Abstract
Introduction:
Coronavirus disease 2019 (COVID-19) was declared a global pandemic in March 2020. Since then, several studies have found COVID-19 patients with recurrent viral polymerase chain reaction (PCR) positivity.
Methods:
On May 6, 2021, an exhaustive literature search of the Web of Science, PubMed, Cochrane Library, Chinese National Knowledge Infrastructure databases, Embase, Wan Fang Data, VIP database, Sinomed database, BioRxiv, MedRxiv, and Research Square was conducted to find describing the laboratory indicators of recurrent and non-recurrent viral PCR positivity in patients with COVID-19. The data were statistically analyzed using STATA version 15.0.
Results:
In total, 22 studies—comprising 5154 laboratory-confirmed COVID-19 cases—were included in the analyses. Patients with less severe COVID-19 illness (i.e. those clinically classified as mild or common-type) seemed to exhibit recurrent PCR positivity more commonly than patients with more severe illness (i.e. those classified as severe or critical). There were also significant differences between the two groups in terms of the rates of headaches and dizziness, in addition to the levels of aspartate aminotransferase, C reactive protein, interleukin-6, and lactate dehydrogenase. Further, there were variations in the ratio of CD4+ T cells/CD8+ T cells on admission to the hospital.
Conclusion:
In comparison to COVID-19 patients with non-recurrent viral PCR positivity, patients with recurrent virus PCR positivity seem to experience more severe immune function suppression upon hospital admission.
Keywords: clinical features, COVID-19, immune function status, meta-analysis, recurrent viral positivity
Introduction
Coronavirus disease 2019 (COVID-19) was declared a global pandemic in March 2020. COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), belonging to the β-coronavirus genus. 1 Currently, in China, COVID-19 patients are discharged from the hospital for observation when they meet the following criteria: (1) normal body temperature for more than three days; (2) significant relief from respiratory symptoms; (3) lung imaging showing significant improvement in prior acute exudative lesions; and (4) two consecutive respiratory tract samples testing negative for virus nucleic acid (sampled at least 24 h apart). 2 In China, patients undergo regular retesting for virus nucleic acid after they are discharged from the hospital. As a result, several studies have found COVID-19 patients with recurrent viral polymerase chain reaction (PCR) positivity. 3 Gidari et al. 4 reported a series of COVID-19 patients with recurrent virus PCR positivity in Italy. The emergence of COVID-19 patients with recurrent virus PCR positivity poses a challenge to the prevention and control of the pandemic. Therefore, it is imperative to investigate the clinical features and corresponding immune function status of COVID-19 patients with recurrent virus PCR positivity.
Although there have been several studies on the clinical manifestations and laboratory test expressions of patients with recurrent virus PCR positivity, there is a lack of meta-analyses summarizing relevant data on patient immune function status. To this end, this study examines immune function status-related manifestations and laboratory indicators of COVID-19 patients with recurrent virus PCR positivity.
