy as a novel management of diabetic foot ulcers: an overview of systematic reviews. J Diabetes Metab Disord 2019;18:625–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Rys P, Borys S, Hohendorff J, et al. NPWT in diabetic foot wounds—a systematic review and meta-analysis of observational studies. Endocrine 2020;68:44–55 [DOI] [PubMed] [Google Scholar]
- 326. Huang Q, Wang JT, Gu HC, Cao G, Cao JC. Comparison of vacuum sealing drainage and traditional therapy for treatment of diabetic foot ulcers: a meta-analysis. J Foot Ankle Surg 2019;58:954–958 [DOI] [PubMed] [Google Scholar]
- 327. Chereddy KK, Coco R, Memvanga PB, et al. Combined effect of PLGA and curcumin on wound healing activity. J Control Release 2013;171:208–215 [DOI] [PubMed] [Google Scholar]
- 328. Kant V, Gopal A, Kumar D, et al. Curcumin-induced angiogenesis hastens wound healing in diabetic rats. J Surg Res 2015;193:978–988 [DOI] [PubMed] [Google Scholar]
- 329. Sidhu GS, Mani H, Gaddipati JP, et al. Curcumin enhances wound healing in streptozotocin induced diabetic rats and genetically diabetic mice. Wound Repair Regen 1999;7:362–374 [DOI] [PubMed] [Google Scholar]
- 330. Zhang Y, McClain SA, Lee HM, et al. A novel chemically modified Curcumin “Normalizes” wound-healing in rats with experimentally induced type I diabetes: initial studies. J Diabetes Res 2016;2016:5782904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Zhou J, Ni M, Liu X, Ren Z, Zheng Z. Curcumol promotes vascular endothelial growth factor (VEGF)-mediated diabetic wound healing in streptozotocin-induced hyperglycemic rats. Med Sci Monit 2017;23:555–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Amin MA, Abdel-Raheem IT. Accelerated wound healing and anti-inflammatory effects of physically cross linked polyvinyl alcohol-chitosan hydrogel containing honey bee venom in diabetic rats. Arch Pharm Res 2014;37:1016–1031 [DOI] [PubMed] [Google Scholar]
- 333. Badr G, Hozzein WN, Badr BM, Al Ghamdi A, Saad Eldien HM, Garraud O. Bee venom accelerates wound healing in diabetic mice by suppressing activating transcription factor-3 (ATF-3) and inducible nitric oxide synthase (iNOS)-mediated oxidative stress and recruiting bone marrow-derived endothelial progenitor cells. J Cell Physiol 2016;231:2159–2171 [DOI] [PubMed] [Google Scholar]
- 334. Molan PC. The role of honey in the management of wounds. J Wound Care 1999;8:415–418 [DOI] [PubMed] [Google Scholar]
- 335. Moghazy AM, Shams ME, Adly OA, et al. The clinical and cost effectiveness of bee honey dressing in the treatment of diabetic foot ulcers. Diabetes Res Clin Pract 2010;89:276–281 [DOI] [PubMed] [Google Scholar]
- 336. Jull AB, Cullum N, Dumville JC, Westby MJ, Deshpande S, Walker N. Honey as a topical treatment for wounds. Cochrane Database Syst Rev 2015:CD005083. DOI: 10.1002/14651858.CD005083.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Wang C, Guo M, Zhang N, Wang G. Effectiveness of honey dressing in the treatment of diabetic foot ulcers: a systematic review and meta-analysis. Complement Ther Clin Pract 2019;34:123–131 [DOI] [PubMed] [Google Scholar]
- 338. McLennan SV, Bonner J, Milne S, et al. The anti-inflammatory agent Propolis improves wound healing in a rodent model of experimental diabetes. Wound Repair Regen 2008;16:706–713 [DOI] [PubMed] [Google Scholar]
