Abstract
Triple combination of anti-PD-1/PD-L1 immunotherapy and anti-BRAF plus anti-MEK targeted therapy is a promising antitumor strategy and is increasingly being used in clinical trials. To evaluate the safety and efficacy of triple combination of PD-1/PD-L1, BRAF, and MEK inhibition in patients diagnosed with stage III-IV melanoma, we performed a systematic review and meta-analysis of randomized controlled trials (RCTs). The PubMed, EMBASE, and Cochrane Library were searched for all studies published from inception to January 2021. The progression free survival (PFS), overall survival (OS), overall response rate (ORR), and risk of adverse events (AEs) were extracted by two independent investigators and pooled hazard ratio (HR) or risk ratio (RR) with 95% CI were determined using the random-effects model for data synthesis. Overall, five randomized controlled trials encompassing 1,266 patients with stage III-IV melanoma were selected. Triple combination therapy significantly improved PFS (HR = 0.71; 95% CI = 0.59 to 0.86; P = 0.0005) and 2-year OS (RR = 1.12; 95% CI = 1.03 to 1.23; P = 0.01), but had no impact on ORR (RR = 1.09; 95% CI = 0.91 to 1.30; P = 0.37) when compared with controlled treatment group. In addition, triple combination therapy was associated with increased risks of hypothyroidism, arthralgia, myalgia, ALT increased, AST increased, asthenia, and pyrexia compared with control group. Triple combination therapy of PD-1/PD-L1, BRAF, and MEK inhibition achieved better survival benefits but had higher incidence of some adverse events over two-drug combination or monotherapy. Further randomized controlled clinical trials are needed to verify our results.
Systematic Review Registration
PROSPERO 2021 CRD42021235845 Available from https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021235845.
Keywords: triple combination therapy, PD-1/PD-L1, BRAF inhibition, MEK inhibition, melanoma, meta-analysis
Introduction
Melanoma is a very aggressive form of skin cancer with the fastest growing incidence rate among all cancers. About 1.7% of all cases of newly diagnosed malignant cancers are cutaneous melanoma (1, 2). Once melanoma has spread, it becomes the deadliest type of cutaneous cancer. As a result of its high heterogeneity and ability to the elude the body’s immune system, melanoma cells often display a multidrug resistance phenotype and are extremely difficult to treat (3, 4).
Recently, the therapies for patients with advanced melanoma have been upgraded with the development of small molecule inhibitors targeting B-Raf proto-oncogene serine/threonine-kinase (BRAF) and/or MAPK/ERK kinase (MEK), as well as immunotherapy drugs targeting the programmed death-1/programmed death-ligand-1 (PD-1/PD-L1) and the cytotoxic T lymphocyte antigen-4 (CTLA-4) (5, 6). Targeted therapy including a BRAF inhibitor (vemurafenib, dabrafenib, and encorafenib) in combination with a MEK inhibitor (trametinib, cobimetinib, binimetinib, and selumetinib) was the first-line therapy for metastatic or advanced melanoma (7, 8). With deeper understanding of cancer immunology and immunotherapy, PD-1/PD-L1 immune checkpoint inhibitors (pembrolizumab, pidilizumab, nivolumab, atezolizumab, durvalumab, avelumab, or spartalizumab), and CTLA-4 inhibitors also served as first-line therapy for advanced melanoma (2, 9, 10).
Immunotherapy and targeted therapy both have significant advantages and disadvantages. A benefit of targeted therapy over immunotherapy is the high objective response rates, which means more people respond to targeted therapy. However, the main disadvantage is that the duration of response (DOR) is short-lived (11). A clear advantage of immunotherapy over targeted therapy is providing more durable responses and inhibitory effects on cancer growth which may continue to exist after the drugs have been discontinued (12). However, the biggest drawback is the relatively lower response rate, as only a small percentage of people respond to immunotherapy (13). Thus, there is a continuous need to develop new treatment strategies.
