Abstract
Objective
To compare the diagnostic performance between Prostate Imaging-Reporting and Data System version 2.0 (PI-RADSv2.0) and version 2.1 (PI-RADSv2.1) for clinically significant prostate cancer (csPCa) in the peripheral zone (PZ).
Materials and Methods
This retrospective study included 317 patients who underwent multiparametric magnetic resonance imaging and targeted biopsy for PZ lesions. Definition of csPCa was International Society of Urologic Pathology grade ≥ 2 cancer. Area under the curve (AUC), sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy for csPCa were analyzed by two readers. The cancer detection rate (CDR) for csPCa was investigated according to the PI-RADS categories.
Results
AUC of PI-RADSv2.1 (0.856 and 0.858 for reader 1 and 2 respectively) was higher than that of PI-RADSv2.0 (0.795 and 0.747 for reader 1 and 2 respectively) (both p < 0.001). Sensitivity, specificity, PPV, NPV, and accuracy for PI-RADSv2.0 vs. PI-RADSv2.1 were 93.2% vs. 88.3% (p = 0.023), 52.8% vs. 76.6% (p < 0.001), 48.7% vs. 64.5% (p < 0.001), 94.2% vs. 93.2% (p = 0.504), and 65.9% vs. 80.4% (p < 0.001) for reader 1, and 96.1% vs. 92.2% (p = 0.046), 34.1% vs. 72.4% (p < 0.001), 41.3% vs. 61.7% (p < 0.001), 94.8% vs. 95.1% (p = 0.869), and 54.3% vs. 78.9% (p < 0.001) for reader 2, respectively. CDRs of PI-RADS categories 1–2, 3, 4, and 5 for PI-RADSv2.0 vs. PI-RADSv2.1 were 5.9% vs. 5.9%, 5.8% vs. 12.5%, 39.8% vs. 56.2%, and 88.9% vs. 88.9% for reader 1; and 4.5% vs. 4.1%, 6.1% vs. 11.1%, 32.5% vs. 53.4%, and 85.0% vs. 86.8% for reader 2, respectively.
Conclusion
Our data demonstrated improved AUC, specificity, PPV, accuracy, and CDRs of category 3 or 4 of PI-RADSv2.1, but decreased sensitivity, compared with PI-RADSv2.0, for csPCa in PZ.
Keywords: Prostate cancer, Peripheral zone, Magnetic resonance imaging, PI-RADS
INTRODUCTION
Recently, Prostate Imaging-Reporting and Data System version 2.1 (PI-RADSv2.1) was released [1]. Researchers have reported the usefulness of Prostate Imaging-Reporting and Data System version 2.0 (PI-RADSv2.0) in the detection of clinically significant prostate cancer (csPCa). Consequently, the information obtained through interpretation using PI-RADSv2.0 can be used to guide biopsies or for treatment planning [2]. Currently, urologic guidelines accept the use of PI-RADS as a diagnostic tool, to arrive at decisions involving the diagnosis or management of prostate cancer [3,4].
Reportedly, the cancer detection rate (CDR) of PI-RADSv2.0 categories 1–2, 3, 4, and 5 for csPCa (Gleason score [GS] 3 + 4 or greater) were approximately less than 10%, less than 25%, 20–60%, and 70–90%, respectively [5,6,7,8]. Hence, although PI-RADS categories 3 or 4 are defined as having intermediate or high probabilities of detecting csPCa, respectively, the actual probabilities of csPCa in these categories seem to be lower than the expected values. Various benign conditions mimicking PCa or the considerable inter-reader variability regarding the interpretation of PI-RADSv2.0 may be associated with the limited positive predictive value (PPV) of PI-RADSv2.0 in targeted biopsies [2,9].
PCa typically (70–80%) manifests in the peripheral zone (PZ) [10]. Diffusion-weighted (DW) magnetic resonance imaging (MRI) plays a major role in the evaluation of the PZ and it was revised in PI-RADSv2.1 as follows: category 2, linear/wedge-shaped hypointense on apparent diffusion coefficient (ADC) and/or linear/wedge-shaped hyperintense on high b-value DW: category 3, focal (discrete and different from the background) hypointense on ADC and/or focal hyperintense on high b-value DW; may be markedly hypointense on ADC or markedly hyperintense on high b-value DW, but not both [1]. However, data regarding the performance of PI-RADSv2.1 in the assessment of PZ remain insufficient.
Therefore, the objective of the present study was to compare the diagnostic performance of PI-RADSv2.0 and PI-RADSv2.1 for csPCa in the PZ.
MATERIALS AND METHODS
Study Subjects
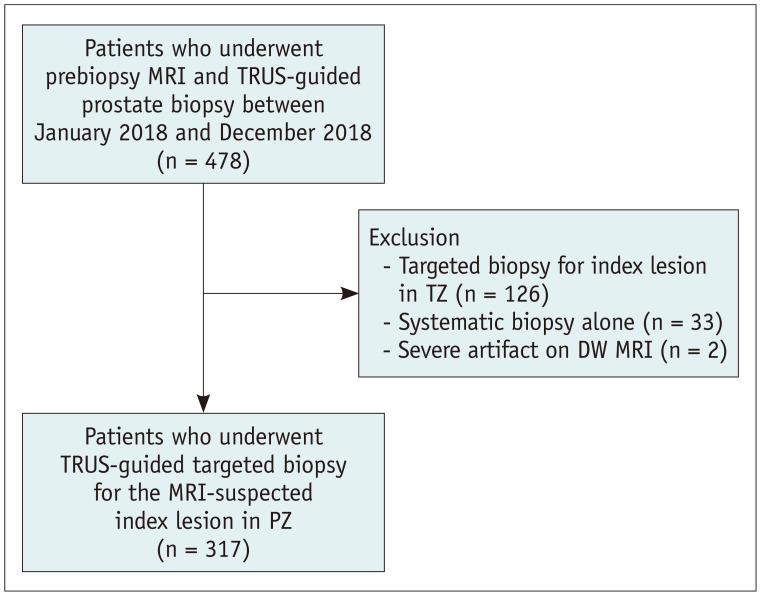
This retrospective study was approved by the Institutional Review Board of our institution, and the requirement for informed consent was waived (IRB No. 2019-08-101). The medical records of a consecutive series of 478 patients who underwent prebiopsy prostate MRI and transrectal ultrasound (TRUS)-guided prostate biopsies from January 2018 to December 2018 were obtained from the institutional database (Fig. 1). Among these, 161 patients were excluded, owing to the following reasons: targeted biopsies for the index lesion in transition zone (TZ) lesions (n = 126); systematic biopsy alone (n = 33); and severe artifacts on DW MRI (n = 2). Hence, 317 patients (median age, 64 years; range, 44–82 years) who underwent prebiopsy MRI and TRUS-guided targeted biopsy for the MRI-suspected index lesion in the PZ were analyzed retrospectively. All the patients had a time interval of less than six months between the MRI scan and the biopsy procedure (range: 1–159 days).
