ABSTRACT
This study compared vitamin D sufficiency between indoor and outdoor elite athletes. We also evaluated the association between vitamin D level, body composition, and stress fractures incidence. 27 outdoor elite male collegiate athletes (field hockey players) and 21 indoor elite male collegiate athletes (fencing players) were enrolled. Participants’ demographic information including past fractures were recorded. Furthermore, all the athletes’ body compositions including percentage of body fat were measured. Blood samples were collected to test serum calcium, phosphorus, and 25(OH)D. levels. Participants were classified into three groups: vitamin D sufficiency (serum 25(OH)D levels of ≥30 ng/ml), vitamin D insufficiency (serum 25(OH)D levels of <30 ng/ml), and vitamin D deficiency (serum 25(OH)D levels of <20 ng/ml). The indoor athletes showed significantly higher mean percentage of body fat than outdoor athletes, 12.2 ± 3.2% and 9.7 ± 3.7%, respectively. The serum 25(OH)D levels of indoor athletes were significantly lower than those of outdoor athletes, 15.3 ± 3.3 ng/mL and 24.9 ± 4.5 ng/ml, respectively (P < 0.001). Furthermore, the indoor athletes showed a significantly higher rate of vitamin D deficiency than the outdoor athletes, 19 of 21 (90.5%) and 5 of 27 (18.5%), respectively (P < 0.001). The cohort of outdoor athletes with stress fractures’ history had significantly lower serum 25(OH)D levels than those without history of any fractures, 21.1 ± 4.3 ng/ml and 26.4 ± 3.0 ng/ml, respectively (P < 0.05). In conclusion, a majority of the indoor elite athletes were vitamin D-deficient. The serum 25(OH)D levels were significantly higher in outdoor elite athletes. However, lower serum 25(OH)D levels might be associated with stress fractures among outdoor athletes.
Key Words: serum 25(OH)D, indoor athletes, outdoor athletes, stress fracture
INTRODUCTION
Vitamin D has proposed roles in bone health, the inflammatory response, immunity, neuromuscular function, and the reduction of incidence of carcinomas.1 In addition, vitamin D deficiency has been correlated to the risk of bone stress fractures.2,3 Bone stress fracture is a common overuse injury in elite athletes.4,5 On the other hand, it is reported that obesity is one of the risk factors of vitamin D deficiency.6 Furthermore, vitamin D level is inversely related to body weight, BMI, and percentage of body fat.7,8,9 Vitamin D is believed to directly affect the skeletal muscles through a specific vitamin D receptor.10 Therefore, the importance of adequate vitamin D levels in athletes has become a trending topic of interest.11
The sun emits ultraviolet B radiation, converting 7-dehydrocholesterol to previtamin D3 in the skin.12 Therefore, natural sunlight is the major source of vitamin D in humans.13 Hence, indoor sports might be associated with vitamin D insufficiency.10,14 However, the precise difference of vitamin D levels between indoor and outdoor elite athletes remains unclear.
The primary aim of this cross-sectional study is to compare vitamin D sufficiency between indoor and outdoor elite athletes. The secondary aim is to evaluate the association between vitamin D level, the incidence of stress fractures, and body composition including body fat.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board and Ethics Committee of Asahi University prior to the study (IRB number: 31023). A cross-sectional analysis was performed. Inclusion criteria for the participants were the following: participants should be collegiate athletes who are members of national-champion-level teams in the authors’ university and of the male sex. However, those who are receiving any medications were excluded from this study. A total of 48 elite male collegiate athletes between 19 and 21 years old were enrolled, including 27 outdoor athletes (field hockey players) and 21 indoor athletes (fencing players).
Participation was voluntary, and the participants were provided a thorough explanation of the objectives, methods, and ethical considerations of this study. A written informed consent was obtained from all the participants included in the study.
The participants’ demographic information was prospectively recorded, including age, height, practice time with sunlight exposure, time of sleeping, and past medical history of stress fractures and other fractures by written questionnaire. The participants were divided into the following three groups according to past medical history of fractures: with stress fractures group, with other fractures group, and without any fractures group. Furthermore, in February, all the athletes’ body weight, body mass index, body fat percentage, muscle mass, and bone mass were measured using MC-980A-N plus (Tanita Corporation, Tokyo, Japan), a body composition analyzer.
