Abstract
Objective
To evaluate the feasibility, safety, and effectiveness of CT-guided microcoil localization of solitary pulmonary nodules (SPNs) for guiding video-assisted thoracoscopic surgery (VATS).
Materials and Methods
Between June 2016 and October 2019, 454 consecutive patients with 501 SPNs who received CT-guided microcoil localization before VATS in our institution were enrolled. The diameter of the nodules was 0.93 ± 0.49 cm, and the shortest distance from the nodules to the pleura was 1.41 ± 0.95 cm. The distal end of the microcoil was placed less than 1 cm away from the nodule, and the proximal end was placed outside the visceral pleura. VATS was performed under the guidance of implanted microcoils without the aid of intraoperative fluoroscopy.
Results
All 501 nodules were marked with microcoils. The time required for microcoil localization was 12.8 ± 5.2 minutes. Microcoil localization-related complications occurred in 179 cases (39.4%). None of the complications required treatment. A total of 463 nodules were successfully resected under the guidance of implanted microcoils. VATS revealed 38 patients with dislocated microcoils, of which 28 underwent wedge resection (21 cases under the guidance of the bleeding points of pleural puncture, 7 cases through palpation), 5 underwent direct lobectomy, and the remaining 5 underwent a conversion to thoracotomy. In 4 cases, a portion of the microcoil remained in the lung parenchyma.
Conclusion
CT-guided microcoil localization of SPNs is safe and reliable. Marking the nodule and pleura simultaneously with microcoils can effectively guide the resection of SPNs using VATS without the aid of intraoperative fluoroscopy.
Keywords: Tomography, X-Ray computed; Thoracic surgery, video-assisted; Solitary pulmonary nodule; Localization
INTRODUCTION
The widespread use of chest CT for lung cancer screening in high-risk populations and routine follow-up of oncologic patients have led to frequent detection of solitary pulmonary nodules (SPNs). Video-assisted thoracoscopic surgery (VATS) has become preferred for diagnosis and, in selected cases, treatment of indeterminate small SPNs [1]. However, small and deep SPNs, especially ground-glass nodules (GGNs), may be invisible and impalpable during VATS [2].
CT-guided microcoil localization is a reliable method [3,4,5,6,7,8,9,10]. For SPNs that cannot be seen and palpated during VATS, microcoil localization can help surgeons accurately locate SPNs and facilitate easier and quicker resection [3,4,5]. In previous studies, wedge resection of SPNs using VATS after microcoil localization was usually performed with the aid of intraoperative fluoroscopy [3,4,5,6,7,8,9]. Intraoperative fluoroscopy requires the use of a mobile fluoroscopy system or a hybrid operating room, and it is time-consuming, costly, and associated with increased radiation exposure of both the patients and the surgeons.
The first objective of this study was to evaluate the feasibility, safety, and effectiveness of CT-guided microcoil localization of SPNs for guiding resection using VATS without the aid of intraoperative fluoroscopy. The secondary objective was to evaluate the feasibility and safety of the extended interval (> 24 hours) between CT-guided microcoil localization and VATS. A total of 454 cases were analyzed retrospectively.
MATERIALS AND METHODS
Materials
The criteria for CT-guided microcoil localization for SPNs before VATS were as follows: 1) SPNs persisted for more than 3 months; 2) SPNs were invisible and impalpable during VATS; 3) the distance from the SPNs to the pleura was less than 4 cm. Between June 2016 and October 2019, 454 consecutive patients with 501 peripheral SPNs who received CT-guided microcoil localization before VATS at our institution were enrolled. The general conditions of patients and nodules are shown in Table 1. The diameter of the lesion was 0.93 ± 0.49 cm, and the shortest distance from the lesion to the pleura was 1.41 ± 0.95 cm. This study was approved by the ethics committee of China-Japan Friendship Hospital (approval number: 2019051). Before CT-guided microcoil localization, all patients were informed of the procedure, possible complications, and the use of relevant clinical and imaging data for clinical studies under anonymous conditions. All patients provided written informed consent.
