Abstract
Objective
To determine the associations between matrix metalloproteinase-2 (MMP-2, encoded by the MMP2 gene) 1306C/T and 735C/T polymorphisms and first and recurrent ischemic stroke in a Chinese population.
Methods
Patients with first and recurrent ischemic stroke were included. Serum MMP-2 was measured, and MMP2 1306C/T and 735C/T polymorphisms were detected. The associations between MMP2 1306C/T and 735C/T polymorphisms and first and recurrent ischemic stroke were analyzed.
Results
Serum MMP-2 in patients with first and recurrent ischemic stroke was significantly higher compared with controls, and patients with recurrent ischemic stroke had higher MMP-2 than those with first ischemic stroke. The frequency of the CC genotype and C allele of MMP2 735C/T was highest in patients with recurrent ischemic stroke, followed by patients with first ischemic stroke, and controls. Conversely, the genotype and allele of MMP2 1306C/T did not significantly differ between groups. The CC genotype of MMP2 735C/T was independently associated with first and recurrent ischemic stroke (odds ratios = 1.45 and 1.64, respectively), as was the C allele of MMP2 735C/T (odds ratios = 1.68 and 1.77, respectively).
Conclusions
The CC genotype and C allele of MMP2 735C/T were associated with first and recurrent ischemic stroke in a Chinese population.
Keywords: Metalloproteinase-2, polymorphism, ischemic stroke, recurrence, genotype, allele
Introduction
Ischemic stroke is a common cause of morbidity and mortality worldwide. 1 Epidemiological data have shown that ischemic stroke accounts for 60% to 80% of all strokes,2,3 and cumulative deaths in Chinese patients hospitalized for ischemic stroke are 3.3% to 5.2% at 1 month and 9% to 9.6% at 3 months. 4 Moreover, patients with first ischemic stroke are vulnerable to recurrence, with a reported average recurrence of over 40% in Chinese patients. Currently, ischemic stroke is considered a complex disease that involves interactions between genetic and environmental factors. 5 Atherosclerosis is the key pathophysiological feature of the disease. 6 The pathogenesis of atherosclerosis involves many cellular processes and molecular mechanisms, and genetic factors also play important roles. 7 , 8 Therefore, the identification of genetic factors related to ischemic stroke is of clinical significance for the early identification of a high-risk population, or even for revealing novel treatment targets.
Matrix metalloproteinase (MMP) is a calcium-dependent zinc-containing endopeptidase that is synthesized and secreted by macrophages; it functions to degrade the extracellular matrix (ECM). 1 Previous studies have demonstrated that MMP-2, encoded by the MMP2 gene, is involved in the formation and rupture of atherosclerotic plaques, thereby contributing to the pathogenesis of vascular diseases, including ischemic stroke. 9 Among MMP family members, MMP-2 is suggested to be the main enzyme involved in the pathogenesis of ischemic stroke. 7 At present, uncertainty remains regarding the relationship between MMP2 polymorphisms (1306C/T and 735C/T) and ischemic stroke; however, inactivation of the MMP2 gene has protective effects against the pathogenesis of atherosclerosis. 10 Furthermore, mutations of 1306C/T and 735C/T in the promoter region of MMP2, at positions 1306 and 735, have been identified, which may lead to increased oxidative stress and atherosclerotic cerebral infarction via the regulation of MMP-2 expression. However, the associations between MMP2 1306C/T and 735C/T polymorphisms and first and recurrent ischemic strokes have not been fully determined by previous studies.
We performed a case–control study to evaluate the associations between MMP2 1306C/T and 735C/T polymorphisms and the risk of first and recurrent ischemic stroke in a Chinese population.
Patients and methods
Consecutive patients with ischemic stroke who were admitted to the Department of Neurology in Suining Central Hospital (Suining, Sichuan, China) from April 2012 to April 2017 were screened for enrollment. All subjects were unrelated, and each of them provided their signed informed consent before inclusion. The study protocol was approved by the Ethics Committee of Suining Central Hospital before initiation (IRB number, 20120324).
