Abstract
The Notch signaling pathway is an evolutionarily conserved cell signaling system known to be involved in vascular development and function. Recent evidence suggests that dysfunctional Notch signaling could play a critical role in the pathophysiology of neurodegenerative diseases. We reviewed current literature on the role of Notch signaling pathway, and specifically Notch receptor genes and proteins, in aging, cerebrovascular disease and Alzheimer’s disease. We hypothesize that Notch signaling may represent a key point of overlap between age-related vascular and Alzheimer’s pathophysiology contributing to their comorbidity and combined influence on cognitive decline and dementia. Numerous findings from studies of genetics, neuropathology and cell culture models all suggest a link between altered Notch signaling and Alzheimer’s pathophysiology. Age-related changes in Notch signaling may also trigger neurovascular dysfunction, contributing to the development of neurodegenerative diseases; however, additional studies are warranted. Future research directly exploring the influence of aberrant Notch signaling in the development of Alzheimer’s disease is needed to better understand this mechanism.
Keywords: Notch Signaling, Vascular Aging, Alzheimer’s Disease
1. Introduction
The Notch signaling pathway is an evolutionarily conserved cell signaling and interaction system [1] involved in a various processes including neuronal development and function [2,3], angiogenesis [4], vasculogenesis [5], cardiac development and function [6,7], and maintenance of neural stem cells [8-10]. The system is activated by the interaction between five Notch ligands encoded by JAG1, JAG2, and DLL1, DLL3 and DLL4, and four transmembrane receptors encoded by Notch genes (NOTCH1-4) [11]. The importance of this pathway in cerebrovascular and neurological function has been highlighted by the critical role of Notch signaling in conditions such as cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), Down syndrome, and stroke [12-15]. Each of these conditions is linked to cognitive decline and dementia, and further analysis of Notch signaling pathways involved in these conditions reveals a striking pattern of overlap that implicates Notch signaling in the interface between cerebrovascular disease, characterized by vascular brain lesions, and Alzheimer’s disease (AD), characterized by amyloid-β (Aβ)-containing plaques and tau-containing neurofibrillary tangles. Cerebrovascular disease and AD are the two primary contributors to age-related cognitive decline and dementia.
Vascular dementia caused by cerebrovascular lesions is the second most common cause of dementia after AD [16]. Cerebrovascular lesions are also the most common secondary contributor to mixed dementia, being present in an estimated 30-75% of AD patient brains at autopsy [17]. Moreover, up to 100% of AD brains exhibit vascular lesions in the form of cerebral amyloid angiopathy (CAA)[18]. Thus, the link between vascular disease and AD is well established, but the underlying mechanisms remain unclear. Clues regarding mechanisms involved in vascular dementia and AD dementia have been uncovered through study of genetically-determined cases. CADASIL is the most common genetic form of vascular dementia and is caused by NOTCH3 mutations, strongly implicating Notch signaling in vascular dementia. Further supporting the importance of Notch signaling in vascular dementia, polymorphisms in NOTCH3 have been linked to age-related cerebrovascular disease and stroke [19]. Fascinatingly, case reports have suggested links between CADASIL and AD pathophysiology [20-22], potentially implicating Notch signaling in AD in addition to its established role in cerebrovascular disease.
Genetic forms of AD dementia include autosomal dominant mutations in the amyloid precursor protein gene (APP) and associated enzymes, such as presenilin-1 (PSEN1) and presenilin-2 (PSEN2), termed autosomal dominant AD (ADAD). Study of these genetically-determined ADAD cases have implicated cerebral amyloidosis in the pathoetiology of AD. However, recent brain imaging studies revealed that ADAD patients also exhibit cerebrovascular lesions during the earliest stages of the disease process [23], and these lesions cannot be fully accounted for by the presence of CAA [24], potentially implicating cerebrovascular disease in the pathophysiology of AD. Down syndrome is another genetically-determined cause of AD, with 100% of Down syndrome patients ultimately developing early onset AD dementia. Down syndrome is caused by trisomy 21, leading to increased amyloid precursor protein (APP) production, which may account for the connection between Down syndrome and AD dementia. As with other genetic forms of AD, recent evidence from neuroimaging and autopsy studies now indicates all Down syndrome patients with AD dementia also exhibit extensive cerebrovascular lesions [25,26]. Interestingly, molecular studies have revealed that increased APP production drives competitive inhibition of Notch-1 cleavage by their shared presenilin-1 enzyme, potentially suggesting Notch signaling abnormalities could impact all forms of ADAD, including Down syndrome, which may account for the presence of cerebrovascular disease in these populations [26].
Collectively, these studies of genetically-determined vascular and AD dementias place Notch signaling directly within the pathways to both vascular dementia and AD dementia, the two most common and overlapping neuropathological factors accounting for the vast majority of dementia cases [17]. Thus, emerging evidence suggests that dysfunctional Notch signaling could play a critical role in the pathophysiology of both vascular and neurodegenerative diseases forms of cognitive impairment and dementia [27]. What remains unclear is whether Notch signaling is important in the age-related, sporadic forms of vascular disease and AD that represent the majority of dementia cases since genetic forms of dementia are rare.
The present work provides an overview of the current literature on the role of Notch signaling pathway, and specifically Notch receptor genes and proteins, in aging, cerebrovascular disease and AD. We hypothesize that age-related Notch signaling changes may represent a key point of overlap between vascular and Alzheimer’s pathophysiology contributing to their comorbidity and combined influence on cognitive decline and dementia.
2. Notch Signaling and Neurovascular Aging
The Notch signaling pathway is known to play a key role in numerous cellular processes during nervous system development as well as in the adult nervous system. Although studies examining Notch signaling and vascular smooth muscle cells in aging vessels are scarce, decline in skeletal muscle stem cells has been linked to loss of Notch signaling and impaired regenerative capacity of aged muscles, suggesting that altered Notch signaling may occur with aging [28-30]. The Notch pathway is known to interact with numerous other important pathways, including the Wnt pathway, which is involved in cell-fate determination, survival and proliferation [31]. The interaction and balance between these different pathways may be disrupted in aging and aging-related diseases. For instance, a balance between Notch and Wnt signaling is required for regeneration of adult skeletal muscle [32] and angiogenesis [33,34], however advancing age may disrupt such interactions and limit regenerative capacity [30]. The Notch and Wnt signaling pathways interaction may play a key role in vascular sprouting and regression in angiogenesis [34]. Moreover, the Wnt/β-catenin signaling pathway is known to be involved in triggering angiogenic factors, such as VEGF [35,36]. Although there are few studies examining angiogenesis in AD, altered angiogenesis has previously been observed [37], with some studies suggesting that angiogenesis may contribute to the pathogenesis of AD [38], and others showing that it may protect against memory impairment [39]. Similar to other pathological states, angiogenesis leading to healthy vessel formation is likely beneficial for brain function, whereas angiogenesis leading to aberrant vessel outgrowth may contribute to neurodegeneration through vessel leakage [40-42]. Angiogenesis may be a potential mechanism by which Notch signaling may influence AD risk since alterations in Notch signaling could derail healthy new vessel formation in response to angiogenic signaling[43]. Studies examining age-related alterations in Notch signaling and associated pathways in vascular smooth muscle cells are warranted and could elucidate the role of the vasculature in the development of aging-related neurodegenerative conditions.
