Abstract
Background
Vitamin-D is an immune-modulator which might be linked to disease severity by SARS-CoV-2.
Methods
Meta-analysis of RCTs and quasi-experimental studies, evaluating the role of vitamin-D supplementation in COVID patients was done.
Results
Total 5 studies (3 RCTs and 2 Quasi-experimental) including n = 467 patients were included. Vitamin D didn't reduce mortality (RR 0.55, 95%CI 0.22 to 1.39, p = 0.21), ICU admission rates (RR 0.20, 95% CI 0.01–4.26, p = 0.3) and need for invasive ventilation (RR 0.24, 95% CI 0.01–7.89, p = 0.42).
Conclusion
No significant difference with vitamin-D supplementation on major health related outcomes in COVID-19. Well-designed RCTs are required addressing this topic.
Keywords: Vitamin-D, COVID-19, COVID pneumonia, SARS-CoV-2
1. Introduction
Currently the entire globe is suffering from third major outbreak caused by the family of beta-Coronavirus. Globally, till 18th May 2021 there have been 163,312,429 confirmed cases and 3,386,825 case fatality incidences, among which, 8.23% (2,78,719) case fatality incidences have been reported by India [1]. Despite being a tropical country and having plenty of sunshine, most of the Indian population are deficient in vitamin D [2]. The Severe Acute Respiratory syndrome virus (SARS-CoV-2) induces an inflammatory state, evidenced by raised acute markers like interleukin-6 (IL-6), c-reactive protein (CRP), ferritin, d-dimer etc. and may lead to lung damage especially in the second week of illness owing to the ‘cytokine storm’ [3]. This upsurge of inflammation has been linked to the occurrence of coronavirus induced acute respiratory distress syndrome (COVID ARDS) [4,5]. Vitamin D acts as an immune-modulator, which via various mechanisms e.g. ACE-2 receptor modulation, maintenance of pulmonary barrier function, enhancing neutrophil activity, reduces the damage caused by pro-inflammatory cytokines [6]. This vitamin boosts the innate immune response during the initial viremic phase and shifts the adaptive immune response to a more T helper cell-2 (Th2) type [7]. Thus, it's regular supplementation was found to have beneficial role in preventing acute respiratory infections (ARI) as well as reduce its complications [8,9]. Different observational studies have shown a decreased COVID severity in patients without vitamin D deficiency, however contradictory evidences also exist [10,11]. Although few small scale studies showed vitamin D supplementation to reduce disease severity and/or attain earlier recovery [[12], [13], [14], [15]], a large trial showed no added benefit with vitamin D supplementation [16]. Therefore, we aim to investigate the role of supplementation of Vitamin D among COVID-19 patients on their disease outcomes.
2. Aims and objectives
The study is aimed to assess the impact of Vitamin-D supplementation on clinical outcomes (mortality, ICU admission, mechanical ventilation, and change in inflammatory markers) in patients diagnosed with COVID-19.
3. Methodology
Preferred reporting items for systematic review and meta-analysis (PRISMA) statement for conducting meta-analysis was followed [17].
3.1. Search strategy
A comprehensive literature search was carried out using the pre-defined keywords (mentioned below) of articles published on PubMed, Embase and Scopus from inception till May 18, 2021.
Manual search was done to retrieve other articles using the keywords: (((covid 19) AND (vitamin D)) AND (supplementation)); ((covid 19 [Title/Abstract]) AND (vitamin D [Title/Abstract])) AND (supplementation [Title/Abstract]); (((vitamin D) AND (COVID)) AND (Coronavirus)) AND (covid-19).
3.2. Inclusion criteria
We wanted to know the true effect of vitamin D supplementation in COVID infected individuals, hence, we included randomized trials and quasi-experimental studies published in English language, where vitamin D supplementation was done prospectively i.e. after the diagnosis of COVID. Observational and retrospective studies were excluded due to the risk of their biasness. Patients diagnosed with COVID-19 irrespective of the disease severity, who received vitamin D supplementation in any form and dose in addition to prevailing standard therapy, comprised the intervention arm. Patients who did not receive any form of vitamin D constituted the control arm. We assessed major health related outcomes which are, mortality till the longest follow up mentioned, ICU admission rates, need for mechanical ventilation and change in acute inflammatory markers.
3.3. Exclusion criteria
Articles/pre-prints which are not published in peer reviewed journals, study protocols and trials with incomplete data, retrospective & observational studies were excluded. Studies where vitamin D supplementation was done retrospectively were also excluded to avoid recall bias.