Patient enrollment
Patients were included if they met the following criteria: adult patients (older than 18 years) diagnosed with first or recurrent ischemic stroke during hospitalization, within 3 days of disease onset. The diagnosis of ischemic stroke was in accordance with the criteria from the Fourth National Academic Conference on Cerebrovascular Diseases in 1995, 11 and a clinical diagnosis of ischemic stroke included the comprehensive evaluation of clinical symptoms, brain computed tomography, or magnetic resonance imaging. Patients with atherosclerotic ischemic stroke were selected according to the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification criteria. 12 The inclusion criteria for recurrent ischemic stroke were: 1) new neurological deficit symptoms; 2) initial symptoms were aggravated, with an interval from first ischemic stroke to symptom recurrence of more than 1 month; and 3) imaging examinations confirmed the presence of new ischemic lesions. The exclusion criteria were: 1) uncooperative patients, or patients and family members who did not agree to participate in the study or refused to have specimens collected; 2) patients with arteritis and other vasculitis, cerebral artery malformation, or cardiogenic cerebral embolism; 3) patients who had a serious respiratory infection or other serious infectious disease; 4) patients who had severe liver disease and/or liver function impairment; 5) patients with severe renal disease and/or renal impairment, with creatinine > 220 µmol/L as indicated by renal function examination; 6) patients with severe anemia, routine blood test indicating hemoglobin < 80 g/L, coagulation dysfunction, or other serious diseases of the blood system; 7) patients with recent trauma, surgery, pregnancy, infectious diseases, or parasitic diseases; 8) patients who were taking lipid-lowering medications and/or estrogen or birth control pills in the previous 6 months; and 9) patients with severe hepatorenal dysfunction, autoimmune disease, and/or malignant tumors. In addition, healthy individuals who had undergone a physical examination in the Department of Physical Examination in our hospital were included as controls.
Measurement of MMP2 polymorphisms
A blood sample under a fasting state was obtained from each participant on the morning of their second day after admission. Serum MMP-2 levels were measured using an enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. The 1306C/T and 735C/T polymorphisms of MMP2 were measured using polymerase chain reaction (PCR) restriction fragment length polymorphism methods. Briefly, Primer 5.0 software (Premier Biosoft, San Francisco, CA, USA) was used for primer design, and the primers were synthesized by Shanghai Bioengineering Co. Ltd. (Shanghai, China). The upstream primer sequence of MMP2 735C/T was 5′-ATCCTGTGACGGAGAATC-3′ and the downstream primer sequence was 5′-TAAAATGAGGCTGAGACC-3′, which amplified a sequence of 391 base pairs (bp). The upstream and downstream sequences of the primers for MMP2 1306C/T were 5′-CTTACCCCGAGTCCTATCTGCC-3′ and 5′-TCTTGGGAACGCCTGACTTCAG-3′, respectively, which amplified a sequence of 193 bp. Genomic DNA samples were extracted using a blood genomic extraction kit (Beijing Baitaike Biotechnology Co. Ltd., Beijing, China) according to the manufacturer’s instructions. Subsequently, the PCR reaction was performed at 95°C for 5 minutes, followed by 30 cycles of 94°C for 45 s, 56°C for 30 s, and 72°C for 35 s, and then 72°C for 10 minutes. The reaction system included 2 µL of 10× buffer, 1 µL deoxynucleoside triphosphate (10 mmol/L), 1 µL upstream and downstream primers, 0.1 µg DNA template, 2 U DNA polymerase, and double-distilled H2O to a total volume of 20 µL. After PCR, the specificity of the PCR product was detected using a gel imager after 2% agarose gel electrophoresis, and the PCR product was purified using PCR Purification Reagent (Qiagen, Hilden, Germany). Finally, an enzyme-digested reaction was performed using an anti-reaction system that included 1 µg PCR-purified product, 1 µL 10× buffer, 1.5 U (1306C/T) XspI endonuclease or 1.5 U (735C/T) HinfI endonuclease (New England Biolabs, Ipswich, MA, USA), and double-distilled H2O to a total volume of 10 µL, followed by incubation at 37°C for 16 hours. The results were determined using a gel imager after 2% agarose gel electrophoresis.
Statistical analyses
Continuous parameters are presented as the mean ± standard deviation, and categorical data are expressed as the percentage (%). Comparisons of continuous parameters among multiple groups were performed using one-way analysis of variance with the pair-wise comparison method (least significant difference), whereas comparisons of categorical data among multiple groups were performed using the chi-square test. The count data were compared among the three groups using the Bonferroni-corrected chi-square test, and because two single nucleotide polymorphisms (SNPs) were analyzed in this study, P < 0.0085 was considered statistically significant. The Hardy–Weinberg equilibrium method was used to detect the population representative of the samples. Multivariate logistic regression was used to evaluate the correlation between each factor and initial and recurrent ischemic stroke, and each association was presented as the odds ratio (OR) and its 95% confidence interval (CI). P < 0.05 indicated a statistically significant difference. The statistical analyses were performed using IBM SPSS Statistics for Windows, version 21.0 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
In this study, 380 cases each of first ischemic stroke and recurrent ischemic stroke were included, of which 70 cases were excluded because of the exclusion criteria and excessive bias of the experimental data. Overall, 350 patients with first ischemic stroke, 340 patients with recurrent ischemic stroke, and 360 healthy controls were included. The characteristics of the included participants are summarized in Table 1. Age, sex, body mass index, hypertension, diabetes, smoking, alcohol drinking, and serum levels of triglyceride, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and fasting plasma glucose were not significantly different among the included patients (Table 1). Total cholesterol (TC) levels were significantly higher in patients with first and recurrent ischemic stroke compared with the control group (P < 0.05; Table 1). However, there were no significant differences in TC levels between patients with recurrent and first ischemic stroke (Table 1).