Notch signaling has also been implicated in age-related vascular diseases such as atherosclerosis [29], which have also been associated with AD. For instance, animal models suggest that Notch-1 is reduced by a high-fat diet, and reduction in endothelial Notch-1 may be a predisposing factor in the onset of vascular inflammation and diet-induced atherosclerosis [44]. Balistreri et al. (2016) similarly propose age-related cardiovascular disease may be due to the altered interactions between the Notch pathway and major inflammatory pathways, resulting in endothelial dysfunction and vascular remodeling which may precipitate cardiovascular disease [29]. Cardiovascular diseases are also known to play a role in AD [45], and emerging evidence similarly suggests a role for Notch signaling in neurogenerative diseases [46]. Further research on the association between angiogenesis, atherosclerosis, and AD pathology could yield new insights into the role of vascular functioning in AD.
3. Notch Signaling and Alzheimer’s Disease
Multiple Notch gene mutations and proteins have previously been linked to neurodegenerative conditions. Here, we review current evidence demonstrating the potential involvement of Notch signaling in AD.
3.1. NOTCH3
CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy) is a hereditary stroke disorder that results from mutations in the Notch receptor 3 gene (NOTCH3; Chromosome 19p13.12), which is required for function and maturation of vascular smooth muscle cells [47]. The CADASIL neuropathology is characterized by vascular brain injury caused by abnormal accumulation of granular osmiophilic material (GOM) and Notch-3 protein in the cytoplasmic membrane of vascular smooth muscle cells and pericytes [48]. While increased production or impaired clearance of Notch-3 could both result in Notch-3 protein accumulation, previous findings suggest that impaired clearance is more likely [48]. Brain Magnetic Resonance Imaging (MRI) of CADASIL patients often reveals ischemic infarcts and severe small vessel disease, including white matter hyperintensities, lacunes and microbleeds [47] and neuropathological studies show vascular smooth muscle cell degeneration, GOM deposition and intimal thickening and narrowing of cerebral vessels [49]. Key clinical features of CADASIL include migraines, transient ischemic attacks, mood disturbances, and cognitive and motor impairments [49].
Initial evidence for the association between NOTCH3 mutations and AD came from rare case studies of CADASIL patients showing colocalization of GOM pathological deposits characteristic of CADASIL with Aβ plaques characteristic of AD [20-22], as well as observations of precocious AD pathophysiology in younger CADASIL patient brains [20-22]. Parenchymal deposition of β-amyloid peptide (Aβ) is considered pathognomonic of Alzheimer’s pathophysiology [50] and vascular deposition of Aβ is the cause of cerebral amyloid angiopathy (CAA) [51]. The APP is an important protein located at the synapses of neurons that undergoes processing by β- and γ-secretase and ultimately releases Aβ [52,53]. Studies highlighting coexistence of CADASIL and AD suggest that presenilin proteins (γ-secretases), which regulate the proteolytic cleavage of APP as well as Notch proteins, may play a role in both conditions [22,54,55]. The cleaved ectodomain of the Notch-3 receptor which accumulates within the cytoplasmic membrane of vascular smooth muscle cells [48] has been proposed to potentially also induce amyloid deposition in vascular smooth muscle cells [21,22,56], similar to that observed in CAA [57]. Thijs et al. (2003) have previously reported a case of related disease processes where GOM deposits (characteristic of CADASIL) and Aβ deposits were both present, with the Aβ surrounding the granular deposits [21]. The presence of advanced AD pathology characterized by extensive neocortical Aβ plaques and neurofibrillary tangles in a relatively young person (64 years) indicates that AD as well as CADASIL-related pathology likely contributed to early-onset dementia in this patient [21].
While large studies examining the role of Notch-3 specifically are limited, cerebrovascular disease more generally also contributes to diminished perivascular clearance of Aβ [58,59]. In an atypical case of CADASIL with severe arteriolar changes in the cerebral cortex, failure of protein elimination and diffuse Aβ deposition in the cerebral cortex has been reported [60], further suggesting that degradation of vessels—which is associated with Notch gene mutations— may facilitate amyloid accumulation, and ultimately trigger AD. Both CAA and CADASIL involve entrapment of proteins along capillary and arterial walls which serve as the lymphatic drainage pathways of the brain [58,61] and CAA is observed among 80%–100% of individuals with AD [62]. Carare et al. (2013) propose that such protein elimination failure angiopathies (PEFA) involve common mechanisms resulting in protein accumulation in blood vessels which could contribute to neurodegenerative conditions [61]. Reduced pulsatility and increased stiffness in damaged arteries decreases the motive force required for perivascular drainage of proteins including Aβ [59,61,63]. The reduction in vascular tone and pulsation amplitude due to GOM accumulation-related damage to smooth muscle cells in CADASIL may facilitate further aggregation of other proteins, including Aβ, within blood vessels [61] and alter Aβ distribution in the cerebral cortex [59].
CADASIL offers a “pure” vascular dementia model in the absence of confounding effects of aging. Thus, CADASIL studies allow examination of how vascular dysfunction may drive AD pathophysiology and provide evidence of increased amyloid accumulation characteristic of AD in the context of NOTCH3 mutations. Studies of cerebrospinal fluid (CSF) biomarkers of AD, including β-amyloid 1-42 (Aβ42), total tau protein (t-tau) and phosphorylated tau protein (p-tau), in CADASIL patients may provide insight into age-independent vascular-AD interactions [64,65]. Findings indicate that compared to controls, CADASIL patients exhibit lower levels of Aβ42 in the CSF, indicating increased cerebral Aβ retention characteristic of AD pathophysiology. Levels of CSF t-tau or p-tau were similar between both groups [64]. Formichi et al. (2008) propose that lower Aβ42 may be associated with subcortical vascular lesions, independent from aging-related changes [65,66]. Together these findings support a role for vascular damage in the accumulation of cerebral Aβ pathology linked to AD dementia.
In addition to potentially facilitating Aβ accumulation, NOTCH3 has also been proposed as a disease modifier, influencing susceptibility to AD due to mutations which result in vascular smooth muscle cell damage [67]. NOTCH3 is essential for function, maturation and maintenance of vascular smooth muscle cells [68,69]. The majority of pathological variants (CADASIL-causing) involve loss or gain of a cysteine residue in the epidermal growth factor (EGF)-like protein repeats in the Notch-3 extracellular domain [56]. In a transgenic mouse model of CADASIL, Notch-3 aggregation is associated with neurovascular unit injury, including loss of pericytes and separation of astrocytic end-feet from cerebral microvessels, and resulting increase in blood-brain barrier permeability [70]. Cerebral small vessel damage, and subsequent alterations in blood flow, as well as pericyte loss and blood brain barrier breakdown have all been implicated in the development of AD [71-73]. Small vessel damage triggered by NOTCH3 mutations [74] may therefore be a contributing factor to the development of AD [72]. Similarly, aberrant Notch signaling worsens brain damage after ischemic stroke [75] and may expedite progression or development of post-stroke dementia [76]. Thus, Notch signaling may be implicated in the development of AD through the role of Notch in the functioning of the blood-brain barrier and development of the cerebrovasculature [15]. Neurovascular dysfunction—including blood brain barrier breakdown [73,77], altered cerebral blood flow [78] and cerebrovascular reactivity [79,80], and small vessel damage [81]—have all been associated with risk of AD and proposed to occur in the early stages of the disease [71,72]. However, further research is warranted to disentangle the role of NOTCH3 as a disease modifier of AD.