3.4. Study selection
The articles retrieved initially from the databases were screened for the titles and abstracts to identity all eligible articles bearing the above MeSH (medical sub-heading) terminologies. Two independent researchers AR and DR screened all the articles for the titles and abstract eliminating all irrelevant or duplicate articles, followed by full text assessment. Any disagreement on the inclusion of article was sought by another co-author (SM/VS).
3.5. Data extraction
Relevant data of each articles pertaining to the population studied, intervention implemented, and outcomes (acute serum marker change, mechanical ventilation days, ICU stay days, mortality) were extracted independently by AR and DR. A search was made for supplementary data against each article. A qualitative approach was used for evaluation and synthesis of key findings. A consensus was reached when conflict occurred.
3.6. Quality assessment
Two authors (AR and DR) independently assessed the methodological quality of the included studies as per Cochrane Systematic Review Guidelines, any disagreements were resolved through a discussion with third co-author (SM/VS.) Individual quality analysis were expressed via GRADE-PRO approach and risk of bias graph was made using Review Manager 5.4.
3.7. Statistical analysis
Effect measure used in this review was risk ratio (RR). Presence of heterogeneity among the included studies was assessed by I2. We employed random-effect model and a p-value less than 0.05 was considered to be statistically significant. Methodological quality of studies was assessed by the Guidelines recommended by the Cochrane guidelines using the Review Manager version. 5.4.
4. Results
4.1. Study characteristics
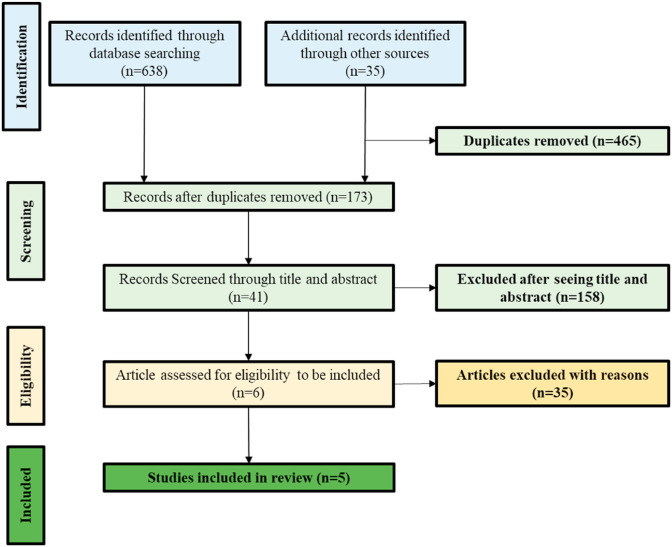
For the present review, our initial search identified a total of 673 articles using the database searching and thorough manual search strategies. 465 duplicate references to the same papers were removed manually. After screening the titles and abstracts of remaining articles (n = 173) with irrelevant topics or not fulfilling the selection criteria, irrelevant articles were excluded, resulting in a potential total of 41 articles. After evaluation of the full text of all selected articles among 41, 5 articles were eventually included the present review, meeting the inclusion criteria (Fig. 1 ; Table 1 ).
Fig. 1.
Study flow diagram.
Table 1.
Summary of the included articles in this review.
