Abstract
Objective
To evaluate current evidence and results of cell-free scaffold techniques for knee chondral lesions.
Design
A systematic review was conducted on 3 medical electronic databases according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines, and the methodological quality was assessed with a modified Coleman Methodology Score. A meta-analysis was performed on the articles reporting results for visual analogue scale (VAS), Lysholm, and International Knee Documentation Committee (IKDC) scores. In order to investigate the clinical results improvement over time of cell-free cartilage scaffold implantation, all scores were reported and analyzed as improvement from basal scores at 1, 2, and ≥3 years’ follow-up.
Results
A total of 23 studies involving 521 patients were included in the qualitative data synthesis. The Coleman score showed an overall poor study quality with the majority of studies reporting results at short-/mid-term follow-up. Sixteen studies were included in the meta-analysis, showing a significant improvement from basal score at 1, 2, and ≥3 years’ follow-up. The improvement reached at 1 year remained stable up to the last follow-up for all scores.
Conclusions
The current literature suggests that cell-free scaffolds may provide good clinical short-/mid-term results; however, the low evidence of the published studies and their short mean follow-up demand further evidence before more definitive conclusions can be drawn on their real potential over time and on their advantages and disadvantages compared to the cell-based strategies for the treatment of cartilage lesions.
Keywords: cartilage, cell-free, scaffold, matrix, knee
Introduction
Articular chondral lesions have always represented a challenging pathology leading to functional impairment, pain, and eventually the development of end-stage osteoarthritis. In the past decades, a variety of surgical techniques were developed aiming at restoring articular surface and preventing joint degeneration. Among these, regenerative scaffold-based procedures have emerged as a potential therapeutic option for the treatment of these kinds of lesions. 1
The rationale for using a scaffold is to have a temporary 3-dimensional (3D) structure of biodegradable polymers to allow the growth of living cells. 2 In this light, scaffolds have been introduced in the clinical practice to improve results previously achieved with autologous chondrocyte implantation (ACI), 3 while overcoming the drawback, like the periosteal overgrowth and the dedifferentiation into fibroblasts typical of 2D culture,4,5 and simplifying the procedure.2,6 ACI scaffold-based evolution (matrix-assisted autologous chondrocyte transplantation [MACT]), applied with various materials in different physical forms, 2 demonstrated equally good results in long-term follow-ups, while avoiding the complications reported for the first-generation ACI Nevertheless, MACT was still burdened by the need of a 2-step procedure and by the high costs of cell cultures. Moreover, MACT presented the same issues in terms of regulatory requirements due to the need for cell expansions. Therefore, in the past years both researchers and clinicians have been looking for solutions to overcome the aforementioned limitations and regenerate the articular surface. 6
In the past 15 years an increasing awareness of the role of scaffolds has grown: They are not considered just carrier system for cell delivery, but they also present an intrinsic ability to promote chondral or osteochondral regeneration by exploiting the self-regenerative potential of the body.6-8 Accordingly, chondral scaffolds started to be used alone or as augmentation for microfractures, providing a substrate to take advantage of bone marrow mesenchymal cells obtained through the perforation of the subchondral bone plate. Microfractures technique was demonstrated to provide good results at short-term follow-up, but a subsequent worsening of clinical score has been shown at mid-term follow-up. Consequently, the mayor concern about cell-free procedures regards the durability over time of the obtained results Thus, it would be important to document if the implant of biomaterial without cells could lead to better results and understanding if the cell-free scaffold approach could offer a positive and durable outcome in the treatment of cartilage lesions.
The aim of this study was to systematically review the current literature, in order to provide an updated insight on the potential of cell-free scaffolds, evaluating their results over time with a meta-analysis.
Materials and Methods
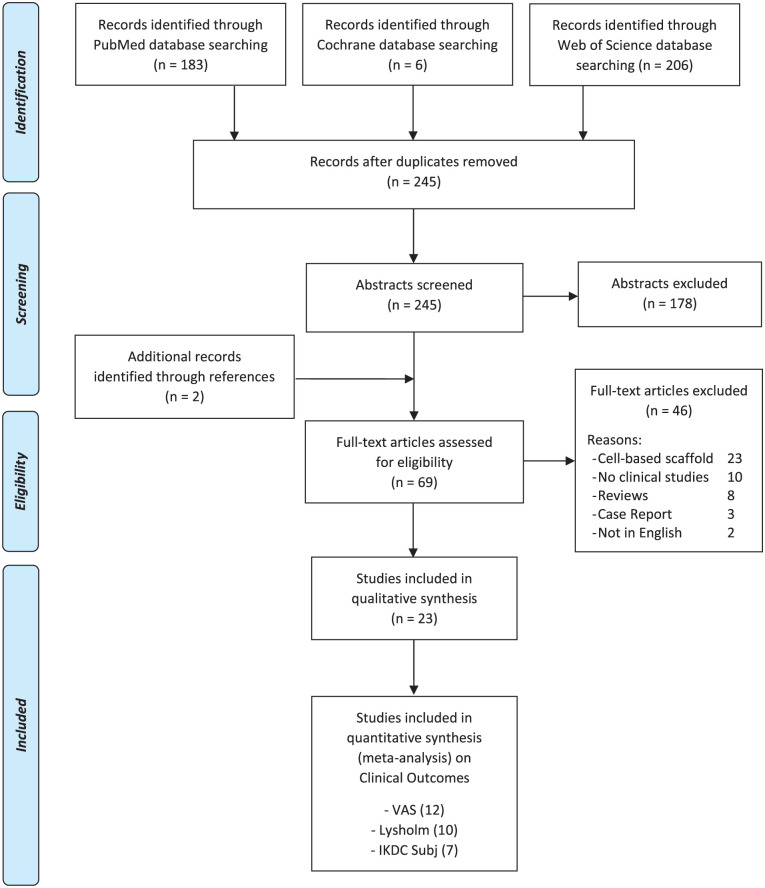
A systematic review and a meta-analysis were performed on the literature of cell-free chondral scaffold implantation for cartilage knee lesions. The search was conducted on PubMed, Web of Science and Cochrane databases on January 14, 2019, using of the following parameters: ((cartilage OR chondral) AND (defect OR defects OR lesion OR lesions)) AND (AMIC OR ACIC OR (cell-free AND (scaffold OR implant))). The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines were used. 9 A flowchart of the studies selection for qualitative and quantitative data synthesis is reported in Figure 1 . The screening process and analysis were conducted separately by 2 independent observers (D.R. and A.B.). In the first step, the articles were screened by title and abstract. The following inclusion criteria for relevant articles were used during the initial screening of titles and abstracts: clinical reports of any level of evidence, written in the English language, on cells-free scaffolds for the treatment of cartilage lesion of the knee. Exclusion criteria were articles written in other languages, preclinical studies, studies reporting other chondral and osteochondral not cell-free procedures such as cell-based scaffolds, autologous blood- or platelet-rich plasma–augmented techniques, and osteochondral scaffolds, and reviews. In the second step, the full texts of the selected articles were retrieved and screened, with further exclusions according to the previously described criteria. Moreover, articles not reporting clinical results were excluded. Reference lists from the selected papers and from the systematic reviews, found with the first and second screening, were also checked, and all selected studies were included in the qualitative data synthesis.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) flowchart of the systematic literature review.
Relevant data (year, type of study, number of patients, sex, age, body mass index, follow-up, lesion size, lesion location, lesion grade, type of scaffold, scores reported, results) were then extracted and collected in a database with consensus of the 2 observers, to be analyzed for the purposes of the present study. To assess the methodological quality of the collected data, the subscales of a Coleman Methodology Score (CMS), modified by Kon et al. 10 to better suit to the cartilage repair field, were determined for each study. The articles reporting at least one of specific clinical outcomes (visual analogue score [VAS], Lysholm score, International Knee Documentation Committee [IKDC] subjective scores) were selected and included in the meta-analysis. The articles included in the systematic review were excluded from the meta-analysis in the following cases: the same survey was reported at different follow-up times and the most recent articles also reported the intermediate follow-up results; basal scores or follow-up scores (including standard deviation) not reported; results reported according to other clinical outcome measurements. All scores were reported and analyzed as improvement from basal scores at 1, 2, and ≥3 years’ follow-up, in order to investigate any clinical results improvement over time of cell-free cartilage scaffold implantation.
