Abstract
Preeclampsia is an obstetric disorder that affects 3–8% of pregnant women and remains a leading cause of short- and long-term neonatal and maternal morbidity and mortality. Professional societies recommend the use of low dose aspirin to prevent preeclampsia in high-risk women. However, interest in prevention of this disease and better understanding of its pathophysiology have led to growing research on other agents. This review focuses on the main therapeutic agents evaluated or in use for preeclampsia prevention.
Keywords: Preeclampsia, Aspirin, Statin, Metformin, Esomeprazole, Pregnancy
Introduction, Epidemiology, and Risk Factors
Preeclampsia is a multisystem disorder that affects 3–8% of pregnancies in the US and 1.5 and 16.7% worldwide, and results in 60,000 maternal deaths and >500,000 preterm birth worldwide each year. Geographic, social, economic, and racial differences may explain the different rates of preeclampsia seen in different populations. Worldwide, preeclampsia is the second leading cause of maternal death, with estimates of at least 16% among low-middle income countries up to more than 25% in certain countries in Latin America1–3.
Preeclampsia is characterized by new-onset hypertension which usually occurs after 20 weeks’ gestation, and evidence of end-organ dysfunction3. The end-organ disease resulting from the preeclampsia is varied, and can include proteinuria, acute kidney injury, hepatic dysfunction, hemolysis, thrombocytopenia, and – less frequently – liver rupture, seizures (eclampsia), stroke, and death4. There are a number of risk factors for developing preeclampsia such as history of preeclampsia in a prior pregnancy, diabetes, hypertension, obesity, and multiple pregnancy3. The reported incidence of preeclampsia for twin gestation ranges from 8–20% and for triplets’ ranges from 12–34%, however if the index pregnancy affected by preeclampsia was a multiple gestation, the risk of recurrence in a subsequent pregnancy is lower than if the index pregnancy was a singleton (6.8% vs. 14.1%, p < 0.001)5,6.
Preeclampsia remains a major cause of maternal mortality and morbidity including seizures, acute kidney injury, pulmonary edema, severe hypertension, cerebrovascular events, and liver injury3. However, the consequences of preeclampsia are long-arching, and preeclampsia has been associated with an increased risk of future maternal cardiovascular, metabolic, and cerebrovascular diseases and premature mortality7. Preeclampsia is also associated with adverse neonatal outcomes, usually secondary to iatrogenic preterm delivery and increased risk of fetal growth restriction and placental abruption. These include respiratory distress syndrome, bronchopulmonary dysplasia, retinopathy of prematurity, necrotizing enterocolitis, neonatal intensive care units (NICU) admission, neurodevelopmental delay, and fetal or neonatal death8.
The adverse intrauterine environment associated with preeclampsia is thought to contribute to the association between maternal preeclampsia and childhood and adult chronic disease in the offspring, such as obesity, diabetes, hypertension, and neurodevelopmental abnormalities 9,10. Due to the above adverse outcomes, preeclampsia adds a substantial financial burden on the health care system in the United States, estimated more than $1.03 billion in maternal costs and $1.15 billion in infant costs, in the first year after delivery 11.
The only current cure for preeclampsia is delivery of the placenta and fetus, however this is commonly associated with iatrogenic preterm delivery. In an effort to prevent that and improve outcomes for mothers, children and adult offspring, research efforts are currently focused not only on treatment of preeclampsia, but on ways to prevent preeclampsia from occurring. Recent advances in understanding the pathogenesis of preeclampsia and need to reduce its short- and long-term morbidities, led to research in novel agents for either the prevention or treatment of preeclampsia. These include agents such as anti-digoxin antibodies, antithrombin, relaxin, proton pump inhibitors, 3-hydroxy-3-methylglutaryl-coenzyme-A reductase inhibitors (statins), use of apheresis and others. Discussion of all these therapeutics is beyond the scope of this paper, which will review the most promising therapeutics aimed at prevention of preeclampsia, including aspirin, statins, metformin, and esomeprazole.
Pathophysiology of Preeclampsia
While not fully understood, the pathophysiology of preeclampsia is likely a multifactorial combination of genetic and environmental factors, and abnormal placentation3,12. The genetic and environmental components to the disease are evident by epidemiological studies suggesting a hereditary component to preeclampsia, and an apparent contribution of risk factors such as low socioeconomic status, maternal obesity and geographical variations to the risk of preeclampsia. 1,3,13.
