Abstract
Objective
This study aimed to determine predictors of microvascular occlusion (MVO) in patients with ST elevation myocardial infarction (STEMI) after primary percutaneous coronary intervention (pPCI).
Methods
This retrospective, observational study consecutively included 113 patients with STEMI undergoing pPCI. Cardiac magnetic resonance imaging was used to determine the presence of MVO in these patients. Biomarkers in serum were routinely tested 1 day after pPCI. Multivariable logistic regression analysis was used to evaluate significant predictors of occurrence of MVO.
Results
There were 62 patients in the MVO group and 51 patients in the non-MVO group. C-reactive protein (CRP), thrombomodulin, lymphatic vessel endothelial hyaluronan receptor-1, syndecan-1, and troponin T (TnT) levels after the procedure were significantly higher in the MVO group than in the non-MVO group. CRP (hazard ratio [HR]=1.036), TnT (HR=1.316), and syndecan-1 (HR=1.986) levels were independently associated with MVO in patients with acute myocardial infarction after pPCI.
Conclusions
Levels of CRP, TnT, and syndecan-1 can be used as serum biomarkers for MVO in patients with STEMI receiving pPCI.
Keywords: Microvascular occlusion, ST elevation myocardial infarction, C-reactive protein, troponin T, syndecan-1, primary percutaneous coronary intervention
Introduction
ST elevation myocardial infarction (STEMI) is a clinical syndrome caused by acute occlusion of the coronary artery due to thrombosis on the basis of coronary atherosclerosis and plaque rupture. 1 Primary percutaneous coronary intervention (pPCI) is one of the most effective methods for maintaining the integrity of function and structure of the coronary artery microcirculation. 2 However, the patency of epicardial coronary flow does not imply complete recovery of myocardial reperfusion at the microvascular level. 3
Microvascular occlusion (MVO) is a phenomenon characterized by “no reflow” caused by myocardial microvascular injury and dysfunction in the infarct area and blood flow cannot pass through the myocardial capillary beds. 4 Cardiac magnetic resonance imaging (CMR) is considered as the gold standard for assessing MVO in the first week after pPCI. 5 Other assessments, including electrocardiography, emission computed tomography, and ultrasonic cardiography, have a poor ability for assessing MVO. Therefore, serum markers for detecting high-risk patients for MVO after pPCI need to be determined.
This study aimed to investigate serum biomarkers and establish a prediction model of MVO. We selected syndecan-1, syndecan-4, lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1), thrombomodulin, C-reactive protein (CRP), and troponin T (TnT) for potential biomarkers of MVO because these markers are elevated in microvascular damage.
Methods
Study population
Patients with STEMI who were referred for pPCI in the Drum Tower Hospital affiliated with the Medical School of Nanjing University from June 2018 to May 2019 were consecutively enrolled in this retrospective, observational study. The definition of STEMI was based on the European Society of Cardiology/American College of Cardiology Foundation/American Heart Association/World Heart Federation consensus document, including levels of cardiac biomarkers that are increased or decreased more than the reference value of healthy people. 6 Patients who were >85 years, those who had renal insufficiency or cardiac shock, and those with contraindications for CMR were excluded. Patients were included after written informed consent was provided, and all details of the patients were de-identified. The reporting of this study conforms to the STROBE statement. The Ethics Committee of Drum Tower Hospital approved the study protocol (2019-190-01).
pPCI procedure
All patients received aspirin (300 mg orally), clopidogrel (600 mg orally), or ticagrelor (180 mg orally) before pPCI. The percutaneous method was used to perform coronary angiography and determine the infarct-related artery. After collecting angiographic data, all patients were treated with vascular interventional therapy. Drug-eluting stents were subsequently implanted using direct techniques whenever possible. Starting from 1 minute after the initial reperfusion using thrombus aspiration, the balloon was inflated at the same position to a pressure of 4 to 6 atm for 60 s, and post-dilation was performed. The balloon was located at the proximal end of the lesion (to avoid embolization of the ruptured plaque). Selection of the inflation-deflation sequence was based on selection of the surgeon in the actual operation. The choice of IIb/IIIa inhibitors (abciximab or eptifibatide) was based on the physician’s intraoperative thrombotic load and disease severity. All patients signed a consent form for treatment.