Statistical Analysis
The statistical analysis and the forest plots were carried out according to Neyeloff et al. 11 using Microsoft Excel. The comparisons among the follow-up times was based on the analysis of variance 12 of the difference between basal and follow-up score (MD). With no heterogeneity, the estimation of the MD and its 95% confidence interval was based on fixed effect analysis of variance; the random effect model was preferred otherwise. A P value of 0.05 was used as the level of statistical significance. Statistical heterogeneity was evaluated by t using Cochran’s Q statistic and I2 metric and was considered significant when I2 > 25%.
Results
The search identified 245 records, whose abstracts were screened and selected according to the inclusion/exclusion criteria ( Fig. 1 ): 178 abstracts were excluded and 2 articles were identified through the reference lists, which gave a total of 69 full-text articles assessed for eligibility. Forty-six full-text articles were also excluded for the following reasons: 23 articles reported on cell-based techniques, 10 articles did not report clinical results, 8 articles were reviews, 3 articles were case reports, and 2 articles did not present the full-text in English language.
Therefore, a total of 23 studies13-35 were included in the qualitative data synthesis and reported in detail in Table 1 . The scores used to assess results in these articles are summarized in Table 2 . Among the different scores used in the literature, we selected the most common ones reported at every follow-up time (VAS, Lysholm, IKDC subjective score) for the meta-analysis: 12 articles reported subjective outcome evaluated with VAS, 10 articles with the Lysholm score, and 7 articles with the IKDC subjective score.
Table 1.
Characteristics of the 23 Articles Included in the Qualitative Data Synthesis, with Study Quality Assessment.
| Author(s) | Type of Study | No. of Initial Patients/Patients at Follow-up | Age Sex BMI |
Follow-up | Size Location Classification |
Type of Treatment | Results | Coleman Methodology Score |
|---|---|---|---|---|---|---|---|---|
| Benthien et al. AOB 2010 |
Retrospective case series | 3/3 | — | 18 months | — | MFX and glued AMIC (Chondro-Gide) | Improvement of the Oxford Knee Score after 18 months in 3 patients with retropatellar osteochondral defects treated | 32 |
| Gille et al. KSSTA 2010 |
Prospective case series | 27/24 | 39 years (16-50 years) 16 M/11 F 26 (30-32) |
37 months | 4.2 cm2 (range 1.3-8.8) 7 MFC/3 LFC/2 Tro/9 Pat/6 Pat + CF 100% IV |
MFX and glued AMIC (Chondro-Gide) | Significant improvement (P < 0.05) of all scores was observed as early as 12 months after AMIC, and further increased values were notable up to 24 months postoperatively | 59 |
| Pascarella et al. KSSTA 2010 |
Prospective case series | 19/19 | 26 years (18-50 years) 12 M/7 F — |
24 months | 3.6 cm2 (2.8-3.9) 12 MFC/5 LFC/2 Pat III: 12 – IV: 7 |
MFX and modified AMIC technique (Chondro-Gide) | Good to excellent outcome for the majority of the patients at 24 months postoperatively | 56 |
| Schiavone Panni et al. IJIP 2011 |
Prospective case series | 17/17 | 39 years (22-52 years) 6 M/9 F 28 (23-33) |
36 moths | 4.6 cm2 (2.5-8.0) 8 MFC/3 LFC/5 Tro/1 Pat — |
Modified AMIC (Chondro-Gide) | At an average follow-up of 36 months, mean IKDC score and Lysholm score improved in all patients | 61 |
| Efe et al. KSSTA 2012 |
Prospective case series | 15/15 | 26 ± 8 years 6 M/9 F — |
24 months | <11 mm (diameter) 8 MFC/3 LFC/4 Pat III-IV |
Debridement + press-fit with autologous cancellous bone from, then cell-free collagen type I gel (CaReS-1S) | Cell-free collagen type I matrix repair of small articular cartilage lesions in the knee leads to good clinical results at a follow-up of 2 years | 58 |
| Kusano et al. KSSTA 2012 |
Retrospective comparative study | 11/11 | 26 ± 3 years 5 M/6 F 26 ± 2 |
27 months | 4.2 ± 0.4 cm2
11 MFC III-IV |
MFX and sutured and glued AMIC (Chondro-Gide) | Significant improvements in clinical outcome scores were noted in the 3 groups at a follow-up of 28 months. The largest improvements were seen in the osteochondral subgroup. | 50 |
| 20/20 | 39 ± 3 years 10 M/10 F 25 ± 1 |
29 months | 4.4 ± 0.6 cm2
20 Pat III-IV |
MFX and sutured and glued AMIC (Chondro-Gide) | ||||
| 9 / 9 | 39 Y ± 4 8M/1F 26 ± 1 |
30 m | 2.3 cm2 ± 0.4 5 MFC / 4 LFC III - IV |
MFX and Sutured & glued AMIC (Chondro-Gide) | ||||
| Anders et al. OOJ 2013 |
Randomized controlled trial | 13/8 | 33 years 11 M/2 F 28 |
24 months | 3.7 cm2
— III: 7 - IV: 6 |
MFX and sutured AMIC (Chondro-Gide) | This study confirms the effectiveness and safety of AMIC glued or sutured and demonstrates that the good results observed at 1-year post-operation are maintained at 2-years. | 62 |
| 15/13 | 38 years 12 M /3 F 28 |
24 months | 3.5 cm2
— III: 6 - IV: 9 |
MFX and glued AMIC (Chondro-Gide) | ||||
| Gille et al. AOTS 2013 |
Retrospective case series | 57/57 | 37 years (1761) 38 M/19 F — |
24 months | 3.4 cm2 (1.0-12.0) 32 MFC/6 LFC/4 Tro/15 Pat III: 20 - IV: 37 |
MFX and AMIC (Chondro-Gide) | The majority of patients were satisfied with the postoperative outcome, reporting a gradual and significant clinical improvement at follow-ups 1 and 2 years after surgery | 51 |
| Shetty et al. Orthopedics 2013 |
Retrospective case series | 10/10 | 45 ± 11 years — — |
24 months | 2-8 cm2
— III-IV |
Microdrilling, Autologous collagen-induced chondrogenesis (ACIC): Arthroscopically injection of 1 mL of fibrinogen (Tisseel) and 0.9 mL of atelocollagen (CartiFill) and 0.1 mL of thrombin under CO2 insufflation | A single-stage arthroscopic procedure using microdrilling combined with ACIC appears to be an effective cartilage regeneration method | 48 |
| Dhollander et al. AOB 2014 |
Prospective case series | 10/10 | 37 ± 7 years 8 M/2 F — |
24 months | 4.2 ± 1.9 cm2
8 Pat/2 Tro III-IV |
MFX and sutured AMIC (Chondro-Gide) | The patients included in this study showed a significant gradual clinical improvement after the osteochondral scaffold plug. However, this clinical improvement was not confirmed by the MRI findings | 58 |
| Schuttler et al. KSSTA 2014 |
Prospective case series | 15/15 | 24 years 6 M/9 F 25 ± 5 |
48 months | 0.82 ± 0.19 cm2
MFC 8/LFC 3/Pat 4 III-IV |
Debridement, defect filled with autologous cancellous bone, then implanted cell-free collagen type I gel matrix (CaReS-1S) | This study showed that the use of cell-free collagen type I matrix implants led to a significant and durable improvement in all the clinical and imaging scores investigated 4 years after implantation | 61 |
| Roessler et al. IntOrth 2015 |
Prospective case series | 28/28 | 35 years 17 M/11 F — |
24 months | 3.71 ± 1.93 cm2
MFC 18/LFC 3/Pat 7 — |
Debridement and cell-free collagen type I gel matrix (CaReS-1S) | Cell-free collagen type I matrices appear to be a safe and suitable treatment option even for large cartilage defects of the knee. Results of this study were comparable to the better-established findings for small cartilage defects | 62 |