Considering that immunotherapy and targeted therapy are complementary in terms of advantages and disadvantages, combinations of immunotherapy and targeted therapy are proposed and applied to clinical trials. Data show that treatment with BRAF and MEK inhibitors increases T cell numbers, CD8+ T cell infiltration, downregulates immunosuppressive cytokines and upregulates PD-1/PD-L1 expressions (14, 15), implying that adding PD-1/PD-L1 inhibitor to BRAF and MEK targeted therapy could help to prevent the spread of cancer. Despite some exciting results from the recent clinical studies (16–18), the question of whether triple combination therapy is better than other drug therapy is still open. To solve this puzzle, we conducted a systematic review and meta-analysis of clinical trials to determine whether the triple therapy combined with PD-1/PD-L1, BRAF, and MEK inhibitor has better outcomes than two-drug combination or monotherapy in metastatic melanoma treatment.
Methods
The study was performed in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines (19). The protocol was registered in PROSPERO (CRD42021235845).
Search Strategy
Literature searches were done without language restrictions using PubMed, EMBASE, and Cochrane Library from inception to January 2021. The key search terms with Boolean operators (AND, OR) were as follows: (“melanoma” OR “skin neoplasms”) AND (“PD-1” OR “PD-L1” OR “pembrolizumab” OR “pidilizumab” OR “nivolumab” OR “atezolizumab” OR “durvalumab” OR “avelumab” OR “spartalizumab”) AND (“BRAF” OR “vemurafenib” OR “zelboraf” OR “dabrafenib” OR “encorafenib”) AND (“MEK” OR “trametinib” OR “cobimetinib” OR “binimetinib” OR “selumetinib”). The detailed search strategies for each database are available in Table S1 .
Selection Criteria
Titles and abstracts were initially screened for relevance, and then full-text screening was carried out. Screening was performed in duplicate by two investigators (YL and XLZ). Studies were considered eligible if the selection criteria were met: (1) The study design of literature was a randomized clinical trial. (2) Enrolled patients with stage III-IV melanoma had histologically confirmed diagnosis of unresectable Stage III or metastatic stage IV melanoma and had at least one measurable lesion as defined by RECIST 1.1 on CT or MRI imaging studies. (3) Intervention treatments were triple combination therapy combined with PD-1/PD-L1 inhibition, BRAF inhibition and MEK inhibition versus two-drug combination therapy or monotherapy. (4) Study results included progression-free survival (PFS), overall survival (OS), overall response rate (ORR), and adverse events (AEs). Studies with unavailable or incomplete data were excluded.
Data Extraction
Two investigators (YL and GW) independently reviewed the full text of eligible studies and extracted data using a prespecified data-collection form, including the following information: publication reference, study type, clinical trial registry numbers, sample size, age, gender, treatment regimen, and outcomes (including PFS, OS, ORR and AEs). For the IMPemBra study [Rozeman et al. (20)] and COMBI-i study [Nathan et al. (21)], there were only descriptive words of outcomes without detailed survival curves and adverse events, therefore, we referred to the clinical data presented at American Society of Clinical Oncology (ASCO) and European Society for Medical Oncology (ESMO) conferences, respectively. When data of PFS and 2-year OS were not reported in the text, it was independently calculated from survival curves using graph data extraction software Engauge Digitizer.
Quality Assessment
Methodologic quality of included studies was assessed independently by two investigators (GYW and XCC) using the Cochrane Collaboration’s risk-of-bias tool (22). Bias assessment was generated by Review Manager Version 5.4 (Cochrane Collaboration).
Data Analysis
The risk ratio (RR) with 95% confidence interval (CI) was used to summarize the dichotomous outcomes, including 2-year OS, ORR, complete response (CR), partial response (PR), and adverse events (i.e., nausea, arthralgia, diarrhea, asthenia), while hazard ratio (HR) with 95% CI was used to summarize results for PFS. We generated forest plots using Review Manager 5.4. The random-effects model was used for all meta-analysis, and a value of P < 0.05 was considered as statistically significant. Heterogeneities between the studies were evaluated by I2 statistic, and I2 > 50% represented significant heterogeneity (23). Sensitivity analysis was conducted using Stata/SE 16.0 to evaluate the influence of every single study on the overall estimate via omitting study in turn. Because of the limited number (<10) of included studies, we did not assess the publication bias.
