Abstract
Objective:
Adolescence is a sensitive period for the development and emergence of anxiety and mood disorders. Research suggests that symptoms ranging from subclinical to clinical levels are associated with pathological developmental changes in the neocortex. However, much of this research has been cross-sectional, limiting the field’s ability to identify the neurodevelopmental impacts of these symptoms. The present study examined how early reported symptoms predict baseline cortical thickness and surface area, and trajectories of change in these measures during adolescence.
Method:
205 typically-developing 9- to 15-year-olds (103 male participants) completed 3T structural MRI annually for three years. From these, we extracted mean cortical thickness and total surface area for each year. Youth self-reported their anxiety, depressive, and posttraumatic stress symptoms during their first visit. We employed latent growth curve modeling to determine how these symptoms along with sex interactions predicted baseline thickness and surface area, and rates of change in these measures over the three-year period.
Results:
Higher anxiety was associated with lower baseline thickness and slowed cortical thinning over time. Conversely, greater posttraumatic stress predicted higher baseline thickness and accelerated thinning over time. Sex interactions suggested that the effects were dampened among female compared to male participants. Depressive symptoms were not related to cortical thickness or surface area.
Conclusion:
Female adolescents may express more regionally specific effects of symptoms sets on cortical thickness, though this requires further investigation. Cortical thickness in male adolescents appears to be preferentially susceptible to anxiety and posttraumatic stress symptoms, exhibiting global changes across multiple years.
Keywords: adolescent development, MRI, subclinical symptoms, longitudinal, structural equation modeling
Introduction
Adolescence is a dynamic phase of development and is a noted sensitive period for the development and emergence of mental health disorders. Anxiety (e.g., generalized anxiety disorder, posttraumatic stress disorder) and mood disorders (e.g., major depressive disorder) are among the most prevalent diagnoses attributed to youth in the United States. In a study of over 10,000 youth, 31.9% of adolescents ages 13-to-18 years-old had a lifetime history of an anxiety disorder diagnosis, while 14.3% had a history of a mood disorder.1 Importantly, multiple large-scale studies have found that: A) symptom severity increases throughout adolescence, B) youth with anxiety and mood disorders are at a significantly higher risk of developing additional psychopathologies throughout their lifetimes, and C) there are substantial societal and interpersonal burdens associated with these diagnoses, including school problems, parenting challenges, and markedly-increased health care costs.1–5
Aside from these broader impacts, evidence suggests that anxiety and mood disorders are associated with morphological brain alterations across distributed cortical and subcortical networks.6–10 Changes in cortical gray matter are associated with cognitive abilities,11–13 as well as the emergence of neurodevelopmental disorders in childhood and adolescence,6,14,15 making gray matter metrics a prime target for investigation. Past studies have primarily focused on gray matter volume, surface area, or cortical thickness in regions involved in social-emotional processes, and compared individuals with or without clinical diagnoses.14,16–19 More recent studies have examined the effects of anxiety and depressive symptom severity to better understand full spectra of symptom sets and their unique impacts on brain structure and function.9,20–24
Studies have generally shown that increasing anxiety or depressive symptom severity is associated with changes in gray matter, though the specific regions implicated are disparate across studies.25 For instance, studies in adults have shown that gray matter alterations are associated with symptom severity within the amygdala, hippocampus, frontal, prefrontal, parietal, inferior temporal, anterior and posterior cingulate, and occipital cortices.18,20,22,26–30 The lack of consistent regions may suggest more systemic, global associations between symptom sets and brain morphology. The majority of studies examining anxiety, posttraumatic stress, and/or depressive symptom severity focus on adults, despite mounting evidence that anxiety and mood disorders are particularly salient in childhood and adolescence.1,2 Moreover, studies of developing youth are predominantly cross-sectional. For example, Boes et al. reported that increased depressive symptom severity is associated with decreased rostral anterior cingulate cortex volume in male, but not female youth.23 A small study of children with and without posttraumatic stress disorder indicated that posttraumatic stress symptoms were associated with increased prefrontal cortex volume.31 A larger study in adolescents indicated that ventromedial prefrontal cortex and global mean cortical thickness predict generalized anxiety symptom severity, and vice versa.24 However, because of the cross-sectional nature of these studies, the direction of causality remains ambiguous.
Few studies have examined the link between symptom severity and cortical structure longitudinally, a necessary endeavor to fully understand the neurodevelopmental impacts of psychopathological symptoms.25 One such study examined changes in cortical thickness in a large study of 8-to-25 year-olds during three visits approximately two years apart, with an in-depth assessment of depressive symptoms occurring only during the third visit.32 Individuals with more depressive symptoms at the third time point had different trajectories of thinning in the orbitofrontal cortex and precentral gyrus compared to those with fewer depressive symptoms. Volume and thickness changes in the hippocampus and amygdala did not relate to depressive symptoms. Another longitudinal investigation in 4-to-18 year-olds showed that parent-reported anxiety and depressive symptoms were related to greater amygdala volume over time.21
Overall, data on the associations between gray matter and anxiety, depressive, and posttraumatic stress symptoms are widely variable and suggest gross effects across the brain, though the trajectory of these effects during childhood and adolescence is poorly understood. Few studies have examined the longitudinal changes in brain structure associated with these symptom clusters, and almost no studies assess the concomitant effects of multiple symptom sets, despite the comorbid nature of depression, anxiety, and posttraumatic stress.1,25,33,34 Moreover, there has been relatively little investigation of sex-specific patterns of neurobiological change associated with psychopathological symptoms despite evidence that male and female youth experience these symptoms at different prevalence rates.35–37 Evidence suggests that male and female youth may be differentially susceptible to developing symptoms following trauma exposure depending on the timing and severity of the incident.9,38,39 Given the prevalence of anxiety and mood disorders emerging in childhood and adolescence, it is paramount to better understand how early co-occurring symptom sets may affect the nature of brain development over time.
In this study, we conduct an exploratory investigation to determine whether self-reported anxiety, depressive, and posttraumatic stress symptoms are associated with baseline cortical thickness and/or surface area, as well as the rate of change in these metrics over a three-year period with annual follow-ups in a large cohort of typically developing youth. Due to the broadly distributed neural systems involved in mental health symptoms, we examined global mean cortical thickness and surface area, as well as that in specific brain regions, to better understand the associations between concomitant symptom sets and brain structure. We also explore the interactions between sex and each symptom to better interrogate any sexually-divergent associations between symptoms and cortical development.
Method
Participants
We collected data from a total of 212 typically-developing youth (106 male participants) as part of the Developmental Chronnecto-Genomics study. Of the total sample, 112 youth were recruited at the University of Nebraska Medical Center (UNMC), and 100 at the Mind Research Network (MRN). During their first visit, children were between the ages of 9–15 years-old. All participants were invited to return annually for repeated neuroimaging and neuropsychological testing for three years. Of the original sample, 175 youth returned and had complete data for year 2 (time between tests: Msite 1 = 1.09 years, SD = .16; Msite 2 = 1.16 years, SD = .23), and 127 returned and had complete data for year 3 (time between tests: Msite 1 = 1.02 years, SD = .084; Msite 2 = 1.13 years, SD = .32). Exclusionary criteria, assessed by parent report, included history of concussion or head injury, any medical illness affecting neural functioning, neurological or psychiatric disorder, current substance abuse, or current use of medications that affect brain function. All parents signed informed consent forms, and youth signed assent forms before proceeding with the study. The appropriate institutional review board for each study site approved all procedures.
