Abstract
Objective
Spasticity is a frequent complication after spinal cord injury (SCI), but the existing therapies provide only limited relief and are associated with adverse reactions. Therefore, we aimed to develop a novel strategy to ameliorate the spasticity induced by SCI.
Methods
This nonrandomized controlled study used a repeated measurement design. The study involved four monkeys, two of which served as controls and only underwent spinal cord hemisection surgery at the T8 spine level. The other two monkeys underwent transplantation of sural nerve segments into the injured sites and long-term infusion of acidic fibroblast growth factor (aFGF). All monkeys received postoperative exercise training and therapy.
Results
The combined therapy substantially reduced the spasticity in leg muscle tone, patella tendon reflex, and fanning of toes. Although all monkeys showed spontaneous recovery of function over time, the recovery in the controls reached a plateau and started to decline after 11 weeks.
Conclusions
The combination of peripheral nerve grafting and aFGF infusion may serve as a complementary approach to reduce the signs of spasticity in patients with SCI.
Keywords: Spinal cord injury, spasticity, peripheral nerve grafting, acidic fibroblast growth factor, physical therapy, animal model
Introduction
Spasticity is a frequent complication after spinal cord injury (SCI). As a component of upper motoneuron syndrome, spasticity is a motor disorder characterized by a velocity-dependent increase in tonic stretch reflexes, exaggerated tendon jerks, and hyperexcitability of the stretch reflex.1–5 It occurs a few weeks after acute SCI and may develop over months or years. 6 Spasticity is not a static phenomenon, and if untreated, it can lead to secondary reorganization of both the nervous system and musculature. 7 Patients with serious SCI may develop paresis, leading to adaptive shortening of muscles that change afferent input to the spinal cord. This exacerbates the spasticity and causes the development of contractures, abnormal positioning, and further loss of function.
Early medication and physical activity can reportedly interfere with the development of spasticity.8,9 The pharmacological agents prescribed as first-line treatment for individuals with SCI typically reduce spinal cord excitability. 10 Baclofen, a GABA analog, is the most widely used medication for this purpose and is known to inhibit monosynaptic and polysynaptic spinal reflexes.11,12 However, the effects of baclofen are limited because only a small portion of the active substance penetrates the blood–brain barrier, and the drug frequently causes adverse effects such as sedation, nausea, dizziness, and difficulty breathing. 11 Sudden termination of drug treatment may also induce epileptic seizures, psychosis, and hyperthermia. 13 Other agents, including tizanidine, 14 benzodiazepines, 11 clonidine, and gabapentin, 12 may reduce spasticity; however, their effectiveness as spasmolytics in clinical practice is even more limited than that of baclofen.
Surgical treatments are suitable for patients with nonprogressive conditions.15,16 Orthopedic interventions are applied as a form of physiotherapy for local spasticity, botulinum toxin injections and tendon plasty are used to treat intractable local spasticity with joint deformation, and rhizotomy is used for pronounced regional spasticity. However, these surgical treatments entail irreversible changes and reportedly have variable and unpredictable therapeutic effects. 17 Numerous physical strategies have also been reported to effectively treat spasticity and improve motor function.18,19 Although various guidelines have recommended exercise to counteract the spasticity, the effect of physical therapy on spasticity needs to be evaluated in detail.
The currently available therapies only provide limited relief and are associated with a high risk of adverse effects, and the resultant substantial enduring disability has necessitated the identification of therapeutic measures to ameliorate the spasticity in patients with SCI. Therefore, the present study was performed to develop a novel strategy to ameliorate SCI-associated spasticity. We performed spinal cord hemisection surgery in four monkeys and then conducted a series of surgical procedures for transplantation of sural nerve fragments into the injured site for structural support in two of these monkeys, who also simultaneously received a fibrin-based mixture containing nerve acidic fibroblast growth factor (aFGF) into the site of damage to stabilize the peripheral nerve grafts. The other two monkeys, which served as controls, only underwent right unilateral surgical hemisection at the T8 spine level with a 5-mm gap. All four monkeys received postoperative exercise training and physical therapy. Clinical evaluation was performed to test the spasticity values during the recovery period. We hope that this approach can yield improvements in terms of reduced spasticity and/or improved residual motor control with few or no adverse effects of anti-spastic medications.
Materials and methods
Animals
Four male rhesus monkeys (Macaca mulatta) weighing 5 to 7 kg were used in this study. The monkeys were housed in a vivarium accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. The experiments were conducted in accordance with the National Institutes of Health Policy on Humane Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Arizona State University (Permit number: 07-944R).
Primate surgery
Food and water restrictions were initiated 12 hours before the surgery. All surgical procedures were performed under general anesthesia. Anesthesia was induced with an intramuscular injection of a mixture of ketamine hydrochloride (10 mg/kg) and xylazine (2 mg/kg). Endotracheal intubation was then performed, and anesthesia was subsequently maintained with a mixture of isoflurane (1%–2%) and oxygen (100%), which was administered with a standard anesthesia machine. Throughout the surgery, all animals’ heart rate, blood pressure, oxygen saturation, respiratory rate, respiratory pressure, tidal volume, and core body temperature were monitored using a medical multiparameter monitor and maintained within physiological limits by a specialty veterinarian.
All monkeys underwent spinal cord hemisection at the T8 level. The two monkeys in the repair group underwent transplantation of sural nerve segments as well as aFGF infusion into the injured spinal cord. The other two monkeys (control group) underwent exactly the same hemisection operation to induce lesions but received no repair treatment.