| S. No. | Author and Year | Study design | Country (Study Setting) | Age (Mean ± SD) | Sample Size (I/C) | Participants | Intervention | Control/Placebo | Outcome | Remark | Outcomes studied |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rastogi et al. 2020 [12] | Randomised, placebo controlled, study | India (Tertiary care hospital in north India) | – | 16/24 | Asymptomatic or mildly symptomatic SARS-CoV-2 RNA positive vitamin D deficient (25(OH) D < 20 ng/ml) individuals. |
Daily 60,000 IU of cholecalciferol (5 ml oral solution in nano droplet form) for 7 days. Then weekly supplementation of 60,000 IU (if 25(OH)D > 50 ng/ml) else continued 60,000 IU for another 7 days up until day-14 in participants with 25(OH)D < 50 ng/ml | Placebo (5 ml distilled water for 7 Days) | Proportion of patients with SARS CoV-2 RNA negative before day-21 and change in inflammatory markers (D-dimer, fibrinogen, CRP, Prolactin) | Short term high-dose cholecalciferol supplementation |
|
|
| Murari et al. 2020 [16] | Multicentre, double-blind, parallel-group, randomized, placebo-controlled trial | Brazil (Clinical Hospital of the School of Medicine of the University of Sao Paulo (a quaternary referral teaching hospital) and from the Ibirapuera field hospital) | 56.2 ± 14.4 | 119/118 | Hospitalized patients with COVID-19 who were moderately to severely ill at the time of enrollment. | A single, oral dose of 200,000 IU of vitamin D3 dissolved in a 10-ml peanut oil solution | Placebo (10 ml of a peanut oil solution) | Length of stay, in-hospital mortality, admission to ICU, mechanical ventilation requirement | Single high dose of vitamin D3 |
|
|
| Castillo et al. 2020 [13] | Parallel Pilot randomized, open label, double-masked clinical study | Spain (Reina Sofia University Hospital, Cordoba, Spain EU) | 53 ± 10 | 50/26 | Hospitalized patients with COVID-19 clinical picture of acute respiratory infection, confirmed by a radiographic pattern of viral pneumonia and by a positive SARS-CoV-2 PCR with CURB65 severity scale. | Oral Calcifediol (0.532 mg soft capsules on day of admission; and 0.266 mg on day 3 and 7, and then weekly until discharge or ICU admission | Usual Care | ICU admission; Death | – |
|
|
| Annweiler C. et al. 2020 [14] | Quasi-experimental study | France (Nursing home in Rhone, South East of France) | 87.7 ± 9.3 | 57/9 | Elderly nursing-home residents with COVID-19 and/or with physical disabilities, major neurocognitive and psychiatric disorders. | An oral bolus of 80,000 IU vitamin D3 either in the week following the suspicion or diagnosis of COVID-19, or during the previous month. | Usual Care | Mortality and Ordinal Scale for Clinical Improvement (OSCI) score in acute phase. | Single oral dose of 80,000 IU vitamin D3, either in the week following the suspicion or diagnosis of COVID-19, or during the previous month |
|
|
| Annweiler G. et al. 2020 [15] | Quasi-Experimental Study | France (Angers University Hospital, France) | 88 ± 5 | 16/32 | Patients admitted for COVID-19 in a geriatric unit | Oral Vitamin D3 supplement of 80,000 IU within a few hours of the diagnosis of COVID-19 | Usual Care | 14-Day COVID-19 Mortality; Ordinal Scale for Clinical Improvement (OSCI) Score for COVID-19 in Acute Phase | 80,000 IU vitamin D3 within a few hours of the diagnosis of COVID-19. |
|
4.2. Description of the included studies
In the present review among all included studies, three were RCTs [12,13,16] and two were Quasi experimental [14,15], involving details from 467 subjects (sample size range 40–237). Among all, one study was from India [12] one from Brazil [16], one from Spain [13] and two from France [14,15].
In one of the study, the population were divided into three groups: group 1 received vitamin D supplementation in the preceding year, group 2 received vitamin D after diagnosis and group 3 no supplementation [15]. Owing to our study inclusion criteria, we extracted data of group 2 and 3 of the above study. One RCT [12] included mild/asymptomatic middle aged vitamin D deficient individuals, whereas other two RCTs [13,16] included moderately to severely ill patients aged between 50 and 60 years. Both the quasi-experimental study groups included elderly nursing home individuals aged >80 years [14,15].
Considerable heterogeneity was noted in the dosages applied in the intervention arm, whereas control arm received standard therapy i.e., steroids, antibiotics etc. as per available guidelines. The follow up period ranged from a minimum of 7 days [12] to a maximum of around 37 days [14].
One RCT [12] measured SARS-CoV-2 RNA negative status (tested twice with a gap of 24 h within 3 weeks) as primary outcome and change of inflammatory markers as secondary outcomes, whereas rest two RCTs [13,16] analysed ICU admission rates, mortality as outcome measures. The quasi-experimental studies measured an objective Ordinal Scale of Clinical Improvement (OSCI) score to signify disease severity along with mortality [14,15,18]. Detailed characteristics are given in Table 1.
