Abstract
Background:
Delayed walking is common in intellectual disability (ID) but may be less common when ID occurs with autism spectrum disorder (ASD). Previous studies examining this were limited by reliance on clinical samples and exclusion of children with severe motor deficits.
Objective:
To examine in a population-based sample if age of walking is differentially related to intellectual ability in children with ASD versus other neurodevelopmental disorders (NDD).
Methods:
Participants were from the nested Autism Birth Cohort Study of the Norwegian Mother, Father and Child Cohort Study (MoBa). Cox proportional hazards regression assessed if diagnosis (ASD n=212 versus NDD n=354), continuous nonverbal IQ, and their interaction, were associated with continuous age of walking.
Results:
The relationship between nonverbal IQ and age of walking was stronger for NDD than for ASD (Group x nonverbal IQ interaction, χ2=13.93, p=0.0002). This interaction was characterized by a 21% decrease in the likelihood of walking onset at any given time during the observation period per 10-point decrease in nonverbal IQ (hazard ratio=0.79, 95% CI: 0.78–0.85) in the NDD group compared to 8% (hazard ratio=0.92, 95% CI: 0.86–0.98) in the ASD group.
Conclusions:
Replicating the finding in a population-based sample that late walking is less strongly related to low intellectual ability in children with ASD than in children without other NDDs supports the hypothesis that ID in ASD may result from heterogeneous developmental pathways. Late walking may be a useful stratification variable in etiological research focused on ASD and other NDDs.
Keywords: Intellectual disability, Gross motor milestones, Late walking, Epidemiology
Introduction
Low intellectual ability is associated with delays in attainment of early developmental milestones, including walking (Flensborg-Madsen & Mortensen, 2015; Hamadani, Tofail, Cole, & Grantham-McGregor, 2013; Murray, Jones, Kuh, & Richards, 2007; Poranen-Clark et al., 2015; Taanila, Murray, Jokelainen, Isohanni, & Rantakallio, 2005; Vlasblom et al., 2019). Especially among individuals with severe and profound intellectual disability (ID), late walking is very common, with one study showing that less than 40% of children with profound ID walked before 18 months (Kaminer & Jedrysek, 1983). Thus, late onset of walking is an early marker of potential global developmental delay and/or ID.
Autism spectrum disorder (ASD) is often diagnosed with co-occurring ID (Baio et al., 2018; Fombonne, 2005; Ritvo et al., 1989), and late walking does occur in children with ASD at all cognitive levels (Reindal et al., 2020). However, compared to children with ID without ASD, late walking appears to be much less common among children with ASD , even in those with severe to profound ID (Bishop, Thurm, Farmer, & Lord, 2016; Kaminer & Jedrysek, 1983; Kokubun, Haishi, Okuzumi, & Hosobuchi, 1995). This finding is intriguing, as it indicates that, among children with ASD, ID is not always associated with the same types of early developmental predictors characteristic of other groups with ID.
A major limitation of previous studies of the association between ASD, age of walking, and ID, is that participants were recruited from ASD specialty clinics (Bishop et al., 2016). If delayed walking is observed prior to any other developmental concern, services may be more likely to be rendered by pediatrics, neurology, or genetics clinics than by ASD clinical-research centers. As a result, the low rates of late walking and/or strength of associations between late walking and ID in ASD clinical samples could be explained by sampling bias. Furthermore, specific study exclusion criteria may have affected the rates of late walking (e.g., excluding children who cannot walk independently (Thurm, Farmer, Salzman, Lord, & Bishop, 2019). Finally, while the onset of walking may be less susceptible than other milestones to telescoping effects (i.e., recalling distant events as closer to the present and therefore more delayed than they were) (Hus, Taylor, & Lord, 2011), the accuracy of parental retrospective recall is a concern (Ozonoff, Li, Deprey, Hanzel, & Iosif, 2018).
This is the first study to use a prospective population-based cohort to test the hypothesis that age of walking is less predictive of intellectual ability in children with ASD than in children with other neurodevelopmental disorders (NDDs). Secondarily, to examine the specificity of this relationship to walking, we tested for a weaker association in ASD (compared to other NDDs) between intellectual ability and other potential indicators of early global functioning which have been robustly associated with developmental delay/ID (i.e., pre and perinatal factors such as birth weight, gestational age, APGAR scores, as well as early delays in words and phrases) (Bilder et al., 2013; Huang, Zhu, Qu, & Mu, 2016; Modabbernia, Mollon, Boffetta, & Reichenberg, 2016; Murray et al., 2007).
Methods
Participants
The Norwegian Mother, Father and Child Cohort Study (MoBa) is a prospective population-based pregnancy cohort study conducted by the Norwegian Institute of Public Health (NIPH) (Magnus et al., 2016; Magnus et al., 2006). Participants were recruited from all over Norway from 1999–2008. Women consented to participation in 40.6% of the pregnancies. The cohort now includes 114,500 children, 95,200 mothers and 75,200 fathers. The current study is based on version nine of the quality-assured data files. The children were born August 1999-July 2009. Parents in MoBa were invited for an in-person assessment of their child in the Autism Birth Cohort (ABC) Study research clinic based on criteria related to early developmental delays, including: parent endorsement that the child has been referred due to concern about language delay (3-year-questionnaire), or parent concern that the child shows little interest in playing with peers (3-year-questionnaire), or having a sibling invited to the ABC Study, or high score on the Social Communication Questionnaire (study-specific criteria in the 3-year-questionnaire) (Rutter, Bailey, & Lord, 2003; Surén et al., 2014), or parent concern about autistic traits or ASD (3/5/7-year-questionnaire), or referral to the ABC Study by parents or professional for concern about ASD, or having a diagnosis of ASD in the national patient registry. In total, parents of 1,306 children were invited based on any of these criteria. In addition, 915 children randomly selected from the MoBa cohort were invited for assessment as controls. Participation rate for the clinical assessment in the ABC Study was approximately 50% for all those invited (1,033 participated). Figure 1 shows the participant flow and eTable 2 the comparison of participants and non-participants on the study variables. The ABC Study assessments were carried out during 2005–2012 at Lovisenberg Diaconal Hospital in Oslo, in collaboration with the NIPH and Columbia University (Stoltenberg et al., 2010). Further information about the ABC Study is available in Surén et al., 2014. The children were aged between 3 and 11 years at the time of assessment (see means and standard deviations in Table 1). Data from MoBa and the nested ABC study are available to researchers by application to the Norwegian Regional Committee for Medical and Health Research Ethics (REK) and MoBa (https://www.fhi.no/en/studies/moba/for-forskere-artikler/research-and-data-access/).
Figure 1. Participant flow.