The existence of co-occurring AD and CADASIL may be particularly difficult to differentiate without pathological confirmation, genetic testing and detailed neuropsychological assessment. While previous studies have observed and reported co-occurring CADASIL and AD, vascular lesions underlying CADASIL are usually defined specifically as nonarteriosclerotic, and amyloid-negative angiopathy [82]. This distinction allows differentiation of angiopathies but may limit identification of overlapping pathologies. Both AD and CADASIL may not only share similar molecular mechanisms based on pathological evidence [20,21], but also result in cognitive deficits that may be difficult to differentiate without detailed neuropsychological testing. More than half of all patients with CADASIL ultimately develop cognitive deficits and dementia [83,84]. Early stage cognitive deficits are in individuals with AD tend to involve episodic memory, early stage deficits in CADASIL disproportionately involve working memory and executive function [85]. However, as both diseases increase in severity, deterioration of multiple cognitive domains becomes increasingly common and cognitive deficits unique to each disease may be difficult to differentiate on global cognitive measures [86,87]. Moreover, cognitive assessment may be particularity challenging in CADASIL in the context of multiple strokes and lesions involving different regions [86,88]. Thus, further biomarker studies in addition to neuropsychological studies may benefit efforts to disentangle AD in the context of CADASIL could elucidate the role of Notch signaling in the development of AD pathophysiology.
Findings reviewed to date suggest that NOTCH3 mutations trigger changes in vascular smooth muscle cells and the overall cerebral microvasculature which may play a role in increased amyloid accumulation—a key hallmark of AD. Numerous mechanisms suggest a relationship between Notch-3 and amyloid accumulation. Both APP and Notch proteins undergo proteolytic cleavage by γ-secretases and result in protein elimination failure angiopathies. The effects of NOTCH3 mutations on the cerebrovasculature further illustrate a mechanism through which Notch proteins may be involved in AD and other dementias as a disease modifier. Current literature demonstrating these associations are primarily based on limited case studies, and animal and human studies directly examining these mechanisms are lacking. As an early onset monogenic disease caused by NOTCH3 mutations, CADASIL provides a unique opportunity to study the effects of Notch signaling and related vascular dysfunction in the development of AD prior to the onset of age-related vascular changes. Future studies are warranted to directly explore these mechanistic pathways.
3.2. NOTCH1
Another genetic cause of Alzheimer’s pathology, which has been previously been linked to Notch signaling, is Down syndrome. Down syndrome is genetic disorder resulting from trisomy 21—three copies of chromosome 21 in the genome [89]. The key neuropathological features of AD, including amyloid plaques and neurofibrillary tangles, have been observed in most individuals with Down syndrome by the age of 40 years [90]. Similar to AD, vascular changes have previously been observed in individuals with Down syndrome. Microvessel density and endothelial integrity have been found to be significantly diminished in Down syndrome [26], and white matter damage, enlarged perivascular spaces, microbleeds and infarcts are commonly evident [91].
Similar to studies linking CADASIL and AD, Drachman et al. (2017) have proposed that the relationship between Notch signaling and AD may be due to age or Down syndrome-mediated alterations in presenilin cleavage of both APP and Notch-1 [26,92-94]. Based on observations of microvascular changes in Down syndrome with Alzheimer’s-type pathology, they propose that microvascular changes and eventual neuronal loss in AD may be due to disrupted angiogenesis, and competition between APP and Notch-1 for cleavage by presenilin [26]. The cleaved active Notch intracellular domain is required for angiogenesis. Greater cleavage of APP by presenilin—resulting in Aβ accumulation—may therefore also reduce Notch cleavage, further restricting angiogenesis [26]. However, it is unclear whether angiogenesis would be beneficial or detrimental in the context of microvascular disease and AD, and further research is warranted to establish the consequences of diminished angiogenesis on AD pathophysiology and progression. Angiogenesis leading to healthy vessel formation is likely beneficial for brain function; however, previous studies propose that angiogenic activation of endothelium in AD may lead to amyloid plaque deposition and others suggest that amyloid may trigger aberrant angiogenesis leading to blood brain barrier permeability in AD [40-42]. Although the mechanism remains is unclear, a link between increased angiogenesis and AD has been observed. Moreover, the crosstalk of Notch and APP proposed by Drachman et al. (2017) has also previously been examined by gene expression analysis of components of the Notch signaling pathway in the adult Down syndrome brain. Overexpression of Notch-1 as well as other components of the Notch signaling pathway were observed in AD and Down syndrome patients, potentially due to increased APP cleavage in AD and Down syndrome [14].
Evaluation of Notch-1 expression in postmortem human brain tissue from sporadic AD patients has yielded similar findings. Compared to age-matched control individuals, overexpression of Notch-1 is particularly increased in the hippocampus in AD [27], an area known to be critical in memory function and early stage AD pathophysiology. Similarly, presenilin 1—encoded by PSEN1 and known to play a role in AD through its role in the release of Aβ from APP—has been found to colocalize with Notch-1 in the adult mammalian brain [27]. Berezovska et al. (1998) suggest that these findings raise the possibility of Notch-1 involvement in AD pathophysiology via presenilin-1 function. Another study has reported reduced Notch-1 levels in the pyramidal neurons of the hippocampus in AD [95], but displaced Notch-1 accumulation in fibrillary tangles and plaques [95]. Although both increased and decreased levels of Notch-1 have been observed, these studies both suggest alterations in Notch signaling in AD [95]. Greater accumulation of Notch-1 in the brain may in fact be associated with reduced Notch signaling [95], which may impede angiogenesis needed to maintain cerebrovascular function [26]. Similarly, evaluation of plasma and CSF levels have demonstrated dysregulation of the Notch pathway in AD. Reduced clearance of Notch-1 in CSF has been observed in AD patients, suggesting greater Notch-1 accumulation in the brain [95]. Consistently, increased Notch-1 deposition in brain tissue may be associated with lower plasma levels of soluble Notch-1, as observed in AD patients compared to controls [96].
In vitro studies have demonstrated increased Notch-1 protein in Aβ-treated human brain microvascular endothelial cells [96] and impaired Notch signaling induced by mutations in the PSEN1 gene (PSEN1 L166P mutation) which cause an aggressive form of familial AD [97]. In vitro studies have also found a role for presenilin-1 in endothelial progenitor-mediated angiogenesis via intracellular β-catenin [98], suggesting that presenilin-1 dysfunction in endothelial cell lineage could trigger vascular pathology. Interestingly, Notch-1 has been found to interact with the Wnt/β-catenin signaling pathway [99]—which is also involved in angiogenesis and the development of the blood brain barrier [100,101]—and known to regulate β-catenin protein in stem and progenitor cells [102]. Progenitor senescence has also been reported in AD [103]. Examining the associations among Notch-1, Wnt/β-catenin signaling and presenilin-1 could provide further insight into the role of this system in AD.
Finally, Brai et al. (2016) have also found that Notch-1 localizes in neurofibrillary tangles in AD brains and overlaps with p-tau in plaque-like structures [95]. This is consistent with previous studies showing a role for Notch signaling in microtubule stabilization [104,105]. Vascular risk is known to trigger neocortical p-tau deposition when coupled with high Aβ burden [106]. Notch proteins have similarly been proposed to be linked to both amyloid and p-tau deposition [95]. These findings warrant further investigation to examine whether altered Notch signaling may trigger AD pathophysiology.
