Abstract
Purpose
The aim of this study is to evaluate the prognostic value of pre-treatment advanced lung cancer inflammation index (ALI) in patients with HPV-negative HNSCC undergoing up-front surgical treatment.
Methods
The present multi-centre, retrospective study was performed in a consecutive cohort of patients who underwent upfront surgery with or without adjuvant (chemo)-radiotherapy for head and neck squamous cell carcinoma (HNSCC). Patients were stratified by ALI, and survival outcomes were compared between groups. In addition, the prognostic value of ALI was compared with two other indices, the prognostic nutritional index (PNI) and systemic inflammatory index (SIM).
Results
Two hundred twenty-three patients met the inclusion criteria (151 male and 72 female). Overall and progression-free survival were significantly predicted by ALI < 20.4 (HR 3.23, CI 1.51–6.90 for PFS and HR 3.41, CI 1.47–7.91 for OS). Similarly, PNI < 40.5 (HR = 2.43, 95% CI: 1.31–4.51 for PFS and HR = 2.40, 95% CI: 1.19–4.82 for OS) and SIM > 2.5 (HR = 2.51, 95% CI: 1.23–5.10 for PFS and HR = 2.60, 95% CI: 1.19–5.67 for OS) were found to be significant predictors. Among the three indices, ALI < 20.4 identified the patients with the worst 5-year outcomes. Moreover, patients with a combination of low PNI and low ALI resulted to be a better predictor of progression (HR = 5.26, 95% CI: 2.01–13.73) and death (HR = 5.68, 95% CI: 1.92–16.79) than low ALI and low PNI considered alone.
Conclusions
Our results support the use of pre-treatment ALI, an easily measurable inflammatory/nutritional index, in daily clinical practice to improve prognostic stratification in surgically treated HPV-negative HNSCC.
Keywords: Head and neck cancer, Nutrition, Advanced lung cancer inflammation index, Inflammatory indexes, Survival
Introduction
In recent years, the role of the inflammatory system and immunity in head and neck squamous cell carcinoma (HNSCC) tumorigenesis has been the subject of intense interest among researchers, with the focus being primarily on tumor infiltrating immune cells (TIICs) and immunoediting mechanisms [1, 2].
Several subtypes of TIICs have been observed to be associated with HNSCC prognosis and treatment response [3]. Interestingly, tumors seem to induce systemic immune changes in peripherical blood cells in order to promote cancer progression [4], meaning that immune cells in the peripheral blood could be as important as those in the tumor microenvironment (TME) [5]. This is consistent with a systemic disease interpretation of cancer. The tumor-associated immune landscape may be reflected in peripheral blood leucocyte counts via the interaction between tumor cells, TIICs, and stromal cells, which promotes proinflammatory cytokine production (i.e., TNFα, interleukins, TGFβ, CXCLs) leading to immune cell recruitment, as has been described in the case of neutrophils and monocytes [6, 7]. Evidence for specific mechanisms are now emerging, for example the description of raised granulocyte-colony stimulating factor (G-CSF) in tumor development and consequent bone marrow reprogramming, with activation of a myeloid differentiation program in the early hematopoietic compartment, and an expansion of T cell-suppressive myeloid cells [8]. These mechanisms are yet to be fully understood, although their possible clinical implications are significant, as evidenced by the finding that lower lymphocyte and higher platelet, neutrophil, and monocyte counts are associated with poor prognosis [9].
Several inflammatory indices based on peripherical white blood cell counts, including lymphocyte-to-neutrophil ratio (LNR) [10], systemic inflammatory marker (SIM) [11], prognostic nutritional index (PNI) [12], and H-Index [13], have been proposed to provide health researchers with more comprehensive and accurate prognostication.
In recent years, inflammatory indices have been supplemented with nutritional information to produce novel indices. Among them, the advanced lung cancer inflammation index (ALI) is a novel prognostic index designed for metastatic non-small cell lung cancer (NSCLC) and also found to be associated with OS in small cell lung cancer, large B cell lymphoma, esophageal squamous carcinoma, and colorectal cancer [14]. Described for the first time in 2013 by Jafri et al. [15], ALI is calculated using neutrocyte-to-lymphocyte ratio (NLR) as well as body mass index (BMI) and baseline serum albumin. Thus, ALI includes both inflammatory and nutritional aspects, the latter being another well-investigated prognostic factor for HNSCC [16]. The prognostic value of ALI in HNSCC has been observed only in one study and found to predict both overall and disease-free survival [17]; however, the cohort was small, and no comparison was made between ALI and other inflammatory indices.
The aim of this study is to evaluate the prognostic value of pre-treatment ALI in patients with HPV-negative HNSCC undergoing up-front surgical treatment, and to compare its prediction accuracy with two other indices, the PNI and SIM.
