Abstract
Objectives: An expert panel reviewed and summarized the literature related to the evidence for the 4Ms—what matters, medication, mentation, and mobility—in supporting care for older adults. Methods: In 2017, geriatric experts and health system executives collaborated with the Institute for Healthcare Improvement (IHI) to develop the 4Ms framework. Through a strategic search of the IHI database and recent literature, evidence was compiled in support of the framework’s positive clinical outcomes. Results: Asking what matters from the outset of care planning improved both psychological and physiological health statuses. Using screening protocols such as the Beers’ criteria inhibited overprescribing. Mentation strategies aided in prevention and treatment. Fall risk and physical function assessment with early goals and safe environments allowed for safe mobility. Discussion: Through a framework that reduces cognitive load of providers and improves the reliability of evidence-based care for older adults, all clinicians and healthcare workers can engage in age-friendly care.
Keywords: goal-directed care, quality, safety
Background
Remarkable improvements in health and health care have led to a longevity “bonus” in this country and around the world. Currently, there are more than 46 million older adults aged 65 years and older in the United States, and by 2050, that number is expected to grow to almost 90 million. By 2030, one in five Americans is projected to be in this demographic (Ortman et al., 2014).
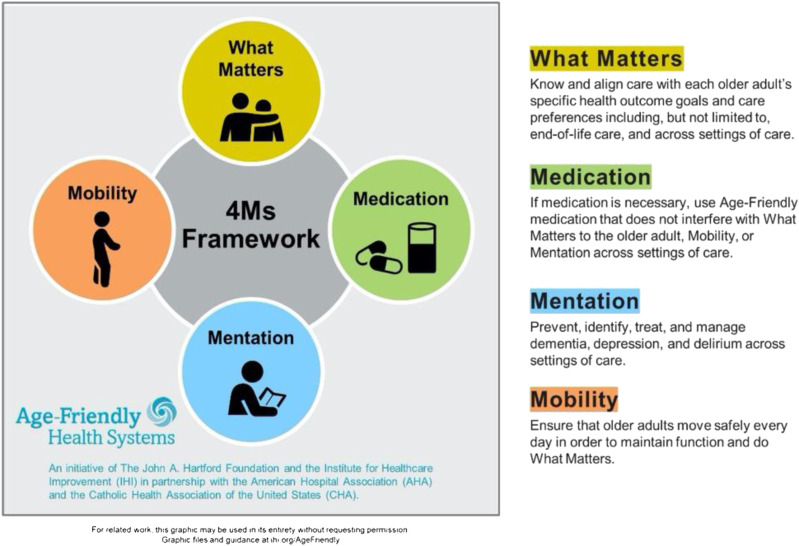
The current system of care in the United States is fragmented and plagued by discontinuity and alarming rates of error. We are not prepared to provide reliable continuity of care for older adults. And we need a sense of urgency to reimagine our care with this population (Tinetti et al., 2016). In 2017, the John A. Hartford Foundation and the Institute for Healthcare Improvement (IHI) partnered with the American Hospital Association and the Catholic Health Association of the United States to develop a social movement for Age-Friendly Health Systems (AFHSs) (Mate et al., 2018). Age-friendly health systems utilize a person-centered approach to maintain the health of older adults based on evidence-based care that improves health outcomes and prevents avoidable harm. Age-friendly health systems employ a framework called the 4Ms (what matters, medication, mentation, and mobility) to ensure reliable, evidence-based care. When we refer to an AFHS, we are describing every point of care that older adults access ranging from emergency rooms to nursing homes to convenient care clinics. The 4Ms have equal importance in any care domain. We continue to test the 4Ms set with providers in all settings.
Focusing on what matters means to prioritize the older adult’s specific health outcome goals and care preferences across all settings of care. The second of the 4Ms, medication, means using age-friendly medication or dose adjusting if medication is needed, and avoiding or deprescribing high-risk medications that may interfere with what matters, mentation, or mobility. The third of the 4Ms, mentation, also referred to as mood and memory, focuses on the prevention, identification, treatment, and management of dementia, depression, and delirium. The final of the 4Ms, mobility, ensures the appropriate approach to assisting or encouraging older adults to move safely every day in order to maintain functional ability and do what matters. The simultaneous integration of all aspects of this framework into care in every setting improves health outcomes for older adults, reduces waste from low-quality services, and increases the utilization of cost-effective services (Mate et al., 2018).
The aim of this study is to document the evidence base and rationale for assessing and addressing the 4Ms across the care continuum. We provide evidence for why we have selected the screening tools and related actions, used in AFHSs. There are some site-specific examples, but the evidence for the AFHS model and the 4Ms applies across all settings. We will also discuss the impact of the interaction of the 4Ms.
Methods
The 4Ms framework was developed by a team led by the IHI in August 2016, which included experts in aging and geriatrics along with health system leaders (Mate et al., 2018). Using a consensus panel approach, the experts were queried regarding the most salient and essential care elements using email as a platform for their replies in order to determine what the strongest evidence-based care models were in the care of older adults. Each of the seventeen care models with level 1 or 2a evidence of impact was then examined to determine the essential elements of care (Table 1). Across the models, over 90 elements were identified, and the expert group was then asked to review them and distill them down to a critical few, what we now call the 4Ms (Figure 1). It should be noted that we recognize the 4Ms exist within a broader age-friendly ecosystem, and progress is being made on that larger context (Fulmer et al., 2020).
Table 1.
Seventeen Care Models with Level 1 or 2a Evidence of Impact.
| 1. ACE Unit |
| 2. CM+ |
| 3. Care Transitions Program |
| 4. Center to Advance Palliative Care |
| 5. Geriatric Emergency Department |
| 6. Geriatric Interdisciplinary Team Training |
| 7. GRACE |
| 8. Guided Care |
| 9. HomeMeds |
| 10. Hospital at Home and Mount Sinai’s MACT |
| 11. HELP |
| 12. IMPACT |
| 13. NICHE |
| 14. Patient Priority Care |
| 15. PACE |
| 16. TCM |
| 17. University of California at Los Angeles Alzheimer’s and Dementia Care Program |
Note. ACE = Acute Care for Elders; CM+ = Care Management Plus; GRACE = Geriatric Resources for Assessment and Care of Elders; MACT = Mobile Acute Care Team; HELP = Hospital Elder Life Program; IMPACT = Improving Mood–Promoting Access to Collaborative Treatment; NICHE = Nurses Improving Care for Health System Elders; PACE = Program for All-Inclusive Care of the Elderly; TCM = Transitional Care Model.
Figure 1.
4Ms interaction. Source. Reproduced with permission from the Institute of Healthcare Improvement. (Institute for Healthcare Improvement, 2020).
Results
What Matters
Age-friendly health systems are centered on person-centered care, and they seek to understand and act on “what matters” to older adults. The essential starting place is an understanding of the context of the person’s life and awareness of each older adult’s personalized health preferences and goals. Identifying these priorities is especially important to older adults with multiple chronic conditions who may otherwise receive fragmented or even unwanted care (Chevarley, 2017). Annually, providers in AFHSs take the time to directly talk with the older adults in their care about what matters most to them. More frequent conversations need to occur when there are changes in health status—which may be daily, or even hourly. The intent is for these conversations to optimize care planning that respects the older adult’s preferences.
The first step is to establish the core values of the older adult. These values are the fundamentals in which a person’s beliefs are rooted, including ideas about happiness and fulfillment (Tinetti et al., 2016). Next steps involve dialog with a clinician who can then take those values and incorporate them into the treatment plan, such that the health priorities of the older adult are respected (Naik et al., 2018). Asking what matters from a clinician’s perspective can be especially difficult as the framing of the question is critical. Given the complexity of such a question, there is the potential for oversimplification or erroneous interpretation from the older adult (Olsen et al., 2020). But when the conversation is navigated successfully, the impacts can be resounding. People have differing ideas about what they consider important to them and about their treatment (Wiering et al., 2017). And when compared to the usual standard of care, studies have shown that collaborative goal setting between providers and older adults can directly result in improvements in health outcomes, both physical and psychological (Coulter et al., 2015). Given the variability of workforce resources and skill sets across care settings, we intentionally did not make recommendations about which member of the clinical team should complete the values assessment.
