Abstract
Introduction
Orthodontic relapse occurs after orthodontic treatment and shifting of teeth to unfavorable positions. Bisphosphonates’ effects on bone resorption and relapse prevention have been extensively investigated. However, topical administration, which results in local effect, is still a problem.
Objective
This study aimed to investigate the effect of risedronate with gelatin hydrogel as a carrier to prevent relapse movement by inhibiting osteoclast activity.
Methods
Lower incisors of 75 guinea pigs were moved distally using an orthodontic appliance until ±3 mm length. Gelatin hydrogel was fabricated to obtain a semisolid controlled release of 250 (Bis-CR250) and 500 mmol/L risedronate (Bis-CR500) and then applied intrasulcularly into the mesial subperiosteal area of 50 guinea pigs (25 in each group) every 3 days; the rest were the control (Bis-CR000). After 14 days of stabilization, the apparatus was removed. The distance decrease between incisors and the osteoclast number with TRAP staining at 0, 3, 7, 14, and 21 days were measured. ANOVA was used to determine the differences among the different time and experimental groups.
Results
Both treatments showed significantly less relapse movement compared to the control (p < 0.05) at 14 and 21 days. Bis-CR500 more effectively inhibited the relapse movement than Bis-CR250 on day 21, indicating a dose dependency in the inhibition. Both treatments showed less osteoclast numbers than control (p < 0.05).
Conclusion
Controlled release of bisphosphonate risedronate with a topically administered gelatin hydrogel has shown to be effective in decreasing the tooth relapse movement and osteoclast activity.
Keywords: Risedronate, Controlled release, Gelatin hydrogel, Orthodontics, Relapse movement
1. Introduction
Several studies evaluated the benefits of bisphosphonates in dentistry using different models, i.e., implantology (Peter et al., 2004), periodontics (Juan et al., 2006), oral surgery (Lustosa-Pereira et al., 2006), and orthodontics (Fujimura et al., 2009, Krishnan et al., 2015). Two major concerns in orthodontic treatment are the anchorage loss (mesial movement of anchored teeth) and post-treatment relapse (Krishnan et al., 2015). After orthodontic appliance removal, the binding forces are released and the teeth begin to return to their original positions (relapse) which is hard to predict at the individual level (Franzen et al., 2013, Van Leeuwen et al., 2003). Since the relapse movement is a complex process, its etiology remains unclear. Van Leeuwen et al. (2003) reported that relapse is commonly caused by intrinsic factors related to the periodontal ligament and alveolar bone (Kim et al., 1999). A previous study indicated the periodontal fiber force is the main cause of tooth relapse, although the resorption and formation of osteoclasts and osteoblasts around the alveolar bone also influence relapse (Han et al., 2010). Franzen et al. (2013) concluded that alveolar bone remodeling is an important element in relapse processes as both relapse and tooth movement undergo a similar process; osteoclast differentiation increases in compression areas and decreases in tension areas.
Previous studies showed that bisphosphonates can be used to inhibit the relapse movement (Adachi et al., 1994, Igarashi et al., 1994, Kim et al., 1999). Bisphosphonates have affinity to the calcium in the bone and remains as the p-chlorophenyl moiety on the hydroxyapatite, which is then phagocytized by osteoclasts, leading to the apoptosis of osteoclast and effectively inhibiting bone resorption and preventing relapse (Fujimura et al., 2009, Licata, 2005, Shetty et al., 2006).
All previously mentioned studies used pure bisphosphonates without any carrier, yet reported the successful treatment of periodontal diseases. Adachi et al. (1994) reported that local injection of risedronate effectively inhibits relapse, but with systemic effects, including the increase of the bone mineral density of the tibia. Given that bisphosphonates affect the entire skeleton, a topical application would be the best to treat periodontal bone loss (Juan et al., 2006). In addition, finding a proper way to deliver bisphosphonates with optimal bioavailability by taking the risk into the account has been a major challenge.
Drug delivery system is a drug administration system using a carrier (Omedian and Park, 2010). This system can prevent the drug from being metabolized by the digestive system while also increasing the effectiveness and duration of the drug release, create a localized action, and the amount and rate of drug release can be tailored (Ranade et al., 2003, Troy, 2006). Hydrogel is one of the carrier material which is biocompatible and biodegradable (Saito and Tabata, 2012). Hydrogels are porous so that a substance can be inserted into the gel matrix with the adjustable release rate. Gelatin is often used as hydrogel base material because it is available with a wide range of physicochemical properties and biodegradable, make it suitable as a carrier (Tabata and Ikada, 1998, Bregg, 2005).