Blood samples were collected to check the levels of serum calcium, phosphorus, and 25(OH)D at the same day as the body composition monitoring. Serum 25(OH)D levels were analyzed using electrochemiluminescence immunoassay (ECLIA) kits by SRL, Inc. (Tokyo, Japan). Serum calcium levels were measured through an enzymatic method using phospholipase D. Serum inorganic phosphate levels were measured through an enzymatic method using purine nucleoside phosphorylase.
According to the previous study, participants were divided into the following three items according to serum vitamin D levels: vitamin D sufficiency (serum 25(OH)D levels greater than or equal to 30 ng/ml), vitamin D insufficiency (serum 25(OH)D levels less than 30 ng/ml but not less than 20 ng/ml), and vitamin D deficiency (serum 25(OH)D levels less than 20 ng/ml) groups.15
The data were analyzed using the Student’s t-test, chi-squared test to identify associations. P values of <0.05 were considered significant.
RESULTS
No athlete was excluded, and a total of 48 elite male collegiate athletes were included in the analysis. The demographic and clinical characteristics of the indoor and outdoor athletes were similar, with the exception of practice time with sunlight exposure and body fat percentage (Table 1). The indoor athletes showed a significantly lower mean practice time with sunlight exposure than the outdoor athletes, 1.3 ± 1.5 h/wk and 17.0 ± 2.0 h/wk, respectively. Furthermore, the indoor athletes showed a significantly higher mean percentage of body fat than the outdoor athletes, 12.2 ± 3.2% and 9.7 ± 3.7%, respectively.
Table 1.
Characteristics of the participants
| All athletes
(n=48) |
Indoor athletes
(n=21) |
Outdoor athletes
(n=27) |
P Value | |
| Age, y | 19.8±0.9 | 20.0±1.0
(19–21) |
19.7±0.8
(19–21) |
NS♰ |
| Height, cm | 171.5±4.8 | 172±4.8
(163–181) |
171±4.8
(163–186) |
NS♰ |
| Body weight, kg | 62.8±7.4 | 64.7±5.9
(57.1–80.2) |
61.3±8.2
(52.6–91.9) |
NS♰ |
| BMI, kg/m2 | 21.3±2.5 | 21.9±2.2
(19.2–29.3) |
20.8±2.6
(17.0–29.7) |
NS♰ |
| Body fat percentage, % | 10.8±3.7 | 12.2±3.2
(6.6–20.2) |
9.7±3.7
(3.0–22.6) |
<0.05♰ |
| Muscle mass, kg | 52.9±4.6 | 53.8±3.7
(48.3–62.3) |
52.2±5.1
(45.9–67.4) |
NS♰ |
| Bone mass, kg | 2.9±0.2 | 2.9±0.2
(2.7–3.4) |
2.9±0.3
(2.5–3.7) |
NS♰ |
| Practice time with sunlight exposure, h/wk | 10.1±8.0 | 1.3±1.5
(0–2.0) |
17.0±2.0
(11.0–21.0) |
<0.001♰ |
| Time of sleeping, h/dy | 6.2±1.0 | 6.4±0.8
(5–8) |
6.1±1.2
(3–9) |
NS♰ |
| Past history of stress fracture | 6/48 | 3/21
(14.3%) |
3/27
(11.1%) |
NS* |
| Past history of other fracture | 21/48 | 7/21
(33.3%) |
14/27
(51.9%) |
NS* |
| No history of fracture | 21/48 | 11/21
(52.4%) |
10/27
(37.0%) |
NS* |
The values are given as means±standard deviation.
♰ Stundent’s t-test.
* Chi-squared test.