Table 1. The General Conditions of Patients and Nodules.
| Number of patients | 454 | |
| Age, year | 59.28 ± 10.22 | |
| Sex | ||
| Male | 165 | |
| Female | 289 | |
| Number of patient-marked nodules | ||
| One nodule | 411 | |
| Two nodules | 39 | |
| Three nodules | 4 | |
| Number of nodules | 501 | |
| Size of nodules, cm | 0.93 ± 0.49 | |
| Depth of nodules, cm | 1.41 ± 0.95 | |
| Location of nodules | ||
| Right upper lobe | 190 | |
| Right middle lobe | 41 | |
| Right lower lobe | 90 | |
| Left upper lobe | 109 | |
| Left lower lobe | 71 | |
| Type of nodule | ||
| Pure GGN | 330 | |
| Mixed GGN | 69 | |
| Solid nodules | 102 | |
Data are presented as number or mean ± standard deviation. GGN = ground-glass nodule
Marking Method
All CT-guided microcoil localizations were performed by two radiologists who had been engaged in CT-guided intervention for 24 and 3 years, respectively. An 18G 10-cm-long Chiba puncture needle (Cook) and an MWCE-18S-6/2 vascular embolization microcoil set (Cook) were used for localization. The microcoil was 7 cm long when straightened, and it had a diamond shape in its natural state, with a small end diameter of 2 mm and a large end diameter of 6 mm. It was installed in the loading cannula. The loading cannula fitted exactly into the 18G Chiba needle sheath. All CT scans were performed on an Aquillion-one 16 Slice CT Scanner (Toshiba) using 100 KV, 120 mAs, and a 1.25-mm thickness. We used the coaxial needle technique of the CT puncture to implant the microcoil. First, the Chiba needle was inserted in the planned position. A local CT scan confirmed that the positional relationship between the Chiba needle tip and the nodule was satisfactory (the distance between the needle tip and the nodule was ≤ 1.0 cm) (Figs. 1A, 2A, B). The Chiba needle core was pulled out, and the loading cannula with a microcoil was inserted into the Chiba needle sheath. The microcoil was slightly pushed out of the loading cannula with the stylet, and a 2–3-cm microcoil was deployed into the lung parenchyma where it assumed a tightly coiled helical configuration adjacent to the nodule. Second, the Chiba needle and loading cannula were simultaneously retracted from around the nodule to outside the pleura while holding the stylet in place. This was done to deploy a straight segment of the microcoil along the needle path from the nodule to the pleural surface. Third, a local CT scan confirmed that the needle tip was outside the pleura. The Chiba needle and loading cannula were kept stationary. Meanwhile, the stylet was used to push the remaining microcoil out of the loading cannula. Subsequently, the Chiba needle, empty loading cannula, and stylet were removed from the chest wall. CT scans of the whole lung were performed, and axial, sagittal, coronal, and three-dimensional volume-rendered images were used to identify the relationship between the microcoil, nodule, and chest wall (Figs. 1B, 2C-E) and any procedure-related complications. If the microcoil position was found to be unsatisfactory, another microcoil was inserted immediately. If more than one microcoil was used for nodule localization, only the microcoil with a satisfactory location was counted during the statistical analysis. After the CT-guided localization, the patients were transferred to the operating room for VATS. The patients who were not scheduled for VATS on the same day were sent back to the ward without restricting their daily activities and diet. The patients were instructed to inform the doctors if there were obvious symptoms. A chest radiograph was performed immediately after the patient developed symptoms. The time required for microcoil localization was defined as the time interval from obtaining the first CT image to obtaining the last CT image.
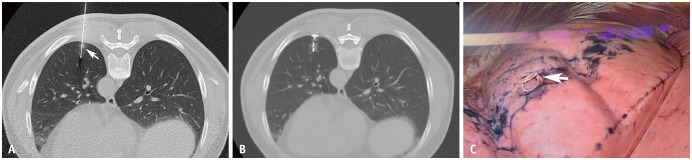
Fig. 1. A 63-year-old male patient with a 0.9-cm ground-glass nodule in the left lower lobe underwent CT-guided microcoil localization followed by VATS.