Methods
Search strategy and selection criteria
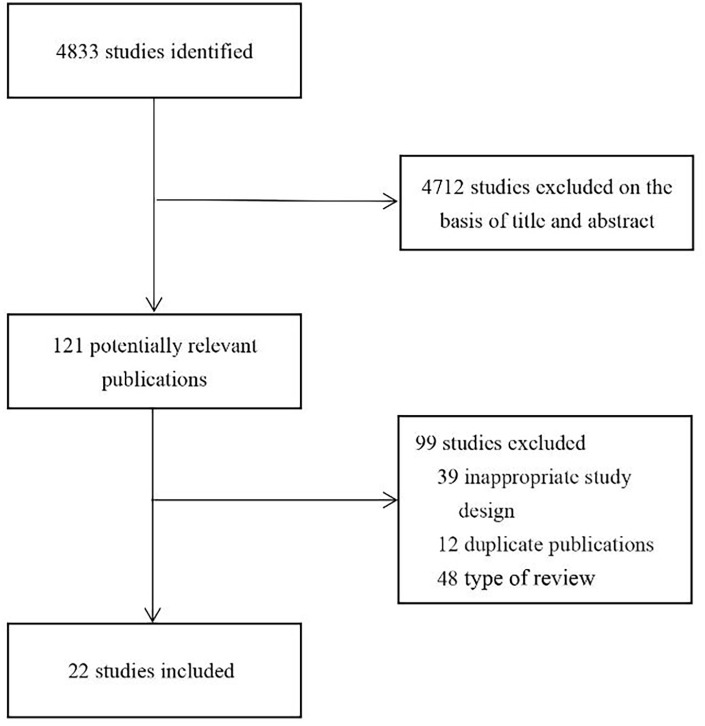
On May 6, 2021, a comprehensive literature search was conducted; the search included English databases such as Embase, Pubmed, Web of Science, and Cochrane Library; Chinese databases such as Wanfang Data, China National Knowledge Infrastructure Database, Traditional Chinese Medicine Database, and VIP Database; and pre-printed platforms such as BioRxiv, MedRxiv, and Research Square. The keywords included “2019-nCoV infection,” “2019 novel coronavirus disease,” “COVID-19,” “COVID-19 pandemic,” “COVID-19 virus disease,” “coronavirus disease 2019,” “2019 novel coronavirus infection,” “2019-nCoV disease,” “coronavirus disease-19,” “COVID-19 virus infection,” “SARS-CoV-2 infection,” “reactivation,” “recurrent,” and “relapse.” A flow-chart of the literature review process is shown in Figure 1. The protocol for this research was registered on the PROSPERO platform (CRD42020206385).
Figure 1.
Flow diagram of the review process.
Study selection and data extraction
The inclusion criteria for this meta-analysis were as follows: 1. the studied patients must meet one or more of the following diagnostic criteria: (1) COVID-19 RNA detected in the patients’ specimens by PCR or (2) virus isolated from the patients’ blood samples; (2) the study must be prospective or retrospective; (3) the study must be related to recurrent PCR positivity in COVID-19 patients; (4) laboratory data must be available for the recurrent and non-recurrent groups separately upon hospital admission. The exclusion criteria were as follows: (1) non-Chinese or non-English studies; (2) reviews; (3) studies with ambiguous definitions of COVID-19; (4) duplicate publications.
Two independent reviewers (Ren and Wang) reviewed the titles and abstracts of each retrieved study. If these studies satisfied the inclusion criteria, the two reviewers separately and independently read the entire text. The inclusion or exclusion of each study was determined through a discussion and consensus between the two reviewers; disagreements were resolved by a third investigator.
The following data were extracted from each article that met the inclusion criteria: sample size; general information on the patients, including sex, age, smoking history, epidemiological history, and complications; and clinical data, including initial symptoms, treatment time, vital signs, therapeutic drugs, and dosage.
Quality assessment
The quality of each included study was determined by referring to the Newcastle-Ottawa Scale (NOS).
Meta-analysis
The meta-analysis was performed using STATA version 15.0. I 2 analysis and the Q tests were used to evaluate the heterogeneity among the included studies. When I 2 < 50% and p > 0.1, it can be concluded that there is no statistical heterogeneity among the included studies, and a fixed-effect model can be used; if heterogeneity is present, a random-effect model should be used.
Results
Literature search, basic information, and quality assessments
The literature search revealed a total of 4833 related articles. After removal of duplicates and application of the inclusion and exclusion criteria, 22 articles5–26 remained. The detailed process of study screening is shown in Figure 1. Basic information on all included studies is provided in Table 1. Each study was rated according to the NOS scale, each of which scored over six stars. The star rating of each study, according to the NOS scale, is provided in Table 1.
Table 1.