A tool kit was developed to give practical guidance to health systems on eliciting and acting on what matters to older adults. The resource, What Matters to Older Adults: A Toolkit for Health Systems to Design Better Care with Older Adults, was created as a resource for the AFHS movement and is available for free download on the IHI website (Institute for Healthcare Improvement, 2019). The tool kit includes guidance on how to prepare an older adult for a what matters conversation, how to understand the context of an older adults life that might impact the design of the what matters conversation, how to conduct and document and act on a what matters conversation, and case studies and sample conversations.
When personalized care is more thoroughly integrated into every aspect of care and everyday practice, the effects on health status indicators can be significant (Whitehead, 2016). This type of care is especially important for those with multiple chronic conditions, for whom care is often fragmented and not centered on what matters to them (Blaum et al., 2018). Current guideline-based clinical decision-making may overrule personal preferences, resulting in treatment that is not holistic (Tinetti et al., 2016). Given the various complex implications of multiple comorbidities, integrated treatment that follows what matters allows the older adults and family to weigh in on the potential benefits and harms of various treatments to find out what is right for them (Boyd et al.,2019). Priority-aligned, person-centered care using what matters right at the start promotes age-friendly care that results in a better physiological and psychological health status for older adults, from improved hypertension and diabetes management to overall better treatment adherence (Berlowitz et al., 2017; Naik & McCullough, 2014; Naik et al., 2011). The SMART criteria—specific, measurable, actionable, realistic, and timely—have been shown to be a strong framework to establish goals that reflect what matters to the older adults (Tinetti et al., 2016). In circumstances where the older adult is unable to state preferences, the healthcare proxy is vital to the plan.
To “act on” what matters, care providers need to align the care plan with what matters to the older adult. They also need to incorporate what matters into the goal-oriented care plan and align the care plan with the older adult’s goals and preferences (Mate et al., 2018).
Medication
The avoidance of high-risk medication and when medication is necessary, establishing a plan for it to be safely dose adjusted or deprescribed are key actions of the 4Ms framework. When medication is necessary, the aim in any care setting is to ensure that what matters, mentation, and mobility are not negatively influenced by medication. Regular screening for the seven following drug categories known to harm older adults is evidence-based best practice: benzodiazepines, opioids, anticholinergic medications, all prescription and over-the-counter sedatives and sleep medications, muscle relaxants, tricyclic antidepressants, and antipsychotics (Motter et al., 2018).
The overprescribing of medication to older adults is a common phenomenon, and it comes with dire consequences. As many as 50% of older adults are overprescribed medication that is not medically necessary, with those in nursing homes being particularly susceptible and vulnerable (Maher et al., 2014). The condition of therapeutic duplication or medical inefficacy through the use of five or more drugs—also known as polypharmacy—has been shown in studies to be correlated with negative clinical outcomes (Guaraldo et al., 2011). Polypharmacy has serious implications for the health of older adults, including increased risk of adverse drug events (ADEs), drug-drug interactions, medication nonadherence, reduced functional capacity, multiple geriatric syndromes, and higher costs (Lau et al., 2010). For example, studies showed an 88% increased risk of experiencing an ADE for outpatients taking five or more medications, compared to those who were taking fewer medications (Dormann et al., 2013). Almost 10% of emergency department visits are due to ADEs, with an estimated 4.3 million healthcare visits in 2005 attributed to ADEs (Dedhiya et al., 2010). Another consideration of using multiple medications is the additive effect of the different drugs, with interactions that can prove deleterious. For those taking between five and nine medications, the risk of a drug-drug interaction is 50%. If that number rises to more than 20 medications, the risk doubles to 100% (Storms et al., 2017). Perhaps the most consequential effect of these issues is that they can lead to decreased physical functioning and capability to perform daily activities, which are an essential consideration of what matters (Lyu et al., 2017). However, any of the aforementioned outcomes can dramatically affect the other components of the 4Ms framework. Proper deprescribing for older adults helps promote age-friendly care across care settings, especially at home.
Important screening protocols for evaluating age-friendly medication include the Beers Criteria for Potentially Inappropriate Medication Use in Older Adults, first established by the American Geriatrics Society (AGS) in 2002, and the screening tool of older person’s potentially inappropriate prescriptions (STOPP) and screening tool of alert doctors to the right treatment (START) criteria (Grace et al., 2014). The Beers Criteria include lists of medications that are potentially harmful to older adults and should be avoided (Campanelli, 2012). Through careful study, it has been shown that drugs listed in the Beers Criteria have directly led to adverse health outcomes, from falls and delirium to, in severe cases, mortality (Kanaan et al., 2013). A more recent iteration of the Beers Criteria in 2015 also takes a deeper look into medications that might have negative effects on kidney function as well as drug–drug interactions that may prove harmful in older adults (Fick, Semla et al., 2015). Given its prevalence as one of the most frequently consulted sources in geriatric clinical care, the AGS Beers Criteria is an important resource for the evaluation of age-friendly medication (Levy et al., 2010). The STOPP/START criteria have also been utilized as a powerful tool for preventing inappropriate prescribing. The criteria are especially more prevalent in Europe, with greater sensitivity than the 2002 Beers Criteria for evaluating ADEs (Hill-Taylor et al., 2013). A great deal of literature synthesis has gone into establishing the lists published in the different criteria, validating their importance as tools for the assessment of age-friendly medication.
Studies document that it is essential to ensure that older adults and their family caregivers are aware of and avoid high-risk medications; medications, when clinically appropriate, are safely deprescribed through dose reduction and medication discontinuation, or other pathways and procedures that significantly act on the assessment of age-friendly medication (Elshaug et al., 2017; Gallagher et al., 2011; Kolanowski et al., 2010).
Specifically, providers should avoid, safely deprescribe, or dose adjust the following high-risk medications: benzodiazepines, opioids, high-anticholinergic medications (e.g., diphenhydramine), all prescription and over-the-counter sedatives and sleep medications, muscle relaxants, tricyclic antidepressants, and antipsychotics (Campanelli, 2012). If the older adult takes one or more of these medications, discuss any concerns the person or family may have, assess for adverse effects, and discuss deprescribing with the older adult (Kanaan et al., 2013). Also, assess the economic impact of the cost of the medications.
Mentation
Mentation refers to the health of the mind. An AFHS prevents, identifies, treats, and manages dementia and depression in community-based settings and prevents, identifies, treats, and manages delirium in the hospital setting (Institute for Healthcare Improvement, 2020).
Mentation: Dementia
There are modifiable risk factors associated with cognitive impairment that are the basis for prevention. Older adults who experience depression are at twice the risk for developing cognitive impairment (Diniz et al., 2013). There is an indication that addressing the depression through behavior modifications, such as mobility, and/or medications, may reduce risk of developing cognitive impairment (Maes et al., 2011). Increased physical activity is also a modifiable risk factor for cognitive impairment (Kivipelto et al., 2018). These demonstrate the interconnectedness of mobility and mentation.
An AFHS screens older adults at least one time per year for cognitive impairment and, if screen is positive, refers for further evaluation and manages manifestations of cognitive impairment (Institute for Healthcare Improvement, 2020). An AFHS can select which evidence-based tool to utilize including but not limited to: Mini-Cog, Saint Louis University Mental Status (SLUMS), and Montreal Cognitive Assessment (MoCA). The Mini-Cog is a three-minute test consisting of two components: a three-item recall test and a clock drawing (Borson et al., 2000). The SLUMS is an 11-item, 7-minute exam consisting of animal naming, clock drawing, figure differentiation, numeric calculation, and assessment of recall ability (Morley & Tumosa, 2002). The MoCA is a 30-question, 10- to 15-minute test that measures visuospatial and executive functions, as well as various cognitive domains, such as naming, memory, attention, language, abstraction, delayed recall, and orientation. Finally, the Mini-Mental Status Exam (MMSE) tests seven domains—orientation, registration, attention, calculation, recall, language, and visual construction—through a 30-item questionnaire (Folstein et al., 1983).