Contemporary evidence suggests that preeclampsia is a two-stage disease. The first stage is an early pregnancy asymptomatic stage, resulting from poor placentation due to abnormal trophoblast invasion and spiral artery remodeling. This results in the second stage of the disease, characterized by a placental ischemia/reperfusion injury and a maternal immune-mediated response. Consequently, there is a release of anti-angiogenic factors and placental debris into the maternal circulation and an inadequate release of pro-angiogenic factors. This leads to an angiogenic imbalance, immune-mediated exaggerated inflammatory response, and endothelial cell dysfunction which result in enhanced platelet aggregation, abnormal activation of the coagulation system, and increased systemic vascular. The overall consequence of this cascade is the clinical manifestations such as elevated blood pressure, proteinuria and other end-organ injury 3,12,14,15. The poor placentation results in abnormal fetal perfusion, which is evident by abnormal uterine artery blood flow and a 22.2% incidence of fetal growth restriction in pregnancies affected by preeclampsia, especially among preterm gestations 16,17. This abnormal perfusion is often seen on uterine artery Doppler evaluation as notching. However, the utility of this finding to predict preeclampsia is limited17.
The placental anti-angiogenic factors most commonly studied and thought to contribute to the disease are soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble endoglin (sEng)18,19. These molecules cause maternal vasoconstriction and hypertension, possibly in an effort to improve placental perfusion20. The pro-angiogenic placental factors inhibited in the disease process include placental growth factor (PlGF) and vascular endothelial growth factor (VEGF). This inhibition is likely due to a combination of placental ischemia and the anti-angiogenic inhibitory effects of sFlt-1 and sEng18,21.
The inflammatory response resulting from preeclampsia activates cyclooxygenase (COX)22, which increases thromboxane A2 (TxA2) levels and reduces endothelial cell prostacyclin levels (PGI2)22,23. TxA2 increases platelet aggregation and causes vasoconstriction, and PGI2 counteracts these effects24. The inflammatory response is therefore also a contributor to the disease phenotype. (Figure)
Figure:
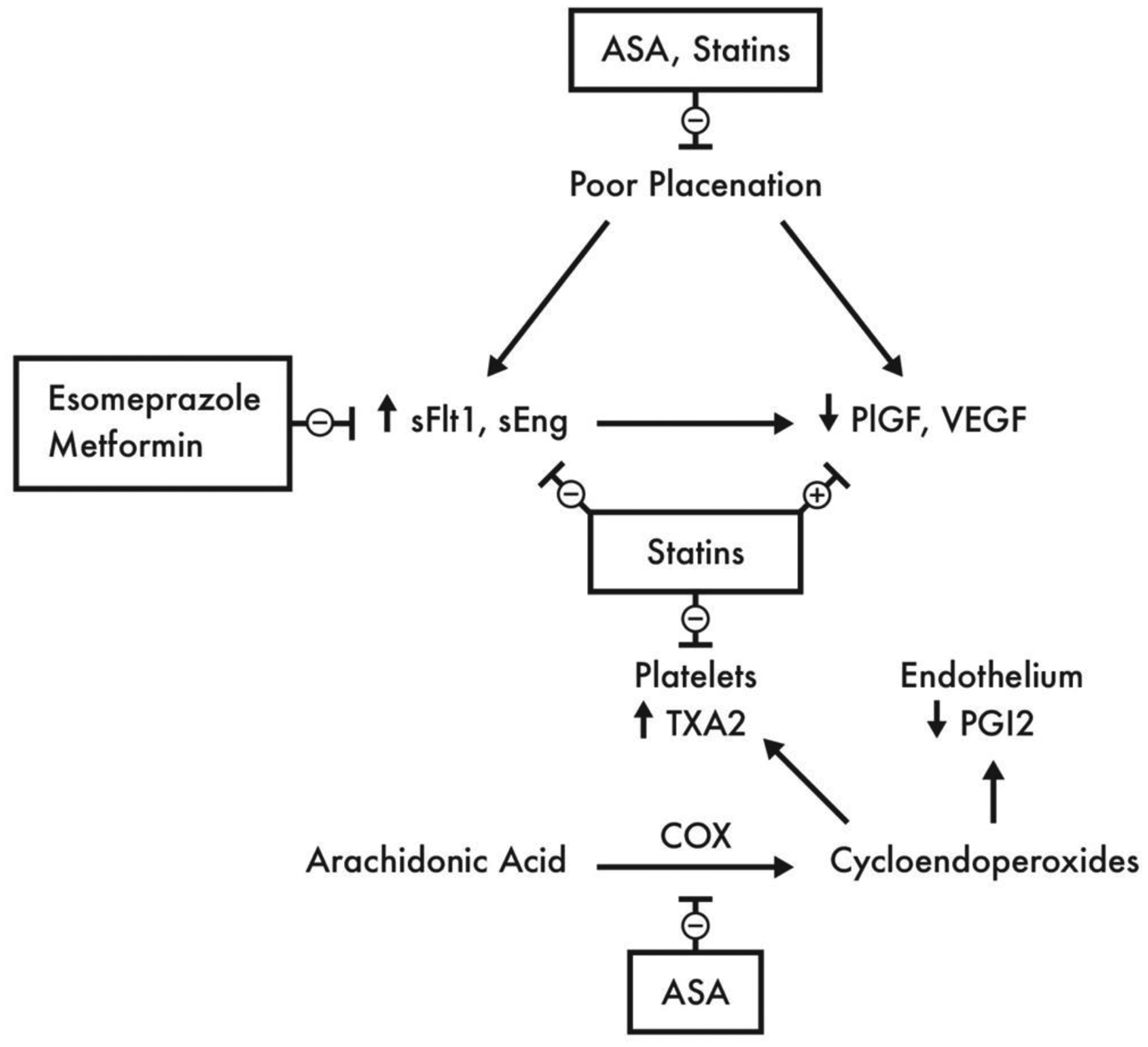
Pathophysiology of Preeclampsia and Proposed Mechanisms of Actions of Preventive Agents
ASA, Aspirin; COX, cyclooxygenase; sEng, soluble endoglin; sFlt1, soluble fms-like tyrosine kinase 1; PGI2, prostaglandin I2 (prostacyclin); PlGF, placental growth factor; TXA2, thromboxane A2; VEGF, vascular endothelial growth factor
Aspirin
Aspirin is a non-steroidal anti-inflammatory drug, which acts by non-selectively and irreversibly inhibiting COX, resulting in anti-platelet and anti-inflammatory effects by preventing the conversion of arachidonic acid to thromboxane and prostaglandins, including TxA2 and PGI223,25. PGI2, unlike TxA2, is rapidly repleted, and the overall net effect of aspirin is therefore a preferential inhibition of TxA223,26. Aspirin also inhibits hypoxia-induced sFlt-1 overexpression by inhibiting COX-1 as an added mechanism of counteracting preeclampsia 27,28. (Figure)