Methods
The present multi-centre, retrospective study was performed in a cohort of consecutive patients diagnosed with HNSCC who underwent up-front surgery and met the inclusion criteria from April 2004 to April 2017. The study network included General and University Hospitals in north-eastern Italy, located in Treviso, Padova, Verona, Trieste, Brescia, Pordenone, Ferrara, and Pavia. Inclusion criteria were (a) HNSCC arising from the oral cavity, oropharynx, hypopharynx, or larynx; (b) curative up-front surgery as the primary treatment modality; and (c) availability of body mass index (BMI) and blood parameters for ALI calculation. Patients were specifically excluded if (a) they were diagnosed with nasopharyngeal carcinoma or T1 glottic SCC; (b) they had any coexisting conditions or hematological conditions that could alter inflammatory parameters; (c) they had previous malignancy or additional synchronous primary tumors; (d) their pre-treatment blood test results were not available; (e) they had metastatic disease; and (f) HPV-driven SCC.
Participants and data
Medical records were reviewed to collect socio-demographic and clinical characteristics of enrolled patients. Baseline characteristics, including body mass index (BMI), Adult Comorbidity Evaluation 27 (ACE-27) comorbidity index, clinical stage, histology, and grading were retrieved. For oropharyngeal carcinomas, HPV status was assessed by p16 immunostaining and/or HPV-DNA. Blood parameters were collected at baseline and before treatment, including red blood cell (103/μL), white blood cell (103/μL), platelet (103/μL), hemoglobin (Hb, g/L), hematocrit (%), mean corpuscular volume (MCV, fL), mean platelet volume (MPV, fL), neutrophils (103/μL), lymphocytes (103/μL), monocytes (103/μL), basophils (103/μL), eosinophils (103/μL), serum albumin (g/dL), and C-reactive protein (CRP).
Patients were routinely followed-up according to consensus guidelines [18] with endoscopic examination of the upper aerodigestive tract every 1–3 months for the first year, 3–4 months during the second year, 4–6 months during the 3rd year, and every 6 months after that. A dedicated CT scan of the chest was done annually. Additional dedicated head and neck imaging was arranged based on clinical features and local protocol.
Inflammatory indices
Pre-treatment ALI [15], PNI [12], and SIM [11] indices were calculated as illustrated in Appendix Table 5.
Table 5.
Definition of inflammatory and nutritional indexes
| Index | Formula |
|---|---|
| ALI | |
| PNI | 10 · Albumin + 0.005 · Lymphocytes |
| SIM |
Statistics
Median values of blood markers and corresponding interquartile ranges (Q1–Q3) were reported; differences in blood markers across socio-demographic and clinical characteristics were evaluated through the Kruskal-Wallis test.
For each patient, the time at risk was computed from the date of surgery to the date of locoregional recurrence, death, or last follow-up, whichever occurred first according to the outcome of interest. xProgression-free survival (PFS) was defined as the time from surgery to any type of recurrence/progression or death from any cause. Overall survival (OS) was defined as the time from surgery to death from any cause. The Kaplan-Meier method was used to generate crude survival probabilities and the log-rank test was used to assess the heterogeneity in time to event in strata of selected covariates [19], censoring follow-up at 10 years. Hazard ratios (HR) and the corresponding 95% CI were calculated using Cox proportional hazards models [19], adjusting for gender and age, plus covariates significantly associated to OS in the multivariable analysis (i.e., education and cN). ALI, PNI, and SIM were categorized in three levels; the optimal cut-offs were determined according to a recursive algorithm that maximizes the model predictability in OS, measured through Harrell’s C-index [20].
Results
Population
Overall, 223 patients met the inclusion criteria (median age, 66 years; interquartile range, 59–73 years); the majority of patients (n = 151, 67.7%) were male, with stage III–IV cancer (n = 158, 63.7%) and with moderately differentiated SSC (n = 155, 69.5%; Table 1). Negative surgical margins were achieved in 176 patients (78.9%) and extracapsular extension was absent in 177 patients (89.4%). Adjuvant (chemo)radiotherapy was administered to 98 patients (43.9%).
Table 1.