Immediate frozen-resection histopathology revealed inflammatory lesions. A. Needle passes through the lesion (arrow). B. The proximal end of the microcoil is located within the chest wall with minor bleeding in the lung parenchyma around the microcoil. C. The proximal end of the microcoil (arrow) is visibly exposed outside the pleura during VATS. VATS = video-assisted thoracoscopic surgery
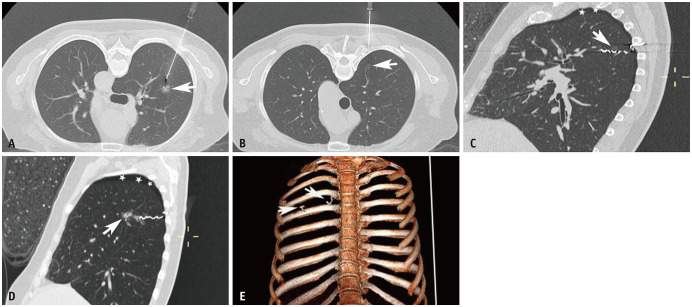
Fig. 2. A 66-year-old female patient with 2 GGNs in the right upper lobe underwent CT-guided microcoil localization before video-assisted thoracoscopic surgery.
The larger GGN was 1.2 cm in length and immediate frozen-resection histopathology revealed invasive adenocarcinoma. The smaller GGN was 0.4 cm and immediate frozen-resection histopathology showed atypical adenomatous hyperplasia. A. Needle is adjacent to the larger GGN (arrow). B. The needle path for the smaller GGN (arrow) is correct. C, D. The intrapulmonary part of the microcoil is adjacent to the lesions (arrows), and the proximal ends of the microcoils are located within the chest wall on post-marking CT. E. A three-dimensional volume-rendered image reveals the relationship between the microcoils (arrows) and the ribs. GGN = ground-glass nodule
SPNs Resection by VATS
VATS was performed under the guidance of implanted microcoils without the aid of intraoperative fluoroscopy. The patient was placed in the lateral position with the affected side facing up and ventilated with a double-lumen endotracheal tube while under general anesthesia. Routine disinfection was performed, and an incision of approximately 1.0 cm in length was made on the anterior axillary line of the 4th intercostal space to place the thoracoscope. Under the guidance of post-marking CT, an incision of approximately 1.5 cm in length was made at the appropriate site to reach the lesion. The proximal end of the microcoil exposed outside the visceral pleura could be observed with the naked eye, and the intrapulmonary part of the microcoil was touched using the index finger. Combined with the relationship between the nodule and microcoil on post-marking CT, the position of the lesion was determined. Wedge resection for a range of more than 2 cm from the edge of the lesion was performed using a cutting suture device under the guidance of a microcoil. The specimen was immediately opened to confirm that the lesion was in the resected specimen, followed by rapid frozen-section examination. If the intraoperative pathology confirmed invasive lung cancer without contraindications, the patient underwent further VATS lobectomy and lymph node dissection or sampling. If it was a benign lesion, metastasis, or noninvasive lung cancer, surgery was terminated after wedge resection of the lesion.
Grouping and Statistical Analysis
The patients were grouped based on the time interval between VATS and microcoil localization and the position of the microcoil proximal end. The statistical analyses were performed using SPSS software (SPSS Inc.), version 17.0. The correlation between the dislocation and the position of the proximal end of the microcoil and the waiting time of VATS after localization was analyzed using chi-squared analysis. P values of < 0.05 were considered statistically significant.
RESULTS
Post-Marking CT Findings
All 501 nodules were marked with microcoils. A total of 526 microcoils were used, of which 25 nodules used two microcoils. The reason for implanting the second microcoil was that the position of the first microcoil was unsatisfactory on post-marking CT. In 65 out of the 501 nodules, the distal end of the microcoil was inserted in the nodule (Fig. 1A), and the remaining microcoils were placed within 1.0 cm of the nodule (Figs. 3, 4). The proximal end of the implanted microcoils was located within the pleural cavity (Fig. 3B) and chest wall (Fig. 4) in 82 and 419 nodules, respectively. The time required for microcoil implantation was 12.8 ± 5.2 minutes. Post-marking CT showed complications in 179 cases (39.4%), including small pneumothorax in 86 cases (18.9%) (Fig. 2C, D), mild hemorrhage around the lesion or along the needle track in 124 cases (27.3%) (Figs. 1B, 3B), and hemoptysis in 8 cases (1.8%). No special treatment was required for any of the complications based on clinical medical records.