Fig. 1. Flowchart of the study subjects.
DW = diffusion-weighte, MRI = magnetic resonance imaging, PZ = peripheral zone, TRUS = transrectal ultrasound, TZ = transition zone
MRI Protocols
Multiparametric MRI (mpMRI) was performed using a 3T scanner (Intera Archieva TX or Ingenia, Philips Medical Systems) with a phased-array body coil. MRI protocols are summarized in Supplementary Table 1. MRI scans consisted of T2-weighted (T2W) MRI in the axial and sagittal planes, axial DW MRI with the highest b-value of 1500 s/mm2, and axial dynamic contrast-enhanced (DCE) MRI. The ADC map was generated from the DW MRI data with b-values of 0, 100, and 1000 s/mm2 through a mono-exponential fit. DCE MRI was performed immediately after a bolus injection of gadopentetate dimeglumine (Magnevist, Bayer Schering Pharma) or gadobutrol (Gadovist, Bayer Schering Pharma) (dose: 0.1 mmoL/kg; rate: 2–3 mL/s) using a power injector, followed by a 20 mL saline flush. Prior to the MRI scan, 20 mg of hyoscine butylbromide (Buscopan, Boehringer Ingelheim) was administered as an intramuscular injection to reduce bowel peristalsis. However, it was not administered in patients with myasthenia gravis, glaucoma, cardiac arrhythmia, severe urinary retention, or obstructive ileus (16 patients in our study; 9 due to glaucoma and 7 due to arrhythmia). Subtraction DCE images were generated from the data pertaining to post-contrast and precontrast enhanced images, to assess the actual prostatic enhancement. All MR images were archived using PACS (PathSpeed Workstation, GE Healthcare) for image analyses.
Clinical, Radiologic, and Pathologic Analyses
The current study analyzed the baseline clinical characteristics of the study subjects, namely, the age and serum prostate-specific antigen (PSA) levels, and the time interval between the MRI scan and biopsy procedure.
Two independent readers (reader 1, faculty of genitourinary radiology; experience: eight years in the field of prostate MRI; reader 2, clinical fellow of genitourinary radiology; experience: one year in the field of prostate MRI) re-analyzed prostate MR images of the PZ, in accordance with PI-RADSv2.0 or PI-RADSv2.1 [1,11]. Both readers were blinded to the clinical information (PSA) and biopsy results (the presence of PCa or the histologic grade of tumor). However, both the readers were provided with information regarding the site of targeted biopsy in the PZ, to perform a lesion-based analysis. They were unaware of the results of each other's image analysis. Both readers re-assessed prostate MRIs for the PZ lesions that were subjected to targeted biopsy. The two PI-RADS versions were interpreted in the same session without any interval. This was because if there is a significant time interval in the image interpretations for a patient, the reader may interpret the same MRI lesion differently (e.g., mass shape at first session and wedge shape at second session for the same lesion). Thus, we assumed that analyzing the two PI-RADS versions at the same session would help minimize risks of variability in image interpretation, leading to a better comparison. Consequently, each patient was assigned two types of PI-RADS categories for the corresponding PZ index lesion. One of the readers (i.e., reader 1) had performed the prostate biopsy for 15% of the study patients. However, there was an interval of more than 6 months between biopsy and retrospective MRI reading.
The reference standard in the present study was the formal pathologic results of targeted biopsies involving PZ lesions. At our institution, urologists are the deciding authority on the performance of biopsies, and their decisions are based on clinical or radiographic information (high serum PSA, abnormal digital rectal examination, or MRI findings). One of three experienced radiologists (experience: more than five years in the field of prostate MRI and biopsy) performed either the TRUS-guided targeted biopsy under cognitive registration (n = 233) or the MRI-TRUS fusion technique (n = 84). According to existing literature, there is no significant difference in cancer detection between cognitive registration and the MRI-TRUS fusion technique despite some debates [12]. One of two ultrasound scanners (IU22, Philips Healthcare; Aplio 500, Toshiba) was used for image guidance and an 18-gauge, 20-cm biopsy gun (ACECUT, TSK Laboratory) was used for tissue sampling. In case of targeted biopsy-proven PCa, the tumor grade was assigned to the corresponding International Society of Urological Pathology (ISUP) grade in each patient as follows: grade 1 = GS 3 + 3; grade 2 = GS 3 + 4; grade 3 = GS 4 + 3; grade 4 = GS 8; and grade 5 = GS 9 − 10 [13]. In the present study, csPCa was defined as PCa in the PZ with an ISUP grade of 2 or greater. Additionally, the core number of the targeted biopsy was recorded. A genitourinary pathologist (experience: more than 10 years in the field of PCa) evaluated the biopsy specimens.
