Abstract
Background
Transurethral resection of the prostate (TURP) has been the gold‐standard treatment for alleviating urinary symptoms and improving urinary flow in men with symptomatic benign prostatic hyperplasia (BPH). However, the morbidity of TURP approaches 20%, and less invasive techniques have been developed for treating BPH. Transurethral microwave thermotherapy (TUMT) is an alternative, minimally‐invasive treatment that delivers microwave energy to produce coagulation necrosis in prostatic tissue. This is an update of a review last published in 2012.
Objectives
To assess the effects of transurethral microwave thermotherapy for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia.
Search methods
We performed a comprehensive search using multiple databases (the Cochrane Library, MEDLINE, Embase, Scopus, Web of Science, and LILACS), trials registries, other sources of grey literature, and conference proceedings published up to 31 May 2021, with no restrictions by language or publication status.
Selection criteria
We included parallel‐group randomized controlled trials (RCTs) and cluster‐RCTs of participants with BPH who underwent TUMT.
Data collection and analysis
Two review authors independently assessed studies for inclusion at each stage and undertook data extraction and risk of bias and GRADE assessments of the certainty of the evidence (CoE). We considered review outcomes measured up to 12 months after randomization as short‐term and beyond 12 months as long‐term. Our main outcomes included: urologic symptoms scores, quality of life, major adverse events, retreatment, and ejaculatory and erectile function.
Main results
In this update, we identified no new RCTs, but we included data from studies excluded in the previous version of this review. We included 16 trials with 1919 participants, with a median age of 69 and moderate lower urinary tract symptoms. The certainty of the evidence for most comparisons was moderate‐to‐low, due to an overall high risk of bias across studies and imprecision (few participants and events).
TUMT versus TURP
Based on data from four studies with 306 participants, when compared to TURP, TUMT probably results in little to no difference in urologic symptom scores measured by the International Prostatic Symptom Score (IPSS) on a scale from 0 to 35, with higher scores indicating worse symptoms at short‐term follow‐up (mean difference (MD) 1.00, 95% confidence interval (CI) −0.03 to 2.03; moderate certainty). There is likely to be little to no difference in the quality of life (MD −0.10, 95% CI −0.67 to 0.47; 1 study, 136 participants, moderate certainty). TUMT likely results in fewer major adverse events (RR 0.20, 95% CI 0.09 to 0.43; 6 studies, 525 participants, moderate certainty); based on 168 cases per 1000 men in the TURP group, this corresponds to 135 fewer (153 to 96 fewer) per 1000 men in the TUMT group. TUMT, however, probably results in a large increase in the need for retreatment (risk ratio (RR) 7.07, 95% CI 1.94 to 25.82; 5 studies, 337 participants, moderate certainty) (usually by repeated TUMT or TURP); based on zero cases per 1000 men in the TURP group, this corresponds to 90 more (40 to 150 more) per 1000 men in the TUMT group. There may be little to no difference in erectile function between these interventions (RR 0.63, 95% CI 0.24 to 1.63; 5 studies, 337 participants; low certainty). However, TUMT may result in fewer cases of ejaculatory dysfunction compared to TURP (RR 0.36, 95% CI 0.24 to 0.53; 4 studies, 241 participants; low certainty).
TUMT versus sham
Based on data from four studies with 483 participants we found that, when compared to sham, TUMT probably reduces urologic symptom scores using the IPSS at short‐term follow‐up (MD −5.40, 95% CI −6.97 to −3.84; moderate certainty). TUMT may cause little to no difference in the quality of life (MD −0.95, 95% CI −1.14 to −0.77; 2 studies, 347 participants; low certainty) as measured by the IPSS quality‐of‐life question on a scale from 0 to 6, with higher scores indicating a worse quality of life. We are very uncertain about the effects on major adverse events, since most studies reported no events or isolated lesions of the urinary tract. TUMT may also reduce the need for retreatment compared to sham (RR 0.27, 95% CI 0.08 to 0.88; 2 studies, 82 participants, low certainty); based on 194 retreatments per 1000 men in the sham group, this corresponds to 141 fewer (178 to 23 fewer) per 1000 men in the TUMT group. We are very uncertain of the effects on erectile and ejaculatory function (very low certainty), since we found isolated reports of impotence and ejaculatory disorders (anejaculation and hematospermia).
There were no data available for the comparisons of TUMT versus convective radiofrequency water vapor therapy, prostatic urethral lift, prostatic arterial embolization or temporary implantable nitinol device.
Authors' conclusions
TUMT provides a similar reduction in urinary symptoms compared to the standard treatment (TURP), with fewer major adverse events and fewer cases of ejaculatory dysfunction at short‐term follow‐up. However, TUMT probably results in a large increase in retreatment rates. Study limitations and imprecision reduced the confidence we can place in these results. Furthermore, most studies were performed over 20 years ago. Given the emergence of newer minimally‐invasive treatments, high‐quality head‐to‐head trials with longer follow‐up are needed to clarify their relative effectiveness. Patients' values and preferences, their comorbidities and the effects of other available minimally‐invasive procedures, among other factors, can guide clinicians when choosing the optimal treatment for this condition.
Plain language summary
Transurethral microwave thermotherapy for lower urinary tract symptoms in men with benign prostatic hyperplasia
Review question
Does transurethral microwave thermotherapy (TUMT) improve bothersome urinary symptoms without unwanted side effects in men with an enlarged prostate?
Background
An enlarged prostate may cause bothersome urinary tract symptoms, such as having to urinate often during the day or night, having a weak stream, and the feeling of not completely emptying the bladder. When lifestyle changes (like drinking fewer liquids) or medications do not help, men may choose to have surgery, such as transurethral resection of the prostate. However, this procedure may cause unwanted effects, such as erection and ejaculation problems, or require retreatment. This review looks at the results of transurethral microwave thermotherapy, which is an alternative, less invasive procedure that uses microwave energy to reduce prostatic tissue.
Study characteristics We found no study comparing transurethral microwave thermotherapy with the other newer and less invasive treatments for this condition.
We found 16 studies with 1919 men that compared transurethral microwave thermotherapy with a simulated procedure (participants are made to believe they received treatment, while in reality, they did not) or with traditional surgery (transurethral resection of the prostate (TURP)). Participants’ average age was 69 years, and most had a moderate degree of bothersome urinary symptoms.
Key results
Compared to the traditional surgery (TURP), transurethral microwave thermotherapy probably results in little to no difference in urinary symptoms at short‐term follow‐up, but we are uncertain about its long‐term effects. There may be little to no difference in quality of life or problems with erections between these interventions both short‐term and long‐term. This procedure likely results in fewer serious side effects and problems with ejaculation compared to surgery. However, it likely results in an increase in the need for retreatment (including surgery).
Compared to a simulated procedure, transurethral microwave thermotherapy probably improves urinary symptoms and the need for retreatment at short‐term follow‐up (less than 12 months). This treatment may make little to no difference in the quality of life. We are very uncertain whether or not serious unwanted side effects, including problems with erection and ejaculation, are more common.
Findings of this review are up‐to‐date until 31 May 2021.
Certainty of the evidence
The certainty of the evidence for the outcomes ranged mostly from moderate to low due to shortcomings in how the studies were conducted and small study size. This means that we have either moderate or limited confidence in the results.
Summary of findings
Background
Description of the condition
The prostate gland is an organ approximately the size of a walnut located below the urinary bladder encircling the urethra (Leissner 1979). Benign prostatic hyperplasia (BPH) is a histological diagnosis defined as an increased number of epithelial and stromal cells in the prostate; this may cause prostatic enlargement and subsequently compression of the urethra and obstruction (Roehrborn 2008). BPH may therefore develop with or without lower urinary tract symptoms (LUTS) in men aged over 40 years (Dunphy 2015). BPH acquires clinical significance when associated with bothersome LUTS (Roehrborn 2008). 'Symptom bother' typically correlates with the increased number and severity of symptoms, which relate to both the quality‐of‐life impairment and treatment‐seeking (Agarwal 2014). Self‐administered questionnaires, (e.g. the International Prostate Symptom Score (IPSS)), include the quality‐of‐life domain to evaluate the relative degree of bother across all LUTS (Barry 1995). Chapple 2017 reported that increasing LUTS severity was associated with worsening men's overall distress using the patient perception of bladder condition, which is a single‐item global question (ranging from 1 (causes no problems at all) to 6 (causes severe problems)). In this Cochrane Review, we consider the term BPH as prostatic enlargement with LUTS to define the disease condition and potential need for intervention.
BPH can progress and cause serious consequences such as acute urinary retention, urinary tract infection, and upper urinary tract deterioration. BPH also negatively impacts public health and a reduction in a person's quality of life (Kozminski 2015; Martin 2014). In Europe, 30% of men over 50 years of age, equivalent to 26 million men, are affected by bothersome LUTS, including storage symptoms (such as urinary frequency, urgency, and nocturia) or voiding symptoms (such as urinary hesitancy, weak urinary stream, straining to void, and prolonged voiding), or both. The yearly reported associated number of medical prescriptions is estimated to be around 11.6 million for 74 million people at risk from 2004 to 2008 (Cornu 2010). According to an international study involving 7588 men, the prevalence of LUTS was 18% in 40‐year‐olds, 29% in the 50s, 40% in the 60s, and 56% in the 70s (Homma 1997). In the USA, an estimated eight million men over 50 years of age have BPH (Roehrborn 2008). More recent data show that the lifetime prevalence of BPH was 26.2% (95% confidence interval 22.8 to 29.6%) (Lee 2017).
Diagnosis
Initial evaluation of LUTS suggestive of BPH includes patient history, physical examination including a digital rectal examination, urinalysis, prostate‐specific antigen (PSA) blood test, voiding diary, and IPSS (EAU 2021; McVary 2011). A digital rectal examination is performed to assess the prostate for size and any lesions suspicious of cancer. PSA is secreted by the prostate gland and is found to be abnormally elevated in conditions such as prostate cancer, BPH, infection, or inflammation of the prostate (EAU 2021; McVary 2011). The IPSS is used to assess urinary symptom severity and quality of life. It is also used to document subjective responses to treatment (Barry 1992; EAU 2021; McVary 2011). Measurements of maximum flow rate (Qmax) and postvoid residual (PVR) are also often used in diagnosis and treatment decisions (EAU 2021; McVary 2011). A low Qmax and a large PVR predict an increased risk of symptom progression (Crawford 2006). Other tests include radiological imaging, urodynamic evaluation, and cystoscopy to determine appropriate treatment and predict treatment response (Egan 2016; McVary 2011).
Treatment
Treatment decisions are based on symptoms and the degree of bother noted by the patient. Initial treatment options for BPH include conservative management (watchful waiting and lifestyle modification) and medication (alpha‐blockers and 5‐alpha reductase inhibitors) (EAU 2021; McVary 2011). If patients have been refractory to conservative and medical treatment, and BPH causes subsequent complications, such as acute urinary retention, recurrent urinary tract infection, bladder stones or diverticula, hematuria, or renal insufficiency, surgical options are considered (EAU 2021; McVary 2011). Until the 1970s, the only option available to treat this condition and relieve LUTS was an open or endoscopic surgery to remove or resect prostatic tissue to open up the blocked urethra (Pariser 2015). Clinical guidelines recommend monopolar or bipolar transurethral resection of the prostate (TURP) as a standard treatment modality for subjective symptom relief and objective improvements in urinary flow (EAU 2021; McVary 2011), but this procedure is also associated with significant morbidity and long‐term complications, including hematuria requiring blood transfusion, urethral stricture, recurrent urinary tract infection, and urinary incontinence. Moreover, men may experience ejaculatory (65%) and erectile dysfunction (10%) related to TURP (AUA 2003). Furthermore, BPH is a disease common in elderly men who have an increased risk of complications for general anesthesia and the surgery itself (Dunphy 2015; Yoo 2012). Some alternatives to TURP include laser enucleation, vaporization, and Aquablation, but they all require spinal anesthesia (EAU 2021). In recent years, the number of men undergoing TURP has steadily declined due to increasing pharmacologic treatments (alpha‐blockers and 5‐alpha‐reductase inhibitors) and minimally‐invasive treatments that are usually performed under local anesthesia (Dahm 2021), such as convective radiofrequency water vapor therapy (Hwang 2019), prostatic urethral lift (Jung 2019), prostatic arterial embolization (Jung 2020) which are covered in current evidence‐based guidelines (Parsons 2020).
Description of the intervention
Transurethral microwave thermotherapy (TUMT) uses microwave‐induced heat to ablate prostatic tissue and is designed to have fewer major complications than TURP (Walmsley 2004). The patient is treated in an outpatient setting. Once the patient's bladder is emptied by straight catheterization, a local lidocaine gel is inserted for local anesthesia. The treatment catheter is then placed within the urethra, confirmed by the return of sterile water and transabdominal or transrectal ultrasound, and the balloon is inflated. The catheter is composed of a curved tip, a temperature sensor and a microwave unit. The distal port contains the bladder balloon, allowing for urine drainage and cooling. A rectal probe may be inserted to monitor the rectal temperature (Rubeinstein 2003).
TUMT has evolved over the past decades. Initial systems worked at lower energy or heat settings, and treatment would take around an hour with minimal discomfort, but results were disappointing. Subsequent systems incorporated catheters that provided urethral cooling, thus allowing higher energy delivery. These advances reduced the procedure time to around 30 minutes and improved outcomes, but the higher energy leads to more significant discomfort during the procedure, in which patients often require sedation and analgesia, with continued risk of urinary retention (Walmsley 2004).
While TUMT was once the most widely‐used procedure for minimally‐invasive surgical therapies among the USA's Medicare population (Yu 2008), its use has declined since its peak in 2006 (Malaeb 2012). A recent study in Australia highlighted that TUMT currently constitutes only 0.26% of all procedures performed for BPH (Morton 2020).
How the intervention might work
TUMT uses a special transurethral catheter that transmits heat into the prostate using microwaves' electromagnetic radiation, penetrating water‐rich tissue. The energy transferred by the microwave to the tissue in the form of heat‐induces coagulation necrosis, reducing prostatic volume. This mechanism may also cause denervation of receptors, decreasing smooth muscle tone of the prostatic urethra (Walmsley 2004). Temperatures lower than 45 ºC seemed ineffective in producing this effect, so higher‐energy devices were developed to reach more than 70 ºC, causing thermoablation of the prostatic tissue (Aoun 2015).
Why it is important to do this review
A review was published in 2012 (Hoffman 2012). The Cochrane Urology Review Group commissioned a network meta‐analysis of minimally‐invasive treatments for lower urinary tract symptoms (Franco 2020) that draws its evidence from individual reviews of these interventions. It therefore became necessary to update the previous version of the review in search of the latest evidence and using the latest Cochrane guidance and methodological standards. This review in its updated format intends to guide clinicians, patients, and guideline developers when assessing the available options for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia, especially considering the critical outcomes of the summary of findings table, which are now comparable with other reviews on this topic published by the Cochrane Urology Group (Hwang 2019; Jung 2019; Jung 2020; Kang 2020).
Objectives
To assess the effects of transurethral microwave thermotherapy for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia.
Methods
Criteria for considering studies for this review
Types of studies
The methods for this update have been extensively modified since its last publication to meet current methodological expectations; please refer to the Differences between protocol and review section. We included parallel‐group RCTs and cluster‐RCTs. We excluded cross‐over trials, as these study designs are not relevant in this setting. We did not include single‐armed studies. We included studies regardless of their publication status or language.
Types of participants
We defined the eligible participant population as men over the age of 40 years with a prostate volume of 20 mL or greater (as assessed by ultrasound or cross‐sectional imaging), with lower urinary tract symptoms (LUTS) as determined by International Prostate Symptom Scores (IPSS) of eight or over, and a maximum flow rate (Qmax) of less than 15 mL/second, as measured by non‐invasive uroflowmetry, invasive pressure flow studies, or both (Dunphy 2015; EAU 2021; McNicholas 2016; McVary 2011). We based the age limit on the fact that the prevalence of BPH increases in middle‐aged and older men and is infrequent in younger men (Barry 1997; EAU 2021; Egan 2016). We included studies in which only a subset of participants was relevant to this review (i.e. studies with more than 75% of participants only as relevant to the review) if data were available separately for the relevant subset.
We excluded studies of men with active urinary tract infection, bacterial prostatitis, chronic renal failure, untreated bladder calculi or large diverticula, prostate cancer, and urethral stricture disease, as well as those who had undergone prior prostate, bladder neck, or urethral surgery. We also excluded studies of people with other conditions that affect urinary symptoms, such as neurogenic bladder due to spinal cord injury, multiple sclerosis, or central nervous system disease.
Types of interventions
Experimental intervention
Transurethral microwave thermotherapy (TUMT)
Comparator interventions
Sham control (or no intervention)
Transurethral resection of the prostate (TURP) (monopolar or bipolar)
Minimally‐invasive therapies: convective radiofrequency water vapor thermal therapy (CRFWVT, also known as Rezum); prostatic urethral lift (PUL), prostatic arterial embolization (PAE), temporary implantable nitinol device (TIND)
We planned to investigate the following comparisons of experimental intervention versus comparator interventions. Concomitant interventions must be the same in the experimental and comparator groups to establish fair comparisons.
Comparisons
TUMT versus TURP
TUMT versus sham control (or no intervention)
TUMT versus CRFWVT
TUMT versus PUL
TUMT versus PAE
TUMT versus TIND
Types of outcome measures
We did not use the measurement of the outcomes assessed in this review as an eligibility criterion.
Primary outcomes
Urologic symptom scores (continuous outcome)
Quality of life (continuous outcome)
Major adverse events (dichotomous outcome)
Secondary outcomes
Retreatment (dichotomous outcome)
Erectile function (continuous outcome)
Ejaculatory function (continuous outcome)
Minor adverse events (dichotomous outcome)
Acute urinary retention (dichotomous outcome)
Indwelling urinary catheter (continuous outcome)
Method and timing of outcome measurement
We considered the clinically important differences for the review outcome measures to rate the overall certainty of evidence in the Table 1 and Table 2 (Jaeschke 1989; Johnston 2013).
Summary of findings 1. Transurethral microwave thermotherapy compared to transurethral resection of the prostate for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia.
| Transurethral microwave thermotherapy compared to transurethral resection of the prostate for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia | |||||
| Patient or population: men with lower urinary tract symptoms due to benign prostatic hyperplasia Setting: outpatient (TUMT) / inpatient (TURP) ‐ UK, Netherlands, Scandinavia, USA Intervention: Transurethral microwave thermotherapy (TUMT) Comparison: Transurethral resection of the prostate (TURP) | |||||
| Outcomes | № of participants (studies) Follow up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with transurethral resection of the prostate (TURP) | Risk difference with Transurethral microwave thermotherapy | ||||
|
Urologic symptom scores Assessed with: IPSS Scale from 0 (best: not at all) to 35 (worst: almost always) Follow‐up: 6 ‐ 12 months |
306 (4 RCTs) | ⊕⊕⊕⊝ MODERATEa | ‐ | The mean urologic symptoms score (IPSS) was 5.63 | MD 1 higher (0.03 lower to 2.03 higher) |
|
Quality of life Assessed with: IPSS‐QoL Scale from 0 (best: delighted) to 6 (worst: terrible) Follow‐up: 12 months |
136 (1 RCT) | ⊕⊕⊕⊝ MODERATEa | ‐ | The mean quality of life was 1.5 | MD 0.10 lower (0.67 lower to 0.47 higher) |
|
Major adverse events Assessed with: Clavien‐Dindo classification system (Grade III, IV and V complications) Follow‐up: 6 ‐ 12 months |
525 (6 RCTs) | ⊕⊕⊕⊝ MODERATEa | RR 0.20 (0.09 to 0.43) | Study population | |
| 168 per 1000 | 135 fewer per 1000 (153 fewer to 96 fewer) | ||||
|
Retreatment Participants requiring additional procedures or surgery Follow‐up: 6 ‐ 12 months |
463 (5 RCTs) | ⊕⊕⊕⊝ MODERATEa,b | RR 7.07 (1.94 to 25.82) | Study population | |
| 0 per 1000 | 90 more per 1000 (40 more to 150 more) | ||||
|
Erectile function (sexually‐active men only) Assessed with: issues related to erectile function Follow‐up: 6 ‐ 12 months |
337 (5 RCTs) | ⊕⊕⊝⊝ LOWa,c | RR 0.63 (0.24 to 1.63) | Study population | |
| 129 per 1000 | 48 fewer per 1000 (98 fewer to 82 more) | ||||
|
Ejaculatory function (sexually‐active men only) Assessed with: issues related to ejaculatory function Follow‐up: 6 ‐ 12 months |
241 (4 RCTs) | ⊕⊕⊝⊝ LOWa,c | RR 0.36 (0.24 to 0.53) | Study population | |
| 523 per 1000 | 335 fewer per 1000 (397 fewer to 246 fewer) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: mean difference; RCT: randomized controlled trial; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded by one level for study limitations: studies at an overall high risk of bias. bWe did not downgrade for imprecision since we used a minimally conceptualized approach: although the confidence interval is wide, there are no concerns about whether the effect results in a moderate to a large increase in the retreatment rate. cDowngraded by one level for imprecision: the incidence is mostly reported in a subset of sexually‐active participants.
Summary of findings 2. Transurethral microwave thermotherapy compared to sham treatment for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia.
| Transurethral microwave thermotherapy compared to sham treatment for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia | |||||
| Patient or population: men with lower urinary tract symptoms due to benign prostatic hyperplasia Setting: outpatient ‐ France, USA, UK, Sweden, Netherlands Intervention: Transurethral microwave thermotherapy Comparison: Sham treatment | |||||
| Outcomes | № of participants (studies) Follow up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with sham treatment | Risk difference with Transurethral microwave thermotherapy | ||||
|
Urologic symptom scores Assessed with: IPSS Scale from 0 (best: not at all) to 35 (worst: almost always) Follow‐up: 3 ‐ 6 months |
483 (4 RCTs) | ⊕⊕⊕⊝ MODERATEa | ‐ | The mean urologic symptom scores was 16.2 | MD 5.40 lower (6.97 lower to 3.84 lower) |
|
Quality of life Assessed with: IPSS‐QoL Scale from 0 (best: delighted) to 6 (worst: terrible) Follow‐up: 6 months |
347 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b | ‐ | The mean quality of life score was 3.05 | MD 0.95 lower (1.14 lower to 0.77 lower) |
|
Major adverse events Assessed with: Clavien‐Dindo classification system (Grade III, IV and V complications) Follow‐up: 6 ‐ 12 months |
924 (8 RCTs) | ⊕⊝⊝⊝ VERY LOWa,c | ‐ | Six studies reported that there were no major adverse events. The two remaining studies reported four isolated cases of lesions of the urinary tract related to the procedure in both groups. | |
|
Retreatment Participants requiring additional procedures or surgery Follow‐up: 6 ‐ 12 months |
82 (2 RCTs) | ⊕⊕⊝⊝ LOWa,d | RR 0.27 (0.08 to 0.88) | Study population | |
| 194 per 1000 | 141 fewer per 1000 (178 fewer to 23 fewer) | ||||
|
Erectile function (sexually‐active men only) Assessed with: issues related to erectile function Follow‐up: 6 ‐ 12 months |
375 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWa,c | ‐ | Two studies reported normal erections. One study reported one case of impotence. | |
|
Ejaculatory function (sexually‐active men only) Assessed with: issues related to ejaculatory function Follow‐up: 6‐12 months |
727 (5 RCTs) | ⊕⊝⊝⊝ VERY LOWa,c | ‐ | Three studies reported no issues related to ejaculatory function. The two remaining studies reported isolated cases of loss of ejaculate and hematospermia. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: mean difference; RCT: randomized controlled trial; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded by one level for study limitations: studies at an overall high risk of bias. bDowngraded by one level for imprecision: confidence interval crosses assumed threshold of minimal clinically important difference. cDowngraded by two levels for imprecision: very few events (isolated reports). dDowngraded by one level for imprecision: few events.
Urologic symptom scores
Mean change from baseline or final mean value, measured using a validated scale (such as IPSS)
We considered the improvement of an IPSS score of three points as the minimal clinically important difference (MCID) to assess the efficacy and comparative effectiveness (Barry 1995). If possible, we used different thresholds of MCID based on the severity of IPSS, with a threshold of three points for men with mild LUTS, five for moderate LUTS, and eight for severe LUTS (Barry 1995).
Quality of life
Mean change from baseline or final mean value measured as a validated scale (such as IPSS‐quality of life or BPH Impact Index)
A BPH Impact Index score of one as an MCID was used to indicate improvement (Barry 2013; Rees 2015).
Major adverse events
Example: postoperative hemorrhage requiring admission or intervention
We used the Clavien‐Dindo classification system to assess surgical complications (Dindo 2004), and categorized grade III, IV and V complications as major adverse events. If the study authors of eligible studies did not use the Clavien‐Dindo system, we judged the adverse events by severity using the available information described in the studies.
Retreatment
Events requiring other surgical treatment modalities (e.g. TURP) after the intervention.
Erectile function
Mean change from baseline or final mean value measured as a total score on the International Index of Erectile Function (IIEF)‐5 questionnaire, also known as Sexual Health Inventory for Men (Rosen 1997)
We considered the MCID an erectile function domain score of four on the IIEF (Rosen 2011). If possible, we used different thresholds of MCID based on the severity of erectile dysfunction, with a threshold of two for men with mild erectile dysfunction, five for moderate erectile dysfunction, and seven for men with severe erectile dysfunction (Rosen 2011). We considered a difference in IIEF‐5 score of over five points as the MCID (Spaliviero 2010).
Ejaculatory function
Mean change from baseline or final mean value measured using the Male Sexual Health Questionnaire for Ejaculatory Dysfunction (MSHQ‐EjD) or the four‐item version of the MSHQ‐EjD (Rosen 2004; Rosen 2007)
We considered the MCID as an ejaculatory function domain score of two on the MSHQ or a four‐item version of the MSHQ‐EjD (Rosen 2004; Rosen 2007).
Minor adverse events
Example: postoperative fever or pain requiring medication
We used the Clavien‐Dindo classification system to assess surgical complications (Dindo 2004) and categorized grade I and II complications as minor adverse events. If the authors of eligible studies did not use the Clavien‐Dindo system, we judged the severity of adverse events using the available information described in these studies.
Acute urinary retention
Events requiring catheterization after the intervention
Indwelling urinary catheter
Measured in hours from intervention to urinary catheter removal (as a continuous outcome) or the need for urinary catheterization (as a dichotomous outcome)
Hospital stay
Measured in days from admission to discharge
There were no reported thresholds in adverse events, retreatment, acute urinary retention, indwelling urinary catheter, or hospital stay. We considered a clinically important difference for adverse events, retreatment, acute urinary retention, and indwelling catheter as risk ratio reductions of at least 25% (Guyatt 2011a). We used a MCID of one day (24 hours) to assess the efficacy and comparative effectiveness for indwelling urinary catheter and hospital stay.
We considered outcomes measured up to and including 12 months after randomization as short‐term, and later than 12 months as long‐term, for urologic symptom scores, quality of life, major adverse events, retreatment, erectile function, ejaculatory function, minor adverse events, and acute urinary retention. We assessed retreatment, indwelling urinary catheter and hospital stay as short‐term only.
Search methods for identification of studies
We performed a comprehensive search with no restrictions by date, by language of publication or publication status.
Electronic searches
We searched the following sources from the inception of each database to the date of search, and placed no restrictions on the language of publication:
CENTRAL (Cochrane Central Register of Controlled Trials) searched 31 May 2021;
MEDLINE (Ovid) searched 31 May 2021;
Embase (Elsevier) searched 31 May 2021;
LILACS ( Bireme) searched 31 May 2021;
Scopus searched 31 May 2021;
Web of Science (Clarivate analytics) searched 31 May 2021;
ClinicalTrials.gov (www.ClinicalTrials.gov) searched 31 May 2021;
World Health Organization International Clinical Trials Registry Platform (ICTRP; www.who.int/trialsearch/) searched 31 May 2021.
For detailed search strategies, see Appendix 1.
Searching other resources
We tried to identify other potentially eligible studies or ancillary publications by searching the reference lists of included studies, reviews, meta‐analyses, and health technology assessment reports. We also contacted the authors of the included studies to identify any further studies that we may have missed. We contacted drug/device manufacturers for ongoing or unpublished studies. We searched only the published abstract proceedings of relevant meetings of the American Urological Association, European Association of Urology, and International Continence Society for the last three years (2018 to 2020) for unpublished studies (see Appendix 2).
Data collection and analysis
Selection of studies
We used Covidence software to identify and remove potential duplicate records. Two review authors (JVAF, LIG) independently scanned abstracts and titles to determine which studies should be assessed further. Two review authors categorized all potentially relevant records as full‐text or mapped records to studies, and classified studies as included studies, excluded studies, studies awaiting classification, or ongoing studies, following the criteria in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). We resolved any disagreements between the two review authors through consensus or by recourse to a third review author (PD). If a resolution was not possible, we designated the corresponding study as 'awaiting classification'. We documented reasons for the exclusion of studies in the Characteristics of excluded studies table. We presented a PRISMA 2020 flow diagram showing the process of study selection (Page 2020).
Data extraction and management
We developed a dedicated data extraction form that we pilot‐tested ahead of time.
For studies that fulfilled our inclusion criteria, two review authors (JVAF and LIG) independently abstracted the following information, which we provide in the Characteristics of included studies table.
Study design
Study dates (if dates are not available, then this was reported as such)
Study settings and country
Participant inclusion and exclusion criteria (e.g. age, baseline IPSS, medical pretreatment)
Participant details, baseline demographics (e.g. age, prostate size, IPSS)
The number of participants by study and by study arm
Details of relevant experimental intervention, such as delivery devices (e.g. size of cystoscope) for the intervention and comparator (e.g. monopolar versus bipolar energy, type of laser)
Definitions of relevant outcomes, and method (e.g. type of instrument, such as IPSS) and timing of outcome measurement (e.g. in months) as well as any relevant subgroups (e.g. based on age, prostate volume, the severity of LUTS)
Study funding sources
Declarations of interest by primary investigators
We extracted outcome data relevant to this Cochrane Review as needed to calculate summary statistics and measures of variance. For dichotomous outcomes, we attempted to obtain numbers of events and totals for the study population in a 2 x 2 table, as well as summary statistics with corresponding measures of variance. We attempted to obtain means and standard deviations or other data necessary to calculate this information for continuous outcomes.
We resolved any disagreements by discussion, or if required by consultation with a third review author (PD).
We have provided information, including study identifiers, about potentially relevant ongoing studies in the Characteristics of ongoing studies table.
We contacted the authors of included studies to obtain key missing data as needed.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents or multiple reports relating to a primary study, we maximized the yield of information by mapping all publications to unique studies and collating all available data. We used the most complete data set aggregated across all known publications. In case of doubt, we gave priority to publications reporting the longest follow‐ups associated with our primary or secondary outcomes.
Assessment of risk of bias in included studies
Two review authors (JVAF and LIG) independently assessed the risks of bias of each included study. We resolved disagreements by consensus, or by consultation with a third review author (PD). We have presented a risk of bias summary figure to illustrate these findings. We further summarize the risk of bias across the studies and domains for each outcome in each included study, in accordance with the approach for the summary assessments of the risk of bias presented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021).
We assessed risk of bias using Cochrane's risk of bias assessment tool (Higgins 2021). We assessed the following domains.
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Other potential sources of bias
We judged risk of bias domains as 'low risk', 'high risk' or 'unclear risk' and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
For selection bias (random sequence generation and allocation concealment), we evaluated risk of bias at study level. For performance bias (blinding of participants and personnel), we considered all outcomes as similarly susceptible to performance bias. For detection bias (blinding of outcome assessment), we grouped outcomes as susceptible to detection bias (subjective) or not susceptible to detection bias (objective).
We defined the following outcomes as subjective outcomes.
Urologic symptom scores
Quality of life
Erectile function
Ejaculatory function
Minor adverse events
We defined the following outcomes as objective outcomes.
Major adverse events
Retreatment
Acute urinary retention
Indwelling urinary catheter
We also assessed attrition bias (incomplete outcome data) on an outcome‐specific basis and present the judgment for each outcome separately when reporting our findings in the risk of bias tables.
For reporting bias (selective reporting), we evaluated the risk of bias at the study level.
Measures of treatment effect
We expressed dichotomous data as risk ratios (RRs) with 95% confidence intervals (CIs). We expressed continuous data as mean differences (MDs) with 95% CIs, unless different studies used different measures to assess the same outcome, in which case we re‐expressed the data as standardized mean differences (SMDs) with 95% CIs.
Unit of analysis issues
The unit of analysis was each individual participant. We planned to take into account the level at which randomization occurred, such as cluster‐randomized trials, and multiple observations of the same outcome. If more than one comparison from the same study was eligible for inclusion in the same meta‐analysis, we either combined study groups to create a single pairwise comparison or appropriately reduced the sample size so that the same participants did not contribute multiple times (if possible, splitting the 'shared' group into two or more groups). While the latter approach offers some solution to adjust the precision of the comparison, it does not account for correlations arising from the same set of participants being in multiple comparisons (Deeks 2021).
Dealing with missing data
We obtained missing data from corresponding study authors, if feasible, and performed intention‐to‐treat analyses if data were available. Otherwise, we performed available‐case analyses. We investigated attrition rates (e.g. dropouts, losses to follow‐up, and withdrawals), and critically appraised issues of missing data. We did not impute missing data.
Assessment of heterogeneity
We planned to assess heterogeneity. We identified heterogeneity (inconsistency) through visual inspection of the forest plots to assess the amount of overlap of CIs, and by using the I2 statistic, which quantifies inconsistency across studies to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003). We would have interpreted the I2 statistic as follows (Deeks 2021).
0% to 40%: may not be important
30% to 60%: may indicate moderate heterogeneity
50% to 90%: may indicate substantial heterogeneity
75% to 100%: considerable heterogeneity
When we identified heterogeneity, we attempted to determine possible reasons by examining individual study and subgroup characteristics.
Assessment of reporting biases
We tried to obtain study protocols to assess selective outcome reporting.
We could not use funnel plots to assess small‐study effects due to the few number of participants in each comparison. If we had included 10 or more studies in a meta‐analysis, we would have used funnel plots to assess small‐study effects (Page 2021). Several explanations can be offered for the asymmetry of a funnel plot, including true heterogeneity of effect with respect to study size, poor methodological design (and hence bias of small studies), and publication bias. We would therefore have interpreted results cautiously.
Data synthesis
Unless there was good evidence for homogeneous effects across studies, we summarized data using a random‐effects model. We interpreted random‐effects meta‐analyses with due consideration of the whole distribution of effects. We also performed statistical analyses according to the statistical guidelines contained in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). For dichotomous outcomes, we used the Mantel‐Haenszel method. For continuous outcomes, we used the inverse variance method. We used Review Manager 5 (RevMan 2020) software to perform analyses.
Subgroup analysis and investigation of heterogeneity
We expected the following characteristics to potentially introduce clinical heterogeneity, and carried out subgroup analyses to investigate interactions.
Participant age (less than 65 years versus 65 years or more)
Prostate volume (less than 50 mL versus 50 mL or more)
Severity of LUTS based on IPSS (score less than or equal to 19 (moderately symptomatic) versus greater than 19 (severely symptomatic))
These subgroup analyses are based on the following observations.
Age is a well‐known risk factor of BPH surgery. Older men have a higher rate of postoperative complications compared with younger men (Bhojani 2014; Pariser 2015). The age cut‐off is based on the World Health Organization (WHO) definition of old age (WHO 2012).
The outcomes and complications of minimally‐invasive procedures, such as TURP, correlate with prostate volume (Reich 2008). We adjusted the prostate volume to 50 mL based on the available evidence.
The relationship between changes in IPSS scores and patient global ratings of improvement is influenced by the baseline scores (Barry 1995).
We planned to limit subgroup analyses to the primary outcomes only.
Sensitivity analysis
We performed sensitivity analyses limited to the primary outcomes to explore the influence of the following factors (when applicable) on effect size.
Restricting the analysis to RCTs by considering risk of bias, excluding studies with at least one domain at 'high risk' or 'unclear risk' of bias for the analyzed outcome.
Restricting the analysis to RCTs with adequately‐described inclusion criteria (prostate size, age, IPSS value, and Qmax).
Summary of findings and assessment of the certainty of the evidence
We presented the overall certainty of the evidence for each outcome according to the GRADE approach (Guyatt 2008). For each comparison, two review authors (JVAF and LIG) independently rated the certainty of the evidence for each outcome as 'high', 'moderate', 'low', or 'very low', using the GRADEpro Guideline Development Tool (GRADEpro GDT). We resolved any discrepancies by consensus or if needed by arbitration from a third review author (PD). For each comparison, we presented a summary of the evidence for the main outcomes in the summary of findings table, which provides key information about the best estimate of the magnitude of effect in relative terms and absolute differences for each relevant comparison of alternative management strategies; numbers of participants and studies addressing each important outcome; and the rating of our overall confidence in the effect estimates for each outcome (Guyatt 2011b; Schünemann 2021). We considered five criteria, not only related to internal validity (risk of bias, inconsistency, imprecision, and publication bias), but also external validity (directness of results), for downgrading the certainty of the evidence for a specific outcome (Schünemann 2021). We included the following outcomes:
Urologic symptom scores
Quality of life
Major adverse events
Retreatment
Erectile function
Ejaculatory function
Results
Description of studies
Details of included studies are presented elsewhere (see Table 3 and Characteristics of included studies).
1. Characteristics of included studies.
| Study name | Trial period | Setting/country | Description of participants | Duration of follow‐up | Intervention and comparator | Age (mean ± SD)* | IPSS (mean ± SD)* | Prostate volume (mean ± SD)* |
| Abbou 1995 | N/A | France | Men ≥ 50 years with symptoms > 3 months, prostate 30 ‐ 80 g, PFR < 15 mL/s, PVR < 300 mL | 12 months | TUMT (Thermex II, Prostcare, BSD‐50) | 65 ± 8 | N/A | 45 ± 15 g |
| Sham | 66 ± 7 | N/A | 44 ± 11 g | |||||
| Ahmed 1997 | N/A | UK | Men ≥ 55 years with AUA score > 12 > 1 year, prostate 25 ‐ 100 mL, PFR < 15 mL/s and a PVR < 300 mL | 6 months | TUMT (Prostatron) | 69.36 | 18.5 | 36.6 mL |
| TURP | 69.45 | 18.4 | 46.1 mL | |||||
| Albala 2002 | N/A | USA | Men 50 ‐ 80 years, AUA index > 13 and a bother score > 11, PFR < 12 mL/sec and PVR > 125 mL; prostate 30 ‐ 100 mL without a significant intravesical middle lobe | 12 months | TUMT (TMx‐2000) | 65.2 ± 7.3 | 22.2 ± 5.0 | 50.5 ± 18.6 mL |
| Sham | 64.6 ± 7.1 | 22.7 ± 5.7 | 47.1 ± 17.9 mL | |||||
| Bdesha 1994 | N/A | UK | Men with prostatism (WHO score > 14), PVR > 50 mL, PFR < 15 mL/s | 3 months | TUMT (LEO Microthermer) | 63.7 | 19.2 | N/A |
| Sham | 62.6 | 18.8 | N/A | |||||
| Blute 1996 | N/A | USA | Men suffering from urinary symptoms (Madsen Symptom score > 8), PVR 10000 mL, PFR < 10 mL/s, prostate length 30 ‐ 50 mm | 12 months | TUMT (Prostatron) | 66.9 ± 7.8 | 19.9 ± 7.2 | 37.4 ± 14.2 mL |
| Sham | 66.9 ± 7.1 | 20.8 ± 6.7 | 36.1 ± 13.4 mL | |||||
| Brehmer 1999 | N/A | Sweden | Men suffering from lower urinary tract symptoms and with an enlarged prostate | 12 months | TUMT (30' ‐ 60' ‐ ECP system) | 70.4 | N/A | N/A |
| Sham | ||||||||
| D'Ancona 1998 | 1994 ‐ 1995 | Netherlands | Men ≥ 45 years with Madsen score > 8 months, prostate 2.5 ‐ 5 cm/30 ‐ 100 mL, PFR < 15 mL/s PRV < 350 mL | 24 months | TUMT (Prostatron) | 69.6 ± 8.5 | 16.7 ± 5.6 | 45 ± 15 mL |
| TURP | 69.3 ± 5.9 | 18.3 ± 6.3 | 43 ± 12 mL | |||||
| Dahlstrand 1995 | N/A | Sweden | Men ≥ 45 years with Madsen score > 8 months, prostate 3.5 ‐ 5 cm, PFR < 15 mL/s PRV > 150 mL | 24 months | TUMT (Prostatron) | 68 | N/A | 33 mL |
| TURP | 79 | N/A | 37 mL | |||||
| De Wildt 1996 | 1991 ‐ 1992 | Netherlands/UK | Men ≥ 45 years with Madsen score > 8 months, PFR < 15 mL/s PRV > 150 mL | 12 months | TUMT (Prostatron) | 63.3 ± 8.1 | N/A | 48.6 ± 16.6 mL |
| Sham | 66.9 ± 6.0 | N/A | 49.0 ± 20.0 mL | |||||
| Floratos 2001 | 1996 ‐ 1997 | Netherlands | Men ≥ 45 years, prostate ≥ 30 cm3, prostatic urethral length ≥ 25 mm, a Madsen symptom score ≥ 8, PFR ≤ 15 mL/s, PVR ≤ 350 mL | 36 months | TUMT (Prostatron) | 68 | 21 | 42 mL |
| TURP | 66 | 20 | 48 mL | |||||
| Larson 1998 | 1994 ‐ 1996 | USA | Men ≥ 45 years with AUA score > 9, enlarged prostate (3 ‐ 5 cm TRUS), PFR < 12 mL/s without a significantly enlarged middle lobe | 12 months | TUMT (Targis) | 66 | 20.8 | 38.1 mL |
| Sham | 65.9 | 21.3 | 44.7 mL | |||||
| Nawrocki 1997 | N/A | UK | Men with a Madsen symptom score ≥ 8, PFR ≤ 15 mL/s, PVR > 150 mL, detrusor pressure > 70 cm H2O | 6 months | TUMT (Prostatron) | 70 (56 ‐ 80) | 19 (7 ‐ 31) | 41.2 ± 14.6 mL |
| Sham | 17.5 (7 ‐ 28) | 46.7 ± 16.8 mL | ||||||
| Nørby 2002a | 1996 ‐ 1997 | Denmark | Men ≥ 50 years, IPSS ≥ 7, PFR ≤ 12 mL/s | 6 months | TUMT (Prostatron) | 66 ± 7 | 20.5 ± 5.7 | 43 (35 – 79) mL |
| TURP/TUIP | 68 ± 7 | 21.3 ± 6.6 | 44 (35 – 50) mL | |||||
| Roehrborn 1998 | N/A | USA | Men ≥ 55 years, AUA‐SI ≥ 13, PFR ≤ 12 mL/s, prostate volume 25 ‐ 100 mL | 6 months | TUMT (Dornier) | 66.3 ± 6.5 | 23.6 ± 5.6 | 48.1 ± 16.2 mL |
| Sham | 66.0 ± 5.8 | 23.9 ± 5.6 | 50.5 ± 18.1 mL | |||||
| Venn 1995 | N/A | UK | Men with a Madsen symptom score ≥ 8, PVR < 250 mL | 6 months | TUMT (Microwave Engineering Designs) | 70.5 | 19.2 | 40.4 mL |
| Sham | 68 | 20.1 | 40.6 mL | |||||
| Wagrell 2002 | 1998 ‐ 1999 | Scandinavia/USA | Men IPSS ≥ 13, PFR ≤ 13 mL/s, prostate volume 30 ‐ 100 mL | 5 years | TUMT (ProstaLund Feedback) | 67 ± 8 | 21.0 ± 5.4 | 48.9 ± 15.8 mL |
| TURP | 69 ± 8 | 20.4 ± 5.9 | 52.7 ± 17.3 mL |
TUMT: transurethral microwave thermotherapy; TURP: transurethral resection of the prostate; IPSS: International Prostate Symptom Score; SD: standard deviation; N/A: not available. (*) SD when available.
Results of the search
We identified 3635 records from electronic databases, including 445 records from trial registers. We found no relevant records in the grey literature repository. After removing duplicates, we screened the titles and abstracts of the remaining 1995 records, 1935 of which we excluded. We assessed 60 full‐text articles: we were unable to find six full‐text articles (see Characteristics of studies awaiting classification) and we excluded 22 studies (23 records) for various reasons (see Excluded studies). Finally, we included 16 studies (37 reports) in this review. There were no ongoing studies that met the inclusion criteria or were relevant to the review question. We have shown the flow of literature through the assessment process in the PRISMA 2020 flowchart (Figure 1).
1.
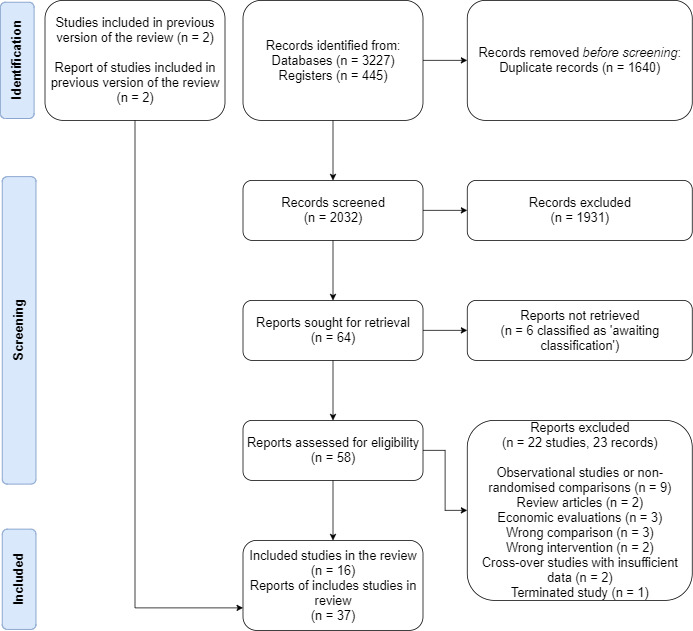
PRISMA 2020 flow diagram.
Included studies
Study design and settings
We included 16 randomized controlled trials. The median sample size was 117 (range 40 to 220). The studies were mostly performed in Europe and the USA: one in France (Abbou 1995), four in the USA (Albala 2002; Blute 1996; Larson 1998; Roehrborn 1998), two in the Netherlands (D'Ancona 1998; Floratos 2001) four in the United Kingdom (Ahmed 1997; Bdesha 1994; Nawrocki 1997; Venn 1995), three in Scandinavian countries (Brehmer 1999; Dahlstrand 1995; Nørby 2002a) and two international studies (De Wildt 1996; Wagrell 2002).
Participants
The included studies randomized 1919 participants with a median age of 69 years. All studies included participants with moderate symptoms, with a median IPSS score of 21 points (range 17 to 29 points); however, four studies did not provide a baseline IPSS score (Abbou 1995; Brehmer 1999; Dahlstrand 1995; De Wildt 1996). The median prostate size was 45 mL (range 33 to 53 mL), but two studies did not provide a baseline prostate size (Bdesha 1994; Brehmer 1999).
Major exclusion criteria relevant to all trials were urethra (e.g. urethral stricture) or bladder disorders (e.g. neurogenic bladder, bladder calculi or diverticula), renal failure, history of prostate, bladder neck, or urethral surgery, and suspected prostate cancer.
Interventions and comparisons
All TUMT procedures were performed in an outpatient setting under local anesthesia. Each device's software and programs varied (most studies used the Prostatron device with the Prostasoft v2.0); however, they delivered a temperature between 45 ºC and 55 ºC in a 60‐ to 90‐minute session through a urethral catheter. The temperature was monitored through the urethral catheter with a rectal probe that triggered a power cut‐off when it reached a certain temperature (usually 42.5 ºC in the rectum). Some studies routinely catheterized participants for two to four days, whereas others only when the participants presented with voiding difficulties or acute urinary retention. Antibiotic prophylaxis across studies was poorly described.
The comparators included:
Sham: the participants were catheterized with the TUMT system, but a sham procedure took place with activation of the monitors in a simulated program. Furthermore, sometimes heat was externally irradiated to the perineum to maintain blinding of participants.
TURP: this was poorly described throughout studies; however, most studies reported that senior surgeons performed this surgery under spinal anesthesia. Participants were usually routinely catheterized for some days.
Ten studies with 1287 randomized participants compared TUMT with sham. The devices used to deliver TUMT by these studies included:
Thermex II (Abbou 1995)
LEO Microthermer (Bdesha 1994)
Prostatron (Blute 1996; De Wildt 1996; Nawrocki 1997)
TherMatrx TMx‐2000 (Albala 2002)
ECP system (Brehmer 1999)
Targis Microwave (Larson 1998)
Dornier Urowave (Roehrborn 1998)
Microwave Engineering Designs (Venn 1995)
Six studies with 632 randomized participants compared TUMT with TURP. The devices used to deliver TUMT by these studies included:
Prostatron (Ahmed 1997; D'Ancona 1998; Dahlstrand 1995; Floratos 2001; Nørby 2002a)
ProstaLund Feedback (Wagrell 2002)
Outcomes
Most studies reported urologic symptom scores and quality of life by IPSS and IPSS‐quality of life, respectively. Adverse events were poorly reported, and in many cases we had to infer whether they were minor or major according to the Clavien‐Dindo classification system. None of the studies reported sexual function as we had predefined, so we extracted data on adverse sexual function instead (i.e. impotence and retrograde ejaculation). Moreover, this information was usually reported in the subset of sexually‐active participants. The reporting of indwelling catheter duration was very scarce across studies and influenced by routine versus selective catheterization during the procedure. Data on acute urinary retention were extracted from data on adverse events. Finally, information on the retreatment rates was scattered, and we had to infer it from the sections reporting the flow of participants or accompanying adverse events.
All studies reported short‐term follow‐up outcomes and only four studies in the TUMT versus TURP comparison reported long‐term outcomes (D'Ancona 1998; Dahlstrand 1995; Floratos 2001; Wagrell 2002). In many cases, long‐term outcomes were only reported in one arm of the study and without sufficient statistical details.
Funding sources
Most studies did not report their funding sources. Three studies were funded by their manufacturers (Larson 1998; Roehrborn 1998; Wagrell 2002), two by public institutions (Nawrocki 1997; Nørby 2002a) and one by a combination of manufacturers and public funders (Abbou 1995).
Excluded studies
We excluded 22 studies (23 records) for the following reasons:
Two studies addressed transrectal thermotherapy (Zerbib 1992; Zerbib 1994; Albala 2000)
Three studies provided economic data on published trials (Kobelt 2004; Norby 2002b; Waldén 1998)
Cross‐over studies with insufficient data (Albala 2000; Tan 2005)
Observational studies and other non‐randomized comparisons (Arai 2000; D'Ancona 1997; Hahn 2000; Hansen 1998; Mulvin 1994; Ohigashi 2007; Servadio 1987; Trock 2004; Vesely 2006)
Review articles identified through full‐text assessment (Dahlstrand 2003; Nørby 2004)
Ineligible comparison (Djavan 1999; Schelin 2006; Shore 2010)
Terminated study (ISRCTN23921450)
Risk of bias in included studies
The summary of the risks of bias by study and domain is available in Figure 2.
2.