| Shetty et al. JCOT 2016 |
Prospective case series | 30/30 | 18-65 years — — |
48 months | 2-8 cm2
— III-IV |
Microdrilling, autologous collagen-induced chondrogenesis (ACIC): Arthroscopically injection of 1 mL of fibrinogen (Tisseel) and 0.9 mL of atelocollagen (Coltrix) and 0.1 mL of thrombin under CO2 insufflation | A single-stage arthroscopic procedure using microdrilling combined with atelocollagen and fibrin gel appears to be an effective cartilage regeneration method | 65 |
| Sofu et al. Arthroscopy 2017 |
Retrospective comparative study | 19/19 | 40 ± 10 years 9 M/19 F 23 ± 2 |
24 months | 3.6 ± 1.3 cm2
13 MFC/6 LFC III-IV |
Debridement, MFX + hyaluronic acid-based cell-free scaffold (Hyalofast) | Single-stage regenerative cartilage surgery using hyaluronic acid–based cell free scaffold in combination with MFX revealed promising clinical outcomes at 24 months of follow-up, but the clinical significance of the differences seen is simply not known. | 51 |
| Sadlik et al. JKS 2017 |
Prospective case series | 12/12 | 36 years (22-52) 7 M/5 F — |
38 months | 2.4 cm2 (0.8-4.0) 12 Pat III: 7 - IV: 5 |
Debridement and AMIC-aided: Collagen matrix (Chondro-Gide) fixated with fibrin glue | Significantly improved clinical and radiological results after all-arthroscopic AMIC repair of patellar lesions | 61 |
| Volz et al. IntOrth 2017 |
Randomized controlled trial | 17/16 | 34 ± 11 years 12 M/5 F 27 ± 4 |
60 months | 3.8 ± 2.1 cm2
— — |
MFX and sutured AMIC (Chondro-Gide) | AMIC is an effective cartilage repair procedure in the knee resulting in stable clinical results significantly better than the MFx group at 5 years | 73 |
| 17/14 | 39 ± 9 years 15 M/2 F 27 ± 4 |
60 months | 3.9 ± 1.1 cm2
— — |
MFX and glued AMIC (Chondro-Gide) | ||||
| Schagemann et al. AOTS 2018 |
Retrospective comparative study | 20/20 | 38 years (18-70) 13 M/7 F 27 (19-35) |
24 months | 3.1 cm2(1.0-6.0) 10 MFC/2 LFC/2 Tro/6 Pat III-IV |
MFX and arthroscopic glued AMIC (Chondro-Gide) | Our results suggest that mini-open AMIC is equivalent to the arthroscopic procedure at a follow-up of 1 and 2 years | 36 |
| 30/30 | 34 years (14-53) 17 M/13 F 24 (18-29) |
24 months | 3.4 cm2 (1.5-12.0) 13 MFC/6 LFC/1 Tro/9 Pat/1 TP III-IV |
Mini-open glued AMIC (Chondro-Gide) | ||||
| Bertho et al. OTSR 2018 |
Prospective case series |
13 / 13 | 29 Y (15-51) 8M/5F - |
24 m | 3.7 cm² (2.2 - 6.6) 8 MFC / 4 LFC / 1 Pat III: 1 - IV: 12 |
Drilling, AMIC sutured (Chondro-Gide) | AMIC significantly improves knee function scores in patients with large osteochondral defects due to advanced osteochondritis of the knee | 46 |
| Hoburg et al. AOTS 2018 |
Prospective case series | 15/15 | 26 years (17-41) 9 M/6 F 25 (21-35) |
49 months | 4.98 ± 3.02 cm2
15 MFC III: 11 - IV: 4 |
Debridement, drilling, bone grafting + Chondro-Gide | Significant improvement of knee function could be achieved with simultaneous AMIC procedure and bone grafting in 2/3 of patients with large osteochondral lesions at 4 years | 59 |
| Lahner et al. BMRI 2018 |
Prospective case series | 9/9 | 45 ± 10 years — 29 |
15 months | 2.1 ± 1.2 cm2
— III-IV |
MFX and glued AMIC (Chondro-Gide) | The AMIC procedure enhances pain reduction and gain of knee function for cartilage defects of overweight patients | 55 |
| Schiavone Panni et al. KSSTA 2018 |
Retrospective case series | 21/21 | / | 84 months | 4.3 cm2(2.9-8.0) 11 MFC/3 LFC/6 Tro/1 Pat IV |
MFX and glued AMIC (Chondro-Gide) | AMIC was found to be an effective method to treat full-thickness knee chondral defects larger than 2 cm2, with significant clinical and functional improvement maintained over a 7-year follow-up | 58 |
| Schuttler et al. Arthroscopy 2018 |
Prospective case series | 28/28 | 35 ± 7 years — 27 ± 3 |
60 months | 3.7 ± 1.9 cm2
13 MFC/3 LFC/5 Pat/2 Tro III-IV |
Debridement and cell-free collagen type I gel matrix (CaReS-1S) | The use of this cell-free collagen type I scaffold for large defects showed increased wear of the repair tissue and clinical failure in 18% of cases at 5-year follow-up | 57 |
| Sofu et al. KSSTA 2018 |
Retrospective comparative study | 21/21 | 39 ± 10 years 9M /12 F 23 ± 1 |
24 months | 3.3 ± 0.7 cm2
MFC 16/LFC 5 III-IV |
Debridement, MFX + hyaluronic acid–based cell-free scaffold (Hyalofast) | Single-stage regenerative cartilage surgery using hyaluronic acid–based cell free scaffold in combination with MFX showed improvement of symptoms at final follow-up | 63 |
BMI = body mass index; MFC = medial femoral condyle; LFC = lateral femoral condyle; Pat = patella; Tro = trochlea; MFX = microfractures; AMIC = autologous matrix-induced chondrogenesis.
Table 2.
List of the Different Scores Used in the Articles Included in the Qualitative Data Synthesis.
| 1-Year Follow-up | 2-Year Follow-up | ≥3-Years Follow-up | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors, Journal, Year | VAS | Lysholm | IKDC Subjective | KOOS Subscales | Cincinnati | Tegner | Kujala | SF-36 | VAS | Lysholm | IKDC Subjective | KOOS subscales | Cincinnati | Tegner | Kujala | SF-36 | VAS | Lysholm | IKDC Subjective | KOOS Subscales | Cincinnati | Tegner | Kujala | SF-36 |
| Benthien et al. AOB 2010 | ||||||||||||||||||||||||
| Gille et al. KSSTA 2010 | X | X | X | X | X | X | X | X | X | |||||||||||||||
| Pascarella et al. KSSTA 2010 | X | X | ||||||||||||||||||||||
| Schiavone Panni et al. IJIP 2011 | X | X | ||||||||||||||||||||||
| Efe et al. KSSTA 2012 | X | X | X | X | X | |||||||||||||||||||
| Kusano et al. KSSTA 2012 | X | X | X | X | ||||||||||||||||||||
| Anders et al. OOJ 2013 | X | X | X | X | ||||||||||||||||||||
| Gille et al. AOTS 2013 | X | X | X | X | ||||||||||||||||||||
| Shetty et al. Orthopedics 2013 | X | |||||||||||||||||||||||
| Dhollander et al. AOB 2014 | X | X | X | X | X | X | X | X | ||||||||||||||||
| Schuttler et al. KSSTA 2014 | X | X | X | X | X | X | X | X | X | |||||||||||||||
| Roessler et al. IntOrth 2015 | X | X | X | X | X | X | X | X | ||||||||||||||||
| Shetty et al. JCOT 2016 | X | X | X | |||||||||||||||||||||
| Sofu et al. Arthroscopy 2017 | X | X | X | X | X | X | ||||||||||||||||||
| Sadlik et al. JKS 2017 | X | X | X | |||||||||||||||||||||
| Volz et al. IntOrth 2017 | X | X | X | |||||||||||||||||||||
| Schagemann et al. AOTS 2018 | X | X | X | X | X | X | ||||||||||||||||||
| Bertho et al. OTSR 2018 | X | X | X | X | X | X | ||||||||||||||||||
| Hoburg et al. AOTS 2018 | X | X | X | X | X | X | X | X | X | X | ||||||||||||||
| Lahner et al. BMRI 2018 | X | X | X | |||||||||||||||||||||
| Schiavone Panni et al. KSSTA 2018 | X | X | ||||||||||||||||||||||
| Schuttler et al. AOTS 2018 | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||
| Sofu et al. KSSTA 2018 | X | X | X | X | X | X | ||||||||||||||||||
| 13 | 7 | 6 | 6 | 3 | 8 | 1 | 1 | 12 | 8 | 7 | 5 | 3 | 9 | 1 | 0 | 4 | 5 | 7 | 4 | 2 | 4 | 0 | 0 | |
VAS, visual analogue scale; IKDC, International Knee Documentation Committee; KOOS, Knee Injury and Osteoarthritis Outcome Score; SF-36, 36-item Short Form health questionnaire.