While the MoCA and the MMSE are both accurate tools for detecting dementia and mild cognitive impairment (MCI), the MoCA (0.63) has a higher Youden index than the MMSE (0.55) for differentiating individuals with MCI from healthy individuals (Roalf et al., 2013). However, there is a lack of consensus regarding the ability of the MoCA to detect dementia as compared with the MMSE among culturally diverse populations. There are different optimal MoCA cutoffs among different racial/ethnic minorities, particularly non-Hispanic Blacks, such that the cutoff for minority groups should be below the widely used cutoff of 26 (Milani et al., 2018). A study on a Chinese population showed the MoCA’s greater specificity in assessing MCI. However, the MMSE had a higher sensitivity, specificity, and predictive values for dementia (Tsai et al., 2016). For a Taiwanese population, the results were reversed; the MoCA had a greater ability to detect dementia as compared with the MMSE. When adjusting for education and age, the MoCA continues to be the recommended tool (Hsu et al., 2015). The effect of education and age on the MoCA score also holds within a Chinese–American population for both Mandarin and Cantonese speakers, such that the score increases with higher education and decreases with advancing age (Hsu et al., 2015; Zheng et al., 2012). The MoCA was also shown to be effective for diagnosing MCI and mild dementia in a Spanish population and showed significantly greater discriminant validity than the MMSE at differentiating MCI from dementia (Delgado et al., 2019). Among a Brazilian population, the MoCA is a valid and reliable tool for the screening of MCI among older adults with at least four years of education (Memória et al., 2013). Although there are inconsistencies in the research regarding the efficiency of the MoCA in comparison with the MMSE in detecting dementia, the research nonetheless supports that the MoCA is a useful screening tool for dementia among diverse populations. Research also suggests that the SLUMS test is an effective screening tool for detecting MCI and dementia among culturally and linguistically diverse populations (Kaya et al., 2016; Szcześniak & Rymaszewska, 2016). Compared to the MMSE, the SLUMS test has a higher sensitivity in detecting MCI and dementia within a Polish population. The SLUMS test also has high discriminatory power in differentiating MCI from dementia and thus is an effective alternative to the MMSE (Szcześniak & Rymaszewska, 2016). Although the SLUMS test and the MMSE are equally efficient at detecting dementia in a Turkish population, the SLUMS test is more effective at detecting MCI. This advantage is due to the difference in activities between the two exams: the SLUMS test more extensively evaluates cognitive function through clock drawing, animal naming, and recall from a paragraph (Kaya et al., 2016).The research supports that the Mini-Cog, MoCA, and SLUMS test are effective screening tools for early detection of dementia. There is a fee associated with the MMSE and MoCA, whereas the Mini-Cog and SLUMS test are freely available.
To meaningfully engage older adults and families with screening and assessment results, it is important to review the results and provide educational materials. Studies have shown that despite the ease of accessing information in the digital age, clinicians and caregivers are often uninformed in regard to dementia identification and care, and they are receptive to instruction in the area, whether it is from a physician or other referral resources (Peterson et al., 2016). Proper education of family or professional caregivers on different rehabilitation strategies can result in positive impacts for older adults with dementia as they are offered memory and cognitive support (Smith et al., 2011).
Addressing cognitive impairment includes managing symptoms, monitoring progression, and making medication decisions in light of the assessment findings (Petersen et al., 2018). Another useful step is to refer the older adult, family, and/or other caregivers to supportive resources, such as the Alzheimer’s Association (Alzheimer’s Association, 2020).
Mentation: Depression
An AFHS prevents, identifies, treats, and manages depression (Institute for Healthcare Improvement, 2020). Mobility and physical activity are linked to prevention of depression, thus further demonstrating the interconnectedness of mentation and mobility (Marques et al., 2020).
Screening for depression is recommended annually or when symptoms arise. Evidence supports using one of the following four screening tools: Patient Health Questionnaire-2 (PHQ-2), Patient Health Questionnaire-9 (PHQ-9), Geriatric Depression Scale (GDS), and Geriatric Depression Scale - short form (GDS - 15).
The PHQ-9 and PHQ-2 are both effective depression screening tools (Dadfar & Lester, 2017; Randall et al., 2013; Suzuki et al., 2015). Both have high validity and reliability for culturally and linguistically diverse populations such as older adults from China, Iran, Japan, and Taiwan (Chen et al., 2016; Dadfar & Lester, 2017; Liu et al., 2016; Suzuki et al., 2015). The PHQ-9 and PHQ-2 have lower specificities in older adults with cognitive impairment (Boyle et al., 2011). However, research validating their effectiveness in minority groups in the United States such as non-Hispanic Black and Hispanic populations is largely lacking. Although cognitive function can affect the GDS-15 score, it is still an appropriate tool for older adults with different levels of cognitive function (Conradsson et al., 2013; Shin et al., 2019). It is important to note that the GDS-15 has a higher validity among older adults with less cognitive impairment (Conradsson et al., 2013). Nevertheless, the GDS and GDS-15 have proven to be effective in assessing depression and are appropriate for culturally diverse populations and older adults with different levels of education (Dias et al., 2017; Durmaz et al., 2018). The impact that cognitive function may have on the validity of these tools emphasizes the importance of testing for dementia as well as depression to develop a comprehensive understanding of an older adult’s mentation.
To act on a positive depression screen, identify and manage factors that contribute to depressive symptoms, including sensory limitations (vision and hearing), social isolation, losses associated with aging (job, income, and societal roles), loneliness, and bereavement. Consider the need for counseling and/or pharmacological treatment of depression or refer to a mental health provider if and when appropriate (Institute for Healthcare Improvement, 2020). Psychotherapy and physical activity (mobility) may be effective treatments, as well as medication, when risks of polypharmacy and other negative impacts are carefully considered (Kok & Reynolds, 2017). Prevention and treatment of depression demonstrate the intersectionality of medication, mentation, and mobility.
Mentation: Delirium
An AFHS prevents, identifies, treats, and manages delirium including screening at least every 12 hours, and more often as indicated, when an older adult is in the hospital (Institute for Healthcare Improvement, 2020). The prevention, rapid identification, and management of delirium are critical aspects of supporting the health of the mind (Fick, 2016). Older adults with dementia are at increased risk for delirium and worse outcomes and can pose unique screening challenges (Morandi et al., 2017).
An AFHS can select which evidence-based screening tool to utilize including but not limited to: Confusion Assessment Method (CAM), 3-Minute Diagnostic Assessment for Delirium using the CAM algorithm (3D-CAM), CAM for the Intensive Care Unit (CAM-ICU), Brief CAM (bCAM), Ultrabrief Two-Item Bedside Test for Delirium (UB-2), and Nursing Delirium Symptom Checklist (NuDESC).
The CAM is a 5-minute diagnostic tool that consists of nine operationalized criteria from the Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition: acute onset and fluctuating course, inattention, disorganized thinking, altered level of consciousness, disorientation, memory impairment, perceptual disturbances, increased or decreased psychomotor activity, and disturbance of the sleep-wake cycle. The CAM is a useful tool for detecting delirium in older adults with dementia, depression, or normal mental status with a sensitivity of 100% and specificity of 95% (Inouye et al., 1999).
The 3D-CAM is a 20-item tool, derived from the four CAM diagnostic features, that consists of mental status testing, symptom probes, and guided interviewer observations for signs of delirium. It is an effective tool for those with normal baseline cognition or MCI, and it has a sensitivity and specificity of 93% and 96%, respectively. It is also useful for those with dementia, with a sensitivity of 96% and specificity of 86% (Marcantonio, 2017).
