Abstract
Purpose
Our previous work demonstrated that miRNA-495 targets SOX9 to inhibit chondrogenesis of mesenchymal stem cells. In this study, we aimed to investigate whether miRNA-495-mediated SOX9 regulation could be a novel therapeutic target for osteoarthritis (OA) using an in vitro cell culture model.
Materials and Methods
An in vitro model mimicking the OA environment was established using TC28a2 normal human chondrocyte cells. Interleukin-1β (IL-1β, 10 ng/mL) was utilized to induce inflammation-related changes in TC28a2 cells. Safranin O staining and glycosaminoglycan assay were used to detect changes in proteoglycans among TC28a2 cells. Expression levels of COX-2, ADAMTS5, MMP13, SOX9, CCL4, and COL2A1 were examined by qRT-PCR and/or Western blotting. Immunohistochemistry was performed to detect SOX9 and CCL4 proteins in human cartilage tissues obtained from patients with OA.
Results
miRNA-495 was upregulated in IL-1β-treated TC28a2 cells and chondrocytes from damaged cartilage tissues of patients with OA. Anti-miR-495 abolished the effect of IL-1β in TC28a2 cells and rescued the protein levels of SOX9 and COL2A1, which were reduced by IL-1β. SOX9 was downregulated in the damaged cartilage tissues of patients with OA, and knockdown of SOX9 abolished the effect of anti-miR-495 on IL-1β-treated TC28a2 cells.
Conclusion
We demonstrated that inhibition of miRNA-495 alleviates IL-1β-induced inflammatory responses in chondrocytes by rescuing SOX9 expression. Accordingly, miRNA-495 could be a potential novel target for OA therapy, and the application of anti-miR-495 to chondrocytes could be a therapeutic strategy for treating OA.
Keywords: Chondrocytes, inflammation, osteoarthritis, miRNA-495, SOX9
INTRODUCTION
Osteoarthritis (OA) is a painful disease that affects approximately 10% of adults above 60 years of age.1 OA is characterized by synovitis, chondrocyte apoptosis, and inflammation, resulting in the destruction of articular cartilage. Apart from these characteristics, chondrocytes can respond to external stimuli and tissue damage and are responsible for OA.2 Chondrocytes in articular cartilage tissue play a pivotal role in maintaining tissue health by secreting extracellular matrix (ECM) proteins.3 Chondrocyte dysfunction leads to matrix proteinase activation4 and the degradation of the ECM,5 resulting in the deterioration of cartilage integrity and the development of OA. In an OA environment, chondrocytes undergo physiological changes and secrete proteolytic enzymes, such as MMP136 or ADAMTS5,7 that, in turn, cause damage to the cartilage tissue, leading to joint dysfunction.8 Therefore, it is important to understand the changes in chondrocyte behavior in an osteoarthritic environment in order to establish a successful strategy for OA therapy.
Investigation of genes involved in OA pathogenesis can provide molecular insights, and related genes can be considered as candidates with which to identify novel therapeutic targets for treating OA. In particular, non-coding RNAs (ncRNAs) are excellent targets for OA gene therapy since they do not encode proteins, and thus, targeting ncRNAs may have fewer unexpected side effects than targeting protein-coding genes.9 ncRNAs are classified based on their transcript length: small ncRNAs are less than 200 nucleotides and long non-coding RNAs are more than 200 nucleotides. MicroRNAs (miRNAs) are a class of small ncRNAs that inhibit mRNA translation of target genes by binding to the 3′-untranslated region (UTR) of target mRNAs. miRNAs play a role in multiple cellular functions and can have multiple targets, and a single gene can be regulated by multiple miRNAs.10 To date, many miRNAs have been described as novel targets for OA therapy and are anticipated to be of use as biomarkers for the diagnosis and prognosis of OA.11
In this study, we aimed to evaluate the effects of anti-miR-495 on immortalized human chondrocyte cells (TC28a2 cells) under inflammatory conditions. Our previous study demonstrated that introduction of anti-miR-495 improved the differentiation potential of the chondrogenic lineage among mesenchymal stem cells (MSCs) by disrupting the binding between miRNA-495 and the 3′-UTR of SOX9 mRNA.12 Recent studies have shown that miRNA-495 is involved in chondrocyte apoptosis by regulating the apoptotic signaling pathways, such as AKT/mTOR and NF-κB.13,14 These studies have also indicated that miRNA-495 is highly expressed in the cartilage of rats with OA, compared with rats in sham groups. Therefore, it might be meaningful to investigate whether miRNA-495 could be a novel target molecule for OA therapeutics. In the current study, we showed that miRNA-495 is upregulated in interleukin-1β (IL-1β)-treated TC28a2 cells and primary-cultured chondrocytes obtained from damaged cartilage tissues of patients with OA. Transfection of anti-miR-495 into IL-1β-treated TC28a2 cells decreased the upregulation of inflammatory markers and rescued reductions in chondrocyte marker levels. We also examined the protein levels of miRNA-495 targets, such as SOX9 and CCL4, in the cartilage tissues of patients with OA. We observed that the anti-miR-495-mediated prophylactic effects on OA in vitro were dependent on the presence of SOX9. Based on these results, we suggest that miRNA-495 may be a valuable target for designing novel therapies for OA treatment.
MATERIALS AND METHODS
Cell culture
The TC28a2 normal human chondrocyte cell line was purchased from Sigma-Aldrich (St. Louis, MO, USA). Human primary chondrocytes were obtained from articular cartilage tissues from the knee joints of patients with OA with approval from the Institutional Review Board (IRB) (2019-1374-002) of Yonsei University College of Medicine. The cartilage tissue was divided into two groups to separate the chondrocytes: the site of intact tissues and the site of damaged tissues. The tissues were thoroughly minced and subsequently digested with 0.1% collagenase type II (Sigma-Aldrich) for 6 h. TC28a2 cells and primary chondrocytes were maintained in Dulbecco's modified Eagle's medium with high glucose (DMEM-HG; Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Gibco) and 1% (v/v) antibiotic antimycotic solution (Gibco) at 37℃ and 5% CO2.
Monolayer micromass culture and IL-1β treatment
To mimic the OA environment in in vitro cell culture conditions, TC28a2 cells were seeded in the center of the individual wells of 24-well plates at 1×105 cells per well in a 10-µL volume. The cells were then allowed to adhere for 2 h in a cell culture incubator. Next, DMEM-HG supplemented with 1% insulin transferrin selenium-A (ITS-A; Invitrogen, Carlsbad, CA, USA) was added gently. IL-1β (R&D Systems, Minneapolis, MN, USA) was chosen to induce and maintain inflammatory conditions, and the concentration of IL-1β was 10 ng/mL, based on our previous results.15 The cells were maintained for 5 d in the absence or presence of IL-1β and stained with 1% safranin O (Sigma) solution to visualize proteoglycan content produced by the chondrocytes. Crystal violet (CV; Merck, Darmstadt, Germany) staining was performed to compare the density of the cells stained by safranin O. The CV staining method has been described previously.16 For quantitative analysis, absorbance was detected at 490 nm after destaining with 50% acetic acid solution for 20 min. Each safranin O value was normalized to absorbance from CV staining.