T8 spinal cord hemisection
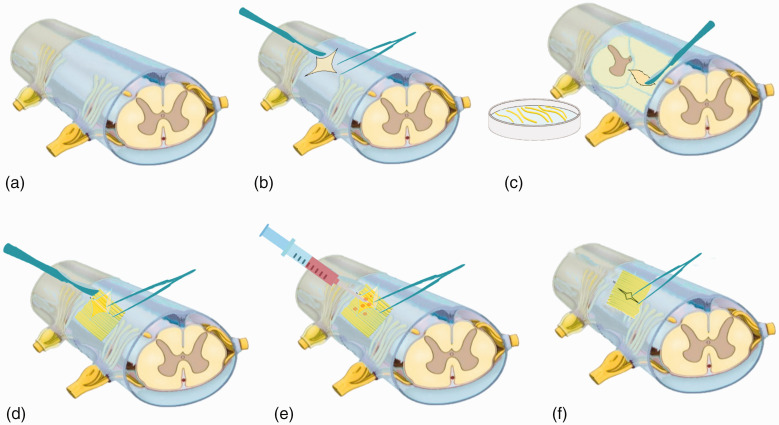
All animals were maintained in the prone position on a surgical table. The dorsal hair was shaved, and 70% alcohol and iodine were used to disinfect the skin. A midline incision was made above the T8 spinous process, and the paraspinal muscles were dissected (Figure 1(a)). Total laminectomy was subsequently conducted under a surgical microscope. The dura mater was then exposed and opened longitudinally at the midline to uncover the spinal cord (Figure 1(b)). Right-side spinal hemisection of 5 mm in length was performed with micro-scissors, and a surgical microscope was used to ensure that neural tissue was completely removed from the gap.
b. Sural nerve transplantation and spinal cord repair
Figure 1.
Images showing the spinal cord surgery performed in the monkeys. (a) Unmanipulated spinal cord after total laminectomy at the T8 level. (b) The dura mater was opened longitudinally at the midline to uncover the spinal cord. (c) The sural nerve was harvested, cut into several segments, and placed in Hank’s balanced salt solution. (d) Sural nerve segments were extracted and used to fill the space of the spinal cord stumps. (e) Acidic fibroblast growth factor was infused into the grafted site. (f) The dura mater was sutured.
The right soleus and gastrocnemius of the monkeys in the repair group were fully exposed and separated. The sural nerve was excised, harvested, and cut into several segments with lengths of approximately 6 mm; these segments were then placed in Hank’s balanced salt solution before grafting (Figure 1(c)). After T8 spinal cord hemisection, several minced pieces of the sural nerve segments were extracted and used to fill the space containing the stumps of the spinal cord; this generated a base that could provide structural support to the nerve segments as they extended from the proximal sites of white matter to the distal sites of gray matter (Figure 1(d)). The peripheral nerve graft was then stabilized by using a fibrin-based mixture to fill the sites of injury. First, 5 µg of aFGF (R&D Systems, Minneapolis, MN, USA) was added to a solution containing fibrinogen (100 mg/mL) and aprotinin (200 Kallikrein Inhibitor Unit [KIU]/mL). To this solution, 10 µL of thrombin (40 IU/mL) plus calcium chloride was added to produce an aFGF-containing (10 µg/mL) mixture (final volume of approximately 10 µL) that was immediately placed in the grafted site (Figure 1(e)). In all cases, the dura mater was then closed in a watertight manner (Figure 1(f)). The procedures for nerve transplantation were consistent with those performed in previous studies on rats, 20 cats, 21 and monkeys. 22
The dura mater was then sutured with 4-0 monofilament sutures. After surgery, the spinal column was stabilized with transpedicular screws and plates at the T6 and T10 level, respectively. After all surgical procedures were completed, 1-0 monofilament sutures were used to suture the fascia and skin in layers, covering the laminectomized region.
c. Postoperative care
Analgesic treatment was initiated immediately after completion of all surgical procedures and continued for 5 days. After recovery from anesthesia, all animals were returned to postoperative care and then allowed to recover for 1 month prior to behavioral testing. Antibiotics (intramuscular cefazolin at 20 mg/day for 5 days postoperatively) and analgesics (oral pentazocine tablets at 2 mg/kg body weight once daily or intramuscular buprenorphine at 0.3 mg/kg twice daily) were administered for 1 week following surgery. Corticosteroid therapy (intramuscular dexamethasone at 1 mg/kg once daily) was used to reduce inflammation after surgery. Colon and bladder autonomic emptying functions were not compromised by the hemisection procedure. The monkeys’ food, fruit, vegetable, and water intake was closely monitored on a daily basis until their normal appetite resumed.
Postoperative physical therapy
All monkeys received postoperative exercise training and therapy to prevent muscle mass loss, maintain the range of motion of all joints, and promote functional recovery. The training included regular stretching exercises to help maintain flexibility and temporarily reduce muscle tightness, standing with a supportive monkey chair to help stretch the muscles, and progressive adjustment to the desired position to help maintain flexibility and assume a position that did not trigger a spasm. They were also allowed to freely ambulate upright on a treadmill with their collar fixed in a specially designed monkey chair, with their hands supporting their body weight. The therapy was conducted in two 20-minute sessions per day for 5 days per week.
Clinical evaluation of spasticity during postoperative recovery
Spasticity measurements were performed using clinical evaluations. We selected the triceps surae muscle tone, patella tendon reflex, and fanning of toes as the clinical parameters for evaluation of spasticity. The modified Ashworth scale is the most widely used method for assessing muscle spasticity in clinical practice and research. In the present study, we applied the modified Ashworth scale to measure the triceps surae muscle tone using the following 6-grade system.