NS: not significant
The mean serum 25(OH)D level of all the athletes was 20.7 ± 6.3 ng/ml (Table 2). Overall, of the 48 athletes, 24 (50.0%) were classified as vitamin D deficiency, 20 (41.7%) were classified as vitamin D insufficiency, 4 (8.3%) were classified as vitamin D sufficiency. The serum 25(OH)D levels of the indoor athletes were significantly lower than those of the outdoor athletes, 15.3 ± 3.3 ng/ml and 24.9 ± 4.5 ng/ml, respectively (P < 0.001). Furthermore, the indoor athletes showed a significantly higher rate of vitamin D deficiency than the outdoor athletes, 19 of 21 (90.5%) and 5 of 27 (18.5%), respectively (P < 0.001). The serum calcium levels of the indoor athletes were significantly lower than those of the outdoor athletes, 9.67 ± 0.28 ng/ml and 9.86 ± 0.35 ng/ml, respectively (P < 0.05).
Table 2.
Laboratory results of the indoor and outdoor athletes
| All athletes
(n=48) |
Indoor athletes
(n=21) |
Outdoor athletes
(n=27) |
P Value | |
| S-25(OH) D, ng/mL | 20.7±6.3 | 15.3±3.3
(8.8–20.8) |
24.9±4.5
(16.9–33.1) |
<0.001♰ |
| equal to or above 30 ng/mL | 4/48 | 0/21 (0%) | 4/27
(14.8%) |
NS* |
| less than 30 ng/ml | 20/48 | 2/21 | 18/27 | <0.001* |
| but not less than 20 ng/ml | (9.5%) | (66.7%) | ||
| less than 20 ng/mL | 24/48 | 19/21
(90.5%) |
5/27
(18.5%) |
<0.001* |
| S-Calcium, mg/dL | 9.78±0.35 | 9.67±0.28 | 9.86±0.35 | <0.05♰ |
| S-Phosphorus, mg/dL | 3.47±0.50 | 3.54±0.46 | 3.41±0.52 | NS♰ |
The values are given as means±standard deviation.
♰ Stundent’s t-test.
* Chi-squared test.
NS: not significant
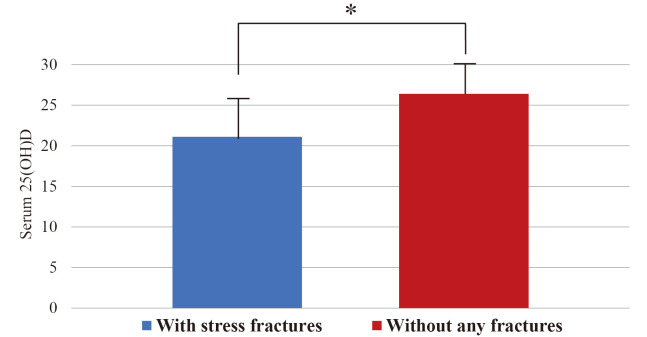
In terms of past medical history of fractures, of the 48 participants, 6 (12.5%) had history of stress fractures, 5 had foot or ankle stress fractures, and 1 had a stress fracture of patella. All stress fractures occurred after they had begun participation in field hockey or fencing. Twenty-one (43.8%) had a history of traumatic fractures other than stress fractures. There were no significant differences in past medical history of stress fractures (3 of 21 (14.3%) and 3 of 27 (11.8%), respectively) and other fractures (7 of 21 (33.3%) and 14 of 27 (51.9%), respectively) between the indoor athletes and outdoor athletes. The mean vitamin D level of the indoor athletes with history of stress fractures was 16.5±3.7 ng/ml. There was no significant difference between this value and the mean vitamin D level of the indoor athletes with history of other fractures (15.1±3.5 ng/ml) and those without history of any fractures (15.1±3.3 ng/ml) (Table 3). On the other hand, the cohort of outdoor athletes with history of stress fractures had significantly lower serum 25(OH)D levels than those without history of any fractures, 21.1±4.3 ng/ml and 26.4±3.0 ng/ml, respectively (P < 0.05) (Table 4) (Figure 1).
Table 3.