Table 1.
Characteristics of the included patients.
| General data | Control group (n = 360) | First ischemic stroke (n = 350) | Recurrent ischemic stroke (n = 340) | P-value |
|---|---|---|---|---|
| Age (years) | 66.76 ± 10.54 | 67.12 ± 10.18 | 66.36 ± 13.29 | 0.69 |
| Male, n (%) | 207 (57.50) | 204 (58.29) | 197 (57.94) | 0.82 |
| BMI (kg/m2) | 26.88 ± 4.27 | 27.17 ± 5.71 | 27.96 ± 3.40 | 0.52 |
| Hypertension, n (%) | 194 (53.89) | 195 (55.71) | 193 (56.76) | 0.26 |
| Diabetes, n (%) | 76 (21.11) | 83 (23.71) | 82 (24.12) | 0.35 |
| Smoking, n (%) | 99 (27.50) | 95(27.14) | 93 (27.35) | 0.21 |
| Alcohol drinking, n (%) | 68 (18.89) | 60 (17.14) | 65 (19.11) | 0.75 |
| TC (mmol/L) | 4.31 ± 1.02 | 4.68 ± 1.05* | 4.72 ± 1.25* | 0.045 |
| TG (mmol/L) | 1.40 ± 0.67 | 1.42 ± 0.56 | 1.44 ± 0.68 | 0.88 |
| LDL-C (mmol/L) | 2.46 ± 0.73 | 2.58 ± 0.56 | 2.52 ± 0.84 | 0.17 |
| HDL-C (mmol/L) | 1.17 ± 0.48 | 1.21 ± 0.45 | 1.12 ± 0.36 | 0.08 |
| Fasting plasma glucose (mmol/L) | 5.18 ± 1.92 | 5.21 ± 1.42 | 5.30 ± 1.02 | 0.96 |
| MMP-2 (mmol/L) | 1.68 ± 0.24 | 2.93 ± 0.53* | 3.32 ± 0.94*▲ | 0.031 |
Data are given as the mean ± standard deviation unless otherwise indicated. *P < 0.05 vs. control group ▲P < 0.05 vs. first ischemic stroke group.
BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MMP-2, matrix metalloproteinase-2; TC, total cholesterol; TG, triglyceride.
Serum MMP-2 and first and recurrent stroke
Serum MMP-2 was significantly higher in patients with first and recurrent ischemic stroke compared with the control group (P < 0.05; Table 1). Moreover, patients with recurrent ischemic stroke had higher serum levels of MMP-2 than those with first ischemic stroke (P < 0.05; Table 1).
MMP2 1306C/T polymorphism and first and recurrent stroke
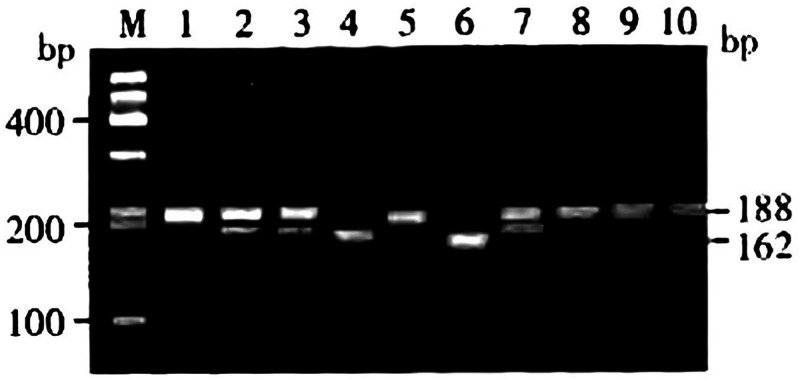
To analyze the MMP2 1306C/T polymorphism, the PCR amplification products were digested by XspI endonuclease and divided into three genotypes: homozygous CC (with only one band of 188 bp), heterozygous CT (with two bands of 188 and 162 bp), and homozygous TT (with only one band of 162 bp; Figure 1). The distribution of the MMP2 1306C/T polymorphism in patients with first and recurrent ischemic stroke and the control group is shown in Table 2. The distribution of MMP2 1306C/T genotypes and alleles were not significantly different among the three groups.