Basic information and quality evaluation of previous research.
| Author | Year | Type | Recurrent group | Non-recurrent group | NOS | |||
|---|---|---|---|---|---|---|---|---|
| Selection | Comparability | Outcome | Scores | |||||
| Chen | 2020 | Case-control study | 986 | 81 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Hui | 2020 | Case-control study | 81 | 17 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Xiao | 2020 | Case-control study | 40 | 29 | ☆☆☆ | ☆☆ | ☆☆ | 7☆ |
| Zheng | 2020 | Cohort study | 27 | 258 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Du | 2020 | Case-control study | 3 | 123 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Yuan | 2020 | Cohort study | 20 | 162 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Hu | 2020 | Case-control study | 11 | 58 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Tong | 2020 | Case-control study | 42 | 58 | ☆☆☆ | ☆☆ | ☆☆ | 7☆ |
| Zhou | 2020 | Case-control study | 6 | 27 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Zhao | 2020 | Case-control study | 7 | 7 | ☆☆ | ☆☆ | ☆☆ | 6☆ |
| Li | 2020 | Case-control study | 11 | 9 | ☆☆☆ | ☆☆ | ☆☆ | 7☆ |
| An | 2020 | Case-control study | 38 | 204 | ☆☆☆ | ☆☆ | ☆☆ | 7☆ |
| Huang | 2020 | Case-control study | 69 | 345 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Chen | 2021 | Cohort study | 29 | 80 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Ao | 2021 | Cohort study | 25 | 26 | ☆☆ | ☆☆ | ☆☆ | 6☆ |
| Zhao | 2021 | Case-control study | 241 | 170 | ☆☆ | ☆☆ | ☆☆☆ | 7☆ |
| Adrielle | 2021 | Case-control study | 33 | 62 | ☆☆☆ | ☆☆ | ☆☆ | 7☆ |
| Hu | 2020 | Case-control study | 30 | 158 | ☆☆ | ☆☆ | ☆☆ | 6☆ |
| Chen | 2020 | Cohort study | 189 | 1093 | ☆☆☆ | ☆☆ | ☆☆☆ | 8☆ |
| Jiang | 2021 | Case-control study | 15 | 28 | ☆☆ | ☆☆ | ☆☆ | 6☆ |
| Liu | 2020 | Case-control study | 11 | 139 | ☆☆☆ | ☆ | ☆☆☆ | 7☆ |
| Wong | 2020 | Case-control study | 21 | 85 | ☆☆ | ☆☆ | ☆☆ | 6☆ |
Gender
A total of 22 studies were included to analyze the role of gender. The meta-analysis returned an odds ratio (OR) of 0.89 (95% CI: 0.74 to 1.07; P = 0.228), indicating no statistical difference in the distribution of gender between the recurrent or non-recurrent group (Supplemental Figure 1a). A funnel plot was then drawn, which was found to be fairly symmetrical (Supplemental Figure 1b).
Severity of illness
A total of six studies were included to analyze the role of the severity of the illness. The results revealed that the occurrence of recurrent PCR positivity in milder patients (clinically classified as mild or common-type illness) was 2.90 times higher than that of severe patients (clinically classified as severe or critical illness) (OR = 2.90, 95% CI: 1.54–5.46, P = 0.001) (Supplemental Figure 2).
Headache and dizziness symptoms
A total of five studies were included for the analysis of headache symptoms and two for the analysis of dizziness symptoms. The occurrence of headache symptoms in patients with recurrent PCR positivity was 2.52 times higher than that of patients with non-recurrent PCR positivity (OR = 2.52, 95% CI: 0.95–6.69; P = 0.064) (Supplemental Figure 3a). The occurrence of dizziness symptoms in patients with recurrent PCR positivity was 5.33 times higher than that of patients with non-recurrent PCR positivity (OR = 5.33, 95% CI: 1.46–19.55; P = 0.009) (Supplemental Figure 3b).