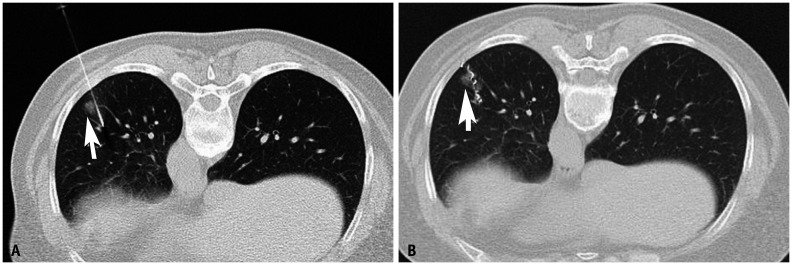
Fig. 3. A 56-year-old female patient with a 0.8-cm ground-glass nodule in the left lower lobe underwent CT-guided microcoil localization before video-assisted thoracoscopic surgery.
Immediate frozen-resection histopathology revealed invasive adenocarcinoma. A. Needle is adjacent to the lesion (arrow). B. The proximal end of the microcoil is within the pleural cavity, whereas the distal end is beyond the nodule (arrow) with minor bleeding in the lung parenchyma around the microcoil.
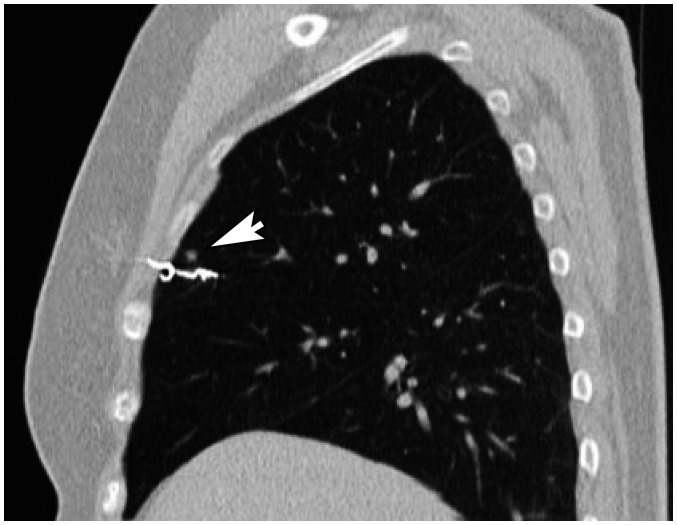
Fig. 4. A 56-year-old female patient with a 0.4-cm solid nodule in the right upper lobe underwent CT-guided microcoil localization before video-assisted thoracoscopic surgery.
Immediate frozen-resection histopathology revealed a reactive lymph node. Post-marking sagittal reconstruction CT revealed that the intrapulmonary part of the microcoil was adjacent to the nodule (arrow), and the proximal end of the microcoil was located within the chest wall.
Intraoperative Findings
VATS was performed on the same day (153 cases), next day (241 cases), third day (38 cases), and fourth day (22 cases) after microcoil implantation. In 416 cases with 463 nodules, the proximal end of the microcoil was exposed outside the pleura, and the intrapulmonary part of the microcoil was palpable. According to the relationship between the lesion and the microcoil on post-marking CT (Fig. 1B, C), the lesion was accurately resected. VATS showed that 38 microcoils were dislocated (Table 2), including 10 microcoils that completely retracted into the lungs and 28 microcoils that were completely withdrawn from the lungs. Of the 38 cases with microcoil dislocation, the location of the proximal end of the coil had been within the pleura cavity on post-marking CT in six cases and the chest wall in 32 cases. In the former group, VATS revealed that the microcoils were completely retracted into the lung in 83.3% (5/6) of the cases, whereas in the latter group, the microcoils were completely withdrawn from the lung in 84.4% (27/32) of the cases. Bleeding points of the pleural puncture were found in 21 cases. Based on the positional relationship between the pleural puncture points and the lesions on post-marking CT, the lesions were successfully resected. On palpation, nodules were found in 4 cases and the intrapulmonary part of the microcoils were found in 3 cases, and wedge resection was completed successfully. In 5 cases with microcoil dislocation, no nodule or intrapulmonary part of the microcoil was found on palpation and no pleural puncture point was found. Preoperative CT raised high suspicions of invasive lung cancer, and lobectomy was performed. The remaining 5 cases had conversions to thoracotomy. A total of 487 nodules were resected with microcoil localization guidance. Microcoil localization was not helpful for the removal of the remaining 14 nodules. A total of 449 cases with 496 (99.0%) nodules underwent successful resection using VATS. The intraoperative pathological studies confirmed a primary invasive lung cancer in a patient that had no contraindications; VATS lobectomy was continued because of the successful wedge resection in 265 cases. Although the nodules were successfully wedge removed, a portion of the microcoil remained in the lung parenchyma in 4 cases.