Grade 0: No increase in muscle tone
Grade 1: Slight increase in tone causing a catch when the leg is moved in extension or flexion
Grade 1+: Slight increase in tone manifesting as a catch followed by minimal resistance throughout the range of motion
Grade 2: More marked increase in tone, although the leg can be mostly easily flexed
Grade 3: Considerable increase in tone making passive movement difficult
Grade 4: Rigidity of leg in extension or flexion
The patella tendon reflex was evaluated using the tendon reflex score with the following 6-grade system. 23
Grade 0: No reflex response
Grade 1: Decreased reflex (lower than normal)
Grade 2: Normal reflex
Grade 3: Increased reflex (brisk)
Grade 4: Very increased reflex (very brisk)
Grade 5: Increased reflex with pathologic reflex such as ankle clonus
Fanning of toes is a classic component of upper motoneuron syndrome.24,25 In the present study, we measured Babinski’s sign using the following 4-grade system:
Grade 0: No response
Grade 1: Slight fanning of toes
Grade 2: Moderate fanning of toes
Grade 3: Severe fanning of toes
Behavioral analysis
Gait analysis was conducted to study the monkeys’ gait patterns. In this analysis, the stepping data were analyzed to identify different gait modes, and the quality of functional recovery was evaluated by determining gait symmetry. Gait symmetry was defined as the ratio of the average number of steps taken using one leg to the average number of steps taking using the ipsilateral leg in multiple 20-s intervals.
Results
Clinical evaluation of triceps surae muscle tone using modified Ashworth scale
All monkeys underwent the standard clinical evaluation of spasticity, and their modified Ashworth scale scores are shown in Figure 2(a). By moving the monkeys’ legs and feet within the range of motion and grading the findings according to the modified Ashworth scale, we found that the treated monkeys showed substantially fewer neurological impairments than the monkeys in the control group.
Figure 2.
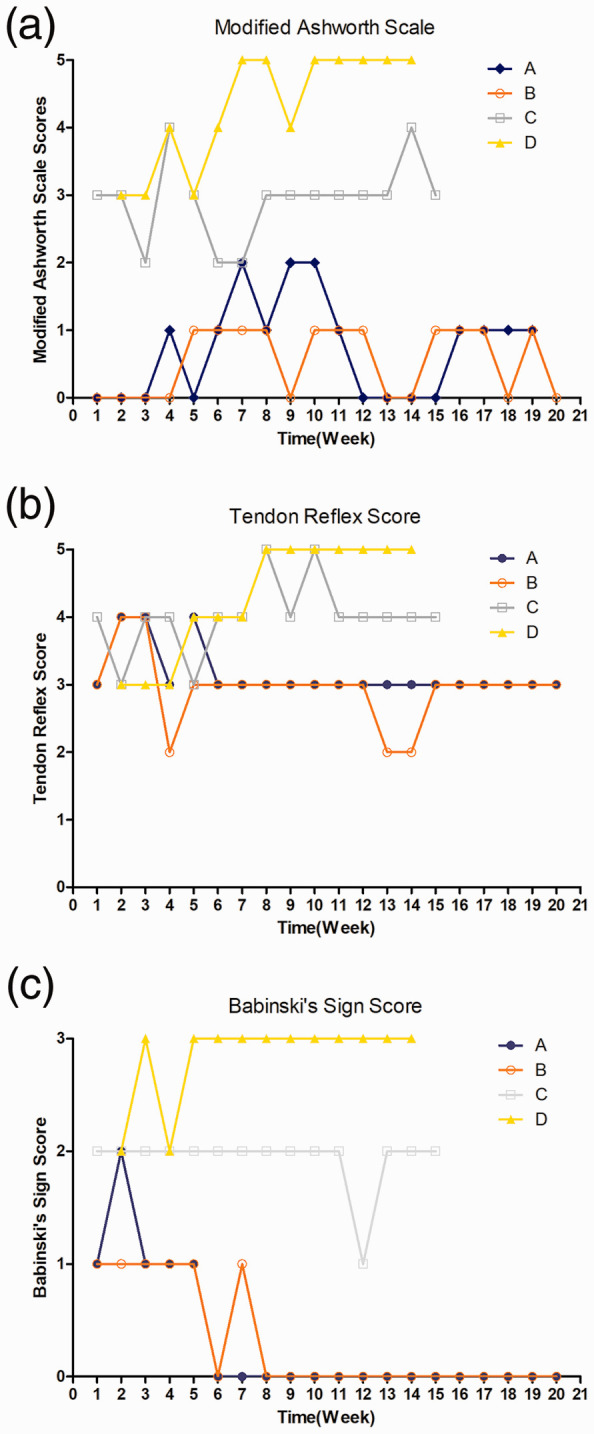
Spasticity in the affected legs of monkeys (A and B, nerve repair group; C and D, control group) was evaluated by moving the legs and feet through the available range of motion. (a) Spasticity evaluation with the modified Ashworth scale. (b) Spasticity evaluation with the tendon reflex scores. (c) Spasticity evaluation with the Babinski’s sign score.
Clinical evaluation of patellar tendon reflex using tendon reflex score
The tendon reflex score was used to evaluate the patellar tendon reflex. Figure 2(b) shows that the tendon reflex scores of the monkeys in the repair group were markedly lower than those of the monkeys in the control group.
Clinical evaluation of fanning of toes using Babinski’s sign score
Babinski’s sign score was used to evaluate fanning of toes in the monkeys. Figure 2(c) shows that the monkeys in the repair group had substantially lower Babinski’s sign scores than the monkeys in the control group.