CMR protocol
CMR was performed at 4.8 ± 1.9 days after pPCI. Images were acquired on a 3.0-T magnetic resonance imaging system (Ingenia; Philips Medical Systems, Best, The Netherlands). Images were acquired using vector cardiograph gating and multi-position spin echo sequences with a 7-mm slice thickness and 2.5-mm slice gaps. Four-chamber long-axis and short-axis views were used for left ventricular (LV) imaging (balanced turbo field echo sequence; time of echo, 1.47 ms; time of repetition, 2.9 ms; and flip angle, 45°). Late gadolinium enhancement imaging was performed 10 minutes after a bolus injection of 0.3 mmol/kg of gadodiamide contrast agent (GE Pharmacy, Shanghai, China) using an inversion recovery gradient echo sequence (time of repetition/time of echo, 6/3; flip angle, 25°; inversion time, 260–350 ms; voxels, 1.6 × 1.9 × 8 mm3; and shot duration, 100–125 ms).
CMR image analysis
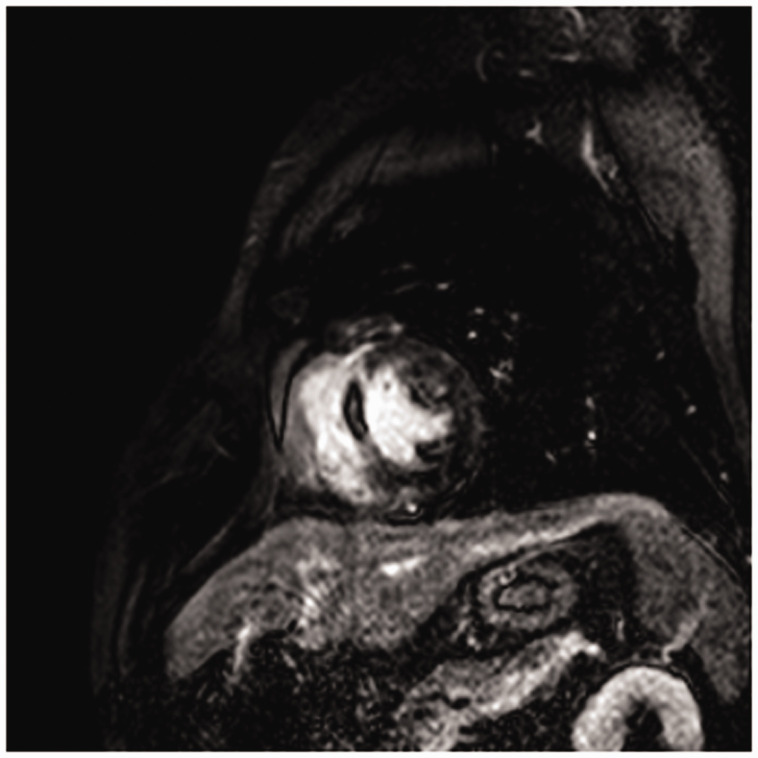
CMR images were analyzed by a cardiac radiologist who was blinded to the patient’s history and clinical outcome, using commercially available software (Extended MR WorkSpace 2.6.3.5; Philips Medical Systems). By tracing out the endocardial and epicardial borders, left ventricular end-diastolic volume, end-systolic volume, stroke volume, the ejection fraction, and end-diastolic mass (LV mass) were determined from short-axis cine images. These parameters were based on the body surface area, and the infarct size was measured by the full width and half maximum method. The infarct size was quantified on late gadolinium enhancement images of the first CMR using a signal intensity threshold of >5 standard deviations above a remote non-infarcted reference region, while the areas of MVO were included in the total infarct size. MVO was defined as the region of central hypo-enhancement within the hyper-enhanced area, which was quantified by tracing the central hypo-enhanced area, and expressed as a percentage of LV mass (% LV) (Figure 1).
Figure 1.
Representative cardiac magnetic resonance image of microvascular occlusion in short-axis late gadolinium enhancement.
Laboratory examinations
A volume of 5 mL of peripheral blood was collected in a procoagulant tube 6 hours after pPCI, placed at room temperature for 30 minutes, and then centrifuged at 1000 ×g for 20 minutes at room temperature. The supernatant plasma was separately packed in an Eppendorf tube, labeled, and stored at −80°C. Routine blood tests, including the white blood cell count, neutrophil count, and levels of hemoglobin, serum creatinine, low-density lipoprotein, CRP, and brain natriuretic peptide, were performed by using an automatic biochemistry analyzer (Afkmed, Guangdong, China). Serum TnT levels were measured by electrochemiluminescence. The levels of serum thrombomodulin, LYVE-1, syndecan-1, and syndecan-4 were detected by an enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN, USA).