Quantitative real-time polymerase chain reaction
Total RNA was isolated from cells using TRIzol reagent (Invitrogen). For cDNA synthesis, 1 µg of RNA was reverse-transcribed using the Mir-X™ miRNA First-Strand Synthesis Kit (Takara Bio, San Jose, CA, USA) and AccuPrep Universal RNA Extraction Kit (Bioneer, Daejeon, South Korea). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using the StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) and 2xqPCRBIO SyGreen Mix (PB20.12-05, PCR Biosystems, London, UK) according to the manufacturer's instructions. The primer sequences are listed in Table 1.
Table 1. Primer Sequences Used in this Study.
| Gene names | Sequence (5′ → 3′) | |
|---|---|---|
| 18S rRNA | Forward | ACACGGACAGGATTGACAGA |
| Reverse | GCCAGAGTCTCGTTCGTTAT | |
| MMP13 | Forward | CCAGACTTCACGATGGCATTG |
| Reverse | GGCATCTCCTCCATAATTTGGC | |
| COL2A1 | Forward | P298511 (Bioneer) |
| Reverse | ||
| PTGES | Forward | P327048 (Bioneer) |
| Reverse | ||
| ADAMTS5 | Forward | GAACATCGACCAACTCTACTCC |
| Reverse | CAATGCCCACCGAACCATCT | |
| SOX9 | Forward | P232240 (Bioneer) |
| Reverse | ||
| CCL4 | Forward | GCTTCCTCGCAACTTTGTGGTAG |
| Reverse | GGTCATACACGTACTCCTGGAC | |
Western blot analysis
Total proteins were isolated from the cells using PRO-PREPTM Protein Extraction Solution (iNtRON Biotechnology, Seongnam, South Korea). Protein concentrations were determined using the Bio-Rad protein assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Protein samples (10 to 30 µg of each sample) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred onto polyvinylidene difluoride membranes. The membranes were blocked with tris-buffered saline with Tween 20 (TBST) containing 5% skim milk (BD Biosciences, Sparks, MD, USA), and subsequently incubated overnight at 4℃ with primary antibodies against SOX9 (1:1000, sc-166505; Santa Cruz Biotechnology, Santa Cruz, CA, USA), CCL4 (1:1000, sc-393441; Santa Cruz Biotechnology), β-actin (1:1000, sc-47778; Santa Cruz Biotechnology), COL2A1 (1:1000, sc-518017; Santa Cruz Biotechnology), COX-2 (1:1000, 610203; BD Biosciences), MMP13 (1:1000, 69926; Cell Signaling Technology, Danvers, MA, USA), and ADAMTS5 (1:1000, PA5-27165; Invitrogen). All membranes were washed three times with TBST for 10 min and incubated with horse-radish peroxidase (HRP)-conjugated secondary antibodies (1:5000, SA001-500 or SA002-500, GenDEPOT, Barker, TX, USA) for 1 h. Finally, all the membranes were washed three times with TBST for 10 min, and the blots were imaged using the LAS 4000 imaging system (Fujifilm, Tokyo, Japan).
Glycosaminoglycan content assay
The amount of sulfated glycosaminoglycans (GAGs) was determined in the culture medium obtained from TC28a2 cells cultured for 5 d using the Blyscan kit (Biocolor, County Antrim, UK) according to the manufacturer's instructions. In brief, 500 µL of the culture medium was mixed with 1 mL of Blyscan dye reagent by shaking for 30 min to complete GAG–dye binding. After centrifugation, the dye bound to GAGs was dissolved in a dissociation reagent. The concentration of the recovered dye was determined at 656 nm, and chondroitin 4-sulfate standard solution (Biocolor) was used to generate standard curves. All samples and standards were tested in triplicate.
Anti-miRNA and siRNA transfection
TC28a2 cells were seeded at a density of 2×105 cells per well in 6-well plates. After 24 h of culture, cells were transfected with negative control anti-miRNA (50 nM), anti-miR-495 (50 nM), negative control siRNA (200 nM), SOX9 siRNA (200 nM), or CCL4 siRNA (200 nM) using CalFectin (Signagen Laboratories, Rockville, MD, USA) according to the manufacturer's instructions. All constructs were purchased from Bioneer (Daejeon, South Korea). The anti-miRNA and siRNA sequences are listed in Table 2.
Table 2. Anti-miRNA and siRNA Sequences Used in This Study.
| Gene names | Sequence (5′ → 3′) | |
|---|---|---|
| Anti-miR-NC | Forward | SMC-2103 (Bioneer) |
| Reverse | ||
| anti-miR-495-3p | Forward | AAA CAA ACA UGG UGC ACU UCU U |
| Reverse | ||
| SOX9 siRNA | Forward | CUC GUA CCC AAA UUU CCA A=tt |
| Reverse | UUG GAA AUU UGG GUA CGA G=tt | |
| CCL4 siRNA | Forward | GCU GAU CCC AGU GAA UCC U=tt |
| Reverse | AGG AUU CAC UGG GAU CAG C=tt | |
Immunohistochemistry
Human cartilage tissue sections were fixed in 10% formalin at room temperature for 7 days. After fixation, the formalin-fixed specimens were embedded in paraffin. The paraffin-embedded sections were deparaffinized, rehydrated, and washed with phosphate-buffered saline, and the tissue sections were evaluated for the disruption of cartilage tissue in the human cartilage. The prepared tissue samples were sliced to a thickness of 4 mm and stained using safranin O solution to observe the progression of OA, or were incubated with anti-SOX9 (Santa Cruz Biotechnology) or anti-CCL4 (Santa Cruz Biotechnology) antibodies to detect SOX9 and CCL4 levels. The signals were detected using the AEC substrate kit (Abcam, Cambridge, UK). The stained tissue sections were observed using a VS120 virtual microscope (Olympus, Tokyo, Japan), and images were analyzed using the OlyVIA 2.5 program (Olympus).
Statistical analysis
All experiments were performed in triplicate using samples from at least three donors. Student's t-test was used to assess the significance of statistical differences for two-group comparisons. The significance of the statistical differences between three or more groups was calculated using one-way analysis of variance and post hoc Bonferroni correction. Data are presented as a mean±standard deviation. For all tests, results with p<0.05 were considered statistically significant.