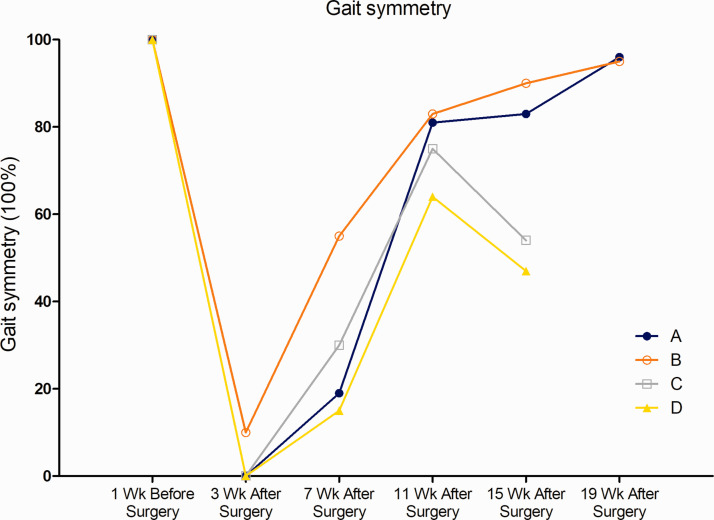
Behavioral effects of grafting on gait symmetry
Figure 3 shows the gait symmetry of the four monkeys. Both treated monkeys were able to start stepping at 4 weeks after the injury. The two monkeys in the control group also showed some step ability at the same time point, but the improvement in their step ability seemed to stop and even started to decline from the 11th week. Notably, the treated monkeys showed fewer symmetrical changes in gait on a daily basis.
Figure 3.
The quality of functional recovery was evaluated by assessing gait symmetry. Both treated monkeys (A and B) and control monkeys (C and D) started to show the ability to take steps at the 4-week point, which likely indicated spontaneous recovery from the injury. This improvement persisted until approximately 11 weeks in the control monkeys, after which their step ability started to deteriorate. In contrast, the treated monkeys (A and B) continued to show improvement in their step ability (shorter bias bars).
Discussion
This study showed that SCI-induced spasticity could be significantly reduced by combining postoperative physical therapy with peripheral nerve grafting into the injured spinal cord sites and the application of a mixture of aFGF growth factors. Because various spinal cord lesions can induce different forms of dysfunction, we used a reproducible hemisection model of SCI in nonhuman primates. An important reason for the use of primates in this study was to facilitate more rigorous evaluation of neurological recovery in the paralyzed leg. All animals, regardless of whether they received a graft, developed flaccid paralysis of the ipsilateral lower extremity and loss of sensation to painful stimuli on the contralateral side immediately after surgery. More specifically, the animals lost their hind limb grasping ability and could not bear weight on the paralyzed limb. Spontaneous recovery of the limb ipsilateral to the side of the hemisection occurred over time in all monkeys.
The treatment for SCI-induced spasticity is focused on rehabilitation of patients to relieve pain and improve daily activities. Baclofen has been widely proven to improve SCI-induced spasticity and is also approved by the United States Food and Drug Administration for effective treatment of spasticity caused by SCI. 11 Baclofen can be taken orally or by injection. However, oral baclofen has limited lipid solubility and therefore crosses the blood–brain barrier poorly. Thus, high doses of oral baclofen are needed to achieve therapeutic effects, and these high doses often lead to adverse effects including sedation, nausea, dizziness, hypotension, confusion, and weakness. 12 Intrathecal baclofen is typically administered by a refillable reservoir and a programmable pump implanted surgically in the abdominal wall, either subcutaneously or subfascially. Although this approach requires much lower doses than oral baclofen, use of the baclofen pump can result in mechanical or infectious complications. 13 Moreover, baclofen is a systemic, not local, therapeutic agent. It can cause weakness of the spastic limbs, sedation, dizziness, fatigue, headache, and ataxia, and its sustained use may cause drug addiction, with sudden withdrawal resulting in hallucinations and seizures. 26 These limitations have necessitated efforts to seek new and innovative alternatives for the treatment of SCI-induced spasticity.
Implantation of Schwann cell-containing peripheral nerve grafts into a traumatically induced defect in the spinal cord has been reported to significantly promote the ingrowth of peripherally myelinated axons in lower mammals. However, the neural mechanisms of injury and neural regeneration in primates may substantially differ from those in lower mammals. Numerous successful applications of a nerve-grafting approach to promote regeneration after SCI have been reported in non-rodent large animal models. Peripheral nerve grafting into the rat and cat spinal cord can support axon regeneration after acute or chronic injury, with synaptic reconnection noted across the lesion site and some level of behavioral recovery. 27 In the present study, we grafted a peripheral nerve into the acute injured spinal cord of monkeys as a preclinical treatment approach to promote regeneration for eventual translational use. We used the autologous sural nerve instead of allotransplants to reduce the possibility of graft rejection.
Segments of the sural nerve may contain intracellular neurotrophic factor and Schwann cells. 28 Thus, placement of these segments directly within the traumatically induced defect in the spinal cord may result in the formation of a bridge across the lesion. First, peripheral nerves are good substrates for bridging in cases of central nervous system trauma. In the present study, the nerve fragments may have reconnected the interrupted neural tracts and promoted nerve repair from the peripheral Schwann cells. Placement of Schwann cell-containing peripheral nerve grafts into the traumatically induced defect in the spinal cord significantly promoted the ingrowth of peripherally myelinated axons. Second, the nerve fragments may have formed a network similar to a sponge. This structure was conducive to the addition of aFGF, which may have diminished the inflammatory response and subsequent scar formation.