Results
Included Trials and Studies
A total of 442 relevant citations were initially retrieved from PubMed, EMBASE, and Cochrane Library databases. After duplicate checking, 309 studies were included. Then, 267 studies were excluded based upon the assessment of titles or abstracts and because of them being reviews, comments, case reports, animal trials, or irrelevant to our inclusion criteria. Screening of the full-text citations resulted in the exclusion of studies that did not fulfill the inclusion criteria (n=14); did not show adequate data (n=7); not randomized clinical studies (n=1) or in the early stage of clinical trials without uploaded results (n=15). Finally, five randomized clinical trials (RCTs) were included in our meta-analysis (20, 21, 24–26)and details about selection of studies were schematically shown in Figure 1 .
Figure 1.
A PRISMA Flow chart of study selection. PubMed, EMBASE and Cochrane Library databases were searched for articles published from inception to January 1, 2021.
Study Characteristics
All the included randomized trials were published in 2020. Of all eligible studies, we gathered a total of 1,266 patients with stage III to IV metastatic melanoma treated with triple therapy of PD-1/PD-L1 inhibition, BRAF inhibition, and MEK inhibition versus two-drug combination or monotherapy as control group. The detailed characteristics of these trials were presented in Table 1 . In terms of patient characteristics, all studies except one enrolled BRAF V600 mutation-positive patients in both triple and control treatment group. Only a study by Ribas et al. enrolled BRAF V600 mutation-positive patients in triple therapy group, while BRAF-wild type patients and a patient with other mutation in two-drug control group. In terms of treatment regimen, patients of the IMSpire 150 study in triple therapy arms were treated with atezolizumab (PD-L1 inhibitor) in combination with vemurafenib and cobimetinib; Ribas et al. study used durvalumab (PD-L1 inhibitor) combined with dabrafenib and trametinib; patients of the Keynote-022 and IMPemBra study received pembrolizumab (PD-1 inhibitor) in combination with dabrafenib and trametinib; and COMBI-i study used PD-1 inhibitor spartalizumab in combination with dabrafenib and trametinib. Besides that, only a study by Rozeman et al. used pembrolizumab monotherapy as control group, while IMSpire 150, Keynote-022, and COMBI-i studies all used BRAF inhibitor plus MEK inhibitor as controlled two-drug combination treatment and Ribas et al. study applied PD-L1 inhibitor and MEK inhibitor in control arm.