4.3. Methodological quality of study
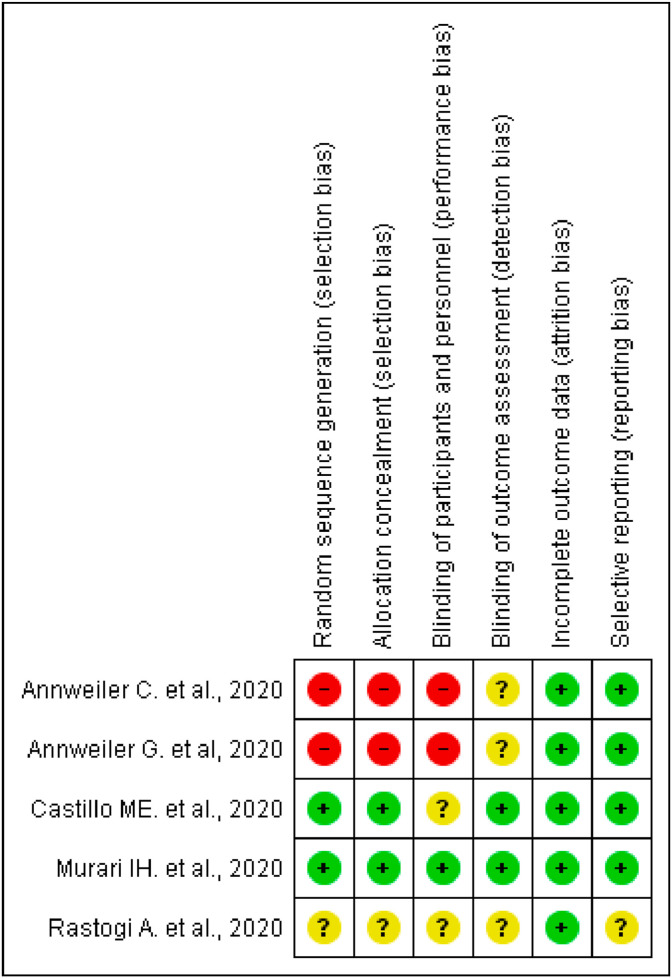
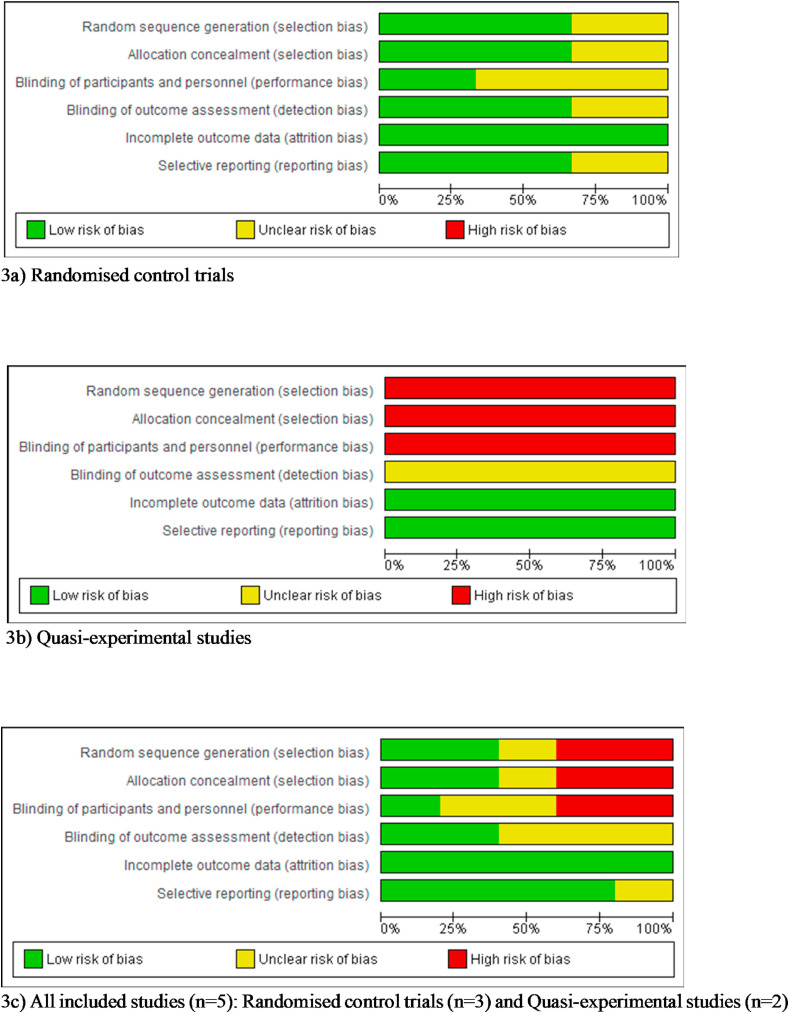
Risk of bias graph, review authors judgements about each risk of bias item presented as percentages and risk of bias summary based on Cochrane Systematic Review Guidelines for each included study (green for low risk of bias, yellow for unclear risk of bias and red for high risk of bias) for randomised control trials and Quasi-experimental study are presented in Fig. 2, Fig. 3 [19].