Few studies have explored the consequences or mechanisms resulting in altered Notch-1 levels in AD. Given the pleiotropic functions of Notch proteins, future studies are needed to better delineate the mechanism through which Notch-1 may be related to AD and whether the role of Notch-1 in angiogenesis and vascular dysfunction may facilitate the development of AD pathology [95].
3.3. NOTCH 2 & 4
Few previous studies have directly examined associations between NOTCH2 and NOTCH4 mutations in relation to AD. Notch-2 has been implicated in the regulation of vascular smooth muscle development, similar to Notch-3 [107]. While lack of Notch-2 delays smooth muscle differentiation, Notch-3 is able to maintain vascular integrity [107]. However, without Notch-3, Notch-2 expression may initiate smooth muscle differentiation but is not able to assist with later maturation, highlighting that Notch-2 and 3 function together to promote vascular smooth muscle development and maintenance [107]. NOTCH4 has previously not been linked to cardiovascular disease [108], however its chromosomal location—which has been demonstrated to have significant linkage with AD—has prompted investigations [109]. The role of NOTCH2 and NOTCH4 gene in cerebrovascular functioning and dementia is currently unclear.
4. Discussion
Burgeoning evidence suggests that altered Notch signaling occurs with advancing age and may be associated with the development of AD through its role in maintaining the cerebral vasculature. Future research directly exploring the influence of aberrant Notch signaling in the development of AD pathology is needed to better understand the related mechanisms (Figure 1). Moreover, the association between Notch signaling and maladaptive or protective vascular mechanisms, such as angiogenesis or mobilization of endothelial progenitor cells could elucidate dysfunctional physiological processes which trigger or protect the vasculature and impact cognition. Previous studies have shown that endothelial progenitor cells are mobilized in response to vascular injury to promote vascular repair [110]. Increased endothelial progenitor cells are observed in response to cerebrovascular injury [111], suggesting a protective role of endothelial progenitor cells. Conversely, depletion of endothelial progenitor cells is associated with cognitive impairment [112], including AD [113] and VaD [114]. Notch signaling may also be involved in homeostatic and regenerative hematopoiesis [115] and in the regulation of endothelial progenitor cells [116]. It is also known to play a role in vessel maturation and angiogenesis [43,117,118]. Further studies are needed, especially to examine the impact of both Notch signaling and angiogenesis on cognition and the development of neurodegenerative conditions.
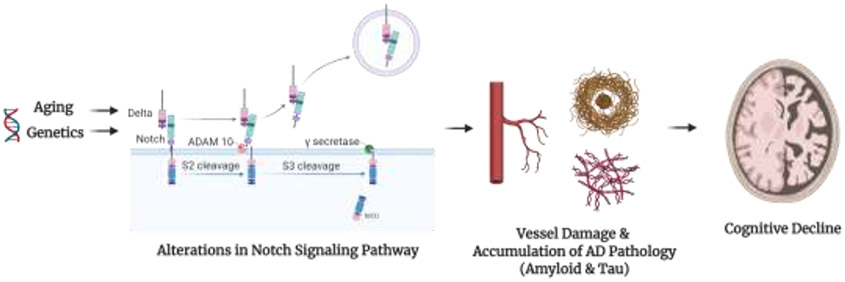
Figure 1. Potential Mechanism of Notch-Mediated Accumulation of Alzheimer's Pathology and Cognitive Decline.
Aging and genetics, among other factors, may alter the Notch signaling pathway. The Notch signaling pathway is an evolutionarily conserved cell signaling and interaction system. The system is activated by the interaction between five Notch ligands (delta-like (DLL)1, DLL3, DLL4, Jagged-1 and Jagged-2) which bind to transmembrane Notch receptors. After interaction with a ligand, a disintegrin and metalloprotease 10 (ADAM10) cleaves the extracellular domain of the notch protein. The γ-secretase protease then cleaves the Notch intracellular domain (NICD) from the transmembrane portion of the Notch receptor. Alterations in this signaling system may induce vascular damage, given the role of Notch proteins in vessel maintenance and lead to development of additional pathologies such as amyloid and p-tau deposition, and subsequently cognitive decline.
Created with and adapted from “Notch Signaling Pathway”, by BioRender.com (2020). Retrieved from https://app.biorender.com/biorender-templates
4.1. Conclusion
Vascular and Alzheimer’s pathologies are the predominant and commonly overlapping causes of age-related cognitive decline and dementia, yet the underlying connection between these diseases remains obscure. The Notch signaling pathway is critical for vascular development and homeostasis, and mutations in Notch genes or pathways impacting Notch physiology have been implicated in multiple genetic forms of both vascular dementia and Alzheimer’s dementia. These findings directly support a potential role for Notch signaling in the connection between vascular and Alzheimer’s pathologies, and suggest a need for further research into the role of Notch signaling changes in age-related neurovascular dysfunction and cognitive decline.
Acknowledgements:
This research was supported by National Institutes of Health grants (DAN R01AG064228, R01AG060049, P01AG052350, P50AG016573), the Alzheimer’s Association (DAN: AA008369), and the Canadian Institutes of Health Research Doctoral Foreign Study Award (AK: DFD-170763).