Aspartate aminotransferase (AST), C reactive protein (CRP), interleukin-6 (IL-6), and lactate dehydrogenase (LDH)
A total of six studies were included for the analysis of CRP level, four for AST level, four for LDH level, and two for IL-6 level. We found the proportion of patients with levated AST levels in the recurrent group was lower than that in the recurrent group (OR = 0.18, 95% CI: 0.06–0.60; P = 0.005) (Supplemental Figure 4a). The proportion of patients with elevated CRP levels in the recurrent group was lower than that in the non-recurrent group (OR = 0.68, 95% CI: 0.45–1.03; P = 0.07) (Supplemental Figure 4b). The proportion of patients with elevated IL-6 levels in the recurrent group was higher than that in the non-recurrent group (OR = 2.28, 95% CI: 1. 18–0.85; P = 0.014) (Supplemental Figure 4c). The proportion of patients with elevated LDH levels in the recurrent group was lower than that in the non-recurrent group (OR = 0.58, 95% CI: 0.31–1.09, P = 0.091) (Supplemental Figure 4d).
T cell subsets
Two studies were included to investigate the T cell subsets. There were no statistically significant differences in CD3+ T cell count (WMD = −18.57, 95% CI: −166.60 to 129.45, P = 0.681) (Supplemental Figure 5a), CD8 + T cell count (WMD = −116.70, 95% CI: −163.85 to −69.54, P = 0.851) (Supplemental Figure 5b), and CD4+ T cell count (WMD = −58.27, 95% CI: −105.94 to −10.59, P = 0.155) (Supplemental Figure 5c) between the two groups. The ratio of CD4+ T cells/CD8+ T cells in the non-recurrent group was higher than that in the recurrent group (WMD = −0.22, 95% CI: −0.35 to −0.09, P = 0.002) (Supplemental Figure 5d).
IgM titer
Two studies were included to analyze the IgM titer. The I2 = 0.0% and P > 0.1, revealing no heterogeneity among the included studies. The WMD = −0.22 (95% CI: −0.35 to −0.09, P = 0.001), indicating that the IgM titer in the recurrent group was lower than that in the non-recurrent group (Supplemental Figure 6).
Discussion
The statistical results showed that patients with mild to moderate illness more commonly exhibited recurrent PCR positivity in comparison to severe and critical patients. The results revealed two possible reasons for this. The first is that previous studies have shown that patients with higher immunoglobulin levels are more likely to experience severe illness, 27 and it can be speculated that high levels of immunoglobulins are more conducive to virus clearance. Second, we argue that severe cases receive more aggressive treatment (such as antiviral treatments and longer hospitalization time), which tends to lead to virus elimination. Additionally, the results revealed that the recurrent group was more likely to develop neurological symptoms (headaches and dizziness) than the non-recurrent group. Previous studies have confirmed that β-coronavirus is generally neuro-invasive in animals and humans.28–33 It is difficult to prevent the virus from entering the central nervous system due to the influence of the blood-brain barrier and craniocerebral structure. This could explain why patients with nervous system-related symptoms have a higher recurrent positivity rate.
The results for the laboratory indicators indicated that the proportion of patients with elevated AST, IL-6, LDH and CRP levels in the recurrent group was lower than those in the non-recurrent group. IL-6 is an important cytokine in the human body, which has a variety of physiological functions, including the regulation of immune cell proliferation and differentiation. 34 Dysregulation of IL-6 signaling is associated with lymphoproliferative and inflammatory diseases, including Castleman disease and rheumatoid arthritis. 34 High IL-6 levels are regarded to be independent risk factors for COVID-19 progression and severity. 35 Herold et al. 36 analyzed 89 COVID-19 patients and found that high CRP levels were a good predictor for the need for mechanical ventilation. Jin et al. 37 studied 651 COVID-19 patients and found that elevated LDH was an independent risk factor for the severity of COVID-19. Non-recurrent group patients have a higher AST level, indicating that non-recurrent group patients are more likely to have liver damage. This may be related to the higher proportion of severe and critically ill patients in the non-recurrent group. Previous studies by Wu et al.38,39 have also shown that abnormal liver biochemical indicators increase the risk of poor prognosis for COVID-19 patients, which is consistent with our conclusion. These results indicate that COVID-19 patients with recurrent PCR positivity experienced milder illness at the time of hospital admission compared to non-recurrent patients. This is consistent with the aforementioned conclusion regarding the severity of disease ratings.