Table 1.
The main characteristics of 5 included studies in the meta-analysis.
| Study | Trial | Study type | Melanoma severity | Triple group Control | Sample size | Age (year) | Female, n% | Treatment regimen |
|---|---|---|---|---|---|---|---|---|
| Gutzmer et al. (25) |
NCT02908672, IMSpire 150 |
Phase III RCT |
Stage IIIc-IV, BRAFV600 mutation-positive | ate+vem+cob | 256 | 54.0 (44.8-64.0) | 106, 41% | Vem 720mg BID + Cob 60mg QD + Ate 840mg |
| vem+cob | 258 | 53.5 (43.0-63.8) | 109, 42% | Vem 960mg BID + Cob 60mg QD + placebo | ||||
| Ferrucci et al. (24) |
NCT02130466, KEYNOTE-022 |
Phase II RCT |
Stage III-IV, BRAFV600 mutation-positive | pem+dab+tra | 60 | 54 (18-82) | 27, 45.0% | Pem Q3W+ Dab 150mg BID + Tra 2mg QD |
| dab+tra | 60 | 58 (21-83) | 24, 40.0% | Placebo Q3W+ Dab 150mg BID + Tra 2mg QD | ||||
| Rozeman et al. (20) |
NCT02625337, IMPemBra |
Phase II RCT |
Stage IIIc-IV, BRAFV600 mutation-positive | pem+dab+tra | 24 | 56 (22-78) | 12, 50% | Pem 200mg Q3W + Dab 150mg BID + Tra 2mg QD |
| pem | 8 | 58 (46-71) | 2, 25% | Pem 200mg Q3W | ||||
| Nathan et al. (21) |
NCT02967692, COMBI-i |
Phase III RCT |
Stage IIIc-IV, BRAFV600 mutation-positive | spa+dab+tra | 267 | 56 (20-86) | 225, 42.3% | Spa 400mg Q4W + Dab 150mg BID + Tra 2mg QD |
| dab+tra | 265 | 55 (23-88) | Placebo Q4W + Dab 150mg BID + Tra 2mg QD | |||||
| Ribas et al. (26) | NCT02027961 | Phase I RCT |
Stage III-IV melanoma | dur+dab+tra | 26 | 49.0 (23-71) | 12, 46.2% | Dur 3 or 10mg/kg Q2W + Dab 150mg BID + Tra 2mg QD |
| dur+tra | 42 | n=20: 68.0 (31-85) n=22: 63.0 (34-84) | 18, 42.9% | Dur 10mg/kg Q2W + Tra 2mg QD (Concurrent n=20; Sequential n=22) |
Data of age were presented as median (range). ate, atezolizumab; vem, vemurafenib; cob, cobimetinib; pem, pembrolizumab; dab, dabrafenib; tra, trametinib; spa, spartalizumab; dur, durvalumab. BID, twice daily; QD, once daily; Q2W, every 2 weeks; Q3W, every 3 weeks; Q4W, every 4 weeks.
Risk-of-Bias Assessment
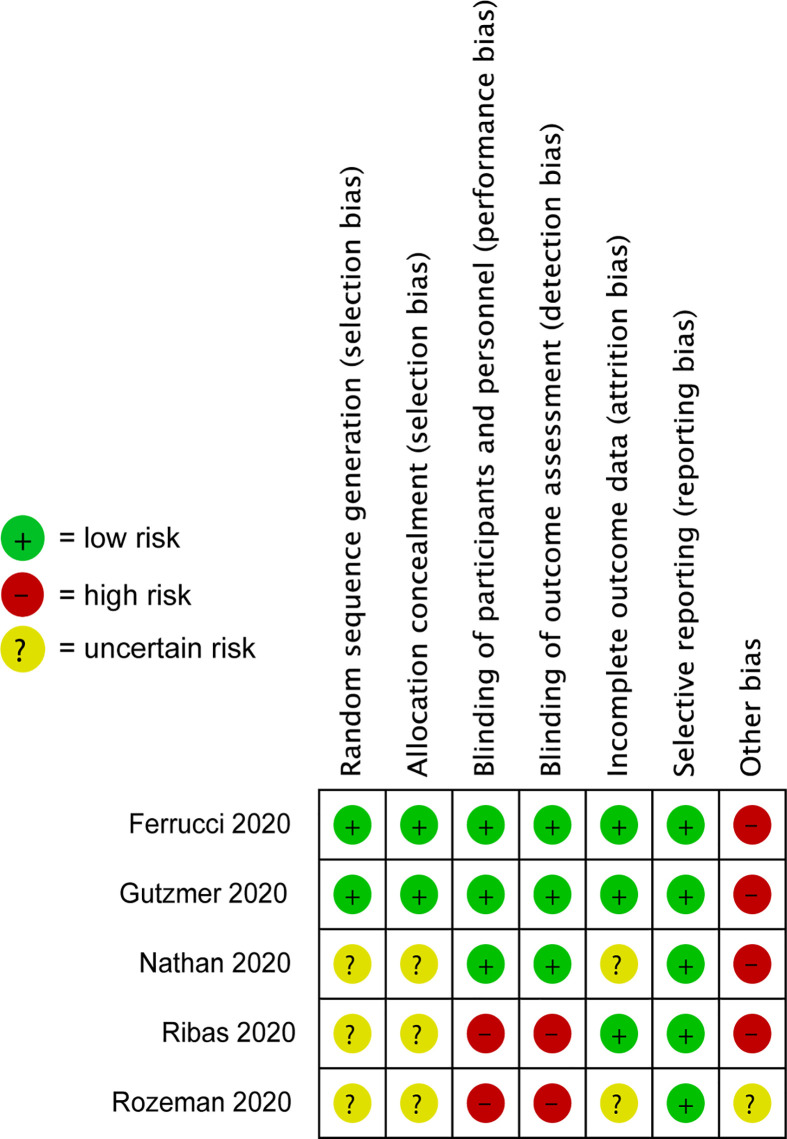
The risk of bias assessment of the included studies has been listed in Figure 2 . Three trials, including Ferrucci et al., Gutzmer et al., and Nathan et al., were double-blind studies, whereas the study by Ribas et al. and Rozeman et al. were open‐label. Four studies were at high risk of other bias, because pharmaceutical companies either sponsored these clinical trials or provided the study drugs.