Footnotes
Conflict of Interest
The authors have no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Artavanis-Tsakonas S Notch Signaling: Cell Fate Control and Signal Integration in Development. Science (80- ) 1999;284:770–6. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- [2].Aguirre A, Rubio ME, Gallo V. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature 2010;467:323–7. doi: 10.1038/nature09347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gaiano N, Fishell G. The Role of Notch in Promoting Glial and Neural Stem Cell Fates. Annu Rev Neurosci 2002;25:471–90. doi: 10.1146/annurev.neuro.25.030702.130823. [DOI] [PubMed] [Google Scholar]
- [4].Liu Z-J, Shirakawa T, Li Y, Soma A, Oka M, Dotto GP, et al. Regulation of Notch1 and Dll4 by Vascular Endothelial Growth Factor in Arterial Endothelial Cells: Implications for Modulating Arteriogenesis and Angiogenesis. Mol Cell Biol 2003;23:14–25. doi: 10.1128/MCB.23.1.14-25.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Patel-Hett S, DAmore PA. Signal transduction in vasculogenesis and developmental angiogenesis. Int J Dev Biol 2011;55:353–63. doi: 10.1387/ijdb.103213sp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Grego-Bessa J, Luna-Zurita L, del Monte G, Bolós V, Melgar P, Arandilla A, et al. Notch Signaling Is Essential for Ventricular Chamber Development. Dev Cell 2007;12:415–29. doi: 10.1016/j.devcel.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gude N, Sussman M. Notch signaling and cardiac repair. J Mol Cell Cardiol 2012;52:1226–32. doi: 10.1016/j.yjmcc.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hitoshi S Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev 2002;16:846–58. doi: 10.1101/gad.975202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Imayoshi I, Sakamoto M, Yamaguchi M, Mori K, Kageyama R. Essential Roles of Notch Signaling in Maintenance of Neural Stem Cells in Developing and Adult Brains. J Neurosci 2010;30:3489–98. doi: 10.1523/JNEUROSCI.4987-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Andersson ER, Sandberg R, Lendahl U. Notch signaling: simplicity in design, versatility in function. Development 2011;138:3593–612. doi: 10.1242/dev.063610. [DOI] [PubMed] [Google Scholar]
- [11].D’Souza B, Meloty-Kapella L, Weinmaster G. Canonical and Non-Canonical Notch Ligands, 2010, p. 73–129. doi: 10.1016/S0070-2153(10)92003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ables JL, Breunig JJ, Eisch AJ, Rakic P. Not(ch) just development: Notch signalling in the adult brain. Nat Rev Neurosci 2011;12:269–83. doi: 10.1038/nrn3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature 1996;383:707–10. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- [14].Fischer DF. Activation of the Notch pathway in Down syndrome: cross-talk of Notch and APP. FASEB J 2005;19:1451–8. doi: 10.1096/fj.04-3395.com. [DOI] [PubMed] [Google Scholar]
- [15].Cai Z, Zhao B, Deng Y, Shangguan S, Zhou F, Zhou W, et al. Notch signaling in cerebrovascular diseases (Review). Mol Med Rep 2016;14:2883–98. doi: 10.3892/mmr.2016.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Knopman DS, Parisi JE, Boeve BF, Cha RH, Apaydin H, Salviati A, et al. Vascular Dementia in a Population-Based Autopsy Study. Arch Neurol 2003;60:569. doi: 10.1001/archneur.60.4.569. [DOI] [PubMed] [Google Scholar]
- [17].Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007;69:2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- [18].Greenberg SM. Cerebral amyloid angiopathy: Prospects for clinical diagnosis and treatment. Neurology 1998;51:690–4. doi: 10.1212/WNL.51.3.690. [DOI] [PubMed] [Google Scholar]
- [19].Rutten JW, Van Eijsden BJ, Duering M, Jouvent E, Opherk C, Pantoni L, et al. The effect of NOTCH3 pathogenic variant position on CADASIL disease severity: NOTCH3 EGFr 1–6 pathogenic variant are associated with a more severe phenotype and lower survival compared with EGFr 7–34 pathogenic variant. Genet Med 2019;21:676–82. doi: 10.1038/s41436-018-0088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gray F, Robert F, Labrecque R, Chrétien F, Baudrimont M, Fallet-Bianco C, et al. Autosomal dominant arteriopathic leuko-encephalopathy and Alzheimer’s disease. Neuropathol Appl Neurobiol 1994;20:22–30. doi: 10.1111/j.1365-2990.1994.tb00953.x. [DOI] [PubMed] [Google Scholar]
- [21].Thijs V. Coexistence of CADASIL and Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2003;74:790–2. doi: 10.1136/jnnp.74.6.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Guerreiro RJ, Lohmann E, Kinsella E, Brás JM, Luu N, Gurunlian N, et al. Exome sequencing reveals an unexpected genetic cause of disease: NOTCH3 mutation in a Turkish family with Alzheimer’s disease. Neurobiol Aging 2012;33:1008.e17–1008.e23. doi: 10.1016/j.neurobiolaging.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lee S, Viqar F, Zimmerman ME, Narkhede A, Tosto G, Benzinger TLS, et al. White matter hyperintensities are a core feature of Alzheimer’s disease: Evidence from the dominantly inherited Alzheimer network. Ann Neurol 2016;79:929–39. doi: 10.1002/ana.24647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lee S, Zimmerman ME, Narkhede A, Nasrabady SE, Tosto G, Meier IB, et al. White matter hyperintensities and the mediating role of cerebral amyloid angiopathy in dominantly-inherited Alzheimer’s disease. PLoS One 2018;13:e0195838. doi: 10.1371/journal.pone.0195838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lao PJ, Gutierrez J, Keator D, Rizvi B, Banerjee A, Igwe KC, et al. Alzheimer’s-related cerebrovascular disease in Down syndrome. Ann Neurol 2020:ana.25905. doi: 10.1002/ana.25905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Drachman DA, Smith TW, Alkamachi B, Kane K. Microvascular changes in Down syndrome with Alzheimer’s-type pathology: Insights into a potential vascular mechanism for Down syndrome and Alzheimer’s disease. Alzheimer’s Dement 2017;13:1389–96. doi: 10.1016/j.jalz.2017.05.003. [DOI] [PubMed] [Google Scholar]
- [27].Berezovska O, Xia MQ, Hyman BT. Notch Is Expressed in Adult Brain, Is Coexpressed with Presenilin-1, and Is Altered in Alzheimer Disease. J Neuropathol Exp Neurol 1998;57:738–45. doi: 10.1097/00005072-199808000-00003. [DOI] [PubMed] [Google Scholar]
- [28].Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005;433:760–4. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- [29].Balistreri CR, Madonna R, Melino G, Caruso C. The emerging role of Notch pathway in ageing: Focus on the related mechanisms in age-related diseases. Ageing Res Rev 2016;29:50–65. doi: 10.1016/j.arr.2016.06.004. [DOI] [PubMed] [Google Scholar]