Psychological Measures
Participants completed the self-report Trauma Symptom Checklist for Children (TSCC).40 Participants indicated the frequency with which they experienced a variety of symptoms on a 0–3 scale. Questions load onto six clinically-oriented subscales, including anxiety, depression, and posttraumatic stress. The anxiety subscale focuses on aspects of generalized worry and specific phobias, whereas the posttraumatic stress scale asks about intrusive thoughts, nightmares, and avoidance behaviors. The depressive symptom scale assesses feelings of sadness and loneliness. We used raw scores given the developmental nature of the study and our selected analysis strategy. The range of raw scores for our three measures of interest was: 0–27 for anxiety, 0–21 for depression (two suicidality symptoms were not assessed per IRB request), and 0–30 for posttraumatic stress. In each scale, higher raw scores indicate a greater number and/or severity of symptoms. Because psychological symptoms are known to vary between sexes, we computed interaction terms for each of the three psychological symptoms of interest. Specifically, we multiplied each individual’s score by a dummy coded variable, where 0 = “male participants” and 1 = “female participants”. Participants also completed a modified version of the Trauma History Profile41–43 assessing traumatic events (questions about sexual abuse were excluded per IRB request). This measure is commonly administered to assess whether it is appropriate to measure posttraumatic symptoms. Questionnaires were completed during the first visit.
Structural MRI Acquisition and Processing
Participants underwent a structural T1-weighted MRI scan during each visit. Children recruited at UNMC were scanned using a Siemens 3T Skyra scanner, and those at MRN using a Siemens 3T TIM Trio. Structural T1-weighted MR images at both data collection sites were acquired with a 32-channel head coil and a MP-RAGE sequence with the following parameters: TR=2400ms; TE=1.94ms; flip angle=8º; FOV=256mm; slice thickness=1mm (no gap); base resolution=256; 192 slices; voxel size=1×1×1mm. The T1-weighted structural brain images of all participants were processed using Freesurfer version 5.3 (http://surfer.nmr.mgh.harvard.edu). Cortical thickness, surface area, and subcortical volume estimates were computed for the 70 Desikan-Killiany atlas regions. We followed the ENIGMA protocol for quality assurance, including performing visual checks on all cortical segmentations (http://enigma.usc.edu/protocols/imaging-protocols) and checking for motion among other artifacts. No cortical segmentations were flagged at this stage of processing. Histograms of all regional values were computed for visual inspection; all data were clean and met criteria for inclusion in further analyses. In this study, we focused on mean cortical thickness and total surface area across the entire brain for each participant, for each year of the study, but also examined multiple a priori regions that are known to be critical to psychological health. Surface area and subcortical volumes were corrected for total intracranial volume.
Statistical Analysis
We began by examining descriptive statistics for each measure of interest. We then fit a series of latent growth curve models (LGCM) to examine changes in either cortical thickness or surface area over time, separately, and how each relates to TSCC measures. The first models fit the base LGCM for changes in cortical structure over the three-year period without any control variables. The intercept was defined by cortical structure (e.g., thickness) at each time point constrained to 1. The slope was defined by cortical structure constrained to 0, 1, and 2 for measures obtained during years 1, 2, and 3, respectively. The second models added in age at time 1, sex (0 = “male participants”, 1 = “female participants”), and data collection site (0 = “site 1”, 1 = “site 2”) as control variables. Specifically, the latent intercept and slope variables were regressed on age, sex, and site to account for potential effects of demographic differences or site-based biases related to the different MRI scanners, all of which influence cortical thickness metrics. Finally, the third models added in TSCC measures and their interactions with sex. Total raw scores for anxiety, posttraumatic stress, and depression assessed at time 1 were all regressed on age at time 1 and site to account for potential maturational effects and demographic differences between study sites. The latent intercept and slope variables were regressed on TSCC measures, their interactions with sex, and the previously introduced control variables. All TSCC scales, sex, and their interactions were allowed to freely correlate. All parameters were freely estimated. These final models allowed us to examine the extent to which subclinical symptom sets were uniquely associated with the baseline and rate of change in cortical structure over time. Results from the LGCM of surface area are described in Supplement 1, available online.
We examined all models for goodness of fit using standard criteria,44 including root mean square error of approximation (RMSEA) < .06, and comparative fit index (CFI) > .95. We also examined the chi-square test of model fit, where a non-statistically significant test indicates good fit. Brief descriptions of each of the model fit indices is provided in the Supplement 1, available online. All models were tested using Mplus version 8.1.
Additional exploratory analyses.
In addition to our models of anxiety, depressive, and posttraumatic stress symptoms, we tested exploratory models examining 1) internalizing versus externalizing symptoms associated with cortical thickness measures, 2) regionally-specific analyses of TSCC measures predicting cortical thickness, surface area, and subcortical volumes, and 3) models of global thickness and surface area predicting changes in TSCC measures. These models and results are described in the Supplement 1, available online.
Missing data.
Of the total sample recruited, 205 children had TSCC and MRI data for year 1 of the study. However, not all children had structural MRI data for years 2 and 3 (N’s for each year are described in the Participants section). We completed all LGCM estimation with and without missing data estimation using full-information maximum likelihood (FIML) and yielded the same conclusions. Thus, we report the results using FIML estimation to reduce potential bias from data missing at random.
Results
Descriptive Statistics
Demographics and descriptive statistics for measures of interest in the final sample after exclusions are detailed in Table 1 for the whole sample, and separately by site. Additional clinical screening data are illustrated in Table S1, available online. Samples from each site were well-matched demographically with one exception; site 2 had a larger proportion of youth who identified as Hispanic/Latino compared to site 1. Most youth (83%) reported experiencing at least one traumatic event (M = 2.19, SD = 1.86; Table S2, available online), supporting our investigation of posttraumatic stress symptoms via the TSCC. The majority of youth in the study reported at least some anxiety, depressive, and/or posttraumatic stress symptoms (Table 1). Youth at site 1 tended to report more anxiety symptoms than those at site 2, whereas youth at site 2 tended to report more depressive and posttraumatic stress symptoms compared to site 1.
Table 1.