The CAM-ICU is a valid and reliable tool for diagnosing delirium in the ICU setting. It has a pooled sensitivity of 80% and specificity of 95.9% in more recent studies (Ely et al., 2001; Gusmao-Flores et al., 2012). Additionally, utilization of the CAM-ICU results in a diagnosis rate of delirium in the emergency department that is three times higher than before implementation of the screening tool (Van de Meeberg et al., 2017).
The bCAM assesses three aspects of mentation: altered mental status or fluctuating course, inattention, and altered level of consciousness. It acts as an effective confirmatory delirium assessment and can be reliably performed by a range of healthcare professionals from different clinical backgrounds and experiences. When implemented in the emergency department, its sensitivity and specificity for physicians are 84% and 95.8%, respectively. Research also suggests that it has high utility in the inhospital setting with a sensitivity and specificity of 81.6% and 95%, respectively (Han et al., 2013).
The UB-2 was developed for situations that require a very brief delirium screening that takes less than one minute. It offers different combinations of items for different circumstances. The best 2-item screen for delirium is the combination of activities that require the older adult to state the “months of the year backwards” and the “day of the week.” This combination has a high sensitivity (93%) but a lower specificity (64%). For those with dementia, the best 2-item screen is the same as that for the overall population of older adults, but sensitivity increases to 96% while specificity drops to 43%. This suggests high efficacy in older adults with cognitive impairment in whom delirium is often missed (Fick et al., 2015).
The NuDESC has high diagnostic accuracy such that it has the capability to identify delirium in 13% of observations when it was not present as screened by the CAM (Gaudreau et al., 2005).
Research on the efficacy of the CAM-ICU and NuDESC to identify delirium in elderly postoperative patients shows low sensitivity for both assessments. The CAM-ICU has a sensitivity of 28% in postanesthesia care units (PACUs) and a sensitivity of 28% as well in recovery rooms and inpatient wards. The NuDESC has a sensitivity of 32% in PACUs and a sensitivity of 29% in recovery rooms and inpatient wards. However, the specificity is 90% for both assessments in all settings (Neufeld et al., 2013).
The recommended delirium prevention and management protocol calls for five key actions. First and foremost it is imperative to ensure sufficient oral hydration. Dehydration is one of the six main delirium risk factors, along with cognitive impairment, sleep deprivation, immobility, visual impairments, and hearing impairment (Inouye et al., 1999). As visual and hearing impairments are also risk factors contributing to delirium, centers must ensure that older adults have their personal adaptive equipment, such as glasses, hearing aids, dentures, and walkers, to facilitate interaction with the environment (Ghaeli et al., 2018). Providers should incorporate routine intake and documentation of each older adults’ personal adaptive equipment.
Too much stimulus, such as noise in the ICU, can negatively affect quality of sleep and increase delirium. Thus, it is important to prevent sleep interruptions and utilize non-pharmacological interventions to support sleep. One such intervention is the use of earplugs, which has proved effective at delaying the onset of delirium and improving sleep perception (Van Rompaey et al., 2012). As previously mentioned, drugs listed in the Beers Criteria have directly led to adverse health outcomes, from falls and delirium to mortality. It is critical to avoid high-risk medications that can cause delirium. Last but not least, to reduce delirium and increase awareness of the environment, staff should orient older adults to the time, place, and situation on every shift. One strategy is to make sure that the day and date are updated on the whiteboard. Staff, home care workers, and family members can place a clock (make sure it is accurate and has a large face visible to older adults) and calendar in the view of the older adult and make changes in lighting to orient the person to the time of day (Ghaeli et al., 2018).
Another useful tool is an “All About Me” board or poster/card that shows preferences of the older adult, what makes them calm and happy, who is important to them, names of pets, etc. It can also be helpful to make newspapers and other periodicals available in the older adult’s room or home and to invite family or other caregivers to use familiar and orienting items (e.g., family pictures and favorite music). Sensory aides should be well maintained. Pharmacological treatment of delirium is not associated with improved outcomes and may increase harm to the older adult (Oh et al., 2017).
Mobility
Screening for safe mobility is a requirement for ensuring that older adults are receiving age-friendly care. The recommended AFHS minimum requirement is to utilize at least one of the following five screening tools: Get Up and Go, Timed Get Up and Go (TUG), Johns Hopkins Highest Level of Mobility (JH-HLM), Tinetti Performance Oriented Mobility Assessment (POMA), and/or referral to physical therapy (PT). Each of these is an excellent evidenced-based screen, and an AFHS is agnostic on the screen chosen.
There is inconsistent research regarding the effectiveness of the TUG to predict fall risk in older adults. While the TUG time performance correlates to a past history of falls, it is less effective at predicting future falls and thus should not be used in isolation to identify older adults who are at high risk of falls (Barry et al., 2014; Beauchet et al., 2011). The TUG may be limited in its ability to predict falls due to additional risk factors that the test fails to account for, such as female gender, poor vision, fear of falling, lower limb proprioception, lifestyle, intake of neuroleptics, and dementia (Rossat et al., 2010; Stenhagen et al., 2013). However, other studies have suggested that the TUG is an accurate measure for screening fall risk among older adults, although a 12.47-second cutoff is a better predictive value for Brazilian older adults (Alexandre et al., 2012; Sharma et al., 2016). It is important to note that there is a lack of research validating the TUG’s effectiveness among non-Hispanic Black and Hispanic populations. To account for the limitations of the TUG, modified versions of the TUG have been developed to include a dual task such as a cognitive or manual supplemental component. The TUGcognitive requires the older adult to count backward by threes from a randomly chosen number between 20 and 100 while walking (Shumway-Cook et al., 2000). The TUGmanual requires the older adult to carry a glass of water while walking and place the glass back on the table at the end of the walk (Lundin-Olsson et al., 1998). While the TUG and TUGmanual may have limited abilities to predict fall risk, the TUGcognitive is a valid assessment for predicting falls in older adults due to its ability to replicate similarly complex multitask situations in everyday life (Hofheinz & Mibs, 2016). It is also important to note that there is an increase in time to perform TUG-dual tasks among older women and those with lower educational levels (Gomes et al., 2015). There is limited research supporting the JH-HLM’s effectiveness at predicting fall risk among older adults.
Studies have shown that the POMA is a valid and reliable tool for assessing balance ability, fall risk, and physical function of older adults who have had strokes (An et al., 2014; Canbek et al., 2013). The POMA has also been assessed in a Turkish population and proves to be reliable and valid among culturally diverse populations (Yücel et al., 2012).
To act on mobility, all caregivers and clinicians should ensure early, frequent, and safe mobility (Klein et al., 2015; Larson, 2017; Wong et al., 2011). If possible, older adults should ambulate three times a day. It is helpful to get all older persons, regardless of setting, out of bed or have them leave the room for meals. Providers should assess and manage impairments that reduce mobility, such as pain; impairments in strength, balance, or gait; and high-risk medications. It may also be helpful to refer older adults to PT or OT to address their mobility and functional challenges.
Older adults, families, and caregivers can create an environment that is safe for mobility by keeping objects off steps, fixing loose or broken stairs, removing throw rugs, and storing frequently used objects on low shelves. The Check for Safety checklist produced by the CDC can help find and fix hazards in the home (Centers for Disease Control and Prevention, 2017).
The identification of daily mobility goals by the older adult is important. Clinicians, caregivers, and family should review and support progress toward the mobility goal in subsequent interactions. Specifically, strategies for enhancing mobility categorized according to setting include:
Ambulatory
1. Multifactorial fall prevention protocol (STEADI) (Eckstrom et al., 2017).
2. Ensure safe home environment for mobility.
Health system (hospital, nursing home, assisted living, and other settings)
1. Avoid restraints.
2. Remove catheters and other tethering devices.
All settings
1. Avoid high risk medications.
2. Identify and set a daily mobility goal with older adult that supports what matters and then review and support progress toward a mobility goal.