Hazard ratio (HR) and corresponding confidence interval (CI) for loco-regional failure, progression, and death according to socio-demographic and clinical characteristics
| Patients | Locoregional failure | PFS | OS | ||
|---|---|---|---|---|---|
| n | (%) | HR (95% CI)a | HR (95% CI) | HR (95% CI) | |
| Gender | |||||
| Male | 151 | (67.7) | Reference | Reference | Reference |
| Female | 72 | (32.3) | 0.81 (0.32–2.05) | 0.77 (0.44–1.36) | 0.68 (0.36–1.29) |
| Age (years) | |||||
| < 65 | 89 | (39.9) | Reference | Reference | Reference |
| 65–74 | 52 | (23.3) | 0.72 (0.26–2.04) | 1.51 (0.78–2.92) | 2.09 (0.95–4.58) |
| ≥ 75 | 82 | (36.8) | 0.41 (0.15–1.15) | 1.24 (0.65–2.34) | 2.10 (1.00–4.42) |
| Educationb | |||||
| Low | 151 | (70.9) | Reference | Reference | Reference |
| High | 62 | (29.1) | 0.45 (0.17–1.20) | 0.46 (0.23–0.92) | 0.38 (0.16–0.90) |
| BMI (kg m−2) | |||||
| < 25 | 124 | (55.6) | Reference | Reference | Reference |
| ≥ 25 | 99 | (44.4) | 1.34 (0.56–3.19) | 0.93 (0.55–1.57) | 0.81 (0.44–1.48) |
| Smoking habitsc | |||||
| Never | 42 | (19.3) | Reference | Reference | Reference |
| Ever | 176 | (80.7) | 1.36 (0.38–4.82) | 1.12 (0.55–2.25) | 0.96 (0.45–2.02) |
| Drinking habitsd | |||||
| Never | 122 | (61.3) | Reference | Reference | Reference |
| Ever | 77 | (38.7) | 0.53 (0.21–1.33) | 0.73 (0.42–1.28) | 0.69 (0.36–1.30) |
| ACE-27 | |||||
| None-Mild | 101 | (45.3) | Reference | Reference | Reference |
| Moderate-Severe | 122 | (54.7) | 0.83 (0.30–2.35) | 1.17 (0.66–2.05) | 1.00 (0.53–1.89) |
| Cancer site | |||||
| Oral cavity | 109 | (47.4) | Reference | Reference | Reference |
| Oropharynx | 26 | (11.3) | 7.93 (2.47–25.50) | 2.01 (0.99–4.07) | 1.11 (0.47–2.60) |
| Hypopharynx/Larynx | 95 | (41.3) | 0.53 (0.14–2.08) | 1.01 (0.55–1.86) | 1.14 (0.59–2.21) |
| pT | |||||
| pT1-pT2 | 104 | (46.6) | Reference | Reference | Reference |
| pT3-pT4 | 119 | (53.4) | 0.51 (0.22–1.14) | 0.81 (0.46–1.41) | 1.11 (0.59–2.09) |
| pNe | |||||
| pN0 | 143 | (65.0) | Reference | Reference | Reference |
| pN1-pN3 | 77 | (35.0) | 1.34 (0.56–3.24) | 2.00 (0.16–3.44) | 2.35 (1.28–4.32) |
| Stage | |||||
| I–II | 90 | (36.3) | Reference | Reference | Reference |
| III–V | 158 | (63.7) | 0.54 (0.22–1.31) | 1.02 (0.58–1.79) | 1.35 (0.70–2.61) |
| Grading (differentiation) | |||||
| Well | 22 | (9.9) | Reference | Reference | Reference |
| Moderately | 155 | (69.5) | 1.32 (0.17–10.46) | 1.35 (0.41–4.49) | 1.52 (0.35–6.54) |
| Poorly | 46 | (20.6) | 1.72 (0.20–15.10) | 2.34 (0.65–8.45) | 2.30 (0.49–10.85) |
| Surgical margins | |||||
| Negative | 176 | (78.9) | Reference | Reference | Reference |
| Close/Positive | 47 | (21.1) | 2.45 (1.00–5.97) | 1.76 (0.99–3.12) | 1.96 (1.04–3.69) |
| Extracapsular extension | |||||
| Absent | 177 | (89.4) | Reference | Reference | Reference |
| Present | 21 | (10.6) | 1.27 (0.57–2.83) | 1.36 (0.63–2.93) | 1.14 (0.49–2.67) |
HRs and CIs were estimated from Cox proportional hazard model, adjusting for gender, age, education, pN, and surgical margins
aAdjusted for competing risks according to Fine-Gray model
bEducation level is missing in 10 patients
cSmoking habit is missing in five patients
dDrinking habit is missing in 24 patients
epN is missing in three patients
During a median follow-up of 58 months (interquartile range, 41–83 months), 59 patients died; cancer was the cause of death for 31 (52.5%) of them. Local recurrence was experienced by 23 patients, while 21 patients had regional recurrence and 11 distant metastases. Second primary tumor was diagnosed during follow-up in 28 patients.