The analysis of the immune status of patients upon admission revealed that the ratio of CD4+ T cells/CD8+ T cells and the IgM titer in recurrent patients were lower than those in non-recurrent patients. The ratio of CD4+ T cells/CD8+ T cells was decreased, which reflects typical dysfunction of cellular immunity. 40 The decreased IgM titer in patients indicates that humoral immunity dysfunction was more obvious in recurrent patients. Cellular and humoral immunity dysfunction could contribute to a decrease in the patient’s immune response to SARS-CoV-2. Current research suggests that the organ and tissue damage observed in patients with COVID-19 is not completely and directly caused by SARS-CoV-2, but more due to an excessive secondary immune-inflammatory response. 41 The findings regarding the aforementioned immune characteristics could explain why the laboratory indicators in recurrent patients that reflect damage to tissues and organs were lower than those of non-recurrent patients. Furthermore, weakened immune function is not conducive to the elimination of the virus, 42 which could explain the emergence of recurrent patients.
Although the included studies clearly stated that the COVID-19 recurrent patients were confirmed by real-time reverse-transcriptase polymerase-chain-reaction (RT-PCR), only a few studies reported the Ct value, which is a limitation of this meta-analysis. In addition, owing to the small number of valid studies, comparisons of the laboratory indicators between the recurrent and non-recurrent PCR positivity patients may be unreliable. With the publication of more relevant studies over the coming year, additional meta-analyses can be conducted to verify these conclusions. Furthermore, most of the studies included in the literature review focus on China. This could lead to issues when attempting to apply the results to other regions.
Supplemental Material
Supplemental material, sj-pdf-1-iji-10.1177_20587384211027679 for Clinical features and corresponding immune function status of recurrent viral polymerase chain reaction positivity in patients with COVID-19 : A meta- analysis and systematic review by Xingxiang Ren, Xiankun Wang, Ziruo Ge, Shuping Cui and Zhihai Chen in International Journal of Immunopathology and Pharmacology
Supplemental material, sj-pdf-2-iji-10.1177_20587384211027679 for Clinical features and corresponding immune function status of recurrent viral polymerase chain reaction positivity in patients with COVID-19 : A meta- analysis and systematic review by Xingxiang Ren, Xiankun Wang, Ziruo Ge, Shuping Cui and Zhihai Chen in International Journal of Immunopathology and Pharmacology
Supplemental material, sj-pdf-3-iji-10.1177_20587384211027679 for Clinical features and corresponding immune function status of recurrent viral polymerase chain reaction positivity in patients with COVID-19 : A meta- analysis and systematic review by Xingxiang Ren, Xiankun Wang, Ziruo Ge, Shuping Cui and Zhihai Chen in International Journal of Immunopathology and Pharmacology
Supplemental material, sj-pdf-4-iji-10.1177_20587384211027679 for Clinical features and corresponding immune function status of recurrent viral polymerase chain reaction positivity in patients with COVID-19 : A meta- analysis and systematic review by Xingxiang Ren, Xiankun Wang, Ziruo Ge, Shuping Cui and Zhihai Chen in International Journal of Immunopathology and Pharmacology
Supplemental material, sj-pdf-5-iji-10.1177_20587384211027679 for Clinical features and corresponding immune function status of recurrent viral polymerase chain reaction positivity in patients with COVID-19 : A meta- analysis and systematic review by Xingxiang Ren, Xiankun Wang, Ziruo Ge, Shuping Cui and Zhihai Chen in International Journal of Immunopathology and Pharmacology
Supplemental material, sj-pdf-6-iji-10.1177_20587384211027679 for Clinical features and corresponding immune function status of recurrent viral polymerase chain reaction positivity in patients with COVID-19 : A meta- analysis and systematic review by Xingxiang Ren, Xiankun Wang, Ziruo Ge, Shuping Cui and Zhihai Chen in International Journal of Immunopathology and Pharmacology
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (82072295). The funders had no role in the study design, data collection and analysis, decision to publish the work, or preparation of the manuscript.