Statistical Analysis
In the present study, the area under the curve (AUC) was assessed using the receiver operating-characteristic (ROC) curve analyses. The AUCs of the two PI-RADS versions were compared by means of the DeLong test. The cutoff maximizing the Youden index was calculated, and the corresponding sensitivity, specificity, and accuracy of the two PI-RADS versions from the same subjects were compared using the McNemar test. The PPV and negative predictive value (NPV) were compared using the Wald test, based on the generalized estimating equation (GEE) [14,15]. In addition, the CDRs of categories 1–2, 3, 4, and 5 for csPCa in the PZ were calculated, and the CDR of each category was compared between the two versions using GEE. The definition of CDR in this study was the proportion of the patients with targeted biopsy-proven csPCa among the study patients who received targeted biopsy for the MRI-suggested index lesion.
We compared the proportions of csPCa between the two targeted biopsy techniques (i.e., cognitive registration vs. MRI-TRUS fusion technique) using the chi-square test.
Regarding the inter-reader agreement, the current study analyzed the kappa value with respect to the following: 1) a category of ≥ 3 or not, 2) ≥ 4 or not, or 3) 5 or not. The degree of inter-reader agreement was interpreted as follows: 0–0.20 = poor; 0.21–0.40 = fair; 0.41–0.60 = moderate; 0.61–0.80 = good; and 0.81–1 = excellent agreement [16].
Finally, a sub-analysis of the DW category change (PI-RADSv2.0 category 3 to PI-RADSv2.1 category 2) was performed.
The MedCalc 18.1 (MedCalc Software) was used to perform the ROC curve analysis with the DeLong test, chi-square test, or kappa, and the SAS version 9.4 (SAS Institute) for the McNemar test and GEE. A p value < 0.05 was considered statistically significant.
RESULTS
Characteristics of the Study Subjects
Targeted biopsies involving PZ lesions were performed in the right, left, and midline regions of the PZ in 120, 194, and 3 subjects, respectively. The median core number of targeted biopsies was three (range, 2–8). The range of serum PSA was 0.4–71.9 mg/dL. The proportion of csPCa was 32.6% (76/233) in cognitive registration and 32.1% (27/84) in MRI-TRUS fusion technique in this study (p = 0.936). The rates of PCa and csPCa in the PZ were 41.0% (130/317) and 32.5% (103/317), respectively (Table 1).
Table 1. Characteristics of the Study Subjects.
| Parameter | Value | ||
|---|---|---|---|
| Age, years | 64.0 (59.0–69.3) | ||
| PSA, mg/dL | 4.9 (3.7–7.2) | ||
| Time interval between MRI and biopsy, days | 36.0 (19.0–57.0) | ||
| PZ lesions | |||
| Laterality, n, right:left:midline | 120:194:3 | ||
| Core number of targeted biopsies, n | 3.0 (2.0–6.0) | ||
| ISUP grade, n | |||
| No cancer | 187 | ||
| 1 | 27 | ||
| 2 | 40 | ||
| 3 | 29 | ||
| 4 | 29 | ||
| 5 | 5 | ||
| Proportion of csPCa, % | 32.5 (103/317) | ||
Data regarding the age, PSA, time interval between MRI and biopsy, and core number of targeted biopsies are presented as median (interquartile range). Data regarding the proportion of csPCa are presented as percentage (number of patients). csPCa = clinically significant prostate cancer, ISUP = International Society of Urologic Pathology, MRI = magnetic resonance imaging, PSA = prostate-specific antigen, PZ = peripheral zone
Performance of PI-RADS
AUC of PI-RADSv2.0 and PI-RADSv2.1
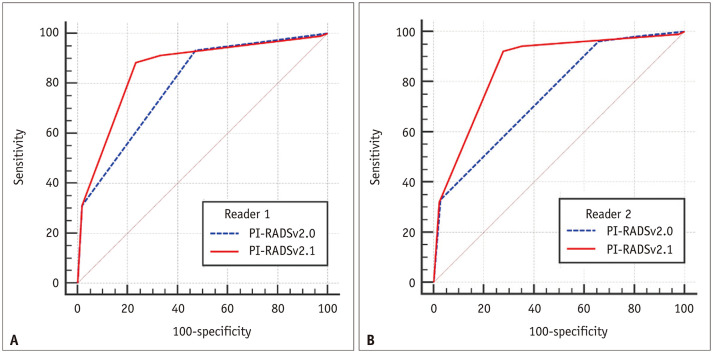
To identify csPCa in the PZ; the AUCs of PI-RADSv2.0 vs. PI-RADSv2.1 were 0.795 (95% CI, 0.746–0.838) vs. 0.856 (95% CI, 0.813–0.893) for reader 1 (p < 0.001) and 0.747 (95% CI, 0.696–0.794) vs. 0.858 (95% CI, 0.814–0.894) for reader 2 (p < 0.001), respectively (Fig. 2).
Fig. 2. In terms of identifying clinically significant prostate cancer in the peripheral zone, area under the curves of PI-RADSv2.0 vs. PI-RADSv2.1 were (A) 0.795 vs. 0.856, for reader 1 (p < 0.001) and (B) 0.747 vs. 0.858, for reader 2 (p < 0.001).
PI-RADSv2.0 = Prostate Imaging-Reporting and Data System version 2.0, PI-RADSv2.1 = Prostate Imaging-Reporting and Data System version 2.1
Sensitivity, Specificity, PPV, NPV, and Accuracy
The current study observed that the cutoff maximizing Youden index for csPCa was consistently at category 4 or greater for both the readers and the two PI-RADS versions. Using this cutoff, it was observed that the specificity PPV, and accuracy of PI-RADSv2.1 were significantly higher compared to that of PI-RADSv2.0 (p < 0.05). The sensitivity of PI-RADSv2.1 was observed to be significantly lower, compared to PI-RADSv2.0 (p < 0.05) (Table 2). The NPV of the two versions were observed to be similar (p >0.05) and the value was greater than 90%, regardless of the reader or version.