Statistical analysis
Continuous variables with a symmetric distribution are expressed as mean ± SD and variables with a skewed distribution are expressed as median with interquartile range. Categorical variables are expressed as frequency with percentage. The Student’s t test or Wilcoxon rank-sum test was used to compare continuous variables between two groups, and the chi-square test or Fisher’s exact test was used to test categorical variables. Logistic regression with stepwise backward selection was used to screen independent variables. A two-sided P < 0.05 was considered significant. All statistical analysis was performed using IBM SPSS software Version 26.0 for Windows (IBM Corp., Armonk, NY, USA).
Results
Clinical characteristics of the study population
A total of 113 patients were enrolled in the current study. Clinical characteristics of the included patients are shown in Table 1. Fifty-one patients with a mean age of 61.4 ± 11.8 years did not show MVO after pPCI (non-MVO group). Sixty-two patients with a mean age of 61.2 ± 12.9 years developed MVO after pPCI (MVO group). The MVO group had a significantly higher Killip class at presentation compared with the non-MVO group (P = 0.032). There were no differences in other clinical parameters, including sex, body mass index, cardiovascular risk factors, previous cardiovascular and cerebrovascular history, a family history of coronary heart disease, heart rate, systolic and diastolic blood pressure, anterior infarction, and ticagrelor administration, between the two groups. The incidence of an anterior descending branch of the culprit artery was significantly higher in the MVO group than in the non-MVO group (P = 0.031). There were no differences in the thrombolysis in myocardial infarction (TIMI) coronary flow grade, corrected TIMI frame count, and SYNTAX (Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery) score between the two groups. These factors were used to assess blood flow and tissue perfusion after pPCI.
Table 1.
Baseline characteristics of the study population based on the presence or absence of MVO.
| Parameters | MVO (−) (n = 51) | MVO (+) (n = 62) | P value |
|---|---|---|---|
| Age (years) | 61.4 ± 11.8 | 61.2 ± 12.9 | 0.937 |
| BMI (kg/m2) | 24.5 ± 2.4 | 24.8 ± 3.4 | 0.598 |
| Male sex (n, %) | 43 (84.3) | 52 (83.9) | 0.949 |
| Hypertension (n, %) | 22 (43.1) | 34 (54.8) | 0.216 |
| DM (n, %) | 6 (11.8) | 10 (16.1) | 0.508 |
| Previous cerebral infarction (n, %) | 1 (2.0) | 2 (3.2) | 1 |
| Previous angina (n, %) | 15 (29.4) | 17 (27.4) | 0.815 |
| Current smoking (n, %) | 29 (56.9) | 36 (58.1) | 0.898 |
| Family history of CHD (n, %) | 2 (3.9) | 2 (3.2) | 1 |
| Previous PCI (n, %) | 1 (2.0) | 3 (4.8) | 0.626 |
| Heart rate (beats/minute) | 78.0 (69.0–85.0) | 81.5 (67.0–92.3) | 0.265 |
| Systolic blood pressure (mmHg) | 130.8 ± 19.3 | 123.6 ± 20.1 | 0.054 |
| Diastolic blood pressure (mmHg) | 84.0 ± 11.3 | 80.3 ± 12.5 | 0.089 |
| Killip class at presentation | 1.1 ± 0.2 | 1.3 ± 0.7 | 0.032 |
| Anterior infarction (n, %) | 23 (45.1) | 38 (61.3) | 0.086 |
| Ticagrelor (n, %) | 36 (70.6) | 47 (75.8) | 0.532 |
| LAD (n, %) | 24 (47.05) | 35 (56.45) | 0.031 |
| No-reflow (n, %) | 1 (1.92) | 0 (0) | 0.212 |
| Number of stents | 1.2 ± 0.7 | 1.3 ± 0.5 | 0.401 |
| Stent diameter (mm) | 3.0 ± 0.2 | 3.0 ± 0.3 | 1.000 |
| Stent length (mm) | 31.5 ± 12.2 | 33.5 ± 5.7 | 0.294 |
| TIMI grade pre-PCI | 0.89 (0.07–1.62) | 0.65 (0.03–1.05) | 0.098 |
| TIMI grade post-PCI | 2.92 ± 0.41 | 2.96 ± 0.45 | 0.623 |
| Corrected TIMI frame count | 27.3 (16.5–36.5) | 22.6 (17.1–27.5) | 0.089 |
| TIMI thrombus score | 3.7 ± 1.3 | 4.3 ± 1.6 | 0.076 |
| SYNTAX score | 13.7 (9.7–17.6) | 17.1 (13.5–20.9) | 0.015 |
Data are mean ± SD, n (%), or median with interquartile range.