Figure 1.
Genotyping of MMP2 1306C/T.
Table 2.
Distribution of MMP2 1306C/T genotype and allele frequencies in the healthy control group, and in patients with first and recurrent ischemic strokes.
| Group | n |
Genotype |
Allele |
|||
|---|---|---|---|---|---|---|
| CC | CT | TT | C | T | ||
| Control group | 360 | 211 (58.61) | 140 (38.88) | 9 (2.50) | 562 (78.06) | 158 (21.94) |
| First ischemic stroke | 350 | 214 (61.14) | 129 (36.86) | 7 (2.00) | 557 (79.57) | 143 (20.42) |
| Recurrent ischemic stroke | 340 | 209 (61.47) | 122 (35.88) | 9 (2.64) | 540 (79.41) | 140 (20.59) |
| χ2 | 1.075 | 1.243 | 2.189 | 1.124 | 1.765 | |
| P-value | 0.32 | 0.26 | 0.19 | 0.28 | 0.22 | |
Data are given as the number (%).
MMP2 735C/T polymorphism and first and recurrent stroke
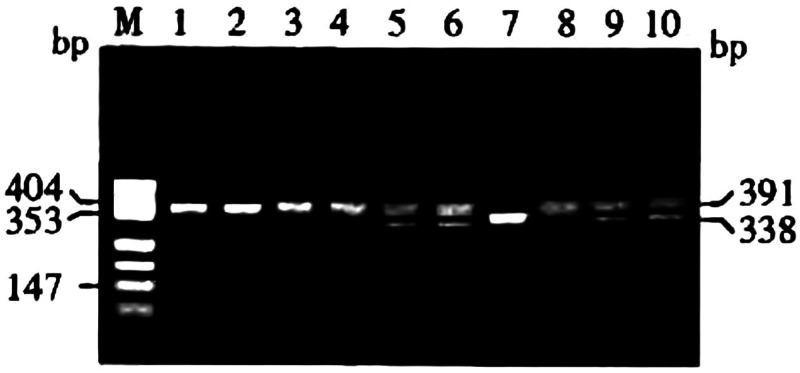
To measure the MMP2 735C/T polymorphism, the PCR amplification products were digested by HinfI endonuclease and divided into three genotypes: homozygous CC (with only one band of 391 bp), heterozygous CT (with three bands of 391 bp, 338 bp, and 53 bp), and homozygous mutant TT (with two bands of 338 bp and 53 bp; Figure 2). The frequency of the CC genotype of MMP2 735C/T was significantly higher in patients with first and recurrent ischemic stroke compared with the control group (P < 0.0085; Table 3). Moreover, the frequency of the CC genotype of MMP2 735C/T in patients with recurrent ischemic stroke was higher than that in patients with first ischemic stroke (P < 0.0085; Table 3). The frequencies of the CT, TT, and CT+TT genotypes of MMP2 735C/T were not significantly different among the three groups. The frequency of the C allele of MMP2 735C/T was higher in patients with recurrent ischemic stroke than in those with first ischemic stroke group, and was higher in patients with first ischemic stroke than in control individuals (P < 0.0085; Table 3). The frequency of the T allele of MMP2 735C/T was not significantly different between the three groups (Table 3). One-way analysis of variance revealed that there were significantly different TC and serum MMP-2 levels among the groups (P < 0.05), and these levels were subsequently included in the multivariate logistic regression analysis. The TC, MMP-2, and MMP2 735C/T genotypes and alleles were used as independent variables, and the first ischemic stroke group was used as the dependent variable for multivariate logistic regression analysis. Multivariate logistic analysis revealed that the CC genotype of the MMP2 735C/T polymorphism was independently associated with first ischemic stroke (OR: 1.45, 95% CI: 1.08–3.78; P = 0.03; Table 4), but the CT and TT genotypes were not. Moreover, multivariate logistic analysis revealed that the C allele of MMP2 735C/T, but not the T allele, was independently associated with first ischemic stroke (OR: 1.68, 95% CI: 1.58–3.29, P = 0.04; Table 4). Neither MMP-2 (OR: 2.37, 95% Cl: 1.85–5.76; Table 4) nor TC (OR: 1.67, 95% Cl: 1.33–3.68; Table 4) levels were independently associated with first ischemic stroke. One-way analysis of variance revealed a significant difference in serum MMP-2 levels (P < 0.05) between the recurrent ischemic stroke group and the control and first ischemic stroke groups; serum MMP-2 levels were thus included in the multivariate logistic regression analysis. The serum MMP-2 levels and MMP2 735C/T genotypes and alleles were used as independent variables, and the first ischemic stroke group was used as the dependent variable for the multivariate logistic regression analysis. Multivariate logistic analysis revealed that the CC genotype of the MMP2 735C/T polymorphism, but not the CT or TT genotypes, was also independently associated with recurrent ischemic stroke (OR: 1.64, 95% CI: 1.15–3.28, P = 0.02; Table 5). In addition, the C allele of MMP2 735C/T, but not the T allele, was independently associated with recurrent ischemic stroke (OR: 1.77, 95% CI: 1.18–2.68, P = 0.01; Table 5). However, serum MMP-2 was not independently associated with recurrent ischemic stroke (OR: 1.42, 95% Cl: 1.21–2.72; Table 5).