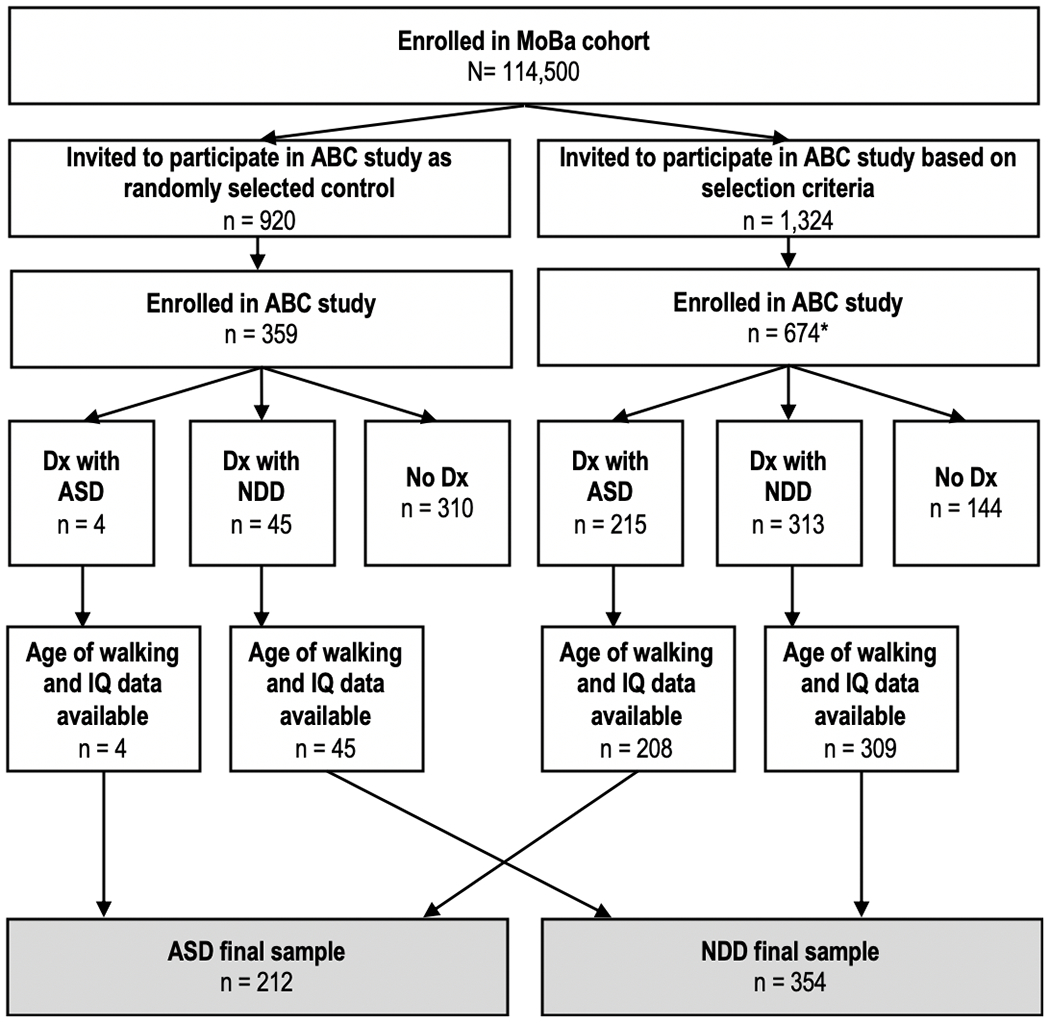
Note: Of the children invited to participate in the ABC study based on selection criteria, one child did not complete the assessment and one child had Rett syndrome. Both were therefore excluded. ASD=autism spectrum disorder. NDD=neurodevelopmental disorders without ASD. IQ=intelligence quotient. MoBa=the Norwegian Mother, Father and Child Cohort Study. Of the children without any clinical diagnosis (n=454), 444 children had data on walking and IQ. ASD included ABC Protocol ASD diagnosis as of 19 Oct 2014 (Autistic Disorder, Asperger Disorder and Pervasive Developmental Disorder Not Otherwise Specified), childhood disintegrative disorder and profound disability with autism.*Rett syndrome was excluded (n=2) because of insufficient information on whether ASD diagnostic criteria were met (i.e., for either Autistic Disorder or PDD-NOS).
Table 1.
Demographic information
| ASD | NDD | ||||
|---|---|---|---|---|---|
| NVIQ> 70 | NVIQ<= 70 | NVIQ> 70 | NVIQ<= 70 | ||
| n | 148 | 64 | 308 | 46 | |
| Age at assessment (months), M ± SD | 71.31 ± 28.15 | 57.08 ± 23.06 | 45.89 ± 14.45 | 45.18 ± 11.96 | |
| Sex (female), n (%) | 22 (14.86) | 17 (26.56) | 82 (26.62) | 12 (26.09) | |
| Walking at time of assessment, n (%) | 148 (100%) | 59 (92.19%) | 307 (99.68%) | 36 (78.26%) | |
| Age of walking*, median (IQR) | 14 (12-16) | 15 (12-18) | 14 (12-16) | 23 (18.50-27.50) | |
| Walking at or after 18 months**], n (%) | 22 (14.86%) | 21 (32.81%) | 41 (13.31%) | 39 (84.78%) | |
| Nonverbal IQ, M ± SD | 99.09 ± 17.05 | 49.48 ± 15.76 | 97.87 ± 12.82 | 48.19 ± 17.23 | |
| Birth weight (grams), M ± SD | 3595.12 ± 734.51 | 3470.17 ± 706.03 | 3460.45 ± 694.95 | 3139.85 ± 613.63 | |
| Low birth weight (<2500g), n (%) | 10 (6.76) | 5 (7.81) | 25 (8.12) | 4 (8.70) | |
| Gestational Age (weeks), n (%) | 22-27 | 1 (0.68) | 1 (1.56) | 1 (0.32) | - |
| 28-36 | 12 (8.11) | 4 (6.25) | 31 (10.06) | 8 (17.39) | |
| 37 | 9 (6.08) | 4 (6.25) | 23 (7.47) | 4 (8.70) | |
| 38 | 19 (12.84) | 9 (14.06) | 42 (13.64) | 9 (19.57) | |
| 39 | 30 (20.27) | 11 (17.19) | 63 (20.45) | 6 (13.04) | |
| 40 | 38 (25.68) | 14 (21.88) | 81 (26.30) | 7 (15.22) | |
| 41 | 24 (16.22) | 16 (25) | 46 (14.94) | 9 (19.57) | |
| 42 | 14 (9.46) | 5 (7.81) | 21 (6.82) | 3 (6.52) | |
| Missing | 1 (0.68) | - | - | - | |
| APGAR 1 minute, Median (IQR) | 9 (8-9) | 9 (8-9) | 9 (9-9) | 9 (8-9) | |
| Low APGAR 1, n (%) | 10 (6.76) | 5 (7.81) | 27 (8.77) | 5 (10.87) | |
| APGAR 5 minutes, Median (IQR) | 9 (9-10) | 9 (9-10) | 9 (9-10) | 9 (9-10) | |
| Low APGAR 5, n (%) | 3 (2.03) | 2 (3.13) | 8 (2.60) | 1 (2.17) | |
| Genetic Syndrome, n (%) | 4 (2.70) | 7 (10.94) | 6 (1.95) | 11 (23.91) | |
| First words by 18 months, n (%) | Yes | 51 (34.46) | 10 (15.63) | 69 (22.40) | 3 (6.52) |
| No | 63 (42.57) | 37 (57.81) | 198 (64.29) | 38 (82.61) | |
| Missing | 34 (22.97) | 17 (26.56) | 41 (13.31) | 5 (10.87) | |
| First phrases by 36 months, n (%) | Yes | 103 (69.59) | 20 (31.25) | 241 (78.25) | 11 (23.91) |
| No | 18 (12.16) | 33 (51.56) | 51 (16.56) | 34 (73.91) | |
| Missing | 27 (18.24) | 11 (17.19) | 16 (5.19) | 1 (2.17) | |
Notes: IQR=interquartile range. Nonverbal IQ was categorized for the purpose of this table only and was entered as a continuous variable in all analyses.