3. Educate older adult and family caregivers.
4. Manage impairments that reduce mobility (pain, balance, gait, and strength).
5. Refer to PT (balance, gait, strength, and exercise program).
Intersectionality
The 4Ms are meant to be used as a set. The evidence is clear that the interactions among and between each is critically important to assess and manage for better care outcomes. Tinetti and colleagues have studied the interaction between antihypertensive medications in increased risk of serious falls and injuries (Tinetti et al., 2014). The association between prescribing anticholinergic medications and delirium has also been well documented (Campbell et al., 2011). The importance of focusing on medications to prevent adverse drug effects, including falls, delirium, depression, change in appetite, or bowel function, has been studied serially.
As noted, it is important to recognize that AFHSs exist within a broader social and economic context. Multiple organizations and government agencies are currently collaborating to define the parameters of an age-friendly ecosystem (Fulmer et al., 2020).
Discussion
We argue that the simplicity and focus for the 4Ms set is starting with what’s most important to the older adult, with the concomitant intersection of all 4Ms driving quality and safety in the plan of care. In the early days of Geriatrics, teams focused on common geriatric syndromes such as falling, delirium, incontinence, failure to thrive, and dementia, but each was addressed in parallel. The AFHS movement is meant to create a quality care framework wherever the older adult is receiving care with a unified 4Ms approach. This means that when transitions of care take place among our Medicare settings, there is a unified language, approach, and meaning that are readily understood by the primary care practice, other ambulatory practices, emergency department, intensive care unit, and so forth.
Each site of care has the latitude to decide on its care team roles in the practice of the 4Ms. Two resources were developed to assist health settings and systems in embedding AFHS’ care into practice. The first is AFHSs: Guide to Using the 4Ms in the Care of Older Adults. The guide provides a summary of evidence-based assessment tools and corresponding actions based on assessment findings (Institute for Healthcare Improvement, 2020). The second resource, The Business Case for Becoming an AFHS, reviews the value proposition related to improvements in cost and quality outcomes for AFHSs and offers real-world findings from inpatient and outpatient settings (Tabbush et al., 2019). Additional guides focused on implementation into the electronic health record, quality measurement, a return on investment calculator for AFHSs, and opportunities to join an action community (learning community with facilitated implementation process) are available on ihi.org/agefriendly.
Limitations
Given the array of geriatric care models available both in this country and globally, it is possible that key constructs may have been overlooked during the course of this expert panel assessment and more work needs to be done to truly understand the cultural, racial, and ethnic specificity that are needed to ensure that this approach is appropriate across populations. However, the rapid cycle plan-do-study-act (PDSA) model employed by IHI and the AFHS community creates a continuous learning process that puts us in good stead as new science is published to inform our work. While the movement has reached across settings of care and specialties, the 4Ms framework has potential to advance evidence-based care and improved outcomes in challenging settings that are under-resourced and struggle with social determinants of health. More work is needed to ensure that teams in these settings are able to adopt the 4Ms and learn from sites such as the Federally Qualified Health Centers that have been recognized. Finally, more research is needed to understand the 4Ms set as an intervention that can be used to rigorously examine and measure the outcomes of 4Ms and care.
Conclusion
The scientific evidence for the 4Ms is robust, and our AFHS model and 4Ms approach create an elegant way to ensure that older adults reliably get the best care possible. Once the assessments are completed, there are evidence-based care protocols for each of the aforementioned geriatric syndromes/conditions that, when used reliably, improve the outcomes for older adults in any given care setting (Boltz et al., 2020; Harper et al, 2019; Reuben et al., 2013). A 4Ms approach to care transcends disciplines, specialties, and disease states. The momentum of the AFHS movement, with more than 700 clinical sites participating in all 50 states, as well as several other countries, is a significant endorsement of the need to act on the evidence and provide age-friendly care.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Jinghan Zhang https://orcid.org/0000-0001-6841-4902
References
- Alexandre T. S., Meira D. M., Rico N. C., Mizuta S. K. (2012). Accuracy of timed up and go test for screening risk of falls among community-dwelling elderly. Brazilian Journal of Physical Therapy, 16(5), 381-388. [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association . (2020). Home. https://www.alz.org/ [Google Scholar]
- An S., Lee Y., Lee G. (2014). Validity of the performance-oriented mobility assessment in predicting fall of stroke survivors: A retrospective cohort study. The Tohoku Journal of Experimental Medicine, 233(2), 79-87. [DOI] [PubMed] [Google Scholar]
- Barry E., Galvin R., Keogh C., Horgan F., Fahey T. (2014). Is the timed up and go test a useful predictor of risk of falls in community dwelling older adults: A systematic review and meta-analysis. BMC Geriatrics, 14(1), 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchet O., Fantino B., Allali G., Muir S. W., Montero-Odasso M., Annweiler C. (2011). Timed up and go test and risk of falls in older adults: A systematic review. The Journal of Nutrition, Health & Aging, 15(10), 933-938. [DOI] [PubMed] [Google Scholar]
- Berlowitz D. R., Foy C. G., Kazis L. E., Bolin L. P., Conroy M. B., Fitzpatrick P., Gure T. R., Kimmel P. L., Kirchner K., Morisky D. E., Newman J., Olney C., Oparil S., Pajewski N. M., Powell J., Ramsey T., Simmons D. L., Snyder J., Supiano M. A., SPRINT Research Group. (2017). Effect of intensive blood-pressure treatment on patient-reported outcomes. New England Journal of Medicine, 377(8), 733-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaum C. S., Rosen J., Naik A. D., Smith C. D., Dindo L., Vo L., Hernandez‐Bigos K., Esterson J., Geda M., Ferris R., Costello D., Acampora D., Meehan T., Tinetti M. E. (2018). Feasibility of implementing patient priorities care for older adults with multiple chronic conditions. Journal of the American Geriatrics Society, 66(10), 2009-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boltz M., Capezuti E., Zwicker D., Fulmer T. T. (2020). Evidence-based geriatric nursing protocols for best practice. Springer Publishing Company. [Google Scholar]
- Borson S., Scanlan J., Brush M., Vitaliano P., Dokmak A. (2000). The mini-cog: A cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. International Journal of Geriatric Psychiatry, 15(11), 1021-1027. [DOI] [PubMed] [Google Scholar]
- Boyd C., Smith C. D., Masoudi F. A., Blaum C. S., Dodson J. A., Green A. R., Kelley A., Matlock D., Ouellet J., Rich M. W., Schoenborn N. L., Tinetti M. E. (2019). Decision making for older adults with multiple chronic conditions: Executive summary for the American geriatrics Society guiding principles on the care of older adults with multimorbidity. Journal of the American Geriatrics Society, 67(4), 665-673. [DOI] [PubMed] [Google Scholar]