Aspirin is currently the only medication recommended for the prevention of preeclampsia. Studies on aspirin use for preeclampsia prevention date back to 1979, and have used doses ranging from 50 to 150mg daily starting at various gestational ages in low- and high-risk women29. Although the results are mixed, more recent systematic reviews and meta-analyses suggest that this may be due to variations in aspirin dosing and timing of drug initiation in the individual studies, with the most beneficial effects seen when aspirin is started before 16 weeks’ gestation29,30. Currently, both the U.S. Preventive Services Task Force (USPSTF) and the American College of Obstetricians and Gynecologists (ACOG) recommend aspirin use for preeclampsia prevention for women at high risk for developing the disease (e.g. those with chronic hypertension, pre-gestational diabetes mellitus, multifetal gestation, renal disease, and autoimmune disease, etc), and to be started between 12 and 28 weeks’ gestation and continued until delivery3,31. In the U.S. aspirin is available as 81-mg formulation; thus, this is the dose most commonly recommended and clinically used. In addition, there are no studies comparing the effects of different doses of aspirin and none of the studies suggesting the benefit from a higher dose were conducted in the US. Moreover, fetal and long-term children safety data of high-dose aspirin use during gestation are limited.
While ACOG and USPSTF utilize risk factors based on history and clinical characteristics to identify women at risk for developing preeclampsia, more recent studies have investigated the use of first-trimester screening tests which include assessment of serum PlGF concentration, uterine artery doppler studies, and other maternal parameters to identify patients at highest risk for developing preeclampsia who would benefit from prophylactic aspirin use32. However, in randomized clinical trials, these screening tests underperformed due to low positive predictive value. In addition, preeclampsia prevention was limited to small number of preterm preeclampsia, and most screen-positive patients would not benefit from interventions. Due to their under-performance and lack of data form the U.S., first trimester screening using biomarkers and ultrasonography remains investigational and is not endorsed by professional societies in the US 33. However, some experts have advocated for universal aspirin use during pregnancy, due to its low cost of approximately $5 throughout pregnancy, its well-studied maternal and neonatal safety profile, and the potential for it to reduce the burden of preeclampsia, improve maternal and fetal outcomes, and reduce healthcare cost34,35. This approach has not been endorsed by professional societies in the U.S.