MVO, microvascular occlusion; BMI, body mass index; DM, diabetes mellitus; CHD, coronary artery heart disease; PCI, percutaneous coronary intervention; LAD, left anterior descending artery; TIMI, Thrombolysis in Myocardial Infarction; SYNTAX, Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery.
Laboratory findings between the non-MVO and MVO groups
Levels of CRP (P = 0.038) and TnT after the procedure (P < 0.001) in the MVO group were significantly higher than those in the non-MVO group (Table 2). Additionally, patients in the MVO group had significantly higher serum thrombomodulin, LYVE-1, and syndecan-1 levels than those in patients in the non-MVO group (all P < 0.05).
Table 2.
Comparison of parameters derived from laboratory examinations.
| Parameters | MVO (−) (n = 51) | MVO (+) (n = 62) | P value |
|---|---|---|---|
| WBCs, 10^9/L | 9.4 (7.8–11.4) | 10.2 (8.6–12.8) | 0.079 |
| Ns (n, %) | 73.7 (60.6–82.7) | 78.0 (69.9–83.5) | 0.086 |
| Hb (g/L) | 140.1 ± 18.8 | 145.2 ± 16.6 | 0.124 |
| Platelet count (10^9/L) | 220.5 ± 79.3 | 214.6 ± 61.2 | 0.8 |
| SCr (µmol/L) | 68.3 ± 17.9 | 70.1 ± 15.4 | 0.565 |
| Cholesterol (mmol/L) | 4.2 ± 0.8 | 4.1 ± 0.9 | 0.327 |
| LDL (mmol/L) | 2.6 (2.0–3.2) | 2.5 (1.8–3.0) | 0.146 |
| CRP (mmol/L) | 4.7 (3.2–9.2) | 6.4 (3.9–15.9) | 0.038 |
| BNP (pg/mL) | 48.9 (22.7–128.0) | 114.0 (42.6–219.0) | 0.114 |
| TnT after procedure (µg/L) | 2.14 (1.16–3.18) | 5.36 (3.99–9.06) | <0.001 |
| Thrombomodulin (ng/mL) | 6.30 (5.16–8.76) | 7.86 (6.11–10.54) | 0.021 |
| LYVE-1 (ng/mL) | 19.89 (16.97–23.49) | 23.09 (17.73–29.37) | 0.037 |
| Syndecan-1 (ng/mL) | 2.07 (1.61–2.84) | 2.85 (1.95–4.18) | 0.003 |
| Syndecan-4 (ng/mL) | 59.00 (37.06–99.50) | 62.98 (43.72–100.18) | 0.467 |
WBCs, white blood cells; N, neutrophils; Hb, hemoglobin; Scr, serum creatinine; LDL, low-density lipoprotein; CRP, C-reactive protein; BNP, brain natriuretic peptide; TnT, troponin T; LYVE-1, lymphatic vessel endothelial hyaluronan receptor-1.