Table 2. The Correlation between the Microcoil Proximal Position on Post-Marking CT and Microcoil Dislocation Revealed by VATS.
| Post-Marking CT | No Dislocation (VATS) | Dislocation (VATS) | Total | |
|---|---|---|---|---|
| Completely Retracted into the Lung | Completely Withdrawn from the Lung | |||
| Proximal end in the pleural cavity | 76 | 5 | 1 | 82 |
| Proximal end in the chest wall | 387 | 5 | 27 | 419 |
| Total | 463 | 10 | 28 | 501 |
VATS = video-assisted thoracoscopic surgery
Correlation between the Dislocation and the Position of the Proximal End of the Microcoil and the Waiting Time between Localization and VATS
For the patients who underwent VATS on the same day, next day, and more than 2 days after microcoil localization, the rates of microcoil dislocation were 7.8% (12/153), 8.7% (21/241), and 8.3% (5/60), respectively (χ2 = 0.093, p = 0.955). The dislocation rates of the microcoils with their proximal ends in the pleural cavity and chest wall were 7.3% (6/82) and 7.6% (32/419), respectively (χ2 = 0.010, p = 0.92).
Pathological Diagnosis of Nodules
All SPNs were pathologically diagnosed after VATS, of which 83.2% (417/501) were malignant. The pathological diagnoses of the nodules are shown in Table 3.
Table 3. Postoperative Pathological Results of 501 Solitary Pulmonary Nodules.
| Number | Percentage | ||
|---|---|---|---|
| Malignant nodules | 417 | 83.23 | |
| Infiltrating adenocarcinoma | 185 | 36.93 | |
| Minimally invasive adenocarcinoma | 154 | 30.74 | |
| Carcinoma in situ | 39 | 7.78 | |
| Atypical adenomatous hyperplasia | 22 | 4.39 | |
| Mucinous adenocarcinoma | 7 | 1.40 | |
| Squamous cell carcinoma | 5 | 1.00 | |
| Metastasis | 3 | 0.60 | |
| Carcinoid | 1 | 0.20 | |
| Scar cancer | 1 | 0.20 | |
| Benign nodules | 84 | 16.77 | |
| Inflammation | 26 | 5.20 | |
| Fibrosis | 17 | 3.39 | |
| Reactive lymph node | 14 | 2.79 | |
| Granuloma | 11 | 2.20 | |
| Adenoma | 7 | 1.40 | |
| Cryptococcus infection | 4 | 0.80 | |
| Hamartoma | 3 | 0.60 | |
| Carbon power deposit | 2 | 0.40 | |
DISCUSSION
Small SPNs, especially GGNs, may be invisible and impalpable during VATS. Accurate and effective preoperative or intraoperative localization techniques are helpful for successful wedge resection using VATS. Different materials have been used for the preoperative localization of SPNs [11,12,13,14,15,16,17,18,19]. Microcoils are relatively ideal for preoperative localization of SPNs. First, microcoils have been used for vascular embolization, and they have good tissue compatibility. Second, the microcoil is soft and does not cause significant damage to the lung parenchyma and pleura after implantation into the lungs or even after falling off. Microcoil localization has a relatively lower complication rate than hook-wire localization [20,21]. Third, the outcome of the microcoil localization was satisfactory. A microcoil has a certain hardness and is radiopaque. It can be located by visual inspection, palpation, and fluoroscopy during surgery. Fourth, microcoils are easy to obtain, and they do not pollute the environment.