Fig. 2.
Risk of bias summary based on Cochrane Systematic Review Guidelines for each included study (green for low risk of bias, blank for unclear risk of bias and red for high risk of bias) included in this review.
Fig. 3.
(3a-3c): Risk of bias graph review authors judgements about each risk of bias item presented as percentages across various study designs.
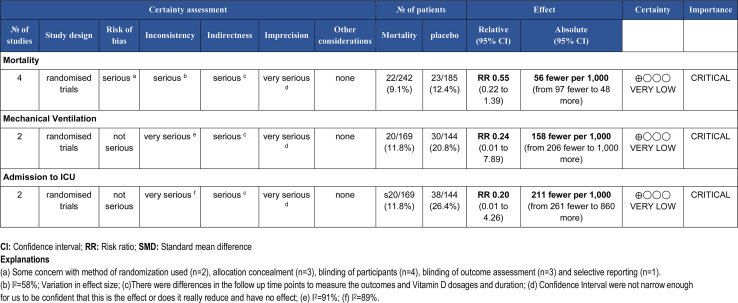
The overall rating for the quality of evidence for the role of vitamin D supplementation in patients with COVID-19 is shown in the GRADE summary of finding Table 2 [20]. GRADE summary reported the certainty of evidence as very low for the outcome's mortality, ICU admission and mechanical ventilation which means that any estimate of effect is very uncertain and we have little confidence in the effect.
Table 2.
The overall rating for the quality of evidence profile for COVID-19 related health outcomes based on the grading of Recommendations Assessment, Development, and Evaluation (GRADE) working group methodology.
4.4. Efficacy outcomes
4.4.1. Heterogeneity
Due to significant heterogeneity random-effects models were used for outcome mortality (I2 = 58%) [[13], [14], [15], [16]] mechanical ventilation (I2 = 91%) [13,16] and ICU admission (I2 = 89%) [13,16] (Fig. 4 ).
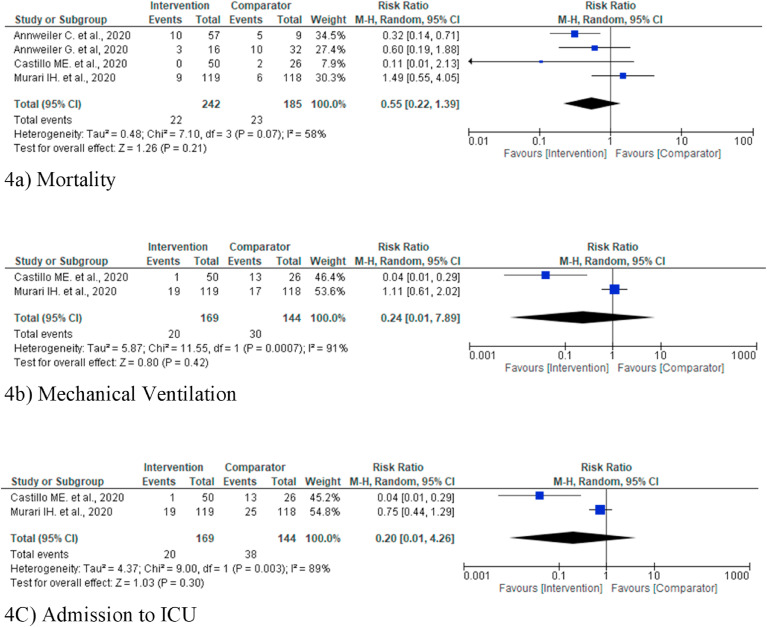
Fig. 4.
(a–c): Forest plot random effect model for vitamin D supplementation for various outcomes.
4.5. Mortality outcome
Mortality was reported in four studies [[13], [14], [15], [16]] involving 427 subjects (242 intervention and 185 control/placebo) among which two studies reported mortality benefit [13,14] and other didn't [15,16]. In the present review pooled analysis of the mortality rate in the intervention group (3.43% less mortality) as compared to placebo/controls was observed to be lower, although it was not statistically significant (Risk Ratio 0.55, 95% CI 0.22 to 1.39; I2 = 0.91; P = 0.21) (Fig. 4a).