In this study, we investigated the effectiveness of gelatin hydrogel as described by Saito and Tabata (2012) as a carrier to transport risedronate to inhibit the tooth relapse movement and osteoclast activity.
2. Material and Methods
2.1. Hydrogel preparation and release measurement
Gelatin (G-2554P, 100286; Nitta Gelatin Inc., Osaka, Japan) was dissolved in distilled water (3% w/v) and homogenized for 3 h at 37 °C. Risedronate sodium (Cat No. 51428; Selleck Chemical, Australia) was added and stirred for 2 h. The mixture was crosslinked with 25% glutaraldehyde (Merck, Darmstadt, Germany) and rinsed with glycine (Merck) three times. The mixture was frozen at 30 °C and lyophilized for 48 h resulting in a hydrogel block which was then ground into microspheres. The microspheres were mixed with distilled water (1:20 w/w) to form a semisolid controlled releasable bisphosphonate.
It was calculated that 400 mg Bis-CR250 and 400 mg Bis-CR500 contained 1.00 mg and 1.92 mg pure risedronate, respectively, after hydrogel preparation. Accordingly, the specimens in Table 1 were prepared in 5 mL phosphate buffer saline (PBS). The prepared specimens were kept at 37 °C for 1 h, spinned down at 3000 rpm for 20 min, and the supernatant was transferred into analytical tubes. Then, the absorbance of risedronate sodium was confirmed using a UV–Vis spectrophotometer at 262 nm. Meanwhile, the tubes with residual microspheres were refreshed with 500 μL PBS at 37 °C for each time interval. The same protocol was applied for 3-, 6-, and 24-h time intervals.
Table 1.
Solid contents in 5 mL PBS for the release experiment.
| Specimen Code | N | Contents in 5 mL PBS (mg) |
||
|---|---|---|---|---|
| Risedronate Sodium | Microsphere Bis-CR250 | Microsphere Bis-CR500 | ||
| Pure Risedronate 250 | 3 | 1.00 | – | – |
| Pure Risedronate 500 | 3 | 1.92 | – | – |
| Bis-CR250 | 3 | – | 400 | – |
| Bis-CR500 | 3 | – | – | 400 |
2.2. Tooth movement in the animal model
In this study, 75 male guinea pigs, weighing 500–600 g, were used. National guidelines for the care and use of laboratory animals were applied in the study. The experimental procedures were approved by the Ethics Committee for Biomedical Research of the Faculty of Dentistry, Gadjah Mada University (clearance number: 355/KKEP/FKG-UGM/EC/2012), which was renewed annually until the end of the study. The guinea pig was used as they are physiologically and immunologically similar to humans than other small animal models and easier to place an orthodontic appliance (Hickey, 2011, Reitan and Kvam, 1971, Ren et al., 2004).
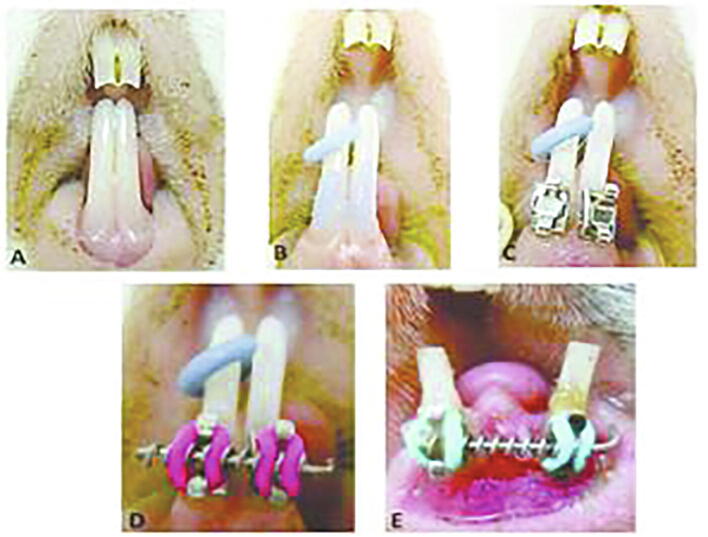
The guinea pigs were anesthetized with 0.1 mg of ketamine and xylazine injection during the orthodontic appliance installation. The bonding cleat was attached to the lower incisor. A Ni-Ti open coil spring was inserted between the bonding cleat with a 0.1-mm stainless steel wire. The smallest diameter (0.254 mm × 1.143 mm) was used with 1.5 times the distance between the lower incisor bonding cleat. With 25% compression of the orthodontic appliance, it was expected to produce 0.25 to 1.30 N force to the tooth. After the tooth movement and when the open coil spring was inactive, the spring was replaced with a new one and the distance between the lower incisor bonding cleat was set to reach ± 3 mm (Fig. 1). The ±3 mm inter-incisor distance was maintained for 14 days as a stabilization period and then the topical application of risedronate was initiated. The animals were divided into Bis-CR250 (250 mmol/L), Bis-CR500 (500 mmol/L), and Bis-CR000 (control) groups, 25 guinea pigs each. The concentration was based on Adachi et al., 1994, Igarashi et al., 1994. The application was performed by applying the hydrogel in the subperiosteal area at the mesial side of tooth with a 30-gauge needle every 3 days.