Children who were not yet walking at the time of assessment (age range 37-46 months) had missing data on age of walking (ASD n=5 (2%), other NDDs n=11 (3%)).
The 97th percentile in the Norwegian data (18 months) was used as a classifier for late walking in this table only (continuous age of walking was used for all analyses).
Procedure
The establishment of MoBa and initial data collection was based on a license from the Norwegian Data Protection Agency and approval from REK. The MoBa cohort is currently regulated by the Norwegian Health Registry Act. Written informed consent was obtained from mothers and fathers. The ABC Study has approval from REK (S-03228) and participation was based on an additional written informed consent. ABC Study participants underwent a multi-disciplinary clinical evaluation over 1 or 2 days, including parent interview about developmental history and current concerns, physical exam, standardized observation of mother-child play interaction, and standardized instruments to assess symptoms of ASD (ADOS and ADI-R; Lord, Rutter, et al., 2012; Lord, Rutter, & Le Couteur, 1994) and other neurodevelopmental and psychiatric conditions (ECI-4, CSI-4 and PAPA; Egger et al., 2006; Gadow & Sprafkin, 1997; Sprafkin et al., 2002). The assessment team did not have access to information from MoBa questionnaires or previous evaluations. Following the assessment, the clinician team met to review all available information, discuss clinical impressions, and assign a best-estimate clinical diagnosis based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV-TR) criteria (American Psychiatric Association, 2000).
Measures
Demographic and medical history data were taken from the Medical Birth Registry (MBRN) and the MoBa questionnaires, and included gestational age, birthweight, and APGAR scores at 1 and 5 minutes. The MBRN is a national health registry containing information about all births in Norway. In the 18-month-questionnaire mothers answered whether the child usually says eight or more single words; in the 36-month-questionnaire mothers answered whether the child usually combines words into phrases. Age of walking was reported by mothers in the 18-month questionnaire, the 36-month-questionnaire, and at the ABC clinic assessment (mean age 54 months; item five from the Autism Diagnostic Interview-Revised (ADI-R) (Lord et al., 1994). Age of walking showed high reliability across the three time points (intraclass correlation coefficient of 0.81, 95% CI 0.80–0.84, n=564; in the full MoBa cohort ICC between age of walking in the 18- and 36-month questionnaires was 0.84, 95% CI 0.84–0.85, n=50,228). Nevertheless, to minimize any recall bias we used the earliest available report of age of walking (18-month-questionnaire > 36-month-questionnaire > clinic assessment). Age of walking was used as a continuous variable in all analyses.
Intellectual ability was estimated from tests administered during the ABC clinic assessment. We used nonverbal IQ because it is a more stable measure of intellectual ability and less confounded with language delay than verbal IQ (Begovac, Begovac, Majić, & Vidovic, 2009; Bishop, Farmer, & Thurm, 2015). A standard hierarchy of cognitive tests was used. Children who were 6 years or older received the Wechsler Abbreviated Scale of Intelligence (WASI, n=88). All children younger than 6 years, and older children with lower developmental level than required for valid completion of the WASI, received the Stanford–Binet Intelligence Scales-5th Edition (SB5; full: n=629, abbreviated version: n=215) (Roid, 2003). Children with lower developmental levels than required for valid completion of the SB5 received the Mullen Scales of Early Learning (MSEL; n=87) (Mullen, 1995). To avoid floor effects on the MSEL, ratio nonverbal IQ was derived from age-equivalent scores from the Visual Reception and Fine Motor subscales (nonverbal mental age/chronological age*100) (Farmer, Golden, & Thurm, 2015). Nonverbal IQ was used as a continuous variable in all analyses.
In line with the current gold standard (Volkmar et al., 2014), each child was assigned a Best-estimate Clinical Diagnosis based on all information obtained as part of the ABC clinic assessment. Diagnoses were further verified by an extended team of experienced clinicians, based on review of all available information from the clinical assessment, as well as information from the Norwegian Patient Registry (20 children who were not diagnosed with ASD at the ABC clinic assessment but registered with ASD in the patient registry by December 2013, were assigned a best-estimate clinical diagnosis of ASD by the extended team upon review of all the ABC clinic assessment results). At the time of the assessment, the DSM-IV-TR was in use (American Psychiatric Association, 1994). ASD diagnosis included Autistic Disorder (n=94), Asperger Disorder (n=34), and Pervasive Developmental Disorder-Not Otherwise Specified (n=78). For consistency with Diagnostic and Statistical Manual of Mental disorders, Fifth Edition (DSM-5) (American Psychiatric Association, 2013) classification, the present analysis also included children diagnosed with childhood disintegrative disorder (n=2) and profound disability with autism (n=4) in the ASD group. Children diagnosed with Rett syndrome (n=2) were excluded because there was insufficient information on whether ASD diagnostic criteria were met (i.e., for either Autistic Disorder or PDD-NOS). As shown in Figure 1, data on walking and IQ was available for 212 children with ASD and 354 with NDD without ASD (primarily diagnosed with developmental language disorder, (n=216), ID (n=45), and attention-deficit/hyperactivity disorder (n=30) (see other diagnoses [n=63] in Supplementary eTable 1)). In addition, 444 children with data on walking and IQ did not meet criteria for any DSM-IV-TR diagnosis (n=301 [68%] of whom had been randomly selected controls).