- 339. Hozzein WN, Badr G, Al Ghamdi AA, Sayed A, Al-Waili NS, Garraud O. Topical application of propolis enhances cutaneous wound healing by promoting TGF-beta/Smad-mediated collagen production in a streptozotocin-induced type I diabetic mouse model. Cell Physiol Biochem 2015;37:940–954 [DOI] [PubMed] [Google Scholar]
- 340. Ciloglu NS, Zeytin K, Aker F. The effects of resveratrol on flap survival in diabetic rats. J Plast Surg Hand Surg 2014;48:234–237 [DOI] [PubMed] [Google Scholar]
- 341. Huang X, Sun J, Chen G, et al. Resveratrol promotes diabetic wound healing via SIRT1-FOXO1-c-Myc signaling pathway-mediated angiogenesis. Front Pharmacol 2019;10:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Atiba A, Ueno H, Uzuka Y. The effect of aloe vera oral administration on cutaneous wound healing in type 2 diabetic rats. J Vet Med Sci 2011;73:583–589 [DOI] [PubMed] [Google Scholar]
- 343. Chithra P, Sajithlal GB, Chandrakasan G. Influence of aloe vera on the healing of dermal wounds in diabetic rats. J Ethnopharmacol 1998;59:195–201 [DOI] [PubMed] [Google Scholar]
- 344. Badr G. Supplementation with undenatured whey protein during diabetes mellitus improves the healing and closure of diabetic wounds through the rescue of functional long-lived wound macrophages. Cell Physiol Biochem 2012;29:571–582 [DOI] [PubMed] [Google Scholar]
- 345. Badr G. Camel whey protein enhances diabetic wound healing in a streptozotocin-induced diabetic mouse model: the critical role of beta-Defensin-1, -2 and -3. Lipids Health Dis 2013;12:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Badr G, Badr BM, Mahmoud MH, Mohany M, Rabah DM, Garraud O. Treatment of diabetic mice with undenatured whey protein accelerates the wound healing process by enhancing the expression of MIP-1alpha, MIP-2, KC, CX3CL1 and TGF-beta in wounded tissue. BMC Immunol 2012;13:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Jacobi J, Jang JJ, Sundram U, Dayoub H, Fajardo LF, Cooke JP. Nicotine accelerates angiogenesis and wound healing in genetically diabetic mice. Am J Pathol 2002;161:97–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Yuan Y, Das SK, Li M. Vitamin D ameliorates impaired wound healing in streptozotocin-induced diabetic mice by suppressing NF-kappaB-mediated inflammatory genes. Biosci Rep 2018;38:BSR20171294. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 349. Shi HP, Most D, Efron DT, Witte MB, Barbul A. Supplemental L-arginine enhances wound healing in diabetic rats. Wound Repair Regen 2003;11:198–203 [DOI] [PubMed] [Google Scholar]
- 350. Crowe MJ, McNeill RB, Schlemm DJ, Greenhalgh DG, Keller SJ. Topical application of yeast extract accelerates the wound healing of diabetic mice. J Burn Care Rehabil 1999;20:155–162 [DOI] [PubMed] [Google Scholar]
- 351. Ahmad M, Ansari MN, Alam A, Khan TH. Oral dose of citrus peel extracts promotes wound repair in diabetic rats. Pak J Biol Sci 2013;16:1086–1094 [DOI] [PubMed] [Google Scholar]
- 352. Ponrasu T, Suguna L. Efficacy of Annona squamosa on wound healing in streptozotocin-induced diabetic rats. Int Wound J 2012;9:613–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Ponrasu T, Suguna L. Efficacy of Annona squamosa L in the synthesis of glycosaminoglycans and collagen during wound repair in streptozotocin induced diabetic rats. Biomed Res Int 2014;2014:124352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Abu-Al-Basal MA. Healing potential of Rosmarinus officinalis L. on full-thickness excision cutaneous wounds in alloxan-induced-diabetic BALB/c mice. J Ethnopharmacol 2010;131:443–450 [DOI] [PubMed] [Google Scholar]