Comparison among the indoor athletes with past stress fractures and without any past fractures
| With stress fractures
(n=3) |
Without any fractures
(n=11) |
P Value♰ | |
| Height, cm | 171.7±3.2 | 172.1±5.2 | NS |
| Body weight, kg | 64.9±2.1 | 64.2±2.7 | NS |
| BMI, kg/m2 | 22.1±3.7 | 21.7±1.1 | NS |
| Body fat percentage, % | 13.1±2.7 | 11.7±2.2 | NS |
| Muscle mass, kg | 53.5±2.9 | 53.8±2.9 | NS |
| Bone mass, kg | 2.9±0.2 | 2.9±0.2 | NS |
| Practice time with | |||
| sunlight exposure, h/wk | 1.3±1.2 | 0.9±1.0 | NS |
| Time of sleeping, h/dy | 6.8±1.3 | 6.3±0.7 | NS |
| S-25(OH) D, ng/mL | 16.5±3.7 | 15.1±3.3 | NS |
The values are given as means±standard deviation.
♰ Stundent’s t-test.
NS: not significant
Table 4.
Comparison among the outdoor athletes with past stress fractures and without any past fractures
| With stress fractures
(n=3) |
Without any fractures
(n=10) |
P Value | |
| Height, cm | 174.3±10.2 | 171.5±3.9 | NS |
| Body weight, kg | 62.7±5.2 | 60.7±11.9 | NS |
| BMI, kg/m2 | 20.6±1.7 | 20.6±3.6 | NS |
| Body fat percentage, % | 11.2±1.8 | 10.8±4.9 | NS |
| Muscle mass, kg | 52.7±4.4 | 50.9±6.4 | NS |
| Bone mass, kg | 2.9±0.3 | 2.8±0.3 | NS |
| Practice time with sunlight
exposure, h/wk |
17.3±1.15 | 16.9±1.9 | NS |
| Time of sleeping, h/dy | 5.3±2.1 | 6.7±1.2 | NS |
| S-25(OH) D, ng/mL | 21.1±4.3 | 26.4±3.0 | <0.05 |
The values are given as means±standard deviation.
♰ Stundent’s t-test.
NS: not significant
Fig. 1.
Comparison among the outdoor athletes with past stress fractures and without any past fractures
Stundent’s t-test. * p < 0.05
DISCUSSION
The present study showed that the serum 25(OH)D levels of the indoor elite athletes were significantly lower than those of the outdoor elite athletes. There exists an extremely high prevalence of vitamin D deficiency in the indoor elite athletes (90.5%). Inadequate exposure to sunlight seems to be the major risk factor of this difference.16 Maruyama-Nagao et al reported that indoor sports athletes are more susceptible to vitamin D insufficiency compared with outdoor sports athletes, especially in winter.17 The results of this study might be influenced by the season of winter.
Vitamin D has a significant impact on bone health, immune function, and physical performance. When there is deficiency, athletes may be at an increased risk of stress fractures, respiratory infections, and muscle injuries.18 Therefore, vitamin D levels are very important for athletes.
In the present study, there was no vitamin-D-sufficient indoor elite athlete. Sunlight exposure can increase vitamin D levels, and a significant amount of active vitamin D circulating in the body is a result of sunlight exposure.18 Therefore, we should promote outdoor training to some extent for indoor athletes.
On the other hand, the outdoor athletes have a low prevalence (18.5%) of vitamin D deficiency in the present study. However, even among the outdoor athletes, the prevalence of vitamin D insufficiency is high, at 66.7%. Hamilton et al reported that 91% of 93 athletes showed vitamin D deficiency or insufficiency.19 Lovell reported vitamin D insufficiency in 33% of female Australian gymnasts.20 These results showed that athletes might be prone to vitamin D deficiency.
Vitamin D deficiency increased the risk of fractures including stress fractures in Finnish military recruits.3 In the present study, the cohort of outdoor elite athletes with history of stress fractures had significantly lower serum 25(OH)D levels than those without history of any fractures. Therefore, the present study implies that vitamin D level is very important in reducing the incidence of fractures even in outdoor elite athletes. Female Navy recruits receiving vitamin D supplementation had a 20% lower incidence of stress fractures than the recruits receiving the placebo.2 Furthermore, low-fat dairy products and the major nutrients in milk (calcium, vitamin D, and protein) were associated with lower stress fracture rates in young female runners.21 Even in outdoor elite athletes, we should check their serum 25(OH)D levels and give them dietary advice to reduce the incidence of fractures.