Among socio-demographic and clinical characteristics, a significant association emerged between higher education and improved both PFS (HR = 0.46, 95% CI: 0.23–0.92) and OS (HR = 0.38, 95% CI: 0.16–0.90; Table 1). Moreover, oropharyngeal primary site was associated with an increased risk of locoregional failure (HR = 7.93, 95% CI: 2.47–25.50), but not of progression or death. Positive surgical margins were associated with higher risk of locoregional failure (HR = 2.45, 95% CI: 1.00–5.97) and lower OS (HR for death 1.96, 95% CI: 1.04–3.69).
Blood samples were obtained a median (IQR) of 18 (10 to 27) days before surgery. Table 2 shows the correlations between blood parameters and cancer outcomes. Patients with serum albumin levels ≥ 4.4 g/dL reported a significant reduced risk of a PFS event (HR = 0.49, 95% CI: 0.25–0.94). Neutrophil, lymphocyte and monocyte counts were not predictors of locoregional failure, PFS, and OS.
Table 2.
Hazard ratio (HR) and corresponding 95% confidence interval (CI) for local failure, regional failure, distant failure, progression, and death according to blood parameters
| Pts | Loco-regional failure | PFS | OS | |
|---|---|---|---|---|
| HR (95% CI)a | HR (95% CI) | HR (95% CI) | ||
| Hemoglobin (g/L) | ||||
| < 139 | 75 | Reference | Reference | Reference |
| 139–151 | 89 | 1.50 (0.55–4.08) | 0.75 (0.42–1.32) | 0.56 (0.29–1.08) |
| ≥ 152 | 59 | 0.80 (0.23–2.75) | 0.43 (0.20–0.95) | 0.41 (0.17–0.98) |
| Albumin (g/dL) | ||||
| < 4.0 | 75 | Reference | Reference | Reference |
| 4.0–4.3 | 74 | 0.80 (0.28–2.23) | 0.59 (0.31–1.10) | 0.59 (0.29–1.22) |
| ≥ 4.4 | 74 | 0.88 (0.34–2.31) | 0.49 (0.25–0.94) | 0.53 (0.25–1.09) |
| Neutrophils (103/μL) | ||||
| < 4.1 | 74 | Reference | Reference | Reference |
| 4.1–5.7 | 75 | 1.29 (0.38–4.32) | 1.30 (0.65–2.57) | 1.10 (0.52–2.34) |
| ≥ 5.8 | 74 | 0.98 (0.31–3.07) | 1.16 (0.58–2.33) | 1.05 (0.48–2.28) |
| Lymphocytes (103/μL) | ||||
| < 1.6 | 72 | Reference | Reference | Reference |
| 1.6–2.0 | 84 | 0.70 (0.24–2.06) | 0.73 (0.39–1.35) | 0.83 (0.42–1.63) |
| ≥ 2.1 | 67 | 1.25 (0.45–3.44) | 0.85 (0.43–1.67) | 1.80 (0.36–1.74) |
| Monocytes (103/μL) | ||||
| < 0.51 | 84 | Reference | Reference | Reference |
| 0.51–0.71 | 71 | 0.64 (0.19–2.20) | 0.93 (0.47–1.82) | 0.96 (0.46–1.98) |
| ≥ 0.72 | 68 | 1.13 (0.42–3.05) | 1.72 (0.91–3.26) | 1.66 (0.78–3.50) |
HRs and CIs were estimated from Cox proportional hazard model, adjusting for gender, age, education, pN, and surgical margins
aAdjusted for competing risks according to Fine-Gray model
Inflammatory indices
Patients were stratified into 3 prognostic groups for each inflammatory index, with 3 ranges of values associated with higher, intermediate or lower survival. The optimal critical values to define these ranges were found to be 20.4 and 50.3 for ALI, 40.5 and 42.4 for PNI, and 1.3 and 2.5 for SIM.