- 355. Rahati S, Eshraghian M, Ebrahimi A, Pishva H. Effect of spinach aqueous extract on wound healing in experimental model diabetic rats with streptozotocin. J Sci Food Agric 2016;96:2337–2343 [DOI] [PubMed] [Google Scholar]
- 356. Liu H, Duan Z, Tang J, Lv Q, Rong M, Lai R. A short peptide from frog skin accelerates diabetic wound healing. FEBS J 2014;281:4633–4643 [DOI] [PubMed] [Google Scholar]
- 357. Zhang Z, Zhao M, Wang J, Ding Y, Dai X, Li Y. Oral administration of skin gelatin isolated from Chum salmon (Oncorhynchus keta) enhances wound healing in diabetic rats. Mar Drugs 2011;9:696–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Michael S, Winters C, Khan M. Acellular fish skin graft use for diabetic lower extremity wound healing: a retrospective study of 58 ulcerations and a literature review. Wounds 2019;31:262–268 [PubMed] [Google Scholar]
- 359. Woodrow T, Chant T, Chant H. Treatment of diabetic foot wounds with acellular fish skin graft rich in omega-3: a prospective evaluation. J Wound Care 2019;28:76–80 [DOI] [PubMed] [Google Scholar]
- 360. Andrade PHM, Portugal LC, Rondon ES, Kadri MCT, Matos MFC. Effect of powdered shells treatment of the snail Megalobulimus lopesi on wounds of diabetic rats. Acta Cir Bras 2018;33:185–196 [DOI] [PubMed] [Google Scholar]
- 361. Ebaid H, Abdel-Salam B, Hassan I, Al-Tamimi J, Metwalli A, Alhazza I. Camel milk peptide improves wound healing in diabetic rats by orchestrating the redox status and immune response. Lipids Health Dis 2015;14:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Kwon AH, Qiu Z, Hashimoto M, Yamamoto K, Kimura T. Effects of medicinal mushroom (Sparassis crispa) on wound healing in streptozotocin-induced diabetic rats. Am J Surg 2009;197:503–509 [DOI] [PubMed] [Google Scholar]
- 363. Yamamoto K, Kimura T. Orally and topically administered Sparassis crispa (Hanabiratake) improved healing of skin wounds in mice with streptozotocin-induced diabetes. Biosci Biotechnol Biochem 2013;77:1303–1305 [DOI] [PubMed] [Google Scholar]
- 364. Bae JS, Jang KH, Jin HK. Polysaccharides isolated from Phellinus gilvus enhances dermal wound healing in streptozotocin-induced diabetic rats. J Vet Sci 2005;6:161–164 [PubMed] [Google Scholar]
- 365. Zhang Z, Cao G, Sha L, Wang D, Liu M. The efficacy of sodium aescinate on cutaneous wound healing in diabetic rats. Inflammation 2015;38:1942–1948 [DOI] [PubMed] [Google Scholar]
- 366. Lodhi S, Singhai AK: Wound healing effect of flavonoid rich fraction and luteolin isolated from Martynia annua Linn. on streptozotocin induced diabetic rats. Asian Pac J Trop Med 2013;6:253–259 [DOI] [PubMed] [Google Scholar]
- 367. Fujita N, Sakaguchi I, Kobayashi H, et al. An extract of the root of Lithospermun erythrorhison accelerates wound healing in diabetic mice. Biol Pharm Bull 2003;26:329–335 [DOI] [PubMed] [Google Scholar]
- 368. Nayak BS, Pinto Pereira LM. Catharanthus roseus flower extract has wound-healing activity in Sprague Dawley rats. BMC Complement Altern Med 2006;6:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Teoh SL, Latiff AA, Das S. The effect of topical extract of Momordica charantia (bitter gourd) on wound healing in nondiabetic rats and in rats with diabetes induced by streptozotocin. Clin Exp Dermatol 2009;34:815–822 [DOI] [PubMed] [Google Scholar]
- 370. Singh R, Garcia-Gomez I, Gudehithlu KP, Singh AK. Bitter melon extract promotes granulation tissue growth and angiogenesis in the diabetic wound. Adv Skin Wound Care 2017;30:16–26 [DOI] [PubMed] [Google Scholar]