ORCID iDs: Shuping Cui  https://orcid.org/0000-0002-8997-9352
https://orcid.org/0000-0002-8997-9352
Zhihai Chen  https://orcid.org/0000-0002-5140-1256
https://orcid.org/0000-0002-5140-1256
Supplemental material: Supplemental material for this article is available online.
References
- 1. Wiersinga WJ, Rhodes A, Cheng AC, et al. (2020) Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA 324: 782–793. [DOI] [PubMed] [Google Scholar]
- 2. China National Health Commission (2020) Diagnosis and treatment of 2019-nCoV pneumonia in China (version 8). China Medicine 15(10): 1494–1499. [Google Scholar]
- 3. Mattiuzzi C, Henry BM, Sanchis-Gomar F, et al. (2020) SARS-CoV-2 recurrent RNA positivity after recovering from coronavirus disease 2019 (COVID-19): A meta-analysis. Acta Bio-Medica: Atenei Parmensis 91: e2020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gidari A, Nofri M, Saccarelli L, et al. (2020) Is recurrence possible in coronavirus disease 2019 (COVID-19)? Case series and systematic review of literature. European Journal of Clinical Microbiology & Infectious Diseases 10: 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen J, Xu X, Hu J, et al. (2020) Clinical course and risk factors for recurrence of positive SARS-CoV-2 RNA: A retrospective cohort study from Wuhan, China. Aging 12: 16675–16689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu H, Fu L, Jin Y, et al. (2020) Clinical features of COVID-19 convalescent patients with re-positive nucleic acid detection. Journal of Clinical Laboratory Analysis 34: e23392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xiao Y, Shi X, She Q, et al. (2020) Exploration of turn-positive RT-PCR results and factors related to treatment outcome in COVID-19: A retrospective cohort study. Virulence 11: 1250–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zheng J, Zhou R, Chen F, et al. (2020) Incidence, clinical course and risk factor for recurrent PCR positivity in discharged COVID-19 patients in Guangzhou, China: A prospective cohort study. PLoS Neglected Tropical Diseases 14: e0008648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Du H, Chen J, Pan X, et al. (2020) Prevalence and outcomes of re-positive nucleic acid tests in discharged COVID-19 patients. European Journal of Clinical Microbiology & Infectious Diseases 31: 100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yuan B, Liu HQ, Yang ZR, et al. (2020) Recurrence of positive SARS-CoV-2 viral RNA in recovered COVID-19 patients during medical isolation observation. Science Reports 10(1): 11887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu R, Jiang Z, Gao H, et al. (2020) Recurrent positive reverse transcriptase-polymerase chain reaction results for coronavirus disease 2019 in patients discharged from a hospital in China. JAMA Network Open 3: e2010475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tong L, Zhang CW, Cao Y, et al. (2020) Study on the relationship between immune function and positive recurrence of nucleic acid test results of recovered patients with Corona Virus Disease 2019. International Journal of Laboratory Medicine 41: 2251–2254. [Google Scholar]
- 13. Zhuo L, Wei F, Zhuo JQ, et al. (2020) Factors influencing the outcome of 34 patients with COVID-19. Journal of Practical Medicine and Medical Science 36: 1861–1865. [Google Scholar]
- 14. Zhao W, Wang Y, Tang YF, et al. (2020) Characteristics of children with reactivation of SARS-CoV-2 infection after hospital discharge. Clinical Pediatrics 59: 929–932. [DOI] [PubMed] [Google Scholar]
- 15. Li Q, Zhang H, Deng SY, et al. (2020) Comparison of the expression and morphological analysis of peripheral blood lymphocytes subsets in patients with positive recovery and negative patients during COVID-19 recovery period. Chong Qing Medicine 19: 3151–3155. [Google Scholar]