Demographic Data and Descriptive Statistics for the Final Sample Combined across Sites and Separately for Each Study Site With Accompanying Site-Based Comparison Statistics
| Full sample | Site 1 | Site 2 | Comparison | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| Variable | N | % | N | % | N | % | χ2 | p | |||
|
| |||||||||||
| Sex (male) | 103 | 50.00% | 52 | 48.60% | 51 | 52.04% | .24 | .62 | |||
| Race | .011 | .92 | |||||||||
| American Indian/Alaska Native | 5 | 2.44% | 0 | 0% | 5 | 5.10% | |||||
| Asian | 1 | 0.49% | 1 | 0.93% | 0 | 0 % | |||||
| Native Hawaiian or Other Pacific Islander | 0 | 0% | 0 | 0% | 0 | 0% | |||||
| Black or African American | 5 | 2.44% | 3 | 2.80% | 2 | 2.04% | |||||
| White | 172 | 83.90% | 88 | 82.24% | 84 | 85.71% | |||||
| More than One Race | 13 | 6.34% | 8 | 7.48% | 5 | 5.10% | |||||
| Unknown/Not Reported | 9 | 4.39% | 7 | 6.54% | 2 | 2.04% | |||||
| Ethnicity | 26.34 | < .001 | |||||||||
| Hispanic or Latino | 47 | 22.93% | 9 | 8.41% | 38 | 38.78% | |||||
| Not Hispanic or Latino | 157 | 76.21% | 97 | 90.65% | 60 | 61.22% | |||||
| Unknown/Not Reported | 1 | 0.49% | 1 | 0.93% | 0 | 0% | |||||
| M | SD | Range | M | SD | Range | M | SD | Range | t | p | |
|
|
|||||||||||
| Age time 1 | 11.81 | 1.78 | 9.02 – 16.29 | 11.79 | 1.72 | 9.02 – 16.29 | 11.83 | 1.85 | 9.07 – 15.12 | −.18 | .86 |
| Age time 2 | 12.96 | 1.79 | 9.99 – 17.48 | 12.84 | 1.67 | 9.99 – 15.99 | 13.08 | 1.91 | 10.18 – 17.48 | −.89 | .38 |
| Age time 3 | 13.92 | 1.77 | 10.98 – 17.42 | 13.85 | 1.65 | 10.98 – 16.99 | 14.00 | 1.91 | 11.19 – 17.42 | .48 | .63 |
| Anxiety | 4.18 | 3.05 | 0 – 15 | 4.69 | 3.31 | 0 – 15 | 3.64 | 2.66 | 0 – 11 | 2.48 | .014 |
| Depressive | 3.95 | 3.75 | 0 – 18 | 2.82 | 2.63 | 0 – 11 | 5.13 | 4.35 | 0 – 18 | −4.54 | < .001 |
| Posttraumatic stress | 5.71 | 4.09 | 0 – 18 | 5.10 | 4.02 | 0 – 17 | 6.35 | 4.09 | 0 – 18 | −2.18 | .03 |
| Cortical thickness time 1 | 2.76 | .11 | 2.41 – 3.07 | 2.73 | .12 | 2.41 – 3.02 | 2.78 | .10 | 2.44 – 3.07 | −3.19 | .002 |
| Cortical thickness time 2 | 2.72 | .11 | 2.28 – 2.98 | 2.71 | .11 | 2.28 – 2.98 | 2.74 | .10 | 2.47 – 2.95 | −1.49 | .14 |
| Cortical thickness time 3 | 2.71 | .10 | 2.42 – 2.94 | 2.73 | .093 | 2.49 – 2.90 | 2.70 | .10 | 2.42 – 2.94 | 1.34 | .18 |
| Surface area time 1 | .118 | .0075 | .08–.25 | .118 | .0083 | .08–.15 | .119 | .0067 | .09–.14 | −.64 | .52 |
| Surface area time 2 | .119 | .014 | .09–.25 | .120 | .020 | .09–.25 | .117 | .0083 | .09–.15 | .19 | .29 |
| Surface area time 3 | .118 | .011 | .09–.17 | .117 | .0095 | .10–.14 | .118 | .0012 | .09–.17 | .99 | .83 |
Note: Chi-square comparisons compare count-based demographic variables between sites, and independent samples t-tests compare means between sites for continuous variables of interest. Reported values for anxiety, depressive, and posttraumatic stress symptoms are based on raw scores from the self-report TSCC, with a maximum range of 0–27 for anxiety, 0–21 for depression, and 0–30 for posttraumatic stress; “Cortical Thickness” is the mean, global cortical thickness measured within each year of the study (i.e., time 1, 2 or 3); “Surface Area” is the total surface area measured within each year of the study, corrected for total intracranial volume per participant within the specified year; Sex, race, and ethnicity variables are reported based on Time 1 with the complete sample.
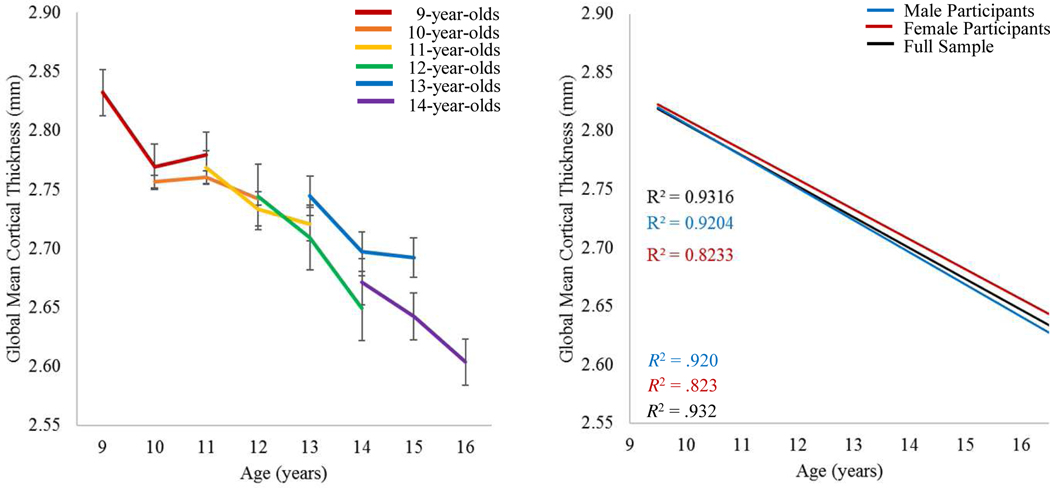
With respect to cortical thickness, male and female participants followed a similar pattern of cortical thinning over time. Figure 1 illustrates the lines of best fit for the trajectory of cortical thinning (see Figure S1, available online for line plots showing individual participants). The plot on the right was projected by calculating the mean cortical thickness of each age bin (e.g., 9-year-olds, 10-year-olds, etc.) collapsed across data from all three time points and estimating a linear regression through those means. The data suggest global mean cortical thickness linearly decreases throughout adolescence, replicating many previous studies.12,13,45,46 Overall, youth at site 2 relative to youth at site 1 tended to have a greater mean cortical thickness at time 1, though the pattern did not continue into years 2 or 3 of the study (Table 1). In regard to cortical surface area, shifts over time were minute, but generally indicated an increase from year 1 to year 2, followed by a decrease in year 3 (Figure S2, available online). There were no significant differences by site, or between sexes.
Figure 1. Trajectories of Change in Global Mean Cortical Thickness.
Note: (Left) Average cortical thickness measures over time, plotted separately for children starting the study at different ages. Participants who were 14-years-old and older were collapsed into a single age bin for this figure because, by design, there were relatively few youth who were over 14 at the start of the study. (Right) Lines of best fit demonstrating the trajectory of cortical thinning throughout adolescence for the full sample, and separately for male versus female participants. The R2 for each line is shown on the graphs. There were relatively few youth over the age of 16 at the final time point of the study; thus, data from youth 16 and older were collapsed across the 16-year-old age bin to avoid biasing the regression.