- [30].Conboy IM. Notch-Mediated Restoration of Regenerative Potential to Aged Muscle. Science (80- ) 2003;302:1575–7. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- [31].Carlson ME, Silva HS, Conboy IM. Aging of signal transduction pathways, and pathology. Exp Cell Res 2008;314:1951–61. doi: 10.1016/j.yexcr.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA. A Temporal Switch from Notch to Wnt Signaling in Muscle Stem Cells Is Necessary for Normal Adult Myogenesis. Cell Stem Cell 2008;2:50–9. doi: 10.1016/j.stem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- [33].Olsen JJ, Pohl SÖ-G, Deshmukh A, Visweswaran M, Ward NC, Arfuso F, et al. The Role of Wnt Signalling in Angiogenesis. Clin Biochem Rev 2017;38:131–42. [PMC free article] [PubMed] [Google Scholar]
- [34].Phng L-K, Potente M, Leslie JD, Babbage J, Nyqvist D, Lobov I, et al. Nrarp Coordinates Endothelial Notch and Wnt Signaling to Control Vessel Density in Angiogenesis. Dev Cell 2009;16:70–82. doi: 10.1016/j.devcel.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Easwaran V, Lee SH, Inge L, Guo L, Goldbeck C, Garrett E, et al. beta-Catenin regulates vascular endothelial growth factor expression in colon cancer. Cancer Res 2003;63:3145–53. [PubMed] [Google Scholar]
- [36].Masckauchán TNH, Shawber CJ, Funahashi Y, Li C-M, Kitajewski J. Wnt/β-Catenin Signaling Induces Proliferation, Survival and Interleukin-8 in Human Endothelial Cells. Angiogenesis 2005;8:43–51. doi: 10.1007/s10456-005-5612-9. [DOI] [PubMed] [Google Scholar]
- [37].Lau S-F, Cao H, Fu AKY, Ip NY. Single-nucleus transcriptome analysis reveals dysregulation of angiogenic endothelial cells and neuroprotective glia in Alzheimer’s disease. Proc Natl Acad Sci 2020;117:25800–9. doi: 10.1073/pnas.2008762117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Jefferies WA, Price KA, Biron KE, Fenninger F, Pfeifer CG, Dickstein DL. Adjusting the compass: new insights into the role of angiogenesis in Alzheimer’s disease. Alzheimers Res Ther 2013;5:64. doi: 10.1186/alzrt230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wang P, Xie Z-H, Guo Y-J, Zhao C-P, Jiang H, Song Y, et al. VEGF-induced angiogenesis ameliorates the memory impairment in APP transgenic mouse model of Alzheimer’s disease. Biochem Biophys Res Commun 2011;411:620–6. doi: 10.1016/j.bbrc.2011.07.003. [DOI] [PubMed] [Google Scholar]
- [40].Vagnucci AH, Li WW. Alzheimer’s disease and angiogenesis. Lancet 2003;361:605–8. doi: 10.1016/S0140-6736(03)12521-4. [DOI] [PubMed] [Google Scholar]
- [41].Biron KE, Dickstein DL, Gopaul R, Jefferies WA. Amyloid Triggers Extensive Cerebral Angiogenesis Causing Blood Brain Barrier Permeability and Hypervascularity in Alzheimer’s Disease. PLoS One 2011;6:e23789. doi: 10.1371/journal.pone.0023789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Desai BS, Schneider JA, Li J-L, Carvey PM, Hendey B. Evidence of angiogenic vessels in Alzheimer’s disease. J Neural Transm 2009;116:587–97. doi: 10.1007/s00702-009-0226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Siekmann AF, Lawson ND. Notch Signalling and the Regulation of Angiogenesis. Cell Adh Migr 2007;1:104–5. doi: 10.4161/cam.1.2.4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Briot A, Civelek M, Seki A, Hoi K, Mack JJ, Lee SD, et al. Endothelial NOTCH1 is suppressed by circulating lipids and antagonizes inflammation during atherosclerosis. J Exp Med 2015;212:2147–63. doi: 10.1084/jem.20150603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tublin JM, Adelstein JM, del Monte F, Combs CK, Wold LE. Getting to the Heart of Alzheimer Disease. Circ Res 2019;124:142–9. doi: 10.1161/CIRCRESAHA.118.313563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Alberi L, Hoey SE, Brai E, Scotti AL, Marathe S. Notch signaling in the brain: In good and bad times. Ageing Res Rev 2013;12:801–14. doi: 10.1016/j.arr.2013.03.004. [DOI] [PubMed] [Google Scholar]
- [47].Di Donato I, Bianchi S, De Stefano N, Dichgans M, Dotti MT, Duering M, et al. Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) as a model of small vessel disease: update on clinical, diagnostic, and management aspects. BMC Med 2017; 15:41. doi: 10.1186/s12916-017-0778-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Joutel A, Andreux F, Gaulis S, Domenga V, Cecillon M, Battail N, et al. The ectodomain of the Notch3 receptor accumulates within the cerebrovasculature of CADASIL patients. J Clin Invest 2000; 105:597–605. doi: 10.1172/JCI8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Craggs LJL, Yamamoto Y, Deramecourt V, Kalaria RN. Microvascular Pathology and Morphometrics of Sporadic and Hereditary Small Vessel Diseases of the Brain. Brain Pathol 2014;24:495–509. doi: 10.1111/bpa.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Murphy MP, LeVine H. Alzheimer’s Disease and the Amyloid-β Peptide. J Alzheimer’s Dis 2010;19:311–23. doi: 10.3233/JAD-2010-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bates KA, Verdile G, Li Q-X, Ames D, Hudson P, Masters CL, et al. Clearance mechanisms of Alzheimer’s amyloid-β peptide: implications for therapeutic design and diagnostic tests. Mol Psychiatry 2009;14:469–86. doi: 10.1038/mp.2008.96. [DOI] [PubMed] [Google Scholar]
- [52].Nunan J, Small DH. Regulation of APP cleavage by α-, β- and γ-secretases. FEBS Lett 2000;483:6–10. doi: 10.1016/S0014-5793(00)02076-7. [DOI] [PubMed] [Google Scholar]
- [53].Priller C, Bauer T, Mitteregger G, Krebs B, Kretzschmar HA, Herms J. Synapse Formation and Function Is Modulated by the Amyloid Precursor Protein. J Neurosci 2006;26:7212–21. doi: 10.1523/JNEUROSCI.1450-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Konishi J, Kawaguchi KS, Vo H, Haruki N, Gonzalez A, Carbone DP, et al. γ-Secretase Inhibitor Prevents Notch3 Activation and Reduces Proliferation in Human Lung Cancers. Cancer Res 2007;67:8051–7. doi: 10.1158/0008-5472.CAN-07-1022. [DOI] [PubMed] [Google Scholar]
- [55].De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, et al. A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature 1999;398:518–22. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- [56].Joutel A, Vahedi K, Corpechot C, Troesch A, Chabriat H, Vayssière C, et al. Strong clustering and stereotyped nature of Notch3 mutations in CADASIL patients. Lancet 1997;350:1511–5. doi: 10.1016/S0140-6736(97)08083-5. [DOI] [PubMed] [Google Scholar]
- [57].Greenberg SM, Charidimou A. Diagnosis of Cerebral Amyloid Angiopathy. Stroke 2018;49:491–7. doi: 10.1161/STROKEAHA.117.016990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Weller RO, Subash M, Preston SD, Mazanti I, Carare RO. SYMPOSIUM: Clearance of Aβ from the Brain in Alzheimer’s Disease: Perivascular Drainage of Amyloid-β Peptides from the Brain and Its Failure in Cerebral Amyloid Angiopathy and Alzheimer’s Disease. Brain Pathol 2007;18:253–66. doi: 10.1111/j.1750-3639.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Weller RO, Yow HY, Preston SD, Mazanti I, Nicoll JA. Cerebrovascular Disease Is a Major Factor in the Failure of Elimination of Aβ from the Aging Human Brain. Ann N Y Acad Sci 2002;977:162–8. doi: 10.1111/j.1749-6632.2002.tb04812.x. [DOI] [PubMed] [Google Scholar]