- 16. An J, Liao X, Xiao T, et al. (2020) Clinical characteristics of recovered COVID-19 patients with re-detectable positive RNA test. Annals of Translational Medicine 8: 1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang J, Zheng L, Zhen L, et al. (2020) Recurrence of SARS-CoV-2 PCR positivity in COVID-19 patients: A single center experience and potential implications. MedRxiv 10: 1–32. [Google Scholar]
- 18. Chen Z, Xie W, Ge Z, et al. (2021) Reactivation of SARS-CoV-2 infection following recovery from COVID-19. Journal of Infection and Public Health 14: 620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ao Z, Li Y, Wei J, et al. (2021) Clinical characteristics and potential factors for recurrence of positive SARS-CoV-2 RNA in convalescent patients: A retrospective cohort study. Clinical and Experimental Medicine. Epub ahead of print 4 February 2021. DOI: 10.1007/s10238-021-00687-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao H, Zhang C, Chen X, et al. (2021) The relationship between SARS-COV-2 RNA positive duration and the risk of recurrent positive. Infectious Diseases of Poverty 10: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adrielle DSL, Filho PG, Silva AMF, et al. (2021) Recurrent COVID-19 including evidence of reinfection and enhanced severity in thirty Brazilian healthcare workers. The Journal of Infection 82: 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hu XX, Ni L, Chen Y, et al. (2020) Clinical characteristics of discharged COVID-19 patients with reappeared positive nucleic acid test. Medical Journal of Wuhan University 5: 1–5. [Google Scholar]
- 23. Chen SL, Xu H, Feng HY, et al. (2020) Epidemiological and clinical findings of short-term recurrence of severe acute respiratory syndrome coronavirus 2 ribonucleic acid polymerase chain reaction positivity in 1282 discharged coronavirus disease 2019 cases: A multicenter, retrospective, observational study. Open Forum Infectious Diseases 7(10): aa432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jiang F, Ding K, Li SX, et al. (2021) Analysis of clinical characteristics ofre-detectable SARS-CoV-2 nucleic acidpositive patients and results of nucleic acid test in different specimens. Infectious Disease Information 34(1): 20–24+31. [Google Scholar]
- 25. Liu T, Wu SY, Zeng G, et al. (2020) Recurrent positive SARS-CoV-2: Immune certificate may not be valid. Journal of Medical Virology 92(11): 2384–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wong J, Koh WC, Momin RN, et al.(2020) Probable causes and risk factors for positive SARS-CoV-2 test in recovered patients: Evidence from Brunei Darussalam. Journal of Medical Virology 92: 2847–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang B, Zhou X, Zhu C, et al. (2020) Immune phenotyping based on the neutrophil-to-lymphocyte ratio and IgG level predicts disease severity and outcome for patients with COVID-19. Frontiers in Molecular Biosciences 7: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Giraudon P, Bernard A. (2020) Inflammation in neuroviral diseases. Journal of Neural Transmission 117: 899–906. [DOI] [PubMed] [Google Scholar]
- 29. Desforges M, Le Coupanec A, Dubeau P, et al. (2019) Human coronaviruses and other respiratory viruses: Underestimated opportunistic pathogens of the central nervous system? Viruses 12: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arbour N, Day R, Newcombe J, et al. (2000) Neuroinvasion by human respiratory coronaviruses. Journal of Virology 74: 8913–8921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Perlman S, Netland J. (2000) Coronaviruses post-Sars: Updateon replication and pathogenesis. Nature Reviews: Microbiology 7: 439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lau KK, Yu WC, Chu CM, et al. (2004) Possible central nervous system infection by Sarscoronavirus. Emerging Infectious Diseases 10: 342–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Montalvan V, Lee J, Bueso T, et al. (2020) Neurological manifestations of Covid-19 and other coronavirus infections: A systematic review. Clinical Neurology and Neurosurgery 194: 105921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Garbers C, Heink S, Korn T, et al. (2018) Interleukin-6: Designing specific therapeutics for a complex cytokine. Nature Reviews Drug Discovery 17: 395–341. [DOI] [PubMed] [Google Scholar]