Qualitative Data Synthesis
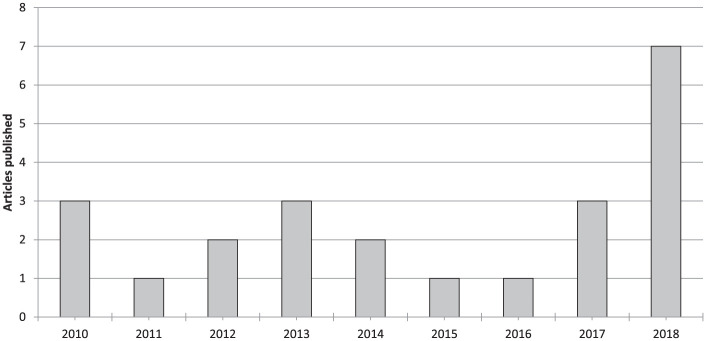
Among the 23 articles included in the qualitative data synthesis, the evaluation of study type showed 2 articles reporting the outcome of 1 randomized clinical trial (RCT), 4 comparative studies, and 17 case series (with 2 articles reporting the outcome of the same cohort at different follow-ups) ( Table 1 ). In 19 articles the scaffolds were used combined with microfractures or drilling, in 4 articles the surgical technique entailed only the debridement of the defect. Fifteen articles analyzed the results of Chondro-Gide scaffold (2 articles reporting on 1 RCT, 2 comparative studies, and 11 case series), 4 of CaReS-1S (4 case series), 2 of Hyalofast (2 comparative studies), and 1 case series each for Coltrix and CartiFill. Since the first reports in 2010, the publication trend did not significantly increase over time, with 9 articles published from 2010 to 2013 and 7 articles from 2014 to 2017, with the exception of 2018, with the highest value of 7 articles published ( Fig. 2 ).
Figure 2.
Number of articles per year dealing with cell-free scaffold techniques.
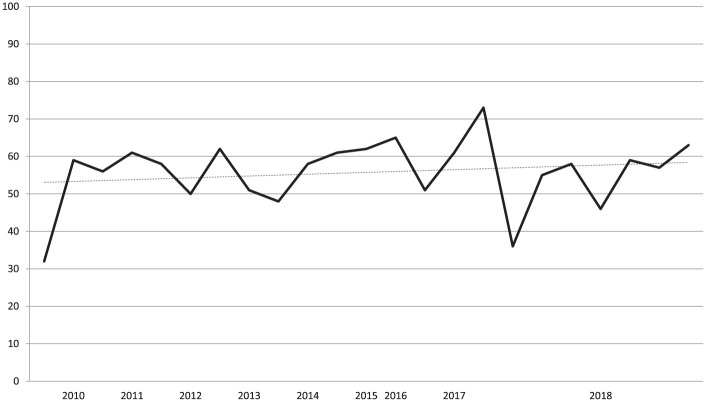
Similarly, even the level of evidence of the literature did not increase, with only 1 RCT reported in 2 articles published by the same group, the first one being only an interim analysis of the same RCT. The evaluation with the CMS showed an overall poor quality of the included studies. In fact, only 1 study scored higher than 70 and only 7 studies reached a score between 60 and 69, whereas 11 studies had a score between 50 and 59, and 4 studies obtained a score lower than 50. No improvement over time was found for CMS score ( Fig. 3 ).
Figure 3.
Trend over time of the Coleman Methodology Score (CMS) of the articles dealing with cell-free scaffold techniques.
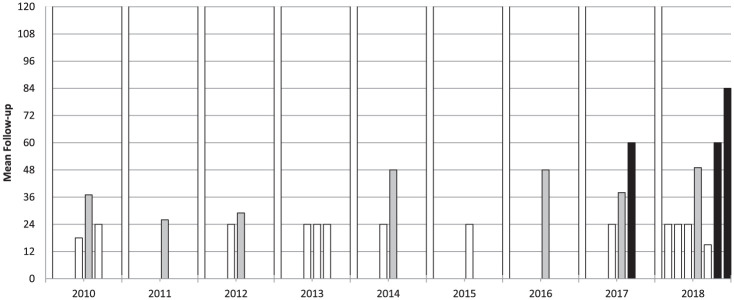
A total of 526 patients were treated with cell-free scaffolds, and the outcomes of 507 patients were reported (a detailed description of the analyzed data, with the number of patients and the specific data available is provided in Table 3 ). Patients were evaluated using a wide range of heterogeneous scores ( Table 2 ), at a mean of 34.4 months’ follow-up: 13 studies reported the outcome at short-term follow- up (≤24 months), 7 at short-/mid-term follow-up (24-60 months), and only 3 at mid-term follow-up (≥60 months), including the longest follow-up available in the literature (84 months). The study follow-up duration over the years did not show an increasing trend and in the last year only 2 mid-term follow-up studies were included in the review, with 1 short-/mid-term and 4 short-term follow-up studies ( Fig. 4 ).
Table 3.
Demographic Data of the Patients Analyzed in the Articles Included in the Qualitative Data Synthesis.
| Data Reported | Available Data | |||
|---|---|---|---|---|
| n | % | No. of Patients with Available Data | No. of Studies Reporting Data | |
| No. of patients | 526 | |||
| No. of patients at follow-up | 507 | |||
| Study design (patients reported) | 450 | 20 | ||
| Randomized controlled trial | 34 | 7.6 | 1 a | |
| Comparative studies | 130 | 28.8 | 4 | |
| Case series | 286 | 63.6 | 15 b | |
| Follow-up, months, mean | 34.4 | 502 | 23 | |
| Sex | 420 | 17 | ||
| Men | 258 | 61.4 | ||
| Women | 162 | 38.6 | ||
| Age, years | 35.3 | 462 | 20 | |
| Body mass index, kg/m2 | 26.1 | 298 | 12 | |
| Location | 402 | 17 | ||
| MFC | 208 | 51.7 | ||
| LFC | 59 | 14.7 | ||
| Patella | 104 | 25.9 | ||
| Trochlea | 24 | 6.0 | ||
| Multi/Tibial Plateau | 7 | 1.7 | ||
| Size, cm2, mean | 3.6 | 458 | 19 | |
| Grade | 192 | 8 | ||
| Outerbridge III | 64 | 33.3 | ||
| Outerbridge IV | 128 | 66.7 | ||
| Scaffold | 516 | 23 | ||
| Chondro-Gide | 355 | 68.8 | 15 | |
| CaReS-1S | 81 | 15.7 | 4 | |
| Hyalofast | 40 | 7.8 | 2 | |
| Coltrix | 30 | 5.8 | 1 | |
| CartiFill | 10 | 1.9 | 1 | |
| Failures | 16 | 5.9 | 271 | 14 |
MFC = medial femoral condyle; LFC = lateral femoral condyle.
One study reported the preliminary results of the same survey was excluded from the total.
Two studies reported the preliminary results of the same survey were excluded from the total.
Figure 4.
Mean follow-up of the articles dealing with cell-free scaffold techniques.
Failures were reported only in 14 articles, with 16 failures among 271 patients evaluated at a mean 34.8 months of follow-up, for an overall 5.9% failure rate.
Clinical Scores Meta-Analysis
Seven studies of the systematic review were excluded from the meta-analysis for the following reasons: preliminary data of the same survey (3 studies),23,29,30 no clinical scores (1 study), 33 no standard deviation reported (3 studies).26,32,35 Thus, 16 studies (for a total of 20 study groups)13-22,24,25,27,28,31,34 were included in the quantitative synthesis.