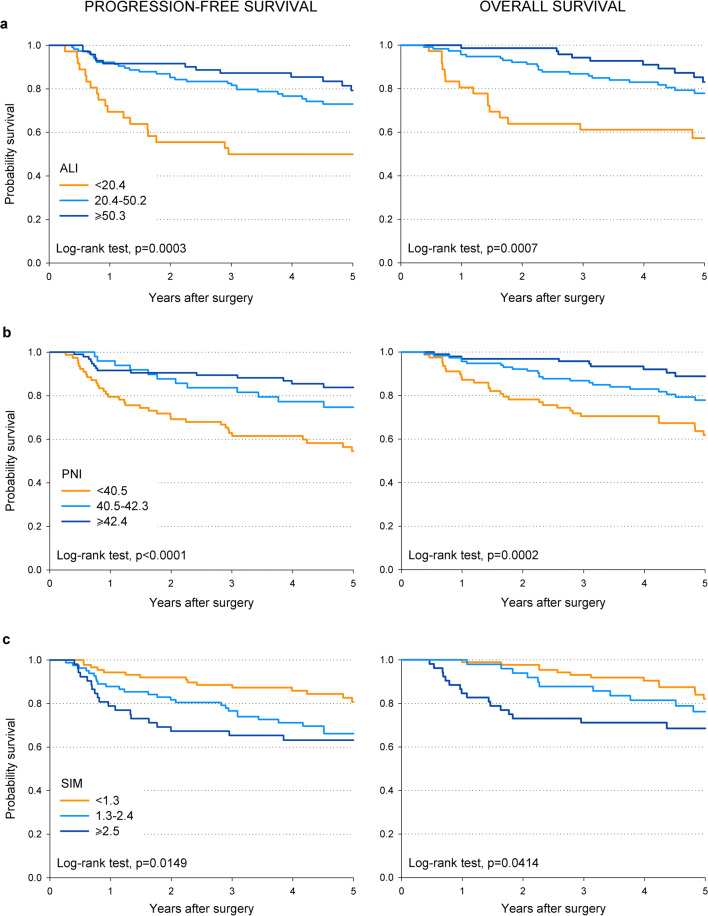
Patients with ALI < 20.4 reported the worst prognosis, with 5-year PFS of 50.0% compared to 79.3% in those with ALI ≥ 50.3 (p = 0.0003; Fig. 1). Similarly, 5-year OS was 57.3% and 83.1%, respectively (p = 0.0007; Fig. 1). The disadvantage in survival for patients with ALI < 20.4 was confirmed by multivariate analysis, with hazard ratios (HR) of 3.23 (95% CI: 1.51–6.90) for PFS and 3.41 (95% CI: 1.47–7.91) for OS (Table 3). Similar patterns emerged for PNI < 40.5 (HR = 2.43, 95% CI: 1.31–4.51 for PFS and HR = 2.40, 95% CI: 1.19–4.82 for OS) and SIM > 2.5 (HR = 2.51, 95% CI: 1.23–5.10 for PFS and HR = 2.60, 95% CI: 1.19–5.67 for OS). No index was significantly associated to loco-regional failure (Table 3).
Fig. 1.
Kaplan-Meier curves showing the correlation of ALI, PNI, and SIM with the 5-year progression-free and overall survival
Table 3.
Hazard ratio (HR) and corresponding 95% confidence interval (CI) for loco-regional failure, progression, and death according to inflammatory and nutritional indexes
| Pts | Locoregional failure | PFS | OS | |
|---|---|---|---|---|
| HR (95% CI)a | HR (95% CI) | HR (95% CI) | ||
| NLR | ||||
| ≥ 3.7 | 49 | Reference | Reference | Reference |
| < 3.7 | 174 | 0.77 (0.30–1.96) | 0.53 (0.30–0.92) | 0.51 (0.28–0.96) |
| ALI | ||||
| ≥ 50.3 | 71 | Reference | Reference | Reference |
| 20.4–50.2 | 116 | 0.83 (0.30–2.32) | 1.32 (0.66–2.66) | 1.30 (0.58–2.91) |
| < 20.4 | 36 | 1.31 (0.40–4.27) | 3.23 (1.51–6.90) | 3.41 (1.47–7.91) |
| PNI | ||||
| ≥ 42.4 | 95 | Reference | Reference | Reference |
| 40.5–42.3 | 49 | 1.49 (0.48–4.62) | 1.53 (0.74–3.16) | 1.48 (0.64–3.41) |
| < 40.5 | 79 | 1.64 (0.65–4.11) | 2.43 (1.31–4.51) | 2.40 (1.19–4.82) |
| SIM | ||||
| < 1.3 | 88 | Reference | Reference | Reference |
| 1.3–2.4 | 83 | 1.39 (0.44–4.44) | 1.67 (0.86–3.27) | 1.35 (0.65–2.83) |
| ≥ 2.5 | 52 | 2.24 (0.73–6.90) | 2.51 (1.23–5.10) | 2.60 (1.19–5.67) |
HRs and CIs were estimated from Cox proportional hazard model, adjusting for gender, age, education, pN, and surgical margins
aAdjusted for competing risks according to Fine-Gray model
Interestingly, among the three indices, ALI < 20.4 identified the patients with the worst 5-year outcomes (i.e., 50.0% for PFS and 57.3% for OS; Fig. 1), whereas PNI ≥ 42.4 identified those with the best prognosis (i.e., 83.8% for PFS and 88.8% for OS; Fig. 1).
We therefore analyzed the combination of PNI and ALI in relation to prognosis (Table 4). Patients with low ALI and low PNI reported a risk of progression (HR = 5.26, 95% CI: 2.01–13.73) and death (HR = 5.68, 95% CI: 1.92–16.79) much higher than ALI and PNI considered alone. Interestingly, BMI, hemoglobin, albumin, and lymphocytes were significantly lower in patients with ALI < 20.4 and PNI < 40.5 compared to patients with ALI ≥ 42.4 and PNI ≥ 50.3 (Table 4). Conversely, neutrophils were significantly higher.