- 35. Wang C, Fei D, Li X, et al. (2020) IL-6 may be a good biomarker for earlier detection of COVID-19 progression. Intensive Care Medicine 46: 1475–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Herold T. (2020) Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. Journal of Allergy and Clinical Immunology 146: 128–136.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jin X, Lian JS, Hu JH, et al. (2020) Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut 69: 1002–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu Y, Ma Z, Guo X, et al. (2021) Characteristics and in-hospital outcomes of COVID-19 patients with abnormal liver biochemical tests. Annals of Hepatology 24: 100349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu Y, Li H, Guo X, et al. (2020) Incidence, risk factors, and prognosis of abnormal liver biochemical tests in COVID-19 patients: A systematic review and meta-analysis. Hepatology International 14(5): 621–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thibaudin M, Fumet JD, Bon MF, et al. (2020) Immunological features of coronavirus disease 2019 in patients with cancer. European Journal of Cancer 139: 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wiersinga WJ, Rhodes A, Cheng AC, et al. (2020) Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA 324: 782–793. [DOI] [PubMed] [Google Scholar]
- 42. Han H, Xu Z, Cheng X, et al. (2020) Descriptive, retrospective study of the clinical characteristics of asymptomatic COVID-19 patients. mSphere 5: 20–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-iji-10.1177_20587384211027679 for Clinical features and corresponding immune function status of recurrent viral polymerase chain reaction positivity in patients with COVID-19 : A meta- analysis and systematic review by Xingxiang Ren, Xiankun Wang, Ziruo Ge, Shuping Cui and Zhihai Chen in International Journal of Immunopathology and Pharmacology
Supplemental material, sj-pdf-2-iji-10.1177_20587384211027679 for Clinical features and corresponding immune function status of recurrent viral polymerase chain reaction positivity in patients with COVID-19 : A meta- analysis and systematic review by Xingxiang Ren, Xiankun Wang, Ziruo Ge, Shuping Cui and Zhihai Chen in International Journal of Immunopathology and Pharmacology
Supplemental material, sj-pdf-3-iji-10.1177_20587384211027679 for Clinical features and corresponding immune function status of recurrent viral polymerase chain reaction positivity in patients with COVID-19 : A meta- analysis and systematic review by Xingxiang Ren, Xiankun Wang, Ziruo Ge, Shuping Cui and Zhihai Chen in International Journal of Immunopathology and Pharmacology
Supplemental material, sj-pdf-4-iji-10.1177_20587384211027679 for Clinical features and corresponding immune function status of recurrent viral polymerase chain reaction positivity in patients with COVID-19 : A meta- analysis and systematic review by Xingxiang Ren, Xiankun Wang, Ziruo Ge, Shuping Cui and Zhihai Chen in International Journal of Immunopathology and Pharmacology
Supplemental material, sj-pdf-5-iji-10.1177_20587384211027679 for Clinical features and corresponding immune function status of recurrent viral polymerase chain reaction positivity in patients with COVID-19 : A meta- analysis and systematic review by Xingxiang Ren, Xiankun Wang, Ziruo Ge, Shuping Cui and Zhihai Chen in International Journal of Immunopathology and Pharmacology
Supplemental material, sj-pdf-6-iji-10.1177_20587384211027679 for Clinical features and corresponding immune function status of recurrent viral polymerase chain reaction positivity in patients with COVID-19 : A meta- analysis and systematic review by Xingxiang Ren, Xiankun Wang, Ziruo Ge, Shuping Cui and Zhihai Chen in International Journal of Immunopathology and Pharmacology