Lastly, aspirin use appears to be safe during pregnancy as there have been multiple studies that failed to identify an association between low-dose aspirin use during pregnancy and higher risk of placental abruption, postpartum hemorrhage, spinal hematoma, congenital anomalies, persistent pulmonary hypertension in the neonate, premature closure of the ductus arteriosus, or neonatal bleeding complications or intracranial hemorrhage36. There have also been no notable adverse neonatal or childhood outcomes following in-utero aspirin exposure, with follow-up to 18 years of age31.
Statins
Statins are competitive inhibitors of the enzyme 5-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, which converts HMG-CoA to mevalonic acid37. Recently, there is growing interest in the role of statins to prevent preeclampsia due to increasing number of studies demonstrating strong biological plausibility to reverse or ameliorate several pathophysiological pathways associated with preeclampsia. Animal models suggest that statins increase the production of PlGF, and reduce sFlt and TxA238. Additional pleiotropic actions of statins include enhanced trophoblastic invasion and improved placental blood flow, anti-inflammatory and antioxidant effects, endometrial protection, inhibition of platelet adhesion, and anticoagulant effects39. These properties are thought to counteract the pathophysiological pathways of preeclampsia, and may result in a protective effect against it or amelioration of its manifestations on maternal, fetal and neonatal wellbeing40. (Figure)
Studies into the use of statins for preeclampsia have evaluated it using a therapeutic approach for patients who develop preeclampsia, and a prophylactic approach for women at high-risk for developing the disease. In animal models of preeclampsia, statins were shown to resolve the clinical manifestations of preeclampsia, and prevent associated fetal growth restriction41,42. Other findings include increased nitric oxide production in the vasculature, reversing angiogenic imbalance, and anti-inflammatory and oxidative actions43. Initial data from case reports demonstrated that, when given to women with preterm preeclampsia, pravastatin use was associated with improvement in blood pressure and reduction in sFlt-1 serum concentrations, and improved pregnancy outcomes44. Other reports demonstrated that pravastatin prevented fetal demise in patient with massive perivillous fibrin deposition in the placenta and improved angiogenic profile45. A pilot multicenter, randomized, placebo-controlled trial enrolled high-risk women with a history of preeclampsia that required delivery before 34 weeks in a prior pregnancy. These women were randomized between 12- and 16-weeks’ gestation to receive either pravastatin 10 mg daily or placebo. Findings from the study demonstrated no identifiable maternal or fetal/neonatal safety risk signals associated with pravastatin therapy. In addition, clinical outcomes including the rates of preeclampsia and indicated preterm delivery tended to be lower, and the angiogenic imbalance reversed in subjects receiving pravastatin46.
However, results from other human studies especially those where pravastatin was used after the development of preeclampsia, are mixed43. In a prospective cohort of women with antiphospholipid syndrome and poor obstetrical history, addition of pravastatin to standard of care in women who developed preeclampsia or intrauterine uterine growth restriction, led to improved pregnancy and neonatal outcomes. On the other hand, a recent randomized double-blinded placebo-controlled proof of concept trial (Statins to Ameliorate early onset Preeclampsia (StAmP) trial) did not show a benefit in using pravastatin in patients who develop early preeclampsia, with no evidence of improved angiogenic biomarkers or lower preeclampsia rates47. Despite the imitations of the study which included small sample size, slow recruitment, overoptimistic sample size assumptions, and lack of power to identify differences in clinical outcomes, the StAmP trial supported the safety of pravastatin as there were no maternal serious adverse events and extremely low drug concentrations in the cord. The latter finding is similar to the pilot randomized trial in the US, which showed that the majority of fetuses exposed to pravastatin, had drug concentration in their cord blood below or close to the detection limit of the assay46.
Statins were initially classified as Category X by the FDA. This was due to poorly designed, small, retrospective studies that suggested an increase in teratogenic effects with statin use39, in addition to the absence of any indications to use statins in pregnancy. More contemporary studies and systematic reviews suggest that statins, and in particular pravastatin, do not demonstrate increased risk in fetal malformations, stillbirth, spontaneous abortions, or effects on fetal cholesterol levels or fetal weight39,46. These findings could be expected by the unique pharmacokinetic and properties of pravastatin. Pravastatin is among the most hepatoselective and hydrophilic statins with limited transplacental transfer, as demonstrated in placental transfer studies and from the observed cord blood drug concentrations in the two clinical trials described above46–48.