Another finding of the present study was the significant difference of body fat percentage between the indoor and outdoor athletes. Arunabh et al reported that the percentage of body fat is inversely related to the serum 25(OH)D level.22 Worstman et al explained that vitamin D insufficiency associated with obesity is due to decreased bioavailability of vitamin D from the cutaneous and dietary sources because of its deposition in body fat compartments.23 However, Salepour et al showed that increasing vitamin D levels by supplementation led to body fat mass reduction in a randomized controlled trial.24 Therefore, the association between body fat and serum 25(OH)D level remains unclear. However, the significant difference of body fat percentage between the indoor and outdoor athletes in this study might come from the difference in vitamin D levels between them, although we cannot deny the possibility that it might arise from differences in sports types.
This study had several limitations. The first is the inherent uncertainty regarding testing of vitamin D levels and the definition of sufficient levels of vitamin D. The second is the lack of investigation of seasonal changes. The histories of fractures were self-reported, which may have introduced error.
Finally, a larger sample size would improve the power of the study. Therefore, further investigation is required.
In conclusion, none of the indoor elite athletes had sufficient vitamin D levels, and 91.5% were vitamin D deficient. Serum 25(OH)D levels were significantly higher in the outdoor elite athletes, but only 14.8% of them had sufficient vitamin D levels. The outdoor elite athletes with history of stress fractures had significantly lower serum 25(OH)D levels than those without history of any fractures. Body fat percentages of indoor athletes are significantly higher than those of outdoor athletes.
ACKNOWLEDGMENTS
Thanks to all the athletes and coaches for their interest and participation in this study.
CONFLICT OF INTEREST
The authors declare that they have no competing interests. There was no funding for the research of this manuscript of the authors.
REFERENCES
- 1.Angeline ME, Gee AO, Shindle M, Warren RF, Rodeo SA. The effects of vitamin D deficiency in athletes. Am J Sports Med. 2013;41(2):461–464. doi: 10.1177/0363546513475787. [DOI] [PubMed]
- 2.Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin D supplementation decreases incidence of stress fractures in female Navy recruits. J Bone Miner Res. 2008;23(5):741–749. doi: 10.1359/jbmr.080102. [DOI] [PubMed]
- 3.Ruohola JP, Laaksi I, Ylikomi T, et al. Association between serum 25(OH)D concentrations and bone stress fractures in Finnish young men. J Bone Miner Res. 2006;21(9):1483–1488. doi: 10.1359/jbmr.060607. [DOI] [PubMed]
- 4.Sterling JC, Edelstein DW, Calvo RD, Webb II R. Stress fractures in the athlete: Diagnosis and management. Sports Med. 1992;14(5):336–346. doi: 10.2165/00007256-199214050-00005. [DOI] [PubMed]
- 5.Ekstrand J, Torstveit MK. Stress fractures in elite male football players. Scand J Med Sci Sports. 2012;22(3):341–346. doi: 10.1111/j.1600-0838.2010.01171.x. [DOI] [PubMed]
- 6.Yanoff LB, Parikh SJ, Spitalnik A, et al. The prevalence of hypovitaminosis D and secondary hyperparathyroidism in obese Black Americans. Clin Endocrinol (Oxf). 2006;64(5):523–529. doi: 10.1111/j.1365-2265.2006.02502.x. [DOI] [PMC free article] [PubMed]
- 7.Rontoyanni VG, Avila JC, Kaul S, Wong R, Veeranki SP. Association between obesity and serum 25 (OH) D concentrations in older Mexican adults. Nutrients. 2017;9(2):97. doi: 10.3390/nu9020097. [DOI] [PMC free article] [PubMed]