Table 2. Comparison of Diagnostic Performances between PI-RADSv2.0 and PI-RADSv2.1 for csPCa.
| Parameter | Cutoff | Sensitivity | Specificity | PPV | NPV | Accuracy | |
|---|---|---|---|---|---|---|---|
| Reader 1 | |||||||
| PI-RADSv2.0 | ≥ 4 | 93.2 (96/103) [86.4–96.7] | 52.8 (113/214) [46.1–59.4] | 48.7 (96/197) [41.8–55.7] | 94.2 (113/120) [88.3–97.2] | 65.9 (209/317) [60.6–70.9] | |
| PI-RADSv2.1 | ≥ 4 | 88.3 (91/103) [80.6–93.3] | 76.6 (164/214) [70.5–81.8] | 64.5 (91/141) [56.3–72.0] | 93.2 (164/176) [88.4–96.1] | 80.4 (255/317) [75.7–84.4] | |
| p value | 0.025 | < 0.001 | < 0.001 | 0.504 | < 0.001 | ||
| Reader 2 | |||||||
| PI-RADSv2.0 | ≥ 4 | 96.1 (99/103) [90.1–98.5] | 34.1 (73/214) [28.1–40.7] | 41.3 (99/240) [35.2–47.6] | 94.8 (73/77) [87.0–98.0] | 54.3 (172/317) [48.8–59.7] | |
| PI-RADSv2.1 | ≥ 4 | 92.2 (95/103) [85.2–96.1] | 72.4 (155/214) [66.1–78.0] | 61.7 (95/154) [53.8–69.0] | 95.1 (155/163) [90.5–97.5] | 78.9 (250/317) [74.0–83.0] | |
| p value | 0.045 | < 0.001 | < 0.001 | 0.869 | < 0.001 | ||
The sensitivity, specificity, PPV, and NPV were calculated using the cutoff category of 4 or greater. Data presented as percentage (numerator/denominator) [95% confidence interval]. csPCa = clinically significant prostate cancer, NPV = negative predictive number, PI-RADSv2.0 = Prostate Imaging-Reporting and Data System version 2.0, PI-RADSv2.1 = Prostate Imaging-Reporting and Data System version 2.1, PPV = positive predictive number
Distribution of PI-RADS Categories and Cancer Detection Rates
The distribution of PI-RADSv2.0 vs. PI-RADSv2.1 in the categories 1–2, 3, 4, and 5 were as follows: 1) reader 1: 68 (category 2, n = 68) vs. 152 (category 1, n = 7; category 2, n = 145), 52 vs. 24, 161 vs. 105, and 36 vs. 36; and 2) reader 2: 44 (category 2, n = 44) vs. 145 (category 1, n = 6; category 2, n = 139), 33 vs. 18, 200 vs. 116, and 40 vs. 38, respectively (Supplementary Table 2).
Regarding the diagnosis of csPCa in the PZ, the CDRs of the PI-RADS categories 1–2, 3, 4, and 5 in PI-RADSv2.0 vs. PI-RADSv2.1 are shown in Table 3. The CDR of categories 3 or 4 of PI-RADSv2.1 was significantly higher compared to that of PI-RADSv2.0.
Table 3. Distribution of PI-RADS Categories and Cancer Detection Rates.
| Parameter | PI-RADSv2.0 | PI-RADSv2.1 | P | |
|---|---|---|---|---|
| Reader 1 | ||||
| Category 1–2 | 5.9 (4/68) | 5.9 (9/152) | 0.985 | |
| Category 3 | 5.8 (3/52) | 12.5 (3/24) | < 0.001 | |
| Category 4 | 39.8 (64/161) | 56.2 (59/105) | < 0.001 | |
| Category 5 | 88.9 (32/36) | 88.9 (32/36) | > 0.999 | |
| Reader 2 | ||||
| Category 1–2 | 4.5 (2/44) | 4.1 (6/145) | 0.868 | |
| Category 3 | 6.1 (2/33) | 11.1 (2/18) | < 0.001 | |
| Category 4 | 32.5 (65/200) | 53.4 (62/116) | < 0.001 | |
| Category 5 | 85.0 (34/40) | 86.8 (33/38) | 0.409 | |
Data presented as percentage (number of patients). PI-RADSv2.0 = Prostate Imaging-Reporting and Data System version 2.0, PI-RADSv2.1 = Prostate Imaging-Reporting and Data System version 2.1
Inter-Reader Agreement
Regarding the interpretation of PI-RADSv2.0, the kappa for the categories of ≥ 3 or not, ≥ 4 or not, and 5 or not were 0.227 (95% CI, 0.100–0.353), 0.387 (95% CI, 0.284–0.490), and 0.791 (95% CI, 0.685–0.896), respectively. Regarding the interpretation of PI-RADSv2.1, the kappa for the categories of ≥ 3 or not, ≥ 4 or not, and 5 or not were 0.462 (95% CI, 0.364–0.560), 0.589 (95% CI, 0.500–0.677), and 0.755 (95% CI, 0.641–0.870), respectively.
Changes from DW Category 3 in PI-RADSv2.0 to Category 2 in PI-RADSv2.1
The proportion of the DW category 3 in PI-RADSv2.0 was 43.2% (137/317) and 50.5% (160/317) for readers 1 and 2, respectively. The DW category 3 of PI-RADSv2.0 was changed to category 2 of PI-RADSv2.1 in 74 and 95 patients for readers 1 and 2, respectively. Among these patients, the proportion of csPCa was less than 5% for both the readers (4.1% for reader 1 and 3.2% for reader 2) (Table 4).