Fig. 1.
Application of orthodontics appliances in guinea pigs. A. Lower incisor, B. Separator and etching, C. The Attached bonding cleat, D. Inserted Open coil spring, E. Before the stabilization period.
2.3. Tooth movement measurement
The orthodontic appliance were removed after 14 days, but the hydrogel application was continued. Relapse movement and interincisal distance were measured on days 3, 7, 14, and 21 using a sliding caliper (Tricle Brand, Shanghai, China). The observation period of tooth movement was based on Noxon et al., 2001, Franzen et al., 2013.
2.4. Histological preparation
The guinea pigs were sacrificed using 0.1 mg ketamine. Intracardial perfusion using NaCl and 4% paraformaldehyde was performed after the sacrifice. The mandibular alveolar bones were dissected and fixed with 4% paraformaldehyde at 4 °C for 12 h. The specimens were then demineralized with 10% ethylenediaminetetraacetic acid at 4 °C for 60 days. After complete demineralization, a series of dehydration steps with stratified alcohol concentrations and paraffin block embedding were performed. A series of 6-µm thick sagittal sections were cut parallel to the tooth long axis. Tartrate-resistant acid phosphatase (TRAP) staining procedure was carried out to count the osteoclasts.
2.5. Histological analysis
Histological analysis was done by calculating the average number of osteoclasts on the mesial (compression side) of the alveolar bone. Data were obtained from five randomized regions of interest from the apical point to the junction, which were taken using a light microscope (Olympus). Active osteoclasts were found in contact with the bone surface, or within the lacunae, and characterized by a multinucleate, irregular shape of the nucleus with a bright red granular cytoplasm. The mean number of osteoclasts was obtained from five regions of interest.
2.6. Statistical analysis
The data were analyzed using the Shapiro–Wilk test and the test of homogeneity of variances to determine the data distribution and homogeneity for both relapse and histological measured data. One-way analysis of variance (ANOVA) and the Kruskal–Wallis test were used to analyze the differences between groups. Least significant difference (LSD) multiple comparison tests were also applied. Each analysis was done using the Statistical Package for the Social Sciences version 21 (IBM, USA).
3. Results
3.1. Release of risedronate
The release of risedronate with gelatin hydrogel was slower than without the hydrogel carrier; 19.9% to 15.7% in pure 250 mg to Bis-CR250 and 39.7% to 22.3% in pure 500 mg to Bis-CR500. Both Bis-CR250 and Bis-CR500 had no detectable release before 1 h of immersion. t-test shows a significant difference in the release rate between groups (p < 0.05) during the initial period (hour 0 and hour 1) (Table 2). The results confirmed the controlled release of risedronate by gelatin hydrogel.
Table 2.
Risedronate release proportion with and without hydrogel carrier.
| Duration (hour) | Release Proportion (mean percentage ± SD) |
|||
|---|---|---|---|---|
| Pure-250 | Pure-500 | Bis-CR250 | Bis-CR500 | |
| 0 | 0.199 ± 0.007 | 0.399 ± 0.014 | No release | No release |
| 1 | 0.199 ± 0.006 | 0.397 ± 0.006 | 0.157 ± 0.012 | 0.223 ± 0.011 |
| 3 | 0.070 ± 0.001 | 0.161 ± 0.053 | 0.094 ± 0.003 | 0.115 ± 0.005 |
| 6 | 0.024 ± 0.001 | 0.047 ± 0.002 | 0.052 ± 0.002 | 0.058 ± 0.004 |
| 24 | 0.006 ± 0.001 | 0.015 ± 0.001 | 0.234 ± 0.022 | 0.205 ± 0.013 |
*All experimental groups were done in triplicate.