Metformin
Metformin, or dimethyl-biguanide hydrochloride, is a biguanide used mainly as an anti-diabetic agent49. Its mechanisms of action include inhibition of hepatic gluconeogenesis, reducing gastrointestinal glucose absorption, and increasing glucose absorption by peripheral tissues49. By inhibiting sFlt-1 and sEng, metformin counteracts their antiangiogenic effects50. This may overall reverse the placental perfusion and imbalance of angiogenic and antiangiogenic factors in the clinical preeclampsia spectrum. However, clinical data on the effectiveness of metformin as a prophylactic agent for preeclampsia prevention are varied51. In a recent randomized, double blind, placebo-controlled trial of pregnant women with a BMI >35 without diabetes, the use of metformin resulted in a 75% reduction in the incidence of preeclampsia52. However, a recent meta-analysis of five randomized trials concluded that metformin use did not result in a significant reduction in the incidence of preeclampsia51.
The safety profile of metformin use in pregnancy is well-established. The most common maternal side effect is transient gastrointestinal symptoms in up to 25% of women53. Although metformin freely crosses the placenta, it is poorly metabolized by the fetus, and multiple studies have shown no evidence of teratogenicity or adverse fetal or neonatal effects, however long-term data on neurodevelopmental or metabolic outcomes associated with its use are limited54–57.
Esomeprazole
The use of proton pump inhibitors including esomeprazole is safe in pregnancy, and has not been found to be associated with fetal teratogenicity, miscarriage or preterm birth58. In preclinical studies, esomeprazole was associated with an inhibition of sFlt-1 and sEng production, with associated vasodilation and a reduction in endothelial dysfunction59. In an sFlt-1 overexpression animal model, esomeprazole was able to counteract preeclampsia symptoms59.
However, human studies using esomeprazole have been less promising. In a randomized, placebo-controlled study, there was no apparent pregnancy prolongation or reduction in sFlt-1 with using 40mg of esomeprazole daily60. Further research is needed to evaluate the use of higher doses of esomeprazole and using the medication as a prophylactic agent for preeclampsia prevention.
Conclusion
Contemporary research into prophylactic and therapeutic interventions for preeclampsia are providing novel and promising modalities. Research into this topic remains a major endeavor, and as our understanding of the disease is evolving, studies will continue to pave the way for new effective therapeutics for preeclampsia.
Practice points:
Aspirin is recommended for the prevention of preeclampsia in high-risk women
First trimester screening for preeclampsia is not recommended in the US
Research directions:
The optimal dose of aspirin in preeclampsia prevention, and the cohort of women who will benefit from higher dose
The effectiveness of statins in preventing preeclampsia
The role of statin in managing pregnancies complicated by early onset preeclampsia
Conflicts of Interest & Funding Source:
Maged Costantine, MD is supported by a grant from The Eunice Kennedy Shriver National Institute of Child Health and Human Development (5 UG1 HD027915-29) and the National Heart, Lung, and Blood Institute (5UH3HL140131). This paper does not necessarily represent the official views of the NICHD, NHLBI, or the National Institute of Health.