Mayo et al. [4] reported their experience using a microcoil to mark the nodule and the visceral pleural surface. They suggested that the distal end of the microcoil passed through the lesion and the proximal end was within the pleural cavity, which is a challenge for CT puncture technology. First, it is not easy to stab nodules smaller than 1 cm, especially when they are located within the lower lobe and affected by breathing movement. Second, it is also challenging to place the proximal end of the microcoil within the pleural cavity rather than the chest wall in the absence of a small pneumothorax. In recent years, there have been several reports on the modification of CT-guided microcoil implantation technology described by Mayo et al. [4]. Su et al. [6] recommended placing the distal microcoil close to the lesion rather than through it. Kha et al. [7] deployed the entire microcoil adjacent to or within the nodule without pleural marking. Their results showed that the localization method of marking nodules could decrease the CT procedure time and radiation dose while maintaining equivalent complete resection rates with the aid of intraoperative fluoroscopy compared with the localization method of marking nodules and visceral pleura simultaneously.
In this study, we used the microcoil implantation method described by Mayo et al. [4] to mark the nodules and the pleura at the same time, but we adjusted the position of the implanted microcoil. The distal end of the microcoil was placed less than 1 cm away from the nodule (within or beyond the nodule), and the proximal end was placed outside the visceral pleura (in the pleural cavity or chest wall). Compared with the microcoil localization method described by Mayo et al. [4], our management of the microcoil position significantly reduced the technical difficulty of CT-guided microcoil localization. Meanwhile, the proximal end of the microcoil was exposed outside the pleura and could be observed by the naked eye; the intrapulmonary part of the microcoil could be touched by hand. The surgeon could accurately judge the location of the microcoil without the aid of intraoperative fluoroscopy. Based on the relationship between the lesion and the microcoil on post-marking CT, the SPNs were accurately resected under the guidance of the implanted microcoils. The time required for microcoil implantation (12.8 ± 5.2 minutes) was significantly shorter than that reported by Mayo et al. [4] (33.0 ± 12.6 minutes) and Kha et al. [7] (59.3 ± 22.2 minutes). The pneumothorax rate (18.9%) was also lower than that reported by Mayo et al. [4] and Kha et al. [7] (75% and 69%, respectively). The relatively low incidence of complications and the relatively short marking time may be related to the reduced technical difficulty and the fixed operator. On the other hand, dislocation occurred in 38 microcoils (7.6%) in this study, which was higher than previously reported. Without intraoperative fluoroscopy, the distal end of the microcoil should be exposed outside the pleura for guidance, which will allow the subliminal shortening of the intrapulmonary part of the microcoil. The unreasonable proportion of the intrapulmonary and extrapulmonary parts can lead to microcoil dislocation.
Microcoil localization and VATS were not scheduled on the same day in 56% of the patients in this study. For 38 and 22 cases, VATS was performed on the third and fourth days after microcoil implantation. To our knowledge, a time interval between microcoil implantation and VATS for more than 24 hours has not been reported. Similar to most researchers, we initially performed preoperative CT-guided microcoil localization of the SPNs on the day of VATS. If VATS was the first operation of the day, we had to complete microcoil implantation before normal working hours. This changed our work schedules from time to time. After initial exploration, we found that most of the patients had no obvious discomfort after microcoil localization, and we tried to gradually increase the time interval between microcoil localization and VATS to reduce the effect of microcoil localization on the work schedule. We routinely performed CT-guided microcoil localization on Monday, Wednesday, and Friday afternoons. For patients who underwent wedge resection of SPNs using VATS on Monday morning, we usually performed microcoil localization on Friday afternoon (VATS was performed on the fourth day after microcoil localization). The results showed that the scheduling of microcoil localization and VATS on different days was safe, and it did not increase the rate of microcoil dislocation.