Figure 2.
Genotyping of MMP2 735C/T.
Table 3.
Distribution of MMP2 735C/T genotype and allele frequencies in the control group, and in patients with first and recurrent ischemic strokes.
| Group | n |
Genotype |
Allele |
||||
|---|---|---|---|---|---|---|---|
| CC | CT | TT | CT+TT | C | T | ||
| Control group | 360 | 255 (70.83) | 99 (27.50) | 6 (1.67) | 105 (29.17) | 609 (84.58) | 111 (15.42) |
| First ischemic stroke | 350 | 279 (79.71)* | 66 (18.86) | 5 (1.43) | 71 (20.29) | 624 (89.14)* | 76 (10.85) |
| Recurrent ischemic stroke | 340 | 293 (86.18)*▲ | 42 (12.35) | 5 (1.47) | 47 (13.82) | 628 (92.35)*▲ | 52 (7.65) |
| χ2 | 7.02 | 2.56 | 2.34 | 4.897 | 6.83 | 2.12 | |
| P-value | 0.001 | 0.72 | 0.87 | 0.75 | 0.002 | 0.98 | |
Data are presented as the number (%). *P < 0.0085 vs. control group; ▲P < 0.0085 vs. first ischemic stroke group.
Table 4.
Multivariate regression analysis for the associations between different genotypes and alleles of MMP2 735C/T and first ischemic stroke.
| B | OR | 95% CI | P-value | |
|---|---|---|---|---|
| TC | 0.42 | 1.67 | 1.33–3.68 | 0.47 |
| MMP-2 | 0.65 | 2.37 | 1.85–5.76 | 0.23 |
| CC | 0.82 | 1.45 | 1.08–3.78 | 0.03 |
| CT | 0.35 | 0.75 | 0.37–1.55 | 0.45 |
| TT | 0.48 | 0.63 | 0.52–5.17 | 0.42 |
| C | 0.76 | 1.68 | 1.58–3.29 | 0.04 |
| T | 0.62 | 0.87 | 0.51–1.61 | 0.32 |
OR, odds ratio; CI, confidence interval.
Table 5.
Multivariate regression analysis for the associations between different genotypes and alleles of MMP2 735C/T and recurrent ischemic stroke.
| B | OR | 95% CI | P-value | |
|---|---|---|---|---|
| MMP-2 | 0.73 | 1.42 | 1.21–2.72 | 0.17 |
| CC | 0.68 | 1.64 | 1.15–3.28 | 0.02 |
| CT | 0.46 | 0.39 | 0.16–1.01 | 0.34 |
| TT | 0.76 | 2.32 | 0.79–3.75 | 0.42 |
| C | 0.87 | 1.77 | 1.18–2.68 | 0.01 |
| T | 0.58 | 0.96 | 0.53–1.72 | 0.09 |
OR, odds ratio; CI, confidence interval.
Discussion
In this case–control study of patients with first and recurrent ischemic stroke and control individuals, although the serum levels of MMP-2 were significantly higher in patients with first and recurrent ischemic stroke than in the control group, the increase in MMP-2 was not independently associated with first or recurrent ischemic stroke. Moreover, the MMP2 1306C/T polymorphism genotypes and alleles were not different among patients with first ischemic stroke, recurrent ischemic stroke, and the control group. Notably, however, both the CC genotype and C allele of MMP2 735C/T were independently associated with first and recurrent ischemic stroke after adjusting for conventional risk factors for atherosclerosis. These results need to be validated in prospective cohort studies, and future studies should also determine the clinical implications of the CC genotype and C allele of the MMP2 735C/T polymorphism in ischemic stroke.