Numerous studies have shown that the addition of growth factors to a grafted peripheral nerve bridge can promote axonal growth, reduce glial scarring and neurite outgrowth, and result in functional recovery. 29 Favorable outcomes have been obtained in experimental studies focused on members of the neurotrophin family, such as nerve growth factor, brain-derived neurotrophic factor, and neurotrophin-3. Apart from neurotrophins, the fibroblast growth factor (FGF) family also plays an important role in these processes. FGFs are a family of growth factors that are involved in embryonic development, wound healing, angiogenesis, and various endocrine signaling pathways. Among them, aFGF has been investigated for its role in nerve repair; it is mitogenic and pluripotent in nature and can mitigate glial scarring and enhance axonal growth. Li et al. 30 found that aFGF can improve functional recovery by inducing peroxiredoxin 1 to regulate autophagy and anti-reactive oxygen species after SCI in a rat model. Tsai et al. 31 used a proteomics approach and showed that aFGF can downregulate expression of the proteins involved in the process of secondary injury after SCI.
Furthermore, aFGF has already been used in clinical practice. As described in a review by Ko et al., 28 Dr. Henrich Cheng and his team have conducted multiple human and animal trials using aFGF to repair spinal cord or peripheral nerve injuries in Taiwan during the last couple of decades, and these studies showed that aFGF was effective. In 2004, a patient with chronic paraplegia due to spinal cord hemisection from a stab injury 3 years previously obtained significant motor and sensory recovery 2.5 years after therapy involving four sural nerve grafts and aFGF at the T11 level. 32 Additionally, a 20-µg aFGF bolus injection was administered via intradural lumbar puncture for treatment of a 28-year-old patient who presented with a 4-year history of spastic paralysis, and the authors described the details of the treatment and the functional recovery of sensation, muscle activities, and gait pattern. 33 In a clinical trial, 49 patients with SCI were treated by application of aFGF with fibrin glue and duraplasty via lumbar puncture at 3 and 6 months post-laminectomy surgery. 34 The patients attained significant improvements in motor, sensory, and neurological levels as well as in functional independence measures at 2 years after treatment. Another clinical trial of aFGF with an extended 4-year follow-up period also showed that all patients with SCI attained recovery of motor function and a decrease in dependence, with no major adverse events or other oncological problems. 35 In brief, aFGF with or without nerve grafting shows advantages in terms of neurological function improvement in human patients with SCI. Moreover, aFGF has shown promising results for neural regeneration in human patients with cervical root injury, 36 brachial plexus injury, 37 and common peroneal nerve injury. 38 Growing evidence has also shown that aFGF can ameliorate hyperglycemia, increase insulin sensitivity, and relieve neuropathic impairment. 39
The existing imaging methods have limited effectiveness for evaluating acute SCI during transient axonal dysfunction and spinal shock. However, neuroelectrophysiological techniques show high sensitivity and accuracy and can allow quantitative measurement of the degree of SCI and clarify the characteristics of SCI-induced functional loss. As the new standard for examining nerve function, neuroelectrophysiological evaluations have received increasing attention as auxiliary clinical examinations for evaluating the severity of SCI. Neuroelectrophysiological evaluations are also widely used to assess and quantify spinal cord function in laboratory animals. 40
Mekhael et al. 41 found that transcranial direct current stimulation can relieve spasticity in anesthetized and awake mice with SCI through testing of velocity-dependent ankle resistance and the associated electromyographical activities. Sadlaoud et al. 42 analyzed the association between the glycinergic receptor expression rate and dependent depression of Hoffmann’s reflex (the H-reflex) in mice and verified that alterations of glycinergic inhibition of lumbar spinal motoneurons are involved in the mechanisms underlying spasticity after SCI. Corleto et al. 43 characterized a thoracic complete transection-induced spasticity model in rats and described the corresponding features using systematic electromyography and histopathological methods. Côté et al. 21 demonstrated the successful application of a nerve-grafting approach to promote regeneration after SCI in a non-rodent large animal model. Although they recorded motor-evoked potentials and somatosensory potentials, their results only served as an indicator of nerve repair and did not elucidate the relationship between electrophysiology and spasticity. Levi et al. 44 and Ko et al. 22 both developed reproducible models of SCI in nonhuman primates and verified the effectiveness of a combination of peripheral nerve grafts and aFGF infusion for repairing incomplete SCI. Although the magnetic motor-evoked potentials and somatosensory evoked potentials recorded in the latter study provided evidence of repair, the authors did not discuss the relationship between electrophysiologic changes and spasticity.
The neural mechanisms underlying injury and neural regeneration in primates may substantially differ from those in lower mammals. However, there are still relatively few studies on monkeys with SCI, especially monkeys with SCI-induced spasticity. Electrophysiological assessments of animals after peripheral nerve grafting presents major technical challenges because the physiologic effects obtained by direct electrical activation of neuronal fibers within the graft and those caused by current spread or conduction along intact tracts are difficult to distinguish. The H-reflex, which is the electrophysiological equivalent of the monosynaptic stretch reflex, has long been used to study the excitability of motor neurons induced by the activation of Ia afferents. 45 Generally, the rate-dependent depression of the H-reflex is viewed as the only electrophysiological measurement correlated with the degree of spasticity in patients.