Allocation
Random sequence generation
Only five studies reported adequately how the random sequence was generated (Abbou 1995; Blute 1996; Nawrocki 1997; Roehrborn 1998; Venn 1995). The other studies did not provide sufficient information for this domain.
Allocation concealment
Only two studies reported an adequate method for allocation concealment (Blute 1996; Roehrborn 1998). One study used an inadequate method to conceal the allocation (Nawrocki 1997). The other studies did not provide sufficient information for this domain.
Blinding
Blinding of participants and personnel
For the TUMT versus sham comparison, we rated most studies as low risk of bias, since they used an adequate method for blinding (Abbou 1995; Bdesha 1994; Blute 1996; De Wildt 1996; Larson 1998; Nawrocki 1997; Roehrborn 1998). However, three studies did not specify whether personnel were blinded (Albala 2002; Brehmer 1999; Venn 1995), and are rated at unclear risk.
For the TUMT versus TURP comparison, we rated all studies at high risk of bias since blinding was not possible (Ahmed 1997; D'Ancona 1998; Dahlstrand 1995; Floratos 2001; Nørby 2002a; Wagrell 2002).
Blinding of outcome assessment
Subjective outcomes (urologic symptom scores, quality of life, major adverse events, erectile function, ejaculatory function, and minor adverse events): we judged all unblinded studies for the TUMT versus TURP comparison as high risk of bias.
Objective outcomes (retreatment, acute urinary retention, and indwelling urinary catheter): we rated all studies as low risk of bias for these outcomes that are not likely to be affected by lack of blinding.
Incomplete outcome data
We rated four studies (Abbou 1995; Blute 1996; D'Ancona 1998; Larson 1998) as high risk of bias due to high and unbalanced attrition affecting all outcomes. Three studies did not provide details on outcome data lost at follow‐up (Ahmed 1997; Brehmer 1999; Roehrborn 1998). The rest of the studies were rated as low risk of bias.
Selective reporting
We rated all studies at unclear risk of bias, given the lack of available protocols. Two studies were reported as high risk of bias since they selectively reported outcomes for one of the arms of the study or only graphically (Albala 2002; Blute 1996).
Other potential sources of bias
We rated all studies at low risk of bias; no other sources of bias were identified.
Effects of interventions
1. TUMT versus TURP
Six studies (Ahmed 1997; D'Ancona 1998; Dahlstrand 1995; Floratos 2001; Nørby 2002a; Wagrell 2002) with 632 randomized participants were included under this comparison. See Table 3 for a summary of the characteristics of participants, interventions and comparisons. See Table 1.
1.1. Urologic symptom scores
Based on four studies (Ahmed 1997; D'Ancona 1998; Nørby 2002a; Wagrell 2002) with 306 participants, TUMT probably results in little to no difference in urologic symptom scores measured by IPSS scores when compared to TURP at 6 to 12 months follow‐up (mean difference (MD) 1.00, 95% confidence interval (CI) −0.03 to 2.03; Analysis 1.1). In two studies (D'Ancona 1998; Dahlstrand 1995) with 108 participants that assessed this outcome with the Madsen‐Iversen score (range 0 to 28) a small difference was found favoring TURP (MD 1.59, 95% CI 0.69 to 2.48; 2 studies, 108 participants; I2 = 0%, Analysis 1.2). The certainty of the evidence is moderate, due to an overall high risk of bias.
1.1. Analysis.