Table 4.
Median values of baseline parameters and hazard ratio (HR) and corresponding confidence intervals (CI) of oncological outcomes in 223 patients with head and neck cancer undergoing surgery, according to combination of prognostic nutritional index (PNI) and advanced lung inflammation index (ALI)
| ALI ≥ 42.4 and PNI ≥ 50.3 | 20.4 ≤ ALI < 42.4 or 40.5 ≤ PNI < 50.4 | ALI < 20.4 and PNI < 40.5 | ||
|---|---|---|---|---|
| (n = 42) | (n = 156) | (n = 25) | ||
| Oncological outcomes | ||||
| Loco-regional failure (HR, 95% CI)a | Reference | 1.20 (0.30–4.74) | 1.25 (0.25–6.35) | |
| Progression-free survival (HR, 95% CI) | Reference | 1.43 (0.61–3.32) | 5.26 (2.01–13.73) | |
| Overall survival (HR, 95% CI) | Reference | 1.39 (0.52–3.75) | 5.68 (1.92–16.79) | |
| Baseline parameters | ||||
| BMI (kg m−2) | 26.6 (23.1–28.8) | 24.1 (21.7–26.5) | 23.8 (19.9–27.1) | p = 0.0014 |
| Hemoglobin (g/L) | 152 (140–160) | 140 (130–150) | 121 (117–140) | p < 0.0001 |
| Albumin (g/dL) | 4.50 (4.35–4.70) | 4.10 (3.92–4.38) | 3.40 (3.26–3.70) | p < 0.0001 |
| Neutrophils (103/μL) | 3.95 (3.32–4.80) | 4.68 (3.77–6.50) | 6.90 (5.30–8.65) | p < 0.0001 |
| Lymphocytes (103/μL) | 2.12 (1.84–2.35) | 1.89 (1.40–2.22) | 1.25 (0.90–1.46) | p < 0.0001 |
| Monocytes (103/μL) | 0.60 (0.40–0.70) | 0.60 (0.50–0.80) | 0.68 (0.50–1.11) | p = 0.1482 |
HRs and CIs were estimated from Cox proportional hazard model, adjusting for gender, age, education, pN, and surgical margins
aAdjusted for competing risks according to Fine-Gray model
Discussion
In the present study, we report the prognostic value of ALI in patients with HPV-negative HNSCC treated by upfront surgery. Irrespective of other stage-related prognostic parameters, a low ALI was associated with a poor prognosis.
To date, the most robust prognostic factor in head and neck oncology is HPV-status which was recently incorporated in the 8th edition of TNM staging system by its surrogate biomarker p16 [21]. However, its role is limited to oropharyngeal SCC, and reliable biomarkers for the stratification of prognosis in non-oropharyngeal HNSCC and HPV-negative oropharyngeal SCC are lacking. For these reasons and in order to study a more homogeneous population, we selected for the present analysis only HPV-negative HNSCCs.
Inflammatory indices are a prognostic tool that reflects the immunity of the host response to cancer progression. Cancer influences the immune system in a pro-tumorigenic way, increasing neutrophil and monocyte count and decreasing lymphocyte count. The former are involved in tumor initiation, growth, proliferation, or metastasis, the latter suppresses tumor development and growth through immune surveillance mechanisms [11]. An association between higher ALI and survival has been described in several different cancer types (non-small cell lung cancer, small cell lung cancer, diffuse large B cell lymphoma, and colorectal cancer) [14] but until now has only been reported in HNSCC by a small-cohort retrospective study, which found a correlation with prognosis [11].
Among standard socio-demographic and clinical parameters, only age, low educational status, neck node metastases, and close/positive margins were independently associated with worse OS in the present study. However, in addition, we identified a significant independent association between several inflammatory/nutritional indices, including lower ALI, PNI, and higher SIM with both inferior PFS and OS. Of these, the ALI was found to be a more reliable prognostic index, with stronger associations with PFS and OS compared with PNI and SIM. This may be due to the more complete representation and synthesis of the inflammatory and nutritional status of the patient. We also found that higher serum albumin level was the only blood parameter significantly associated with a better PFS when considered alone, which highlights the importance of preoperative nutritional status and the potential value of nutrient supplementation [16]. Particularly, early nutrition intervention in patients with HNSCC was observed to result in an improved treatment tolerance and outcome [22].
Moreover, patients with both an ALI < 20.4 and a PNI < 40.5 had a significantly lower PFS and OS than those with a low score on either index alone. The reason for this synergy between ALI and PNI is unclear, given the common parameters composing ALI and PNI.