References
- 1.World Health Organization. Geographic variation in the incidence of hypertension in pregnancy. World Health Organization International Collaborative Study of Hypertensive Disorders of Pregnancy. Am J Obstet Gynecol. 1988;158(1):80–3. [PubMed] [Google Scholar]
- 2.Firoz T, Sanghvi H, Merialdi M, Von Dadelszen P. Pre-eclampsia in low and middle income countries. Best Pract Res Clin Obstet Gynaecol. 2011;25:537–48. [DOI] [PubMed] [Google Scholar]
- 3.Espinoza J, Vidaeff A, Pettker CM, Simhan H. ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet Gynecol. 2019;133(1):e1–25. [DOI] [PubMed] [Google Scholar]
- 4.Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet Gynecol. 2017;133(1):e1–25. [DOI] [PubMed] [Google Scholar]
- 5.Barton JR, Sibai BM. Prediction and prevention of recurrent preeclampsia. Obstet Gynecol. 2008;112:359–72. [DOI] [PubMed] [Google Scholar]
- 6.Trogstad L, Skrondal A, Stoltenberg C, Magnus P, Nesheim B-I, Eskild A. Recurrence risk of preeclampsia in twin and singleton pregnancies. Am J Med Genet. 2004;126A:41–5. [DOI] [PubMed] [Google Scholar]
- 7.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. BMJ. 2007;335(7627):974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Backes CH, Markham K, Moorehead P, Cordero L, Nankervis CA, Giannone PJ. Maternal preeclampsia and neonatal outcomes. J Pregnancy. 2011;2011:214365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alsnes IV, Vatten LJ, Fraser A, Bjørngaard JH, Rich-Edwards J, Romundstad PR, et al. Hypertension in Pregnancy and Offspring Cardiovascular Risk in Young Adulthood: Prospective and Sibling Studies in the HUNT Study (Nord-Trøndelag Health Study) in Norway. Hypertension. 2017;69(4):591–8. [DOI] [PubMed] [Google Scholar]
- 10.Wu CS, Nohr EA, Bech BH, Vestergaard M, Catov JM, Olsen J. Health of children born to mothers who had preeclampsia: a population-based cohort study. Am J Obstet Gynecol. 2009;201(3):269.e1–269.e10. [DOI] [PubMed] [Google Scholar]
- 11.Stevens W, Shih T, Incerti D, Ton TGN, Lee HC, Peneva D, et al. Short-term costs of preeclampsia to the United States health care system. Am J Obstet Gynecol. 2017;217(3):237–48. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham F, Leveno K, Bloom F, Spong C, Dashe J, Hoffman B. Williams Obstetrics. 24th ed. New York: McGraw-Hill Education; 2014. [Google Scholar]
- 13.Esplin M, Fausett M, Fraser A, Kerber R, Mineau G, Carrillo J, et al. Paternal and maternal components of the predisposition to preeclampsia. N Engl J Med. 2011;344(12):867–72. [DOI] [PubMed] [Google Scholar]
- 14.Sargent IL, Germain SJ, Sacks GP, Kumar S, Redman CWG. Trophoblast deportation and the maternal inflammatory response in pre-eclampsia. J Reprod Immunol. 2003;59:153–60. [DOI] [PubMed] [Google Scholar]
- 15.Dekker GA, Sibai BM. Etiology and pathogenesis of preeclampsia: Current concepts. Am J Obstet Gynecol. 1998;179:1359–75. [DOI] [PubMed] [Google Scholar]
- 16.Villar J, Carroli G, Wojdyla D, Abalos E, Giordano D, Ba’aqeel H, et al. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions? Am J Obstet Gynecol. 2006;194:921–31. [DOI] [PubMed] [Google Scholar]
- 17.Sciscione AC, Hayes EJ. Uterine artery Doppler flow studies in obstetric practice. Am J Obstet Gynecol. 2009;201(2):121–6. [DOI] [PubMed] [Google Scholar]
- 18.Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennström M, et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N Engl J Med. 2016;374(1):13–22. [DOI] [PubMed] [Google Scholar]
- 19.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. [DOI] [PubMed] [Google Scholar]
- 20.Roberts JM. Pathophysiology of ischemic placental disease. Semin Perinatol. 2014;38(3):139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee Kim Y, Gonçalves LF, et al. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia: Young Investigator Award. Am J Obstet Gynecol. 2004;190:1541–50. [DOI] [PubMed] [Google Scholar]
- 22.Walsh SW. Eicosanoids in preeclampsia. Prostaglandins, Leukot Essent Fat Acids. 2004;70:223–32. [DOI] [PubMed] [Google Scholar]
- 23.Walsh SW. Preeclampsia: An imbalance in placental prostacyclin and thromboxane Production. Am J Obstet Gynecol. 1985;152(3):335–40. [DOI] [PubMed] [Google Scholar]