Comparison 1: Transurethral microwave thermotherapy versus transurethral resection of the prostate (TURP), Outcome 1: Urologic symptoms score (IPSS)
1.2. Analysis.

Comparison 1: Transurethral microwave thermotherapy versus transurethral resection of the prostate (TURP), Outcome 2: Urologic symptoms score (Madsen‐Iversen)
Long‐term data
Three studies (D'Ancona 1998; Dahlstrand 1995; Wagrell 2002) with 187 participants reported long‐term data. We are uncertain of the effect of TUMT on urologic symptom scores when compared to TURP at 2‐ to 5‐year follow‐up (SMD 0.32, 95% CI 0.03 to 0.62; I2 = 0%; Analysis 1.3). Another study with 155 participants (Floratos 2001) was not incorporated in meta‐analysis due to missing data. It reported that the TUMT group had a reduction in IPSS scores from 20 to 12 at three years, whereas the TURP group had a reduction from 20 to 3 in the same period (P < 0.001). The certainty of the evidence is very low due to an overall high risk of bias (severe attrition at long‐term follow‐up) and imprecision.
1.3. Analysis.

Comparison 1: Transurethral microwave thermotherapy versus transurethral resection of the prostate (TURP), Outcome 3: Urologic symptoms score (SMD) ‐ long‐term
Subgroup analysis
Since heterogeneity was extremely low, subgroup analysis by baseline severities found no significant differences across subgroups.
1.2. Quality of life
Based on one study with 136 participants (Wagrell 2002), TUMT likely results in little to no difference in the quality of life when compared to TURP at 12 month follow‐up (MD −0.10, 95% CI −0.67 to 0.47; Analysis 1.5). Another study (Nørby 2002a) with 66 participants reported similar scores in quality of life in the TUMT group (median 2, IQR 1 to 3) and in the TURP group (median 1, IQR 1 ‐ 2) at six‐month follow‐up (P = 0.64 from a three‐arm comparison with interstitial laser coagulation). The certainty of the evidence is moderate, due to an overall high risk of bias.
1.5. Analysis.

Comparison 1: Transurethral microwave thermotherapy versus transurethral resection of the prostate (TURP), Outcome 5: Quality of life
Long‐term data
Long‐term data from Wagrell 2002 indicated that TUMT may result in little to no difference in the quality of life when compared to TURP at 60‐month follow‐up (MD 0.00, 95% CI −0.46 to 0.46; Analysis 1.6). Floratos 2001 (155 participants) reported that quality‐of‐life scores decreased from 4 to 2 at three years in the TUMT group and from 4 to 1 in the TURP group (P < 0.001).
1.6. Analysis.

Comparison 1: Transurethral microwave thermotherapy versus transurethral resection of the prostate (TURP), Outcome 6: Quality of life ‐ long term
1.3. Major adverse events
Based on six studies (Ahmed 1997; D'Ancona 1998; Dahlstrand 1995; Floratos 2001; Nørby 2002a; Wagrell 2002) with 525 participants, TUMT probably results in significantly fewer major adverse events when compared to TURP at 6‐ to 12‐month follow‐up (RR 0.20, 95% CI 0.09 to 0.43; I2 = 0%; Analysis 1.7). Based on 168 cases per 1000 men in the TURP group, this corresponds to 135 fewer (153 to 96 fewer) per 1000 men in the TUMT group. These events primarily included: hospitalization due to bleeding, clot retention, serious infection, TURP syndrome, and urethral stricture (requiring another surgical intervention). The certainty of the evidence is moderate, due to an overall high risk of bias.
1.7. Analysis.

Comparison 1: Transurethral microwave thermotherapy versus transurethral resection of the prostate (TURP), Outcome 7: Major adverse events
Subgroup analysis
Since heterogeneity was extremely low, subgroup analysis by baseline severities found no significant differences across subgroups.
1.4. Retreatment
Based on five studies (D'Ancona 1998; Dahlstrand 1995; Floratos 2001; Nørby 2002a; Wagrell 2002) with 463 participants, TUMT probably results in a large increase in the need for retreatment at 6‐ to 36‐month follow‐up (RR 7.07, 95% CI 1.94 to 25.82; I2 = 0%; Analysis 1.9). Retreatment was usually TURP, TUMT, or TUMT and then TURP. Based on no cases per 1000 men in the TURP group, this corresponds to 90 more (40 to 150 more) per 1000 men in the TUMT group. The certainty of the evidence is moderate, due to an overall high risk of bias.
1.9. Analysis.

Comparison 1: Transurethral microwave thermotherapy versus transurethral resection of the prostate (TURP), Outcome 9: Retreatment
1.5. Erectile function
Based on five studies (Ahmed 1997; Dahlstrand 1995; Floratos 2001; Nørby 2002a; Wagrell 2002) with 337 participants, TUMT may result in little or no difference in erectile function when compared to TURP at 6‐ to 12‐month follow‐up (RR 0.63, 95% CI 0.24 to 1.63; I2 = 35%; Analysis 1.10). The certainty of the evidence is low due to an overall high risk of bias and imprecision (the incidence is mostly reported in a subset of sexually‐active participants).
1.10. Analysis.

Comparison 1: Transurethral microwave thermotherapy versus transurethral resection of the prostate (TURP), Outcome 10: Erectile function
Long‐term data
One study (Wagrell 2002) reported five‐year data on erectile dysfunction with an incidence of 7.5% in the TUMT group and 15.4% in the TURP group (data were available for 119/154 randomized participants). The certainty of the evidence is very low due to an overall high risk of bias and imprecision (the incidence is mostly reported in a subset of sexually‐active participants with high attrition).
1.6. Ejaculatory function
Based on four studies (Ahmed 1997; Dahlstrand 1995; Floratos 2001; Nørby 2002a) with 241 participants, TUMT may result in fewer cases of retrograde ejaculation when compared to TURP at 6‐ to 12‐month follow‐up (RR 0.36, 95% CI 0.24 to 0.53; I2 = 0%, Analysis 1.11). The certainty of the evidence is low, due to an overall high risk of bias and imprecision (the incidence mostly reported in a subset of sexually‐active participants).
1.11. Analysis.

Comparison 1: Transurethral microwave thermotherapy versus transurethral resection of the prostate (TURP), Outcome 11: Ejaculatory function
1.7. Minor adverse events
Based on five studies (Ahmed 1997; D'Ancona 1998; Dahlstrand 1995; Nørby 2002a; Wagrell 2002) with 397 participants, TUMT may result in little to no difference in the incidence of minor adverse events when compared to TURP at 6‐ to 12‐month follow‐up (RR 1.27, 95% CI 0.75 to 2.15; I2 = 0%, Analysis 1.12). These events primarily included urinary tract infection. The certainty of the evidence is low due to an overall high risk of bias and imprecision.
1.12. Analysis.

Comparison 1: Transurethral microwave thermotherapy versus transurethral resection of the prostate (TURP), Outcome 12: Minor adverse events
1.8. Acute urinary retention
Based on four studies (Ahmed 1997; D'Ancona 1998; Nørby 2002a; Wagrell 2002) with 343 participants, TUMT may result in an increased incidence of acute urinary retention when compared to TURP at 6‐ to 12‐month follow‐up (RR 2.61, 95% CI 1.05 to 6.47; I2 = 40%; Analysis 1.13). The certainty of the evidence is low due to an overall high risk of bias and imprecision (the incidence mostly reported in a subset of sexually‐active participants). In many cases, we highlight that participants undergoing TURP were routinely catheterized after surgery and for shorter periods of time than TUMT (see below).
1.13. Analysis.

Comparison 1: Transurethral microwave thermotherapy versus transurethral resection of the prostate (TURP), Outcome 13: Acute urinary retention
1.9. Indwelling urinary catheter
The evidence is very uncertain about the effect of TUMT on the duration of catheterization when compared to TURP. This outcome was not adequately reported across the included studies. Furthermore, one study (Floratos 2001) reported that per‐protocol all participants were catheterized for 2 to 4 days. Most of the information we found was narrative:
Ahmed 1997 reported that three participants required an indwelling catheter for 10 days to six weeks in the TUMT group and two participants for four weeks in the TURP group.
D'Ancona 1998 reported that the mean days of catheterization were 12.7 (range 6 to 35) in the TUMT group and 4.1 (range 4 to 5) in the TURP group.
Dahlstrand 1995 reported that eight participants required catheterization for less than one week in the TUMT group and two participants in the TURP group required prolonged catheterization.
Nørby 2002a reported that the median catheterization time in the TUMT group was seven days for those treated with Prostasoft v2.0 and 14 in those with Prostasoft v2.5, whereas the median in the TURP group was two days.
Wagrell 2002 reported that the mean catheterization time was 14 days (SD 8) after TUMT and 3 days (SD 4) after TURP.
The certainty of the evidence is very low, due to an overall high risk of bias, inconsistency and imprecision.
2. TUMT versus sham
Ten studies with 1287 randomized participants were included under this comparison (Abbou 1995; Albala 2002; Bdesha 1994; Blute 1996; Brehmer 1999; De Wildt 1996; Larson 1998; Nawrocki 1997; Roehrborn 1998; Venn 1995). See Table 3 for a summary of the characteristics of participants, interventions and comparisons. Refer to the Table 2 for the main outcomes.
2.1. Urologic symptom scores
Based on four studies (Bdesha 1994; Blute 1996; Larson 1998; Roehrborn 1998) with 483 participants, TUMT probably reduces urologic symptom scores measured by IPSS at three to six months when compared to sham (MD −5.40, 95% CI −6.97 to −3.84; I2 = 45%; Analysis 2.1). Similar results were obtained in two studies (Blute 1996; De Wildt 1996) with 196 participants that used the Madsen‐Iversen score (range 0 to 28) (MD −5.10, 95% CI −6.42 to −3.79; I2 = 0%; Analysis 2.2). The certainty of the evidence is moderate, due to an overall high risk of bias.
2.1. Analysis.

Comparison 2: Transurethral microwave thermotherapy versus sham treatment, Outcome 1: Urologic symptom scores (IPSS/AUA)
2.2. Analysis.

Comparison 2: Transurethral microwave thermotherapy versus sham treatment, Outcome 2: Urologic symptom scores (Madsen score)
Responder rate
Based on four studies (Abbou 1995; Bdesha 1994; De Wildt 1996; Venn 1995) with 322 participants, TUMT may cause little to no difference in the responder rate, defined as a large decrease in symptom scores at three months (RR 2.50, 95% CI 0.57 to 10.86; Analysis 2.4.1), but it may increase the responder rate at 12 months (RR 3.10, 95% CI 1.34 to 7.17, see Analysis 2.4.2). The certainty of the evidence is low, due to imprecision (few events) and overall high risk of bias.
2.4. Analysis.

Comparison 2: Transurethral microwave thermotherapy versus sham treatment, Outcome 4: Urologic symptom score (responder analysis)
Two studies were not included in the meta‐analysis, since they did not report standard deviations or exact P values:
Albala 2002 with 183 participants reported that the mean AUA score in the active treatment group was 12.4 and 17 in the control group ("statistically significant", P value not available).
Nawrocki 1997 with 78 participants reported that the mean score in the TUMT group was 9.5 (range 1 to 27) and 9.5 (range 0 to 30) in the sham group (P = 0.81).
2.2. Quality of life
Based on two studies (Larson 1998; Roehrborn 1998) with 347 participants, TUMT may result in little to no difference in quality of life at six months as measured by IPSS subscore (MD −0.95, 95% CI −1.14 to −0.77; I2 = 25%; Analysis 2.5). The certainty of the evidence is low, due to an overall high risk of bias and imprecision.
2.5. Analysis.

Comparison 2: Transurethral microwave thermotherapy versus sham treatment, Outcome 5: Quality of Life
2.3. Major adverse events
The evidence is very uncertain about the effect of TUMT on adverse events.
Most studies did not comprehensively report adverse events during their 6‐ to 12‐month follow‐up. Six studies (Abbou 1995; Albala 2002; Bdesha 1994; Brehmer 1999; Nawrocki 1997; Roehrborn 1998) with 662 participants reported that all adverse events were minor, but one participant in one study (Bdesha 1994) underwent TURP after persistent acute urinary retention. One multicenter study (De Wildt 1996) with 93 participants did not adequately describe major adverse events, but one of the reports of a single centre of the same study (n = 40) reported that one participant in the TUMT group received TURP due to persistent urinary tract retention and one participant in the sham group received TUMT due to a lesion in the verumontanum. Another study (Larson 1998) with 169 participants reported that two participants were hospitalized after TUMT due to urethral stricture and urinary tract infection. The remaining two studies (Blute 1996; Venn 1995) did not report the incidence of adverse events.
The certainty of the evidence is very low due to an overall high risk of bias and severe imprecision.
2.4. Retreatment
Based on two studies (Bdesha 1994; Brehmer 1999) with 82 participants, TUMT may reduce the incidence of retreatment at 6 to 12 months (RR 0.27, 95% CI 0.08 to 0.88; I2 = 0%; Analysis 2.6). Based on 194 retreatments per 1000 men in the sham group, this corresponds to 141 fewer (178 to 23 fewer) per 1000 men in the TUMT group. The certainty of the evidence is low, due to an overall high risk of bias and imprecision (few events).
2.6. Analysis.

Comparison 2: Transurethral microwave thermotherapy versus sham treatment, Outcome 6: Retreatment
One study (Abbou 1995) reported that 9/66 (14%) in the TUMT group, 6/31 (19%) in the sham group withdrew due to lack of improvement to seek other treatments, but they comprised either medical or surgical treatment. Another study (Larson 1998) reported that 7/42 (17%) participants in the sham group and 2/125 (2%) in the TUMT group required a subsequent therapeutic procedure or medication.
2.5. Erectile function
The evidence is very uncertain about the effect of TUMT on erectile function at 6 to 12 months.
Three studies (Bdesha 1994; Blute 1996; Roehrborn 1998) with 375 participants reported this outcome within the description of adverse events. Bdesha 1994 and Blute 1996 reported that there were normal erections and no report of sexual dysfunction respectively. Roehrborn 1998 reported that 44 (28.9%) participants in the TUMT group and one (1.4%) in the sham group suffered sexual dysfunction, including one case of impotence due to corporeal fibrosis.
The certainty of the evidence is very low, due to an overall high risk of bias and severe imprecision.
2.6. Ejaculatory function
The evidence is very uncertain about the effect of TUMT on ejaculatory function at 6 to 12 months.
Five studies (Albala 2002; Bdesha 1994; Blute 1996; Larson 1998; Roehrborn 1998) with 727 participants reported this outcome within the description of adverse events. Albala 2002, Bdesha 1994, and Blute 1996 reported that there were normal erections and no report of sexual dysfunction. Roehrborn 1998 reported that 44 (28.9%) participants in the TUMT group and one (1.4%) in the sham group suffered sexual dysfunction, including mostly participants with hematospermia and other ejaculatory abnormalities. Larson 1998 reported that five participants (4%) had a loss of ejaculate after TUMT and no cases in the sham group.
The certainty of the evidence is very low, due to an overall high risk of bias and severe imprecision.
2.7. Minor adverse events
Most studies did not comprehensively report adverse events during their 6‐ to 12‐month follow‐up. Based on three studies (Abbou 1995; Blute 1996; Larson 1998) with 378 participants, TUMT may increase the incidence of minor adverse events compared to sham (RR 1.42, 95% CI 1.00 to 2.01; I2 = 31%; Analysis 2.7). The most commonly‐described adverse events were: hematuria, urethral bleeding, acute urinary retention and urinary tract infection. Six studies were not included in the meta‐analysis, since they did not report the global incidence of minor adverse events, but the narrative description of the findings are similar to the main analysis of this outcome.
2.7. Analysis.

Comparison 2: Transurethral microwave thermotherapy versus sham treatment, Outcome 7: Minor adverse events
Albala 2002 (200 participants) reported that both the active treatment arm (6.6%) and the sham arm (4.8%) suffered from dysuria. Gross hematuria (9.1%) and bladder spasm (4.1%) were only reported in the active treatment arm.
Bdesha 1994 (40 participants) reported that 65% of the active treatment and 60% of the sham‐treated participants experienced bladder spasm, while 82% and 83% respectively reported mild or moderate discomfort during treatment. Thirty percent of all participants reported transient dysuria, urgency, frequency or bloodstained urethral discharge lasting up to 48 hours (no disaggregated data).
Brehmer 1999 (44 participants) reported that two participants contracted bacterial cystitis (no disaggregated data).
De Wildt 1996 (93 participants) reported that most participants had some hematuria for up to three days. However, one of the reports of a single centre of the same study (n = 40) said that five participants required treatment for urinary tract infection in the TUMT group and one in the sham group.
Nawrocki 1997(120 participants) reported that all participants treated by standard or simulated TUMT experienced some hematuria and dysuria following treatment, and that these symptoms were self‐limiting and none required specific treatment.
Roehrborn 1998 (220 participants) reported that the main difference in minor adverse events was pain on the day of the treatment (87.8% of the actively treated and 65.8% of sham‐treated participants). Others included: bladder spasms, urethral bleeding, and hematuria and other transient adverse events that were distributed similarly across groups.
The remaining study (Venn 1995) did not report the incidence of adverse events. The certainty of the evidence is low, due to an overall high risk of bias and imprecision.
2.8. Acute urinary retention
Based on eight studies (Abbou 1995; Albala 2002; Bdesha 1994; Blute 1996; De Wildt 1996; Larson 1998; Nawrocki 1997; Roehrborn 1998) with 995 participants, TUMT probably results in a large increase in the incidence of acute urinary retention at 6‐ to 12‐month follow‐up (RR 9.02, 95% CI 3.31 to 24.63; I2 = 0%; Analysis 2.8). Based on six cases per 1000 men in the sham group, this corresponds to 54 more (20 to 148 more) per 1000 men in the TUMT group. The certainty of the evidence is moderate, due to high risk of bias.
2.8. Analysis.