ALI has been previously investigated in HNSCC in only one small single-centre retrospective study [17]. The present multicentre study provides a relatively large cohort of highly selected patients with strict inclusion criteria. Follow-up was accurate, with regular clinical radiological examination as recommended by the American Cancer Society [23]. Despite these strengths, this study does also have some limitations. Firstly, the retrospective design may have biased the results. Secondly, blood parameters were collected pre-operatively in order to avoid the influence of surgery itself on the baseline values. However, it was not always possible to exclude the influence of any other systemic condition (such as inflammatory or infectious conditions) as ALI, PNI, and SIM are nonspecific tumor markers. Thirdly, 122 patients reported abstinence from alcohol, which does not reflect the drinking prevalence of the region. Finally, given the period of time during which included patients were diagnosed and treated, HNSCC were staged according to the 7th edition of the AJCC TNM. However, considering that we excluded HPV-positive patients, the discrepancy between the 7th and the 8th editions of AJCC TNM was limited.
The present study supports the use of ALI as a prognostic marker, offering evidence of a strong correlation with prognosis. ALI can be easily calculated in routine clinical practice using standard blood tests and clinical parameters to help inform clinicians and patients on prognosis. Further research is required to confirm the usefulness of blood parameters in the assessment of inflammatory and nutritional status, and their importance in prognostication and eventually perhaps in therapeutic strategy.
At present, the AJCC TNM staging system for HNSCC includes only tumor-associated factors in risk stratification. However, heterogeneity is still evident within staging groups, as reflected by the present analysis. The encouraging performance of inflammatory and nutritional indices in stratifying outcomes in patients with HNSCC supports further large and prospective research to verify whether the integration of host-related factors with tumor-related parameters increases the performance of the staging system.
In conclusion, the present study supports the use of pre-treatment ALI, an easily measurable inflammatory/nutritional index, in daily clinical practice to improve prognostic stratification in surgically treated HPV-negative HNSCC.
Appendix
Authors’ contributions
Piergiorgio Gaudioso and Paolo Boscolo-Rizzo had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Piergiorgio Gaudioso, Daniele Borsetto, Jonathan Fussey, Paolo Boscolo-Rizzo. Acquisition, analysis, or interpretation of data: Piergiorgio Gaudioso, Daniele Borsetto, Margherita Tofanelli, Fiordaliso Cragnolini, Anna Menegaldo, Cristoforo Fabbris, Chiara Bianchini, Simone Mauramati, Vittorio Giacomarra, Roberto Di Carlo, Jerry Polesel, Jonathan Fussey, Paolo Boscolo-Rizzo. Drafting of the manuscript: Piergiorgio Gaudioso, Daniele Borsetto, Jerry Polesel, Jonathan Fussey, Paolo Boscolo-Rizzo. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Jerry Polesel. All authors read and approved the final manuscript.
Funding information
Open access funding provided by Università degli Studi di Trieste. (Information that explains whether and by whom the research was supported)—The authors did not receive support from any organization for the submitted work.
Data availability
Yes.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Treviso and Belluno provinces (Date March 23th 2020/No. 773/CE Marca).
Consent for publication
Given.
Code availability
(Software application or custom code)—Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang HC, Chan LP, Cho SF. Targeting the immune microenvironment in the treatment of head and neck squamous cell carcinoma. Front Oncol. 2019;9:1084. doi: 10.3389/fonc.2019.01084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horton JD, Knochelmann HM, Day TA, Paulos CM, Neskey DM. Immune evasion by head and neck cancer: foundations for combination therapy. Trends in Cancer. 2019;5:208–232. doi: 10.1016/j.trecan.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen N, Bellile E, Thomas D, McHugh J, Rozek L, Virani S, Peterson L, Carey TE, Walline H, Moyer J, Spector M, Perim D, Prince M, McLean S, Bradford CR, Taylor JMG, Wolf GT, Head and Neck SPORE Program Investigators Tumor infiltrating lymphocytes and survival in patients with head and neck squamous cell carcinoma. Head & Neck. 2016;38:1074–1084. doi: 10.1002/hed.24406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang L, Simons DL, Lu X, Tu TY, Avalos C, Chang AY, Dirbas FM, Yim JH, Waisman J, Lee PP. Breast cancer induces systemic immune changes on cytokine signaling in peripheral blood monocytes and lymphocytes. EBioMedicine. 