Magnetic resonance imaging, histologic examination, immunostaining, and other methods have also been used to evaluate the effects and explore the underlying mechanisms. Ko et al. 22 analyzed the spinal cord cephalic to, at, and caudal to the lesion site between repair and control monkeys. In both groups, the lesion center sustained the largest area of inflammation, and the repair group demonstrated smaller inflammatory areas at each corresponding position compared with the control group. Staining showed that hypercellularity, suggesting an inflammatory response, after spinal cord hemisection was significantly suppressed in the repair group with respect to both the extending area and the thickness. Côté et al. 21 demonstrated the presence of c-Fos immunoreactive neurons close to the distal apposition site by immunohistochemical analysis, indicating that regenerated axons formed functional synapses with host neurons. Four months after grafting, Levi et al. 44 sectioned and immunostained the spinal cord–graft site of monkeys and found that the grafts significantly enhanced the regeneration of myelinated axons into the region of the hemisected spinal cord.
Muscle relaxants are good therapeutic options because they usually decrease muscle tone contractions of paralyzed muscles and hyperreflexia. Physical therapy, including stretching, 46 range-of-motion exercises, 47 static weight-bearing, 48 and passive cycling, 49 have been considered useful methods to reduce spasticity and avoid the adverse reactions associated with pharmacological treatments. Physical treatments have been shown to reduce atrophy-related changes in muscle architecture and reduce passive stiffness. 50 Body weight-supported treadmill training consistently reduced ankle clonus across various studies, in addition to reducing flexor and extensor spasms and co-contraction. 51 In a recent pilot study, resistance training at maximal intensity also improved walking and balance and tended to decrease the modified Ashworth score. 52 However, we found that physical therapy, including general stretching and treadmill training, was not sufficient for preventing spasticity after SCI in monkeys.
Because spontaneous neurological recovery occurs in animals that have undergone partial transection, analysis of the results obtained in peripheral nerve graft-treated monkeys must be carefully compared with those demonstrated in non-grafted animals.16,53 The results of our study confirmed spontaneous recovery of ipsilateral hind limb function in both graft- and non-graft-treated monkeys over time; however, the functional recovery of non-graft-treated monkeys reached a plateau after 11 weeks and started to decline thereafter. Whether the functional improvement other than spontaneous recovery after SCI is related to neural regeneration through neural repair therapy remains unclear. Although some studies have shown positive results of nerve regeneration in rats, 54 similar studies of neural regeneration in nonhuman primates are still rare. Primates have more complex nerves and a higher density of nerve fibers than rodents.
Limitations and outlook
This study had some limitations. First only the three clinical scales and gait symmetry analyses that are most commonly used for patients were applied to evaluate the effectiveness of the treatment approach for SCI-induced spasticity. The most frequently used assessment scale is the modified Ashworth scale, which is used to rate the resistance of a relaxed single joint to movement imposed by the evaluator throughout its full available range. 55 The tendon reflex score was used to grade the patella tendon reflex, 23 and the Babinski sign score was applied to measure fanning of toes.23,24 However, like most of the other assessment methods, these scales are ordinal, and their reliability, validity, and responsiveness have not been adequately explored.56 Thus, these results may not be as reliable and objective as electrophysiological examinations. More objective measures of spasticity based on actual muscle activation patterns recorded over many hours or days are needed. Second, the small sample size may have influenced the findings. Third, this study focused on describing the effect of a combination of peripheral nerve grafting and aFGF infusion after SCI in monkeys and did not delve into the possible mechanisms of nerve repair and spasticity reduction. In a follow-up study, we will conduct a carefully designed tracing experiment on a larger sample to provide more convincing objective evidence by using electrophysiological detection. In addition, immunohistochemical analysis, magnetic resonance imaging, and other methods will be used to further explore the mechanisms responsible for axonal growth or sprouting and spasticity reduction.
Conclusions
Three major conclusions can be drawn from the present study. First, SCI-induced spasticity was substantially reduced by a treatment strategy combining physical therapy with peripheral nerve grafting and the application of a mixture of growth factors. Second, spontaneous recovery of ipsilateral leg function occurred in both graft- and non-graft-treated monkeys over time, but the functional recovery of the non-graft-treated monkeys reached a plateau and started to decline after 11 weeks. Third, physical therapy, including general stretch and treadmill training, may be necessary but is not sufficient for preventing spasticity after SCI.
Acknowledgments
The authors would like to acknowledge Dr. Henrich Cheng and his team in Taipei Veterans General Hospital for performing the surgeries as well as Dr. Hang Zhang, Mr. Jose J. Aguayo, and other researchers in the Neural Control Laboratory for their collaboration in the data analysis.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This work was supported in part by the National Natural Science Foundation of China (31760276, 31960171) and the Jiangxi Natural Science Foundation (20171BAB204019, 20192ACB20022, 20171ACB20002).