Comparison 2: Transurethral microwave thermotherapy versus sham treatment, Outcome 8: Acute urinary retention
2.9. Indwelling urinary catheter
This outcome was not adequately reported across the included studies. Four studies reported that participants that suffered from acute urinary retention (see section above) were catheterized for one to six weeks (Abbou 1995; Bdesha 1994; De Wildt 1996; Nawrocki 1997). In some studies (Albala 2002; Larson 1998; Roehrborn 1998), catheterization after each procedure was routinely maintained for two to four days. One study (Brehmer 1999) reported that four participants were catheterized for four days (no disaggregated data by group).
Secondary analyses
Subgroup analysis based on age
We were unable to conduct this analysis due to the lack of data.
Subgroup analysis based on prostate volume
We were unable to conduct this analysis due to the lack of data.
Subgroup analysis based on baseline severity of LUTS
Our predefined subgroup analysis suggests that participants with more severe symptoms (MD −5.07, 95% CI −5.97 to −4.18) may experience less symptom improvement compared to those with moderate symptoms at baseline (MD −9.10, 95% CI −12.83 to −5.37, test for subgroup differences: P = 0.04, I2 = 76.4%; Analysis 2.3). There were insufficient data to perform these subgroup analyses on other primary outcomes.
2.3. Analysis.

Comparison 2: Transurethral microwave thermotherapy versus sham treatment, Outcome 3: Urologic symptom scores (IPSS/AUA) ‐ subgroup (severity)
3. Other comparisons
We found no trials for the following comparisons:
TUMT versus CRFWVT
TUMT versus PUL
TUMT versus PAE
TUMT versus TIND
Discussion
Summary of main results
We found evidence for our two main comparisons.
TUMT versus TURP
Based on data from six studies with 414 participants, when compared to TURP, TUMT probably results in little to no difference in urologic symptom scores in the short term, but due to the lack of any eligible study with follow‐up longer than 12 months, we are uncertain about the long‐term effects. There may be little to no difference in minor adverse events, quality of life or erectile function between these interventions. TUMT likely results in significantly fewer major adverse events and less ejaculatory dysfunction compared to TURP. TUMT, however, likely results in a large increase in the need for retreatment (usually by repeated TUMT or TURP) and acute urinary retention. The duration of indwelling catheterization was not adequately reported across studies.
TUMT versus sham
Based on data from 10 studies with 679 participants, we found that, compared to sham, TUMT probably reduces urologic symptoms scores at short‐term follow‐up and may result in a higher responder rate at long‐term follow‐up. TUMT may also reduce the need for retreatment, but it may cause little to no difference in the quality of life. We are very uncertain of the effects on major adverse events, or on erectile and ejaculatory functions. TUMT probably results in a large increase in the incidence of acute urinary retention. The incidence of minor adverse events and the duration of indwelling catheterization was not adequately reported across studies.
Overall completeness and applicability of evidence
The studies did not consistently define or report on adverse events, particularly dysuria, hematuria, and sexual dysfunction, and our estimates for these complications may be unreliable. Few studies evaluated the quality of life. Although studies usually reported the occurrence of urinary retention, they did not consistently or uniformly indicate its duration or the use of catheterization. One important complication that was not reported in the clinical trial literature was thermal injury. On 11 October 2000, the United States Food and Drug Administration (FDA) published a Public Health Notification because they had received 16 reports of severe thermal injury associated with TUMT, including 10 resulting in fistula formation and six resulting in tissue damage to the penis or urethra (Henney 2000). The FDA noted that the injuries could take hours or days to develop. Although the FDA recommended several corrective measures for physicians, they considered TUMT to be safe and effective based on the performance of over 25,000 procedures.
The current American Urological Association guidelines for the management of LUTS considered TUMT to be an appropriate alternative for treating men with lower urinary tract symptoms with small‐ to average‐size prostate (Parsons 2020), with the warning that patients should be advised that surgical retreatment rates are higher compared to TURP, which corresponds with the findings of our review. The Canadian guidelines considered TUMT an optional treatment for men with moderate symptoms, with similar considerations about retreatment (Nickel 2018). The European Association of Urology does not list TUMT as one of their alternatives for managing LUTS (EAU 2021).
Quality of the evidence
The certainty of the evidence was primarily affected by:
High risk of bias across studies: most studies did not report the randomization process adequately, and for the TUMT versus TURP comparison none of the included studies was blinded.
Imprecision: details on ejaculatory and erectile function were only reported as binary outcomes in a subset of sexually‐active participants.
Furthermore, our interpretation of the retreatment data was cautious, since this was not consistently reported across studies. In some cases, it was described in the initial flow of participants across the studies, in some studies as a comment about follow‐up, and in other cases within adverse events. The urinary catheterization data were inconsistently reported, since some studies included them as a standard procedure, and some measured them selectively.
Potential biases in the review process
This update changed the original protocol and replaced it with current methods applied to a suite of other reviews by the Urology Review Group on lower urinary tract symptoms due to benign prostatic hyperplasia (Hwang 2019; Jung 2019; Jung 2020; Kang 2020). This allowed us to secure comparability across interventions and to include the findings of this review in our upcoming network meta‐analysis (Franco 2020).
Considering that review methods have improved over time, including the details of the search strategy, we decided to run our searches from inception using the original inclusion criteria but excluding the comparison to alpha‐blockers. While our search identified more references for the included studies in the previous review, it failed to identify the included studies Abbou 1995 and Brehmer 1999. Furthermore, we identified the citations of some additional reports of the included studies, including long‐term data on one of the studies, but we were unable to retrieve the full text through different means, including the use of Task Exchange (Albala 2000a; Dahlstrand 1994; Dahlstrand 1997; Dahlstrand 1998; Roehrborn 1997). We also identified another randomized study that was cited in the Background of the included studies (Devonec 1994) but again we were unable to retrieve the full text.
Finally, reporting on some of the outcomes was scattered and not thoroughly detailed. For some outcomes, including adverse events, retreatment, acute urinary retention, ejaculatory and erectile function, we had to interpret the data available in the flow of participants and in the section describing “complications.” It is unclear whether the studies reported all events or only those they considered relevant, especially with a lack of a prespecified protocol.
Agreements and disagreements with other studies or reviews
The previous version of this Cochrane Review yielded similar results for the global effects of TUMT in relation to sham and TURP (Hoffman 2012). The main difference from the previous version of the review is that we pooled the data for more outcomes in each comparison, with additional critical outcomes in the summary of findings tables. This provided us with a greater understanding of the differences between TURP and TUMT. In this version, we favor an interpretation of similar urinary symptoms scores at short‐term follow‐up, considering that long‐term data from selected studies provided very low‐certainty evidence to highlight substantial differences between these interventions. We also found important differences in the incidence of major adverse events and the incidence of retrograde ejaculation between these interventions, favoring TUMT.
We found a few additional systematic reviews on this topic. A health technology assessment from Sweden assessed the average IPSS score, and concluded that TUMT was inferior to TURP in the improvement of symptoms, which does not take into account the confidence interval and minimally important differences (SBU 2011). Furthermore, the authors stated that they could not determine the differences in major adverse events, as we found in our review, which could be explained by the lack of grouping of serious events. Nevertheless, the findings related to retreatment were similar. Another systematic review reported similar results for urinary symptoms and retreatment, but highlighted the lower incidence of serious adverse events with TURP than with TUMT (Barry Delongchamps 2012). They state that the rate of retreatment for TUMT may vary from 20% to 80% (focusing on observational data), but at the same time highlight that the rate of retreatment is lower in long‐term randomized trials such as the one included in our review (Wagrell 2002). Finally, two systematic reviews focusing on sexual outcomes reported a lower incidence of sexual adverse events (especially retrograde ejaculation) for men undergoing TUMT compared to TURP, which agrees with our findings (Frieben 2010; Marra 2016). None of these studies followed Cochrane methods for high‐quality reviews.
Authors' conclusions
Implications for practice.
TUMT provides a similar reduction in urinary symptoms compared to the standard treatment (TURP), with fewer major adverse events and fewer cases of ejaculatory dysfunction at short‐term follow‐up. However, TUMT probably results in a large increase in retreatment rates. Most of the evidence is short‐term and from studies with a high risk of bias. Patients' values and preferences, their comorbidities and the effects of other available minimally‐invasive procedures, among other factors, can guide clinicians when choosing the optimal treatment for this condition.
Implications for research.
Relatively few patients have been studied in controlled clinical trials of TUMT, and there is a paucity of research on this procedure in the last 20 years. Further studies with better reporting, using randomized treatment allocation, larger sample sizes, and comprehensive measures of relevant outcomes, including adverse events, are still needed to better define the role of TUMT techniques for treating lower urinary tract symptoms in men with benign prostatic hyperplasia. With the emergence of newer minimally‐invasive treatments, head‐to‐head comparisons between them could clarify their relative effectiveness.
What's new
| Date | Event | Description |
|---|---|---|
| 31 May 2021 | New search has been performed | The review was updated following the latest methodological standards by a new author team. Minor changes in conclusions. |
| 31 May 2021 | New citation required but conclusions have not changed | The review was updated following the latest methodological standards by a new author team. Minor changes in conclusions. |
History
Protocol first published: Issue 2, 2003 Review first published: Issue 4, 2007
| Date | Event | Description |
|---|---|---|
| 25 July 2012 | New citation required but conclusions have not changed | No new conclusions |
| 14 January 2012 | New search has been performed | Updated |
| 3 June 2008 | Amended | Converted to new review format. |
| 21 August 2007 | New citation required and conclusions have changed | Substantive amendment |
Notes
We have based parts of the Methods section of this review on a standard template developed by the Cochrane Metabolic and Endocrine Disorders Group, which has been modified and adapted for use by Cochrane Urology. General concepts on benign prostatic hyperplasia and review methods have been adapted from one of the reviews of the suite on this topic (Hwang 2019).
Acknowledgements
Juan Víctor Ariel Franco is a PhD candidate in the Programme of Methodology of Biomedical Research and Public Health, Universitat Autònoma de Barcelona (Spain).
The authors acknowledge the previous authors and contributors to the first versions of the review: Richard M Homan, Manoj Monga, Sean P Elliott, Roderick MacDonald, Jens Langsjoen, James Tacklind, and Timothy J Wilt.
The authors would like to thank several peer reviewers who provided invaluable feedback that has improved the review. These include: Deepak Agarwal, Kymora Scotland, and Armin Secker. Additional reviewers chose not to be acknowledged by name; we nevertheless appreciate their critical input.
Appendices
Appendix 1. Search strategies
| CENTRAL |
| #1 MeSH descriptor: [Prostatic Hyperplasia] explode all trees #2 MeSH descriptor: [Prostatism] explode all trees #3 MeSH descriptor: [Urinary Bladder Neck Obstruction] explode all trees #4 (Prostat* near/3 hyperplasia*):ti,ab,kw #5 (Prostat* near/3 hypertroph*):ti,ab,kw #6 (Prostat* near/3 adenoma*):ti,ab,kw #7 (BPH OR BPO OR BPE):ti,ab,kw #8 (prostat* near/3 enlarg*):ti,ab,kw #9 (Prostatism):ti,ab,kw #10 (Bladder* near/3 obstruct*):ti,ab,kw #11 (BOO):ti,ab,kw #12 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 #13 MeSH descriptor: [Microwaves] explode all trees #14 (microwave*):ti,ab,kw #15 #13 OR #14 #16 #12 AND #15 |
| MEDLINE (Ovid) |
| #1 exp Prostatic Hyperplasia/ #2 exp Prostatism/ #3 exp Urinary Bladder Neck Obstruction/ #4 (Prostat* adj3 hyperplasia*).tw. #5 (Prostat* adj3 hypertroph*).tw. #6 (Prostat* adj3 adenoma*).tw. #7 (BPH or BPO or BPE).tw. #8 (prostat* adj3 enlarg*).tw. #9 Prostatism.tw. #10 (Bladder* adj3 obstruct*).tw. #11 BOO.tw. #12 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 #13 exp Microwaves/ #14 microwave*.tw. #15 #13 OR #14 #16 #12 AND #15 |
| Embase (Elsevier) |
| #1. 'prostate hypertrophy'/exp #2. 'prostatism'/exp #3. 'bladder obstruction'/exp #4. (prostat* NEAR/3 hyperplasia*):ti,ab,kw #5. (prostat* NEAR/3 hypertroph*):ti,ab,kw #6. (prostat* NEAR/3 adenoma*):ti,ab,kw #7. bph:ti,ab,kw OR bpo:ti,ab,kw OR bpe:ti,ab,kw #8. (prostat* NEAR/3 enlarg*):ti,ab,kw #9. prostatism:kw,ti,ab #10. (bladder* NEAR/3 obstruct*):ti,ab,kw #11. boo:ti,ab,kw #12. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 #13. 'microwave thermotherapy'/exp #14. microwave*:ti,ab,kw #15. 'transurethral microwave thermotherapy'/exp #16. #13 OR #14 OR #15 #17. #12 AND #16 |
| Scopus (Elsevier) |
| TITLE‐ABS‐KEY ( "Prostatic Hyperplasia" OR prostat* OR "Urinary Bladder Neck Obstruction" ) AND TITLE‐ABS‐KEY ( microwave* ) |
| Web of Sceince (Clarivate Analytics) |
| #1 TI=("Prostatic Hyperplasia" OR Prostat* OR "Urinary Bladder Neck Obstruction") #2 TS=("Prostatic Hyperplasia" OR Prostat* OR "Urinary Bladder Neck Obstruction") #3 #1 OR #2 #4 TS= microwave* OR TI=microwave* #5 #3 AND #4 |
| LILACS (Bireme) |
| (tw:(“prostatic hyperplasia” OR “hiperplasia prostática” OR prostat* OR “urinary bladder neck obstruction” OR “obstrucción del cuello de la vejiga urinaria” OR “obstrução do colo da bexiga urinária” OR bph OR bpo OR bpe) AND tw:( microondas OR microwaves OR micro‐ondas)) |
Appendix 2. Searches in conferences
| Conference | Website (last access February 2021) |
| American Urology Association 2020 | www.aua2020.org/abstracts |
| American Urology Association 2019 | www.aua2019.org/abstracts |
| American Urology Association 2018 | www.aua2018.org/abstracts |
| International Continence Society 2020 | www.ics.org/2020/ |
| International Continence Society 2019 | www.ics.org/2019/ |
| International Continence Society 2018 | www.ics.org/2018/ |
| European Association of Urology 2020 | resource‐centre.uroweb.org/resource‐centre/eau20v |
| European Association of Urology 2019 | urosource.uroweb.org/resource‐centre/eau19 |
| European Association of Urology 2018 | urosource.uroweb.org/resource‐centre/eau18 |
Appendix 3. Previous version of the methods section (2012)
Types of studies
Randomized controlled trials, with or without blinding, of at least three months duration and a minimum of 10 participants in each treatment arm.
Types of participants
Men with symptomatic BPH as determined by elevated urinary symptom scores with or without documented decreased urinary flow rates.
Types of interventions
Microwave thermotherapy techniques that were reviewed included transurethral thermotherapy and transrectal thermotherapy. Control interventions could have included sham thermotherapy, transurethral resection of the prostate (TURP), open prostatectomy, laser prostatectomy, transurethral incision of the prostate, pharmacologic therapy, watchful waiting, electrovaporization of the prostate, prostate stents, radiofrequency transurethral needle ablation, or high‐intensity focused ultrasound.
Types of outcome measures
The primary outcome was the efficacy of microwave thermotherapy in improving urinary tract symptoms based on changes in urologic symptom scale scores (American Urological Association (AUA) Symptom Index, International Prostate Symptom Score (IPSS), Madsen‐Iversen, Boyarsky). Secondary outcomes included mean and peak urinary flow, post‐void residual, prostate volume, and quality of life. Measures of mortality and morbidity included perioperative death, bleeding requiring transfusion, urinary tract infections, epididymitis or orchitis, dysuria, clot retention, urinary retention, erectile dysfunction, retrograde ejaculation, urethral and bladder neck strictures, urinary incontinence, transurethral resection syndrome, and the need for retreatment either surgical or pharmacologic. Hospital length‐of‐stay and catheter duration were also evaluated. Baseline covariates included age, race or ethnicity, prostate size, residual volume, and prostate‐specific antigen (PSA) levels.
Search methods for identification of studies
The search began with The Cochrane Library of randomized trials. MEDLINE and EMBASE were searched from 1989 through July 2011 using validated Cochrane Collaboration strategies for identifying randomized controlled trials. Search terms included prostatectomy, prostatic hyperplasia/surgery, and microwave thermotherapy. Additional studies were identified from bibliographies of retrieved articles and reviews, Science Citation Index, expert trialists, microwave manufacturers, handsearching of the Journal of Urology and also Urology, systematic reviews, and technical reviews.
Selection of studies
Two independent review authors evaluated titles and abstracts of the electronic search results. From the results of the electronic searches, bibliography searches, handsearches, and contact with experts and manufacturers, two review authors independently selected trials that met previously defined inclusion criteria. Trials selected by at least one review author were retrieved.
Data extraction and management
Two review authors then independently abstracted study characteristics and outcomes, including information on study design, participant characteristics, interventions, follow‐up, treatment outcomes, and adverse events. Differences were resolved by discussion among the review authors or using an independent arbitrator. Reasons for study exclusion were documented.
Assessment of risk of bias in included studies
As a measure of overall methodologic study quality, and bias, we assessed scales and criteria developed by Schulz and The Cochrane Collaboration (Higgins 2011; Schulz 1995). The seven criteria addressed were:
selection bias I (Was there an articulated rule for allocating interventions based on chance?);
selection bias II (Was there any foreknowledge of the allocation of interventions by anyone?);
blinding bias I (During the course of the trial were study participants and personnel blinded to the knowledge of who received which intervention?);
blinding bias II (Were the outcome assessors blinded to who received the intervention and who did not?);
attrition bias (Did the trial assess all patients, or account for those not assessed?);
reporting bias (Were outcomes selectively reported?);
other bias (Were arms assessed differently?).
Each criterion was answered by 'low risk', 'unclear risk', and 'high risk', and summarized here (Figure 2). For the main therapeutic efficacy outcomes, we also assessed the quality of evidence in the 'Summary of findings' table using GRADEpro (GRADEpro GDT).
Measures of treatment effect
We calculated relative risks (RR) and absolute risk differences (RD) with associated 95% confidence intervals (CI) for dichotomous data using an intention‐to‐treat principle (we assumed that people who dropped out had negative outcomes, with the exception of death). Weighted mean differences (WMD) with 95% CI were calculated for continuous data.
Assessment of heterogeneity
We used a fixed‐effect model unless heterogeneity was present. Heterogeneity was defined as I2 > 50%.
Data and analyses
Comparison 1. Transurethral microwave thermotherapy versus transurethral resection of the prostate (TURP).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Urologic symptoms score (IPSS) | 4 | 306 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐0.03, 2.03] |
| 1.2 Urologic symptoms score (Madsen‐Iversen) | 2 | 108 | Mean Difference (IV, Random, 95% CI) | 1.59 [0.69, 2.48] |
| 1.3 Urologic symptoms score (SMD) ‐ long‐term | 3 | 187 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [0.03, 0.62] |
| 1.4 Urologic symptoms score (IPSS) ‐ subgroup analysis (severity) | 4 | 306 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐0.03, 2.03] |
| 1.4.1 Baseline IPSS score < 19 points | 2 | 104 | Mean Difference (IV, Random, 95% CI) | 0.95 [‐0.51, 2.40] |
| 1.4.2 Baseline IPSS score > 19 points | 2 | 202 | Mean Difference (IV, Random, 95% CI) | 1.17 [‐1.34, 3.68] |
| 1.5 Quality of life | 1 | 136 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.67, 0.47] |
| 1.6 Quality of life ‐ long term | 1 | 97 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.46, 0.46] |
| 1.7 Major adverse events | 6 | 525 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.09, 0.43] |
| 1.8 Major adverse events ‐ subgroup analysis (severity) | 5 | 456 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.10, 0.50] |
| 1.8.1 Baseline IPSS score < 19 points | 2 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.02, 0.61] |
| 1.8.2 Baseline IPSS score > 19 points | 3 | 344 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.10, 0.78] |
| 1.9 Retreatment | 5 | 463 | Risk Ratio (M‐H, Random, 95% CI) | 7.07 [1.94, 25.82] |
| 1.10 Erectile function | 5 | 337 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.24, 1.63] |
| 1.11 Ejaculatory function | 4 | 241 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.24, 0.53] |
| 1.12 Minor adverse events | 5 | 397 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.75, 2.15] |
| 1.13 Acute urinary retention | 4 | 343 | Risk Ratio (M‐H, Random, 95% CI) | 2.61 [1.05, 6.47] |
1.4. Analysis.

Comparison 1: Transurethral microwave thermotherapy versus transurethral resection of the prostate (TURP), Outcome 4: Urologic symptoms score (IPSS) ‐ subgroup analysis (severity)
1.8. Analysis.