- Boyle L. L., Richardson T. M., He H., Xia Y., Tu X., Boustani M., Conwell Y. (2011). How do the phq-2, the phq-9 perform in aging services clients with cognitive impairment? International Journal of Geriatric Psychiatry, 26(9), 952-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanelli C. M. (2012). American geriatrics society updated beers criteria for potentially inappropriate medication use in older adults: The American Geriatrics Society 2012 beers criteria update expert panel. Journal of the American Geriatrics Society, 60(4), 616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell N., Perkins A., Hui S., Khan B., Boustani M. (2011). Association between prescribing of anticholinergic medications and incident delirium: A cohort study. Journal of the American Geriatrics Society, 59, S277-S281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canbek J., Fulk G., Nof L., Echternach J. (2013). Test-retest reliability and construct validity of the tinetti performance-oriented mobility assessment in people with stroke. Journal of Neurologic Physical Therapy, 37(1), 14-19. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . (2017). Check for Safety: A Home Fall Prevention Checklist for Older Adults. [Google Scholar]
- Chen I.-P., Liu S.-I., Huang H.-C., Sun F.-J., Huang C.-R., Sung M.-R., Huang Y.-P. (2016). Validation of the patient health questionnaire for depression screening among the elderly patients in Taiwan. International Journal of Gerontology, 10(4), 193-197. [Google Scholar]
- Chevarley F. M. (2017). Health expenditures for adults by number of treated chronic conditions, race/ethnicity, and age, 2012. Agency for Healthcare Research and Quality. [PubMed] [Google Scholar]
- Conradsson M., Rosendahl E., Littbrand H., Gustafson Y., Olofsson B., Lövheim H. (2013). Usefulness of the geriatric depression scale 15-item version among very old people with and without cognitive impairment. Aging & Mental Health, 17(5), 638-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter A., Entwistle V. A., Eccles A., Ryan S., Shepperd S., Perera R. (2015). Personalised care planning for adults with chronic or long‐term health conditions. Cochrane Database of Systematic Reviews, 2015(3), CD010523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadfar M., Lester D. (2017). Psychometric characteristics of patient health questionnaire-2 (PHQ-2) in Iranian psychiatric outpatients. Austin Journal of Psychiatry and Behavioral Sciences, 4(1), 1059. [Google Scholar]
- Dedhiya S. D., Hancock E., Craig B. A., Doebbeling C. C., Thomas J., III. (2010). Incident use and outcomes associated with potentially inappropriate medication use in older adults. The American Journal of Geriatric Pharmacotherapy, 8(6), 562-570. [DOI] [PubMed] [Google Scholar]
- Delgado C., Araneda A., Behrens M. I. (2019). Validation of the Spanish-language version of the Montreal Cognitive Assessment test in adults older than 60 years. Neurología (English Edition), 34(6), 376-385. [DOI] [PubMed] [Google Scholar]
- Dias F. L. d. C., Teixeira A. L., Guimarães H. C., Barbosa M. T., Resende E. d. P. F., Beato R. G., Carmona K. C., Caramelli P. (2017). Accuracy of the 15-item Geriatric Depression Scale (GDS-15) in a community-dwelling oldest-old sample: The Pietà Study. Trends in Psychiatry and Psychotherapy, 39(4), 276-279. [DOI] [PubMed] [Google Scholar]
- Diniz B. S., Butters M. A., Albert S. M., Dew M. A., Reynolds C. F. (2013). Late-life depression and risk of vascular dementia and Alzheimer’s disease: Systematic review and meta-analysis of community-based cohort studies. British Journal of Psychiatry, 202(5), 329-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormann H, Sonst A, Müller F, Vogler R, Patapovas A, Pfistermeister B, Plank-Kiegele B, Kirchner M, Hartmann N, Bürkle T, Maas R. 2013). Adverse drug events in older patients admitted as an emergency: The role of potentially inappropriate medication in elderly people (PRISCUS). Deutsches Arzteblatt International, 110(13), 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durmaz B, Soysal P, Ellidokuz H, Isik AT. (2018). Validity and reliability of geriatric depression scale-15 (short form) in Turkish older adults. Northern Clinics of Istanbul, 5(3), 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstrom E., Parker E. M., Lambert G. H., Winkler G., Dowler D., Casey C. M. (2017). Implementing STEADI in academic primary care to address older adult fall risk. Innovation in Aging, 1(2), igx028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshaug A. G., Rosenthal M. B., Lavis J. N., Brownlee S., Schmidt H., Nagpal S., Littlejohns P., Srivastava D., Tunis S., Saini V. (2017). Levers for addressing medical underuse and overuse: achieving high-value health care. The Lancet, 390(10090), 191-202. [DOI] [PubMed] [Google Scholar]
- Ely E. W., Inouye S. K., Bernard G. R., Gordon S., Francis J., May L., Truman B., Speroff T., Gautam S., Margolin R., Hart R. P., Dittus R. (2001). Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the intensive care unit. JAMA, 286(21), 2703-2710. [DOI] [PubMed] [Google Scholar]
- Fick D. M.. (2016). Promoting cognitive health: Some good news and a brief summary of the institute of medicine report cognitive aging: Progress in understanding and oportunities for action. Journal of Gerontological Nursing, 42(7), 4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick D. M., Inouye S. K., Guess J., Ngo L. H., Jones R. N., Saczynski J. S., Marcantonio E. R. (2015). Preliminary development of an ultrabrief two-item bedside test for delirium. Journal of Hospital Medicine, 10(10), 645-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick D. M., Semla T. P., Beizer J., Brandt N., Dombrowski R., DuBeau C. E., Eisenberg W., Epplin J. J., Flanagan N. (2015). American geriatrics society 2015 updated beers criteria for potentially inappropriate medication use in older adults. Journal of the American Geriatrics Society, 63(11), 2227-2246. [DOI] [PubMed] [Google Scholar]
- Folstein M. F., Robins L. N., Helzer J. E. (1983). The mini-mental state examination. Archives of General Psychiatry, 40(7), 812. [DOI] [PubMed] [Google Scholar]
- Fulmer T., Patel P., Levy N., Mate K., Berman A., Pelton L., Beard J., Kalache A., Auerbach J. (2020). Moving toward a global age‐friendly ecosystem. Journal of the American Geriatrics Society, 68(9), 1936-1940. [DOI] [PubMed] [Google Scholar]
- Gallagher P. F., O’connor M. N., O’mahony D. (2011). Prevention of potentially inappropriate prescribing for elderly patients: A randomized controlled trial using STOPP/START criteria. Clinical Pharmacology & Therapeutics, 89(6), 845-854. [DOI] [PubMed] [Google Scholar]
- Gaudreau J.-D., Gagnon P., Harel F., Tremblay A., Roy M.-A. (2005). Fast, systematic, and continuous delirium assessment in hospitalized patients: The nursing delirium screening scale. Journal of Pain and Symptom Management, 29(4), 368-375. [DOI] [PubMed] [Google Scholar]
- Ghaeli P., Shahhatami F., Mojtahed Zade M., Mohammadi M., Arbabi M. (2018). Preventive intervention to prevent delirium in patients hospitalized in intensive care unit. Iranian Journal of Psychiatry, 13(2), 142. [PMC free article] [PubMed] [Google Scholar]
- Gomes G. d. C., Teixeira-Salmela L. F., Fonseca B. E., Freitas F. A. S. d., Fonseca M. L. M., Pacheco B. D., Gonçalves M. R., Caramelli P. (2015). Age and education influence the performance of elderly women on the dual-task timed up and go test. Arquivos de neuro-psiquiatria, 73(3), 187-193. [DOI] [PubMed] [Google Scholar]
- Grace A. R., Briggs R., Kieran R. E., Corcoran R. M., Romero-Ortuno R., Coughlan T. L., O'Neill D., Collins R., Kennelly S. P. (2014). A comparison of beers and STOPP criteria in assessing potentially inappropriate medications in nursing home residents attending the emergency department. Journal of the American Medical Directors Association, 15(11), 830-834. [DOI] [PubMed] [Google Scholar]
- Guaraldo L., Cano F. G., Damasceno G. S., Rozenfeld S. (2011). Inappropriate medication use among the elderly: A systematic review of administrative databases. BMC Geriatrics, 11(1), 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusmao-Flores D., Salluh J. I. F., Chalhub R., Quarantini L. C. (2012). The confusion assessment method for the intensive care unit (CAM-ICU) and intensive care delirium screening checklist (ICDSC) for the diagnosis of delirium: a systematic review and meta-analysis of clinical studies. Critical Care, 16(4), R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J. H., Wilson A., Vasilevskis E. E., Shintani A., Schnelle J. F., Dittus R. S., Graves A. J., Storrow A. B., Shuster J., Ely E. W. (2013). Diagnosing delirium in older emergency department patients: validity and reliability of the delirium triage screen and the brief confusion assessment method. Annals of Emergency Medicine, 62(5), 457-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper G., Lyons W., Potter J. (2019). Geriatrics review syllabus: A core curriculum in geriatric medicine. New York, NY: American Geriatrics Society. [Google Scholar]