Univariate regression analysis
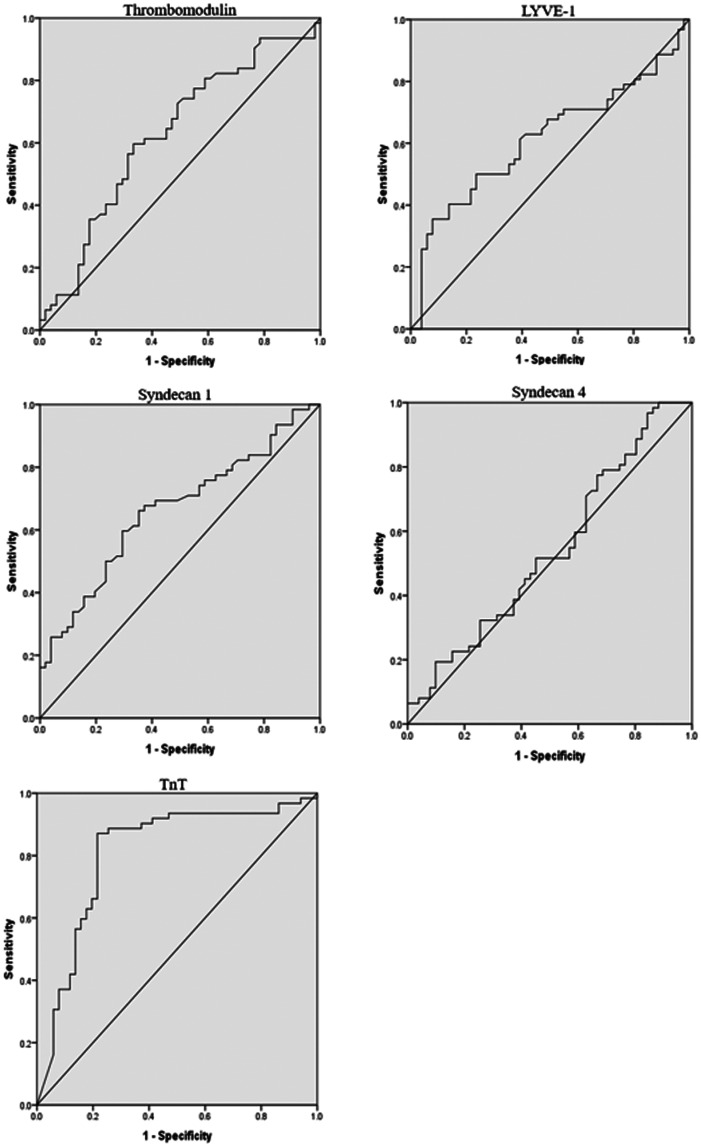
We used receiver operating characteristics curve analysis to evaluate the diagnostic ability of five cytokines. The area under the curve values for thrombomodulin, LYVE-1, syndecan-1, syndecan-4, and TnT after the procedure were 0.626 (P = 0.021), 0.614 (P = 0.037), 0.662 (P = 0.003), 0.540 (P = 0.467), and 0.806 (P < 0.001), respectively (Figure 2). The cut-off points for thrombomodulin, LYVE-1, syndecan-1, syndecan-4, and TnT to predict MVO were 7.505 (sensitivity: 0.597, specificity: 0.333), 28.01 (sensitivity: 0.355, specificity: 0.078), 2.315 (sensitivity: 0.661, specificity: 0.353), 25.01 (sensitivity: 0.968, specificity 0.843), and 3.34 (sensitivity: 0.862, specificity: 0.212), respectively (Figure 2).
Figure 2.
Receiver operating characteristics curves of cytokines for the non-microvascular occlusion and microvascular occlusion groups.
LYVE-1, lymphatic vessel endothelial hyaluronan receptor-1; TnT, troponin T.
Multivariate regression analysis
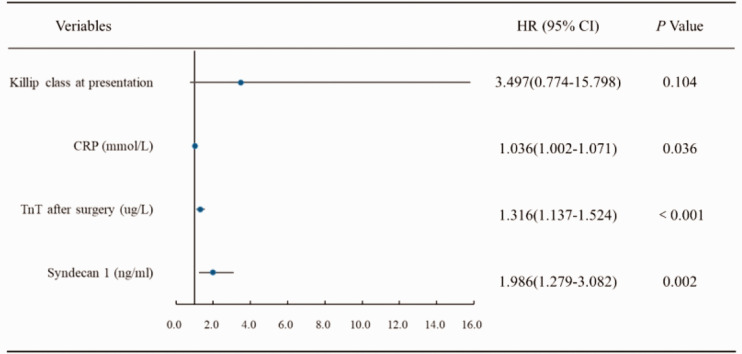
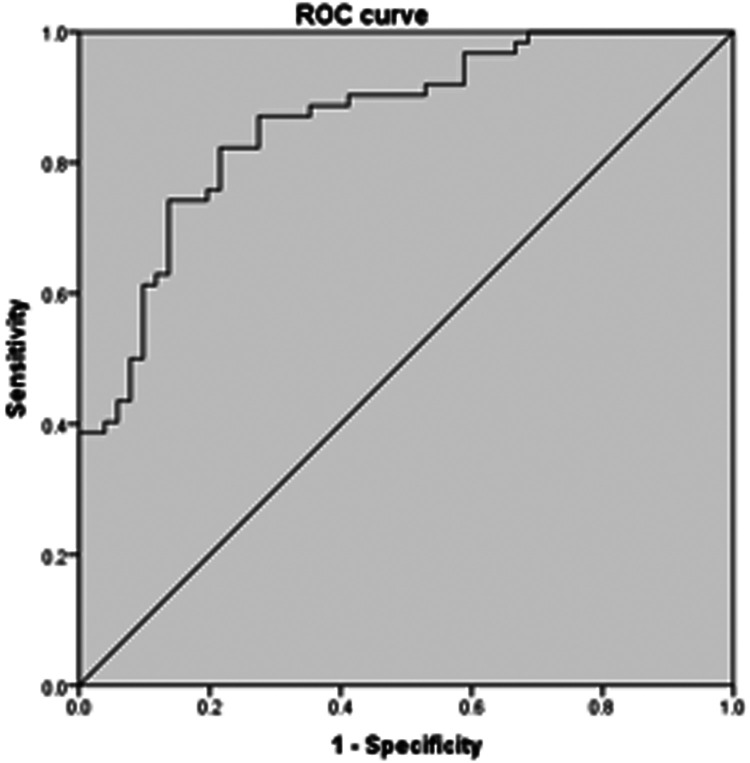
Figure 3 shows a forest map based on the multivariable logistic regression model with stepwise backward selection for the effect of variables on the probability of MVO. CRP (hazard ratio [HR] 1.036, 95% confidence interval [CI] 1.002–1.071, P = 0.036), syndecan-1 (HR 1.986, 95% CI 1.279–3.082, P = 0.002), and TnT levels after the procedure (HR 1.316, 95% CI 1.137–1.524, P < 0.001) were independent predictors of MVO. However, Killip class was not a significant susceptibility factor for MVO in patients with acute myocardial infarction after pPCI. Receiver operating characteristic curve analysis (Figure 4) showed that the area under the curve was 0.862 (95% CI 0.796–0.929, P < 0.001) for the combination of CRP, syndecan-1, and TnT.