Comparison 1: Transurethral microwave thermotherapy versus transurethral resection of the prostate (TURP), Outcome 8: Major adverse events ‐ subgroup analysis (severity)
Comparison 2. Transurethral microwave thermotherapy versus sham treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Urologic symptom scores (IPSS/AUA) | 4 | 483 | Mean Difference (IV, Random, 95% CI) | ‐5.40 [‐6.97, ‐3.84] |
| 2.2 Urologic symptom scores (Madsen score) | 2 | 196 | Mean Difference (IV, Random, 95% CI) | ‐5.10 [‐6.42, ‐3.79] |
| 2.3 Urologic symptom scores (IPSS/AUA) ‐ subgroup (severity) | 4 | 483 | Mean Difference (IV, Random, 95% CI) | ‐5.40 [‐6.97, ‐3.84] |
| 2.3.1 Baseline IPSS score > 19 points | 3 | 443 | Mean Difference (IV, Random, 95% CI) | ‐5.07 [‐5.97, ‐4.18] |
| 2.3.2 Baseline IPSS score < 19 points | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐9.10 [‐12.83, ‐5.37] |
| 2.4 Urologic symptom score (responder analysis) | 4 | 322 | Risk Ratio (M‐H, Random, 95% CI) | 2.60 [0.82, 8.24] |
| 2.4.1 3 to 6‐month follow‐up | 3 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 2.50 [0.57, 10.86] |
| 2.4.2 12‐month follow‐up | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 3.10 [1.34, 7.17] |
| 2.5 Quality of Life | 2 | 347 | Mean Difference (IV, Fixed, 95% CI) | ‐0.95 [‐1.14, ‐0.77] |
| 2.6 Retreatment | 2 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.08, 0.88] |
| 2.7 Minor adverse events | 3 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.00, 2.01] |
| 2.8 Acute urinary retention | 8 | 995 | Risk Ratio (M‐H, Random, 95% CI) | 9.02 [3.31, 24.63] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abbou 1995.
| Study characteristics | ||
| Methods |
Study design: prospective, randomized study. Study dates: study dates not available Setting: outpatient, multicenter, national Country: France |
|
| Participants |
Inclusion criteria: male participants:
Exclusion criteria: men with
Total number of participants randomized: 200 Group 1: n = 66 Transurethral route Hyperthermia
Group 2: n = 31 transurethral sham
Group 3: n = 65 Transrectal route hyperthermia
Group 4: n = 38 transrectal sham
|
|
| Interventions |
Group 1 (n = 66) TUMT 3 devices were used for transurethral treatment (Thermex II, Technorex, Israel: Prostcare, Brucker Spectrospin, France; BSD‐50. BSD Medical Corp, USA). Prostate temperature was monitored by an integrated microwave generator and controlled in each device through a fibreoptic temperature monitor. All devices were used according to the manufacturer's instructions to deliver a temperature compatible with hyperthermia treatment (45 °C). Treatment was delivered in 1 session of 1 to 3 hs (depending on the device used) Group 2 (n = 31) Sham TUMT: Sham treatment consisted of a single session with the temperature maintained at 37 °C Group 3 (n = 65) Transrectal route hyperthermia: 3 devices were used for transrectal treatment (Prostathermer system, Biodan Medical Systems, Israel: Prostcare, Brucker Spectrospin, France: Primus, Tecnomatix Medical, Belgium). Prostate temperature was monitored by an integrated microwave generator and controlled in each device through a fibreoptic temperature monitor. All devices were used according to the manufacturer's instructions to deliver a temperature compatible with hyperthermia treatment (45 °C). Treatment was delivered in 6 sessions of 1 to 3hs (depending on the device used) for each session over 3 weeks Group 4 (n = 38) transrectal sham: sham treatment consisted of a single session with the temperature maintained at 37 °C Co‐interventions: not reported |
|
| Outcomes |
Urologic symptom scores How measured: Madsen score. Additionally, responders were participants showing excellent, good or moderate responses according to each of the criteria analyzed separately (Madsen score decrease > 30%; a PFR > 10 mL/s with a PFR increase > 30%) Time points measured: baseline, 3, 6 and 12 months Time points reported: baseline and 12 months Subgroups: none Retreatment How measured: number of participants with medical or surgical procedure (reported the numbers separately for each) Time points measured: during treatment and 1 to 4 weeks after treatment (early post‐treatment complications) Time points reported: during treatment and 1 to 4 weeks after treatment (early post‐treatment complications) Subgroups: none Major and minor adverse event/acute urinary retention How measured: number of participants with urethral bleeding, pain and urinary tract infection, acute urinary retention Time points measured: during treatment and 1 to 4 weeks after treatment (early post‐treatment complications) Time points reported: during treatment and 1 to 4 weeks after treatment (early post‐treatment complications) Subgroups: none Relevant outcomes not reported in this study
|
|
| Funding sources | This study was supported by a grant from the Comite d’Evaluation et de Diffusion des Innovations Technologiques (CEDIT), Assistance Publique‐Hopitaux de Paris. Devices were lent by the following companies: Biodan, Brucker, BSD, Direx and Tecnomatix | |
| Declarations of interest | Not available | |
| Notes | We only included transurethral active and sham groups for the purpose of this review. No contact information available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was stratified by the investigating centre and by approach (transrectal or transurethral), and was performed using permutation tables such that equal sample sizes were obtained for each type of approach, device and sham group” Comment: The investigators describe a random component in the sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Patients were randomly allocated to a treatment in a single treatment centre after verification of the inclusion criteria.” Comment: Insufficient information to permit judgment of ‘Low risk’ or ‘High risk’. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Patients were not informed of their treatment, nor was the investigator who enrolled the patients.” Comment: Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: “Patients were not informed of their treatment, nor was the investigator who enrolled the patients.” Comment: Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: “Patients were not informed of their treatment, nor was the investigator who enrolled the patients.” Comment: Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There is an imbalance in numbers or reasons for missing data across intervention groups and potentially inappropriate application of simple imputation. Quote: “Patients lost to follow‐up were classified according to maximum bias (in the sham groups as 'responders' and in the hyperthermia groups as 'non‐responders').” Comment: Missing data only in group 2. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Insufficient information to permit judgment of ‘Low risk’ or ‘High risk’ |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Ahmed 1997.
| Study characteristics | ||
| Methods |
Study design: prospective, randomized parallel study Study dates: study dates not available Setting: outpatient, single‐centre, national Country: United Kingdom |
|
| Participants |
Inclusion criteria: men with:
Exclusion criteria: men with:
Total number of participants randomized: 60 Group 1: n = 30 transurethral microwave thermotherapy (TUMT)
Group 2: n = 30 transurethral resection of the prostate (TURP)
|
|
| Interventions |
Group 1 (n = 30): TUMT Done by a single operator using the Prostatron treatment catheter using the Prostasoft software (TechnoMed, Lyon, France) in a single 60‐min session under topical anesthesia with Instillagel(r) (FarcoPharma GmBH, Cologne, Germany) Group 2 (n = 30): TURP Performed on the routine operating lists by a surgeon of Senior Registrar grade or above using a standard technique. No post‐operative irrigation was used and all the resected tissue was submitted for histological examination. The urethral catheter was removed 3 or 4 days after surgery Co‐interventions: “Intramuscular gentamicin (80 mg) was given before the treatment and oral trimethoprim (200 mg twice daily) was continued for 5 days. The participants were followed up at 6 weeks, 3 and 6 months, with a detailed evaluation performed at the last assessment.” |
|
| Outcomes |
Urologic symptom scores How measured: AUA symptom score Time points measured: baseline, 6 weeks, 3 and 6 months Time points reported: not reported (probably 6 months) Subgroups: none Indwelling urinary catheter/acute urinary retention How measured: number of participants requiring an indwelling catheter after treatment due to acute urinary retention Time points measured: 6 weeks, 3 and 6 months Time points reported: not reported Subgroups: none Major adverse event How measured: number of participants requiring blood transfusions after treatment. Time points measured: not reported Time points reported: not reported Subgroups: none Minor adverse event / erectile function / ejaculatory function How measured: number of participants developing urinary tract infections or meatal narrowing that required dilatation. Adverse events related to erectile function and ejaculation are described under adverse events Time points measured: not reported Time points reported: not reported Subgroups: none Relevant outcomes not reported in this study
|
|
| Funding sources | Not available | |
| Declarations of interest | Not available | |
| Notes | No contact information available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “[...] patients were randomized to each treatment by selecting a sealed envelope. [...] Patients failing to complete treatment or return for follow‐up were substituted.” Comment: Insufficient information to permit judgment of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | High risk | Quote: “[...] patients were randomized to each treatment by selecting a sealed envelope. [...] Patients failing to complete treatment or return for follow‐up were substituted.” Comment: Whereas envelopes might be sealed, substitution might indicate tampering of allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | While blinding was not mentioned, the interventions were visibly different (surgery versus outpatient procedure). |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | While blinding was not mentioned, the interventions were visibly different (surgery versus outpatient procedure). The objective outcomes were unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | While blinding was not mentioned, the interventions were visibly different (surgery versus outpatient procedure). The subjective outcomes were likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Due to “substitution” noted above, the number of participants with missing outcome data was not provided. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Insufficient information to permit judgment of ‘Low risk’ or ‘High risk’. |
| Other bias | Low risk | No other sources of bias were detected. |
Albala 2002.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomized trial Study dates: study dates not available Setting: outpatient/inpatient – national/multicenter Country: USA |
|
| Participants |
Inclusion criteria:
Exclusion criteria: none described Total number of participants randomly assigned: 190 Group 1: 125 (TUMT)
Group 2: 65 (Sham)
All participants were men |
|
| Interventions |
Group 1 (n = 125): TUMT The TherMatrx TMx‐2000 device with the RX‐200 prostate applicator was used for heating and monitoring (with 2 thermo‐sensor tracks on the surface of the catheter). The RX‐200 was inserted, balloon inflated, and a drainage lumen connected to a collection bag. The length from the bladder neck to the verumontanum was measured by ultrasound. Temperature reached a peak of 50 ºC to 55 ºC with a monitoring of rectal temperature (< 42.5 ºC). A Foley catheter inserted into the bladder was left in place from 2 to 4 days Group 2 (n = 65): Sham Participants underwent placement of the microwave catheter for the treatment period without energy delivery and received the same post‐treatment care as the active‐treatment participants Co‐interventions: ketorolac 10 mg, narcotic agents, lorazepam 2 mg before treatment. Lidocaine jelly was applied to the urethra for 15 minutes Alpha‐blockers were not permitted |
|
| Outcomes |
Urologic symptoms score How measured: AUA‐SI score Time points measured: baseline, 1, 3, 6, 9 and 12 months Time points reported: baseline, 3, 6, 12 months (for Group 1), baseline and 3 months (for Group 2) Subgroups: none Major and minor adverse event / ejaculatory function / acute urinary retention How measured: major and minor adverse events, including ejaculatory adverse events and recatheterization; all described narratively Time points measured: not reported Time points reported: at 3 months Relevant outcomes not reported in this study:
|
|
| Funding sources | Not available | |
| Declarations of interest | Not available | |
| Notes | Patients were unblinded at 3‐month follow‐up and then crossed over to active treatment in a second phase. Only the first phase was included in our review. Other measured outcomes: AUASI bother, urinary flow rates, PUR, PSA and TRUS. The 5‐year follow‐up study (presented at a conference) only included data on the active treatment arm. Contact information Dr. Albala: albaloo2@mc.duke.edu |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 2:1 randomizations. No other information available. Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. We wrote to study authors. |
| Allocation concealment (selection bias) | Unclear risk | No information available. Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. We wrote to study authors. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “All patients were blinded as to their group assignment, and outcome analysis was performed by individuals blinded to the randomisation.” Comment: it is unclear whether personnel were blinded. We wrote to study authors. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: “All patients were blinded as to their group assignment, and outcome analysis was performed by individuals blinded to the randomisation.” Comment: it is unclear whether personnel were blinded, but the outcomes are unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: "All patients were blinded as to their group assignment, and outcome analysis was performed by individuals blinded to the randomisation." Comment: participants (outcome assessors of subjective outcomes) were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Urologic symptom score: outcome data available for 125/125 participants in Group 1 and 63/65 participants in Group 2. Presumably similar attrition in other outcomes. |
| Selective reporting (reporting bias) | High risk | Protocol not available ‐ outcome data (urologic symptom score) was not available for Group 2 at time points beyond 3 months. Quality‐of‐life data were not available for Group 2. We wrote to study authors. |
| Other bias | Low risk | No other sources of bias were identified. |
Bdesha 1994.
| Study characteristics | ||
| Methods |
Study design: prospective, randomized parallel study Study dates: study dates not available Setting: outpatient, single‐center, national Country: United Kingdom |
|
| Participants |
Inclusion criteria: men with:
Exclusion criteria: men with:
Total number of participants randomized: 40 Group 1: n = 22 microwave treatment
Group 2: n = 18 sham treatment
|
|
| Interventions |
Group 1 (n = 22):TUMT LEO Microthermer was used in all participants in a single active 90‐min treatment. This machine delivers a maximum power output of 20 watts at 915 MHz. and incorporates an automatic power cut‐off, which operates if the rectal temperature increases to > 42.5 ºC Group 2 (n = 18) Sham: Same procedure, participants received 90‐min sham treatment with no power delivered. Participants received a heating pad to simulate hyperthermia Co‐interventions: topical lidocaine gel was used alongside flexible cystoscopy to exclude a coexisting lower urinary tract pathological condition and to measure the prostate |
|
| Outcomes |
Urologic symptom scores How measured: AUA symptom score and WHO symptom score. Also as the proportion of participants with a decrease of 50% or more in symptom scores. Time points measured: baseline and 3 months Time points reported: baseline and 3 months Subgroups: none Minor and major adverse events / erectile function / ejaculatory function How measured: Narratively (including sexual adverse events) Time points measured: not reported Time points reported: not reported Subgroups: none Acute urinary retention How measured: narratively Time points measured: not reported Time points reported: not reported Subgroups: none Retreatment How measured: narratively (TURP after sham) Time points measured: not reported Time points reported: not reported Subgroups: none Relevant outcomes not reported in this study
|
|
| Funding sources | Not available | |
| Declarations of interest | Not available | |
| Notes | Study unblinded with cross‐over at 3 months and follow‐up to 1 year. 16 participants in the sham group were offered active treatment at 3 months (this was not considered retreatment). No contact information available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available. Insufficient information to permit judgment of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | The study describes only "sealed envelope." Insufficient information to permit judgment of ‘Low risk’ or ‘High risk’. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study. Participants and study personnel were blinded. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Double‐blind study. Participants and study personnel were blinded. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Double‐blind study. Participants and study personnel were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2 participants (10%) in the sham group were lost at follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Insufficient information to permit judgment of ‘Low risk’ or ‘High risk’. |
| Other bias | Low risk | No other sources of bias were identified. |
Blute 1996.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomized trial Study dates: study dates not available Setting: outpatient Country: USA |
|
| Participants |
Inclusion criteria: men with:
Exclusion criteria: men receiving medication for:
Total number of participants randomized: 115 Group 1 (n = 78) TUMT
Group 2 (n = 37) sham
|
|
| Interventions |
Group 1 (n = 78): TUMT Prostatron device is inserted by a 20F transurethral applicator (with 2 cooling channels) catheter and a rectal probe confirmed by ultrasonography. The treatment catheter emits a radiofrequency of 1296 MHz. The treatment consist of 3 stages: 1) cooling (to 27 ºC); 2) microwave emission to a threshold of 42.5 ºC rectal temperature; 3) progressive cooling. These details were provided in the report of a previous non‐randomized study (Blute 1993) Group 2 (n = 37): Sham This consisted of circulation of urethral coolant without application of microwave power while a sham treatment was displayed on the computer monitor. and the program run for 60 minutes Co‐interventions: Participants were given anti‐inflammatory agents and prophylactic antibiotics before and after (7 days) the procedure. If the participant experiences difficulties, a Foley catheter was inserted. Sedation was used at discretion (no sedation in 89% of TUMT sessions, and 100% of sham sessions) |
|
| Outcomes |
Urologic symptom scores How measured: Madsen Symptom score / AUA symptom score Time points measured: baseline, 6 weeks, 3, 6 and 12 months Time points reported: baseline, 6 weeks, 3, 6 and 12 months (mostly graphically; comparative outcome data were only available at 3 months) Minor adverse events (including erectile/ejaculatory function) How measured: narratively including sexual adverse events Time points measured: at complete follow‐up (12 months) Time points reported: at complete follow‐up (12 months) Acute urinary retention/Indwelling urinary catheter How measured: narratively Time points measured: at complete follow‐up (12 months) Time points reported: at complete follow‐up (12 months) Relevant outcomes not reported in this study:
|
|
| Funding sources | Not available | |
| Declarations of interest | Not available | |
| Notes | Whereas the blinding lasted for 3 months, the follow‐up time was 12 months. The reporting of outcomes was not disaggregated by group (intervention versus sham, but for the entire population) for most outcomes and time points. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The patients were randomized to TUMT or sham treatment in a 2:1 ratio based on a permuted‐blocks procedure.” |
| Allocation concealment (selection bias) | Low risk | Quote: “Randomization assignments were distributed in sealed envelopes identified only by a unique patient number. The treating physician opened the envelope after completing all screening tests just prior to treatment.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “To blind the evaluating physician to the patient's actual treatment, data on the treatment received (including post‐treatment PSA values) were not entered in the patient's study chart until after the 3‐month evaluation.” “Physicians and paramedical personnel behaved in the same fashion they would have during real thermotherapy sessions.” Comment: There was also “blinding verification” at 1 week after procedure: Quote: “When patients were queried about the treatment they had received, only half of the TUMT patients (51.3%; 40 of 78) guessed correctly, and in the sham‐treatment group, less than half of the patients (44.4%; 16 of 36) guessed correctly (Table 2).” |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Double‐blind study ‐ see above. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Double‐blind study ‐ see above. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “Of the 150 patients treated 118 had Madsen symptom score data at 12 months, since 11 discontinued the study or were lost to follow up, 16 were re‐treated with the Prostatron unit, 4 received alternative therapy (3 underwent transurethral procedures, and 1 received terazosin) and 1 was missing a Madsen score at followup.” Comment: High attrition date for 'Urinary Symptoms Score' (21%). There is no specification about attrition by group. |
| Selective reporting (reporting bias) | High risk | No protocol available. Data were presented graphically for most time points. Comparative outcome data were only available at 3 month‐follow up for some outcomes. |
| Other bias | Low risk | No other sources of bias were detected. |
Brehmer 1999.
| Study characteristics | ||
| Methods |
Study design: prospective, randomized parallel study Study dates: study dates not available Setting: outpatient, single‐center, national Country: Sweden |
|
| Participants |
Inclusion criteria: men with low urinary tract symptoms dominated by:
Exclusion criteria: men with:
Total number of participants randomized: 44 Age, mean (range): 70.4 (53 – 83) years. (No disaggregated data by group reported) Group 1: n = 16 60‐min TUMT ICS questionnaire A: 49 (of a maximum of 124) (see notes) ICS questionnaire B: 36 (of a maximum of 92) (see notes) Qmax: 7mL/s Group 2: n = 14 30‐min TUMT ICS questionnaire A: 58 (of a maximum of 124) (see notes) ICS questionnaire B: 40 (of a maximum of 92) (see notes) Qmax: 8.7 mL/s Group 3: n = 14 Sham ICS questionnaire A: 46 (of a maximum of 124) (see notes) ICS questionnaire B: 36 (of a maximum of 92) (see notes) Qmax: 7.9 mL/s |
|
| Interventions |
Group 1 (n = 16): 60‐min TUMT ECP system (Comair, Sweden) equipped with a microwave antenna (915 MHz), a fibreoptic system for measuring the temperature in the urethra and, by a rectal probe, in the rectum. It contained a circulating cooling system that reduced the heat delivered to the urethral wall with a maximum heating at 30 s and a temperature limit of 46 °C in the urethra and of 43 °C in the rectum. After treatment, a voiding trial was attempted; if difficulties arose, a urethral catheter was inserted and left in place for three days. Group 2 (n = 14): Similar intervention as group 1, except that the duration of the session was 30 min. Group 3 (n = 14): Sham “Only water at 20 °C was circulated in the treatment catheter and a computer monitor, visible to the patient, showed a simulated heat‐treatment curve, similar to that produced during TUMT.” Co‐interventions: Antibiotics (norfloxacin). |
|
| Outcomes |
Minor and major adverse event How measured: number of participants suffering a bacterial cystitis despite antibiotic treatment Time points measured: not reported Time points reported: not reported Subgroups: none Retreatment How measured: number of participants requiring other treatment within the follow‐up year Time points measured: not reported Time points reported: not reported Subgroups: none Relevant outcomes not reported in this study:
|
|
| Funding sources | Not available | |
| Declarations of interest | Not available | |
| Notes | ICS questionnaire consists of 32 questions, most of which comprise an ‘A’ question about the actual symptom and a ‘B’ question about the bother related to the symptom. The questionnaire also includes several questions about sexual function (nos 24 – 27); these were all excluded from the instrument used in the present study. The maximum A and B scores are 124 and 92, respectively; a high score indicates worse symptoms. 2 participants withdrew during the 1‐year study period, leaving 42 for the final evaluation. No contact information available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The patients were randomised to undergo 30 or 60 min of TUMT, or to sham treatment (14, 16 and 14 men, respectively).” Comment: Insufficient information about the sequence generation process to permit judgment of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgment of ‘Low risk’ or ‘High risk’ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information about blinding of personnel. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | The participants were blinded: “study where the patients were unaware of the type of treatment given.” Comment: Outcomes are unlikely to be affected by lack of blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | The participants were blinded: “study where the patients were unaware of the type of treatment given.” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgment of ‘Low risk’ or ‘High risk’. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Insufficient information to permit judgment of ‘Low risk’ or ‘High risk’. |
| Other bias | Low risk | No other sources of bias were identified. |
D'Ancona 1998.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomized trial Study dates: January 1994 to August 1995 Setting: outpatient Country: Netherlands |
|
| Participants |
Inclusion criteria: men:
Exclusion criteria:
Sample size: 52 participants were randomized Group 1: n = 31 transurethral microwave thermotherapy (TUMT)
Group 1: n = 21 transurethral resection of the prostate (TURP)
|
|
| Interventions |
Group 1 (n = 31): TUMT Delivered using Prostatron device with software version 2.5, for 60 minutes increasing thermal dose up to 70 watts. Urethral and rectal thermal sensors provided feedback to prevent harms. Preparation included 100 mg diclofenac suppository and 2 mg of midazolam intramuscularly. If necessary, further intravenous sedation was administered. All participants left with an indwelling urinary catheter Group 2 (n = 21): TURP Performed by 2 experienced urologists with use of spinal anesthesia. The surgical capsule was reached circumferentially from the bladder neck to the verumontanum using 24 Ch. Resectoscopes Co‐interventions: not described |
|
| Outcomes |
Urologic symptom scores How measured: Madsen symptom score and IPSS Time points measured: 1, 3, 6 and 12 months Time points reported: 3, 6, 12 months Subgroups: none Major and minor adverse events How measured: episodes of urinary tract infection, hematuria Time points measured: not reported Time points reported: not reported Subgroups: none Retreatment How measured: “repeat treatment” Time points measured: not reported Time points reported: not reported Subgroups: none Indwelling urinary catheter How measured: days of catheterization Time points measured: not reported Time points reported: median days and range Subgroups: none Relevant outcomes not reported in this study:
|
|
| Funding sources | Not available | |
| Declarations of interest | Not available | |