- 24.Moncada S, Vane JR. Arachidonic Acid Metabolites and the Interactions between Platelets and Blood-Vessel Walls. N Engl J Med. 1979;300:1142–7. [DOI] [PubMed] [Google Scholar]
- 25.Atallah A, Lecarpentier E, Goffinet F, Doret-Dion M, Gaucherand P, Tsatsaris V. Aspirin for Prevention of Preeclampsia. Drugs. 2017;77:1819–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Majed BH, Khalil RA. Molecular Mechanisms Regulating the Vascular Prostacyclin Pathways and Their Adaptation during Pregnancy and in the Newborn. Pharmacol Rev. 2012;64(3):540–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin L, Li G, Zhang W, Wang Y, Yang H. Low-dose aspirin reduces hypoxia-induced sFlt1 release via the JNK/AP-1 pathway in human trophoblast and endothelial cells. J Cell Physiol. 2019;234(10):18928–41. [DOI] [PubMed] [Google Scholar]
- 28.Li C, Raikwar NS, Santillan MK, Santillan DA, Thomas CP. Aspirin inhibits expression of sFLT1 from human cytotrophoblasts induced by hypoxia, via cyclo-oxygenase 1. Placenta. 2015;36(4):446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberge S, Bujold E, Nicolaides KH. Aspirin for the prevention of preterm and term preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol. 2018;218(3):287–293.e1. [DOI] [PubMed] [Google Scholar]
- 30.Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol. 2017;216(2):110–120.e6. [DOI] [PubMed] [Google Scholar]
- 31.LeFevre ML. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(11):819–26. [DOI] [PubMed] [Google Scholar]
- 32.Rolnik DL, O’Gorman N, Roberge S, Bujold E, Hyett J, Uzan S, et al. Early screening and prevention of preterm pre-eclampsia with aspirin: time for clinical implementation. Ultrasound Obstet Gynecol. 2017;50:551–6. [DOI] [PubMed] [Google Scholar]
- 33.Rolnik DL, Wright D, Poon L, O’Gorman N, Syngelaki A, Matallana C de P, et al. Aspirin versus placebo in pregnancies at high risk for preterm pre-eclampsia. N Engl J Med. 2017;377(7):613–22. [DOI] [PubMed] [Google Scholar]
- 34.Ayala NK, Rouse DJ. A Nudge Toward Universal Aspirin for Preeclampsia Prevention. Obstet Gynecol. 2019;133(4):725–8. [DOI] [PubMed] [Google Scholar]
- 35.Werner EF, Hauspurg AK, Rouse DJ. A cost-benefit analysis of low-dose aspirin prophylaxis for the prevention of preeclampsia in the United States. Obstet Gynecol. 2015;126(6):1242–50. [DOI] [PubMed] [Google Scholar]
- 36.Duley L, Meher S, Hunter KE, Seidler AL, Askie LM. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2019;2019(10):CD004659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caniggia I, Post M, Ermini L. Statins, Mevalonate Pathway and its Intermediate Products in Placental Development and Preeclampsia. Curr Mol Pharmacol. 2016;10:152–60. [DOI] [PubMed] [Google Scholar]
- 38.Costantine MM, Tamayo E, Lu F, Bytautiene E, Longo M, Hankins GDV, et al. Using pravastatin to improve the vascular reactivity in a mouse model of soluble Fms-like tyrosine kinase-1-induced preeclampsia. Obstet Gynecol. 2010;116(116):114–20. [DOI] [PubMed] [Google Scholar]
- 39.Esteve-Valverde E, Ferrer-Oliveras R, Gil-Aliberas N, Baraldès-Farré A, Llurba E, Alijotas-Reig J. Pravastatin for Preventing and Treating Preeclampsia: A Systematic Review. Obstet Gynecol Surv. 2018;73(1):40–55. [DOI] [PubMed] [Google Scholar]
- 40.Garrett N, Pombo J, Umpierrez M, Clark JE, Simmons M, Girardi G. Pravastatin therapy during preeclampsia prevents long-term adverse health effects in mice. JCI Insight. 2018;3(8):e120147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumasawa K, Ikawa M, Kidoya H, Hasuwa H, Saito-Fujita T, Morioka Y, et al. Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc Natl Acad Sci. 2010;108(4):1451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bauer AJ, Banek CT, Needham K, Gillham H, Capoccia S, Regal JF, et al. Pravastatin attenuates hypertension, oxidative stress, and angiogenic imbalance in rat model of placental ischemia-induced hypertension. Hypertension. 2013;61:1103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma’ayeh M, Rood KM, Kniss D, Costantine MM. Novel Interventions for the Prevention of Preeclampsia. Curr Hypertens Rep. 2020;22(2):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brownfoot FC, Tong S, Hannan NJ, Binder NK, Walker SP, Cannon P, et al. Effects of Pravastatin on Human Placenta, Endothelium, and Women with Severe Preeclampsia. Hypertension. 2015;66:687–97. [DOI] [PubMed] [Google Scholar]