RESULTS
Osteoarthritic environment upregulates miRNA-495 expression in chondrocytes
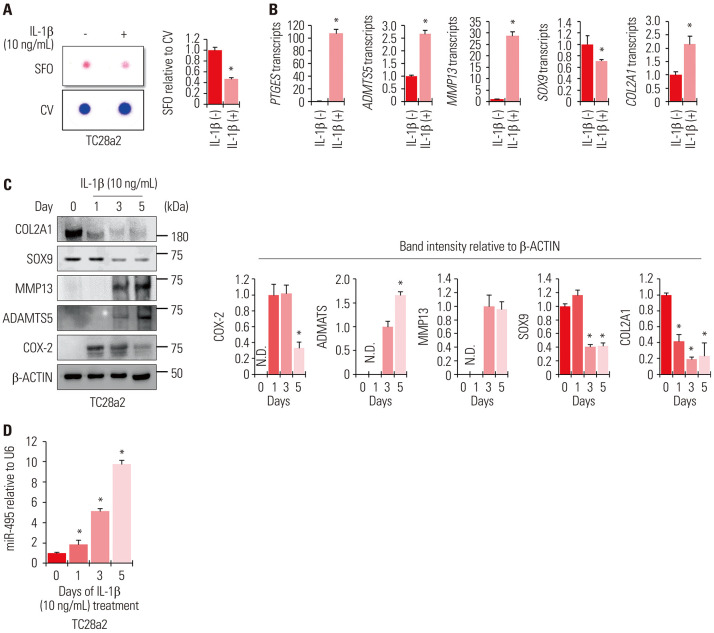
We first sought to mimic the OA environment in a cell culture model to evaluate the expression levels of miRNA-495. IL-1β and TC28a2 cells were used to mimic OA under in vitro cell culture conditions. TC28a2 cells were treated with 10 ng/mL of IL-1β daily, and the cells were maintained for 5 days. Safranin O staining was performed to confirm the effect of IL-1β on inhibiting the synthesis of proteoglycans produced by chondrocytes. Compared with the control group, IL-1β-treated cells showed weaker safranin O staining intensity (Fig. 1A). The mRNA levels of contributors to cartilage matrix protein depletion, such as prostaglandin E synthase (PTGES), a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS5), and matrix metalloproteinase 13 (MMP13), were strongly upregulated by IL-1β treatment (Fig. 1B). The protein levels of cyclooxygenase-2 (COX-2), ADAMTS5, and MMP13 were also upregulated upon IL-1β treatment in a time-dependent manner. Meanwhile, however, the protein levels of chondrogenic markers, such as SOX9 and COL2A1, gradually decreased (Fig. 1C). The mRNA levels of SOX9 and COL2A1 were inconsistent with their respective protein levels under IL-1β-induced inflammatory conditions, which might be due to the roles of collagenases, such as MMP13 and ADAMTS5, at the post-translational level.17,18,19 Based on these results, we confirmed the successful mimicking of the OA environment in vitro using TC28a2 cells. Further, to determine whether the expression levels of miRNA-495 are altered in the in vitro OA environment, TC28a2 cells were treated with IL-1β for 5 days, and a change in the expression of miRNA-495 was observed through qRT-PCR. The results showed that the expression levels of miRNA-495 gradually increased upon treatment with IL-1β (Fig. 1D). These results indicated that upregulation of miRNA-495 may be involved in OA pathogenesis under IL-1β-mediated inflammatory conditions.
Fig. 1. miRNA-495 is upregulated during osteoarthritis progression in an in vitro model generated using the human normal chondrocyte cell line TC28a2. (A) TC28a2 cells were seeded at 1×105 cells per well in 24-well culture plates. The cells were treated with 10 ng/mL of IL-1β or with no IL-1β as a control. Safranin O staining was performed to detect glycosaminoglycans (GAGs). Stained cells were destained with 10% cetylpyridinium for quantitative analysis. Absorbance was measured at 490 nm. *p<0.05 compared to the control groups (n=3 experimental replicates). (B) The graphs represent the results of quantitative real-time polymerase chain reaction (qRT-PCR) using RNA extracted from TC28a2 cells that were treated with 10 ng/mL of IL-1β or with no IL-1β as a control (n=3 experimental replicates). The expression levels of all transcripts tested were normalized to those of 18S rRNA. The data are presented as means±SD. *p<0.05 compared to the IL-1β (−) groups. (C) Protein levels of SOX9, COL2A1, COX-2, ADAMTS5, and MMP13 were analyzed in TC28a2 cells using Western blotting, and the proteins were isolated on days 0, 1, 3, and 5 after treatment with 10 ng/mL of IL-1β. The graphs represent the average values of band intensity for each blot, and each blot was normalized to the band intensity value of β-actin (n=3 experimental replicates). The data are presented as means±SD [COX-2 (*p<0.05 vs. Day 1), ADAMTS5 (*p<0.05 vs. Day 3), SOX9 and COL2A1 (*p<0.05 vs. Day 0)]. (D) The graphs represent the results of qRT-PCR using RNA extracted from TC28a2 cells treated with 10 ng/mL of IL-1β or with no IL-1β as a control (n=3 experimental replicates). The expression levels of miR-495 were normalized to those of U6. The data are presented as means±SD. *p<0.05 vs. Day 0. IL-1β, interleukin-1β; SFO, safranin O; CV, crystal violet; ND, not detectable.