Data analysis
Complete-case analysis was used. Age of walking is a time-to-event variable with incomplete time-to-event data on children not yet walking at the end of follow-up. Therefore, we applied a Cox proportional hazards regression model to the data from children with ASD or NDD (n=566), using age of walking in months as timescale, to test whether the relationship between continuous nonverbal IQ (centered at the group mean 88 to ease interpretation) and onset of walking differed by diagnosis (ASD versus other NDD). Children who had not begun walking at the time of the assessment were considered as censored (n=16; end of follow-up age range: 37–46 months), meaning that these cases were included in the time-to-event analysis until the end of their follow-up time. Sex was entered as a covariate. We used robust sandwich covariance estimation to account for eleven sibships observed in the data. Model assumptions were evaluated via visual inspection of Martingale residuals. The analyses above were separately performed for children who did not receive any diagnosis (n=444 with valid age of walking and nonverbal IQ data), purely for comparison purposes given that we had no prior expectation for an association between age of walking and nonverbal IQ in typically developing children.
Generalized linear models were used to test whether the relationship of nonverbal IQ to the secondary early predictors of ID (birth weight, gestational age, APGAR scores, first words by 18 months, first phrases by 36 months) differed by diagnostic group. Complete-case analysis was used; in all cases, the rate of missing data was very similar between groups. The appropriate linkage function was selected depending on the variable type (i.e., binary: logit; ordinal: cumulative logit; continuous: identity). Initially, a random effect of mother was used to account for sibships; the limited amount of clustering led to convergence problems and this effect was discarded.
As the analyses were planned, alpha was set at 5% for all comparisons and raw p-values are reported.
Results
Demographics for the 566 children with ASD or NDD are found in Table 1. The results of the initial Cox Proportional Hazards model with a linear effect of nonverbal IQ indicated that the functional form of nonverbal IQ was mis-specified. The model fit was improved (−2LL Δ = 24) by the addition of the quadratic term, and this model was selected. Our primary hypothesis was supported by the results of this model; the relationship between nonverbal IQ and age of walking was stronger among NDD participants than among ASD participants (Group x nonverbal IQ interaction, χ2=13.93, p=0.0002). For example, a 10-point decrease in IQ was associated with a 21% decrease in the likelihood of walking onset at any given time during the observation period (hazard ratio=0.79, 95% CI: 0.72–0.85) in the NDD group compared to 8% (hazard ratio=0.92, 95% CI: 0.86–0.98) in the ASD group (results for the “no diagnosis” group are shown in eFigure 1). The interaction is illustrated in Figure 2 by showing the model-estimated probability of walking at various values of nonverbal IQ. As a sensitivity analysis we excluded children with known genetic syndromes (n=11 ASD, n=17 NDD) or cerebral palsy (n=3 ASD, n=7 NDD) from the Cox model and found nearly identical results.
Figure 2. Illustration of differential relationship between nonverbal IQ and age of walking for NDD and ASD groups.
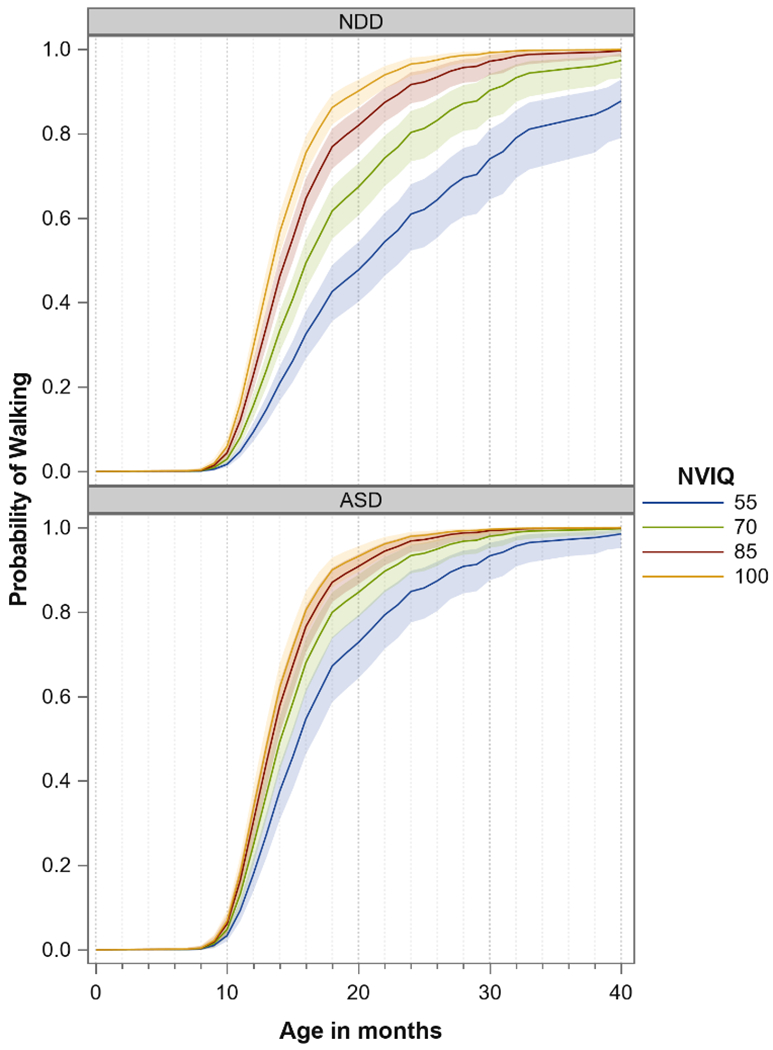
Note: Plotted values are model-estimated probabilities of walking at a given age (X-axis) and given value for nonverbal IQ (line color). IQ was not categorized for analysis or for plotting; instead, the model was used to generate the estimated probability of walking at the given IQ values.
Our secondary analyses addressed the hypothesis that nonverbal IQ should be less strongly related to other potential predictors of developmental delay/ID among children with ASD than children with NDD (Table 2). Although there were differences by diagnostic group on several of these variables, the interaction between diagnostic group and nonverbal IQ was negligible in all cases.
Table 2.