Acute ischemic stroke is considered to be a complex disease that results from interactions between genetic and environmental factors. 13 Previous studies have demonstrated that MMP-2 is closely related to angiogenesis and the inflammatory response, and that it plays an important role in the pathogenesis and development of ischemic stroke. 14 , 15 Pathophysiologically, MMP-2 degrades collagen fibers, elastic fibers, and other ECMs, resulting in weakened fibrous cap function and unstable carotid plaques, 16 and the increased vulnerability of these plaques. Moreover, MMP-2 may penetrate the basement membrane and blood–cerebrospinal fluid barrier and participate in vascular intima cellular aggregation, infiltration, and activation, leading to the rupture of atherosclerotic plaques. In addition, MMP-2 is involved in the inflammatory response after stroke, which may lead to secondary brain injury and impaired nerve repair processes. 17 Brouns et al. 18 reported that serum MMP-2 is significantly increased in patients with ischemic stroke in the acute phase, thus promoting ECM degradation. This results in the opening of the blood–brain barrier and increased vascular permeability, finally leading to vasogenic brain edema. Halder et al. 19 demonstrated that serum levels of MMP-2 are significantly correlated with the degree of neurological impairment after ischemic stroke. Elevated serum levels of MMP-2 can be observed even many years after a stroke occurs. 20 However, it remains unknown whether increased MMP-2 is associated with first stroke or recurrent stroke after controlling for conventional risk factors for atherosclerosis. In the present study, although the serum levels of MMP-2 were significantly higher in first and recurrent ischemic stroke than in the control group, increased MMP-2 was not independently associated with first or recurrent ischemic stroke. These findings suggest that polymorphisms in MMP2 may modify the association between MMP-2 levels and the risk of first and recurrent stroke.
The MMP2 gene is located on chromosome 16q13–21, has a molecular weight of 72 kD, and contains 13 exons and l2 introns. Multiple sequence variations exist in MMP2. However, few studies have evaluated the correlations between the polymorphic sites of 1306C/T and 735C/T and atherosclerotic vascular diseases. The polymorphic variability of MMP2 1306C/T and 735C/T refers to the substitution of C by T at positions 1306 and 735, upstream of the transcription promoter of MMP2. The promoter is directly involved in the transcriptional regulation of the gene, and the occurrence of these polymorphisms may have a direct impact on the transcriptional ability of MMP2. The linkage imbalance of MMP2 1306C/T results in the appearance of the 1306T haplotype. Compared with the 1306T/735T haplotype, the 1306C/735C haplotype is associated with significantly increased MMP2 mRNA levels. Previous functional analyses of MMP2 735C/T have revealed that 735C→T modifies the binding site of Spl (CCACC box), 21 , 22 leading to a decrease in transcriptional activity. Manso et al. 23 reported that MMP2 gene polymorphisms are associated with the outcome of ischemic stroke after 3 months; however, other studies have failed to show a significant association between MMP2 1306C/T and 735C/T sites and carotid intima–media thickness. 24 Liu et al. demonstrated that the 735C/T polymorphism of MMP2 is associated with ischemic stroke in a Chinese population. 25 Additionally, Xu et al. reported that the 1306C/T polymorphism of MMP2 is associated with atherosclerotic cerebral infarction. 26 In the present study, however, the MMP2 1306C/T polymorphism and its alleles were not different among patients with first ischemic stroke, patients with recurrent ischemic stroke, and the control group. In contrast, the CC genotype and C allele of MMP2 735C/T were independently associated with first and recurrent ischemic stroke after adjusting for conventional risk factors for atherosclerosis. The potential mechanisms underlying our findings remain unknown. However, it can be hypothesized that the CC genotype and C allele of MMP2 735C/T may increase the risk of ischemic stroke via MMP-2-dependent mechanisms. On the one hand, locally accumulated MMP-2 degrades the ECM of the vascular wall, induces the proliferation of macrophages to form foam cells, and causes the inflammatory response of the vascular wall, promoting the formation of atherosclerotic plaque. On the other hand, it can also destroy the fibrous cap on the plaque surface, promote the rupture of atherosclerotic plaque, and lead to the rapid progression of atherosclerotic injury and plaque expansion secondary to thrombosis and pathogenesis, resulting in another ischemic stroke. Concurrently, the cerebral blood–brain barrier is destroyed, vascular permeability is increased, and locally elevated MMP-2 enters the peripheral circulation system, resulting in elevated serum MMP-2 levels. This may aggravate both the inflammatory and oxidative stress responses, which are closely related to recurrent ischemic stroke. 25 Future studies are needed to determine the precise mechanisms and clinical implications of the CC genotype and C allele of the MMP2 735C/T polymorphism for ischemic stroke.