Figure 3.
Association between Killip class at presentation, CRP, TnT, and syndecan-1 after the procedure and microvascular occlusion.
CRP, C-reactive protein; TnT, troponin T; HR, hazard ratio; CI, confidence interval.
Figure 4.
Receiver operating characteristics curve for the combination of C-reactive protein, troponin T, and syndecan-1.
Discussion
Application of pPCI has undoubtedly led to revolutionary changes in the treatment of coronary heart disease. 7 pPCI can improve myocardial perfusion and cardiac function rapidly and effectively to treat STEMI, and has become the primary treatment of coronary heart disease in clinical practice. However, after pPCI treatment, the thrombus load increases and embolism of the distal microcirculation and damage to the microcirculation are still common. 8 A high thrombus load leads to slow blood flow and even no reflow, which aggravate myocardial perfusion, and myocardial perfusion is related to the long-term risk of stent restenosis and thrombus. 9 Previous studies have shown that 30% of patients with STEMI after pPCI still develop coronary MVO. 10 Coronary MVO damages heart function and leads to adverse cardiovascular events in the long term, even if coronary flow reaches TIMI level 3. Therefore, prediction of coronary MVO has been a major goal in the treatment of STEMI, which has important clinical significance. 11
Diagnostic indices of MVO can be classified as invasive or non-invasive. Notably, the incidence of MVO is variable using different diagnostic tools. 12 Invasive indices, including the index of microcirculatory resistance, myocardial blush grade, and TIMI myocardial perfusion grade, focus more on myocardial perfusion by using angiographic assessment. 13 Non-invasive indices, especially incorporation of CMR, are currently the gold standard for comprehensive assessment of MVO. CMR can accurately quantify and locate MVO. MVO detected by CMR has the best predictive value for adverse LV remodeling and clinical outcomes. 14
The mechanism of MVO is complex and not yet fully understood. During pPCI, atherosclerotic plaques rupture because of compression of the balloon and stent. MVO can be caused by microembolus and microthrombosis after plaque rupture. The mechanisms guiding MVO include not only mechanical obstruction and spasm caused by the embolism, 15 but also participation of a large number of inflammatory transmitters. 16 Previous studies have emphasized the role of inflammatory biomarkers in MVO in patients with STEMI, such as the platelet to lymphocyte ratio 17 and the systemic immune-inflammation index. 18
To date, only a few studies have focused on biomarkers of MVO. Among these, vascular endothelial growth factor A, which is actively produced in the damaged myocardium to foster angiogenesis and tissue repair, has a predictive effect on the occurrence of MVO. 19 In this study, we tested several factors related to thrombosis, vascular endothelial function, and inflammation. We evaluated the diagnostic ability of thrombomodulin, LYVE-1, syndecan-1, syndecan-4, and TnT for MVO. Thrombomodulin is a specific molecular marker of endothelial cell damage. Inflammatory factors and neutrophil infiltration, directly or indirectly, lead to vascular endothelial cell damage and a release of a large amount of thrombomodulin. 20 LYVE-1 is a cell surface receptor on lymphatic endothelial cells, and it can be used as a marker of lymphatic endothelial cells. 21 Syndecan-1 is a member of the syndecan proteoglycan family and it binds to the heparan sulfate chain, which is a receptor of growth factors and chemokines, 22 indicating the degree of disturbance of the microcirculation 23 Syndecan-4 is bound to regulatory factors and stromal cell-derived factor 1 secreted by normal cells, and affects aggregation of granulocytes. This process is closely related to the inflammatory response after vascular injury. 24 TnT is a sensitive index for showing myocardial injury and is significantly better than creatine kinase MB isoenzyme. Evidence shows that TnT levels are significantly increased in patients with a high index of microvascular resistance after pPCI, 25 which is consistent with the results of our study. In the present study, CRP, TnT, and syndecan-1 were biomarkers for MVO.