Results of secondary analyses
| Group | Nonverbal IQ | Group x Nonverbal IQ | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| df | F | p | F | p | F | p | |
| Dependent variable | |||||||
| Gestational age (category) | 555 | 4.12 | 0.04 | 1.67 | 0.20 | 2.43 | 0.12 |
| Birthweight (square-root) | 561 | 10.37 | 0.00 | 14.24 | 0.00 | 1.55 | 0.21 |
| APGAR score (1 min) | 551 | 0.55 | 0.46 | 3.61 | 0.06 | 0.1 | 0.75 |
| APGAR score (5 min) | 553 | 0.34 | 0.56 | 3.64 | 0.06 | 0.1 | 0.75 |
| First words by 24 months | 465 | 17.41 | <.0001 | 31.81 | <.0001 | 2.48 | 0.12 |
| First phrase by 36 months | 507 | 1.81 | 0.18 | 86.36 | <.0001 | 0.42 | 0.52 |
Note: Some missing data; see Table 1.
Discussion
Interest in understanding differences in early developmental trajectories among children with ASD is growing. Much attention has been given to phenotypic subtyping efforts within ASD (Hus, Pickles, Cook, Risi, & Lord, 2007), but reliance on cross-sectional data limits the extent to which such studies inform our understanding of an inherently developmental disorder (Amaral et al., 2019; Klin, 2008; Lord, Bishop, & Anderson, 2015). Although apparently equivalent outcomes may be measured in two individuals, discrepant pathways to that outcome may suggest differing causes and therefore different etiologies and/or intervention opportunities.
Consistent with reports from clinically ascertained samples, our results suggest that age of walking is less predictive of later low intellectual ability among children with ASD than among those with other types of NDDs. While the explanation for this phenomenon is unknown, it is possible that the timing and severity of problems associated with the onset of ASD (e.g., in the context of skill loss or worsening trajectories (Lord, Luyster, Guthrie, & Pickles, 2012; Ozonoff & Iosif, 2019)) might contribute to low intellectual ability in some children. Thus, for at least a subset of children with ASD, cognitive development diverges from what might have been predicted based on early motor skill attainment. Prospective studies of infants at familial risk for ASD provide evidence for this assertion with respect to other early milestones; a substantial minority of children diagnosed with ASD with significant language and/or cognitive impairments appeared to show typical language and cognitive development in the first two years of life (Landa, Gross, Stuart, & Faherty, 2013; Lord, Luyster, et al., 2012; Ozonoff, Young et al., 2018).
Delayed age of walking is a robust predictor of later intellectual impairment and is associated with certain risk factors for intellectual disability (ID) (Flensborg-Madsen & Mortensen, 2015; Hamadani et al., 2013; Jeng, Yau, Liao, Chen, & Chen, 2000; Murray et al., 2007; Oudgenoeg-Paz, Mulder, Jongmans, van der Ham, & Van der Stigchel, 2017; Poranen-Clark et al., 2015; Taanila et al., 2005; Vlasblom et al., 2019). For example, it is well-documented that neurogenetic syndromes are often associated with both early motor delays and ID (Mithyantha, Kneen, McCann, & Gladstone, 2017; Moeschler & Shevell, 2014; Moss, Howlin, & Oliver, 2011; Noritz, Murphy, & Neuromotor Screening Expert Panel, 2013). It is also true that among individuals diagnosed with ASD, those with rare genetic mutations are more likely to exhibit both motor delays (e.g., late walking) and ID (Bishop et al., 2017; Buja et al., 2018; Weiner et al., 2017). The current results may suggest that for some children with ASD with apparently intact early development, at least in the attainment of major motor skills, autistic symptoms could also interfere with learning and increase the likelihood of “delayed-onset” intellectual impairment. This is in line with the argument that early impairments in social attention can affect learning and intellectual development, and there may be a particular window of opportunity for early intervention to reduce the likelihood of “secondary” learning disabilities in ASD (Wetherby et al., 2018; Zwaigenbaum et al., 2015). However, further studies using causally informative designs are needed to directly examine this.
Our secondary aim was to examine if a differential relationship between walking and ID in ASD has specificity in comparison with other individual factors apparent in early life that have been previously associated with ID. The findings suggest specificity. Birth weight, gestational age, and APGAR scores were designated as secondary precisely because they may be more strongly influenced by external factors like maternal health and are more distally related to later development of the child. Age of first words and first phrases are direct indicators of developmental pathways, but in contrast to walking, delays in achieving these language milestones are very common among children with ASD, regardless of intellectual ability (Anderson et al., 2007; Hill, Zuckerman, & Fombonne, 2014; Kjelgaard & Tager-Flusberg, 2001; Norbury et al., 2017; Pickles, Anderson, & Lord, 2014). Thus, the lack of differential association by diagnosis between these predictors and IQ may result from the fact that language milestone attainment is related more to language delay (which may be transient) than low intellectual ability.
While some large-scale studies exclude non-walkers (Fischbach & Lord, 2010), a strength of the current study was the inclusion and standardized assessment and diagnostic evaluation of children recruited from a general population who were not walking. The fact that major diagnostic instruments require independent walking has resulted in a dearth of data on ASD symptoms in these children. Moving forward, it will be important to strive to include children with serious motor impairments in large-scale data collection efforts and to make ASD symptom measures applicable to children of all levels of motor abilities.
Limitations
Although the sample was ascertained through population-based methods, selection bias may remain. Compared to the nationwide population in Norway, there is underrepresentation in MoBa of the youngest mothers and of mothers who are single, smoking during pregnancy, or non-users of folic acid in pregnancy (Nilsen et al., 2013). Selection bias is also likely to pertain to participation in the nested ABC Study, as the response rate was only about 50%. We examined potential differences between invited participants and invited non-participants. As detailed in eTable 2, maternal and paternal education was lower for a higher percentage of non-participating families. However, invited participants and invited non-participants were very similar in age of walking and other main study variables, except for 5-minute Apgar score, which was low for a higher percentage of non-participants. Although the comparison group of children with NDD without ASD were recruited to a study of ASD, the screening criteria captured general neurodevelopmental difficulties not necessarily specific to ASD (e.g., it was sufficient to have been seen by a specialist for language delay) and ASD screening tools have relatively low predictive value for ASD specifically (high predictive value for the broader category of neurodevelopmental disorders) (Corsello et al., 2007; Marvin, Marvin, Lipkin, & Law, 2017; Suren et al., 2019). Therefore, the comparison group is likely representative of children with early onset NDD. However, the findings might not generalize to later onset NDD such as many children with mild ID, who simply were too young for their condition to be diagnosed.
We are not able to fully rule out the possibility that clinician bias affected the current results. It is possible, for example, that clinicians are less comfortable diagnosing ASD in children with profiles less prototypically associated with ASD (e.g., children with significant motor delays). However, the proportion of children who were not walking at the time of evaluation was similar across the ASD and NDD groups (2.4% [95% CI=0.3%-4.5%] and 3.1% [95% CI 1.3%-4.9%], respectively, p=0.603), suggesting that ASD diagnosis was not incompatible with very late walking. Nevertheless, it will be important for future work to consider the role of clinician training and potential bias in diagnostic assessment of children with extreme motor, cognitive, and other delays (Thurm et al., 2019).