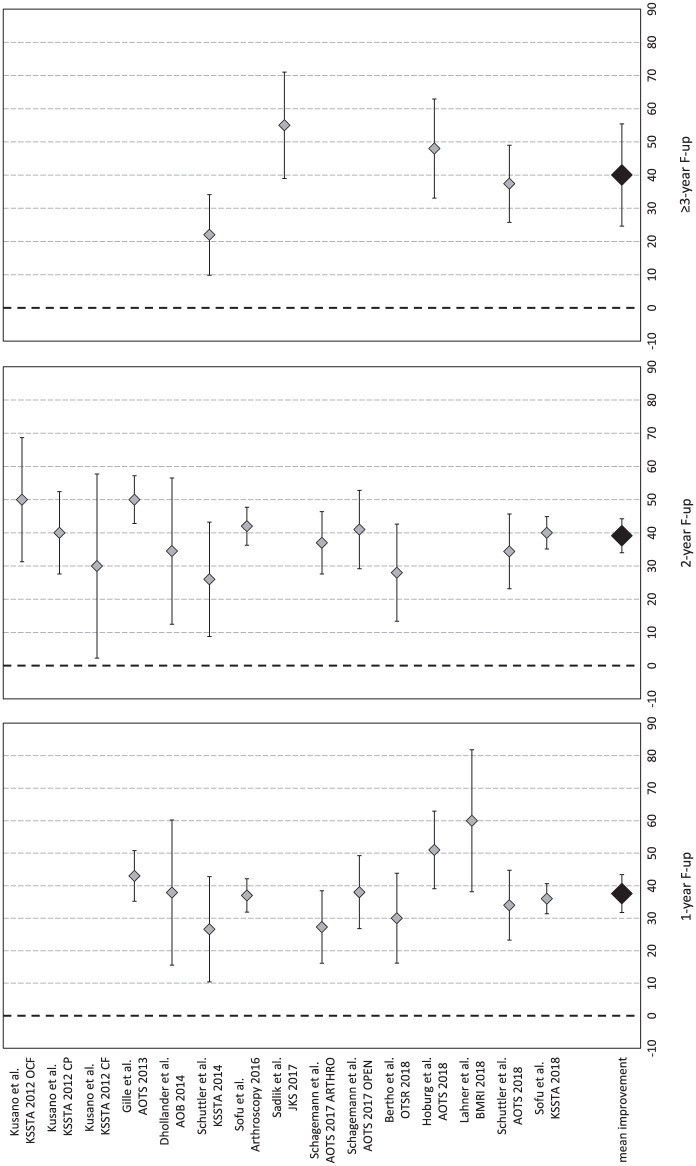
Pain evaluated with VAS was available for 289 patients in 12 studies (15 study groups).13-18,20,22,24,27,28,31 In detail, 1-year VAS follow-up was available for 237 patients (11 study groups in 10 studies), 2-year follow-up for 253 patients (12 study groups in 9 studies), and ≥3-year follow-up (mean 48.8 ± 9.0 months from surgery) for 58 patients (4 study groups in 4 studies). Compared with the basal score, the meta-analysis on VAS showed a mean improvement of 37.6 (95% CI 31.8-43.4, I2 = −29%) at 1-year follow-up, 39.1 (95% CI 34.0-44.3, I2 = −64%) at 2-year follow-up, and 40.0 (95% CI 24.7-55.4, I2 = −20%) at ≥3-year follow-up ( Fig. 5 ), all significantly higher than baseline (P < 0.05), but without any significant difference among follow-up times. That is, the improvement reached in the first year remained stable up to the final follow-up, average 49 months.
Figure 5.
Forest plot of mean improvement of visual analogue scale (VAS) score at 1, 2, and ≥3 years’ follow-up.
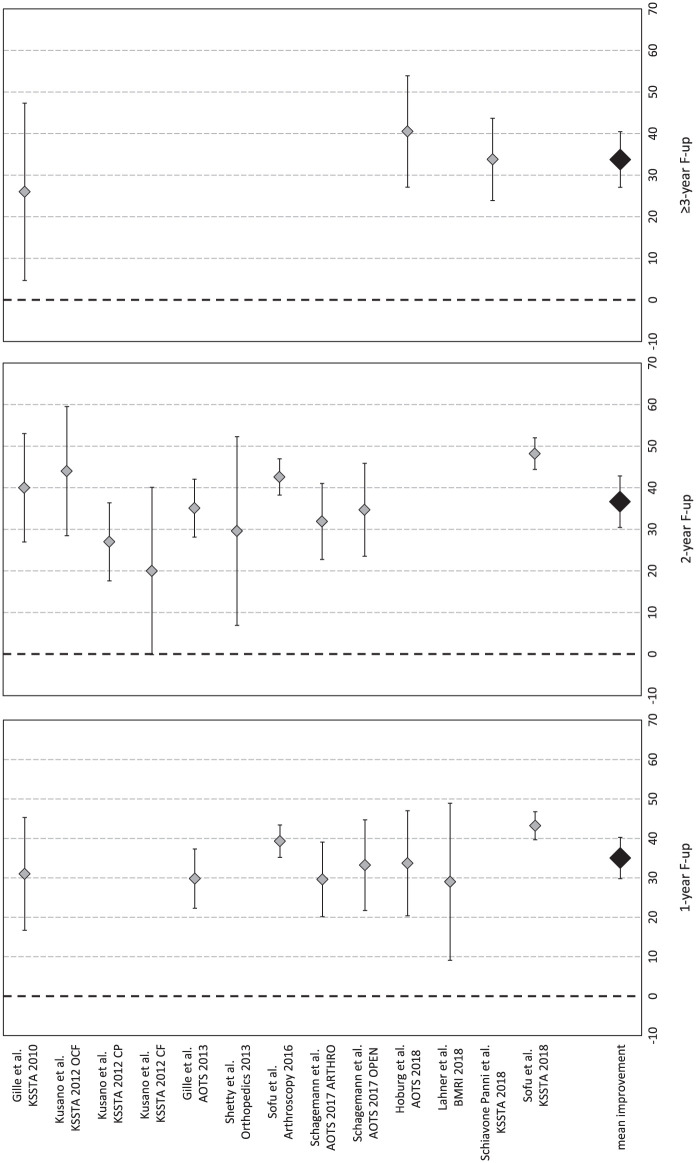
The Lysholm score was available for 265 patients in 10 studies (13 study groups).14,16-19,22,25,27,31,34 In detail, 1-year follow-up was available for 194 patients (8 study groups in 7 studies), 2-year follow-up for 220 patients (10 study groups in 7 studies), and ≥ 3-year follow-up (mean 56.0±25.0 months from surgery) for 43 patients (3 study groups in 3 studies). Compared with the basal score, the meta-analysis showed a mean improvement of 35.0 (95% CI 29.8-40.3, I2 = −76%) at 1-year follow-up, 36.7 (95% CI 30.5-42.9, I2 = −48%) at 2-year follow-up, and 33.8 (95% CI 27.1-40.5, I2 = 17%) at ≥3-year follow-up ( Fig. 6 ), all significantly higher than baseline (P < 0.05), but without any significant difference among follow-up times. That is, the improvement reached in the first year remained stable up to the final follow-up, average 56 months.
Figure 6.
Forest plot of mean improvement of Lysholm score at 1, 2, and ≥3 years’ follow-up.