| Notes | No contact information available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Participants were randomised”. Comment: No information available. Insufficient information to permit judgment of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Participants were randomised.” Comment: No information available. Insufficient information to permit judgment of ‘Low risk’ or ‘High risk’. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Participants and personnel were not blinded. Outcomes are unlikely to be affected by lack of blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants and personnel were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome data were available for 44/52 participants at 1 year follow‐up, 2 were lost in the TURP group (bladder cancer and bladder neck sclerosis) and 6 in the TUMT group (1 underwent TURP, 1 died, 1 lost to follow up, 3 refused follow‐up). Unbalanced attrition. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Insufficient information to permit judgment of ‘Low risk’ or ‘High risk’. |
| Other bias | Low risk | No other sources of bias were detected. |
Dahlstrand 1995.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomized trial Study dates: study dates not available Setting: outpatient (TUMT), inpatient (TURP), single‐center, national Country: Sweden |
|
| Participants |
Inclusion criteria: men:
Exclusion criteria:
Total number of participants randomized: 93 Group 1 (n = 46) TUMT
Group 2 (n = 47) TURP
|
|
| Interventions |
Group 1 (n = 39): TUMT 1‐hour treatment in a single session performed by a single physician using the Prostatron (Technomed International, France) only with topical anesthesia and oral analgesia. The urethral catheter delivered up to 60 W of microwave energy and monitored temperature (as well as the rectal probe) through software. The urethral temperature could reach a maximum temperature of 44.5 °C and the rectal temperature could reach a maximum temperature of 42.5 °C. Postoperatively oral norfloxacin 400 mg twice a day was administered for 5 days. An indwelling urethral catheter was left in place for 3 ‐ 5 days if the participant was unable to void after treatment Group 2 (n = 44): TURP Urologists who were at the level of senior registrar or above resected the prostate, using resectoscopes with a Charrière of 24 ‐ 28, down to the surgical capsule circumferentially and extended from the bladder neck to the verumontanum Co‐interventions: not reported. |
|
| Outcomes |
Urologic symptom scores How measured: Madsen symptom score Time points measured: baseline, 2, 3, 6, 12 months, 2 years Time points reported: baseline, 2, 3, 6, 12 months, 2 years Subgroups: none Major and minor adverse events (including erectile and ejaculatory function) How measured: not reported Time points measured: not reported Time points reported: not reported Subgroups: none Retreatment How measured: number of participants that required another session of TUMT or TURP Time points measured: not reported Time points reported: not reported Subgroups: none Indwelling urinary catheter How measured: number of participants that required catheterization after the procedure. Time points measured: not reported Time points reported: not reported Subgroups: none Relevant outcomes not reported in this study
|
|
| Funding sources | Not available | |
| Declarations of interest | Not available | |
| Notes | There are 2 reports of this study by the same authors. In the first report there are 83 randomized participants, whereas in the second report there are 72. We accounted this as attrition. Email for the contact author was not available, so we wrote to his coauthor Dr. Fall (magnus.fall@urology.gu.se) for details, but he did not have this information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “patients were recruited for the study and blindly randomised.” Comment: Insufficient information to permit judgment of ‘Low risk’ or ‘High risk’. We wrote to study authors. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “patients were recruited for the study and blindly randomised.” Comment: Insufficient information to permit judgment of ‘Low risk’ or ‘High risk’. We wrote to study authors. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | While blinding was not mentioned, the interventions were visibly different (surgery versus outpatient procedure). |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | While blinding was not mentioned, the interventions were visibly different (surgery versus outpatient procedure). Comment: The objective outcomes were unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | While blinding was not mentioned, the interventions were visibly different (surgery versus outpatient procedure). Comment: The subjective outcomes were likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12‐month follow‐up, 78 participants (93%) had available data (first report). Quote: “Four patients were excluded; 1 patient because he contracted severe hepatitis while abroad precluding follow‐up; 2 patients because cancer was discovered at the time of histological examination of the TUR specimen requiring orchiectomy, and 1 patient who refused randomizations to TURP.” Judgment (12 months): low risk of bias. 2‐year follow‐up, 61 participants (73%) had available data (second report). Quote: “All patients were followed for 2 years but in 10 patients the follow‐up was incomplete. In the TURP group, one patient died from a brain tumour after his 6‐month follow‐up. At the 2‐year follow‐up, one patient underwent an operation for a lumbar disc hernia and was unavailable. In the TUMT group, one patient was abroad at the 3‐month follow‐up and after the 6‐month follow‐up, two patients had a TURP and were excluded from the study, one patient refused further follow‐up and another suffered severe pancreatitis which precluded that visit. Two patients who had undergone a second TUMT after the 6‐month follow‐up took part in the 1‐year follow‐up but had not improved and, after undergoing TURP, they were excluded before the 2‐year follow‐up. One patient was disabled due to severe neurological disease after the 1‐year follow‐up.” Judgment (2 years): high risk of bias. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Insufficient information to permit judgment of ‘Low risk’ or ‘High risk’. We wrote to study authors. |
| Other bias | Low risk | No other sources of bias were identified. |
De Wildt 1996.
| Study characteristics | ||
| Methods |
Study design: parallel group randomized trial Study dates: Start date June 1991 – End date December 1992 Setting: outpatient, multicenter, international Country: Netherlands and the United Kingdom |
|
| Participants |
Inclusion criteria: men:
Exclusion criteria:
Total number of participants randomized:93 men recruited but 90 were randomized (there is no further detail on the report) Group 1: n = 46 TUMT
Group 2: n = 47 Sham
|
|
| Interventions |
Group 1 (n = 46): TUMT A single session of Prostatron treatment unit which consisted of a microwave generator, urethral applicator/cooler, fiberoptic temperature‐monitor, and couch. This study used the lower energy thermotherapy protocol (Prostasoft 2.0) Group 2 (n = 47): Sham Same procedure as in TUMT with a simulated program Co‐interventions: Not described |
|
| Outcomes |
Urologic symptoms score How measured: Madsen symptom score. Responder analysis (> 50% decrease in Madsen score) Time points measured: baseline, 6, 12, 26, 52 weeks Time points reported: baseline, 6, 12, 26, 52 weeks (cross‐over after 3 months) Subgroups: none Major and minor adverse event How measured: major and minor adverse events Time points measured: not reported Time points reported: at 3 months Acute urinary retention How measured: number of participants that required a catheter after the procedure due to urinary retention Time points measured: not reported Time points reported: at 3 months Relevant outcomes not reported in this study
|
|
| Funding sources | Not available | |
| Declarations of interest | Not available | |
| Notes | This study reports the trial by location and globally. The quality‐of‐life results are only available for the Netherlands report. After 3 months participants were offered TUMT. 27 participants in the Sham group and 4 participants in the TUMT group received a verum procedure, thus the results of this trial beyond 3 months are not included in this review. No contact information available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients were randomised after informed consent was obtained.” Comment: Insufficient information to permit judgment of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Patients were randomised after informed consent was obtained.” Comment: Insufficient information to permit judgment of ‘Low risk’ or ‘High risk’. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “As far as possible, the patient and the investigator were kept unaware as to the treatment administered.” (first three months) Comment: Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: “As far as possible, the patient and the investigator were kept unaware as to the treatment administered” (first three months). Comment: Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: “As far as possible, the patient and the investigator were kept unaware as to the treatment administered” (first three months). Comment: Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were not available at 3 months for 3 participants in the Sham group (2 losses at follow‐up and 1 technical failure) and 2 participants in the TUMT group (underwent TURP). |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Insufficient information to permit judgment of ‘Low risk’ or ‘High risk’. |
| Other bias | Low risk | No other sources of bias were identified. |
Floratos 2001.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomized trial Study dates: start date January 1996 – end date March 1997 Setting: outpatient/inpatient, national, single‐center Country: The Netherlands |
|
| Participants |
Inclusion criteria: Male participants:
Exclusion criteria: men with:
Total number of participants randomly assigned: 155 Group 1 (n = 82) TUMT
Group 2 (n = 73) TURP
|
|
| Interventions |
Group 1 (n = 74): TUMT A 1‐hour session was administered by the Prostatron device (EDAP Technomed, Lyon, France) with a second‐generation, high‐energy protocol (Prostasoft 2.5) with a maximum power of 70 W and a rectal threshold set at 43.5 °C. Participants were administered 40 mg of morphine sulphate orally 2 hours before treatment. All participants received an indwelling Foley catheter following an outpatient voiding trial. Participants also received co‐trimoxazole 960 mg twice a day for 5 days after treatment as prophylaxis Group 2 (n = 73): TURP It was performed under spinal anesthesia and intended to remove as much prostate tissue as possible; all participants received an indwelling Foley catheter, which was removed when hematuria decreased sufficiently, and the participant completed a successful voiding trial Co‐interventions: not described |
|
| Outcomes |
Urologic symptoms score How measured: IPSS score and Madsen score Time points measured: baseline, 3, 6, 12, 18, 24 and 36 months Time points reported: baseline, 12, 24 and 36 months Subgroups: none Quality of life How measured: 41‐item questionnaire designed for BPH patients Time points measured: baseline, 1, 3, 6 and 12 months Time points reported: baseline, 12 and 52 weeks Subgroups: none Retreatment How measured: narratively Time points measured: baseline, 3, 6, 12, 18, 24 and 36 months Time points reported: 6, 12, 18, 24, 30 and 26 months Major and minor adverse events How measured: major and minor adverse events Time points measured: not reported Time points reported: at 3 months Erectile function/ejaculatory function ("Sexual function") How measured: ad‐hoc questionnaire that assessed erections, sexual activities, orgasms, and satisfactions, among other aspects Time points measured: baseline, 3 months and 1 year Time points reported: baseline, 3 months and 1 year Relevant outcomes not reported in this study:
|
|
| Funding sources | Not available | |
| Declarations of interest | Not available | |
| Notes | No contact information available. We found a secondary report on sexual function with a greater attrition of data and with a slightly lower number of randomized individuals (147 participants versus 155 in the original report). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “All patients were randomised after informed consent had been obtained.” Comment: Insufficient information to permit judgment of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “All patients were randomised after informed consent had been obtained.” Comment: Insufficient information to permit judgment of ‘Low risk’ or ‘High risk’. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Open‐label study. However, the outcomes are unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Although […] 155 patients initially randomised, unfortunately because of the 10 who skipped the assigned treatment and 1 who died before the scheduled treatment, we have no follow up information.” Comment: Attrition was documented and was balanced (7 in the thermotherapy group and 11 in the TURP group) (low risk of bias) Sexual function report: "A total of 66 patients undergoing transurethral microwave thermotherapy and 56 undergoing transurethral prostatic resection were evaluated" (high risk of bias). |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Insufficient information to permit judgment of ‘Low risk’ or ‘High risk’. |
| Other bias | Low risk | No other sources of bias were identified. |
Larson 1998.
| Study characteristics | ||
| Methods |
Study design: prospective, randomized parallel study Study dates: September 1994 to June 1996 Setting: outpatient, multicenter, national Country: USA |
|
| Participants |
Inclusion criteria: men with:
Exclusion criteria: men with:
Total number of participants randomized: 169 Group 1: n = 125 transurethral microwave thermotherapy (TUMT)
Group 2: n = 44 Sham
|
|
| Interventions |
Group 1 (n = 125): TUMT Power was applied with a Targis device in increments to achieve a target urethral temperature of 40 ± 1 °C with measurement by the catheter’s fibreoptic thermo sensor. Microwave treatment was administered continuously for 1 hour, with the circulation of coolant at 8 °C Group 2 (n = 44): Sham The same procedure as TUMT group, with the exception that microwave power was not applied, and coolant temperature was increased in increments from 8 °C to 20 °C over the same time period as microwave power was increased in the microwave group. It was not feasible to increase the urethral temperature further in the sham group because the Targis cooling system is not designed or equipped to provide active heating of coolant other than that occurring as the result of the application of microwave energy. The sham‐group participants experienced rising urethral temperatures rather than unchanging low temperatures Co‐interventions: All participants underwent insertion of a Targis (formerly T3) transurethral thermoablation system treatment catheter (Urologix, Inc., Minneapolis, Minn). It is a compact and portable unit equipped with a 21F silicone treatment catheter containing a helical dipole microwave antenna operating in the range 902 to 928 MHz. This provides urethral cooling via circumferential cooling compartments and also includes a urine drainage canal and a fibreoptic thermo sensor for monitoring urethral catheter interface temperatures. The thermoablation system automatically interrupts microwave power if urethral temperatures reach 44.5 °C or higher or rectal temperatures reach 42.5 °C or higher. Catheterization was carried out under topical lidocaine anesthesia. The positioning of the catheter balloon and antenna was confirmed by TRUS. The catheter was then secured in the proper spatial orientation with respect to the posteroanterior prostatic axis. A rectal thermal unit equipped with 5 thermocouples was used to monitor rectal temperatures. All participants received a 3‐day prescription of prophylactic oral antibiotics and catheterization for 36 to 60 hours |
|
| Outcomes |
Urologic symptom scores How measured: AUA score Time points measured: baseline, 6 weeks, 3 months and 6 months Time points reported: baseline, 6 weeks, 3 months and 6 months Subgroups: none Quality of life How measured: QOL score was evaluated by participant responses to the question of how they would feel if their current urinary symptoms were to continue indefinitely. Time points measured: Baseline and 6 months Time points reported: baseline, 6, 9 and 12 months follow‐up (these last 2 time points were not reported In group 2) Subgroups: none Minor and major adverse event (including ejaculatory function) How measured: number of participants with UTI confirmed by urine culture and resolved with antibiotics, among other adverse events Time points measured: not reported Time points reported: not reported Subgroups: none Retreatment How measured: number of participants requiring other treatment within the 6 months follow‐up Time points measured: 6 months Time points reported: 6 months Subgroups: none Acute urinary retention How measured: number of participants with urinary retention > 1 week after the procedure Time points measured: > 1 week Time points reported: > 1 week Subgroups: none Relevant outcomes not reported in this study
|
|
| Funding sources | This study was supported by a grant from Urologix Inc | |
| Declarations of interest | Not available | |
| Notes | Blinding was broken after 6 months (we included data from the blinded phase in this review). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients were randomised in a 3:1 target ratio to the microwave (n = 125) or sham (n = 44) group.” Comment: Insufficient information about the sequence generation process to permit judgment of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Patients were randomised in a 3:1 target ratio to the microwave (n = 125) or sham (n = 44) group.” Comment: Insufficient information about the sequence generation process to permit judgment of ‘Low risk’ or ‘High risk’. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “The study was double‐blind: Neither the patients nor any of the investigators and support staff involved in carrying out the study procedures had knowledge of group assignment (microwave versus sham).” Comment: Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: “The study was double‐blind: Neither the patients nor any of the investigators and support staff involved in carrying out the study procedures had knowledge of group assignment (microwave versus sham).” Comment: Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: “The study was double‐blind: Neither the patients nor any of the investigators and support staff involved in carrying out the study procedures had knowledge of group assignment (microwave versus sham).” Comment: Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “Of the 169 patients enrolled, 155 were evaluable at the conclusion of the 6‐month blinded phase of the study (Table III) and 114 at the end of the full 12‐month follow‐up period. Analyses of efficacy results are presented for the 155 subjects evaluable at the conclusion of the blinded phase.” Comment: Unbalanced attrition at 6 months (20% vs 4%). |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Insufficient information to permit judgment of ‘Low risk’ or ‘High risk’. |
| Other bias | Low risk | No other sources of bias were identified. |
Nawrocki 1997.
| Study characteristics | ||
| Methods |
Study design: prospective, randomized parallel study Study dates: not reported Setting: outpatient, single‐center, national Country: United Kingdom |
|
| Participants |
Inclusion criteria: men with:
Exclusion criteria: men with:
Total number of participants randomized: 120 Age, median (range): 70 (56 ‐ 80) years (no disaggregated data by group available) Group 1: n = 38 transurethral microwave thermotherapy (TUMT) AUA score, median (range): 19 (7 ‐ 31) Qmax, mean (SD): 8.83 (2.32) mL/s Prostate volume, mean (SD): 41.2 (14.6) mL Group 2: n = 40 sham transurethral microwave thermotherapy (TUMT) AUA score, median (range): 17.5 (7 ‐ 28) Qmax, mean (SD): 9.44 (2.78) mL/s Prostate volume, mean (SD): 46.7 (16.8) mL Group 3: n = 42 no treatment AUA score, median (range): 18 (10 ‐ 29) Qmax, mean (SD): 8.79 (2.66) mL/s Prostate volume, mean (SD): 46.4 (19.9) mL |
|
| Interventions |
Group 1 (n = 38): TUMT It was delivered for an hour under local anesthesia, through a urethral catheter using Prostatron. The temperature was measured through the catheter and a rectal probe and guided the cooling of the urethra through a software (Prostasoft v2.0) which was not under the control of the operator Group 2 (n = 40): Sham A technically identical procedure to standard TUMT with no microwaves, with similar noise and appearance with simulated heat using a heat pad Group 3 (n = 42): No treatment (they received treatment after completion of the study) Co‐interventions: not reported |
|
| Outcomes |
Urologic symptom scores How measured: AUA score Time points measured: baseline and 6 months Time points reported: baseline and 6 months Subgroups: none Major and minor adverse events How measured: not reported Time points measured: not reported Time points reported: not reported Subgroups: none Acute urinary retention How measured: number of participants developing acute urinary retention in the first 24 hrs after treatment Time points measured: not reported Time points reported: not reported Subgroups: none Relevant outcomes not reported in this study
|
|
| Funding sources | LORS grant from the South East Thames Regional Research Committee | |
| Declarations of interest | Not available | |
| Notes | We included that TUMT and sham arms of the study in our review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: The investigators describe a random component in the sequence generation process. Quote: “Randomization was carried out by selecting one of three differently numbered but otherwise identical balls from a sealed bag.” |
| Allocation concealment (selection bias) | High risk | Comment: The allocation could be tampered with, considering that the balls could be re‐inserted to the bag and pulled out again. Quote: “Randomization was carried out by selecting one of three differently numbered but otherwise identical balls from a sealed bag.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. Quote: “The treatment of the standard and simulated TUMT groups was designed and carried out as a double‐blind, so that neither the operator nor the patient was aware of which treatment was being per‐ formed. Patients randomised to group 3 were treated after completion of the study.” |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes: No apparent missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. There is a trial registry (ISRCTN24866285), but it was retrospectively registered and there is no information about the outcomes. |
| Other bias | Low risk | No other sources of bias were identified. |
Nørby 2002a.
| Study characteristics | ||
| Methods |
Study design: prospective, randomized stud. Study dates: May 1996 and November 1999 Setting: outpatient, multicenter, national Country: Denmark |
|
| Participants |
Inclusion criteria: symptomatic benign prostatic hyperplasia (BPH) and:
Exclusion criteria: men with:
Total number of participants randomized: 118 Group 1: 48 Interstitial laser coagulation (ILC)
Group 2: 46 transurethral microwave thermotherapy (TUMT)
Group 3: 24 (control: TURP or TUIP)
|
|
| Interventions |
Group 1 (n = 48): “ILC was delivered by a MediLas 4100 Fibertom (Dornier, Germany), a Nd‐YAG laser with a wavelength of 1064 nm. The energy was delivered using an applicator with a quartz glass tip (length 20 mm, diameter 1.9 mm). The 3‐min radiation was used, thus applying 20 W for 30 s, 15 W for 30 s, 10 W for 30 s and 7 W for 90 s. Treatments were undertaken with a laser cystoscope (18 F) using saline as the irrigant. The fibre was placed deep within the lateral lobes at an angle in the plane of the urethra of 30º (to avoid heating the urethral mucosa). If a median lobe was present it was treated with one or two punctures in the direction of the bladder. Initially the intent was to apply one puncture per 10 mL of prostate tissue, but later the regimen became more aggressive, aiming at one puncture per 5 mL. All patients had a suprapubic tube placed at the start of the procedure and most also had a transurethral catheter for 12–24 h to reduce prostatic oedema. All patients received prophylactic antibiotics. Patients were discharged after removing the urethral catheter and scheduled to visit the outpatient clinic for removal of the suprapubic tube, generally at fixed intervals of 1–2 weeks.” Group 2 (n = 46): “TUMT was administered using the Prostatron® system; before treatment cystoscopy was used to exclude bladder pathology. Prostasoft v2.0 was chosen when the prostatic volume was < 30 mL and v2.5 in larger prostates. Treatment comprised 1 h sessions under local anaesthesia with Installagel® (Farco‐Pharma GmbH, Cologne, Germany); 1 h beforehand, 100 mg of diclofenac and 500 mg ciprofloxacin was administered. During treatment pethidine was given if necessary. If patients developed urinary retention after treatment a suprapubic or a transurethral catheter was inserted and the patient seen at weekly intervals until spontaneous voiding with an acceptable PVR (in general < 100 mL) was achieved.” Group 3 (n = 24): “Patients underwent TUIP or TURP according to the surgeons’ decision. The prostate was resected using a 26 F Iglesias resectoscope with a standard resection loop and 1.5% glycine for irrigation. TUIP comprised a unilateral incision in the 7 o’clock position starting proximal to the bladder neck and extending distally to the verumontanum. After surgery a three‐way irrigation catheter was inserted and first removed when bleeding had stopped. Prophylactic antibiotics were given according to the routine of the department.” Co‐interventions: “All treatments were administered by one of the two consultants or the senior registrar. Patients were treated under spinal or general anaesthesia.” |
|
| Outcomes |
Urologic symptom scores How measured: IPSS Time points measured: baseline, 1, 3 and 6 months Time points reported: baseline and 6 months Subgroups: none Quality of life How measured: not reported Time points measured: baseline, 1, 3 and 6 months Time points reported: baseline and 6 months Subgroups: none Major and minor adverse event How measured: number of participants with bleeding necessitating transfusion Time points measured: 6 months Time points reported: 6 months Subgroups: none Retreatment How measured: number of participants undergoing TURP or other treatment Time points measured: 6 months Time points reported: 6 months Subgroups: none Erectile function How measured: To evaluate erectile function participants scoring 0 or 1 (i.e. normal or slightly reduced erectile capacity) were defined as ‘normal’, whereas participants scoring 2 or 3 (i.e. greatly reduced or no erectile function) were defined having decreased erectile capacity Time points measured: 6 months Time points reported: 6 months Subgroups: none Ejaculatory function How measured: number of patients with retrograde ejaculation Time points measured: 6 months Time points reported: 6 months Subgroups: none Acute urinary retention How measured: number of participants with persistent retention after treatment Time points measured: 6 months Time points reported: 6 months Subgroups: none Indwelling urinary catheter How measured: not reported Time points measured: 6 months Time points reported: 6 months (narratively) Subgroups: none |
|
| Funding sources | The study was supported by a grant from Vejle County, Denmark | |
| Declarations of interest | Not available | |