In 4 cases, a portion of the microcoil inadvertently remained within the lung parenchyma in this study. In all 4 cases, the depth of the distal end of the microcoil exceeded the lesion. The microcoil was cut off during wedge resection. Although the partial retention of the microcoil within the lung did not cause significant damage and require special treatment, it should be avoided. CT interventional doctors need to communicate closely with thoracic surgeons. In the above four cases, if the surgeon had carefully analyzed the relationship between the microcoil and the lesion or CT intervention doctors had reminded the surgeon that the depth of the distal end of the microcoil exceeded the lesion, the surgeon could have pulled out the microcoil a little before wedge resection or adjusted the wedge resection range appropriately to avoid its occurrence.
This study has some limitations. First, this was a single-center retrospective study. Second, the radiation dose received by the patient during microcoil localization was not recorded.
In conclusion, CT-guided microcoil localization is safe and reliable, and it can be used to effectively mark peripheral SPNs. Marking the nodules and pleura simultaneously using microcoils can accurately guide VATS to remove SPNs without the aid of intraoperative fluoroscopy. Moreover, the extension of the duration (> 24 hours) between the CT-guided microcoil localization and VATS is safe, and it does not increase the rate of microcoil dislocation.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Zhen-guo Huang, Cun-li Wang, Hong-liang Sun.
- Data curation: all authors.
- Formal analysis: Zhen-guo Huang, Chuan-dong Li, Bao-xiang Gao, Min-xing Yan.
- Investigation: Zhen-guo Huang, Hong-liang Sun, Chuan-dong Li, Bao-xiang Gao, He Chen, Min-xing Yan.
- Methodology: Zhen-guo Huang, Cun-li Wang, Hong-liang Sun.
- Project administration: Zhen-guo Huang, Hong-liang Sun.
- Supervision: Zhen-guo Huang.
- Writing—original draft: Zhen-guo Huang.
- Writing—review & editing: all authors.
References
- 1.Burdine J, Joyce LD, Plunkett MB, Inampudi S, Kaye MG, Dunn DH. Feasibility and value of video-assisted thoracoscopic surgery wedge excision of small pulmonary nodules in patients with malignancy. Chest. 2002;122:1467–1470. doi: 10.1378/chest.122.4.1467. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki K, Nagai K, Yoshida J, Ohmatsu H, Takahashi K, Nishimura M, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest. 1999;115:563–568. doi: 10.1378/chest.115.2.563. [DOI] [PubMed] [Google Scholar]
- 3.Wang ZX, Li L, Zhang Z, Wang GH, Kong DM, Wang XD, et al. High-resolution computed tomography features and CT-guided microcoil localization of subcentimeter pulmonary ground-glass opacities: radiological processing prior to video-assisted thoracoscopic surgery. J Thorac Dis. 2018;10:2676–2684. doi: 10.21037/jtd.2018.04.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayo JR, Clifton JC, Powell TI, English JC, Evans KG, Yee J, et al. Lung nodules: CT-guided placement of microcoils to direct video-assisted thoracoscopic surgical resection. Radiology. 2009;250:576–585. doi: 10.1148/radiol.2502080442. [DOI] [PubMed] [Google Scholar]
- 5.Liu L, Zhang LJ, Chen B, Cao JM, Lu GM, Yuan L, et al. Novel CT-guided coil localization of peripheral pulmonary nodules prior to video-assisted thoracoscopic surgery: a pilot study. Acta Radiol. 2014;55:699–706. doi: 10.1177/0284185113506136. [DOI] [PubMed] [Google Scholar]
- 6.Su TH, Fan YF, Jin L, He W, Hu LB. CT-guided localization of small pulmonary nodules using adjacent microcoil implantation prior to video-assisted thoracoscopic surgical resection. Eur Radiol. 2015;25:2627–2633. doi: 10.1007/s00330-015-3676-5. [DOI] [PubMed] [Google Scholar]
- 7.Kha LC, Hanneman K, Donahoe L, Chung T, Pierre AF, Yasufuku K, et al. Safety and efficacy of modified preoperative lung nodule microcoil localization without pleural marking: a pilot study. J Thorac Imaging. 2016;31:15–22. doi: 10.1097/RTI.0000000000000188. [DOI] [PubMed] [Google Scholar]