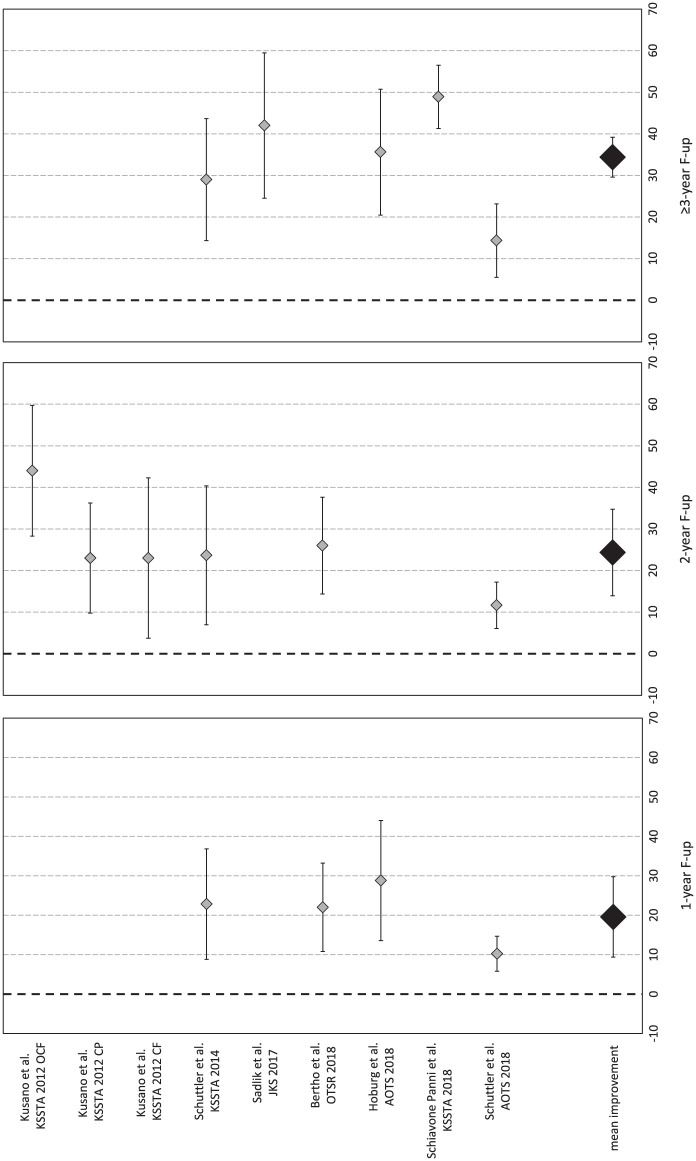
The IKDC subjective score was available for 144 patients in 7 studies (9 study groups).13,15,17,19,20,28,31 In detail, 1-year follow-up was available for 71 patients (4 study groups in 4 studies), 2-year follow-up for 96 patients (6 study groups in 4 studies), and ≥3-year follow-up (mean 56.0 ± 17.6 months from surgery) for 81 patients (5 study groups in 5 studies). Compared with the basal score, the meta-analysis showed a mean improvement of 19.6 (95% CI 9.4-29.8, I2 = −62%) at 1-year follow-up, 24.4 (95% CI 14.0-34.8, I2 = −48%) at 2-year follow-up, and 34.4 (95% CI 29.6-39.3, I2 = 92%) at ≥3-year follow-up ( Fig. 7 ), all significantly higher than baseline (P < 0.05), but without any significant difference among follow-up times. That is, the improvement reached in the first year remained stable up to the final follow-up, average 56 months.
Figure 7.
Forest plot of mean improvement of International Knee Documentation Committee (IKDC) subjective score at 1, 2, and ≥3 years’ follow-up.
Discussion
The main finding of the present study was that cell-free chondral scaffolds provided significant improvement at short-/mid-term follow-up, but the evidence level was limited and studies at longer follow-up were missing.
Surgical treatments of cartilage lesions have the main indication for young active patients, 36 where the recovery time is an important aspect; therefore, early follow-ups are useful to provide correct expectations to patients undergoing these procedures. In this light, this meta-analysis showed that at 1-year follow-up all evaluated scores improved significantly compared with the baseline scores, demonstrating the efficacy of the technique. Moreover, the evaluation at 2 years’ follow-up did not show a further improvement, suggesting that most of the benefit had already been achieved in the first year. This is in line with studies about microfractures, which led to a relatively fast recovery. 37 Interestingly, this trend differs from what reported for cell-based techniques, where a further improvement has been documented from 1 to 2 years of follow-up. 38 This shows that the scaffold-based cell-free approach presents an improvement trend more similar to microfracture than cell-based procedures, as also confirmed by the only RCT comparing MFX and cell-free scaffold approach.21,29
Another important aspect to evaluate in surgical chondral procedures is the stability of clinical results over time. Cell-based cartilage procedures have already been evaluated at long-term follow-up, and even if results are not univocal in the literature, a general trend can be depicted. ACI and MACT generally showed stable results up to a long-term follow-up.36,38-43 Microfractures, on the other hand, showed more controversial results. While short-term results are supported by good-level evidence, long-term results are not 37 : some studies reported good long-term results for microfractures,44-47 but other articles reported worst results and an increasing number of failures versus mosaicplasty.48-50 Moreover, some studies documented a worsening trend over time, with positive short-term results and a decrease at mid-/long-term.51,52 This meta-analysis showed that clinical results after scaffold-based cell-free approach remained stable for up to ≥3 years’ follow-up, and a RCT reporting mid-term results demonstrated significant better outcome for cell-free scaffold compared to microfractures at 5 years’ follow-up. 21 Unfortunately, the current literature does not allow to draw conclusions on long-term follow-ups and the reported findings on results duration have to be taken with caution. Besides the low number of studies, mainly reporting on small series at a short-follow-up, the overall study quality is limited, as evaluated with the modified CMS.
The relatively short follow-up of the published studies should be underlined, also considering that this surgical approach cannot be considered recent. The first articles included in this review are dated 2010,33-35 and the cell-free techniques had actually been already described in a report dated 2006. 53 Moreover, since 2005, patients treated with Chondro-Gide (Geistlich Pharma, Switzerland), a scaffold-based cell-free approach, started to be enrolled in the “AMIC Registry.” 27 Consequently, considering the high number of patients treated and initially followed, and the time elapsed from those documented treatments, these techniques can no longer be considered new and long-term follow-up studies are lacking to confirm the duration of documented benefits of these procedures.
The quality of the overall literature on this topic is very low, as confirmed by the CMS of the published studies and by the presence of only 1 RCT on AMIC, reported in 2 different articles included in the systematic review. This aspect has been previously underlined, 54 and the current update does not show any improvement in the evidence level of the scientific literature over time. High-level comparative studies are needed to demonstrate the advantage of implementing microfractures with a cell-free scaffold or of using cell-free scaffolds alone. Moreover, since currently cell-based therapies are the most documented regenerative techniques, comparative studies including also the economic evaluation are needed to fully evaluate advantages and disadvantages of cell-based and cell-free strategies for the treatment of cartilage lesions. The limitations of the current systematic review and meta-analysis reflect the abovementioned limitations of the literature: paucity of studies, generally of low quality, including a low number of patients evaluated at short- to mid-term follow-up with heterogeneous scores. Accordingly, a weakness of the meta-analysis is represented by the high proportion of evidence level IV studies and this is also the reason why it was not possible to perform further subanalysis (e.g., techniques with microfractures/drilling vs. cell-free scaffold alone). Moreover, the inclusion of heterogeneous surgical techniques and scaffolds (e.g., collagen scaffolds, hyaluronic acid scaffolds, atelocollagen, with or without microfractures) may weaken the results of this study. Nonetheless, all these studies use the cell-free scaffold-based approach, proposed as alternative to the cell-based one, which also includes variants related to techniques and scaffolds applied. Until comparative studies specifically focused on this issue will prove differences among the several proposed variants of these approaches, the literature will only allow a broad comparison of the 2 strategies, while it cannot demonstrate if and which techniques emerges as the most suitable cell-based or cell-free options.
In conclusion, the current literature suggests that cell-free scaffolds may provide good clinical short-/mid-term results, but the low evidence of the published studies and their short mean follow-up, demand further evidence before drawing more definitive conclusions on the real potential of this technique over time and on its advantages and disadvantages compared with cell-based strategies for the treatment of cartilage lesions.