| Notes | ILC group data are not included in this review. Antibiotic regimen in ILC group was changed during the study because there was a high rate of UTI. “The study had to be stopped at the final date because of financial restrictions.” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: 2:1:1: Randomization ‐ “A weighted randomisation was therefore chosen as the object was to gain maximum information about the new treatments.” Comment: Insufficient information about the sequence generation process to permit judgment of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Patients were recruited from two centres and randomised at a 2:2:1 to TUMT, ILC or the control group.” Comment: Method of allocation concealment is not described in sufficient detail to allow a definite judgment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding, but the outcomes ar not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding, and the outcomes are likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Analyses are presented on an intention‐to‐treat basis.” Group 1: “Before ILC but after randomisation two patients had prostate cancer diagnosed and one had a urethral stricture. A further two patients declined surgery. One of these patients completed the IPSS at 6 months by mail contact. Thus, 44 patients were available for evaluation at 6 months.” Group 2: “All patients were followed at 6 months except one who developed an apoplexy at 4 months. One patient had TURP.” Group 3: “23 of 24 patients were treated according to the randomisation. One patient declined surgery. Two patients were excluded as the pathology revealed T1 prostate cancer.” |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Insufficient information to permit judgment of ‘Low risk’ or ‘High risk’. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Roehrborn 1998.
| Study characteristics | ||
| Methods |
Study design: prospective, randomized study Study dates: not reported Setting: outpatient, multicenter centre, national Country: USA |
|
| Participants |
Inclusion criteria: men with:
Exclusion criteria: not reported Total number of participants randomized: 220 Group 1 (n = 147) TUMT
Group 2 (n = 73) Sham
|
|
| Interventions |
Group 1 (n = 147) TUMT The Dornier Urowave (second‐generation microwave therapy device), can deliver up to 90 W of power and has an integrated water‐cooling circuit. The safety threshold was set at 50 °C in the urethra and at 42.5 °C in the rectum Group 2 (n = 73) Sham: sham‐treated participants received a 60‐minute, preprogrammed sham treatment cycle with the catheter in place Co‐interventions: All participants had negative urine cultures before treatment and were given peritreatment antibiotic prophylaxis (investigators’ choice). After treatment, an indwelling Foley catheter was inserted and left in place for 2 to 5 days, depending on logistics |
|
| Outcomes |
Urologic symptom scores How measured: AUA‐SI (0 to 35 points) Time points measured: baseline, 1, 3, and 6 months Time points reported: baseline, 1, 3, and 6 months Subgroups: none Quality of Life How measured: AUA‐SI subscore (0 to 6 points) Time points measured: baseline, 1, 3, and 6 months Time points reported: baseline, 1, 3, and 6 months Subgroups: none Major and minor adverse events (including ejaculatory and erectile function) How measured: Adverse events were solicited from participants during and after treatment as well as at each follow‐up visit. Adverse events were designated as treatment‐related or unrelated to treatment by the investigator Time points measured: during treatment, 72 hrs after treatment and up to 6 months Time points reported: during treatment, 72 hrs after treatment and up to 6 months Subgroups: none Acute urinary retention How measured: not reported Time points measured: baseline, 1, 3, and 6 months Time points reported: 6 months Subgroups: none Relevant outcomes not reported in this study
|
|
| Funding sources | Funded by Dornier MedTech, Atlanta, Georgia | |
| Declarations of interest | Not available | |
| Notes | A secondary report states that quality of life was also measured by another scale (0 ‐ 21), but it is not clear which scale was used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: The investigators describe a random component in the sequence generation process. Quote: “The physician administering the treatment opened the centrally provided randomisation envelope immediately before treatment.” |
| Allocation concealment (selection bias) | Low risk | Comment: Participants and investigators enrolling participants could not foresee assignment. Quote: “The physician administering the treatment opened the centrally provided randomisation envelope immediately before treatment.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: Blinding of participants and key study personnel was ensured, and it was unlikely that the blinding could have been broken. Quote: “They were made aware that in this trial there would be an active/sham randomizations at a ratio of 2:1. Furthermore, patients were made aware that a ‘‘subset’’ of patients would have interstitial temperature monitoring by way of inserting a needle through the perineum into the prostate. However, for ethical reasons, only actively treated patients received such monitoring. Thus, the patients were effectively blinded as to whether or not they underwent active or sham treatment despite the fact that only the actively treated patients had interstitial temperature monitoring.” |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: Blinding of participants and key study personnel was ensured, and it was unlikely that the blinding could have been broken. Quote: “The treating physician and assistant were excluded from the follow‐up evaluation of the patient. The physician and/or nurse involved in the follow‐up evaluation was not present in the room during treatment.” |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Comment: Blinding of participants and key study personnel was ensured, and it was unlikely that the blinding could have been broken. Quote: “The treating physician and assistant were excluded from the follow‐up evaluation of the patient. The physician and/or nurse involved in the follow‐up evaluation was not present in the room during treatment.” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Insufficient reporting of attrition/exclusions to permit judgment of ‘Low risk’ or ‘High risk'. Quote: “For the various parameters, between 124 and 130 of the actively treated patients (86% to 88%) were available for 6‐month follow‐up; in the sham‐treated group, between 65 and 67 (89% to 92%) of patients were available for 6‐month follow‐up.” |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Insufficient information to permit judgment of ‘Low risk’ or ‘High risk’. |
| Other bias | Low risk | No other sources of bias were identified. |
Venn 1995.
| Study characteristics | ||
| Methods |
Study design: prospective, randomized study Study dates: not reported Setting: outpatient, multicenter, national Country: United Kingdom |
|
| Participants |
Inclusion criteria: men with:
Exclusion criteria: not reported Total number of participants randomized: 96 Group 1: n = 48 Transurethral microwave Hyperthermia
* no SD or 95% CI reported Group 2: n = 48 transurethral sham
* no SD or 95% CI reported |
|
| Interventions |
Group 1 (n = 48) TUMT Participants in the treated group underwent 1 hr of microwave hyperthermia, with a maximum urethral temperature of 46 °C or a maximum rectal temperature of 42.5 °C. The machine was designed and constructed in conjunction with Microwave Engineering Designs, Newport, Isle of Wight, UK (434 MHz, maximum power of 50 W). The antenna was a helical coil, loaded in a modified eyeless 22F Foley Simplastic catheter fitted with water cooling Group 2 (n = 48) Sham Treated with the same procedure but without the use of heat Co‐interventions: After selection for inclusion in the trial a treatment catheter was inserted under antibiotic cover (gentamicin 80 mg) |
|
| Outcomes |
Urologic symptom scores How measured: AUA scores (percentage of improvement). Madsen score response rate (responders as those with a score < 8) Time points measured: baseline,3 and 6 months Time points reported: baseline,3 and 6 months (responder data only at 3 months) Subgroups: none Relevant outcomes not reported in this study
|
|
| Funding sources | Not available | |
| Declarations of interest | Not available | |
| Notes | Participants were selected from waiting lists for transurethral resection of the prostate (TURP) at St Thomas's Hospital and Worthing Hospital, or by direct referral. Cross‐over: after 3 months, 47 participants in the treated group and 46 of the controls were assessed. After 6 months, 42 treated participants and 20 control participants were assessed, because 24 participants in the control group had been made aware of the sham treatment and so were not included in the analysis Protocol: not available Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: The investigators describe a random component in the sequence generation process. Quote: “patients were then randomly assigned to either a treated or control group by selection of sealed envelopes prepared before the trial.” |
| Allocation concealment (selection bias) | Unclear risk | Comment: Participants and investigators enrolling participants could not foresee assignment, although it is not clear if the envelopes were opaque. Quote: “patients were then randomly assigned to either a treated or control group by selection of sealed envelopes prepared before the trial.” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: It is unclear if personnel was blinded (first 3 months). Quote: “The patients were not aware of the group to which they were assigned.” |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: These outcomes are unlikely to be affected by blinding. Quote: “The patients were not aware of the group to which they were assigned.” |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Comment: These outcomes are likely to be affected by blinding. Quote: “The patients were not aware of the group to which they were assigned.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes: outcome data was available for nearly all participants. After 3 months, 47/48 patients in the treated group and 46/48 of the controls were assessed (6‐month data not included in this review, see “Notes”) |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Insufficient information to permit judgment of ‘Low risk’ or ‘High risk’. |
| Other bias | Low risk | No other sources of bias were identified |
Wagrell 2002.
| Study characteristics | ||
| Methods |
Study design: prospective, randomized study Study dates: October 1998 to November 1999 Setting: outpatient, multicenter, international Country: Scandinavia and USA |
|
| Participants |
Inclusion criteria: men with:
Exclusion criteria: not reported Total number of participants randomized: 154 Group 1: n = 103 Microwave Treatment
Group 2: n = 51 Transurethral resection of the prostate
|
|
| Interventions |
Group 1 (n = 103) TUMT ProstaLund feedback measured temperatures and were continuously displayed on the device computer. Using the heat equation, the device also calculates the extent of the coagulation necrosis continuously during the treatment, stopping at 55 °C Group 2 (n = 51): TURP TURP was performed as a clinical standard inpatient procedure according to the routines at each centre Co‐interventions: A washout period of at least 6 weeks preceded the treatment for participants who had been using any alpha‐receptor blocker or finasteride |
|
| Outcomes |
Urologic symptom scores How measured: IPSS Time points measured: baseline, 3, 6, 12, 24, 36, 48 and 60 months Time points reported: baseline, 3, 6, 12, 24, 36, 48 and 60 months Subgroups: none Quality of life How measured: QoL domain of IPSS score Time points measured: baseline, 3, 6, 12, 24, 36, 48 and 60 months Time points reported: baseline, 3, 6, 12, 24, 36, 48 and 60 months Subgroups: none Major adverse events How measured: All adverse events occurring during the entire study period were reported. A serious adverse event was defined according to International Congress on Harmonization as any untoward medical event that resulted in death, was life‐threatening, required inpatient hospitalization or prolongation of existing hospitalization, resulted in persistent or significant disability or incapacity, was cancer, or required intervention to prevent permanent damage to body functions or structure Time points measured: during treatment and up to 12 months Time points reported: during treatment and up to 12 months Subgroups: none Minor adverse events (includes acute urinary retention and erectile dysfunction) How measured: not reported Time points measured: during treatment or up to 12 months, and from 12 to 60 months Time points reported: during treatment or up to 12 months, and from 12 to 60 months Subgroups: none Indwelling urinary catheter How measured: time with the catheter Time points measured: after the procedure Time points reported: after the procedure Subgroups: none Retreatment How measured: number of participants with additional medical or surgical treatment Time points measured: after the procedure Time points reported: after the procedure Subgroups: none Relevant outcomes not reported in this study
|
|
| Funding sources | Funded by ProstaLund. | |
| Declarations of interest | Wagrell L, Schelin S, Larson TR, and Mattiasson A were paid consultants to the sponsor of this study. | |
| Notes | A total of 154 participants were included on an intention‐to‐treat basis. Eight participants (5 in the TURP and 3 in the PLFT group) were withdrawn before treatment, resulting in a total of 146 treated participants; 100 in the PLFT arm and 46 in the TURP arm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgment of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient information to permit judgment of ‘Low risk’ or ‘High risk’. Quote: “The randomisation ratio between PLFT and TURP was 2:1.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | While blinding was not mentioned, the interventions were visibly different (surgery versus outpatient procedure). |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | While blinding was not mentioned, the interventions were visibly different (surgery versus outpatient procedure). The objective outcomes were unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | While blinding was not mentioned, the interventions were visibly different (surgery versus outpatient procedure). The subjective outcomes were likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 months: balanced attrition, and outcome data were available for 133/154 (86%) Judgment: low risk of bias 24 months: outcome data was available for 79/103 in the TUMT group and 39/51 in the TURP group (76%) 36 months: outcome data was available for 69/103 in the TUMT group and 35/51 in the TURP group 60 months: outcome data was available for 62/103 in the TUMT group and 34/51 in the TURP group Judgment: high risk of bias |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Insufficient information to permit judgment of ‘Low risk’ or ‘High risk’. |
| Other bias | Low risk | No other sources of bias were identified. |
IPSS: International prostate symptom score; PSA: prostate‐specific antigen
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Albala 2000 | Ineligible intervention (Variant technique: periurethral); cross‐over at 3 months with no interpretable outcome data. |
| Arai 2000 | Prospective observational study comparing TUMT with other modalities. |
| D'Ancona 1997 | Observational non‐comparative study. |
| Dahlstrand 2003 | Review article (full‐text assessment). |
| Djavan 1999 | Ineligible comparison: TUMT ± neoadjuvant alpha‐blocker. |
| Hahn 2000 | Observational study on cardiovascular complications of TUMT. |
| Hansen 1998 | Methods paper on the symptoms scores. The TUMT data come from an observational study. |
| ISRCTN23921450 | "Please note that this trial was terminated due to poor recruitment." |
| Kobelt 2004 | Economic data only from the Wagrell 2002 trial. |
| Mulvin 1994 | Non‐randomized comparative study of TUMT and transurethral catheter therapy. |
| Norby 2002b | Economic data only of the Nørby 2002a study. |
| Nørby 2004 | Review article (full‐text assessment). |
| Ohigashi 2007 | Prospective observational study comparing TUMT with other modalities. |
| Schelin 2006 | Ineligible comparison: Compares TUMT to a group of participants that underwent TURP and enucleation surgery (no disaggregated data available). |
| Servadio 1987 | Observational study of the use of TUMT for various diseases of the prostate. |
| Shore 2010 | Ineligible comparison: Compared 2 similar energy TUMT systems that differed only by an adjunct balloon dilator. |
| Tan 2005 | Long‐term follow‐up of the sham crossed‐over group. Ten out of 12 participants in the sham group had crossed over to the active treatment group and no disaggregated data were available for this group before crossing over. |
| Trock 2004 | Pooled observational with previously extracted RCT data. |
| Vesely 2006 | Non‐randomized comparative study: participants were assigned by severity to TUMT or TURP. |
| Waldén 1998 | Economic data only on the Dahlstrand 1995 study. |
| Zerbib 1992 | Ineligible intervention: Transrectal hyperthermia. |
| Zerbib 1994 | Ineligible intervention: Transrectal hyperthermia. |
Characteristics of studies awaiting classification [ordered by study ID]
Albala 2000a.
| Methods | Technical report of the TUMT intervention (with a summary of a randomized controlled trial, possibly this is a secondary report of the Albala 2002 study). |
| Participants | Likely men with benign prostatic hyperplasia. |
| Interventions | TherMatrx TMx‐2000 TUMT device |
| Outcomes | Not available |
| Notes | Full‐text not available |
Dahlstrand 1994.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Possibly a secondary report of the Dahlstrand 1995 study. Full‐text not available. |
Dahlstrand 1997.
| Methods | Randomized controlled trial |
| Participants | Men with benign prostatic hyperplasia |
| Interventions | Prostatron TUMT device |
| Outcomes | Not available |
| Notes | 5‐year follow‐up of the Dahlstrand 1995 study. Full‐text not available. |
Dahlstrand 1998.
| Methods | Randomized controlled trial |
| Participants | Men with benign prostatic hyperplasia |
| Interventions | Prostatron TUMT device |
| Outcomes | Not available |
| Notes | 7‐year follow‐up of the Dahlstrand 1995 study. Full‐text not available. |
Devonec 1994.
| Methods | Randomized controlled trial |
| Participants | Men with benign prostatic hyperplasia |
| Interventions | Thermotherapy device |
| Outcomes | Not available |
| Notes | This is a trial that is referenced in various included studies, but the full text is not available. |
Roehrborn 1997.
| Methods | Randomized controlled trial |
| Participants | Men with benign prostatic hyperplasia |
| Interventions | Dornier Urowave TUMT device |
| Outcomes | AUA‐SI, Qmax, PSA, BPH QoL, adverse events, prostate volume |
| Notes | Presumably a secondary report of Roehrborn 1998 (this abstract reported 205 participants while the included study reported 220). Full‐text not available. |
Differences between protocol and review
We changed the original title Microwave thermotherapy for benign prostatic hyperplasia to Transurethral microwave thermotherapy for lower urinary tract symptoms in men with benign prostatic hyperplasia to harmonize the suite of reviews on this topic by the Urology Review Group. We synthesized the objective of the review in a single sentence. We decided not to include transrectal thermotherapy, since this procedure is no longer used.
The Methods section has been extensively modified to fit current methodological standards (we chose a 'replacement approach' as defined by MECIR criteria UR3). The previous version of the protocol can be found in Appendix 3. We restricted the comparisons, excluding comparisons of invasive treatments (e.g. radical prostatectomy), pharmacological treatments (e.g. alpha‐blockers) and incorporating other minimally‐invasive therapies (e.g. Rezum). We therefore excluded a study that was included in the previous version of this review (Djavan 2001). This was because transurethral resection of the prostate is currently the gold standard for surgery (replacing radical prostatectomy), and minimally‐invasive procedures are considered for those with moderate‐to‐severe symptoms that have not responded to medical therapy (including alpha‐blockers) (EAU 2021).
In the previous version of the review, secondary references for the included studies were categorized as 'excluded studies'. We have incorporated these secondary references into the included studies for this update. Furthermore, we incorporated one additional study that was excluded in the previous review (Brehmer 1999).
This version of the review was harmonized to fit a suite of reviews on treatments for benign prostatic hyperplasia (see Published notes). We therefore modified the core set of outcomes and suppressed surrogate outcomes such as peak flow (Qmax).
We were unable to perform the following analyses:
Subgroup analysis: we were unable to perform subgroup analysis considering prostate size and age, since all studies included participants with an average prostate size < 50 mL and an average age > 65 years. However, we were able to perform a subgroup analysis considering baseline IPSS scores (severity). We rated it severe when the mean score in both groups was > 19 (as prespecified in the Methods). This was only possible for urologic symptoms score since insufficient information was available for other primary outcomes (quality of life and major adverse events).
Sensitivity analysis: all studies were at an overall high risk of bias, and there were no substantial differences in the description of their inclusion criteria.
Contributions of authors
JVAF: Conceived, designed, and wrote the protocol and full review, and performed all aspects of the data abstraction, analysis, risk of bias assessment and certainty of evidence ratings. LG: Performed all aspects of the data abstraction, analysis, risk of bias assessment and certainty of evidence ratings, and drafted the review. CMEL: Designed and ran the electronic searches, drafting the full review. MF: Reviewed critical content, and gave final approval for the draft of the review. PD: Conceived, designed and wrote the protocol for the update, reviewed the methods and the critical content, and gave final approval for the draft of the review.
Sources of support
Internal sources
-
Department of Urology, University of Minnesota, USA
Support in kind for Philipp Dahm
-
Minneapolis VA Health Care System, USA
Salary support for Philipp Dahm
-
Instituto Universitario Hospital Italiano, Argentina
Salary support for Juan Franco, Luis Garegnani, Camila Micalea Escobar Liquitay
External sources
-
None, Argentina
N/A
Declarations of interest
JVAF: none known. LG: none known. CMEL: none known. MB: Boston Scientific (consultant for endourology and stone management), Auris Health (consultant for robotic surgery and endourology). PD: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Abbou 1995 {published data only}
- Abbou CC, Payan C, Viens-Bitker C, Richard F, Boccon-Gibod L, Jardin A, et al. Transrectal and transurethral hyperthermia versus sham treatment in benign prostatic hyperplasia: a double-blind randomized multicentre clinical trial. British Journal of Urology 1995;76(5):619-24. [DOI] [PubMed] [Google Scholar]
Ahmed 1997 {published data only}
- Ahmed M, Bell T, Lawrence WT, Ward JP, Watson GM. Transurethral microwave thermotherapy (Prostatron version 2.5) compared with transurethral resection of the prostate for the treatment of benign prostatic hyperplasia: a randomized, controlled, parallel study. British Journal of Urology 1997;79(2):181-5. [DOI] [PubMed] [Google Scholar]
Albala 2002 {published data only}
- Albala DM, Andriole G, Davis BE, Eure GR, Kabalin JN, Lingeman JE, et al. Transurethral microwave thermotherapy (TUMT) using the TherMatrx TMX-2000: Durability exhibited in a study comparing tumt with a sham procedure in patients with benign prostatic hyperplasia (BPH). Journal of Urology 2003;169(4):465. [Google Scholar]
- Albala DM, Andriole GL, Davis B, Eure G, Kabalin JN, Lingeman JE, et al. Transurethral microwave thermotherapy (TUMP) using the thermatrx TMx-2000(tm) for treatment of benign prostatic hyperplasia: Five year follow-up of multicenter randomized pivotal trial. Journal of Endourology 2006;20:A71. [Google Scholar]
- Albala DM, Andriole GL, Davis B, Eure G, Kabalin JN, Lingeman JE, et al. Transurethral microwave thermotherapy (TUMT) using the thermatrx TMX-2000 (TM): Long-term results in a study comparing TUMT with a sham procedure in patients with benign prostatic hyperplasia (BPH). Journal of Urology 2005;173(4):420. [Google Scholar]
- Albala DM, Fulmer BR, Turk TM, Koleski F, Andriole G, Davis BE, et al. Office-based transurethral microwave thermotherapy using the TherMatrx TMx-2000. Journal of Endourology 2002;16(1):57-61. [DOI] [PubMed] [Google Scholar]
Bdesha 1994 {published data only}
- Bdesha AS, Bunce CJ, Kelleher JP, Snell ME, Vukusic J, Witherow RO. Transurethral microwave treatment for benign prostatic hypertrophy: a randomised controlled clinical trial. BMJ 1993;306(6888):1293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bdesha AS, Bunce CJ, Snell ME, Witherow RO. A sham controlled trial of transurethral microwave therapy with subsequent treatment of the control group. Journal of Urology 1994;152(2 Part 1):453-8. [DOI] [PubMed] [Google Scholar]
Blute 1996 {published data only}
- Blute ML, Patterson DE, Segura JW, Tomera KM, Hellerstein DK. Transurethral microwave thermotherapy v sham treatment: double-blind randomized study. Journal of Endourology 1996;10(6):565-73. [DOI] [PubMed] [Google Scholar]
Brehmer 1999 {published data only}
- Brehmer M, Wiksell H, Kinn A. Sham treatment compared with 30 or 60 min of thermotherapy for benign prostatic hyperplasia: a randomized study. BJU International 1999;84(3):292-6. [DOI] [PubMed] [Google Scholar]
D'Ancona 1998 {published data only}
- D'Ancona FC, Francisca EA, Witjes WP, Welling L, Debruyne FM, De la Rosette JJ. High energy thermotherapy versus transurethral resection in the treatment of benign prostatic hyperplasia: results of a prospective randomized study with 1 year of followup. Journal of Urology 1997;158(1):120-5. [DOI] [PubMed] [Google Scholar]
- D'Ancona FC, Francisca EA, Witjes WP, Welling L, Debruyne FM, De La Rosette JJ. Transurethral resection of the prostate vs high-energy thermotherapy of the prostate in patients with benign prostatic hyperplasia: long-term results. British Journal of Urology 1998;81(2):259-64. [DOI] [PubMed] [Google Scholar]
- D'Ancona FCH, Francisca EAE, Debruyne FMJ, De La Rosette JJMCH. TURP versus high-energy microwave thermotherapy (HE-TUMT) of the prostate in patients with BPH: Long-term results. British Journal of Urology 1998;81(SUPPL. 4):52. [DOI] [PubMed] [Google Scholar]
- Rosette JJ, Ancona FC, Francisca EA, Debruyne FM. High energy transurethral microwave thermotrherapy (HE-TUMT) versus transurethral resection of the prostate (TURP) in the treatment of benign prostatic hyperplasia (BPH); Results of a randomized prospective study with a 1-year follow-up. British Journal of Urology 1997;80(SUPPL. 2):214. [Google Scholar]
Dahlstrand 1995 {published data only}
- Dahlstrand C, Geirsson G, Fall M, Pettersson S. Transurethral microwave thermotherapy versus transurethral resection for benign prostatic hyperplasia: preliminary results of a randomized study. European Urology 1993;23(2):292-8. [DOI] [PubMed] [Google Scholar]
- Dahlstrand C, Walden M, Geirsson G, Pettersson S. Transurethral microwave thermotherapy versus transurethral resection for symptomatic benign prostatic obstruction: a prospective randomized study with a 2-year follow-up. British Journal of Urology 1995;76(5):614-8. [DOI] [PubMed] [Google Scholar]
- Dahlstrand C, Walden M, Geirsson G, Sommar S, Pettersson S. Transurethral microwave thermotherapy versus transurethral resection for BPH. Transurethral microwave thermotherapy versus transurethral resection for BPH. Progress in Clinical and Biological Research 1994;386:455-61. [PubMed] [Google Scholar]
De Wildt 1996 {published data only}
- De La Rosette JJ, De Wilt MJ, Alivizatos G, Froeling FM, Debruyne FM. Transurethral microwave thermotherapy (TUMT) in benign prostatic hyperplasia: placebo versus TUMT. Urology 1994;44(1):58-63. [DOI] [PubMed] [Google Scholar]
- De Wildt MJ, Hubregtse M, Ogden C, Carter SS, Debruyne FM, De la Rosette JJ. A 12-month study of the placebo effect in transurethral microwave thermotherapy. British Journal of Urology 1996;77(2):221-7. [DOI] [PubMed] [Google Scholar]
- Francisca EA, D Ancona FC, Hendriks JC, Kiemeney LA, Debruyne FM, De la Rosette JJ. Quality of life assessment in patients treated with lower energy thermotherapy (Prostasoft 2.0): results of a randomized transurethral microwave thermotherapy versus sham study. Journal of Urology 1997;158(5):1839-44. [DOI] [PubMed] [Google Scholar]