- [60].Paquet C, Jouvent E, Mine M, Vital A, Hugon J, Chabriat H, et al. A cortical form of CADASIL with cerebral Aβ amyloidosis. Acta Neuropathol 2010;120:813–20. doi: 10.1007/s00401-010-0758-y. [DOI] [PubMed] [Google Scholar]
- [61].Carare RO, Hawkes CA, Jeffrey M, Kalaria RN, Weller RO. Review: Cerebral amyloid angiopathy, prion angiopathy, CADASIL and the spectrum of protein elimination failure angiopathies (PEFA) in neurodegenerative disease with a focus on therapy. Neuropathol Appl Neurobiol 2013;39:593–611. doi: 10.1111/nan.12042. [DOI] [PubMed] [Google Scholar]
- [62].Ringman JM, Sachs MC, Zhou Y, Monsell SE, Saver JL, Vinters HV. Clinical Predictors of Severe Cerebral Amyloid Angiopathy and Influence of APOE Genotype in Persons With Pathologically Verified Alzheimer Disease. JAMA Neurol 2014;71:878. doi: 10.1001/jamaneurol.2014.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Schley D, Carare-Nnadi R, Please CP, Perry VH, Weller RO. Mechanisms to explain the reverse perivascular transport of solutes out of the brain. J Theor Biol 2006;238:962–74. doi: 10.1016/j.jtbi.2005.07.005. [DOI] [PubMed] [Google Scholar]
- [64].Formichi P, Parnetti L, Radi E, Cevenini G, Dotti MT, Federico A. CSF levels of β-amyloid 1-42, tau and phosphorylated tau protein in CADASIL. Eur J Neurol 2008;15:1252–5. doi: 10.1111/j.1468-1331.2008.02277.x. [DOI] [PubMed] [Google Scholar]
- [65].Formichi P, Parnetti L, Radi E, Cevenini G, Dotti MT, Federico A. CSF Biomarkers Profile in CADASIL—A Model of Pure Vascular Dementia: Usefulness in Differential Diagnosis in the Dementia Disorder. Int J Alzheimers Dis 2010;2010:1–6. doi: 10.4061/2010/959257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Stenset V, Johnsen L, Kocot D, Negaard A, Skinningsrud A, Gulbrandsen P, et al. Associations between white matter lesions, cerebrovascular risk factors, and low CSF A 42. Neurology 2006;67:830–3. doi: 10.1212/01.wnl.0000234030.77831.5a. [DOI] [PubMed] [Google Scholar]
- [67].Sassi C, Nalls MA, Ridge PG, Gibbs JR, Lupton MK, Troakes C, et al. Mendelian adult-onset leukodystrophy genes in Alzheimer’s disease: critical influence of CSF1R and NOTCH3. Neurobiol Aging 2018;66:179.e17–179.e29. doi: 10.1016/j.neurobiolaging.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Domenga V Notch3 is required for arterial identity and maturation of vascular smooth muscle cells. Genes Dev 2004;18:2730–5. doi: 10.1101/gad.308904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ragot H, Monfort A, Baudet M, Azibani F, Fazal L, Merval R, et al. Loss of Notch3 Signaling in Vascular Smooth Muscle Cells Promotes Severe Heart Failure Upon Hypertension. Hypertension 2016;68:392–400. doi: 10.1161/HYPERTENSIONAHA.116.07694. [DOI] [PubMed] [Google Scholar]
- [70].Ghosh M, Balbi M, Hellal F, Dichgans M, Lindauer U, Plesnila N. Pericytes are involved in the pathogenesis of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Ann Neurol 2015;78:887–900. doi: 10.1002/ana.24512. [DOI] [PubMed] [Google Scholar]
- [71].Sweeney MD, Montagne A, Sagare AP, Nation DA, Schneider LS, Chui HC, et al. Vascular dysfunction-The disregarded partner of Alzheimer’s disease. Alzheimers Dement 2019;15:158–67. doi: 10.1016/j.jalz.2018.07.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol 2019;18:684–96. doi: 10.1016/S1474-4422(19)30079-1. [DOI] [PubMed] [Google Scholar]
- [73].Nation DA, Sweeney MD, Montagne A, Sagare AP, D’Orazio LM, Pachicano M, et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med 2019;25:270–6. doi: 10.1038/s41591-018-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Yoon CW, Kim Y-E, Seo SW, Ki C-S, Choi SH, Kim J-W, et al. NOTCH3 variants in patients with subcortical vascular cognitive impairment: a comparison with typical CADASIL patients. Neurobiol Aging 2015;36:2443.e1–2443.e7. doi: 10.1016/j.neurobiolaging.2015.04.009. [DOI] [PubMed] [Google Scholar]
- [75].Arumugam TV, Chan SL, Jo D-G, Yilmaz G, Tang S-C, Cheng A, et al. Gamma secretase-mediated Notch signaling worsens brain damage and functional outcome in ischemic stroke. Nat Med 2006;12:621–3. doi: 10.1038/nm1403. [DOI] [PubMed] [Google Scholar]
- [76].Woo H-N, Park J-S, Gwon A-R, Arumugam TV., Jo D-G. Alzheimer’s disease and Notch signaling. Biochem Biophys Res Commun 2009;390:1093–7. doi: 10.1016/j.bbrc.2009.10.093. [DOI] [PubMed] [Google Scholar]
- [77].Sweeney MD, Sagare AP, Zlokovic BV. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 2018;14:133–50. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM. Mild Cognitive Impairment and Alzheimer Disease: Patterns of Altered Cerebral Blood Flow at MR Imaging. Radiology 2009;250:856–66. doi: 10.1148/radiol.2503080751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Suri S, Mackay CE, Kelly ME, Germuska M, Tunbridge EM, Frisoni GB, et al. Reduced cerebrovascular reactivity in young adults carrying the APOE ε4 allele. Alzheimer’s Dement 2015;11:648–657.e1. doi: 10.1016/j.jalz.2014.05.1755. [DOI] [PubMed] [Google Scholar]
- [80].Montagne A, Nation DA, Pa J, Sweeney MD, Toga AW, Zlokovic BV. Brain imaging of neurovascular dysfunction in Alzheimer’s disease. Acta Neuropathol 2016;131:687–707. doi: 10.1007/s00401-016-1570-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Makedonov I, Black SE, Macintosh BJ. Cerebral small vessel disease in aging and Alzheimer’s disease: a comparative study using MRI and SPECT. Eur J Neurol 2013;20:243–50. doi: 10.1111/j.1468-1331.2012.03785.x. [DOI] [PubMed] [Google Scholar]
- [82].Ruchoux MM, Maurage CA. CADASIL: Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. J Neuropathol Exp Neurol 1997;56:947–64. [PubMed] [Google Scholar]
- [83].Dichgans M, Mayer M, Uttner I, Brüning R, Müller-Höcker J, Rungger G, et al. The phenotypic spectrum of CADASIL: clinical findings in 102 cases. Ann Neurol 1998;44:731–9. doi: 10.1002/ana.410440506. [DOI] [PubMed] [Google Scholar]
- [84].Opherk C, Peters N, Herzog J, Luedtke R, Dichgans M. Long-term prognosis and causes of death in CADASIL: a retrospective study in 411 patients. Brain 2004;127:2533–9. doi: 10.1093/brain/awh282. [DOI] [PubMed] [Google Scholar]
- [85].Amberla K, Wäljas M, Tuominen S, Almkvist O, Pöyhönen M, Tuisku S, et al. Insidious cognitive decline in CADASIL. Stroke 2004;35:1598–602. doi: 10.1161/01.STR.0000129787.92085.0a. [DOI] [PubMed] [Google Scholar]
- [86].Peters N, Herzog J, Opherk C, Dichgans M. A Two-Year Clinical Follow-Up Study in 80 CADASIL Subjects. Stroke 2004;35:1603–8. doi: 10.1161/01.STR.0000131546.71733.f1. [DOI] [PubMed] [Google Scholar]
- [87].Buffon F, Porcher R, Hernandez K, Kurtz A, Pointeau S, Vahedi K, et al. Cognitive profile in CADASIL. J Neurol Neurosurg Psychiatry 2006;77:175–80. doi: 10.1136/jnnp.2005.068726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Dichgans M Cognition in CADASIL. Stroke 2009;40:S45–7. doi: 10.1161/STROKEAHA.108.534412. [DOI] [PubMed] [Google Scholar]
- [89].Epstein CJ. Down Syndrome. Abnorm. States Brain Mind, Boston, MA: Birkhäuser Boston; 1989, p. 43–4. doi: 10.1007/978-1-4899-6768-8_18. [DOI] [Google Scholar]