LGCM Results for Cortical Thickness
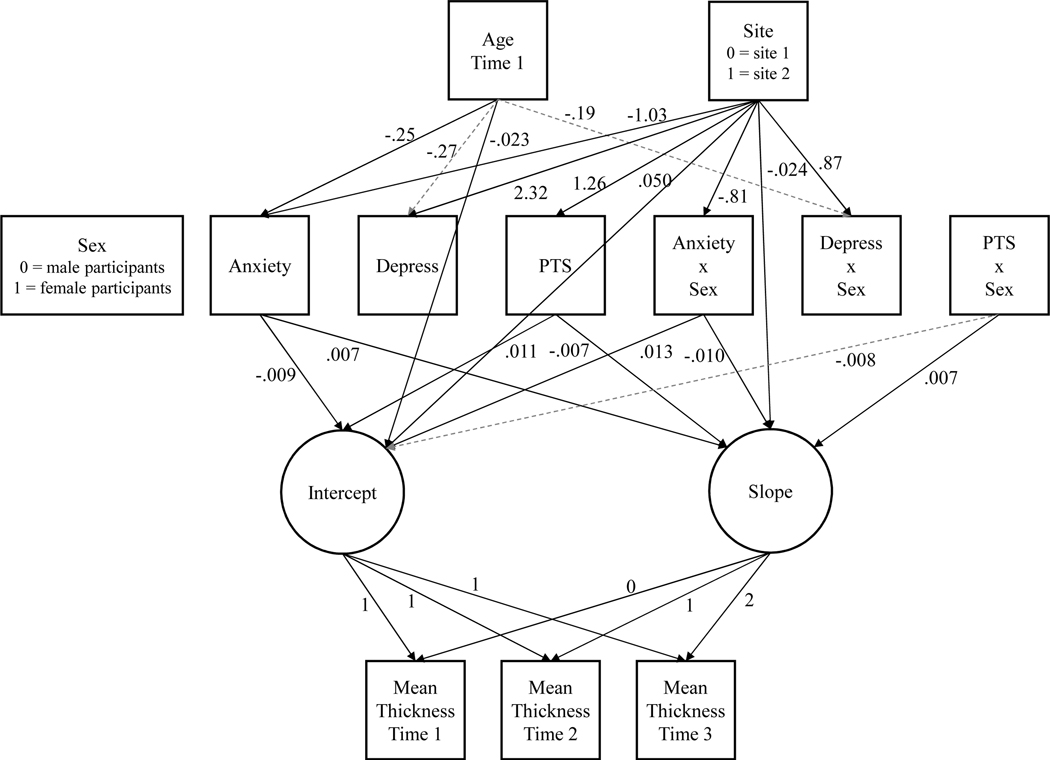
Results of the baseline model and the model including only control variables are described in the Supplement 1, available online. Model fit comparisons at each step of the analysis are illustrated in Table S3. Our final model included age, site, sex, raw scores of self-reported anxiety, depressive, and posttraumatic stress symptoms, and each symptom score’s interaction with sex as predictors of the intercept and slope of change in cortical thickness. The final model had excellent fit (χ2(14) = 17.10, p = .25; RMSEA = .032, 90% CI[.00, .08]; CFI = 1.00). We provide a complete correlation table for all measures included in the model in Table S4, available online. Significant and trending results are shown in Figure 2.
Figure 2. Latent Growth Curve Model Results.
Note: Results of the final model, in which age at time 1, study site, sex, psychological symptoms, and their interactions with sex predict the latent intercept and slope of change in global mean cortical thickness. Solid lines indicate statistically significant estimates at the p < .05 level, while dashed gray lines indicate non-significant, but trending (p < .10) relationships. For simplicity and visibility, relationships that were non-significant at the p > .10 level, and correlations among variables (not of interest) are not shown on the figure. All estimates are unstandardized. “Anxiety” = anxiety symptoms; “Depress” = depressive symptoms; “PTS” = posttraumatic stress symptoms; “…x Sex” = interaction terms; Dummy coding schemes for categorical variables are shown in the figure with the respective variable.
Age at time 1 was significantly associated with baseline cortical thickness (b = −.023, p < .001) and anxiety symptoms (b = −.25, p = .03), and indicated trending associations with both depressive symptoms (b = −.27, p = .06) and their interaction with sex (b = −.19, p = .06). Put simply, older youth tended to have a smaller baseline cortical thickness, and self-reported fewer anxiety and depressive symptoms. However, the trending effect of age on depressive symptoms was reduced for female participants. Site also showed numerous significant effects throughout the model, such that youth at site 2 tended to self-report greater depressive (b = 2.32, p < .001) and posttraumatic stress symptoms (b = 1.26, p = .03), and fewer anxiety symptoms (b = −1.03, p = .01) relative to youth at site 1. Among female participants, the effects of site on anxiety were reduced (b = −.81, p = .005), whereas the effects of site on depressive symptoms were exacerbated (b = .87, p = .015). There was also an effect of site on the latent slope of change in cortical thickness, suggesting that youth at site 2 generally had a steeper slope of change relative to youth at site 1 (b = −.024, p = .018). Sex did not show any unique associations with the latent intercept or slope.
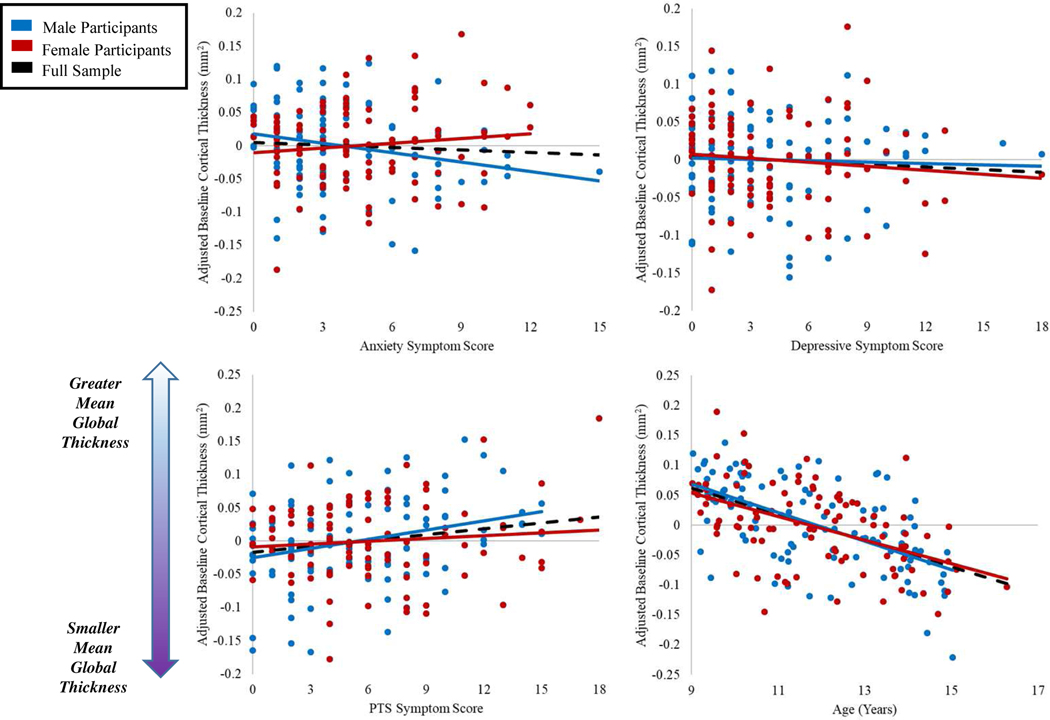
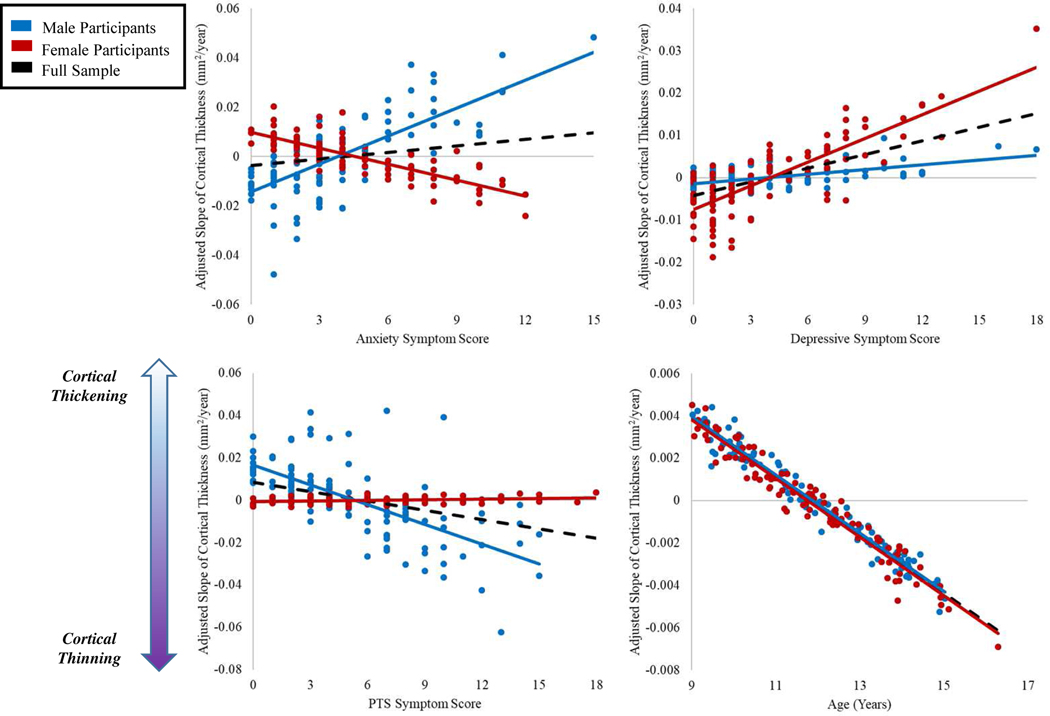
Turning to the effects of interest, anxiety symptoms were significantly associated with both the latent intercept and slope variables, such that youth who self-reported more anxiety symptoms tended to have smaller baseline cortical thickness (b = −.009, p = .047) coupled with slowed cortical thinning over time (b = .007, p = .008). Sex did modulate these relationships, with female participants showing dampened effects of anxiety on both baseline global cortical thickness (b = .013, p = .046), and changes in thickness over time (b = −.10, p = .013). Interestingly, posttraumatic stress symptoms showed the opposite pattern. Youth who self-reported more posttraumatic stress symptoms tended to have higher baseline cortical thickness (b = .011, p = .004), coupled with accelerated cortical thinning (b = −.007, p = .002). These relationships were modulated by sex, with female participants showing a trend toward dampened effects on baseline thickness (b = −.008, p = .093), and a significant decrement to the relationship between posttraumatic stress and the latent slope (b = .007, p = .032). Surprisingly, neither depressive symptoms nor their interaction with sex showed any significant or trending associations with either the latent intercept or slope (b’s = −.002 to .002, p’s = .59 to .72). Figures 3 and 4 demonstrate the effects of psychological symptoms and age on intercept and slope estimates.