2020;52:102631. doi: 10.1016/j.ebiom.2020.102631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi J, Maeng HG, Lee SJ, Kim YJ, Kim DW, Lee HN, Namgung JH, Oh HM, Kim TJ, Jeong JE, Park SJ, Choi YM, Kang YW, Yoon SG, Lee JK. Diagnostic value of peripheral blood immune profiling in colorectal cancer. Annals of Surgical Treatment and Research. 2018;94:312–321. doi: 10.4174/astr.2018.94.6.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17:559–572. doi: 10.1038/nri.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masucci MT, Minopoli M, Carriero MV. Tumor associated neutrophils. Their role in tumorigenesis, metastasis, prognosis and therapy. Front Oncol. 2019;9:1146. doi: 10.3389/fonc.2019.01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casbon AJ, Reynau D, Park C, et al. Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc Natl Acad Sci U S A. 2015;112:E566–E575. doi: 10.1073/pnas.1424927112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valero C, Pardo L, López M, García J, Camacho M, Quer M, León X. Pretreatment count of peripheral neutrophils, monocytes, and lymphocytes as independent prognostic factor in patients with head and neck cancer. Head Neck. 2017;39:219–226. doi: 10.1002/hed.24561. [DOI] [PubMed] [Google Scholar]
- 10.Mascarella MA, Mannard E, Silva SD, Zeitouni A. Neutrophil-to-lymphocyte ratio in head and neck cancer prognosis: a systematic review and meta-analysis. Head Neck. 2018;40:1091–1100. doi: 10.1002/hed.25075. [DOI] [PubMed] [Google Scholar]
- 11.Zhou S, Yuan H, Wang J, Hu X, Liu F, Zhang Y, Jiang B, Zhang W. Prognostic value of systemic inflammatory marker in patients with head and neck squamous cell carcinoma undergoing surgical resection. Future Oncol. 2020;16:559–571. doi: 10.2217/fon-2020-0010. [DOI] [PubMed] [Google Scholar]
- 12.Bruixola G, Caballero J, Papaccio F, Petrillo A, Iranzo A, Civera M, Moriana M, Bosch N, Maroñas M, González I, Pastor M, Cervantes A. Prognostic nutritional index as an independent prognostic factor in locoregionally advanced squamous cell head and neck cancer. ESMO open. 2018;3(6):e000425. doi: 10.1136/esmoopen-2018-000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valero C, Zanoni DK, Pillai A, Ganly I, Morris LGT, Shah JP, Wong RJ, Patel SG. Host factors independently associated with prognosis in patients with Oral cavity Cancer. JAMA Otolaryngology–Head & Neck Surgery. 2020;146(8):1–9. doi: 10.1001/jamaoto.2020.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hua X, Chen J, Wu Y, Sha J, Han S, Zhu X. Prognostic role of the advanced lung cancer inflammation index in cancer patients: a meta-analysis. World Journal of Surgical Oncology. 2019;17:117. doi: 10.1186/s12957-019-1725-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC Cancer. 2013;13:158. doi: 10.1186/1471-2407-13-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Müller-Richter U, Betz C, Hartmann S, Brands RC. Nutrition management for head and neck cancer patients improves clinical outcome and survival. Nutr Res. 2017;48:1–8. doi: 10.1016/j.nutres.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Jank BJ, Kadletz L, Schnöll J, Selzer E, Perisanidis C, Heiduschka G. Prognostic value of advanced lung cancer inflammation index in head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2019;276(5):1487–1492. doi: 10.1007/s00405-019-05381-0. [DOI] [PubMed] [Google Scholar]
- 18.Simo R, Homer J, Clarke P, Mackenzie K, Paleri V, Pracy P, Roland N. Follow-up after treatment for head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130(S2):S208–S211. doi: 10.1017/S0022215116000645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalbfleisch JDPR. The statistical analysis of failure time data. 2. Hoboken (US): John Wiley & Sons; 2002. [Google Scholar]
- 20.Harrell FE, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247:2543–2546. doi: 10.1001/jama.1982.03320430047030. [DOI] [PubMed] [Google Scholar]
- 21.Boscolo-Rizzo P, Dietz A. The AJCC/UICC eighth edition for staging head and neck cancers: is it wise to de-escalate treatment regimens in p16-positive oropharyngeal cancer patients? Int J Cancer. 2017;141:1490–1491. doi: 10.1002/ijc.30837. [DOI] [PubMed] [Google Scholar]
- 22.Paccagnella A, Morello M, Mosto MCD, et al. Early nutritional intervention improves treatment tolerance and outcomes in head and neck cancer patients undergoing concurrent chemoradiotherapy. Support Care Cancer. 2010;18:837–845. doi: 10.1007/s00520-009-0717-0. [DOI] [PubMed] [Google Scholar]
- 23.Cohen EEW, LaMonte SJ, Erb NL, et al. American Cancer Society head and neck cancer survivorship care guideline. CA Cancer J Clin. 2016;66:203–239. doi: 10.3322/caac.21343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Yes.