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Angelo Boffa  https://orcid.org/0000-0002-1523-6900
https://orcid.org/0000-0002-1523-6900
References
- 1. Filardo G, Kon E, Roffi A, Di Martino A, Marcacci M. Scaffold-based repair for cartilage healing: a systematic review and technical note. Arthroscopy. 2013;29(1):174-86. doi: 10.1016/j.arthro.2012.05.891 [DOI] [PubMed] [Google Scholar]
- 2. Kon E, Roffi A, Filardo G, Tesei G, Marcacci M. Scaffold-based cartilage treatments: with or without cells? A systematic review of preclinical and clinical evidence. Arthroscopy. 2015;31(4):767-75. doi: 10.1016/j.arthro.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 3. Vasiliadis HS, Danielson B, Ljungberg M, McKeon B, Lindahl A, Peterson L. Autologous chondrocyte implantation in cartilage lesions of the knee: long-term evaluation with magnetic resonance imaging and delayed gadolinium-enhanced magnetic resonance imaging technique. Am J Sports Med. 2010;38(5):943-9. doi: 10.1177/0363546509358266 [DOI] [PubMed] [Google Scholar]
- 4. Grigolo B, Lisignoli G, Piacentini A, Fiorini M, Gobbi P, Mazzotti G, et al. Evidence for redifferentiation of human chondrocytes grown on a hyaluronan-based biomaterial (Hyaff 11): Molecular, immunohistochemical and ultrastructural analysis. Biomaterials. 2002;23(4):1187-95. [DOI] [PubMed] [Google Scholar]
- 5. Nehrer S, Domayer S, Dorotka R, Schatz K, Bindreiter U, Kotz R. Three-year clinical outcome after chondrocyte transplantation using a hyaluronan matrix for cartilage repair. Eur J Radiol. 2006;57(1):3-8. doi: 10.1016/j.ejrad.2005.08.005 [DOI] [PubMed] [Google Scholar]
- 6. Kon E, Filardo G, Roffi A, Andriolo L, Marcacci M. New trends for knee cartilage regeneration: from cell-free scaffolds to mesenchymal stem cells. Curr Rev Musculoskelet Med. 2012;5(3):236-43. doi: 10.1007/s12178-012-9135-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kon E, Delcogliano M, Filardo G, Altadonna G, Marcacci M. Novel nano-composite multi-layered biomaterial for the treatment of multifocal degenerative cartilage lesions. Knee Surg Sports Traumatol Arthrosc. 2009;17(11):1312-5. doi: 10.1007/s00167-009-0819-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kon E, Muttini A, Arcangeli E, Delcogliano M, Filardo G, Nicoli Aldini N, et al. Novel nanostructured scaffold for osteochondral regeneration: pilot study in horses. J Tissue Eng Regen Med. 2010;4(4):300-8. doi: 10.1002/term.243 [DOI] [PubMed] [Google Scholar]
- 9. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kon E, Verdonk P, Condello V, Delcogliano M, Dhollander A, Filardo G, et al. Matrix-assisted autologous chondrocyte transplantation for the repair of cartilage defects of the knee: systematic clinical data review and study quality analysis. Am J Sports Med. 2009;37(Suppl 1):156S-166S. doi: 10.1177/0363546509351649 [DOI] [PubMed] [Google Scholar]
- 11. Neyeloff JL, Fuchs SC, Moreira LB. Meta-analyses and forest plots using a Microsoft Excel Spreadsheet: step-by-step guide focusing on descriptive data analysis. BMC Res Notes. 2012;5:52. doi: 10.1186/1756-0500-5-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. Hoboken, NJ: Wiley; 2009. [Google Scholar]
- 13. Schüttler KF, Götschenberg A, Klasan A, Stein T, Pehl A, Roessler PP, et al. Cell-free cartilage repair in large defects of the knee: increased failure rate 5 years after implantation of a collagen type I scaffold. Arch Orthop Trauma Surg. 2019;139(1):99-106. doi: 10.1007/s00402-018-3028-4 [DOI] [PubMed] [Google Scholar]
- 14. Sofu H, Camurcu Y, Ucpunar H, Ozcan S, Yurten H, Sahin V. Clinical and radiographic outcomes of chitosan-glycerol phosphate/blood implant are similar with hyaluronic acid-based cell-free scaffold in the treatment of focal osteochondral lesions of the knee joint. Knee Surg Sports Traumatol Arthrosc. 2019;27:773-81. doi: 10.1007/s00167-018-5079-z [DOI] [PubMed] [Google Scholar]
- 15. Bertho P, Pauvert A, Pouderoux T, Robert H; Orthopaedics and Traumatology Society of Western France (SOO). Treatment of large deep osteochondritis lesions of the knee by autologous matrix-induced chondrogenesis (AMIC): preliminary results in 13 patients. Orthop Traumatol Surg Res. 2018;104(5):695-700. doi: 10.1016/j.otsr.2018.05.008 [DOI] [PubMed] [Google Scholar]
- 16. Lahner M, Ull C, Hagen M, von Schulze Pellengahr C, Daniilidis K, von Engelhardt LV, et al. Cartilage surgery in overweight patients: clinical and mri results after the autologous matrix-induced chondrogenesis procedure. Biomed Res Int. 2018;2018:6363245. doi: 10.1155/2018/6363245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoburg A, Leitsch JM, Diederichs G, Lehnigk R, Perka C, Becker R, et al. Treatment of osteochondral defects with a combination of bone grafting and AMIC technique. Arch Orthop Trauma Surg. 2018;138:1117-26. doi: 10.1007/s00402-018-2944-7 [DOI] [PubMed] [Google Scholar]
- 18. Schagemann J, Behrens P, Paech A, Riepenhof H, Kienast B, Mittelstadt H, et al. Mid-term outcome of arthroscopic AMIC for the treatment of articular cartilage defects in the knee joint is equivalent to mini-open procedures. Arch Orthop Trauma Surg. 2018;138(6):819-25. doi: 10.1007/s00402-018-2887-z [DOI] [PubMed] [Google Scholar]
- 19. Panni AA, Del Regno C, Mazzitelli G, D’Apolito R, Corona K, Vasso M. Good clinical results with autologous matrix-induced chondrogenesis (AMIC) technique in large knee chondral defects. Knee Surg Sports Traumatol Arthrosc. 2018;26(4):1130-6. doi: 10.1007/s00167-017-4503-0 [DOI] [PubMed] [Google Scholar]
- 20. Sadlik B, Puszkarz M, Kosmalska L, Wiewiorski M. All-arthroscopic autologous matrix-induced chondrogenesis-aided repair of a patellar cartilage defect using dry arthroscopy and a retraction system. J Knee Surg. 2017;30(9):925-9. doi: 10.1055/s-0037-1599246 [DOI] [PubMed] [Google Scholar]
- 21. Volz M, Schaumburger J, Frick H, Grifka J, Anders S. A randomized controlled trial demonstrating sustained benefit of autologous matrix-induced chondrogenesis over microfracture at five years. Int Orthop. 2017;41(4):797-804. doi: 10.1007/s00264-016-3391-0 [DOI] [PubMed] [Google Scholar]
- 22. Sofu H, Kockara N, Oner A, Camurcu Y, Issin A, Sahin V. Results of hyaluronic acid-based cell-free scaffold application in combination with microfracture for the treatment of osteochondral lesions of the knee: 2-year comparative study. Arthroscopy. 2017;33(1):209-16. doi: 10.1016/j.arthro.2016.06.035 [DOI] [PubMed] [Google Scholar]
- 23. Roessler PP, Pfister B, Gesslein M, Figiel J, Heyse TJ, Colcuc C, et al. Short-term follow up after implantation of a cell-free collagen type I matrix for the treatment of large cartilage defects of the knee. Int Orthop. 2015;39(12):2473-79. doi: 10.1007/s00264-015-2695-9 [DOI] [PubMed] [Google Scholar]
- 24. Dhollander A, Moens K, Van der Maas J, Verdonk P, Almqvist KF, Victor J. Treatment of patellofemoral cartilage defects in the knee by autologous matrix-induced chondrogenesis (AMIC). Acta Orthop Belg. 2014;80(2):251-9. [PubMed] [Google Scholar]
- 25. Shetty AA, Kim SJ, Bilagi P, Stelzeneder D. Autologous collagen-induced chondrogenesis: single-stage arthroscopic cartilage repair technique. Orthopedics. 2013;36(5):e648-e652. doi: 10.3928/01477447-20130426-30 [DOI] [PubMed] [Google Scholar]
- 26. Shetty AA, Kim SJ, Shetty V, Jang JD, Huh SW, Lee DH. Autologous collagen induced chondrogenesis (ACIC: Shetty-Kim Technique)—a matrix based acellular single stage arthroscopic cartilage repair technique. J Clin Orthop Trauma. 2016;7(3):164-9. doi: 10.1016/j.jcot.2016.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gille J, Behrens P, Volpi P, de Girolamo L, Reiss E, Zoch W, et al. Outcome of autologous matrix induced chondrogenesis (AMIC) in cartilage knee surgery: data of the AMIC Registry. Arch Orthop Trauma Surg. 2013;133(1):87-93. doi: 10.1007/s00402-012-1621-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schüttler KF, Schenker H, Theisen C, Schofer MD, Getgood A, Roessler PP, et al. Use of cell-free collagen type I matrix implants for the treatment of small cartilage defects in the knee: clinical and magnetic resonance imaging evaluation. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1270-6. doi: 10.1007/s00167-013-2747-x [DOI] [PubMed] [Google Scholar]