- Hill-Taylor B., Sketris I., Hayden J., Byrne S., O’sullivan D., Christie R. (2013). Application of the STOPP/START criteria: A systematic review of the prevalence of potentially inappropriate prescribing in older adults, and evidence of clinical, humanistic and economic impact. Journal of Clinical Pharmacy and Therapeutics, 38(5), 360-372. [DOI] [PubMed] [Google Scholar]
- Hofheinz M., Mibs M. (2016). The prognostic validity of the timed up and go test with a dual task for predicting the risk of falls in the elderly. Gerontology and Geriatric Medicine, 2, 1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J.-L., Fan Y.-C., Huang Y.-L., Wang J., Chen W.-H., Chiu H.-C., Bai C.-H. (2015). Improved predictive ability of the montreal cognitive assessment for diagnosing dementia in a community-based study. Alzheimer’s Research & Therapy, 7(1), 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S. K., Bogardus S. T., Jr, Charpentier P. A., Leo-Summers L., Acampora D., Holford T. R., Cooney L. M., Jr. (1999). A multicomponent intervention to prevent delirium in hospitalized older patients. New England Journal of Medicine, 340(9), 669-676. [DOI] [PubMed] [Google Scholar]
- Institute for Healthcare Improvement . (2019). “What matters” to older adults? A toolkit for health systems to design better care with older adults. Age-Friendly Health Systems. [Google Scholar]
- Institute for Healthcare Improvement . (2020). Age-friendly health systems: Guide to using the 4Ms in the care of older adults. Age-Friendly Health Systems. [Google Scholar]
- Kanaan A. O., Donovan J. L., Duchin N. P., Field T. S., Tjia J., Cutrona S. L., Gagne S. J., Garber L., Preusse P., Harrold L. R., Gurwitz J. H. 2013). Adverse drug events after hospital discharge in older adults: Types, severity, and involvement of beers criteria medications. Journal of the American Geriatrics Society, 61(11), 1894-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya D., Isik A. T., Usarel C., Soysal P., Ellidokuz H., Grossberg G. T. (2016). The saint louis university mental status examination is better than the mini-mental state examination to determine the cognitive impairment in Turkish elderly people. Journal of the American Medical Directors Association, 17(4), 370. [DOI] [PubMed] [Google Scholar]
- Kivipelto M., Mangialasche F., Ngandu T. (2018). Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nature Reviews Neurology, 14(11), 653-666. [DOI] [PubMed] [Google Scholar]
- Klein K., Mulkey M., Bena J. F., Albert N. M. (2015). Clinical and psychological effects of early mobilization in patients treated in a neurologic ICU: A comparative study. Critical Care Medicine, 43(4), 865-873. [DOI] [PubMed] [Google Scholar]
- Kok R. M., Reynolds C. F. (2017). Management of depression in older adults: A review. JAMA, 317(20), 2114-2122. [DOI] [PubMed] [Google Scholar]
- Kolanowski A., Fick D., Frazer C., Penrod J. (2010). It’s about time: Use of nonpharmacological interventions in the nursing home. Journal of Nursing Scholarship, 42(2), 214-222. [DOI] [PubMed] [Google Scholar]
- Larson E. B. (2017). Evidence supports action to prevent injurious falls in older adults. JAMA, 318(17), 1659-1660. [DOI] [PubMed] [Google Scholar]
- Lau D. T., Mercaldo N. D., Harris A. T., Trittschuh E., Shega J., Weintraub S. (2010). Polypharmacy and potentially inappropriate medication use among community-dwelling elders with dementia. Alzheimer Disease & Associated Disorders, 24(1), 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy H. B., Marcus E.-L., Christen C. (2010). Adverse reactions/medication safety: Beyond the beers criteria: A comparative overview of explicit criteria. Annals of Pharmacotherapy, 44(12), 1968-1975. [DOI] [PubMed] [Google Scholar]
- Liu Z.-w., Yu Y., Hu M., Liu H.-m., Zhou L., Xiao S.-y. (2016). PHQ-9 and PHQ-2 for screening depression in Chinese rural elderly. PloS One, 11(3), Article e0151042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin‐Olsson L., Nyberg L., Gustafson Y. (1998). Attention, frailty, and falls: The effect of a manual task on basic mobility. Journal of the American Geriatrics Society, 46(6), 758-761. [DOI] [PubMed] [Google Scholar]
- Lyu H., Xu T., Brotman D., Mayer-Blackwell B., Cooper M., Daniel M., Wick E. C., Saini V., Brownlee S., Makary M. A. (2017). Overtreatment in the united states. Plos One, 12(9), Article e0181970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M., Leonard B., Fernandez A., Kubera M., Nowak G., Veerhuis R., Gardner A., Ruckoanich P., Geffard M., Altamura C. (2011). (Neuro) inflammation and neuroprogression as new pathways and drug targets in depression: From antioxidants to kinase inhibitors. Progress in Neuro-psychopharmacology & Biological Psychiatry, 3(35), 659-663. [DOI] [PubMed] [Google Scholar]
- Maher R. L., Hanlon J., Hajjar E. R. (2014). Clinical consequences of polypharmacy in elderly. Expert Opinion on Drug Safety, 13(1), 57-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcantonio E. R. (2017). Delirium in hospitalized older adults. New England Journal of Medicine, 377(15), 1456-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques A., Bordado J., Peralta M., Gouveia E. R., Tesler R., Demetriou Y., Baya D. G. (2020). Cross-sectional and prospective relationship between physical activity and depression symptoms. Scientific Reports, 10(1), 1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mate K. S., Berman A., Laderman M., Kabcenell A., Fulmer T. (2018). Creating age-friendly health systems–A vision for better care of older adults. Healthcare, 6, 4-6. [DOI] [PubMed] [Google Scholar]
- Memória C. M., Yassuda M. S., Nakano E. Y., Forlenza O. V. (2013). Brief screening for mild cognitive impairment: Validation of the Brazilian version of the Montreal cognitive assessment. International Journal of Geriatric Psychiatry, 28(1), 34-40. [DOI] [PubMed] [Google Scholar]
- Milani S. A., Marsiske M., Cottler L. B., Chen X., Striley C. W. (2018). Optimal cutoffs for the Montreal cognitive assessment vary by race and ethnicity. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, 10, 773-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morandi A., Davis D., Bellelli G., Arora R. C., Caplan G. A., Kamholz B., Kolanowski A., Fick D. M., Kreisel S., MacLullich A., Meagher D., Neufeld K., Pandharipande P. P., Richardson S., Slooter A. J. C., Taylor J. P., Thomas C., Tieges Z., Teodorczuk A., Rudolph J. L. (2017). The diagnosis of delirium superimposed on dementia: an emerging challenge. Journal of the American Medical Directors Association, 18(1), 12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley J., Tumosa N. (2002). Saint louis university mental status examination (SLUMS). Aging Successfully, 12(1), 4. [Google Scholar]
- Motter F. R., Fritzen J. S., Hilmer S. N., Paniz É. V., Paniz V. M. V. (2018). Potentially inappropriate medication in the elderly: A systematic review of validated explicit criteria. European Journal of Clinical Pharmacology, 74(6), 679-700. [DOI] [PubMed] [Google Scholar]
- Naik A. D., Dindo L. N., Liew J. R., Hundt N. E., Vo L., Hernandez‐Bigos K., Esterson J., Geda M., Rosen J., Blaum C. S., Tinetti M. E. 2018). Development of a clinically feasible process for identifying individual health priorities. Journal of the American Geriatrics Society, 66(10), 1872-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik A. D., McCullough L. B. (2014). Health intuitions inform patient-centered care. The American Journal of Bioethics, 14(6), 1-3. [DOI] [PubMed] [Google Scholar]