- 371. Keat EC, Razak SS, Fadil NM, et al. The effect of Piper betel extract on the wound healing process in experimentally induced diabetic rats. Clin Ter 2010;161:117–120 [PubMed] [Google Scholar]
- 372. Lu LL, Wan P, Li LZ, Zhao MJ, Hu JY, Zhao YF. Experimental study on topical treatment of diabetic skin ulcers with yi medicine “yi bu a jie” extract. Chin J Integr Med 2013;19:464–467 [DOI] [PubMed] [Google Scholar]
- 373. Furumoto T, Ozawa N, Inami Y, et al. Mallotus philippinensis bark extracts promote preferential migration of mesenchymal stem cells and improve wound healing in mice. Phytomedicine 2014;21:247–253 [DOI] [PubMed] [Google Scholar]
- 374. Aksoy H, Sen A, Sancar M, et al. Ethanol extract of Cotinus coggygria leaves accelerates wound healing process in diabetic rats. Pharm Biol 2016;54:2732–2736 [DOI] [PubMed] [Google Scholar]
- 375. Ramazanov NS, Bobayev ID, Yusupova UY, et al. Phytoecdysteroids-containing extract from Stachys hissarica plant and its wound-healing activity. Nat Prod Res 2017;31:593–597 [DOI] [PubMed] [Google Scholar]
- 376. Zhang XN, Ma ZJ, Wang Y, et al. Angelica dahurica ethanolic extract improves impaired wound healing by activating angiogenesis in diabetes. PLoS One 2017;12:e0177862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. Azevedo IM, Araujo-Filho I, Teixeira MMA, Moreira M, Medeiros AC. Wound healing of diabetic rats treated with Moringa oleifera extract. Acta Cir Bras 2018;33:799–805 [DOI] [PubMed] [Google Scholar]
- 378. Lau TW, Lam FF, Lau KM, et al. Pharmacological investigation on the wound healing effects of Radix Rehmanniae in an animal model of diabetic foot ulcer. J Ethnopharmacol 2009;123:155–162 [DOI] [PubMed] [Google Scholar]
- 379. Lau KM, Lai KK, Liu CL, et al. Synergistic interaction between Astragali Radix and Rehmanniae Radix in a Chinese herbal formula to promote diabetic wound healing. J Ethnopharmacol 2012;141:250–256 [DOI] [PubMed] [Google Scholar]
- 380. Naji S, Zarei L, Pourjabali M, Mohammadi R. The extract of Lycium depressum stocks enhances wound healing in streptozotocin-induced diabetic rats. Int J Low Extrem Wounds 2017;16:85–93 [DOI] [PubMed] [Google Scholar]
- 381. Nayak BS, Isitor GN, Maxwell A, Bhogadi V, Ramdath DD. Wound-healing activity of Morinda citrifolia fruit juice on diabetes-induced rats. J Wound Care 2007;16:83–86 [DOI] [PubMed] [Google Scholar]
- 382. Nayak SB, Pinto Pereira L, Maharaj D. Wound healing activity of Carica papaya L. in experimentally induced diabetic rats. Indian J Exp Biol 2007;45:739–743 [PubMed] [Google Scholar]
- 383. Sarandy MM, Novaes RD, Xavier AA, et al. Hydroethanolic extract of Strychnos pseudoquina accelerates skin wound healing by modulating the oxidative status and microstructural reorganization of scar tissue in experimental type I diabetes. Biomed Res Int 2017;2017:9538351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Cooper R. Honey for wound care in the 21st century. J Wound Care 2016;25:544–552 [DOI] [PubMed] [Google Scholar]
- 385. ClinicalTrials.gov. The TRAfermin in Neuropathic Diabetic Foot Ulcer Study—Northern Europe The TRANS-North Study—Study Results—ClinicalTrials.gov. 2020. https://clinicaltrials.gov/ct2/show/results/NCT01217476 (last accessed February2020) [Google Scholar]