- 8.Yao Y, Zhu L, He L, et al. A metaanalysis of the relationship between vitamin D deficiency and obesity. Int J Clin Exp Med. 2015;8(9):14977–14984. [PMC free article] [PubMed]
- 9.Zittermann A, Ernst JB, Gummert JF, Börgermann J. Vitamin D supplementation, body weight and human serum 25-hydroxyvitamin D response: a systematic review. Eur J Nutr. 2014;53(2):367–374. doi: 10.1007/s00394-013-0634-3. [DOI] [PubMed]
- 10.Grieshober JA, Mehran N, Photopolous C, Fishman M, Lombardo SJ, Kharrazi FD. Vitamin D insufficiency among professional basketball players: a relationship to fracture risk and athletic performance. Orthop J Sports Med. 2018;21;6(5):2325967118774329. doi: 10.1177/2325967118774329. [DOI] [PMC free article] [PubMed]
- 11.Mehran N, Schulz BM, Neri BR, Robertson WJ, Limpisvasti O. Prevalence of vitamin D insufficiency in professional hockey players. Orthop J Sports Med. 2016;23;4(12):2325967116677512. doi: 10.1177/2325967116677512. [DOI] [PMC free article] [PubMed]
- 12.Holick MF. Deficiency of sunlight and vitamin D. BMJ. 2008;336(7657):1318–1319. doi: 10.1136/bmj.39581.411424.80. [DOI] [PMC free article] [PubMed]
- 13.Holick MF. Photosynthesis of vitamin D in the skin: effect of environmental and life-style variables. Fed Proc. 1987;46(5):1876–1882. [PubMed]
- 14.Wolman R, Wyon MA, Koutedakis Y, Nevill AM, Eastell R, Allen N. Vitamin D status in professional ballet dancers: winter vs. summer. J Sci Med Sport. 2013;16(5):388–391. doi: 10.1016/j.jsams.2012.12.010. [DOI] [PubMed]
- 15.Okazaki R, Ozono K, Fukumoto S, et al. Assessment criteria for vitamin D deficiency/insufficiency in Japan - Proposal by an Expert Panel Supported by Research Program of Intractable Diseases, Ministry of Health, Labour and Welfare, Japan, The Japanese Society for Bone and Mineral Research and The Japan Endocrine Society[Opinion]. Endocr J. 2017;64(1):1–6. doi: 10.1507/endocrj.EJ16-0548. [DOI] [PubMed]
- 16.Storlie DM, Pritchett K, Pritchett R, Cashman L. 12-week vitamin D supplementation trial does not significantly influence seasonal 25(OH)D status in male collegiate athletes. Int J Health Nutr. 2011;2(2):8–13.
- 17.Maruyama-Nagao A, Sakuraba K, Suzuki Y. Seasonal variations in vitamin D status in indoor and outdoor female athletes. Biomed Rep. 2016;5(1):113–117. doi: 10.3892/br.2016.671. [DOI] [PMC free article] [PubMed]
- 18.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed]
- 19.Hamilton B, Grantham J, Racinais S, Chalabi H. Vitamin D deficiency is endemic in Middle Eastern sportsmen. Public Health Nutr. 2010;13(10):1528–1534. doi: 10.1017/S136898000999320X. [DOI] [PubMed]
- 20.Lovell G. Vitamin D status of females in an elite gymnastics program. Clin J Sport Med. 2008;18(2):159–161. doi: 10.1097/JSM.0b013e3181650eee. [DOI] [PubMed]
- 21.Nieves JW, Melsop K, Curtis M, et al. Nutritional factors that influence change in bone density and stress fracture risk among young female cross-country runners. PM R. 2010;2(8):740–750;quiz 794. doi: 10.1016/j.pmrj.2010.04.020. [DOI] [PubMed]
- 22.Arunabh S, Pollack S, Yeh J, Aloia JF. Body Fat Content and 25-hydroxyvitamin D Levels in Healthy Women. J Clin Endocrinol Metab. 2003;88(1):157–161. doi: 10.1210/jc.2002-020978. [DOI] [PubMed]
- 23.Worstman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed]
- 24.Salehpour A, Hosseinpanah F, Shidfar F, et al. A 12-week double-blind randomized clinical trial of vitamin d3 supplementation on body fat mass in healthy overweight and obese women. Nutr J. 2012;11:78. doi: 10.1186/1475-2891-11-78. [DOI] [PMC free article] [PubMed]