Table 4. Sub-Analysis Pertaining to the Changes from DW Category 3 in PI-RADSv2.0 to Category 2 in PI-RADSv2.1.
| Category Changes | Reader 1 | Reader 2 | |||
|---|---|---|---|---|---|
| Number of Case | csPCa* | Number of Case | csPCa* | ||
| DW category: 3 → 2 | 73 | 4.1 (3/73) | 95 | 3.2 (3/95) | |
| Final category: 3 + 1 → 2 + 1 | 50 | 6.0 (3/50) | 81 | 3.7 (3/81) | |
| Final category: 3 + 0 → 2 + 0 | 23 | 0.0 (0/23) | 14 | 0.0 (0/14) | |
Final categories of 3 + 1, 2 + 1, 3 + 0, or 2 + 0 indicate the DW category 3 plus positive DCE, DW category 2 plus positive DCE, DW category 3 plus negative DCE, or DW category 2 plus negative DCE MRI findings, respectively. *Data presented as percentage (number of patients). csPCa = clinically significant prostate cancer, DCE = dynamic contrast-enhanced, DW = diffusion-weighted, PI-RADSv2.0 = Prostate Imaging-Reporting and Data System version 2.0, PI-RADSv2.1 = Prostate Imaging-Reporting and Data System version 2.1
Depending on the DCE MRI findings, two types of changes in the final PI-RADS category were observed: 1) from a final category 4 in PI-RADSv2.0 (category 3 plus positive DCE MRI) to category 2 in PI-RADSv2.1 (DW category 2 plus positive DCE MRI); 2) from a final category 3 in PI-RADSv2.0 (DW category 3 plus negative DCE MRI) to category 2 in PI-RADSv2.1 (DW category 2 plus negative DCE MRI). In patients whose final category was changed from 4 to 2, the proportion of csPCa for readers 1 and 2 were 6.0% and 3.7%, respectively (Figs. 3, 4, 5). When the final category was changed from 3 to 2, no csPCa was detected in the PZ.
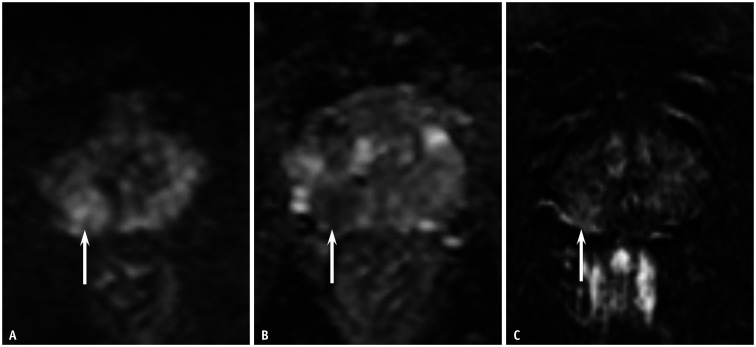
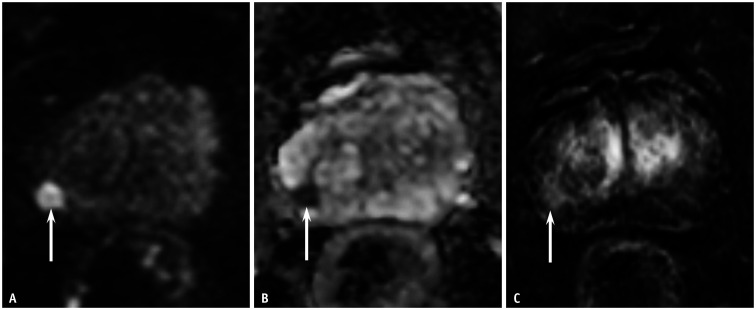
Fig. 3. A 68-year-old male patient with a prostate-specific antigen level of 12.0 mg/dL.
A, B. The DW MRI and apparent diffusion coefficient map depicted a wedge-shaped lesion of moderate diffusion restriction, measuring 0.8 cm in the left PZ of the prostate (arrows). DW categories were 3 for PI-RADSv2.0 and 2 for PI-RADSv2.1 for both the readers. C. Dynamic contrast-enhanced MRI showed positive findings at the corresponding site (arrow). Thus, the PI-RADSv2.0 and PI-RADSv2.1 categories were 4 and 2, respectively, for both readers. Targeted biopsy with four cores revealed chronic inflammation in the PZ lesion. DW = diffusion-weighted, MRI = magnetic resonance imaging, PI-RADSv2.0 = Prostate Imaging-Reporting and Data System version 2.0, PI-RADSv2.1 = Prostate Imaging-Reporting and Data System version 2.1, PZ = peripheral zone
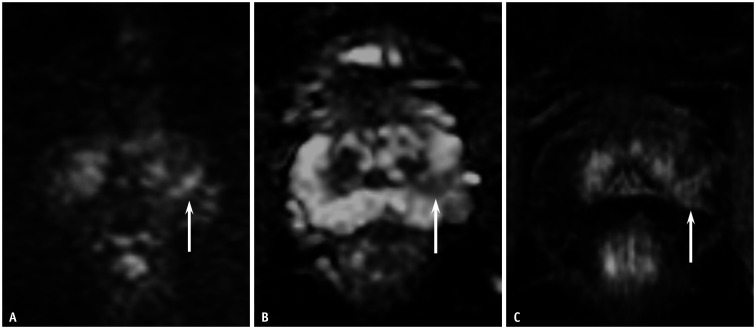
Fig. 4. A 61-year-old male patient with a prostate-specific antigen level of 3.5 mg/dL.
A, B. The DW MRI and apparent diffusion coefficient map depicted a focal lesion of moderate diffusion restriction, measuring 1.2 cm in the right PZ of the prostate (arrows). DW categories were 3 for PI-RADSv2.0 and 3 for PI-RADSv2.1 for both the readers. C. Dynamic contrast-enhanced MRI showed positive findings at the corresponding site (arrow). Thus, the PI-RADSv2.0 and PI-RADSv2.1 categories were identical (category 4) for both readers. Targeted biopsy of the PZ lesion, with five cores, revealed clinically significant prostate cancer of International Society of Urological Pathology grade 2. DW = diffusion-weighted, MRI = magnetic resonance imaging, PI-RADSv2.0 = Prostate Imaging-Reporting and Data System version 2.0, PI-RADSv2.1 = Prostate Imaging-Reporting and Data System version 2.1, PZ = peripheral zone
Fig. 5. An 80-year-old male patient with a prostate-specific antigen level of 4.1 mg/dL.