A hierarchy of cognitive tests was used to achieve valid measurement of nonverbal IQ across age and developmental levels. Although a single cognitive test might provide a more consistent measure, there is a lack of cognitive tests applicable across age and developmental levels (Bishop et al., 2015).
Conclusion
The current study provides further evidence that walking is less strongly related to later low intellectual ability among children with ASD than among those with other NDDs without ASD, and addresses limitations of previous clinic-based studies by utilizing a cohort ascertained from a population-based sample. Future research using prospective data should further elucidate different developmental milestone profiles among individuals diagnosed with ASD and other NDDs.
Supplementary Material
Key Points:
Though delayed walking is a common precursor of low intellectual ability, evidence from clinical samples suggests that late walking may be less common when low intellectual ability occurs with ASD.
To address the concern that previous findings could be due to referral bias and underrepresentation of children with motor disabilities in clinical ASD samples, we examined walking and intellectual ability in a population-based sample of children with ASD and other neurodevelopmental disorders.
Age of walking was indeed less predictive of intellectual ability in children with ASD as compared to those with other neurodevelopmental disorders. The finding suggests potentially differential pathways to low intellectual ability in ASD.
Acknowledgments:
The ABC Study was supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke (U01 NS047537). The Norwegian Mother, Father and Child Cohort Study is supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research. This work was supported by grants from the South-Eastern Norway Regional Health Authority (2018059 to Drs. Øyen and Havdahl), National Institute of Child Health and Human Development (R01HD093012 to Dr. Bishop) and the Autism Science Foundation (P0516945 to Dr. Bishop), and the Intramural Research Program of the NIMH (1ZICMH002961 to Drs. Thurm and Farmer).
We are grateful to all the participating families in Norway who take part in the Norwegian Mother, Father and Child Cohort Study and the nested Autism Birth Cohort Study. We thank the Autism Birth Cohort Study staff and Scientific Advisory Board for their invaluable contributions to the data collection. We thank Sheila Ghods for administrative support.
Abbreviations:
- ASD
Autism Spectrum Disorder
- NDD
Neurodevelopmental Disorders
- ID
Intellectual Disability
- MoBa
The Norwegian Mother, Father and Child Cohort Study
- ABC Study
Autism Birth Cohort Study
- HR
hazard ratio
- IQ
intelligence quotient
Footnotes
Conflicts of Interest:
Drs. Bishop and Lord have received royalties from the ADOS-2. All profits from their research are donated to charity. The other authors report no conflicts of interest.
References
- Amaral DG, Anderson GM, Bailey A, Bernier R, Bishop S, Blatt G, … Whitehouse A (2019). Gaps in Current Autism Research: The Thoughts of the Autism Research Editorial Board and Associate Editors. Autism Res, 12(5), 700–714. doi: 10.1002/aur.2101 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC. [Google Scholar]
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed., text rev.). Washington, DC. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (5th ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Anderson DK, Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, … Pickles A (2007). Patterns of growth in verbal abilities among children with autism spectrum disorder. J Consult Clin Psychol, 75(4), 594–604. doi: 10.1037/0022-006X.75.4.594 [DOI] [PubMed] [Google Scholar]
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, … Dowling NF (2018). Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill Summ, 67(6), 1–23. doi: 10.15585/mmwr.ss6706a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begovac I, Begovac B, Majić G, & Vidovic V (2009). Longitudinal studies of IQ stability in children with childhood autism-Literature survey. Psychiatr Danub, 21(3), 310–319. [PubMed] [Google Scholar]
- Bilder DA, Pinborough-Zimmerman J, Bakian AV, Miller JS, Dorius JT, Nangle B, & McMahon WM (2013). Prenatal and perinatal factors associated with intellectual disability. Am J Intellect Dev Disabil, 118(2), 156–176. doi: 10.1352/1944-7558-118.2.156 [DOI] [PubMed] [Google Scholar]
- Bishop SL, Farmer C, Bal V, Robinson EB, Willsey AJ, Werling DM, … Thurm A (2017). Identification of Developmental and Behavioral Markers Associated With Genetic Abnormalities in Autism Spectrum Disorder. Am J Psychiatry, 174(6), 576–585. doi: 10.1176/appi.ajp.2017.16101115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SL, Farmer C, & Thurm A (2015). Measurement of nonverbal IQ in autism spectrum disorder: scores in young adulthood compared to early childhood. J Autism Dev Disord, 45(4), 966–974. doi: 10.1007/s10803-014-2250-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SL, Thurm A, Farmer C, & Lord C (2016). Autism Spectrum Disorder, Intellectual Disability, and Delayed Walking. Pediatrics, 137(3), 1–8. doi: 10.1542/peds.2015-2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buja A, Volfovsky N, Krieger AM, Lord C, Lash AE, Wigler M, & Iossifov I (2018). Damaging de novo mutations diminish motor skills in children on the autism spectrum. Proceedings of the National Academy of Sciences. doi: 10.1073/pnas.1715427115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsello C, Hus V, Pickles A, Risi S, Cook EH Jr., Leventhal BL, & Lord C (2007). Between a ROC and a hard place: decision making and making decisions about using the SCQ. J Child Psychol Psychiatry, 48(9), 932–940. doi: 10.1111/j.1469-7610.2007.01762.x [DOI] [PubMed] [Google Scholar]
- Egger HL, Erkanli A, Keeler G, Potts E, Walter BK, & Angold A (2006). Test-Retest Reliability of the Preschool Age Psychiatric Assessment (PAPA). J Am Acad Child Adolesc Psychiatry, 45(5), 538–549. doi: 10.1097/01.chi.0000205705.71194.b8 [DOI] [PubMed] [Google Scholar]
- Farmer C, Golden C, & Thurm A (2015). Concurrent validity of the differential ability scales, second edition with the Mullen Scales of Early Learning in young children with and without neurodevelopmental disorders. Child Neuropsychol, 1–14. doi: 10.1080/09297049.2015.1020775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach GD, & Lord C (2010). The Simons Simplex Collection: a resource for identification of autism genetic risk factors. Neuron, 68(2), 192–195. doi: 10.1016/j.neuron.2010.10.006 [DOI] [PubMed] [Google Scholar]