4.6. Mechanical ventilation outcome (MVO)
Only two studies [13,16] reported the MVO outcome involving 313 subjects (169 intervention and 144 control/placebo). In the present meta-analysis pooled data did not show a statistically significant improvement in the intervention group, although reduction in 9% requirement for MVO by the intervention group was observed (Risk Ratio 0.24, 95% CI 0.01 to 7.89; P = 0.42) (Fig. 4b).
4.7. Admission to ICU
This outcome was reported by two studies involving 313 subjects (169 intervention and 144 control/placebo [13,16]. In the present review pooled data failed to show a statistically significant reduction in ICU admission in the intervention group. [Reduction in 14.55% in the intervention group (Risk Ratio 0.20, 95% CI 0.01 to 4.26; P = 0.30)] (Fig. 4c).
4.8. Acute markers
Only one study [12] evaluated all the inflammatory markers like fibrinogen, ferritin, d-dimer, CRP and it showed significant reduction of fibrinogen values [change of fibrinogen Δ fibrinogen −0.64 (−1.41 to 0.11) in intervention group vs. 0.06 (0.01–0.51) in control group]. Rest all values were insignificant. Due to paucity of data, we were unable to include quantitative data for two markers i.e. d-dimer and CRP hence only qualitative data were taken out of two studies [total 277 subjects, n = 135 for intervention and n = 142 for control] [12,16].
5. Discussion
The present systematic review and meta-analysis showed that administration of vitamin D after diagnosis of SARS-CoV-2 infection did not reduce major health outcomes like mortality, ICU admission and need for invasive ventilation. The included studies suffered from significant baseline heterogeneity with respect to drug dosing and population characteristics.
The SARS-CoV-2 may induce a pro-inflammatory state and in a subgroup of patients may lead to ‘cytokine storm’, which has been linked to worse outcomes [3,21]. Dysregulated delayed adaptive immune response leads to raised pro-inflammatory cytokines {interleukin-6, TNF-alpha, interferon etc.) which subsequently may lead to potentially dreaded complications like ARDS, thrombosis of major vascular bed due to pro-coagulant state etc [4,5]. Infection associated molecular response elements e.g. toll-like receptors (TLR) induce 1-alpha hydroxylase to increase the active form of vitamin D i.e. 1,25-hydroxy cholecalciferol (DHCC), which in turn induces defensin group of proteins like beta-defensin 2, cathelicidin via it's receptor (vitamin D receptor-VDR) [22]. These proteins help in autophagy, and apoptosis of infected cell and strengthen innate immune system. Till date multitude of infections both by bacteria and viruses have been linked to vitamin D deficiency (VDD) [23]. Individual patient data (IPD) analysis of nearly 10,000 patients across 25 RCTs by Martineau et al. also revealed almost 11% reduction of incidence of ARI with vitamin D supplementation, and that regular supplementation among the sub-group with VDD may have additional benefit [8]. Researchers have gone on to correlate the historical influenza pandemic of 1918–1919 to VDD [24]. By suppressing delayed dendritic cell activation and driving the ‘T’ cell response towards an anti-inflammatory (Th2/T-reg) domain rather than pro-inflammatory (Th1/Th17) one, vitamin may be instrumental for curtailing maladaptive cytokine storm seen with COVID-19, as observed in vitro studies [25,26].
A recent systematic review and meta-analysis consisting of 43 observational studies and nearly 6 lakh patients concluded that, vitamin D deficient individuals (<20 ng/ml) had 50% higher risk of infection with COVID as compared to replete individuals [OR 1.5, 95% CI 1.08–2.08,p = 0.02] [27]. Observational study including 10 articles and nearly 3 lakh patients done by Liu et al. (Dec 2020) showed that low vitamin D levels were associated more frequently with COVID positivity [10]. In a similar meta-analysis [28] which included two RCTS [13,16] similar to our study along with a retrospective case control study [29], it was concluded that vitamin D supplementation had reduction of ICU admission rates but insignificant effect on mortality [28]. The different result from this study as compared to ours could be attributable to the inclusion of retrospective study [29]. Another study showed reduced ICU admission rates and mortality with vitamin D supplementation [30], however, their study could not include data from the RCT which had the largest weightage and influence in our study [16]. The findings of our study corroborated with one large study which included a total of 34 peer reviewed observational studies and RCTs [31]. Though VDD was positively correlated with COVID disease, there was insignificant reduction of mortality/ICU admission/hospital admission etc [31]. Systematic review and meta-analysis of effect of vitamin D on elderly frail people revealed that vitamin D had protective role against the viral infection and also reduced primary health related outcomes i.e. mortality, ICU admission etc. in them [32].