Figure 3. Relationships Between Baseline Thickness and Psychological Symptoms.
Note: Scatterplots showing the relationships between estimated intercepts (i.e., baseline) global mean cortical thickness and age at time 1, posttraumatic stress, depressive, and anxiety symptom scores. Baseline thickness values have been adjusted by regressing out the effects of additional variables in the LGCM as necessary per scatterplot (e.g., in the plot with age on the x-axis, baseline thickness values were adjusted by regressing out the effects of site, sex, anxiety, depressive, and posttraumatic stress scores and their interactions with sex).
Figure 4. Relationships Between Slope of Change in Cortical Thickness and Psychological Symptoms.
Note: Scatterplots showing the relationships between estimated slope of change in global mean cortical thickness and age at time 1, posttraumatic stress, depressive, and anxiety symptom scores. Slope values have been adjusted by regressing out the effects of additional variables in the LGCM as necessary per scatterplot (eg, in the plot with age on the x-axis, slope values were adjusted by regressing out the effects of site, sex, anxiety, depressive, and posttraumatic stress scores and their interactions with sex).
Finally, we also examined regional effects in the medial orbitofrontal, anterior and posterior cingulate areas, amygdala, hippocampus, and other regions. The key findings linked posttraumatic stress with accelerated thinning in posterior cingulate, depressive symptoms with faster thinning in the caudal anterior cingulate, and anxiety symptoms with lower rates of surface area expansion in medial orbitofrontal and posterior cingulate cortices. These models and the accompanying results are described in detail within the (see Table S5, available online). Lastly, we examined the extent to which cortical thickness predicted changes in psychological symptoms and found a negative association between global thickness and anxiety for the whole sample (Table S6).
Discussion
The present study examined the longitudinal associations between cortical thickness and anxiety, depressive, and posttraumatic stress symptoms in a large cohort of typically developing youth. Our base models corroborated prior literature on the nature of age-related cortical thinning throughout adolescence.e.g., 12 Namely, youth who were older at the start of the study generally had thinner cortical tissue, and global cortical thickness gradually decreased over time in a linear fashion. Critically, our data indicated that the rate of change in cortical thickness throughout adolescence was significantly associated with psychological symptom severities. Our data on cortical surface area also corroborated prior literature on the trajectory of change during adolescence,12 although such surface area measurements were not clearly associated with symptoms. This report contributes to a small, but growing body of work examining how neurodevelopmental trajectories vary among individuals with subclinical levels of comorbid psychological symptoms.
Our key model included anxiety, depressive, and posttraumatic stress symptoms as simultaneous predictors of morphological changes in gray matter thickness over the three-year study period, controlling for age, sex, and data collection site. We also examined sex as a moderator on the effects of psychological symptoms on trajectories of cortical thickness. Our models indicated significant changes in global cortical thickness over time that were specific to anxiety and posttraumatic stress symptoms. Youth who reported more anxiety symptoms at their first visit (e.g., greater generalized anxiety) tended to have lower baseline cortical thickness, and significantly slower cortical thinning over the three-year study period. Conversely, youth who reported greater posttraumatic stress symptoms at their first visit (e.g., more intrusive thoughts) had significantly higher baseline cortical thickness, coupled with accelerated cortical thinning. The opposing directionality of associations between cortical thickness and anxiety versus posttraumatic stress symptoms supports the recent reclassification of trauma and stress-related disorders as separate from anxiety disorders in the DSM-5.33 Surprisingly, our moderations suggested that these effects were significantly dampened among female participants. We did not detect any significant associations between depressive symptoms and indices of global cortical thickness.