- Flensborg-Madsen T, & Mortensen EL (2015). Infant developmental milestones and adult intelligence: A 34-year follow-up. Early Hum Dev, 91(7), 393–400. doi: 10.1016/j.earlhumdev.2015.04.006 [DOI] [PubMed] [Google Scholar]
- Fombonne E (2005). Epidemiology of autistic disorder and other pervasive developmental disorders. J Clin Psychiatry, 66 Suppl 10, 3–8. [PubMed] [Google Scholar]
- Gadow KD, & Sprafkin J (1997). Child Symptom Inventory 4: CSI: Checkmate Plus. [Google Scholar]
- Hamadani JD, Tofail F, Cole T, & Grantham‐McGregor S (2013). The relation between age of attainment of motor milestones and future cognitive and motor development in Bangladeshi children. Matern Child Nutr, 9, 89–104. doi: 10.1111/mcn.12020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AP, Zuckerman KE, & Fombonne E (2014). Epidemiology of autism spectrum disorders. Handbook of Autism and Pervasive Developmental Disorders, Fourth Edition. [Google Scholar]
- How to apply for access to data and biological material from the Norwegian Mother, Father and Child Cohort Study (MoBa) (2010). Retrieved from https://www.fhi.no/en/studies/moba/for-forskere-artikler/research-and-data-access/
- Huang J, Zhu T, Qu Y, & Mu D (2016). Prenatal, Perinatal and Neonatal Risk Factors for Intellectual Disability: A Systemic Review and Meta-Analysis. PloS one, 11(4), e0153655. doi: 10.1371/journal.pone.0153655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hus V, Pickles A, Cook EH, Risi S, & Lord C (2007). Using the Autism Diagnostic Interview—Revised to Increase Phenotypic Homogeneity in Genetic Studies of Autism. Biol Psychiatry, 61(4), 438–448. doi: 10.1016/j.biopsych.2006.08.044 [DOI] [PubMed] [Google Scholar]
- Hus V, Taylor A, & Lord C (2011). Telescoping of caregiver report on the Autism Diagnostic Interview--Revised. J Child Psychol Psychiatry, 52(7), 753–760. doi: 10.1111/j.1469-7610.2011.02398.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng S-F, Yau K-IT, Liao H-F, Chen L-C, & Chen P-S (2000). Prognostic factors for walking attainment in very low-birthweight preterm infants. Early Human Development, 59(3), 159–173. doi: 10.1016/s0378-3782(00)00088-8 [DOI] [PubMed] [Google Scholar]
- Kaminer RK, & Jedrysek E (1983). Age of walking and mental retardation. Am J Public Health, 73(9), 1094–1096. doi: 10.2105/AJPH.73.9.1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelgaard MM, & Tager-Flusberg H (2001). An investigation of language impairment in autism: implications for genetic subgroups. Language and Cognitive Processes, 16(2–3), 287. doi: 10.1080/01690960042000058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A (2008). Three things to remember if you are a functional magnetic resonance imaging researcher of face processing in autism spectrum disorders. Biol Psychiatry, 64(7), 549–551. doi: 10.1016/j.biopsych.2008.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokubun M, Haishi K, Okuzumi H, & Hosobuchi T (1995). Factors affecting age of walking by children with mental retardation. Percept Mot Skills, 80(2), 547–552. doi: 10.2466/pms.1995.80.2.547 [DOI] [PubMed] [Google Scholar]
- Landa RJ, Gross AL, Stuart EA, & Faherty A (2013). Developmental trajectories in children with and without autism spectrum disorders: the first 3 years. Child Dev, 84(2), 429–442. doi: 10.1111/j.1467-8624.2012.01870.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Bishop SL, & Anderson D (2015). Developmental trajectories as autism phenotypes. Am J Med Genet C Semin Med Genet, 169(2), 198–208. doi: 10.1002/ajmg.c.31440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Luyster R, Guthrie W, & Pickles A (2012). Patterns of developmental trajectories in toddlers with autism spectrum disorder. J Consult Clin Psychol, 80(3), 477–489. doi: 10.1037/a0027214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, & Bishop S (2012). Autism Diagnostic Observation Schedule, Second Edition (ADOS-2). Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Lord C, Rutter M, & Le Couteur A (1994). Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685. doi: 10.1007/BF02172145 [DOI] [PubMed] [Google Scholar]
- Magnus P, Birke C, Vejrup K, Haugan A, Alsaker E, Daltveit AK, … Stoltenberg C (2016). Cohort Profile Update: The Norwegian Mother and Child Cohort Study (MoBa). International Journal of Epidemiology, 45(2), 382–388. doi: 10.1093/ije/dyw029 [DOI] [PubMed] [Google Scholar]
- Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C, & MoBa Study, G. (2006). Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol, 35(5), 1146–1150. doi: 10.1093/ije/dyl170 [DOI] [PubMed] [Google Scholar]
- Marvin AR, Marvin DJ, Lipkin PH, & Law JK (2017). Analysis of Social Communication Questionnaire (SCQ) Screening for Children Less Than Age 4. Curr Dev Disord Rep, 4(4), 137–144. doi: 10.1007/s40474-017-0122-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithyantha R, Kneen R, McCann E, & Gladstone M (2017). Current evidence-based recommendations on investigating children with global developmental delay. Arch Dis Child, 102(11), 1071–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modabbernia A, Mollon J, Boffetta P, & Reichenberg A (2016). Impaired Gas Exchange at Birth and Risk of Intellectual Disability and Autism: A Meta-analysis. J Autism Dev Disord, 46(5), 1847–1859. doi: 10.1007/s10803-016-2717-5 [DOI] [PubMed] [Google Scholar]
- Moeschler JB, & Shevell M (2014). Comprehensive Evaluation of the Child With Intellectual Disability or Global Developmental Delays. Pediatrics, 134(3), e903–e918. doi: 10.1542/peds.2014-1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J, Howlin P, & Oliver C (2011). The assessment and presentation of autism spectrum disorder and associated characteristics in individuals with severe intellectual disability and genetic syndromes. In Burack J, Hodapp R, Iarocci G, & Zigler E (Eds.), The Oxford Handbook of Intellectual Disablity and Development (pp. 1–57). New York, NY: Oxford University Press. [Google Scholar]
- Mullen EM (1995). Mullen scales of early learning. Circle Pines, MN: American Guidance Service. [Google Scholar]