- 8.Donahoe LL, Nguyen ET, Chung TB, Kha LC, Cypel M, Darling GE, et al. CT-guided microcoil VATS resection of lung nodules: a single-centre experience and review of the literature. J Thorac Dis. 2016;8:1986–1994. doi: 10.21037/jtd.2016.06.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lempel JK, Raymond DP, Ahmad U, O’Malley S, Bolen MA, Graham R, et al. Video-assisted thoracic surgery resection without intraoperative fluoroscopy after CT-guided microcoil localization of peripheral pulmonary nodules. J Vasc Interv Radiol. 2018;29:1423–1428. doi: 10.1016/j.jvir.2018.01.787. [DOI] [PubMed] [Google Scholar]
- 10.Powell TI, Jangra D, Clifton JC, Lara-Guerra H, Church N, English J, et al. Peripheral lung nodules: fluoroscopically guided video-assisted thoracoscopic resection after computed tomography-guided localization using platinum microcoils. Ann Surg. 2004;240:481–488. doi: 10.1097/01.sla.0000137132.01881.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tseng YH, Lee YF, Hsieh MS, Chien N, Ko WC, Chen JY, et al. Preoperative computed tomography-guided dye injection to localize multiple lung nodules for video-assisted thoracoscopic surgery. J Thorac Dis. 2016;8:S666–S671. doi: 10.21037/jtd.2016.09.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miura H, Yamagami T, Tanaka O, Yoshimatsu R, Ichijo Y, Kato D, et al. CT findings after lipiodol marking performed before video-assisted thoracoscopic surgery for small pulmonary nodules. Acta Radiol. 2016;57:303–310. doi: 10.1177/0284185115576047. [DOI] [PubMed] [Google Scholar]
- 13.Lee NK, Park CM, Kang CH, Jeon YK, Choo JY, Lee HJ, et al. CT-guided percutaneous transthoracic localization of pulmonary nodules prior to video-assisted thoracoscopic surgery using barium suspension. Korean J Radiol. 2012;13:694–701. doi: 10.3348/kjr.2012.13.6.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nardini M, Bilancia R, Paul I, Jayakumar S, Papoulidis P, ElSaegh M, et al. 99mTechnetium and methylene blue guided pulmonary nodules resections: preliminary British experience. J Thorac Dis. 2018;10:1015–1021. doi: 10.21037/jtd.2018.01.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodrigues JCL, Pierre AF, Hanneman K, Cabanero M, Kavanagh J, Waddell TK, et al. CT-guided microcoil pulmonary nodule localization prior to video-assisted thoracoscopic surgery: diagnostic utility and recurrence-free survival. Radiology. 2019;291:214–222. doi: 10.1148/radiol.2019181674. [DOI] [PubMed] [Google Scholar]
- 16.Pang X, Xue L, Chen J, Ding J. A novel hybrid technique for localization of subcentimeter lung nodules. J Thorac Dis. 2017;9:1107–1112. doi: 10.21037/jtd.2017.03.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sui X, Zhao H, Yang F, Li JL, Wang J. Computed tomography guided microcoil localization for pulmonary small nodules and ground-glass opacity prior to thoracoscopic resection. J Thorac Dis. 2015;7:1580–1587. doi: 10.3978/j.issn.2072-1439.2015.09.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li C, Liu B, Jia H, Dong Z, Meng H. Computed tomography-guided hook wire localization facilitates video-assisted thoracoscopic surgery of pulmonary ground-glass nodules. Thorac Cancer. 2018;9:1145–1150. doi: 10.1111/1759-7714.12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tao G, Jingying Y, Tan G, Xiaotao D, Min C. A novel CT-guided technique using medical adhesive for localization of small pulmonary ground-glass nodules and mixed ground-glass nodules (≤20 mm) before video-assisted thoracoscopic surgery. Diagn Interv Radiol. 2018;24:209–212. doi: 10.5152/dir.2018.17315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang S, Kim TG, Song YG. Comparison of hook wire versus coil localization for video-assisted thoracoscopic surgery. Thorac Cancer. 2018;9:384–389. doi: 10.1111/1759-7714.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu L, Gao J, Chen C, Zhi X, Liu H, Hong N. Comparison between the application of microcoil and hookwire for localizing pulmonary nodules. Eur Radiol. 2019;29:4036–4043. doi: 10.1007/s00330-018-5939-4. [DOI] [PubMed] [Google Scholar]