Anti-miR-495 attenuates IL-1β-mediated OA progression in vitro
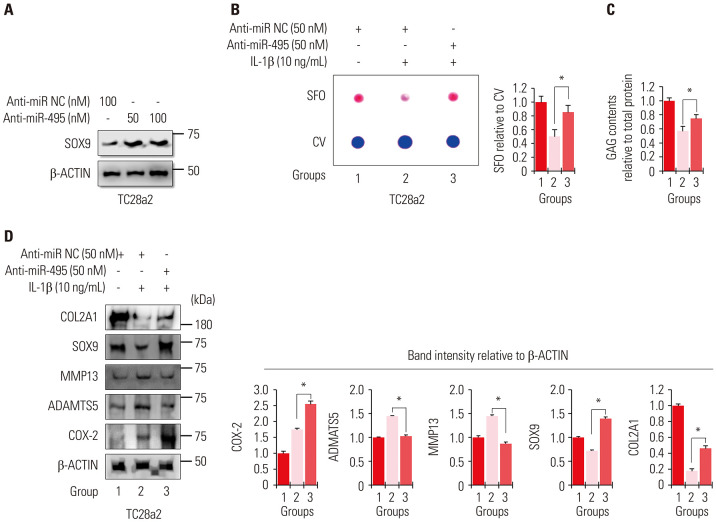
To determine whether the upregulation of miRNA-495 is involved in IL-1β-mediated OA progression in vitro, anti-miR-495 (hsa-miR-495-3p) was used to suppress the upregulation of miRNA-495 upon IL-1β treatment in TC28a2 cells. Our previous study showed that miRNA-495 binds to the 3′-UTR of SOX9 mRNA and inhibits its expression.12 Based on this previous study, the dose and effectiveness of anti-miR-495 were determined according to changes in SOX9 protein levels (Fig. 2A). Transfection with anti-miR-495 protected TC28a2 cells against IL-1β-mediated loss of proteoglycans, as observed by safranin O staining (Fig. 2B), indicating that miRNA-495 is involved in the degradation of matrix proteins by chondrocytes. The results of the GAG content assays also showed that upon anti-miR-495 transfection, chondrocytes retained the ability to produce GAGs even in the presence of IL-1β (Fig. 2C). As expected, the protein levels of SOX9 and COL2A1, which were downregulated by IL-1β, were rescued in the anti-miR-495-transfected group, while the protein levels of MMP13 and ADAMTS5, which were upregulated by IL-1β, were reduced in the anti-miR-495-transfected group (Fig. 2D). However, anti-miR-495 did not affect the protein levels of COX-2, indicating that IL-1β-mediated upregulation of miRNA-495 is independent of COX-2 expression. Accordingly, these data suggest that miRNA-495 could be a target for the treatment of OA, and the anti-miR-495-mediated inhibitory effect of COL2A1 degradation in the presence of IL-1β may depend on the rescue effect of SOX9.
Fig. 2. Inhibition of miRNA-495 expression during in vitro osteoarthritis progression protects chondrocytes from IL-1β-mediated disruption of SOX9 and COL2A1. (A) Protein levels of SOX9 were analyzed in TC28a2 cells transfected with anti-miR negative control (NC, 100 nM), anti-miR-495 (50 or 100 nM) by Western blotting to determine the effective dose of anti-miR-495. (B) TC28a2 cells transfected with anti-miR NC (50 nM) or anti-miR-495 (50 nM) were seeded at 1×105 cells per well in 24-well culture plates. The cells were treated with 10 ng/mL of IL-1β or with no IL-1β as a control. Safranin O staining was performed to detect glycosaminoglycans (GAGs). Stained cells were destained with 10% cetylpyridinium for quantitative analysis. Absorbance was measured at 490 nm. *p<0.05 compared to Group 2 (n=3 experimental replicates). (C) GAG content was measured using the Blyscan sulfate GAG assay. The sulfated GAGs were quantified in the supernatant and standardized using the chondroitin 4-sulfate standard solution. *p<0.05 compared to group 2 (n=3 experimental replicates). (D) Protein levels of SOX9, COL2A1, COX-2, ADAMTS5, and MMP13 were analyzed in 10 ng/mL of IL-1β-treated TC28a2 cells transfected with anti-miR NC (50 nM) or anti-miR-495 (50 nM) using Western blotting. The graphs represent the average values of the band intensity for each blot, and each blot was normalized to the band intensity value of β-actin (n=3 experimental replicates). The data are presented as means±SD. *p<0.05 vs. Group 2. IL-1β, interleukin-1β; SFO, safranin O; CV, crystal violet.
Reduced SOX9 expression is associated with OA progression
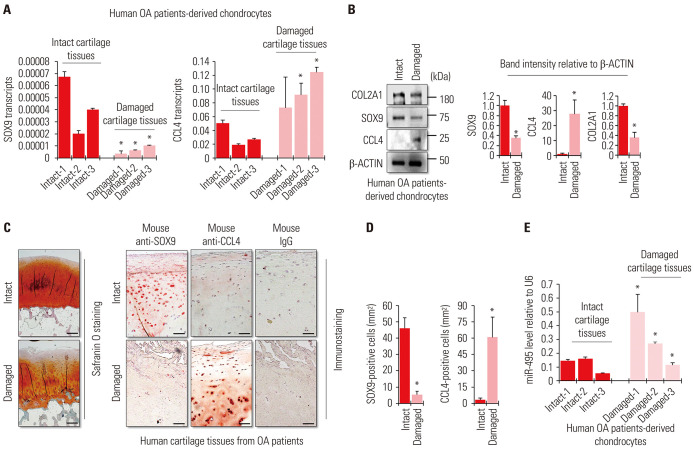
miRNA-495 has been shown to be involved in various developmental and inflammatory processes, as well as in the development of normal or tumor cells.20 Importantly, it has been shown that miRNA-495 targets chemokine ligand 4 (CCL4) to repress the activity of nuclear factor kappa light chain enhancer of activated B cells (NF-κB) in chondrocytes.14 The study suggested that inhibition of miRNA-495 prevents chondrocyte apoptosis and enhances their proliferation through upregulation of CCL4 in the OA environment. Since CCL4 is known as a mediator of IL-1β-mediated inflammation in chondrocytes,21 it is possible that targeting miRNA-495 may increase the inflammatory response in chondrocytes. In the current study, we observed that miRNA-495 is upregulated in chondrocytes treated with IL-1β (Fig. 1D). In addition, the inhibition of miRNA-495 clearly prevented IL-1β-mediated inflammation (Fig. 2). To address this paradoxical mechanism, we first checked the mRNA levels of the targets of miRNA-495, SOX9, and CCL4, in chondrocytes derived from patients with OA. The mRNA levels of SOX9 were clearly decreased in chondrocytes isolated from the damaged cartilage tissues of patients with OA, while the mRNA levels of CCL4 were upregulated (Fig. 3A). Likewise, the protein levels of SOX9 and CCL4 were also increased, similar to their mRNA expression (Fig. 3B). For histological analysis, safranin O staining and immunohistochemistry were performed using cartilage tissues obtained from patients with OA. The results showed that SOX9 was present mostly in intact cartilage tissues and undetectable in damaged cartilage tissues. CCL4 was detected more in damaged cartilage than in the cartilage (Fig. 3C and D). To confirm if this result was clinically meaningful, we checked the levels of miRNA-495 in human OA patient-derived chondrocytes as well. Chondrocytes were isolated from intact or damaged cartilage tissues of patients with OA, and the cells were separated into two groups. miRNA-495 was highly upregulated in chondrocytes isolated from damaged cartilage tissues (Fig. 3E). These results indicated that SOX9, rather than CCL4, is more strongly correlated with miRNA-495, whose expression was increased in the OA environment.