3.2. Effect to relapse distance
The control had the highest relapse rate at days 14 and 21 (Fig. 2). There was a significantly less relapse movement in the treatment group on days 14 and 21 compared to control (p < 0.05). Bis-CR500 inhibited the relapse movement more effectively than Bis-CR250 on day 21, indicating a dose dependency in the bisphosphonate hydrogel application.
Fig. 2.

Interincisor distance measurements on days 3, 7, 14, and 21. Blue: Bis-CR000. Green: Bis-CR250. Yellow: Bis-CR500.
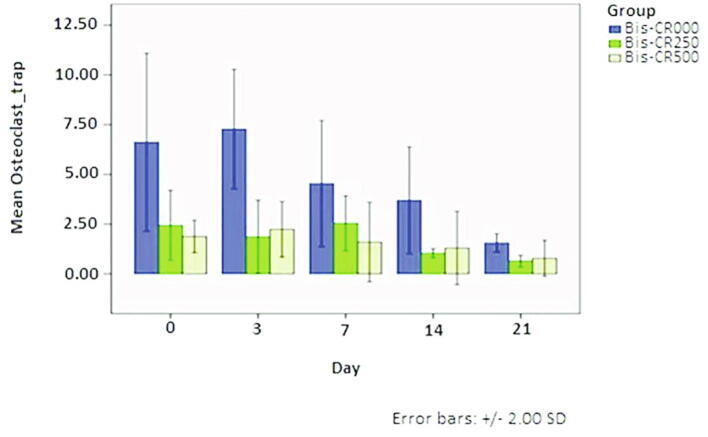
3.3. Number of osteoclasts
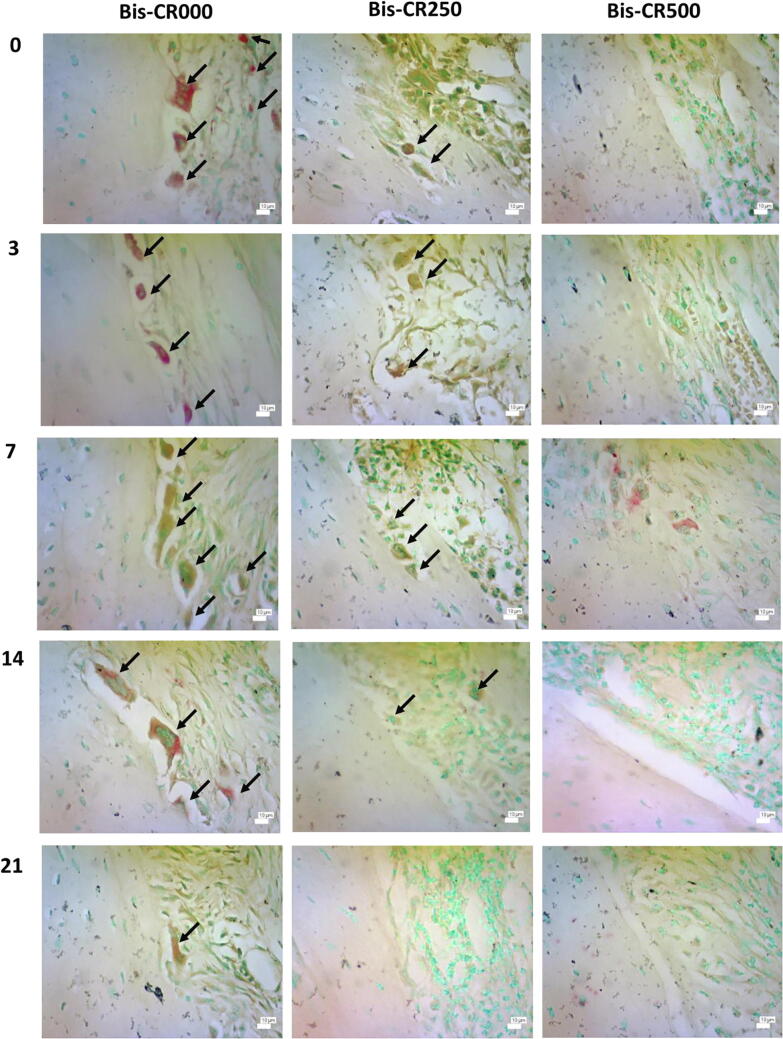
Osteoclasts were abundant along the alveolar bone in Bis-CR000 but decreased in the Bis-CR250 and Bis-CR500 groups, showing the inhibition of osteoclast activity (Fig. 3; Fig. 4). Statistical analysis also showed the significant difference among groups (p < 0.05).