A, B. The DW MRI and apparent diffusion coefficient map depicted a focal lesion of marked diffusion restriction, measuring 1.0 cm, in the right PZ of prostate (arrows). DW categories were 4 for both the versions for both readers. C. Dynamic contrast-enhanced MRI showed positive findings at the corresponding site (arrow). Thus, the PI-RADSv2.0 and PI-RADSv2.1 categories were identical (category 4) for both readers. Targeted biopsy of the peripheral zone lesion, with six cores, revealed clinically significant prostate cancer International Society of Urological Pathology grade 2. DW = diffusion-weighted, MRI = magnetic resonance imaging, PI-RADSv2.0 = Prostate Imaging-Reporting and Data System version 2.0, PI-RADSv2.1 = Prostate Imaging-Reporting and Data System version 2.1, PZ = peripheral zone
DISCUSSION
In the current study, various performance parameters pertaining to the detection of csPCa in the PZ, such as AUC, specificity, PPV, and accuracy were significantly higher in PI-RADSv2.1 compared to that in PI-RADSv2.0. Moreover, the NPV of PI-RADSv2.1 was observed to be excellent (greater than 90% for both the readers) and comparable to that of PI-RADSv2.0. However, sensitivity decreased by approximately 5% in PI-RADSv2.1. The CDR of categories 3 or 4 for csPCa increased in PI-RADSv2.1. These findings were concordantly demonstrated by two board-certified genitourinary radiologists.
Previous studies have suggested that the information obtained using PI-RADSv2.0 can facilitate the decisions involved in the diagnosis or management of PCa [2]. Targeted biopsy of index lesions with high PI-RADS categories can enable the effective diagnosis of csPCa [8]. Furthermore, mpMRI can be incorporated into the practical pathway and guide the decision regarding active surveillance [4]. However, the limited PPV and CDR of PI-RADSv2.0 for csPCa was associated with overestimation, resulting in the risk of unwarranted targeted biopsies [5,7,17].
Many studies on PI-RADSv2.0 have consistently reported categories greater than or equal to 3 or 4 as the optimal cutoff for identifying csPCa [18] and these are currently being utilized to arrive at the decisions regarding targeted biopsies [4,19,20]. In the current study, the cutoff maximizing the Youden index was calculated and the corresponding sensitivity, specificity, PPV, NPV, and accuracy were analyzed. A recent article by Padhani et al. [2] stated that there may be certain uncertainties regarding the use of MR-directed biopsy in PI-RADS category 3 lesions depending on the clinical scenarios, whereas it is generally recommended for PI-RADS category 4–5 lesions. Accordingly, reporting the current performance data at a cutoff of 4 or greater may be clinically meaningful in MR-directed biopsies.
In relation to the PZ, DW category 3 can be a final category of 3 or 4, depending on the DCE MRI findings. Nevertheless, there may be risks of overdiagnosis regarding csPCa, owing to the false positive findings on DCE MRI [21]. Hence, reducing the false positive rate during the initial step of PI-RADS interpretation (i.e., DW MRI reading) can effectively increase the PPV, resulting in a higher CDR using targeted biopsies.
Recently, the criteria for the determination of DW category 3 has become stricter, as the morphologic features and diffusion restriction degree should be considered concurrently in PI-RADSv2.1. In the present study, 55–60% of DW category 3 lesions of PI-RADSv2.0 were reallocated to the category 2 of PI-RADSv2.1, owing to a wedge-shape observed on DW MRI. However, the proportion of csPCa in the reallocated patients was less than 5%, which indicates that the changes pertaining to PZ in PI-RADSv2.1 can facilitate the reduction in false positive results. Nevertheless, the decreased proportion of PI-RADSv2.1 category 4 may be associated with the decreased sensitivity, compared to that of v2.0, despite the increased specificity or PPV.
In the current study, NPV of both the versions were observed to be similar. NPV is closely associated with the rule-out function of MRI in the identification of csPCa [2]. Hence, the exceptional NPV (93–95%) implies that PI-RADSv2.1 can play a major role in decisions involving the omission of targeted biopsy or the selection of optimal candidates for active surveillance. Nonetheless, further studies involving the TZ PCa are mandatory, as the TZ interpretation was also revised in PI-RADSv2.1 [1]. Recently, promising data regarding the performance of PI-RADSv2.1 in the TZ have been reported [22,23]. Hence, the data presented in the current study, which focuses on PZ, supports the evidence concerning the diagnostic performance of PI-RADSv2.1.
This study used a cutoff of category 4 or greater and it was observed that the sensitivity of PI-RADSv2.1 was lower compared to that of PI-RADSv2.0. A recent investigation that analyzed surgical cases reported the proportion of csPCa (ISUP grade ≥ 2) in wedge-shaped PZ lesions as approximately 30% [24]. However, since the proportion of csPCa in surgical cases is much higher compared to biopsy cases, the actual proportion of wedge-shaped csPCa may be lower in the scenarios involving biopsies. The results of the present study indicate that there may be a low risk of overlooking csPCa in linear or wedge-shaped category 2 lesions in the PZ, which could facilitate the prevention of unwarranted targeted biopsies.
Considering the reproducibility, a wide range of the inter-reader agreements regarding PZ evaluation have been reported in literature (kappa, 0.29–0.62 for ≥ 3 or not; 0.40–0.91 for ≥ 4 or not) [25,26,27]. In the current study, inter-reader agreement of PI-RADSv2.0 was observed to be fair for categories ≥ 3 or not and ≥ 4 or not, while that of PI-RADSv2.1 was observed to be moderate. Recognizing linear/wedge shapes may be relatively easier, compared to the identification of indistinct margins of PZ lesions [28]. Furthermore, the current revision reallocated the DW category 3 into category 2 in some patients, which may reduce the possible variability of the final PI-RADS classification resulting from the discordant interpretation of DCE MRI. However, PI-RADSv2.1 seems to have limited reproducibility. As the experience levels of the radiologists might affect the low kappa value in the current study [27], multi-institutional studies involving additional observers with varying levels of experience are required to confirm the reproducibility of PI-RADSv2.1.