- Murray GK, Jones PB, Kuh D, & Richards M (2007). Infant developmental milestones and subsequent cognitive function. Ann Neurol, 62(2), 128–136. doi: 10.1002/ana.21120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen RM, Surén P, Gunnes N, Alsaker ER, Bresnahan M, Hirtz D, … Stoltenberg C (2013). Analysis of Self-selection Bias in a Population-based Cohort Study of Autism Spectrum Disorders. 27(6), 553–563. doi: 10.1111/ppe.12077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbury C, Vamvakas G, Gooch D, Baird G, Charman T, Simonoff E, & Pickles A (2017). Language growth in children with heterogeneous language disorders: a population study. J Child Psychol Psychiatry, 58(10), 1092–1105. doi: 10.1111/jcpp.12793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noritz GH, Murphy NA, & Neuromotor Screening Expert Panel. (2013). Motor delays: Early identification and evaluation. Pediatrics, 131(6), e2016–2027. doi: 10.1542/peds.2013-1056 [DOI] [PubMed] [Google Scholar]
- Oudgenoeg-Paz O, Mulder H, Jongmans MJ, van der Ham IJM, & Van der Stigchel S (2017). The link between motor and cognitive development in children born preterm and/or with low birth weight: A review of current evidence. Neurosci Biobehav Rev, 80, 382–393. doi: 10.1016/j.neubiorev.2017.06.009 [DOI] [PubMed] [Google Scholar]
- Ozonoff S, & Iosif AM (2019). Changing conceptualizations of regression: What prospective studies reveal about the onset of autism spectrum disorder. Neurosci Biobehav Rev, 100, 296–304. doi: 10.1016/j.neubiorev.2019.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Li D, Deprey L, Hanzel EP, & Iosif AM (2018). Reliability of parent recall of symptom onset and timing in autism spectrum disorder. Autism, 22(7), 891–896. doi: 10.1177/1362361317710798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Brian J, Charman T, Shephard E, Solish A, & Zwaigenbaum L (2018). Diagnosis of Autism Spectrum Disorder After Age 5 in Children Evaluated Longitudinally Since Infancy. J Am Acad Child Adolesc Psychiatry, 57(11), 849–857.e842. doi: 10.1016/j.jaac.2018.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles A, Anderson DK, & Lord C (2014). Heterogeneity and plasticity in the development of language: a 17-year follow-up of children referred early for possible autism. J Child Psychol Psychiatry, 55(12), 1354–1362. doi: 10.1111/jcpp.12269 [DOI] [PubMed] [Google Scholar]
- Poranen-Clark T, von Bonsdorff MB, Lahti J, Räikkönen K, Osmond C, Rantanen T, … Eriksson JG (2015). Infant motor development and cognitive performance in early old age: the Helsinki Birth Cohort Study. Age (Dordr), 37(3), 44. doi: 10.1007/s11357-015-9785-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reindal L, Naerland T, Weidle B, Lydersen S, Andreassen OA, & Sund AM (2020). Age of First Walking and Associations with Symptom Severity in Children with Suspected or Diagnosed Autism Spectrum Disorder. J Autism Dev Disord, 50(9), 3216–3232. doi: 10.1007/s10803-019-04112-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritvo ER, Freeman BJ, Pingree C, Mason-Brothers A, Jorde L, Jenson WR, … Ritvo A (1989). The UCLA-University of Utah epidemiologic survey of autism: prevalence. Am J Psychiatry, 146(2), 194–199. doi: 10.1176/ajp.146.2.194 [DOI] [PubMed] [Google Scholar]
- Roid GH (2003). Stanford-Binet Intelligence Scales, Fifth Edition. Itasca, IL: Riverside Publishing. [Google Scholar]
- Rutter M, Bailey A, & Lord C (2003). Social Communication Questionnaire (SCQ). Los Angeles, California: Western Psychological Serives. [Google Scholar]
- Sprafkin J, Volpe RJ, Gadow KD, Nolan EE, Kelly K. J. J. o. t. A. A. o. C., & Psychiatry A (2002). A DSM-IV–referenced screening instrument for preschool children: The Early Childhood Inventory-4. 41(5), 604–612. [DOI] [PubMed] [Google Scholar]
- Stoltenberg C, Schjolberg S, Bresnahan M, Hornig M, Hirtz D, Dahl C, … Group, A. B. C. S. (2010). The Autism Birth Cohort: a paradigm for gene-environment-timing research. Mol Psychiatry, 15(7), 676–680. doi: 10.1038/mp.2009.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suren P, Saasen-Havdahl A, Bresnahan M, Hirtz D, Hornig M, Lord C, … Stoltenberg C (2019). Sensitivity and specificity of early screening for autism. BJPsych Open, 5(3), e41. doi: 10.1192/bjo.2019.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surén P, Schjolberg S, Øyen A, Lie K, Hornig M, Bresnahan M, … Stoltenberg C (2014). The Autism Birth Cohort (ABC): a study of autism spectrum disorders in MoBa (Vol. 24): Norsk Epidemiologi. [Google Scholar]
- Taanila A, Murray GK, Jokelainen J, Isohanni M, & Rantakallio P (2005). Infant developmental milestones: a 31-year follow-up. Dev Med Child Neurol, 47(9), 581–586. doi: 10.1111/j.1469-8749.2005.tb01207.x [DOI] [PubMed] [Google Scholar]
- Thurm A, Farmer C, Salzman E, Lord C, & Bishop S (2019). State of the Field: Differentiating Intellectual Disability From Autism Spectrum Disorder. Front Psychiatry, 10, 526. doi: 10.3389/fpsyt.2019.00526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasblom E, Boere-Boonekamp MM, Hafkamp-de Groen E, Dusseldorp E, van Dommelen P, & Verkerk PH (2019). Predictive validity of developmental milestones for detecting limited intellectual functioning. PLoS One, 14(3), e0214475. doi: 10.1371/journal.pone.0214475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmar FR, Siegel M, Woodbury-Smith M, King B, McCracken J, State M, … Adolescent Psychiatry Committee on Quality, I. (2014). Practice parameter for the assessment and treatment of children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry, 53(2), 237–257. doi: 10.1016/j.jaac.2013.10.013 [DOI] [PubMed] [Google Scholar]
- Weiner DJ, Wigdor EM, Ripke S, Walters RK, Kosmicki JA, Grove J, … Robinson EB (2017). Polygenic transmission disequilibrium confirms that common and rare variation act additively to create risk for autism spectrum disorders. Nature Genetics, 49, 978. doi: 10.1038/ng.3863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherby AM, Woods J, Guthrie W, Delehanty A, Brown JA, Morgan L, … Lord C (2018). Changing Developmental Trajectories of Toddlers With Autism Spectrum Disorder: Strategies for Bridging Research to Community Practice. Journal of Speech, Language, and Hearing Research, 61(11), 2615–2628. doi: 10.1044/2018_JSLHR-L-RSAUT-18-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bauman ML, Choueiri R, Kasari C, Carter A, Granpeesheh D, … Natowicz MR (2015). Early Intervention for Children With Autism Spectrum Disorder Under 3 Years of Age: Recommendations for Practice and Research. Pediatrics, 136 Suppl 1, S60–81. doi: 10.1542/peds.2014-3667E [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