Our study has some limitations. This study was conducted in a single center with a limited number of patients. Therefore, a prospective, multicenter study with a larger number of patients is necessary to confirm the validity our conclusions. We also only evaluated clinical outcomes in the hospital, and did not conduct long-term follow-up outcomes. Finally, factors, such as nursing care and social support, were not considered in this study.
In conclusion, our study shows that postprocedural levels of CRP, TnT, thrombomodulin, LYVE-1, and syndecan-1 are significantly higher in patients with MVO than in those without MVO. Additionally, CRP, TnT, and syndecan-1 might be independent predictors for MVO in patients with STEMI after pPCI.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contributions: HY contributed to conception and design of the study, acquisition of data, analysis, and interpretation of data, and drafting and revising the manuscript. LD and ZC contributed to acquisition of data and revising the manuscript. XB contributed to interpretation of data, and drafting and revising the manuscript. All of the authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Availability of data and materials: The datasets are available from the corresponding author on reasonable request.
ORCID iD: Ziwei Chen https://orcid.org/0000-0001-8211-6805
References
- 1.Singh S, Singh A, Khosla S. Acute Myocardial Infarction. Ann Intern Med 2015; 163: 151–152. doi: 10.7326/L15-5113. [DOI] [PubMed] [Google Scholar]
- 2.Golino M, Spera FR, Manfredonia L, et al. Microvascular ischemia in patients with successful percutaneous coronary intervention: effects of ranolazine and isosorbide-5-mononitrate. Eur Rev Med Pharmacol Sci 2018; 22: 6545–6550. doi: 10.26355/eurrev_201810_16070. [DOI] [PubMed] [Google Scholar]
- 3.Shehata IE, Cheng CI, Sung PH, et al. Predictors of myocardial functional recovery following successful reperfusion of acute ST elevation myocardial infarction. Echocardiography 2018; 35: 1571–1578. doi: 10.1111/echo.14106. [DOI] [PubMed] [Google Scholar]
- 4.Oikonomou E, Mourouzis K, Vogiatzi G, et al. Coronary Microcirculation and the No-reflow Phenomenon. Curr Pharm Des 2018; 24: 2934–2942. doi: 10.2174/1381612824666180911122230. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed N, Carrick D, Layland J, et al. The role of cardiac magnetic resonance imaging (MRI) in acute myocardial infarction (AMI). Heart Lung Circ 2013; 22: 243–255. doi: 10.1016/j.hlc.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation 2007; 116: 2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 7.Head SJ, Milojevic M, Daemen J, et al. Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: a pooled analysis of individual patient data. Lancet 2018; 391: 939–948. doi: 10.1016/S0140-6736(18)30423-9. [DOI] [PubMed] [Google Scholar]
- 8.McAlindon E, Pufulete M, Harris J, et al. Microvascular dysfunction determines infarct characteristics in patients with reperfused ST-segment elevation myocardial infarction: The MICROcirculation in Acute Myocardial Infarction (MICRO-AMI) study. PLoS One 2018; 13: e0203750. doi: 10.1371/journal.pone.0203750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keeble TR, Karamasis GV, Noc M, et al. Effect of Intravascular Cooling on Microvascular Obstruction (MVO) in Conscious Patients with ST-Elevation Myocardial Infarction Undergoing Primary PCI: Results from the COOL AMI EU Pilot Study. Cardiovasc Revasc Med 2019; 20: 799–804. doi: 10.1016/j.carrev.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Bonello L, Ait Mokhtar O, Lemesle G, et al. Incidence and predictors of microvascular dysfunction assessed by the index of microcirculatory resistance following primary PCI for ST-elevation myocardial infarction. Int J Cardiol 2011; 146: 465–467. doi: 10.1016/j.ijcard.2010.10.134. [DOI] [PubMed] [Google Scholar]