Two cases of PI-RADSv2.0 category 5 were falsely interpreted as PI-RADSv2.1 category 2 by reader 2, as these lesions were considered as wide-angled, wedge-shaped lesions. In PI-RADSv2.1, DW category 2 does not specifically describe the degree of diffusion restriction, which might lead to some confusion in the interpretation of PZ lesions of marked diffusion restriction that also exhibit both wide-angle and wedge-shape, when a less-experienced radiologist is involved.
This study has several limitations. First, the study involved the retrospective analysis of the data obtained from a single institution. Consequently, the study design might involve a risk of selection bias. Nonetheless, the current study evaluated a consecutive series of patients who met both the inclusion and exclusion criteria. The current data require external validation. Second, this study did not evaluate patients who did not undergo targeted biopsy for prostatic lesions. However, the initial targeted biopsy results allowed for a lesion-based comparison between the two versions of PI-RADS. This data supports prospective, patient-based studies involving all patients who undergo prostate MRI. Third, the readers were aware of the location of PZ lesions that were confirmed by targeted biopsy to overcome the limitations due to the retrospective study design (i.e., a possible mismatch between the lesion suspected by MRI and the lesion on which targeted biopsy was performed). However, this might be associated with a potential bias because the readers were inevitably aware that the assigned PZ lesions were more likely to be PI-RADS category 3 or higher at the initial MRI interpretation of real practice. Fourth, there might be a risk of expectation bias because many genitourinary radiologists have known the limited capability of PI-RADS in predicting csPCa. Evidence has suggested that PPV or CDR of high PI-RADS categories are somewhat lower than thar initially expected [2]. Thus, in this study, observers might have had risks of reading the PZ lesions showing linear or wedge shape with more emphasis on the specificity, possibly leading to the higher performance of v2.1.
In conclusion, the present study demonstrated improved AUC, specificity, PPV, accuracy, and CDRs of categories 3 or 4 of PI-RADSv2.1, but decreased sensitivity compared with PI-RADSv2.0 in the identification of csPCa in the PZ. Hence, PI-RADSv2.1 may enhance cancer detection through targeted biopsy in the PZ, owing to the improved PPV. The data obtained from the present study requires further prospective and external validation.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Sung Yoon Park.
- Formal analysis: Hyun Soo Kim, Sung Yoon Park.
- Investigation: all authors.
- Methodology: Sung Yoon Park.
- Supervision: Ghee Young Kwon, Sung Yoon Park
- Visualization: Sung Yoon Park.
- Writing—original draft: Hyun Soo Kim, Sung Yoon Park.
- Writing—review & editing: Sung Yoon Park.
Supplements
The Supplement is available with this article at https://doi.org/10.3348/kjr.2020.0837.
MRI Protocols
Distribution of csPCa Stratified by ISUP Grade in Each PI-RADS Category
References
- 1.Turkbey B, Rosenkrantz AB, Haider MA, Padhani AR, Villeirs G, Macura KJ, et al. Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urol. 2019;76:340–351. doi: 10.1016/j.eururo.2019.02.033. [DOI] [PubMed] [Google Scholar]
- 2.Padhani AR, Weinreb J, Rosenkrantz AB, Villeirs G, Turkbey B, Barentsz J. Prostate imaging-reporting and data system steering committee: PI-RADS v2 status update and future directions. Eur Urol. 2019;75:385–396. doi: 10.1016/j.eururo.2018.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fulgham PF, Rukstalis DB, Turkbey IB, Rubenstein JN, Taneja S, Carroll PR, et al. AUA policy statement on the use of multiparametric magnetic resonance imaging in the diagnosis, staging and management of prostate cancer. J Urol. 2017;198:832–838. doi: 10.1016/j.juro.2017.04.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Association of Urology. Prostate cancer. Uroweb. org Web site; Published 2019. [Accessed December 10, 2019]. https://uroweb.org/guideline/prostate-cancer/ [Google Scholar]
- 5.Hofbauer SL, Maxeiner A, Kittner B, Heckmann R, Reimann M, Wiemer L, et al. Validation of prostate imaging reporting and data system version 2 for the detection of prostate cancer. J Urol. 2018;200:767–773. doi: 10.1016/j.juro.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Mehralivand S, Bednarova S, Shih JH, Mertan FV, Gaur S, Merino MJ, et al. Prospective evaluation of PI-RADS™ version 2 using the international society of urological pathology prostate cancer grade group system. J Urol. 2017;198:583–590. doi: 10.1016/j.juro.2017.03.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mertan FV, Greer MD, Shih JH, George AK, Kongnyuy M, Muthigi A, et al. Prospective evaluation of the prostate imaging reporting and data system version 2 for prostate cancer detection. J Urol. 2016;196:690–696. doi: 10.1016/j.juro.2016.04.057. [DOI] [PubMed] [Google Scholar]