Despite its sentinel role in controlling respiratory infections, there is speculation that this late suppression of adaptive response by attenuating dendritic cell mediated pro-inflammatory drive, may be counterproductive and rather increase chance of ARIs [26]. Vitamin D supplementation failed to show all-cause mortality except cancer related deaths in a meta-analysis by Zhang et al. (25). One recently conducted RCT, which is included in the present meta-analysis failed to show beneficial effects of vitamin D in moderate to severe COVID patients [33]. Another study evaluated the effect of recent (<3 month prior) supplementation of vitamin D in hospitalized patients by dividing them into three sub-groups [patients with Parkinson's disease (PD), their caregivers and hospitalized patients (n = 127)], and showed that higher vitamin D levels not associated with either reduced hospital admission or mortality [unadjusted OR 1.30,95% CI 0.51–3.31, P = 0.56 for hospitalization and 1.78, 5%CI 0.64–4.91, P = 0.26 for mortality] [34].
Out of the of 5 studies included, two studies [12,16] showed significant increase in DHCC levels with vitamin D supplementation. While four out of five studies were carried out on symptomatic individuals [[13], [14], [15], [16]], one study included mildly symptomatic patients [12]. All the studies included the active form of vitamin D i.e. DHCC except one [13], who used calcefediol. Although four studies [[13], [14], [15], [16]] reported major outcomes like mortality, ICU admission etc., one study [12] reported COVID negativity as their primary outcome, clinical significance of which are questionable [35,36]. This later study also had discrepancy in the published data and data provided via supplementary material [12]. Therefore, a significant heterogeneity exists in their study design as well as methodology, which rendered them ‘critical/very low’ status as per the GRADE-PRO approach [20].
6. Conclusion
Vitamin D supplementation did not reduce major heath related outcomes like mortality, ICU admission rates and mechanical ventilation. The studies differed significantly with respect to their design, drug dosage, and population characteristics. The intrinsic heterogeneity of the included studies and small sample size make it difficult to interpret and extrapolate this data on to a large population. Thus, well-designed RCTs with uniform study population characteristics, methodology, and uniform drug dosing are warranted to determine the efficacy of treatment with vitamin D on COVID-19 patients.
7. Limitations
Our study has certain limitations. Firstly, outcomes like mortality were assessed without taking into account time frame. A longer follow up, taking into account delayed mortality might have bearing on the result. Secondly, significant heterogeneity across the studies indicate divergent population at baseline. There was heterogeneity in the intervention with respect to dosage of Vitamin-D also. Drawing conclusions based on such heterogeneous population can be fraught with misinformation. Lastly, Studies were limited (<10) for any of outcome measures, hence meta-regression was not possible. In future, if more RCTs are published with different conclusion, findings of this review will be changed.
Hitherto, due to significant non-uniformity regarding various factors governing vitamin D administration, no conclusive evidence could be drawn. Hence, trials comprising of larger sample size and uniform methodology with an aim to focus on specific, time bound clinical outcomes, should pave the way forward.
Funding
Not applicable.