- Ogden CW, Reddy P, Johnson H, Ramsay JW, Carter SS. Sham versus transurethral microwave thermotherapy in patients with symptoms of benign prostatic bladder outflow obstruction. Lancet 1993;341(8836):14-7. [DOI] [PubMed] [Google Scholar]
Floratos 2001 {published data only}
- De La Rosette JJ, Floratos DL, Severens JL, Kiemeney LA, Debruyne FM, Pilar Laguna M. Transurethral resection vs microwave thermotherapy of the prostate: a cost-consequences analysis. BJU International 2003, 2003;92(7):713-8. [DOI] [PubMed] [Google Scholar]
- Floratos DL, Kiemeney LA, Rossi C, Kortmann BB, Debruyne FM, De La Rosette JJ. Long-term followup of randomized transurethral microwave thermotherapy versus transurethral prostatic resection study. Journal of Urology 2001;165(5):1533-8. [PubMed] [Google Scholar]
- Francisca EA, D'Ancona FC, Meuleman EJ, Debruyne FM, De La Rosette JJ. Sexual function following high energy microwave thermotherapy: Results of a randomized controlled study comparing transurethral microwave thermotherapy to transurethral prostatic resection. Journal of Urology 1999;161(2):486-90. [DOI] [PubMed] [Google Scholar]
- Francisca EA, D Ancona FC, Hendriks JC, Kiemeney LA, Debruyne FM, De La Rosette JJ. A randomized study comparing high-energy TUMT to TURP: quality-of-life results. European Urology 2000;38(5):569-75. [DOI] [PubMed] [Google Scholar]
Larson 1998 {published data only}
- Larson TR, Blute ML, Bruskewitz RC, Mayer RD, Ugarte RR, Utz WJ. A high-efficiency microwave thermoablation system for the treatment of benign prostatic hyperplasia: results of a randomized, sham-controlled, prospective, double-blind, multicenter clinical trial. Urology 1998;51(5):731-42. [DOI] [PubMed] [Google Scholar]
Nawrocki 1997 {published data only}
- ISRCTN24866285. A randomised controlled trial of transurethral microwave thermotherapy (TUMT). www.isrctn.com/ISRCTN24866285 (First received 23 January 2004).
- Nawrocki JD, Bell TJ, Lawrence WT, Ward JP. A randomized controlled trial of transurethral microwave thermotherapy. British Journal of Urology 1997;79(3):389-93. [DOI] [PubMed] [Google Scholar]
Nørby 2002a {published data only}
- Nørby B, Nielsen HV, Frimodt-Møller PC. Transurethral interstitial laser coagulation of the prostate and transurethral microwave thermotherapy vs transurethral resection or incision of the prostate: results of a randomized, controlled study in patients with symptomatic benign prostatic hyperplasia. BJU International 2002;90(9):853-62. [DOI] [PubMed] [Google Scholar]
Roehrborn 1998 {published data only}
- Roehrborn CG, Preminger G, Newhall P, Denstedt J, Razvi H, Chin LJ, et al. Microwave thermotherapy for benign prostatic hyperplasia with the Dornier Urowave: results of a randomized, double-blind, multicenter, sham-controlled trial. Urology 1998;51(1):19-28. [DOI] [PubMed] [Google Scholar]
- Trachtenberg J, Roehrborn CG. Updated results of a randomized, double-blind, multicenter sham-controlled trial of microwave thermotherapy with the Dornier Urowave in patients with symptomatic benign prostatic hyperplasia. World Journal of Urology 1998;16(2):102-8. [DOI] [PubMed] [Google Scholar]
Venn 1995 {published data only}
- Venn SN, Montgomery BS, Sheppard SA, Hughes SW, Beard RC, Bultitiude MI, et al. Microwave hyperthermia in benign prostatic hypertrophy: a controlled clinical trial. British Journal of Urology 1995;76(1):73-6. [DOI] [PubMed] [Google Scholar]
Wagrell 2002 {published data only}
- Mattiasson A, Wagrell L, Schelin S, Nordling J, Richthoff J, Magnusson B, et al. Five-year follow-up of feedback microwave thermotherapy versus TURP for clinical BPH: a prospective randomized multicenter study. Urology 2007;69(1):91-7. [DOI] [PubMed] [Google Scholar]
- Wagrell L, Schelin S, Mattiasson A, Magnusson B, Schain M, Ageheim H, et al. Prostalund microwave treatment vs. TURP for LUTS due to BPH. Scandinavian Journal of Urology and Nephrology 2001;35(Suppl 208):18. [Google Scholar]
- Wagrell L, Schelin S, Nordling J, Larsson T, Mattiasson A. Prostalund microwave feedback treatment compared with TURP for treatment of BPH: a prospective randomized multicentre study. Australia and New Zealand Journal of Surgery 2003;73:A146. [Google Scholar]
- Wagrell L, Schelin S, Nordling J, Richthoff J, Magnusson B, Schain M, et al. Three-year follow-up of feedback microwave thermotherapy versus TURP for clinical BPH: a prospective randomized multicenter study. Urology 2004;64(4):698-702. [DOI] [PubMed] [Google Scholar]
- Wagrell L, Schelin S, Nordling J, Richthoff J, Mangnusson B, Schain M, et al. Feedback microwave thermotherapy versus TURP for clinical BPH − a randomized controlled multicenter study. Urology 2002;60:292-9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Albala 2000 {published data only}
- Albala DM, Turk TM, Fulmer BR, Koleski F, Andriole G, Davis BE, et al. Periurethral transurethral microwave thermotherapy for the treatment of benign prostatic hyperplasia: an interim 1-year safety and efficacy analysis using the thermatrx TMx-2000. Techniques in Urology 2000;6(4):288-93. [PubMed] [Google Scholar]
Arai 2000 {published data only}
- Arai Y, Aoki Y, Okubo K, Maeda H, Terada N, Matsuta Y, et al. Impact of interventional therapy for benign prostatic hyperplasia on quality of life and sexual function: A prospective study. Journal of Urology 2000;164(4):1206-11. [PubMed] [Google Scholar]
D'Ancona 1997 {published data only}
- D'Ancona FC, Francisca EA, Debruyne FM, De la Rosette JJ. High-energy transurethral microwave thermotherapy in men with lower urinary tract symptoms. Journal of Endourology 1997;11(4):285-9. [DOI] [PubMed] [Google Scholar]
Dahlstrand 2003 {published data only}
- Dahlstrand C. High-energy microwave therapy with benign prostatic hyperplasia. A good and safe therapeutic choice--for both the patient and the health care. Läkartidningen 2003;100(35):2678-83. [PubMed] [Google Scholar]
Djavan 1999 {published data only}
- Djavan B, Seitz C, Roehrborn CG, Remzi M, Fakhari M, Waldert M, et al. Targeted transurethral microwave thermotherapy versus alpha-blockade in benign prostatic hyperplasia: outcomes at 18 months. Urology 2001;57(1):66-70. [DOI] [PubMed] [Google Scholar]
- Djavan B, Shariat S, Fakhari M, Ghawidel K, Seitz C, Partin AW, et al. Neoadjuvant and adjuvant alpha-blockade improves early results of high-energy transurethral microwave thermotherapy for lower urinary tract symptoms of benign prostatic hyperplasia: a randomized, prospective clinical trial. Urology 1999;53(2):251-9. [DOI] [PubMed] [Google Scholar]
Hahn 2000 {published data only}
- Hahn RG, Farahmand BY, Hallin A, Hannar N, Persson P-G. Incidence of acute myocardial infarction and cause-specific mortality after transurethral treatments of prostatic hypertrophy. Urology 2000;55(2):236-40. [DOI] [PubMed] [Google Scholar]
Hansen 1998 {published data only}
- Hansen BJ, Mortensen S, Mensink HJ, Flyger H, Riehmann M, Hendolin N, et al. Comparison of the Danish Prostatic Symptom Score with the international Prostatic Symptom Score, the Madsen-Iversen and Boyarsky symptom indexes. British Journal of Urology 1998;81(1):36-41. [DOI] [PubMed] [Google Scholar]
ISRCTN23921450 {published data only}
- ISRCTN23921450. A randomised controlled trial comparing the efficacy, safety and cost-effectiveness of transurethral resection (TURP), laser vaporisation (LVAP), transurethral needle ablation (TUNA) and microwave thermoablation (MTA) of the prostate. www.isrctn.com/ISRCTN23921450 (first received 25 April 2003).
Kobelt 2004 {published data only}
- Kobelt G, Spangberg A, Mattiasson A. The cost of feedback microwave thermotherapy compared with transurethral resection of the prostate for treating benign prostatic hyperplasia. BJU International 2004;93(4):543-8. [DOI] [PubMed] [Google Scholar]
Mulvin 1994 {published data only}
- Mulvin D, Creagh T, Kelly D, Smith J, Quinlan D, Fitzpatrick J. Transurethral microwave thermotherapy versus transurethral catheter therapy for benign prostatic hyperplasia. European Urology 1994;26(1):6-9. [DOI] [PubMed] [Google Scholar]
Norby 2002b {published data only}
- Nørby B, Nielsen HV, Frimodt-Møller PC. Cost-effectiveness of new treatments for benign prostatic hyperplasia: results of a randomized trial comparing the short-term cost-effectiveness of transurethral interstitial laser coagulation of the prostate, transurethral microwave thermotherapy and standard transurethral resection or incision of the prostate. Scandinavian Journal of Urology and Nephrology 2002;36(4):286-95. [DOI] [PubMed] [Google Scholar]
Nørby 2004 {published data only}
- Nørby B. Minimally invasive treatment of benign prostatic hyperplasia. Ugeskrift for laeger 2004;166(8):688-90. [PubMed] [Google Scholar]
Ohigashi 2007 {published data only}
- Ohigashi T, Nakamura K, Nakashima J, Baba S, Murai M. Long-term results of three different minimally invasive therapies for lower urinary tract symptoms due to benign prostatic hyperplasia: Comparison at a single institute. International Journal of Urology 2007;14(4):326-30. [DOI] [PubMed] [Google Scholar]
Schelin 2006 {published data only}
- Schelin S, Geertsen U, Walter S, Spångberg A, Duelund-Jacobsen J, Krøyer K, et al. Feedback microwave thermotherapy versus TURP/prostate enucleation surgery in patients with benign prostatic hyperplasia and persistent urinary retention: a prospective, randomized, controlled, multicenter study. Urology 2006;68(4):795-9. [DOI] [PubMed] [Google Scholar]
Servadio 1987 {published data only}
- Servadio C, Leib Z, Lev A. Diseases of prostate treated by local microwave hyperthermia. Urology 1987;30(2):97-9. [DOI] [PubMed] [Google Scholar]
Shore 2010 {published data only}
- Shore ND, Sethi PS. A controlled, randomized, head-to-head comparison of the Prolieve Thermodilation System versus the Targis System for benign prostatic hyperplasia: safety, procedural tolerability, and clinical results. Journal of Endourology 2010;24(9):2469-75. [DOI] [PubMed] [Google Scholar]
Tan 2005 {published data only}
- Tan AH, Nott L, Hardie WR, Chin JL, Denstedt JD, Razvi H. Long-term results of microwave thermotherapy for symptomatic benign prostatic hyperplasia. Journal of Endourology 2005;19(10):1191-5. [DOI] [PubMed] [Google Scholar]
Trock 2004 {published data only}
- Trock BJ, Brotzman M, Utz WJ, Ugarte RR, Kaplan SA, Larson TR, et al. Long-term pooled analysis of multicenter studies of cooled thermotherapy for benign prostatic hyperplasia: results at three months through four years. Urology 2004;63(4):716-21. [DOI] [PubMed] [Google Scholar]
Vesely 2006 {published data only}
- Vesely S, Knutson T, Damber J-E, Dicuio M, Dahlstrand C. TURP and low-energy TUMT treatment in men with LUTS suggestive of bladder outlet obstruction selected by means of pressure-flow studies: 8-Year follow-up. Neurourology and Urodynamics 2006;25(7):770-5. [DOI] [PubMed] [Google Scholar]
Waldén 1998 {published data only}
- Waldén M, Acosta S, Carlsson P, Pettersson S, Dahlstrand C. A cost-effectiveness analysis of transurethral resection of the prostate and transurethral microwave thermotherapy for treatment of benign prostatic hyperplasia: two-year follow-up. Scandinavian Journal of Urology and Nephrology 1998;32(3):204-10. [DOI] [PubMed] [Google Scholar]
Zerbib 1992 {published data only}
- Zerbib M, Steg A, Conquy S, Martinache PR, Flam TA, Debre B. Localized hyperthermia versus the sham procedure in obstructive benign hyperplasia of the prostate: a prospective randomized study. Journal of Urology 1992;147(4):1048-52. [DOI] [PubMed] [Google Scholar]
Zerbib 1994 {published data only}
- Zerbib M, Steg A, Conquy S, Debre B. Hyperthermia: a randomized prospective study applying hyperthermia or a sham procedure in obstructive benign hyperplasia of the prostate. Progress in Clinical and Biological Research 1994;386:439-48. [PubMed] [Google Scholar]
References to studies awaiting assessment
Albala 2000a {published data only}
- Albala DM, Turk TM, Fulmer BR, Koleski F, Andriole G, Davis BE, et al. Periurethral transurethral microwave thermotherapy for the treatment of benign prostatic hyperplasia: An interim 1-year safety and efficacy analysis using the TherMatrx TMx-2000â„¢. Techniques in Urology 2000;6(4):288-93. [PubMed] [Google Scholar]
Dahlstrand 1994 {published data only}
- Dahlstrand C, Waldén M, Geirsson G, Sommar S, Pettersson S. Transurethral microwave thermotherapy versus transurethral resection for BPH. Progress in Clinical and Biological Research 1994;386:455-61. [PubMed] [Google Scholar]
Dahlstrand 1997 {published data only}
- Dahlstrand C, Pettersson S. Prospective randomized study of TURP versus tumt for benign prostatic hyperplasia with a 5-year follow-up. British Journal of Urology 1997;80(SUPPL. 2):211. [DOI] [PubMed] [Google Scholar]
Dahlstrand 1998 {published data only}
- Dahlstrand C, Knutson T, Pettersson S. Seven-year follow-up of transurethral microwave thermotherapy vs transurethral resection for symptomatic benign prostatic hyperplasia. Scandinavian Journal of Urology and Nephrology 1998;33(Suppl 198):19. [DOI] [PubMed] [Google Scholar]
Devonec 1994 {published data only}
- Devonec M, Houdelette P, Colombeau P, Menguy P, Peneau M. A multicentre study off sham versus thermotherapy in benign prostatic hypertrophy. Journal of Urology 1994;151:415A. [Google Scholar]
Roehrborn 1997 {published data only}
- Roehrborn CG, Sech SM, Preminger GM, Cohen T, Perlmutter A, Razvi H, et al. A randomized, blinded study comparing microwave thermotherapy (dornier urowave(tm)) with a sham procedure in patients with clinical benign prostatic hyperplasia (bph). British Journal of Urology 1997;80(SUPPL. 2):192. [Google Scholar]
Additional references
Agarwal 2014
- Agarwal A, Eryuzlu LN, Cartwright R, Thorlund K, Tammela TL, Guyatt GH, et al. What is the most bothersome lower urinary tract symptom? Individual- and population-level perspectives for both men and women. European Urology 2014;65(6):1211-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aoun 2015
- Aoun F, Marcelis Q, Roumeguere T. Minimally invasive devices for treating lower urinary tract symptoms in benign prostate hyperplasia: technology update. Research and Reports in Urology 2015;7:125-36. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
AUA 2003
Barry 1992
- Barry MJ, Fowler FJ, O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. Journal of Urology 1992;148(5):1549-57; discussion 1564. [PMID: ] [DOI] [PubMed] [Google Scholar]
Barry 1995
- Barry MJ, Williford WO, Chang Y, Machi M, Jones KM, Walker-Corkery E, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? Journal of Urology 1995;154(5):1770-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Barry 1997
- Barry MJ, Fowler FJ, Bin L, Pitts JC, Harris CJ, Mulley AG. The natural history of patients with benign prostatic hyperplasia as diagnosed by North American urologists. Journal of Urology 1997;157(1):10-4; discussion 14-5. [PMID: ] [PubMed] [Google Scholar]
Barry 2013
- Barry MJ, Cantor A, Roehrborn CG, CAMUSSG. Relationships among participant international prostate symptom score, benign prostatic hyperplasia impact index changes and global ratings of change in a trial of phytotherapy in men with lower urinary tract symptoms. Journal of Urology 2013;189(3):987-92. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barry Delongchamps 2012
- Barry Delongchamps N, Robert G, Descazeaud A, Cornu JN, Azzouzi AR, Haillot O, et al. Surgical treatment of benign hyperplasia of the prostate with thermotherapy and other emerging techniques; review of the literature of the CTMH of the AFU [Traitement chirurgical de l'hyperplasie bénigne de la prostate par thermothérapie et autres techniques émergentes: Revue de littérature du CTMH de l'AFU]. Progres en Urologie 2012;22(2):87-92. [DOI] [PubMed]
Bhojani 2014
- Bhojani N, Gandaglia G, Sood A, Rai A, Pucheril D, Chang SL, et al. Morbidity and mortality after benign prostatic hyperplasia surgery: data from the American College of Surgeons national surgical quality improvement program. Journal of Endourology 2014;28(7):831-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Blute 1993
- Blute ML, Tornera KM, Hellerstein DK, McKiel CF Jr, Lynch JH, Regan JB, Sankey NE. Transurethral microwave thermotherapy for management of benign prostatic hyperplasia: results of the United States Prostatron Cooperative Study.. Journal of Urology 1993;150:1591. [DOI] [PubMed] [Google Scholar]
Chapple 2017
- Chapple C, Castro-Diaz D, Chuang YC, Lee KS, Liao L, Liu SP, et al. Prevalence of lower urinary tract symptoms in China, Taiwan, and South Korea: results from a cross-sectional, population-based tudy. Advances in Therapy 2017;34(8):1953-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cornu 2010
- Cornu JN, Cussenot O, Haab F, Lukacs B. A widespread population study of actual medical management of lower urinary tract symptoms related to benign prostatic hyperplasia across Europe and beyond official clinical guidelines. European Urology 2010;58(3):450-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation Covidence. Version accessed 14 December 2020. Melbourne, Australia: Veritas Health Innovation, 2013. Available at www.covidence.org.
Crawford 2006
- Crawford ED, Wilson SS, McConnell JD, Slawin KM, Lieber MC, Smith JA, et al. Baseline factors as predictors of clinical progression of benign prostatic hyperplasia in men treated with placebo. Journal of Urology 2006;175(4):1422-6; discussion 1426-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dahm 2021
- Dahm P, MacDonald R, McKenzie L, Jung JH, Greer NL, Wilt T. Newer minimal-invasive treatment modalities to treat lower urinary tract symptoms attributed to benign prostatic hyperplasia. European Urology Open Science 2021;26:72-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). The Cochrane Collaboration, 2021. Available from www.training.cochrane.org/handbook.
Dindo 2004
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of Surgery 2004;240(2):205-13. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Djavan 2001
- Djavan B, Seitz C, Roehrborn CG, Remzi M, Fakhari M, Waldert M, et al. Targeted transurethral microwave thermotherapy versus alpha-blockade in benign prostatic hyperplasia: outcomes at 18 months. Urology 2001;57(1):66-70. [DOI] [PubMed] [Google Scholar]
Dunphy 2015
- Dunphy C, Laor L, Te A, Kaplan S, Chughtai B. Relationship between depression and lower urinary tract symptoms secondary to benign prostatic hyperplasia. Reviews in Urology 2015;17(2):51-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
EAU 2021
- European Association of Urology. Treatment of non-neurogenic male LUTS. uroweb.org/guideline/treatment-of-non-neurogenic-male-luts/ (accessed 27 April 2021).
Egan 2016
- Egan KB. The epidemiology of benign prostatic hyperplasia associated with lower urinary tract symptoms: prevalence and incident rates. Urologic Clinics of North America 2016;43(3):289-97. [PMID: ] [DOI] [PubMed] [Google Scholar]
Franco 2020
- Franco JV, Jung JH, Imamura M, Borofsky M, Omar MI, Escobar Liquitay CM, et al. Minimally invasive treatments for lower urinary tract symptoms in men with benign prostatic hyperplasia: a network meta‐analysis. Cochrane Database of Systematic Reviews 2020, Issue 6. Art. No: CD013656. [DOI: 10.1002/14651858.CD013656] [DOI] [PMC free article] [PubMed] [Google Scholar]
Frieben 2010
- Frieben RW, Lin HC, Hinh PP, Berardinelli F, Canfield SE, Wang R. The impact of minimally invasive surgeries for the treatment of symptomatic benign prostatic hyperplasia on male sexual function: a systematic review. Asian Journal of Andrology 2010;12(4):500-8. [DOI] [PMC free article] [PubMed]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 2 February 2021. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ. What is "quality of evidence" and why is it important to clinicians? BMJ (Clinical Research Ed.) 2008;336(7651):995-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence--imprecision. Journal of Clinical Epidemiology 2011;64(12):1283-93. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
Henney 2000
- Henney JE. Microwave Therapy Warning. JAMA 2000;284(21):2711.
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DA, Sterne JA, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). The cochrane Collaboration, 2021. Available from www.www.training.cochrane.org/handbook.
Homma 1997
- Homma Y, Kawabe K, Tsukamoto T, Yamanaka H, Okada K, Okajima E, et al. Epidemiologic survey of lower urinary tract symptoms in Asia and Australia using the international prostate symptom score. International Journal of Urology : Official Journal of the Japanese Urological Association 1997;4(1):40-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hwang 2019
- Hwang EC, Jung JH, Borofsky M, Kim MH, Dahm P. Aquablation of the prostate for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2019;(2). [DOI: 10.1002/14651858.CD013143.pub2] [CD013143] [DOI] [PMC free article] [PubMed]
Jaeschke 1989
- Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Controlled Clinical Trials 1989;10(4):407-15. [PMID: ] [DOI] [PubMed] [Google Scholar]
Johnston 2013
- Johnston BC, Patrick DL, Busse JW, Schünemann HJ, Agarwal A, Guyatt GH. Patient-reported outcomes in meta-analyses--Part 1: assessing risk of bias and combining outcomes. Health and Quality of Life Outcomes 2013;11:109. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jung 2019
- Jung JH, Reddy B, McCutcheon KA, Borofsky M, Narayan V, Kim MH, et al. Prostatic urethral lift for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2019, Issue 5. Art. No: CD012832. [DOI: 10.1002/14651858.CD012832.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jung 2020
- Jung JH, McCutcheon KA, Borofsky M, Young S, Golzarian J, Reddy B, et al. Prostatic arterial embolization for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2020, Issue 12. Art. No: CD012867. [DOI: 10.1002/14651858.CD012867.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kang 2020
- Kang TW, Jung JH, Hwang EC, Borofsky M, Kim MH, Dahm P. Convective radiofrequency water vapour thermal therapy for lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2020, Issue 3. Art. No: CD013251. [DOI: 10.1002/14651858.CD013251.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kozminski 2015
- Kozminski MA, Wei JT, Nelson J, Kent DM. Baseline characteristics predict risk of progression and response to combined medical therapy for benign prostatic hyperplasia (BPH). BJU International 2015;115(2):308-16. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2017
- Lee SW, Chan EM, Lai YK. The global burden of lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a systematic review and meta-analysis. Scientific Reports 2017;7(1):7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leissner 1979
- Leissner KH, Tisell LE. The weight of the human prostate. Scandinavian Journal of Urology and Nephrology 1979;13(2):137-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Malaeb 2012
- Malaeb BS, Yu X, McBean AM, Elliott SP. National trends in surgical therapy for benign prostatic hyperplasia in the United States (2000-2008). Urology 2012;79(5):1111-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marra 2016
Martin 2014
- Martin S, Lange K, Haren MT, Taylor AW, Wittert G. Risk factors for progression or improvement of lower urinary tract symptoms in a prospective cohort of men. Journal of Urology 2014;191(1):130-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
McNicholas 2016
- McNicholas TA. Benign prostatic hyperplasia and new treatment options - a critical appraisal of the UroLift system. Medical Devices (Auckland, N.Z.) 2016;9:115-23. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McVary 2011
- McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, Donnell RF, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. Journal of Urology 2011;185(5):1793-803. [PMID: ] [DOI] [PubMed] [Google Scholar]
Morton 2020
- Morton A, Williams M, Perera M, Teloken PE, Donato P, Ranasinghe S, et al. Management of benign prostatic hyperplasia in the 21st century: temporal trends in Australian population-based data. BJU International 2020, 2020;126(S1):18-26. [DOI: 10.1111/bju.15098] [DOI] [PubMed] [Google Scholar]
Nickel 2018
- Nickel JC, Aaron L, Barkin J, Elterman D, Nachabé M, Zorn KC. Canadian Urological Association guideline on male lower urinary tract symptoms/benign prostatic hyperplasia (MLUTS/BPH): 2018 update. Canadian Urological Association Journal = Journal de l'Association des urologues du Canada 2018;12(10):303-12. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2020
- Page MJ, McKenzie J, Bossuyt P, Boutron I, Hoffmann T, Mulrow D, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews 2020. Available at: osf.io/preprints/metaarxiv/v7gm2/ 2020 (Last accessed 13 February 2021). [DOI: 10.31222/osf.io/v7gm2] [DOI]
Page 2021
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). The Cochrane Collaboration, 2021. Available from www.training.cochrane.org/handbook..
Pariser 2015
- Pariser JJ, Pearce SM, Patel SG, Bales GT. National trends of simple prostatectomy for benign prostatic hyperplasia with an analysis of risk factors for adverse perioperative outcomes. Urology 2015;86(4):721-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Parsons 2020
- Parsons JK, Dahm P, Köhler TS, Lerner LB, Wilt TJ. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA Guideline amendment 2020. Journal of Urology 2020;204(4):799-804. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rees 2015
Reich 2008
- Reich O, Gratzke C, Bachmann A, Seitz M, Schlenker B, Hermanek P, et al. Morbidity, mortality and early outcome of transurethral resection of the prostate: a prospective multicenter evaluation of 10,654 patients. Journal of Urology 2008;180(1):246-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
RevMan 2020 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Roehrborn 2008
- Roehrborn CG. Current medical therapies for men with lower urinary tract symptoms and benign prostatic hyperplasia: achievements and limitations. Reviews in Urology 2008;10(1):14-25. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Rosen 1997
- Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997;49(6):822-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rosen 2004
- Rosen RC, Catania J, Pollack L, Althof S, O'Leary M, Seftel AD. Male Sexual Health Questionnaire (MSHQ): scale development and psychometric validation. Urology 2004;64(4):777-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rosen 2007
- Rosen RC, Catania JA, Althof SE, Pollack LM, O'Leary M, Seftel AD, et al. Development and validation of four-item version of Male Sexual Health Questionnaire to assess ejaculatory dysfunction. Urology 2007;69(5):805-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rosen 2011
- Rosen RC, Allen KR, Ni X, Araujo AB. Minimal clinically important differences in the erectile function domain of the International Index of Erectile Function scale. European Urology 2011;60(5):1010-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rubeinstein 2003
SBU 2011
- Assessment Swedish Council on Health Technology. Benign Prostatic Obstruction: A Systematic Review. Swedish Council on Health Technology Assessment (SBU), 2011. [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodologically quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408-12. [DOI] [PubMed] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). The Cochrne Collaboration, 2021. Available from www.training.cochrane.org/handbook.
Spaliviero 2010
- Spaliviero M, Strom KH, Gu X, Araki M, Culkin DJ, Wong C. Does Greenlight HPS(™) laser photoselective vaporization prostatectomy affect sexual function? Journal of Endourology 2010;24(12):2051-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Walmsley 2004
- Walmsley K, Kaplan SA. Transurethral microwave thermotherapy for benign prostate hyperplasia: separating truth from marketing hype. Journal of Urology 2004;172(4 Part 1):1249-55. [DOI] [PubMed] [Google Scholar]
Yoo 2012
- Yoo TK, Cho HJ. Benign prostatic hyperplasia: from bench to clinic. Korean Journal of Urology 2012;53(3):139-48. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 2008
- Yu X, Elliott SP, Wilt TJ, McBean AM. Practice patterns in benign prostatic hyperplasia surgical therapy: the dramatic increase in minimally invasive technologies. Journal of Urology 2008;180(1):241-5. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hoffman 2012
- Hoffman RM, Monga M, Elliott SP, MacDonald R, Langsjoen J, Tacklind J, et al. Microwave thermotherapy for benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2012, Issue 9. Art. No: CD004135. [DOI: 10.1002/14651858.CD004135.pub3] [DOI] [PubMed] [Google Scholar]