ORCID iD: Wei-Ming Sun https://orcid.org/0000-0002-2041-0328
References
- 1.Ivanenko Y, Gurfinkel VS. Human postural control. Front Neurosci 2018; 12: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sezer N, Akkuş S, Uğurlu FG. Chronic complications of spinal cord injury. World J Orthop 2015; 6: 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson S, Corbett CJ, Winer JL, et al. Neonatal erythropoietin mitigates impaired gait, social interaction and diffusion tensor imaging abnormalities in a Prat model of prenatal brain injury. Exp Neurol 2018; 302: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nielsen JB, Crone C, Hultborn H. The spinal pathophysiology of spasticity–From a basic science point of view. Acta Physiol (Oxf) 2007; 189: 171–180. [DOI] [PubMed] [Google Scholar]
- 5.Richard-Denis A, Nguyen BH, Mac-Thiong JM. The impact of early spasticity on the intensive functional rehabilitation phase and community reintegration following traumatic spinal cord injury. J Spinal Cord Med 2020; 43: 435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levi R, Hultling C, Nash MS, et al. The Stockholm spinal cord injury study: 1. Medical problems in a regional SCI population. Paraplegia 1995; 33: 308–315. [DOI] [PubMed] [Google Scholar]
- 7.Cha S, Yun JH, Myong Y, et al. Spasticity and preservation of skeletal muscle mass in people with spinal cord injury. Spinal Cord 2019; 57: 317–323. [DOI] [PubMed] [Google Scholar]
- 8.Gracies JM. Pathophysiology of spastic paresis. I: Paresis and soft tissue changes. Muscle Nerve 2005; 31: 535–551. [DOI] [PubMed] [Google Scholar]
- 9.Gracies JM. Pathophysiology of spastic paresis. II: Emergence of muscle overactivity. Muscle Nerve 2005; 31: 552–571. [DOI] [PubMed] [Google Scholar]
- 10.Harvey LA, Dunlop SA, Churilov L, et al. Early intensive hand rehabilitation is not more effective than usual care plus one-to-one hand therapy in people with sub-acute spinal cord injury ('Hands On'): a randomised trial. J Physiother 2016; 62: 88–95. [DOI] [PubMed] [Google Scholar]
- 11.Kita M, Goodkin DE. Drugs used to treat spasticity. Drugs 2000; 59: 487–495. [DOI] [PubMed] [Google Scholar]
- 12.Pittelkow TP, Bendel MA, Lueders DR, et al. Quantifying the change of spasticity after intrathecal baclofen administration: a descriptive retrospective analysis. Clin Neurol and Neurosurg 2018; 171: 163. [DOI] [PubMed] [Google Scholar]
- 13.Yan X, Lan J, Liu Y, et al. Efficacy and safety of botulinum toxin type A in spasticity caused by spinal cord injury: a randomized, controlled trial. Med Sci Monit 2018; 24: 8160–8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nance PW, Bugaresti J, Shellenberger K, et al. Efficacy and safety of tizanidine in the treatment of spasticity in patients with spinal cord injury. North American Tizanidine Study Group. Neurology 1994; 44: S44–S52. [PubMed] [Google Scholar]
- 15.Duffell LD, Brown GL, Mirbagheri MM. Interventions to reduce spasticity and improve function in people with chronic incomplete spinal cord injury: distinctions revealed by different analytical methods. Neurorehabil Neural Repair 2015; 29: 566–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gohritz A, Fridén J. Management of spinal cord injury-induced upper extremity spasticity. Hand Clin 2018; 34: 555–565. [DOI] [PubMed] [Google Scholar]
- 17.Ravindra VM, Ray WZ, Sayama CM, et al. Increased spasticity from a fracture in the baclofen catheter caused by Charcot spine: case report. Arch Phys Med Rehabil 2015; 96: 697–701. [DOI] [PubMed] [Google Scholar]
- 18.Herman R, He J, D'Luzansky S, et al. Spinal cord stimulation facilitates functional walking in a chronic, incomplete spinal cord injured. Spinal Cord 2002; 40: 65–68. [DOI] [PubMed] [Google Scholar]
- 19.Thomaz SR, Cipriano G, Jr, Formiga MF, et al. Effect of electrical stimulation on muscle atrophy and spasticity in patients with spinal cord injury – A systematic review with meta-analysis. Spinal Cord 2019; 57: 258–266. [DOI] [PubMed] [Google Scholar]
- 20.Cheng H, Cao Y, Olson L. Spinal cord repair in adult paraplegic rats: partial restoration of hind limb function. Science 1996; 273: 510–513. [DOI] [PubMed] [Google Scholar]
- 21.Côté MP, Hanna A, Lemay MA, et al. Peripheral nerve grafts after cervical spinal cord injury in adult cats. Exp Neurol 2010; 225: 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ko CC, Tu TH, Chen YT, et al. Monkey recovery from spinal cord hemisection: nerve repair strategies for Rhesus Macaques. World Neurosurg 2019; 129: e343–e351. [DOI] [PubMed] [Google Scholar]
- 23.Hakelius A, Hindmarsh J. The significance of neurological signs and myelographic findings in the diagnosis of lumbar root compression. Acta Orthop Scand 1972; 43: 239–246. [DOI] [PubMed] [Google Scholar]
- 24.Milanov I. Mechanisms of tetrazepam action on spasticity. Acta Neurol Belg 1992; 92: 5–15. [PubMed] [Google Scholar]
- 25.Tederko P, Krasuski M, Czech J, et al . Reliability of clinical spasticity measurements in patients with cervical spinal cord injury. Ortop Traumatol Rehabil 2007; 9: 467–483. [PubMed] [Google Scholar]
- 26.Lake W, Shah H. Intrathecal baclofen infusion for the treatment of movement disorders. Neurosurg Clin N Am 2019; 30: 203–209. [DOI] [PubMed] [Google Scholar]