- Naik A. D., Palmer N., Petersen N. J., Street R. L., Rao R., Suarez-Almazor M., Haidet P. (2011). Comparative effectiveness of goal setting in diabetes mellitus group clinics: Randomized clinical trial. Archives of Internal Medicine, 171(5), 453-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld K. J., Leoutsakos J. S., Sieber F. E., Joshi D., Wanamaker B. L., Rios-Robles J., Needham D. M. (2013). Evaluation of two delirium screening tools for detecting post-operative delirium in the elderly. British Journal of Anaesthesia, 111(4), 612-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E. S., Fong T. G., Hshieh T. T., Inouye S. K. (2017). Delirium in older persons: Advances in diagnosis and treatment. JAMA, 318(12), 1161-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen C. F., Debesay J., Bergland A., Bye A., Langaas A. G. (2020). What matters when asking,“what matters to you?”—perceptions and experiences of health care providers on involving older people in transitional care. BMC Health Services Research, 20, 1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortman J. M., Velkoff V. A., Hogan H. (2014). An aging nation: The older population in the United States (pp. 25-1140). US Census Bureau. [Google Scholar]
- Peterson K., Hahn H., Lee A. J., Madison C. A., Atri A. (2016). In the Information age, do dementia caregivers get the information they need? Semi-structured interviews to determine informal caregivers’ education needs, barriers, and preferences. BMC Geriatrics, 16(1), 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R. C., Lopez O., Armstrong M. J., Getchius T. S. D., Ganguli M., Gloss D., Gronseth G. S., Marson D., Pringsheim T., Day G. S., Sager M., Stevens J., Rae-Grant A. (2018). Practice guideline update summary: Mild cognitive impairment: Report of the guideline development, dissemination, and implementation subcommittee of the american academy of neurology. Neurology, 90(3), 126-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall J. M., Voth R., Burnett E., Bazhenova L., Bardwell W. A. (2013). Clinic-based depression screening in lung cancer patients using the PHQ-2 and PHQ-9 depression questionnaires: A pilot study. Supportive care in cancer, 21(5), 1503-1507. [DOI] [PubMed] [Google Scholar]
- Reuben D. B., Herr K. A., Pacala J. T., Pollock B. G., Potter J. F., Semla T. P. (2013). Geriatrics at your fingertips. [Google Scholar]
- Roalf D. R., Moberg P. J., Xie S. X., Wolk D. A., Moelter S. T., Arnold S. E. (2013). Comparative accuracies of two common screening instruments for classification of Alzheimer's disease, mild cognitive impairment, and healthy aging. Alzheimer’s & Dementia, 9(5), 529-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossat A., Fantino B., Nitenberg C., Annweiler C., Poujol L., Herrmann F. R., Beauchet O. (2010). Risk factors for falling in community-dwelling older adults: Which of them are associated with the recurrence of falls? The Journal of Nutrition, Health & Aging, 14(9), 787-791. [DOI] [PubMed] [Google Scholar]
- Sharma A., Gupta M., Singh S. (2016). Balance and functional assessment in ambulatory elderly patients using timed get up and go test. Journal of Medical College Chandigarh, 6(2), 41-46. [Google Scholar]
- Shin C., Park M. H., Lee S.-H., Ko Y.-H., Kim Y.-K., Han K.-M., Jeong H.-G., Han C. (2019). Usefulness of the 15-item geriatric depression scale (GDS-15) for classifying minor and major depressive disorders among community-dwelling elders. Journal of Affective Disorders, 259, 370-375. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A., Brauer S., Woollacott M. (2000). Predicting the probability for falls in community-dwelling older adults using the timed up & go test. Physical Therapy, 80(9), 896-903. [PubMed] [Google Scholar]
- Smith E. R., Broughton M., Baker R., Pachana N. A., Angwin A. J., Humphreys M. S., Mitchell L., Byrne G. J., Copland D. A., Gallois C., Hegney D., Chenery H. J. (2011). Memory and communication support in dementia: Research-based strategies for caregivers. International Psychogeriatrics, 23(2), 256. [DOI] [PubMed] [Google Scholar]
- Stenhagen M., Nordell E., Elmståhl S. (2013). Falls in elderly people: A multifactorial analysis of risk markers using data from the Swedish general population study ‘good ageing in Skåne’. Aging Clinical and Experimental Research, 25(1), 59-67. [DOI] [PubMed] [Google Scholar]
- Storms H., Marquet K., Aertgeerts B., Claes N. (2017). Prevalence of inappropriate medication use in residential long-term care facilities for the elderly: A systematic review. European Journal of General Practice, 23(1), 69-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Kumei S., Ohhira M., Nozu T., Okumura T. (2015). Screening for major depressive disorder with the Patient Health Questionnaire (PHQ-9 and PHQ-2) in an outpatient clinic staffed by primary care physicians in Japan: A case control study. Plos One, 10(3), Article e0119147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szcześniak D., Rymaszewska J. (2016). The usfulness of the SLUMS test for diagnosis of mild cognitive impairment and dementia. Psychiatria Polska, 50(2), 457-472. [DOI] [PubMed] [Google Scholar]
- Tabbush V., Pelton L., Mate K., Duong T. (2019). The business case for becomimg an age-friendly health system. http://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Documents/IHI_Business_Case_for_Becoming_Age_Friendly_Health_System.pdf [Google Scholar]
- Tinetti M. E., Esterson J., Ferris R., Posner P., Blaum C. S. (2016). Patient priority-directed decision making and care for older adults with multiple chronic conditions. Clinics in Geriatric Medicine, 32(2), 261-275. [DOI] [PubMed] [Google Scholar]
- Tinetti M. E., Han L., Lee D. S. H., McAvay G. J., Peduzzi P., Gross C. P., Zhou B., Lin H. (2014). Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Internal Medicine, 174(4), 588-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J.-C., Chen C.-W., Chu H., Yang H.-L., Chung M.-H., Liao Y.-M., Chou K.-R. (2016). Comparing the sensitivity, specificity, and predictive values of the montreal cognitive assessment and mini-mental state examination when screening people for mild cognitive impairment and dementia in Chinese population. Archives of Psychiatric Nursing, 30(4), 486-491. [DOI] [PubMed] [Google Scholar]
- Van de Meeberg E. K., Festen S., Kwant M., Georg R. R., Izaks G. J., Ter Maaten J. C. (2017). Improved detection of delirium, implementation and validation of the CAM-ICU in elderly emergency department patients. European Journal of Emergency Medicine, 24(6), 411-416. [DOI] [PubMed] [Google Scholar]
- Van Rompaey B., Elseviers M. M., Van Drom W., Fromont V., Jorens P. G. (2012). The effect of earplugs during the night on the onset of delirium and sleep perception: A randomized controlled trial in intensive care patients. Critical Care, 16(3), 1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead L. (2016). The effects of personalized care planning for adults living with chronic conditions. International Journal of Nursing Practice, 22(2), 138-140. [DOI] [PubMed] [Google Scholar]
- Wiering B., de Boer D., Delnoij D. (2017). Asking what matters: The relevance and use of patient-reported outcome measures that were developed without patient involvement. Health Expectations, 20(6), 1330-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C. A., Recktenwald A. J., Jones M. L., Waterman B. M., Bollini M. L., Dunagan W. C. (2011). The cost of serious fall-related injuries at three Midwestern hospitals. The Joint Commission Journal on Quality and Patient Safety, 37(2), 81-87. [DOI] [PubMed] [Google Scholar]
- Yücel S. D., Şahin F., Doğu B., Şahin T., Kuran B., Gürsakal S. (2012). Reliability and validity of the Turkish version of the performance-oriented mobility assessment I. European Review of Aging and Physical Activity, 9(2), 149-159. [Google Scholar]
- Zheng L., Teng E. L., Varma R., Mack W. J., Mungas D., Lu P. H., Chui H. C. (2012). Chinese-language Montreal cognitive assessment for cantonese or Mandarin speakers: age, education, and gender effects. International Journal of Alzheimer’s Disease, 2012, 204623. [DOI] [PMC free article] [PubMed] [Google Scholar]