Fig. 3.
Histological slides stained with TRAP for all groups on days 0, 3, 7, 14, and 21 after the stabilization period (400 × ). Arrows indicate positive osteoclasts.
Fig. 4.
Osteoclasts number on days 0, 3, 7, 14, and 21. Blue: Bis-CR000. Green: Bis-CR250. Yellow: Bis-CR500.
4. Discussion
The results showed that risedronate was released directly to PBS without gelatin hydrogel, indicating that risedronate will be dissolved easily in the body. Hoare and Kohane, 2008 described hydrogel as a porous material able to load drugs and adjust the drug release rate. The loaded substance will be carried by the hydrogel before the gel is degraded (Boateng et al., 2008, Bregg, 2005, Tabata and Ikada, 1998). In this study, gelatin hydrogel controlled the release and degradability by cross-linking to control the system’s water content (Saito and Tabata, 2012, Thilander, 2000). The system was proven effective in prolonging the release of sodium risedronate when applied topically.
The results showed the significant differences in relapse movement between with and without bisphosphonate. Both Bis-CR250 and Bis-CR500 prevented relapse 14 and 21 days after the stabilization period. This due to the rate of remodeling processes after the stabilization period is the forces of the periodontal ligament, showing the reason for dose-dependent rate to fasten bone remodeling process. Franzen et al. (2013) stated that the relapse occurred rapidly at the beginning of the stabilization process due to the periodontal ligament fibers undergo stretching on the tension side by the pressure from orthodontic appliances and in turn are embedded in the new bone. The fibers are elongated or reduced permanently. If the new bone is not yet formed, the rearrangement of the fibers and alveolar bone may cause relapse after the orthodontics appliance removal (Thilander, 2000).
The results were consistent with previous studies that reported the risedronate effectiviness in decreasing osteoclasts and preventing tooth movement. Keles et al. (2007) observed the relapse prevention and osteoclast apoptosis by pamidronate under constant orthodontics forces in mice. Choi et al., 2010, Sirisoontorn et al., 2012 showed similar results for bisphosphonate clodronate and bisphosphonate zoledronate application in rats.
Both treatments showed less osteoclasts than control. This is relevant with the theory mentioning that bisphosphonate reduces osteoclast activity. There are two types of bisphosphonates based on structure. First, the non-nitrogen-containing bisphosphonates or first-generation bisphosphonates, i.e., etidronate and clodronate. The second- and third-generation bisphosphonates (alendronate, risedronate, and zoledronic acid) have nitrogen-containing R2 side chains. The mechanism of nitrogen-containing bisphosphonates promotes the osteoclast apoptosis is distinct from the non-ones. The nitrogen-containing bisphosphonates inhibit farnesyl pyrophosphate synthase activity, a key enzyme in the mevalonic acid pathway in cholesterol, sterols, and isoprenoid lipids production. Then the isoprenylation of small guanosine triphosphate–binding proteins, which is essential in osteoclast cellular activities regulation, is inhibited, leading to osteoclast apoptosis (Ghoneima et al., 2010; Marx, 2003).
5. Conclusion
Topically administered bisphosphonate risedronate with gelatin hydrogel effectively decreases the relapse 7 days after the tooth stabilization period in a dose-dependent manner. The developed gelatin hydrogel system is able to deliver the risedronate to a targeted area in a controlled manner and provide local effects, which is useful in orthodontic practice.
Authors contribution
Tita Ratya Utari: Conceptualization, Data curation, Investigation, Software, Validation, Visualization, Writing - original draft, Writing - review & editing.Ika Dewi Ana: Conceptualization, Data curation, Methodology, Writing - original draft, Writing - review & editing. Pinandi Sri Pudyani: Formal analysis, Funding acquisition, Project administration, Resources. Widya Asmara: Formal analysis, Funding acquisition, Project administration, Resources.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Authors thank the Directorate General of Higher Education (DIKTI) of the Ministry of Education and Culture of the Republic of Indonesia for the support of the study.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Ika Dewi Ana, Email: ikadewiana@ugm.ac.id.