- 8.Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378:1767–1777. doi: 10.1056/NEJMoa1801993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westphalen AC, McCulloch CE, Anaokar JM, Arora S, Barashi NS, Barentsz JO, et al. Variability of the positive predictive value of PI-RADS for prostate MRI across 26 centers: experience of the society of abdominal radiology prostate cancer disease-focused panel. Radiology. 2020;296:76–84. doi: 10.1148/radiol.2020190646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNeal JE, Redwine EA, Freiha FS, Stamey TA. Zonal distribution of prostatic adenocarcinoma. Correlation with histologic pattern and direction of spread. Am J Surg Pathol. 1988;12:897–906. doi: 10.1097/00000478-198812000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, et al. PI-RADS prostate imaging - reporting and data system: 2015, version 2. Eur Urol. 2016;69:16–40. doi: 10.1016/j.eururo.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venderink W, Bomers JG, Overduin CG, Padhani AR, de Lauw GR, Sedelaar MJ, et al. Multiparametric magnetic resonance imaging for the detection of clinically significant prostate cancer: what urologists need to know. Part 3: targeted biopsy. Eur Urol. 2020;77:481–490. doi: 10.1016/j.eururo.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Pierorazio PM, Walsh PC, Partin AW, Epstein JI. Prognostic Gleason grade grouping: data based on the modified Gleason scoring system. BJU Int. 2013;111:753–760. doi: 10.1111/j.1464-410X.2012.11611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, Davis CS, Soong SJ. Comparison of predictive values of two diagnostic tests from the same sample of subjects using weighted least squares. Stat Med. 2006;25:2215–2229. doi: 10.1002/sim.2332. [DOI] [PubMed] [Google Scholar]
- 15.Leisenring W, Alonzo T, Pepe MS. Comparisons of predictive values of binary medical diagnostic tests for paired designs. Biometrics. 2000;56:345–351. doi: 10.1111/j.0006-341x.2000.00345.x. [DOI] [PubMed] [Google Scholar]
- 16.Jakobsson U, Westergren A. Statistical methods for assessing agreement for ordinal data. Scand J Caring Sci. 2005;19:427–431. doi: 10.1111/j.1471-6712.2005.00368.x. [DOI] [PubMed] [Google Scholar]
- 17.Stolk TT, de Jong IJ, Kwee TC, Luiting HB, Mahesh SVK, Doornweerd BHJ, et al. False positives in PIRADS (V2) 3, 4, and 5 lesions: relationship with reader experience and zonal location. Abdom Radiol (NY) 2019;44:1044–1051. doi: 10.1007/s00261-019-01919-2. [DOI] [PubMed] [Google Scholar]
- 18.Woo S, Suh CH, Kim SY, Cho JY, Kim SH. Diagnostic performance of prostate imaging reporting and data system version 2 for detection of prostate cancer: a systematic review and diagnostic meta-analysis. Eur Urol. 2017;72:177–188. doi: 10.1016/j.eururo.2017.01.042. [DOI] [PubMed] [Google Scholar]
- 19.Padhani AR, Barentsz J, Villeirs G, Rosenkrantz AB, Margolis DJ, Turkbey B, et al. PI-RADS Steering Committee: the PI-RADS multiparametric MRI and MRI-directed biopsy pathway. Radiology. 2019;292:464–474. doi: 10.1148/radiol.2019182946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venderink W, van Luijtelaar A, Bomers JGR, van der Leest M, Hulsbergen-van de Kaa C, Barentsz JO, et al. Results of targeted biopsy in men with magnetic resonance imaging lesions classified equivocal, likely or highly likely to be clinically significant prostate cancer. Eur Urol. 2018;73:353–360. doi: 10.1016/j.eururo.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 21.Bae H, Cho NH, Park SY. PI-RADS version 2: optimal time range for determining positivity of dynamic contrast-enhanced MRI in peripheral zone prostate cancer. Clin Radiol. 2019;74:895.e27–895.e34. doi: 10.1016/j.crad.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Tamada T, Kido A, Takeuchi M, Yamamoto A, Miyaji Y, Kanomata N, et al. Comparison of PI-RADS version 2 and PI-RADS version 2.1 for the detection of transition zone prostate cancer. Eur J Radiol. 2019;121:108704. doi: 10.1016/j.ejrad.2019.108704. [DOI] [PubMed] [Google Scholar]
- 23.Byun J, Park KJ, Kim MH, Kim JK. Direct comparison of PI-RADS version 2 and 2.1 in transition zone lesions for detection of prostate cancer: preliminary experience. J Magn Reson Imaging. 2020;52:577–586. doi: 10.1002/jmri.27080. [DOI] [PubMed] [Google Scholar]
- 24.Chatterjee A, Tokdemir S, Gallan AJ, Yousuf A, Antic T, Karczmar GS, et al. Multiparametric MRI features and pathologic outcome of wedge-shaped lesions in the peripheral zone on T2-weighted images of the prostate. AJR Am J Roentgenol. 2019;212:124–129. doi: 10.2214/AJR.18.19742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenkrantz AB, Ginocchio LA, Cornfeld D, Froemming AT, Gupta RT, Turkbey B, et al. Interobserver reproducibility of the PI-RADS version 2 lexicon: a multicenter study of six experienced prostate radiologists. Radiology. 2016;280:793–804. doi: 10.1148/radiol.2016152542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purysko AS, Bittencourt LK, Bullen JA, Mostardeiro TR, Herts BR, Klein EA. Accuracy and interobserver agreement for prostate imaging reporting and data system, version 2, for the characterization of lesions identified on multiparametric MRI of the prostate. AJR Am J Roentgenol. 2017;209:339–349. doi: 10.2214/AJR.16.17289. [DOI] [PubMed] [Google Scholar]
- 27.Mussi TC, Yamauchi FI, Tridente CF, Tachibana A, Tonso VM, Recchimuzzi DZ, et al. Interobserver agreement of PI-RADS v. 2 lexicon among radiologists with different levels of experience. J Magn Reson Imaging. 2020;51:593–602. doi: 10.1002/jmri.26882. [DOI] [PubMed] [Google Scholar]
- 28.Benndorf M, Hahn F, Krönig M, Jilg CA, Krauss T, Langer M, et al. Diagnostic performance and reproducibility of T2w based and diffusion weighted imaging (DWI) based PI-RADSv2 lexicon descriptors for prostate MRI. Eur J Radiol. 2017;93:9–15. doi: 10.1016/j.ejrad.2017.05.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MRI Protocols
Distribution of csPCa Stratified by ISUP Grade in Each PI-RADS Category