- 29. Anders S, Volz M, Frick H, Gellissen J. A randomized, controlled trial comparing autologous matrix-induced chondrogenesis (AMIC®) to microfracture: analysis of 1- and 2-year follow-up data of 2 centers. Open Orthop J. 2013;7:133-43. doi: 10.2174/1874325001307010133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Efe T, Theisen C, Fuchs-Winkelmann S, Stein T, Getgood A, Rominger MB, et al. Cell-free collagen type I matrix for repair of cartilage defects-clinical and magnetic resonance imaging results. Knee Surg Sports Traumatol Arthrosc. 2012;20(10):1915-22. doi: 10.1007/s00167-011-1777-5 [DOI] [PubMed] [Google Scholar]
- 31. Kusano T, Jakob RP, Gautier E, Magnussen RA, Hoogewoud H, Jacobi M. Treatment of isolated chondral and osteochondral defects in the knee by autologous matrix-induced chondrogenesis (AMIC). Knee Surg Sports Traumatol Arthrosc. 2012;20(10):2109-15. doi: 10.1007/s00167-011-1840-2 [DOI] [PubMed] [Google Scholar]
- 32. Schiavone Panni A, Cerciello S, Vasso M. The manangement of knee cartilage defects with modified AMIC technique: preliminary results. Int J Immunopathol Pharmacol. 2011;24(1 Suppl 2):149-52. [DOI] [PubMed] [Google Scholar]
- 33. Benthien JP, Behrens P. Autologous matrix-induced chondrogenesis (AMIC). A one-step procedure for retropatellar articular resurfacing. Acta Orthop Belg. 2010;76(2):260-3. [PubMed] [Google Scholar]
- 34. Gille J, Schuseil E, Wimmer J, Gellissen J, Schulz AP, Behrens P. Mid-term results of autologous matrix-induced chondrogenesis for treatment of focal cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1456-64. doi: 10.1007/s00167-010-1042-3 [DOI] [PubMed] [Google Scholar]
- 35. Pascarella A, Ciatti R, Pascarella F, Latte C, Di Salvatore MG, Liguori L, et al. Treatment of articular cartilage lesions of the knee joint using a modified AMIC technique. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):509-13. doi: 10.1007/s00167-009-1007-6 [DOI] [PubMed] [Google Scholar]
- 36. Filardo G, Andriolo L, Sessa A, Vannini F, Ferruzzi A, Marcacci M, et al. Age is not a contraindication for cartilage surgery: a critical analysis of standardized outcomes at long-term follow-up. Am J Sports Med. 2017;45(8):1822-8. doi: 10.1177/0363546517695088 [DOI] [PubMed] [Google Scholar]
- 37. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053-63. doi: 10.1177/0363546508328414 [DOI] [PubMed] [Google Scholar]
- 38. Zaffagnini S, Vannini F, Di Martino A, Andriolo L, Sessa A, Perdisa F, et al. Low rate of return to pre-injury sport level in athletes after cartilage surgery: a 10-year follow-up study. Knee Surg Sports Traumatol Arthrosc. Epub 2018 Oct 29. doi: 10.1007/s00167-018-5255-1 [DOI] [PubMed] [Google Scholar]
- 39. Kon E, Filardo G, Gobbi A, Berruto M, Andriolo L, Ferrua P, et al. Long-term results after hyaluronan-based mact for the treatment of cartilage lesions of the patellofemoral joint. Am J Sports Med. 2016;44(3):602-8. doi: 10.1177/0363546515620194 [DOI] [PubMed] [Google Scholar]
- 40. Kreuz PC, Kalkreuth RH, Niemeyer P, Uhl M, Erggelet C. Long-term clinical and MRI results of matrix-assisted autologous chondrocyte implantation for articular cartilage defects of the knee. Cartilage. Epub 2018 Feb 1. doi: 10.1177/1947603518756463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schuette HB, Kraeutler MJ, McCarty EC. Matrix-assisted autologous chondrocyte transplantation in the knee: a systematic review of mid- to long-term clinical outcomes. Orthop J Sports Med. 2017;5(6):2325967117709250. doi: 10.1177/2325967117709250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Beck JJ, Sugimoto D, Micheli L. Sustained results in long-term follow-up of autologous chondrocyte implantation (ACI) for distal femur juvenile osteochondritis dissecans (JOCD). Adv Orthop. 2018;2018:7912975. doi: 10.1155/2018/7912975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McCarthy HS, McCall IW, Williams JM, Mennan C, Dugard MN, Richardson JB, et al. Magnetic resonance imaging parameters at 1 year correlate with clinical outcomes up to 17 years after autologous chondrocyte implantation. Orthop J Sports Med. 2018;6(8):2325967118788280. doi: 10.1177/2325967118788280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Knutsen G, Drogset JO, Engebretsen L, Grontvedt T, Ludvigsen TC, Loken S, et al. A randomized multicenter trial comparing autologous chondrocyte implantation with microfracture: long-term follow-up at 14 to 15 years. J Bone Joint Surg Am. 2016;98(16):1332-9. doi: 10.2106/JBJS.15.01208 [DOI] [PubMed] [Google Scholar]
- 45. Kraeutler MJ, Belk JW, Purcell JM, McCarty EC. Microfracture versus autologous chondrocyte implantation for articular cartilage lesions in the knee: a systematic review of 5-year outcomes. Am J Sports Med. 2018;46(4):995-9. doi: 10.1177/0363546517701912 [DOI] [PubMed] [Google Scholar]
- 46. Ossendorff R, Franke K, Erdle B, Uhl M, Sudkamp NP, Salzmann GM. Clinical and radiographical ten years long-term outcome of microfracture vs. autologous chondrocyte implantation: a matched-pair analysis. Int Orthop. 2019;43:553-9. doi: 10.1007/s00264-018-4025-5 [DOI] [PubMed] [Google Scholar]
- 47. Ulstein S, Aroen A, Engebretsen L, Forssblad M, Lygre SHL, Rotterud JH. A controlled comparison of microfracture, debridement, and no treatment of concomitant full-thickness cartilage lesions in anterior cruciate ligament-reconstructed knees: a nationwide prospective cohort study from Norway and Sweden of 368 patients with 5-year follow-up. Orthop J Sports Med. 2018;6(8):2325967118787767. doi: 10.1177/2325967118787767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Solheim E, Hegna J, Inderhaug E. Long-term survival after microfracture and mosaicplasty for knee articular cartilage repair: a comparative study between two treatments cohorts. Cartilage. Epub 2018 Jun 1. doi: 10.1177/1947603518783482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Solheim E, Hegna J, Strand T, Harlem T, Inderhaug E. Randomized study of long-term (15-17 years) outcome after microfracture versus mosaicplasty in knee articular cartilage defects. Am J Sports Med. 2018;46(4):826-31. doi: 10.1177/0363546517745281 [DOI] [PubMed] [Google Scholar]
- 50. Solheim E, Hegna J, Inderhaug E. Long-term clinical follow-up of microfracture versus mosaicplasty in articular cartilage defects of medial femoral condyle. Knee. 2017;24(6):1402-7. doi: 10.1016/j.knee.2017.08.061 [DOI] [PubMed] [Google Scholar]
- 51. Gobbi A, Karnatzikos G, Kumar A. Long-term results after microfracture treatment for full-thickness knee chondral lesions in athletes. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):1986-96. doi: 10.1007/s00167-013-2676-8 [DOI] [PubMed] [Google Scholar]
- 52. Von Keudell A, Atzwanger J, Forstner R, Resch H, Hoffelner T, Mayer M. Radiological evaluation of cartilage after microfracture treatment: a long-term follow-up study. Eur J Radiol. 2012;81(7):1618-24. doi: 10.1016/j.ejrad.2011.04.071 [DOI] [PubMed] [Google Scholar]
- 53. Jakob RP. AMIC technique for cartilage repair, a single-step surgical intervention as compared to other methods. Eur Cell Mater. 2006;12(Suppl 1):26.16941384 [Google Scholar]
- 54. Gao L, Orth P, Cucchiarini M, Madry H. Autologous matrix-induced chondrogenesis: a systematic review of the clinical evidence. Am J Sports Med. 2019;47:222-31. doi: 10.1177/0363546517740575 [DOI] [PubMed] [Google Scholar]