Pinandi Sri Pudyani, Email: pinandi_fkg@ugm.ac.id.
Widya Asmara, Email: wied_as@ugm.ac.id.
References
- Adachi H., Igarashi K., Mitani H., Shinoda H. Effects of topical administration of a bisphosphonate (risedronate) on orthodontic tooth movements in rats. J. Dent. Res. 1994;73:1478–1484. doi: 10.1177/00220345940730081301. [DOI] [PubMed] [Google Scholar]
- Boateng J.S., Matthews K.L., Stevens H.N.E., Eccleston G.M. Wound healing dressing and drug delivery systems: a review. J. Pharm. Sci. 2008;97:2892–2923. doi: 10.1002/jps.21210. [DOI] [PubMed] [Google Scholar]
- Bregg R.K. Nova Science Publishers Inc; New York: 2005. Current topics in polymer research. [Google Scholar]
- Choi J., Baek S.H., Lee J.I., Chang Y.I. Effects of clodronate on early alveolar bone remodeling and root resorption related to orthodontic forces: a histomorphometric analysis. Am. J. Orthod. Dentofacial Orthop. 2010;138 doi: 10.1016/j.ajodo.2010.01.031. [DOI] [PubMed] [Google Scholar]
- Franzen T.J., Brudvik P., Vanderska-Radunovic V. Periodontal tissue reaction during orthodontic relapse in rat molars. Eur. J. Orthod. 2013;35:152–159. doi: 10.1093/ejo/cjr127. [DOI] [PubMed] [Google Scholar]
- Fujimura Y., Kitaura H., Yoshimatsu M., Eguchi T., Kohara H., Morita Y. Influence of bisphosphonates on orthodontic tooth movement in mice. Eur. J. Orthod. 2009;31:572–577. doi: 10.1093/ejo/cjp068. [DOI] [PubMed] [Google Scholar]
- Ghoneima A.A., Allam E.S., Zunt S.L., Windsor L.J. Bisphosphonates treatment and orthodontic considerations. Orthod. Craniofac. Res. 2010;13:1–10. doi: 10.1111/j.1601-6343.2009.01472.x. [DOI] [PubMed] [Google Scholar]
- Han G., Chen Y., Hou J., Liu C., Chen C., Zhuang J. Effects of simvastatin on relapse and remodeling of periodontal tissues after tooth movement in rats. Am. J. Orthod. Dentofacial Orthop. 2010;138 doi: 10.1016/j.ajodo.2010.04.026. [DOI] [PubMed] [Google Scholar]
- Hickey A.J. Guinea Pig Model of Infectious Disease - Viral Infections. Curr. Drug Targets. 2011;12(7):1018–1023. doi: 10.2174/138945011795677827. [DOI] [PubMed] [Google Scholar]
- Hoare T.R., Kohane D.S. Hydrogels in drug delivery: Progress and challenges. Polymer. 2008;49(8):1993–2007. doi: 10.1016/j.polymer.2008.01.027. [DOI] [Google Scholar]
- Igarashi K., Mitani H., Adachi H., Shinoda H. Anchorage and retentive effects of a bisphosphonate (AHBuBP) on tooth movements in rats. Am. J. Orthod. Dentofacial Orthop. 1994;106:279–289. doi: 10.1016/s0889-5406(94)70048-6. [DOI] [PubMed] [Google Scholar]
- Juan A.G., Hugo A.P., Patricia M.M. Effect of topical administration of monosodium olpadronate on experimental periodontitis in rats. J. Periodontol. 2006;77:1–6. doi: 10.1902/jop.2006.77.1.1. [DOI] [PubMed] [Google Scholar]
- Keles A., Grunes B., Di Furia C., Gagari E., Srinivasan V., Darendeliler M.A. Inhibition of tooth movement by osteoprotegerin v/s pamidronate under conditions of constant orthodontic force. Eur. J. Oral Sci. 2007;115:131–136. doi: 10.1111/j.1600-0722.2007.00433.x. [DOI] [PubMed] [Google Scholar]
- Kim T.W., Yoshida Y., Yokoya K., Sasaki T. An ultrastructural study of the effects of bisphosphonate administration on osteoclastic bone resorption during relapse of experimentally moved rat molars. Am. J. Orthod. Dentofacial. Orthop. 1999;115:645–653. doi: 10.1016/s0889-5406(99)70290-8. [DOI] [PubMed] [Google Scholar]