Fig. 3. Contradictory expression levels of SOX9 and CCL4 in damaged cartilage tissues obtained from patients with osteoarthritis (OA). (A) The graphs present the results of quantitative real-time polymerase chain reaction (qRT-PCR) using RNA extracted from primary chondrocytes isolated from the intact or damaged parts of the cartilage tissues from patients with OA. The expression levels of SOX9 and CCL4 mRNAs were normalized to those of 18S rRNA. The data are presented as means±SD. *p<0.05 compared to each intact control group (Damaged-1 vs. Intact-1, Damaged-2 vs. Intact-2, or Damaged-3 vs. Intact-3; n=3 experimental replicates). The number specified for each letter in the bar graphs means that the tissue was obtained from the same patient. For example, “Intact-1” and “Damaged-1” mean that the cartilage tissue obtained from the same patient was divided into "Intact" and "Damaged" parts and then chondrocytes were isolated from each divided tissue. (B) Protein levels of SOX9 and CCL4 were analyzed in primary chondrocytes isolated from the intact or damaged parts of the cartilage tissues from patients with OA using Western blotting. The graphs present the average values of the band intensity for each blot, and each blot was normalized to the band intensity value of β-actin. The data are presented as means±SD. *p<0.05 vs. Intact sample (n=3 experimental replicates). (C) Representative images of SOX9 and CCL4 immunostaining and safranin O staining in intact and damaged cartilage tissues from patients with OA. Scale bar=200 µm. (D) Quantification of SOX9- or CCL4-positive cells from total cell population per field in immunostained sections. The data are presented as means±SD. *p<0.05 vs. Intact sample (n=3 experimental replicates). (E) The graphs represent the results of qRT-PCR using RNA extracted from primary chondrocytes isolated from the intact or damaged parts of the cartilage tissues from patients with OA. The expression levels of miR-495 were normalized to those of U6. The data are presented as means±SD. *p<0.05 compared to each intact control group (Damaged-1 vs. Intact-1, Damaged-2 vs. Intact-2, or Damaged-3 vs. Intact-3; n=3 experimental replicates). The number specified for each letter in bar graphs means that the tissue was obtained from the same patient.
miRNA-495-mediated SOX9 regulation is the target axis for anti-miR-495 in the OA environment
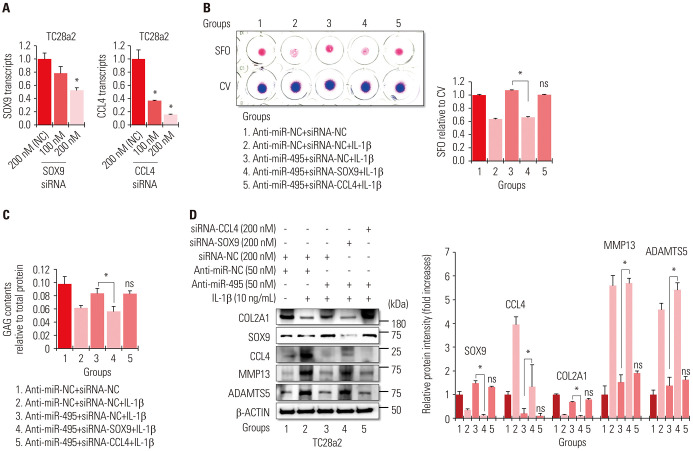
To address the importance of SOX9 or CCL4 in an in vitro OA environment regulated by miRNA-495, we performed an RNA knockdown study and confirmed that 200 nM of siRNA was effective in downregulating the mRNA levels of SOX9 or CCL4 (Fig. 4A). The results of safranin O staining and GAG content assays showed that the anti-miR-495-mediated rescue effect in IL-1β-treated chondrocytes was completely abolished by SOX9 knockdown, while knockdown of CCL4 did not have any effect (Fig. 4B and C). While transfection with anti-miR-495 rescued the protein levels of COL2A1 in IL-1β-treated cells, knockdown of SOX9 completely abolished the rescue effect of anti-miR-495. Protein levels of MMP13 and ADAMTS5 were also dependent on the presence of SOX9 in anti-miR-495-transfected cells. However, CCL4 knockdown did not have any effect in IL-1β-treated TC28a2 cells transfected with anti-miR-495 (Fig. 4D). These results indicated that SOX9 is a crucial mediator of anti-miR-495-mediated therapeutic effects in OA treatment. Taken together, these results suggest that miRNA-495-mediated reduction of SOX9 expression contributes to OA pathogenesis, and therefore, the miRNA-495–SOX9 axis could be a target for the treatment of OA. In this case, the application of anti-miR-495 to patients with OA could be an excellent strategy to restore SOX9 expression through the inhibition of miRNA-495.
Fig. 4. miRNA-495–SOX9 axis is more important than miRNA-495–CCL4 axis in protecting chondrocytes against IL-1β-mediated inflammation. (A) The graphs represent the results of quantitative real-time polymerase chain reaction (qRT-PCR) using RNA extracted from TC28a2 cells transfected with negative control (NC) siRNA (200 nM), SOX9 siRNA (100 or 200 nM), or CCL4 siRNA (100 or 200 nM) (n=3 experimental replicates) to validate the efficiency of the siRNAs used in this study. The expression levels of SOX9 and CCL4 mRNAs were normalized to those of 18S rRNA. The data are presented as means±SD. *p<0.05 compared to the 200 nM of NC siRNA groups. (B) Anti-miR-NC- or anti-miR-495-transfected TC28a2 cells were co-transfected with NC siRNA, SOX9 siRNA, or CCL4 siRNA, and then the cells were seeded at 1×105 cells per well in 24-well culture plates. The cells were treated with 10 ng/mL of IL-1β or with no IL-1β as a control. Safranin O staining was performed to detect glycosaminoglycans (GAGs). Stained cells were destained with 10% cetylpyridinium for quantitative analysis. Absorbance was measured at 490 nm. *p<0.05 for comparison between two groups (n=3 experimental replicates). (C) GAG content was measured using the Blyscan sulfate GAG assay. The sulfated GAGs were quantified in the supernatant and standardized using the chondroitin 4-sulfate standard solution. *p<0.05 compared to group 1 or 3 (n=3 experimental replicates). (D) Protein levels of SOX9, COL2A1, COX-2, ADAMTS5, and MMP13 were analyzed in the cells using Western blotting. The graphs represent the average value of the band intensity for each blot, and each blot was normalized to the band intensity value of β-actin. The data are presented as means±SD. *p<0.05 vs. Group 3 (n=3 experimental replicates). IL-1β, interleukin-1β; SFO, safranin O; CV, crystal violet.