- 45.Chaiworapongsa T, Romero R, Korzeniewski SJ, Chaemsaithong P, Hernandez-Andrade E, Segars JH, et al. Pravastatin to prevent recurrent fetal death in massive perivillous fibrin deposition of the placenta (MPFD). J Matern Neonatal Med. 2016;29(6):855–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Costantine MM, Cleary K, Hebert MF, Ahmed MS, Brown LM, Ren Z, et al. Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high-risk pregnant women: A pilot randomized controlled trial. Am J Obstet Gynecol. 2016;214(720):e1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahmed A, Williams DJ, Cheed V, Middleton LJ, Ahmad S, Wang K, et al. Pravastatin for early-onset pre-eclampsia: a randomised, blinded, placebo-controlled trial. BJOG An Int J Obstet Gynaecol. 2020;127(4):478–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nanovskaya TN, Patrikeeva SL, Paul J, Costantine MM, Hankins GDV, Ahmed MS. Transplacental transfer and distribution of pravastatin. Am J Obstet Gynecol. 2013;209:373.e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: From mechanisms of action to therapies. Cell Metab. 2014;20(6):953–66. [DOI] [PubMed] [Google Scholar]
- 50.Brownfoot FC, Hastie R, Hannan NJ, Cannon P, Tuohey L, Parry LJ, et al. Metformin as a prevention and treatment for preeclampsia: Effects on soluble fms-like tyrosine kinase 1 and soluble endoglin secretion and endothelial dysfunction. Am J Obstet Gynecol. 2016;214:356.e1–15. [DOI] [PubMed] [Google Scholar]
- 51.Alqudah A, McKinley MC, McNally R, Graham U, Watson CJ, Lyons TJ, et al. Risk of pre-eclampsia in women taking metformin: a systematic review and meta-analysis. Diabet Med. 2018;35:160–72. [DOI] [PubMed] [Google Scholar]
- 52.Syngelaki A, Nicolaides KH, Balani J, Hyer S, Akolekar R, Kotecha R, et al. Metformin Versus Placebo in Obese Pregnant Women Without Diabetes Mellitus. N Engl J Med. 2016;374:434–43. [DOI] [PubMed] [Google Scholar]
- 53.McCreight LJ, Bailey CJ, Pearson ER. Metformin and the gastrointestinal tract. Diabetologia. 2016;59:426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nanovskaya TN, Nekhayeva IA, Patrikeeva SL, Hankins GD V, Ahmed MS. Transfer of metformin across the dually perfused human placental lobule. Am J Obstet Gynecol. 2006;195:1081–5. [DOI] [PubMed] [Google Scholar]
- 55.Cassina M, Donà M, Di Gianantonio E, Litta P, Clementi M. First-trimester exposure to metformin and risk of birth defects: A systematic review and meta-analysis. Hum Reprod Update. 2014;20:656–9. [DOI] [PubMed] [Google Scholar]
- 56.Wouldes TA, Battin M, Coat S, Rush EC, Hague WM, Rowan JA. Neurodevelopmental outcome at 2 years in offspring of women randomised to metformin or insulin treatment for gestational diabetes. Arch Dis Child Fetal Neonatal Ed. 2016;101:F488–93. [DOI] [PubMed] [Google Scholar]
- 57.Rowan JA, Rush EC, Plank LD, Lu J, Obolonkin V, Coat S, et al. Metformin in Gestational Diabetes: The Offspring Follow-up (MiG TOFU): Body Composition and Metabolic Outcomes at 7–9 Years of Age. BMJ Open Diabetes Res Care. 2018;6(1):e000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gill SK, O’Brien L, Einarson TR, Koren G. The safety of proton pump inhibitors (PPIs) in pregnancy: A meta-analysis. Am J Gastroenterol. 2009;104(6):1541–5. [DOI] [PubMed] [Google Scholar]
- 59.Onda K, Tong S, Beard S, Binder N, Muto M, Senadheera SN, et al. Proton pump inhibitors decrease soluble fms-like tyrosine kinase-1 and soluble endoglin secretion, decrease hypertension, and rescue endothelial dysfunction. Hypertension. 2017;69(3):457–68. [DOI] [PubMed] [Google Scholar]
- 60.Cluver CA, Hannan NJ, van Papendorp E, Hiscock R, Beard S, Mol BW, et al. Esomeprazole to treat women with preterm preeclampsia: a randomized placebo controlled trial. Am J Obstet Gynecol. 2018;219:388.e1–17. [DOI] [PubMed] [Google Scholar]