In the current study, the associations between MMP2 gene polymorphisms and first and recurrent ischemic stroke were explored, and the risk factors for recurrent ischemic stroke were analyzed; these may be used to predict the outcome and prognosis of cerebral infarction. However, the limitations of this study should be considered when interpreting the results. First, the sample sizes were small. Thus, these findings should be validated in large-scale prospective cohorts in the future. Second, a causative association between the CC genotype and C allele of the MMP2 735C/T polymorphism and ischemic stroke was unable to be derived from our findings because it was an observational study. Moreover, we were unable to exclude the possibility that some residual factors, such as concurrent medications, may confound the association between the CC genotype and C allele of the MMP2 735C/T polymorphism and ischemic stroke. Finally, this study was performed in a Chinese population. The association between the CC genotype and C allele of the MMP2 735C/T polymorphism and ischemic stroke should also be evaluated in patients of other ethnicities.
Conclusion
The results of this study indicate that the CC genotype and C allele of MMP2 735C/T are independently associated with first and recurrent ischemic stroke. These results should be validated in prospective cohort studies, and the clinical implications for ischemic stroke of the CC genotype and C allele of the MMP2 735C/T polymorphism should be determined in future studies.
Footnotes
Author contributions: YL was mainly responsible for writing the manuscript, QO contributed to the collection of the case data, and these two authors contributed equally to this study and share first authorship. MY designed most of the investigation and provided guidance in the manuscript writing. JL provided pathological assistance. XC, LL, and LX contributed to the interpretation of the data. All authors have read and approved the final manuscript.
Availability of data and materials: The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Ming Yu https://orcid.org/0000-0002-5036-0624
References
- 1.Chen L, Yang Q, Ding R, et al. Carotid thickness and atherosclerotic plaque stability, serum inflammation, serum MMP-2 and MMP-9 were associated with acute cerebral infarction. Exp Ther Med 2018; 16: 5253–5257. DOI: 10.3892/etm.2018.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Writing group of guidelines for the diagnosis and treatment of acute ischemic stroke in cerebrovascular disease group: Guidelines for the diagnosis and treatment of acute ischemic stroke in China 2010. Chin J Neurol 2010; 43: 146. DOI: 10.3760/cma.j.issn.1006-7876.2010.02.022. [Google Scholar]
- 3.Kolb B, Saber H, Fadel H, et al. The endocannabinoid system and stroke: a focused review. Brain Circ 2019; 5: 1–7. DOI: 10.4103/bc.bc_29_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neurology Branch of Chinese Medical Association: Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2014. Chin J Neurol 2015; 48: 246–257. DOI: 10.3760/cma.j.issn.1006-7876.2015.04.002. [Google Scholar]
- 5.Mukherjee D, Patil CG. Epidemiology and the global burden of stroke. World Neurosurg 2011; 76: S85–S90. DOI: 10.1016/j.wneu.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 6.Daskalopoulou SS, Daskalopoulos ME, Perrea D, et al. Carotid artery atherosclerosis: what is the evidence for drug action? Curr Pharm Des 2007; 13: 1141–1159. DOI: 10.2174/138161207780619019. [DOI] [PubMed] [Google Scholar]
- 7.Stylianou IM, Bauer RC, Reilly MP, et al. Genetic basis of atherosclerosis: insights from mice and humans. Circ Res 2012; 110: 337–355. DOI: 10.1161/circresaha.110.230854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tu WJ, Dong X, Zhao SJ, et al. Prognostic value of plasma neuroendocrine biomarkers in patients with acute ischaemic stroke. J Neuroendocrinol 2013; 25: 771–778. DOI: 10.1111/jne.12052. [DOI] [PubMed] [Google Scholar]
- 9.Dan L, Yachun W, Hongying S, et al. MMP-2 and MMP-9 gene polymorphisms and clinical classification and prognosis of ischemic stroke. Chin J Pract Neurol Dis 2011; 14: 3–5. DOI: 10.3969/j.issn.1673-5110.2011.07.002. [Google Scholar]