Prior literature shows that changes in cortical gray matter are directly tied to cognitive-behavioral maturation. Declining cortical thickness throughout adolescence into young adulthood is normative and considered a sign of healthy pruning, leading to increased efficiency of the brain.6 Conversely, non-normative rates of thinning (or thickening in some regions) have been associated with deficits in cognitive abilities.15 Studies examining maltreated youth report altered development in limbic areas, which can be associated with abnormal functional connectivity among distributed brain regions.14 Additionally, recent volumetric data suggest that male youth demonstrate smaller limbic volumes subsequent to trauma compared to female peers.42,47 Speculatively, perhaps exaggerated neural plasticity is demanded during the posttraumatic period in otherwise typical children, which could account for observed baseline thickening followed by rapid thinning. Alternatively, perhaps development is arrested in the period immediately following a traumatic event, and thus the normative curve of development is delayed. More concretely, our data suggest that anxiety and posttraumatic stress among children and young adolescents (particularly male adolescents) are associated with disrupted patterns of global cortical thinning, which may in turn have cascading consequences on cognitive and emotional development. Further work is required to determine the extent of the cognitive-behavioral impacts of the noted atypical cortical thinning among young men.
These novel findings complement prior work examining the effects of anxiety symptoms on cortical gray matter. For instance, Newman et al.24 found that generalized anxiety symptoms were associated with reduced global mean cortical thickness in children and adolescents, which is in agreement with our findings. Further, consistent with Newman et al.,24 we found that global cortical thickness was predictive of longitudinal changes in anxiety for a subsample of youth. The phenomenological experience of anxiety includes the inability to halt irrelevant cognitions, but the translation to cortical thickness effects requires additional clinical data on cognitive processing to assess this possibility.
Of note, this is one of only a few studies that examine whole-brain associations between anxiety or mood symptoms and gray matter metrics in youth, as the vast majority have focused on a priori defined regions of interest, e.g., 7,20,21,23,26,30,31 despite existent evidence of widespread, systemic effects of symptoms on cortical morphology. e.g., 18,22,27,28,32 Our own examination of regionally-specific effects largely corroborated this prior work. Specifically, we found associations between posttraumatic stress and accelerated thinning in the posterior cingulate,e.g., 48,49 depressive symptoms and faster thinning in the anterior cingulate, e.g., 50,51 and anxiety symptoms with slower surface area expansion in orbitofrontal and posterior cingulate.e.g., 52,53 In contrast to prior work,16,54 posttraumatic stress was associated with increased expansion of hippocampal volumes in our study. Smaller hippocampal volumes are a known risk factor for developing persistent posttraumatic stress disorder among adults;55 thus, it is possible that the increase in volume over time observed here is neuroprotective and supports resilience in these typically-developing youth.
Depressive symptom severity did not significantly relate to global cortical thickness in the present study. There are indications in the literature that anxiety and depressive symptoms may share similar functional circuitry, though this has yet to be formally tested.25 Prior work has largely focused on a single symptom set at a time, rather than looking at multiple symptom sets simultaneously as we did. That said, perhaps our model did not capture unique effects of anxiety and depressive symptoms on global gray matter because the symptom clusters overlapped in their effects on cortical structure. Further work using wider age ranges should investigate whether there are developmentally-sensitive periods unique to each symptom cluster; such work may provide critical information for intervention/prevention development targeted to specific symptoms.
Importantly, our age range likely contributed to observing minimal effects of depressive symptoms on gray matter. For example, a large study reported that the average age of onset for anxiety disorders was 6-years-old, whereas the onset for mood disorders was 13-years-old.1 Thus, subclinical anxiety symptoms may be reflected in cortical morphology at a much earlier age compared to depressive symptoms. Given that our age range was 9- to 15-years-old, the participants may have been too young for global depression-related morphological changes to be detected. However, sex differences in cortical thickness related to depression should be studied, given that our data suggest directional differences between these variables for male versus female participants, and that sex differences in depression prevalence begins in adolescence.33,34
Finally, we found several significant site effects. It is possible that the difference in MRI scanners used between sites biased the cortical thickness results, however our data show that there were only differences in thickness measures between sites during time 1 of the study. Thus, it is more likely that another intervening factor would better explain the site effects. There were also site differences in self-reported levels of psychological symptoms, as well as a significant site difference in the proportion of participants who identify as Hispanic/Latino. Prior work suggests that, across the lifespan individuals identifying as members of ethnic and/or racial minorities may present with different rates of psychological symptoms, and may actually differ in cortical gray matter metrics such as volume or thickness relative to individuals of ethnic or racial majority groups.e.g., 56–58 Thus, it is possible that sample characteristics which were not directly assessed within our model were drivers of the noted site differences. Unfortunately, the sample was underpowered to further assess these effects in this investigation. However, we do believe that this line of work would be fruitful for future research.
Regarding limitations of this study, our sample was not large enough to investigate additional predictors of cortical thinning, such as interaction effects among the symptom sets. Additionally, our sample included only youth without clinical diagnoses and generalization of our findings to those with clinical levels of psychological symptoms should be done with caution. Future research should examine the developmental trajectories of gray matter among youth with and without clinical diagnoses. We were also unable to properly model the quadratic trend in surface area over time because we had only three time points; thus, our view of associations between surface area and psychological symptoms over time was limited. Finally, our ability to assess changes in both cortical structure and clinical symptom sets over time was limited in this study, though we did detect a significant effect of global cortical thickness on changes in self-reported anxiety over time in a subset of youth. Additional studies would benefit from tracking developmental changes using both metrics in parallel.
To conclude, the present study examined the extent to which subclinical anxiety, depressive, and posttraumatic stress symptoms were associated with trajectories of cortical thinning in youth. Our key findings were that male adolescents may be particularly susceptible to morphological changes associated with anxiety and posttraumatic stress, with each symptom set uniquely associated with baseline cortical thickness, and significantly altering the rate of cortical thinning over time. Future research into functional and structural sex differences regarding psychopathology is essential, especially in the context of variable prevalence rates of mood, anxiety, and stress-related disorders.33
Supplementary Material
Acknowledgments
This work was supported by the National Science Foundation (#1539067 to T.W.W., Y.P.W., J.M.S., and V.D.C.), the National Institutes of Health (R01-MH121101 to T.W.W.; R01-MH103220 to T.W.W.; R01-MH116782 to T.W.W.; R01-MH118013 to T.W.W.; P20-GM103472 to V.D.C.; R01-EB020407 to V.D.C.; and P20-GM130447 to T.W.W.), and At Ease, USA (A.B.B.). Funding agencies had no part in the study design or the writing of this report.
An early version of this work was accepted as a poster presentation at the Society of Biological Psychiatry 75th Annual Meeting; April 30 - May 2, 2020; New York, New York.