DISCUSSION
Many risk factors influence OA or chondrocyte homeostasis, and miRNAs are one of the risk factors that contribute to cartilage-related diseases, including OA.22 Accumulating evidence has shed light on the roles of miRNAs in OA pathogenesis and chondrocyte homeostasis.23 The current study evaluated the potential of anti-miR-495 as a therapeutic molecule in an in vitro OA environment. Our previous finding that miRNA-495 targets SOX9 to inhibit the chondrogenic differentiation of human MSCs12 presented us with the idea that rescuing SOX9 expression by anti-miR-495 might be helpful as a therapeutic strategy for OA pathogenesis. SOX9 is known to repress chondrocyte hypertrophy and osteogenesis, thereby promoting chondrogenesis.24,25 In cartilage tissues with OA, progenitor cells fail to regenerate intact cartilage in the damaged sites,26,27 and decreased SOX9 levels may be one of the causes.28 Some studies have supported the importance of SOX9 in OA therapy to restore chondrocyte function, as well as to secrete COL2A1 and proteoglycans.29,30,31 In addition, lentivirus-mediated overexpression of SOX9 can downregulate the levels of some catabolic proteins, including MMP13 and ADAMTS5.32 Thus, maintaining or reinforcing SOX9 expression in chondrocytes can be a very important therapeutic approach for regenerating cartilage tissue or delaying OA progression. We also showed that inhibition of miRNA-495, a potential repressor of SOX9, prevented the loss of SOX9 expression in IL-1β-treated TC28a2 chondrocytes, indicating that anti-miR-495, which functions as an enhancer of SOX9 expression in OA chondrocytes, could be employed in the development of miRNA-based drugs. CCL4 has also been reported as a target mRNA for miRNA-495 in chondrocytes.14 For this reason, we expected that transfection of anti-miR-495 with chondrocytes (TC28a2 cell line) would upregulate the expression levels of CCL4 mRNA. However, anti-miR-495 reduced the mRNA expression levels of CCL4 in an inflammatory environment. The reason may be due to anti-miR-495-mediated upregulation of SOX9 expression. A recent report showed that SOX9 overexpression alleviates IL-1β-induced inflammatory responses in human chondrocytes,31 which means that SOX9 upregulation by anti-miR-495 inhibited the IL-1β-induced CCL4 expression in chondrocytes. Under the IL-1β-mediated inflammatory condition, siRNA-mediated knockdown of SOX9 rescued the protein levels of CCL4 reduced by anti-miR-495 (Fig. 4D), which support our hypothesis that the anti-miR-495-mediated upregulation of SOX9 expression might inhibit CCL4 expression in chondrocytes. Therefore, we suggest that SOX9, rather than CCL4, is more strongly correlated with miRNA-495, especially in IL-1β-induced inflammation.
Research has shown that miRNA-495 has multiple functions in maintaining the homeostasis of various cells and that it plays a role in various diseases. The role of miRNA-495 in bone homeostasis remains controversial. A previous study showed that miRNA-495 inhibits new bone regeneration by targeting the high mobility group AT-Hook 2 and that anti-miR-495 enhances femur healing in murine experimental models.33 However, a recent study reported a contradictory result showing that miRNA-495 could promote the proliferation and differentiation of osteoblasts in mice with tibial fracture.34 These conflicting results may be due to the age of the experimental animals or different experimental conditions. In addition, inhibition of miRNA-495 has been found to enhance the therapeutic efficacy of angiogenesis in human induced pluripotent stem cells.35 Thus, miR-495 can be considered as a potential therapeutic target for treating skeletal and angiogenesis-related diseases. Nevertheless, the development of miRNA-495-based drugs still faces many obstacles for its clinical application, since miRNA-495 may also act as a tumor suppressor.36 While a few studies have shown that the expression of miRNA-495 is upregulated in hepatocellular carcinoma37 and breast cancer stem cells,38 in which it positively regulates cell proliferation and invasion, thereby promoting tumor progression, miRNA-495 has also been described as a tumor suppressor in many other related studies. miRNA-495-mediated inhibition of cell proliferation and invasion has been detected in different types of cancer, including glioblastoma multiforme39 and myeloid leukemia.40 Thus, identifying the universal role of miRNA-495 in tumors remains controversial, and the reasons why miRNA-495 is either up- or downregulated in different types of cancers also remains unclear. Accordingly, at the moment, it may be too early to develop miRNA-495-based drugs, either as an injectable drug or as an oral drug, and the development of drugs targeting miRNA-495 would require consideration of many risk factors. Despite these risk factors in other diseases, we aimed to determine whether miRNA-495 could be a therapeutic target for the treatment of OA. Since, unlike most tissues, articular cartilage has no blood vessels, nerves, or lymphatics, targeting miRNA-495 in the local area with small cartilage defects would minimize unexpected side effects.
Although we found that miR-495 could be a target for OA treatment in human cells and tissues obtained from patients with OA, there are some limitations to the current study. For example, we could not conclude the role of anti-miR-495 in animal models with OA, which is a weakness of this study. Recent studies have reported a therapeutic effect for anti-miRs in animal cartilage tissue with OA: The intra-articular injection of anti-miR-34a alleviated OA progression in a rat experimental model of OA.41 A miR-146a inhibitor improved the destruction of articular cartilage in surgically induced OA mice.42 Intra-articular injection of antago-miR-483-5p reportedly delayed the progression of experimental OA.43 These results highlight the potential of anti-miRs as novel OA gene therapeutics through inhibition of miRNAs associated with OA pathogenesis. These studies used the lentivirus system as a delivery system for anti-miRs. However, there are still many questions and considerations regarding the use of lentivirus for clinical purposes.44 As anti-miR delivery to cartilage tissue by intra-articular administration may serve as a powerful strategy for OA treatment, an ideal vector system for transducing genes into hard tissues, such as cartilage, should be considered before clinical application. Although this study did not show the therapeutic effect of anti-miR-495 in animal models with OA, we believe that the results contain important experimental results that can be applied to humans, since our study is the first to show that miR-495 can be a target for OA treatment using human cells and patient samples. Overall, we believe this information will help lay the basis for future in vivo experiments.
Taken together, we found that miRNA-495 is upregulated in normal human chondrocyte cells (TC28a2 cells) treated with IL-1β and in primary chondrocytes obtained from the damaged cartilage tissues of patients with OA. Applying anti-miR-495 to inhibit miRNA-495 prevented the loss of the expression of SOX9 and COL2A1, which are key markers of chondrocytes. Thus, we suggest that local application of anti-miR-495 to damaged cartilage tissues could be a novel therapeutic strategy through which to prevent OA progression. In future studies, the use of an appropriate experimental animal model of OA will be necessary to evaluate whether local administration of anti-miR-495 is effective in preventing the progression of OA.
ACKNOWLEDGEMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2019R1F1A1058 391) and by a faculty research grant from Yonsei University College of Medicine (6-2018-0103).
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Dong Suk Yoon, Kwang Hwan Park, Jin Woo Lee, and Sung-Hwan Kim.
- Data curation: Dong Suk Yoon and Sung-Hwan Kim.
- Formal analysis: Dong Suk Yoon, Jin Woo Lee, and Sung-Hwan Kim.
- Funding acquisition: Sung-Hwan Kim.
- Investigation: all authors.
- Methodology: Dong Suk Yoon, Sehee Cho, and Sung-Hwan Kim.
- Project administration: Soyeong Joung, Dong Suk Yoon, Kyoung-Mi Lee, and Sung-Hwan Kim.
- Supervision: Sung-Hwan Kim.
- Validation: Soyeong Joung, Dong Suk Yoon, and Sung-Hwan Kim.
- Visualization: Soyeong Joung, Dong Suk Yoon, and Sehee Cho.
- Writing—original draft: Dong Suk Yoon.
- Writing—review & editing: Kwang Hwan Park, Jin Woo Lee, and Sung-Hwan Kim.
- Approval of final manuscript: all authors.
References
- 1.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26:355–369. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akkiraju H, Nohe A. Role of chondrocytes in cartilage formation, progression of osteoarthritis and cartilage regeneration. J Dev Biol. 2015;3:177–192. doi: 10.3390/jdb3040177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archer CW, Francis-West P. The chondrocyte. Int J Biochem Cell Biol. 2003;35:401–404. doi: 10.1016/s1357-2725(02)00301-1. [DOI] [PubMed] [Google Scholar]
- 4.Kim JH, Jeon J, Shin M, Won Y, Lee M, Kwak JS, et al. Regulation of the catabolic cascade in osteoarthritis by the zinc-ZIP8-MTF1 axis. Cell. 2014;156:730–743. doi: 10.1016/j.cell.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Mobasheri A, Rayman MP, Gualillo O, Sellam J, van der Kraan P, Fearon U. The role of metabolism in the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2017;13:302–311. doi: 10.1038/nrrheum.2017.50. [DOI] [PubMed] [Google Scholar]
- 6.Hoshi H, Akagi R, Yamaguchi S, Muramatsu Y, Akatsu Y, Yamamoto Y, et al. Effect of inhibiting MMP13 and ADAMTS5 by intra-articular injection of small interfering RNA in a surgically induced osteoarthritis model of mice. Cell Tissue Res. 2017;368:379–387. doi: 10.1007/s00441-016-2563-y. [DOI] [PubMed] [Google Scholar]
- 7.Miyauchi A, Kim-Kaneyama Jr, Lei XF, Chang SH, Saito T, Haraguchi S, et al. Alleviation of murine osteoarthritis by deletion of the focal adhesion mechanosensitive adapter, Hic-5. Sci Rep. 2019;9:15770. doi: 10.1038/s41598-019-52301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Kraan PM, van den Berg WB. Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthritis Cartilage. 2012;20:223–232. doi: 10.1016/j.joca.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Yu C, Chen WP, Wang XH. MicroRNA in osteoarthritis. J Int Med Res. 2011;39:1–9. doi: 10.1177/147323001103900101. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez-Garcia I, Miska EA. MicroRNA functions in animal development and human disease. Development. 2005;132:4653–4662. doi: 10.1242/dev.02073. [DOI] [PubMed] [Google Scholar]
- 11.Miyaki S, Asahara H. Macro view of microRNA function in osteoarthritis. Nat Rev Rheumatol. 2012;8:543–552. doi: 10.1038/nrrheum.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S, Yoon DS, Paik S, Lee KM, Jang Y, Lee JW. microRNA-495 inhibits chondrogenic differentiation in human mesenchymal stem cells by targeting Sox9. Stem Cells Dev. 2014;23:1798–1808. doi: 10.1089/scd.2013.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao X, Wang T, Cai B, Wang X, Feng W, Han Y, et al. MicroRNA-495 enhances chondrocyte apoptosis, senescence and promotes the progression of osteoarthritis by targeting AKT1. Am J Transl Res. 2019;11:2232–2244. [PMC free article] [PubMed] [Google Scholar]
- 14.Yang DW, Qian GB, Jiang MJ, Wang P, Wang KZ. Inhibition of microRNA-495 suppresses chondrocyte apoptosis through activation of the NF-κB signaling pathway by regulating CCL4 in osteoarthritis. Gene Ther. 2019;26:217–229. doi: 10.1038/s41434-019-0068-5. [DOI] [PubMed] [Google Scholar]
- 15.Yoon DS, Yoo JH, Kim YH, Paik S, Han CD, Lee JW. The effects of COX-2 inhibitor during osteogenic differentiation of bone marrow-derived human mesenchymal stem cells. Stem Cells Dev. 2010;19:1523–1533. doi: 10.1089/scd.2009.0393. [DOI] [PubMed] [Google Scholar]
- 16.Yoon DS, Kim YH, Jung HS, Paik S, Lee JW. Importance of Sox2 in maintenance of cell proliferation and multipotency of mesenchymal stem cells in low-density culture. Cell Prolif. 2011;44:428–440. doi: 10.1111/j.1365-2184.2011.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, et al. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97:761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Echtermeyer F, Bertrand J, Dreier R, Meinecke I, Neugebauer K, Fuerst M, et al. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat Med. 2009;15:1072–1076. doi: 10.1038/nm.1998. [DOI] [PubMed] [Google Scholar]
- 19.Wang M, Shen J, Jin H, Im HJ, Sandy J, Chen D. Recent progress in understanding molecular mechanisms of cartilage degeneration during osteoarthritis. Ann N Y Acad Sci. 2011;1240:61–69. doi: 10.1111/j.1749-6632.2011.06258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen H, Wang X, Bai J, He A. Expression, regulation and function of miR-495 in healthy and tumor tissues. Oncol Lett. 2017;13:2021–2026. doi: 10.3892/ol.2017.5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z, Bryan JL, DeLassus E, Chang LW, Liao W, Sandell LJ. CCAAT/enhancer-binding protein β and NF-κB mediate high level expression of chemokine genes CCL3 and CCL4 by human chondrocytes in response to IL-1β. J Biol Chem. 2010;285:33092–33103. doi: 10.1074/jbc.M110.130377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malemud CJ. MicroRNAs and osteoarthritis. Cells. 