- 386. Kitamura M, Akamatsu M, Kawanami M, et al. Randomized placebo-controlled and controlled non-inferiority phase III trials comparing trafermin, a recombinant human fibroblast growth factor 2, and enamel matrix derivative in periodontal regeneration in intrabony defects. J Bone Miner Res 2016;31:806–814 [DOI] [PubMed] [Google Scholar]
- 387. DermaPro. Products. 2020. https://cytotools.de/products.html (last accessed February2020) [Google Scholar]
- 388. Taylor N. Phase III Flop Wipes 85% Off the Value of CytoTools. Framingham, MA: FierceBiotech, 2015 [Google Scholar]
- 389. BusinessWire. Derma Sciences Provides Update on Aclerastide Phase 3 Trials. Princeton, NJ: BusinessWire, 2020 [Google Scholar]
- 390. ClinicalTrials.gov. Phase 3 Study Evaluating Efficacy and Safety of DSC127 Compared With Vehicle and With Standard-of-care in Diabetic Foot Ulcers—Full Text View—ClinicalTrials.gov. ClinicalTrials.gov Bethesda, MD: U.S. National Library of Medicine, 2020 [Google Scholar]
- 391. Conroy E. Derma Sciences Terminates Phase III Diabetic Foot Ulcer Healing Clinical Trial. Leipzig, Germany: RegMedNet, 2019 [Google Scholar]
- 392. Dumville JC, Lipsky BA, Hoey C, Cruciani M, Fiscon M, Xia J: Topical antimicrobial agents for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev 2017;6:CD011038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Markakis K, Faris AR, Sharaf H, Faris B, Rees S, Bowling FL: Local antibiotic delivery systems: current and future applications for diabetic foot infections. Int J Low Extrem Wounds 2018;17:14–21 [DOI] [PubMed] [Google Scholar]
- 394. Community PT. Repifermin Phase II Study Fails To Meet Primary Endpoint. Yardley, PA: P&T Community, 2003 [Google Scholar]
- 395. Dipexium's Diabetic Foot Ulcer Candidate Fails Phase III Trials. Genetic Engineering and Biotech News. New Rochelle, NY: Mary Ann Liebert, Inc., 2016 [Google Scholar]
- 396. “Diabetic Foot Ulcer” Clinical Trials—Search Results: U.S. National Library of Medicine. 2020. https://clinicaltrials.gov/ct2/results?cond=Diabetic+Foot+Ulcer&age_v=&gndr=&type=&rslt=&phase=0&phase=1&phase=2&Search=Apply (last ccessed February 2020) [Google Scholar]
- 397. Fang RC, Galiano RD. A review of becaplermin gel in the treatment of diabetic neuropathic foot ulcers. Biologics 2008;2:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Ascierto PA, Marincola FM. Combination therapy: the next opportunity and challenge of medicine. J Transl Med 2011;9:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Edmonds M, Lázaro-Martínez JL, Alfayate-García JM, et al. Sucrose octasulfate dressing versus control dressing in patients with neuroischaemic diabetic foot ulcers (Explorer): an international, multicentre, double-blind, randomised, controlled trial. Lancet Diabetes Endocrinol 2018;6:186–196 [DOI] [PubMed] [Google Scholar]
- 400. Game F, Jeffcoate W, Tarnow L, et al. LeucoPatch system for the management of hard-to-heal diabetic foot ulcers in the UK, Denmark, and Sweden: an observer-masked, randomised controlled trial. Lancet Diabetes Endocrinol 2018;6:870–878 [DOI] [PubMed] [Google Scholar]
- 401. Lavery LA, Fulmer J, Shebetka KA, et al. The efficacy and safety of Grafix® for the treatment of chronic diabetic foot ulcers: results of a multi-centre, controlled, randomised, blinded, clinical trial. Int Wound J 2014;11:554–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402. Rayman G, Vas P, Dhatariya K, et al. Guidelines on use of interventions to enhance healing of chronic foot ulcers in diabetes (IWGDF 2019 update). Diabetes Metab Res Rev 2020;36:e3283. [DOI] [PubMed] [Google Scholar]