Footnotes
Disclosure: Drs. Taylor, Wang, Stephen, Calhoun, Badura-Brack, Wilson, Mr. Eastman, and Mss. Frenzel and Embury have reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Merikangas KR, He J, Burstein M, et al. Lifetime Prevalence of Mental Disorders in U.S. Adolescents: Results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980–989. doi: 10.1016/j.jaac.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bitsko RH, Holbrook JR, Ghandour RM, et al. Epidemiology and Impact of Health Care Provider–Diagnosed Anxiety and Depression Among US Children: J Dev Behav Pediatr. 2018;39(5):395–403. doi: 10.1097/DBP.0000000000000571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kessler RC, Avenevoli S, Costello EJ, et al. Prevalence, Persistence, and Sociodemographic Correlates of DSM-IV Disorders in the National Comorbidity Survey Replication Adolescent Supplement. Arch Gen Psychiatry. 2012;69(4):372–380. doi: 10.1001/archgenpsychiatry.2011.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessler RC, Avenevoli S, McLaughlin KA, et al. Lifetime comorbidity of DSM-IV disorders in the NCS-R Adolescent Supplement (NCS-A). Psychol Med. 2012;42(9):1997–2010. doi: 10.1017/S0033291712000025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conway KP, Swendsen J, Husky MM, He J-P, Merikangas KR. Association of Lifetime Mental Disorders and Subsequent Alcohol and Illicit Drug Use: Results From the National Comorbidity Survey–Adolescent Supplement. J Am Acad Child Adolesc Psychiatry. 2016;55(4):280–288. doi: 10.1016/j.jaac.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 6.Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27(1–2):3–18. doi: 10.1016/S0149-7634(03)00005-8 [DOI] [PubMed] [Google Scholar]
- 7.Bas-Hoogendam JM, van Steenbergen H, Tissier RLM, Houwing-Duistermaat JJ, Westenberg PM, van der Wee NJA. Subcortical brain volumes, cortical thickness and cortical surface area in families genetically enriched for social anxiety disorder – A multiplex multigenerational neuroimaging study. EBioMedicine. 2018;36:410–428. doi: 10.1016/j.ebiom.2018.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Merwe C, Jahanshad N, Cheung JW, et al. Concordance of genetic variation that increases risk for anxiety disorders and posttraumatic stress disorders and that influences their underlying neurocircuitry. J Affect Disord. 2019;245:885–896. doi: 10.1016/j.jad.2018.11.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tozzi L, Garczarek L, Janowitz D, et al. Interactive impact of childhood maltreatment, depression, and age on cortical brain structure: mega-analytic findings from a large multi-site cohort. Psychol Med. 2020;50(6):1020–1031. doi: 10.1017/S003329171900093X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson PM, Jahanshad N, Ching CRK, et al. ENIGMA and global neuroscience: A decade of large-scale studies of the brain in health and disease across more than 40 countries. Transl Psychiatry. 2020;10(1):1–28. doi: 10.1038/s41398-020-0705-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9(2):60–68. doi: 10.1016/j.tics.2004.12.008 [DOI] [PubMed] [Google Scholar]
- 12.Wierenga LM, Langen M, Oranje B, Durston S. Unique developmental trajectories of cortical thickness and surface area. NeuroImage. 2014;87:120–126. doi: 10.1016/j.neuroimage.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 13.Burgaleta M, Johnson W, Waber DP, Colom R, Karama S. Cognitive ability changes and dynamics of cortical thickness development in healthy children and adolescents. NeuroImage. 2014;84:810–819. doi: 10.1016/j.neuroimage.2013.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27(1–2):33–44. doi: 10.1016/S0149-7634(03)00007-1 [DOI] [PubMed] [Google Scholar]
- 15.Baumann PS, Klauser P, Griffa A, et al. Frontal cortical thickness correlates positively with impulsivity in early psychosis male patients. Early Interv Psychiatry. 2019;13(4):848–852. doi: 10.1111/eip.12678 [DOI] [PubMed] [Google Scholar]
- 16.Franz CE, Hatton SN, Hauger RL, et al. Posttraumatic stress symptom persistence across 24 years: association with brain structures. Brain Imaging Behav. Published online March 4, 2019. doi: 10.1007/s11682-019-00059-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kribakaran S, Danese A, Bromis K, Kempton MJ, Gee DG. Meta-Analysis of Structural MRI Studies in Pediatric PTSD and Comparison with Related Conditions. Biol Psychiatry Cogn Neurosci Neuroimaging. Published online August 2019:S2451902219302228. doi: 10.1016/j.bpsc.2019.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lener MS. Cortical abnormalities and association with symptom dimensions across the depressive spectrum. J Affect Disord. Published online 2016:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Wu M, Liao Y, et al. Grey matter reduction associated with posttraumatic stress disorder and traumatic stress. Neurosci Biobehav Rev. 2014;43:163–172. doi: 10.1016/j.neubiorev.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 20.Blackmon K. Structural evidence for involvement of a left amygdala-orbitofrontal network in subclinical anxiety. Psychiatry Res. Published online 2011:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albaugh MD. Age-related volumetric change of limbic structures and subclinical anxious/depressed symptomatology in typically developing children and adolescents. Biol Psychol. Published online 2017:8. [DOI] [PubMed] [Google Scholar]
- 22.Besteher B, Gaser C, Langbein K, Dietzek M, Sauer H, Nenadić I. Effects of subclinical depression, anxiety and somatization on brain structure in healthy subjects. J Affect Disord. 2017;215:111–117. doi: 10.1016/j.jad.2017.03.039 [DOI] [PubMed] [Google Scholar]
- 23.Boes AD, McCormick LM, Coryell WH, Nopoulos P. Rostral Anterior Cingulate Cortex Volume Correlates with Depressed Mood in Normal Healthy Children. Biol Psychiatry. 2008;63:391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newman E, Thompson WK, Bartsch H, et al. Anxiety is related to indices of cortical maturation in typically developing children and adolescents. Brain Struct Funct. 2016;221(6):3013–3025. doi: 10.1007/s00429-015-1085-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Besteher B, Gaser C, Nenadić I. Brain Structure and Subclinical Symptoms: A Dimensional Perspective of Psychopathology in the Depression and Anxiety Spectrum. Neuropsychobiology. 2019;78(3):113–126. [DOI] [PubMed] [Google Scholar]
- 26.Carlson JM. Gender moderates the association between dorsal medial prefrontal cortex volume and depressive symptoms in a subclinical sample. Psychiatry Res. Published online 2015:4. [DOI] [PubMed] [Google Scholar]