2018;7:92. doi: 10.3390/cells7080092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sondag GR, Haqqi TM. The role of microRNAs and their targets in osteoarthritis. Curr Rheumatol Rep. 2016;18:56. doi: 10.1007/s11926-016-0604-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leung VY, Gao B, Leung KK, Melhado IG, Wynn SL, Au TY, et al. SOX9 governs differentiation stage-specific gene expression in growth plate chondrocytes via direct concomitant transactivation and repression. PLoS Genet. 2011;7:e1002356. doi: 10.1371/journal.pgen.1002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao L, Yang F, Liu G, Yu D, Li H, Fan Q, et al. The promotion of cartilage defect repair using adenovirus mediated Sox9 gene transfer of rabbit bone marrow mesenchymal stem cells. Biomaterials. 2011;32:3910–3920. doi: 10.1016/j.biomaterials.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Fellows CR, Williams R, Davies IR, Gohil K, Baird DM, Fairclough J, et al. Characterisation of a divergent progenitor cell sub-populations in human osteoarthritic cartilage: the role of telomere erosion and replicative senescence. Sci Rep. 2017;7:41421. doi: 10.1038/srep41421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell TM, Churchman SM, Gomez A, McGonagle D, Conaghan PG, Ponchel F, et al. Mesenchymal stem cell alterations in bone marrow lesions in patients with hip osteoarthritis. Arthritis Rheumatol. 2016;68:1648–1659. doi: 10.1002/art.39622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cucchiarini M, Thurn T, Weimer A, Kohn D, Terwilliger EF, Madry H. Restoration of the extracellular matrix in human osteoarthritic articular cartilage by overexpression of the transcription factor SOX9. Arthritis Rheum. 2007;56:158–167. doi: 10.1002/art.22299. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Tew SR, Russell AM, Gonzalez KR, Hardingham TE, Hawkins RE. Transduction of passaged human articular chondrocytes with adenoviral, retroviral, and lentiviral vectors and the effects of enhanced expression of SOX9. Tissue Eng. 2004;10:575–584. doi: 10.1089/107632704323061933. [DOI] [PubMed] [Google Scholar]
- 30.Tew SR, Li Y, Pothacharoen P, Tweats LM, Hawkins RE, Hardingham TE. Retroviral transduction with SOX9 enhances re-expression of the chondrocyte phenotype in passaged osteoarthritic human articular chondrocytes. Osteoarthritis Cartilage. 2005;13:80–89. doi: 10.1016/j.joca.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Ouyang Y, Wang W, Tu B, Zhu Y, Fan C, Li Y. Overexpression of SOX9 alleviates the progression of human osteoarthritis in vitro and in vivo. Drug Des Devel Ther. 2019;13:2833–2842. doi: 10.2147/DDDT.S203974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu H, Zeng C, Chen M, Lian L, Dai Y, Zhao H. Lentiviral vector-mediated over-expression of Sox9 protected chondrocytes from IL-1β induced degeneration and apoptosis. Int J Clin Exp Pathol. 2015;8:10038–10049. [PMC free article] [PubMed] [Google Scholar]
- 33.Tian Z, Zhou H, Xu Y, Bai J. MicroRNA-495 inhibits new bone regeneration via targeting high mobility group AT-Hook 2 (HMGA2) Med Sci Monit. 2017;23:4689–4698. doi: 10.12659/MSM.904404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu L, Lin ZW, Wang G, Zhang H, Liu B, Xu QJ. MicroRNA-495 downregulates AQP1 and facilitates proliferation and differentiation of osteoblasts in mice with tibial fracture through activation of p38 MAPK signaling pathway. Sci Rep. 2019;9:16171. doi: 10.1038/s41598-019-50013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang J, Huang W, Cai W, Wang L, Guo L, Paul C, et al. Inhibition of microRNA-495 enhances therapeutic angiogenesis of human induced pluripotent stem cells. Stem Cells. 2017;35:337–350. doi: 10.1002/stem.2477. [DOI] [PubMed] [Google Scholar]
- 36.Zhang B, Yuan F, Liu J, Li Y, Zhou F, Liu X, et al. Hsa-miR-495 acts as a tumor suppressor gene in glioma via the negative regulation of MYB. Mol Med Rep. 2016;14:977–982. doi: 10.3892/mmr.2016.5327. [DOI] [PubMed] [Google Scholar]
- 37.Yang H, Cho ME, Li TW, Peng H, Ko KS, Mato JM, et al. MicroRNAs regulate methionine adenosyltransferase 1A expression in hepatocellular carcinoma. J Clin Invest. 2013;123:285–298. doi: 10.1172/JCI63861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hwang-Verslues WW, Chang PH, Wei PC, Yang CY, Huang CK, Kuo WH, et al. miR-495 is upregulated by E12/E47 in breast cancer stem cells, and promotes oncogenesis and hypoxia resistance via downregulation of E-cadherin and REDD1. Oncogene. 2011;30:2463–2474. doi: 10.1038/onc.2010.618. [DOI] [PubMed] [Google Scholar]
- 39.Chen SM, Chen HC, Chen SJ, Huang CY, Chen PY, Wu TW, et al. MicroRNA-495 inhibits proliferation of glioblastoma multiforme cells by downregulating cyclin-dependent kinase 6. World J Surg Oncol. 2013;11:87. doi: 10.1186/1477-7819-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang X, Huang H, Li Z, He C, Li Y, Chen P, et al. MiR-495 is a tumor-suppressor microRNA down-regulated in MLL-rearranged leukemia. Proc Natl Acad Sci U S A. 2012;109:19397–19402. doi: 10.1073/pnas.1217519109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan S, Wang M, Zhao J, Zhang H, Zhou C, Jin L, et al. MicroRNA-34a affects chondrocyte apoptosis and proliferation by targeting the SIRT1/p53 signaling pathway during the pathogenesis of osteoarthritis. Int J Mol Med. 2016;38:201–209. doi: 10.3892/ijmm.2016.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X, Wang C, Zhao J, Xu J, Geng Y, Dai L, et al. miR-146a facilitates osteoarthritis by regulating cartilage homeostasis via targeting Camk2d and Ppp3r2. Cell Death Dis. 2017;8:e2734. doi: 10.1038/cddis.2017.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang H, Zhang H, Sun Q, Wang Y, Yang J, Yang J, et al. Intra-articular delivery of antago-miR-483-5p inhibits osteoarthritis by modulating matrilin 3 and tissue inhibitor of metalloproteinase 2. Mol Ther. 2017;25:715–727. doi: 10.1016/j.ymthe.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Milone MC, O'Doherty U. Clinical use of lentiviral vectors. Leukemia. 2018;32:1529–1541. doi: 10.1038/s41375-018-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]