- 11.Abanador-Kamper N, Kamper L, Karamani V, et al. Proximal culprit lesion and coronary artery occlusion independently predict the risk of microvascular obstruction in acute myocardial infarction. Int J Cardiovasc Imaging 2016; 32: 1235–1242. doi: 10.1007/s10554-016-0897-x. [DOI] [PubMed] [Google Scholar]
- 12.Niccoli G, Cosentino N, Spaziani C, et al. No-reflow: incidence and detection in the cath-lab. Curr Pharm Des 2013; 19: 4564–4575. doi: 10.2174/1381612811319250005. [DOI] [PubMed] [Google Scholar]
- 13.Niccoli G, Scalone G, Lerman A, et al. Coronary microvascular obstruction in acute myocardial infarction. Eur Heart J 2016; 37: 1024–1033. doi: 10.1093/eurheartj/ehv484. [DOI] [PubMed] [Google Scholar]
- 14.Stiermaier T, Thiele H, Eitel I. Coronary Microvascular Obstruction: Key Factor in the Prognosis of ST-Segment-Elevation Myocardial Infarction. Circ Cardiovasc Imaging 2017; 10: e006568. doi: 10.1161/circimaging.117.006568. [DOI] [PubMed] [Google Scholar]
- 15.Belyaev AV, Panteleev MA, Ataullakhanov FI. Threshold of microvascular occlusion: injury size defines the thrombosis scenario. Biophys J 2015; 109: 450–456. doi: 10.1016/j.bpj.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marinescu MA, Loffler AI, Ouellette M, et al. Coronary microvascular dysfunction, microvascular angina, and treatment strategies. JACC Cardiovasc Imaging 2015; 8: 210–220. doi: 10.1016/j.jcmg.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurtul A, Ornek E. Platelet to Lymphocyte Ratio in Cardiovascular Diseases: A Systematic Review. Angiology 2019; 70: 802–818. doi: 10.1177/0003319719845186. [DOI] [PubMed] [Google Scholar]
- 18.Esenboga K, Kurtul A, Yamanturk YY, et al. Systemic immune-inflammation index predicts no-reflow phenomenon after primary percutaneous coronary intervention. Acta Cardiol 2021: 1–8. doi: 10.1080/00015385.2021.1884786. [DOI] [PubMed] [Google Scholar]
- 19.Garcia R, Bouleti C, Sirol M, et al. VEGF-A plasma levels are associated with microvascular obstruction in patients with ST-segment elevation myocardial infarction. Int J Cardiol 2019; 291: 19–24. doi: 10.1016/j.ijcard.2019.02.067. [DOI] [PubMed] [Google Scholar]
- 20.Remková A, Kováčová E, Príkazská M, et al. Thrombomodulin as a marker of endothelium damage in some clinical conditions. Eur J Intern Med 2000; 11: 79–84. doi: 10.1016/S0953-6205(00)00066-2. [DOI] [PubMed] [Google Scholar]
- 21.Gordon EJ, Gale NW, Harvey NL. Expression of the hyaluronan receptor LYVE-1 is not restricted to the lymphatic vasculature; LYVE-1 is also expressed on embryonic blood vessels. Dev Dyn 2008; 237: 1901–1909. doi: 10.1002/dvdy.21605. [DOI] [PubMed] [Google Scholar]
- 22.Torres Filho IP, Torres LN, Salgado C, et al. Plasma syndecan-1 and heparan sulfate correlate with microvascular glycocalyx degradation in hemorrhaged rats after different resuscitation fluids. Am J Physiol Heart Circ Physiol 2016; 310: H1468–H1478. doi: 10.1152/ajpheart.00006.2016. [DOI] [PubMed] [Google Scholar]
- 23.Burke-Gaffney A, Evans TW. Lest we forget the endothelial glycocalyx in sepsis. Crit Care 2012; 16: 121. doi: 10.1186/cc11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutton A, Friand V, Brule-Donneger S, et al. Stromal cell-derived factor-1/chemokine (C-X-C motif) ligand 12 stimulates human hepatoma cell growth, migration, and invasion. Mol Cancer Res 2007; 5: 21–33. doi: 10.1158/1541-7786.MCR-06-0103. [DOI] [PubMed] [Google Scholar]
- 25.Mangiacapra F, Bressi E, Di Gioia G, et al. Coronary microcirculation and peri-procedural myocardial injury during elective percutaneous coronary intervention. Int J Cardiol 2020; 306: 42–46. doi: 10.1016/j.ijcard.2019.12.042. [DOI] [PubMed] [Google Scholar]