- 27.Herringa R, Phillips M, Almeida J, Insana S, Germain A. Posttraumatic stress symptoms correlate with smaller subgenual cingulate, caudate, and insula volumes in unmedicated combat veterans. Psychiatry Res Neuroimaging. 2012;203(2):139–145. doi: 10.1016/j.pscychresns.2012.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLaren ME, Szymkowicz SM, O’Shea A, Woods AJ, Anton SD, Dotson VM. Vertex-wise examination of depressive symptom dimensions and brain volumes in older adults. Psychiatry Res Neuroimaging. 2017;260:70–75. doi: 10.1016/j.pscychresns.2016.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szymkowicz SM, McLaren ME, Kirton JW, et al. Depressive symptom severity is associated with increased cortical thickness in older adults. Int J Geriatr Psychiatry. 2016;31(4):325–333. doi: 10.1002/gps.4324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szymkowicz SM, McLaren ME, O’Shea A, Woods AJ, Anton SD, Dotson VM. Depressive symptoms modify age effects on hippocampal subfields in older adults. Geriatr Gerontol Int. 2017;17(10):1494–1500. doi: 10.1111/ggi.12901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richert KA, Carrion VG, Karchemskiy A, Reiss AL. Regional differences of the prefrontal cortex in pediatric PTSD: an MRI study. Depress Anxiety. 2006;23(1):17–25. doi: 10.1002/da.20131 [DOI] [PubMed] [Google Scholar]
- 32.Bos MGN, Peters S, van de Kamp FC, Crone EA, Tamnes CK. Emerging depression in adolescence coincides with accelerated frontal cortical thinning. J Child Psychol Psychiatry. 2018;59(9):994–1002. doi: 10.1111/jcpp.12895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 5th Edition.; 2013. [Google Scholar]
- 34.Zahn-Waxler C, Shirtcliff EA, Marceau K. Disorders of Childhood and Adolescence: Gender and Psychopathology. Annu Rev Clin Psychol. 2008;4(1):275–303. doi: 10.1146/annurev.clinpsy.3.022806.091358 [DOI] [PubMed] [Google Scholar]
- 35.Mccabe KM, Lansing AE, Garland A, Hough R. Gender Differences in Psychopathology, Functional Impairment, and Familial Risk Factors Among Adjudicated Delinquents. J Am Acad Child Adolesc Psychiatry. 2002;41(7):860–867. doi: 10.1097/00004583-200207000-00020 [DOI] [PubMed] [Google Scholar]
- 36.Stice E, Presnell K, Bearman SK. Relation of early menarche to depression, eating disorders, substance abuse, and comorbid psychopathology among adolescent girls. Dev Psychol. 2001;37(5):608–619. doi: 10.1037/0012-1649.37.5.608 [DOI] [PubMed] [Google Scholar]
- 37.Graber JA. Pubertal timing and the development of psychopathology in adolescence and beyond. Horm Behav. 2013;64(2):262–269. doi: 10.1016/j.yhbeh.2013.04.003 [DOI] [PubMed] [Google Scholar]
- 38.Marshall AD. Developmental Timing of Trauma Exposure Relative to Puberty and the Nature of Psychopathology Among Adolescent Girls. J Am Acad Child Adolesc Psychiatry. 2016;55(1):25–32.e1. doi: 10.1016/j.jaac.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crick NR, Zahn–Waxler C. The development of psychopathology in females and males: Current progress and future challenges. Dev Psychopathol. 2003;15(3):719–742. doi: 10.1017/S095457940300035X [DOI] [PubMed] [Google Scholar]
- 40.Briere J. Trauma Symptoms Checklist for Children : Professional Manual. Psychological Assessment Resources; 1996. [Google Scholar]
- 41.Hooper LM, Stockton P, Krupnick JL, Green BL. Development, Use, and Psychometric Properties of the Trauma History Questionnaire. J Loss Trauma. 2011;16(3):258–283. doi: 10.1080/15325024.2011.572035 [DOI] [Google Scholar]
- 42.Badura-Brack AS, Mills MS, Embury CM, et al. Hippocampal and parahippocampal volumes vary by sex and traumatic life events in children. J Psychiatry Neurosci JPN. 2020;45(4):288–297. doi: 10.1503/jpn.190013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steinberg AM, Brymer MJ, Kim S, et al. Psychometric Properties of the UCLA PTSD Reaction Index: Part I. J Trauma Stress. 2013;26(1):1–9. doi: 10.1002/jts.21780 [DOI] [PubMed] [Google Scholar]
- 44.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Model Multidiscip J. 1999;6(1):1–55. doi: 10.1080/10705519909540118 [DOI] [Google Scholar]
- 45.Shaw P, Malek M, Watson B, Greenstein D, de Rossi P, Sharp W. Trajectories of cerebral cortical development in childhood and adolescence and adult Attention Deficit Hyperactivity Disorder. Biol Psychiatry. 2013;74(8):599–606. doi: 10.1016/j.biopsych.2013.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal Mapping of Cortical Thickness and Brain Growth in Normal Children. J Neurosci. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colle R, Segawa T, Chupin M, et al. Early life adversity is associated with a smaller hippocampus in male but not female depressed in-patients: a case–control study. BMC Psychiatry. 2017;17(1):71. doi: 10.1186/s12888-017-1233-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nardo D, Högberg G, Looi JCL, Larsson S, Hällström T, Pagani M. Gray matter density in limbic and paralimbic cortices is associated with trauma load and EMDR outcome in PTSD patients. J Psychiatr Res. 2010;44(7):477–485. doi: 10.1016/j.jpsychires.2009.10.014 [DOI] [PubMed] [Google Scholar]
- 49.Kühn S, Gallinat J. Gray Matter Correlates of Posttraumatic Stress Disorder: A Quantitative Meta-Analysis. Biol Psychiatry. 2013;73(1):70–74. doi: 10.1016/j.biopsych.2012.06.029 [DOI] [PubMed] [Google Scholar]
- 50.Frodl T, Jäger M, Born C, et al. Anterior cingulate cortex does not differ between patients with major depression and healthy controls, but relatively large anterior cingulate cortex predicts a good clinical course. Psychiatry Res Neuroimaging. 2008;163(1):76–83. doi: 10.1016/j.pscychresns.2007.04.012 [DOI] [PubMed] [Google Scholar]
- 51.Han K-M, Choi S, Jung J, et al. Cortical thickness, cortical and subcortical volume, and white matter integrity in patients with their first episode of major depression. J Affect Disord. 2014;155:42–48. doi: 10.1016/j.jad.2013.10.021 [DOI] [PubMed] [Google Scholar]
- 52.Strawn JR, Wehry AM, Chu W-J, et al. Neuroanatomic Abnormalities in Adolescents with Generalized Anxiety Disorder: A Voxel-Based Morphometry Study. Depress Anxiety. 2013;30(9):842–848. doi: 10.1002/da.22089 [DOI] [PubMed] [Google Scholar]
- 53.Spampinato MV, Wood JN, De Simone V, Grafman J. Neural Correlates of Anxiety in Healthy Volunteers: A Voxel-Based Morphometry Study. J Neuropsychiatry Clin Neurosci. 2009;21(2):199–205. doi: 10.1176/jnp.2009.21.2.199 [DOI] [PubMed] [Google Scholar]
- 54.Schmaal L, Yücel M, Ellis R, et al. Brain Structural Signatures of Adolescent Depressive Symptom Trajectories: A Longitudinal Magnetic Resonance Imaging Study. J Am Acad Child Adolesc Psychiatry. 2017;56(7):593–601.e9. doi: 10.1016/j.jaac.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 55.van Rooij SJH, Kennis M, Sjouwerman R, van den Heuvel MP, Kahn RS, Geuze E. Smaller hippocampal volume as a vulnerability factor for the persistence of posttraumatic stress disorder. Psychol Med. 2015;45(13):2737–2746. doi: 10.1017/S0033291715000707 [DOI] [PubMed] [Google Scholar]
- 56.Pfefferbaum A, Rohlfing T, Pohl KM, et al. Adolescent Development of Cortical and White Matter Structure in the NCANDA Sample: Role of Sex, Ethnicity, Puberty, and Alcohol Drinking. Cereb Cortex. 2016;26(10):4101–4121. doi: 10.1093/cercor/bhv205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sullivan EV, Lane B, Kwon D, et al. Structural brain anomalies in healthy adolescents in the NCANDA cohort: relation to neuropsychological test performance, sex, and ethnicity. Brain Imaging Behav. 2017;11(5):1302–1315. doi: 10.1007/s11682-016-9634-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jha SC, Xia K, Ahn M, et al. Environmental Influences on Infant Cortical Thickness and Surface Area. Cereb Cortex. 2019;29(3):1139–1149. doi: 10.1093/cercor/bhy020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.