- [90].Cork LC. Neuropathology of Down syndrome and Alzheimer disease. Am J Med Genet 2005;37:282–6. doi: 10.1002/ajmg.1320370756. [DOI] [PubMed] [Google Scholar]
- [91].Lao PJ, Gutierrez J, Keator D, Rizvi B, Banerjee A, Igwe KC, et al. Alzheimer’s-related cerebrovascular disease in Down syndrome. Ann Neurol 2020:ana.25905. doi: 10.1002/ana.25905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Placanica L, Zhu L, Li Y-M. Gender- and Age-Dependent γ-Secretase Activity in Mouse Brain and Its Implication in Sporadic Alzheimer Disease. PLoS One 2009;4:e5088. doi: 10.1371/journal.pone.0005088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Cenini G, Fiorini A, Sultana R, Perluigi M, Cai J, Klein JB, et al. An investigation of the molecular mechanisms engaged before and after the development of Alzheimer disease neuropathology in Down syndrome: a proteomics approach. Free Radic Biol Med 2014;76:89–95. doi: 10.1016/j.freeradbiomed.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Okochi M Presenilins mediate a dual intramembranous gamma-secretase cleavage of Notch-1. EMBO J 2002;21:5408–16. doi: 10.1093/emboj/cdf541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Brai E, Alina Raio N, Alberi L. Notch1 hallmarks fibrillary depositions in sporadic Alzheimer’s disease. Acta Neuropathol Commun 2016;4:64. doi: 10.1186/s40478-016-0327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Cho S-J, Yun S-M, Jo C, Jeong J, Park MH, Han C, et al. Altered expression of Notch1 in Alzheimer’s disease. PLoS One 2019;14:e0224941. doi: 10.1371/journal.pone.0224941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Moehlmann T, Winkler E, Xia X, Edbauer D, Murrell J, Capell A, et al. Presenilin-1 mutations of leucine 166 equally affect the generation of the Notch and APP intracellular domains independent of their effect on A 42 production. Proc Natl Acad Sci 2002;99:8025–30. doi: 10.1073/pnas.112686799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Nakajima M, Ogawa M, Shimoda Y, Hiraoka S, Iida M, Koseki H, et al. Presenilin-1 controls the growth and differentiation of endothelial progenitor cells through its β-catenin-binding region. Cell Biol Int 2006;30:239–43. doi: 10.1016/j.cellbi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- [99].Ishiguro H, Okubo T, Kuwabara Y, Kimura M, Mitsui A, Sugito N, et al. NOTCH1 activates the Wnt/β-catenin signaling pathway in colon cancer. Oncotarget 2017;8:60378–89. doi: 10.18632/oncotarget.19534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, et al. Wnt/β-catenin signaling controls development of the blood–brain barrier. J Cell Biol 2008;183:409–17. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt Signaling Regulates Organ-Specific Assembly and Differentiation of CNS Vasculature. Science (80- ) 2008;322:1247–50. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- [102].Kwon C, Cheng P, King IN, Andersen P, Shenje L, Nigam V, et al. Notch post-translationally regulates β-catenin protein in stem and progenitor cells. Nat Cell Biol 2011; 13:1244–51. doi: 10.1038/ncb2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Zhang P, Kishimoto Y, Grammatikakis I, Gottimukkala K, Cutler RG, Zhang S, et al. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat Neurosci 2019;22:719–28. doi: 10.1038/s41593-019-0372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Bonini SA, Ferrari-Toninelli G, Montinaro M, Memo M. Notch signalling in adult neurons: a potential target for microtubule stabilization. Ther Adv Neurol Disord 2013;6:375–85. doi: 10.1177/1756285613490051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Ferrari-Toninelli G, Bonini SA, Bettinsoli P, Uberti D, Memo M. Microtubule stabilizing effect of notch activation in primary cortical neurons. Neuroscience 2008;154:946–52. doi: 10.1016/j.neuroscience.2008.04.025. [DOI] [PubMed] [Google Scholar]
- [106].Rabin JS, Yang H-S, Schultz AP, Hanseeuw BJ, Hedden T, Viswanathan A, et al. Vascular Risk and β -Amyloid Are Synergistically Associated with Cortical Tau. Ann Neurol 2019;85:272–9. doi: 10.1002/ana.25399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Wang Q, Zhao N, Kennard S, Lilly B. Notch2 and Notch3 Function Together to Regulate Vascular Smooth Muscle Development. PLoS One 2012;7:e37365. doi: 10.1371/journal.pone.0037365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Meester JAN, Verstraeten A, Alaerts M, Schepers D, Van Laer L, Loeys BL. Overlapping but distinct roles for NOTCH receptors in human cardiovascular disease. Clin Genet 2019;95:85–94. doi: 10.1111/cge.13382. [DOI] [PubMed] [Google Scholar]
- [109].Lambert J-C. Association study of Notch 4 polymorphisms with Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2004;75:377–81. doi: 10.1136/jnnp.2003.017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 1999;85:221–8. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- [111].Sobrino T, Hurtado O, Moro MA, Rodríguez-Yáñez M, Castellanos M, Brea D, et al. The Increase of Circulating Endothelial Progenitor Cells After Acute Ischemic Stroke Is Associated With Good Outcome. Stroke 2007;38:2759–64. doi: 10.1161/STROKEAHA.107.484386. [DOI] [PubMed] [Google Scholar]
- [112].Nation DA, Tan A, Dutt S, McIntosh EC, Yew B, Ho JK, et al. Circulating Progenitor Cells Correlate with Memory, Posterior Cortical Thickness, and Hippocampal Perfusion. J Alzheimers Dis 2018;61:91–101. doi: 10.3233/JAD-170587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Lee S-T, Chu K, Jung K-H, Park H-K, Kim D-H, Bahn J-J, et al. Reduced circulating angiogenic cells in Alzheimer disease. Neurology 2009;72:1858–63. doi: 10.1212/WNL.0b013e3181a711f4. [DOI] [PubMed] [Google Scholar]
- [114].Jickling G, Salam A, Mohammad A, Hussain MS, Scozzafava J, Nasser AM, et al. Circulating Endothelial Progenitor Cells and Age-Related White Matter Changes. Stroke 2009;40:3191–6. doi: 10.1161/STROKEAHA.109.554527. [DOI] [PubMed] [Google Scholar]
- [115].Lampreia FP, Carmelo JG, Anjos-Afonso F. Notch Signaling in the Regulation of Hematopoietic Stem Cell. Curr Stem Cell Reports 2017;3:202–9. doi: 10.1007/s40778-017-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Kwon S-M, Alev C, Asahara T. The Role of Notch Signaling in Endothelial Progenitor Cell Biology. Trends Cardiovasc Med 2009;19:170–3. doi: 10.1016/j.tcm.2009.10.002. [DOI] [PubMed] [Google Scholar]
- [117].Pitulescu ME, Schmidt I, Giaimo BD, Antoine T, Berkenfeld F, Ferrante F, et al. Dll4 and Notch signalling couples sprouting angiogenesis and artery formation. Nat Cell Biol 2017;19:915–27. doi: 10.1038/ncb3555. [DOI] [PubMed] [Google Scholar]
- [118].Kofler NM, Shawber CJ, Kangsamaksin T, Reed HO, Galatioto J, Kitajewski J. Notch Signaling in Developmental and Tumor Angiogenesis. Genes Cancer 2011;2:1106–16. doi: 10.1177/1947601911423030. [DOI] [PMC free article] [PubMed] [Google Scholar]