- 27.Theisen CC, Sachdeva R, Austin S, et al. Exercise and peripheral nerve grafts as a strategy to promote regeneration after acute or chronic spinal cord injury. J Neurotrauma 2017; 34: 1909–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ko CC, Tu TH, Wu JC, et al. Acidic fibroblast growth factor in spinal cord injury. Neurospine 2019; 16: 728–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yokota K, Fehlings MG. Acidic fibroblast growth factor in spinal cord injury: a potential therapy which merits further investigation. Neurospine 2019; 16: 739–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Wang Q, Cai H, et al. FGF1 improves functional recovery through inducing PRDX1 to regulate autophagy and anti-ROS after spinal cord injury. J Cell Mol Med 2018; 22: 2727–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai MC, Shen LF, Kuo HS, et al. Involvement of acidic fibroblast growth factor in spinal cord injury repair processes revealed by a proteomics approach. Mol Cell Proteomics 2008; 7: 1668–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng H, Liao KK, Liao SF, et al . Spinal cord repair with acidic fibroblast growth factor as a treatment for a patient with chronic paraplegia. Spine 2004; 29: E284–E288. [DOI] [PubMed] [Google Scholar]
- 33.Lin PH, Chuang TY, Liao KK, et al. Functional recovery of chronic complete idiopathic transverse myelitis after administration of neurotrophic factors. Spinal Cord 2006; 44: 254–257. [DOI] [PubMed] [Google Scholar]
- 34.Wu JC, Huang WC, Chen YC, et al. Acidic fibroblast growth factor for repair of human spinal cord injury: a clinical trial. J Neurosurg Spine 2011; 15: 216–227. [DOI] [PubMed] [Google Scholar]
- 35.Ko CC, Tu TH, Wu JC, et al. Functional improvement in chronic human spinal cord injury: four years after acidic fibroblast growth factor. Sci Rep 2018; 8: 12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsu SP, Shih YH, Huang MC, et al. Repair of multiple cervical root avulsion with sural nerve graft. Injury 2004; 35: 896–907. [DOI] [PubMed] [Google Scholar]
- 37.Wu JC, Huang WC, Huang MC, et al. A novel strategy for repairing preganglionic cervical root avulsion in brachial plexus injury by sural nerve grafting. J Neurosurg 2009; 110: 775–785. [DOI] [PubMed] [Google Scholar]
- 38.Tsai PY, Cheng H, Huang WC, et al. Outcomes of common peroneal nerve lesions after surgical repair with acidic fibroblast growth factor. J Trauma 2009; 66: 1379–1384. [DOI] [PubMed] [Google Scholar]
- 39.Li R, Wang B, Wu C, et al. Acidic fibroblast growth factor attenuates type 2 diabetes-induced demyelination via suppressing oxidative stress damage. Cell Death Dis 2021; 12: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang JP, Liu XY, Zhao F, et al. Three-dimensional bioprinting collagen/silk fibroin scaffold combined with neural stem cells promotes nerve regeneration after spinal cord injury. Neural Regen Res 2020; 15: 959–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mekhael W, Begum S, Samaddar S, et al. Repeated anodal trans-spinal direct current stimulation results in long-term reduction of spasticity in mice with spinal cord injury. J Physiol 2019; 597: 2201–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sadlaoud K, Khalki L, Brocard F, et al. Alteration of glycinergic receptor expression in lumbar spinal motoneurons is involved in the mechanisms underlying spasticity after spinal cord injury. J Chem Neuroanat 2020; 106: 101787. [DOI] [PubMed] [Google Scholar]
- 43.Corleto JA, Bravo-Hernández M, Kamizato K, et al. Thoracic 9 spinal transection-induced model of muscle spasticity in the rat: a systematic electrophysiological and histopathological characterization. PLoS One 2015; 10: e0144642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levi AD, Dancausse H, Li X, et al. Peripheral nerve grafts promoting central nervous system regeneration after spinal cord injury in the primate. J Neurosurg 2002; 96: 197–205. [DOI] [PubMed] [Google Scholar]
- 45.Barss TS, Parhizi B, Mushahwar VK. Transcutaneous spinal cord stimulation of the cervical cord modulates lumbar networks. J Neurophysiol 2020; 123: 158–166. [DOI] [PubMed] [Google Scholar]
- 46.Bovend'Eerdt TJ, Newman M, Barker K, et al. The effects of stretching in spasticity: a systematic review. Arch Phys Med Rehabil 2008; 89: 1395–1406. [DOI] [PubMed] [Google Scholar]
- 47.Harvey LA, Glinsky JV, Bowden JL. The effectiveness of 22 commonly administered physiotherapy interventions for people with spinal cord injury: a systematic review. Spinal Cord 2016; 54: 914–923. [DOI] [PubMed] [Google Scholar]
- 48.Paleg G, Livingstone R. Systematic review and clinical recommendations for dosage of supported home-based standing programs for adults with stroke, spinal cord injury and other neurological conditions. BMC Musculoskel Disord 2015; 16: 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phadke CP, Vierira L, Mathur S, et al. Impact of passive leg cycling in persons with spinal cord injury: a systematic review. Top Spinal Cord Inj Rehabil 2019; 25: 83–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Budini F, Kemper D, Christova M, et al. Five minutes static stretching influences neural responses at spinal level in the background of unchanged corticospinal excitability. J Musculoskelet Neuronal Interact 2019; 19: 30–37. [PMC free article] [PubMed] [Google Scholar]
- 51.Gant KL, Nagle KG, Cowan RE, et al. Body system effects of a multi-modal training program targeting chronic, motor complete thoracic spinal cord injury. J Neurotrauma 2018; 35: 411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Freitas GR, Szpoganicz C, Ilha J. Does neuromuscular electrical stimulation therapy increase voluntary muscle strength after spinal cord injury? A systematic review. Top Spinal Cord Inj Rehabil 2018; 24(1): 6–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galea MP, Darian-Smith I. Corticospinal projection patterns following unilateral section of the cervical spinal cord in the newborn and juvenile macaque monkey. J Comp Neurol 1997; 381: 282–306. [PubMed] [Google Scholar]
- 54.Smith DR, Dumont CM, Park J, et al. Polycistronic delivery of IL-10 and NT-3 promotes oligodendrocyte myelination and functional recovery in a mouse spinal cord injury model. Tissue Eng Part A 2020; 26: 672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 1987; 67: 206–207. [DOI] [PubMed] [Google Scholar]