- 10.Wågsäter D, Zhu C, Björkegren J, et al. MMP-2 and MMP-9 are prominent matrix metalloproteinases during atherosclerosis development in the Ldlr(-/-)Apob(100/100) mouse. Int J Mol Med 2011; 28: 247–253. DOI: 10.3892/ijmm.2011.693. [DOI] [PubMed] [Google Scholar]
- 11.Neurology Society of Chinese Medical Association. Diagnosis of major cerebrovascular diseases in China. Chin J Neurol 2019; 52: 710–715. DOI: 10.3760/cma.j.issn.1006-7876.2019.09.003. [Google Scholar]
- 12.Zeng JS, Pu CO. Evolution and update of the main diagnosis points of various cerebrovascular diseases in China. Chin J Neurol 2019; 52: 681–683. DOI: 10.3760/cma.j.issn.1006-7876.2019.09.001. [Google Scholar]
- 13.Yang N, Lin M, Wang BG, et al. Low level of low-density lipoprotein cholesterol is related with increased hemorrhagic transformation after acute ischemic cerebral infarction. Eur Rev Med Pharmacol Sci 2016; 20: 673–678. [PubMed] [Google Scholar]
- 14.Han SW, Kim SH, Lee JY, et al. A new subtype classification of ischemic stroke based on treatment and etiologic mechanism. Eur Neurol 2007; 57: 96–102. DOI: 10.1159/000098059. [DOI] [PubMed] [Google Scholar]
- 15.Jacob-Ferreira AL, Schulz R. Activation of intracellular matrix metalloproteinase-2 by reactive oxygen-nitrogen species: consequences and therapeutic strategies in the heart. Arch Biochem Biophys 2013; 540: 82–93. DOI: 10.1016/j.abb.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 16.Lin R, Yu K, Li X, et al. Electroacupuncture ameliorates post-stroke learning and memory through minimizing ultrastructural brain damage and inhibiting the expression of MMP-2 and MMP-9 in cerebral ischemia-reperfusion injured rats. Mol Med Rep 2016; 14: 225–233. DOI: 10.3892/mmr.2016.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guangrun X, Qinghai W, Guixiang W. The relationship between matrix metalloproteinase-2 and its inhibitors and the vulnerability of carotid atherosclerotic plaque. Chin J Neurosci 2009; 8: 690–693. DOI: 10.3760/cma.j.issn.1671-8925.2009.07.011. [Google Scholar]
- 18.Brouns R, Wauters A, De Surgeloose D, et al. Biochemical markers for blood-brain barrier dysfunction in acute ischemic stroke correlate with evolution and outcome. Eur Neurol 2011; 65: 23–31. DOI: 10.1159/000321965. [DOI] [PubMed] [Google Scholar]
- 19.Halder AK, Saha A, Jha T. Exploring QSAR and pharmacophore mapping of structurally diverse selective matrix metalloproteinase-2 inhibitors. J Pharm Pharmacol 2013; 65: 1541–1554. DOI: 10.1111/jphp.12133. [DOI] [PubMed] [Google Scholar]
- 20.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 2001; 17: 463–516. DOI: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SK, Kang SW, Park HJ, et al . Meta-analysis of association of the matrix metalloproteinase 2 (-735 C/T) polymorphism with cancer risk. Int J Clin Exp Med 2015; 8: 17096–17101. [PMC free article] [PubMed] [Google Scholar]
- 22.Rahimi Z, Yari K, Rahimi Z. Matrix metalloproteinase-9 -1562T allele and its combination with MMP-2 -735 C allele are risk factors for breast cancer. Asian Pac J Cancer Prev 2015; 16: 1175–1179. DOI: 10.7314/apjcp.2015.16.3.1175. [DOI] [PubMed] [Google Scholar]
- 23.Manso H, Krug T, Sobral J, et al. Variants of the matrix metalloproteinase-2 but not the matrix metalloproteinase-9 genes significantly influence functional outcome after stroke. BMC Med Genet 2010; 11: 40. DOI: 10.1186/1471-2350-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niu F, Wei B, Yan M, et al. Matrix metalloproteinase-2 gene polymorphisms are associated with ischemic stroke in a Hainan population. Medicine (Baltimore) 2018; 97: e12302. DOI: 10.1097/md.0000000000012302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CT WL. Correlation of MMP-2 gene polymorphism with disease relapse as well as inflammatory mediators and oxidative stress molecules in patients with ischemic stroke. J Hainan Med Univ 2017; 23: 1855–1858. DOI: 10.13210/j.cnki.jhmu.20170628.001. [Google Scholar]
- 26.Xu L, Rx Z, Q L. Association between MMP-2 gene polymorphism and atherosclerotic cerebral infarction. Adv Anat Sci 2016; 22: 273–275. DOI: CNKI:SUN:JPKX.0.2016-03-012. [Google Scholar]