- Krishnan S., Pandian S., Kumar S.A. Effect of bisphosphonates on orthodontic tooth movement-an update. J. Clin. Diagn. Res. 2015;9:1–5. doi: 10.7860/jcdr/2015/11162.5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licata A.A. Discovery, clinical development, and therapeutic uses of bisphosphonates. Ann. Pharmacother. 2005;39:668–677. doi: 10.1345/aph.1e357. [DOI] [PubMed] [Google Scholar]
- Lustosa-Pereira A., Garcia R.B., de Moraes I.G., Bernardineli N., Bramante C.M., Bortoluzzi E.A. Evaluation of the topical effect of alendronate on the root surface of extracted and replanted teeth. Microscopic analysis on rats teeth. Dent. Traumatol. 2006;22:30–35. doi: 10.1111/j.1600-9657.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- Noxon S.J., King G.J., Gu G., Huang G. Osteoclast clearance from periodontal tissues during orthodontic tooth movement. Am. J. Orthod. Dentofacial Orthop. 2001;120:466–476. doi: 10.1067/mod.2001.117912. [DOI] [PubMed] [Google Scholar]
- Omedian H., Park K. Introduction to hydrogel. In: Ottenbrite R.M., editor. Biomedical applications of hydrogel handbook. Springer Science; New York: 2010. pp. 1–9. [Google Scholar]
- Peter B., Ramaniraka N., Rakotomanana L.R., Zamelli P.Y., Pioletti D.P. Peri-implant bone remodeling aftertotal hip replacement combined with systemic alendronate treatment: a finite element analysis. Comput. Meth. Biomech. Biomed. Eng. 2004;7:73–78. doi: 10.1080/1025584042000205327. [DOI] [PubMed] [Google Scholar]
- Ranade V.V., Hollinger M.A., Cannon J.B. CRC Press; Florida: 2003. Drug delivery system. [Google Scholar]
- Reitan K., Kvam E. Comparative behaviour of human and animal tissue during experimental tooth movement. Angle Orthod. 1971;41:1–14. doi: 10.1043/0003-3219(1971)041<0001:CBOHAA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Ren Y., Maltha J.C., Kuijpers-Jagtman A.M. The rat as a model for orthodontic tooth movement: a critical review and a proposed solution. Eur. J. Orthod. 2004;26:483–490. doi: 10.1093/ejo/26.5.483. [DOI] [PubMed] [Google Scholar]
- Saito T., Tabata T. Preparation of gelatin hydrogels incorporating low molecular-weight heparin for anti-fibrotic therapy. Acta Biomater. 2012;8:646–652. doi: 10.1016/j.actbio.2011.10.025. [DOI] [PubMed] [Google Scholar]
- Shetty S., Mogra S., Shetty S. The effect of the short term administration of a bisphosphonate upon dental relapse. J. Indian Orthod. Soc. 2006;39:198–203. doi: 10.1345/aph.1e357. [DOI] [Google Scholar]
- Sirisoontorn I., Hotokezaka H., Hashimoto M., Gonzales C., Luppanapornlarp S., Darendeliler M.A. Orthodontic tooth movement and root resorption in ovariectomized rats treated by systemic administration of zoledronic acid. Am. J. Orthod. Dentofacial Orthop. 2012;141:563–573. doi: 10.1016/j.ajodo.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Tabata Y., Ikada Y. Protein release from gelatin matrices. Adv. Drug Deliv. Rev. 1998;31:288–301. doi: 10.1016/s0169-409x(97)00125-7. [DOI] [PubMed] [Google Scholar]
- Thilander B. Orthodontic relapse versus natural development. Am. J. Orthod. Dentofacial. Orthop. 2000;117:562–563. doi: 10.1016/s0889-5406(00)70200-9. [DOI] [PubMed] [Google Scholar]
- Troy D.B. Lippincott Williams-Wilkins; Philadephia: 2006. Remington: the science and practice of pharmacy. [Google Scholar]
- Van Leeuwen E.J., Maltha J.C., Kuijpers-Jagtman A.M., Van Hof M.A. The Effect of retention on orthodontic relapse after the use of small continuous or discontinuous forces: an experimental study in beagle dogs. Eur. J. Oral Sci. 2003;111:111–116. doi: 10.1034/j.1600-0722.2003.00024.x. [DOI] [PubMed] [Google Scholar]