Ethical approval
Not applicable.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.WHO Coronavirus (COVID-19) dashboard. https://covid19.who.int
- 2.Selvarajan S., et al. Systematic review on vitamin D level in apparently healthy Indian population and analysis of its associated factors. Indian J. Endocrinol. Metab. 2017;21:765–775. doi: 10.4103/ijem.IJEM_168_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu F., et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernheim A., et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;295:200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greiller C.L., Martineau A.R. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients. 2015;7 doi: 10.3390/nu7064240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bikle D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21(3):319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martineau A.R., et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginde A.A., et al. High-dose monthly vitamin D for prevention of acute respiratory infection in older long-term care residents: a randomized clinical trial. J Am Geriatr Soc. 2017;65:496–503. doi: 10.1111/jgs.14679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu N., et al. Low vitamin D status is associated with coronavirus disease 2019 outcomes: a systematic review and meta-analysis. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2021;104:58–64. doi: 10.1016/j.ijid.2020.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y., Tong C.H., Bare L.A., Devlin J.J. Assessment of the association of vitamin D level with SARS-CoV-2 seropositivity among working-age adults. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.11634. e2111634–e2111634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rastogi A., et al. Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study) Postgrad. Med. J. postgradmedj- 2020:2020–139065. doi: 10.1136/postgradmedj-2020-139065. [DOI] [PubMed] [Google Scholar]
- 13.Entrenas Castillo M., et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751. doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Annweiler C., et al. Vitamin D and survival in COVID-19 patients: a quasi-experimental study. J Steroid Biochem Mol Biol. 2020;204:105771. doi: 10.1016/j.jsbmb.2020.105771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Annweiler G., et al. Vitamin D supplementation associated to better survival in hospitalized frail elderly COVID-19 patients: the GERIA-COVID quasi-experimental study. Nutrients. 2020;12:3377. doi: 10.3390/nu12113377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murai I.H., et al. Effect of a single high dose of vitamin D 3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. J Am Med Assoc. 2021;325:1053. doi: 10.1001/jama.2020.26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.https://www.who.int/teams/blueprint/covid-19
- 19.https://www.cochranelibrary.com/central/about-central
- 20.https://training.cochrane.org/resource/grade-handbook
- 21.Silberstein M. Correlation between premorbid IL-6 levels and COVID-19 mortality: potential role for Vitamin D. Int Immunopharm. 2020;88:106995. doi: 10.1016/j.intimp.2020.106995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bilezikian J.P., et al. Mechanisms in endocrinology: vitamin D and COVID-19. Eur J Endocrinol. 2020;183:R133–R147. doi: 10.1530/EJE-20-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watkins R.R., Lemonovich T.L., Salata R.A. An update on the association of vitamin D deficiency with common infectious diseases. Can J Physiol Pharmacol. 2015;93:363–368. doi: 10.1139/cjpp-2014-0352. [DOI] [PubMed] [Google Scholar]
- 24.Grant W.B., et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12:988. doi: 10.3390/nu12061620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmed F.A. Network-based analysis reveals the mechanism underlying vitamin D in suppressing cytokine storm and virus in SARS-CoV-2 infection. Front Immunol. 2020;11:3084. doi: 10.3389/fimmu.2020.590459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rudensky A.Y. Regulatory T cells and Foxp3. Immunol Rev. 2011;241:260–268. doi: 10.1111/j.1600-065X.2011.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrelli F., et al. Therapeutic and prognostic role of vitamin D for COVID-19 infection: a systematic review and meta-analysis of 43 observational studies. J Steroid Biochem Mol Biol. 2021;211 doi: 10.1016/j.jsbmb.2021.105883. 105883–105883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah K., Saxena D., Mavalankar D. Vitamin D supplementation, COVID-19 & disease severity: a meta-analysis. QJM Mon. J. Assoc. Physicians hcab009. 2021 doi: 10.1093/qjmed/hcab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernández J.L., Nan D., Fernandez-Ayala M., García-Unzueta M., Hernández-Hernández M.A., López-Hoyos M., et al. Vitamin D status in hospitalized patients with SARS-CoV-2 infection. J Clin Endocrinol Metab. 2020 doi: 10.1210/clinem/dgaa733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nikniaz L., Akbarzadeh M.A., Hosseinifard H., Hosseini M.-S. The impact of vitamin D supplementation on mortality rate and clinical outcomes of COVID-19 patients: a systematic review and meta-analysis. 2021. http://medrxiv.org/lookup/doi/10.1101/2021.01.04.21249219
- 31.Bassatne A., et al. The link between COVID-19 and VItamin D (VIVID): a systematic review and meta-analysis. Metabolism. 2021;119 doi: 10.1016/j.metabol.2021.154753. 154753–154753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dramé M., et al. Relation between vitamin D and COVID-19 in aged people: a systematic review. Nutrients. 2021;13:1339. doi: 10.3390/nu13041339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y., et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. 2019;366:l4673. doi: 10.1136/bmj.l4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cereda E., et al. Vitamin D supplementation and outcomes in coronavirus disease 2019 (COVID-19) patients from the outbreak area of Lombardy. Italy. Nutr. Burbank Los Angel. Cty. Calif. 2021;82 doi: 10.1016/j.nut.2020.111055. 111055–111055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lisboa Bastos M., et al. Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis. BMJ. 2020;370 doi: 10.1136/bmj.m2516. m2516–m2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy A., Rathod P.R., Baidya D.K., Ray B.R. Preoperative testing strategy in discharged COVID-19 patients. Trends Anaesth. Crit. Care. 2021;36:41–42. doi: 10.1016/j.tacc.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]