Figure 2.
Risk-of-bias assessment of randomized controlled trials (RCTs) included in meta-analysis.
Progression-Free Survival
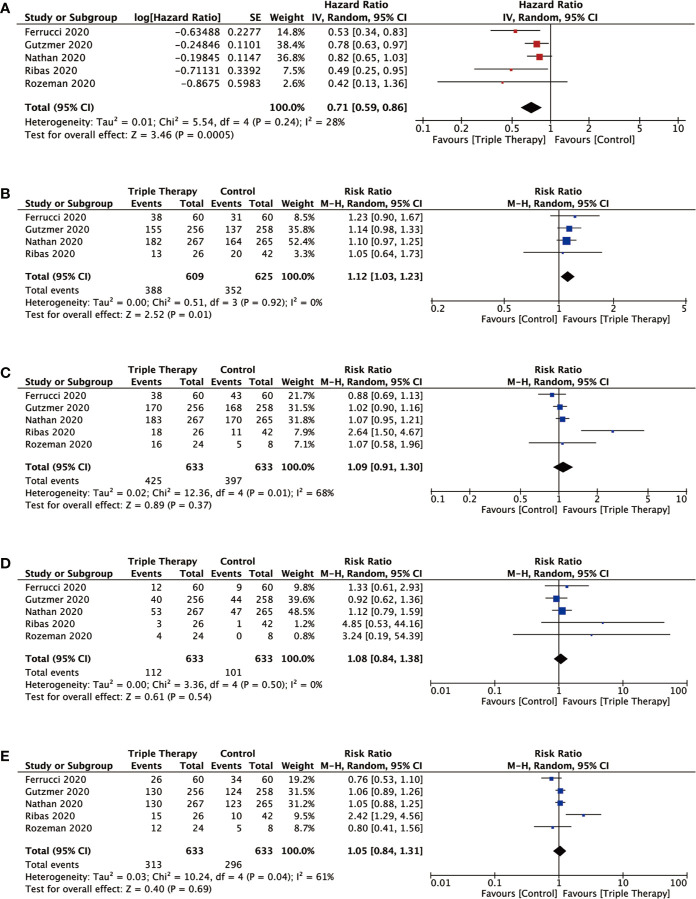
Forest plot of PFS related to triple therapy and control therapy was shown in Figure 3A . The information about HRs for PFS was available from trials or calculated from survival curves given in studies. The pooled HR for PFS based on our random-effects model analysis indicated that triple combination therapy was associated with significantly longer PFS as compared to control (HR = 0.71; 95% CI = 0.59 to 0.86; P = 0.0005; I2 = 28%).
Figure 3.
Forest plots analysis of the efficiency outcomes for triple combination therapy of PD-1/PD-L1, BRAF, and MEK inhibition versus control therapy. Pooled hazard ratio (HR) or risk ratio (RR) with 95% CI were determined using the random-effects model for outcomes. (A) PFS (progression-free survival); (B) OS (overall survival); (C) ORR (overall response rate); (D) CR (complete response); (E) PR (partial response).
Overall Survival
The risk ratio (RR) for 2-year OS was 1.12 (95% CI = 1.03 to 1.23; P = 0.01; I2 = 0%). For the triple therapy group, the pooled 2-year OS rate estimate was 63.7% (n=388/609 responses) and for the control group, it was 56.3% (n=352/625 responses). P value of 2-year OS outcomes demonstrated statistical difference between triple therapy and control therapy ( Figure 3B ).
Objective Response Rate, Complete Response, and Partial Response
Forest plots of ORR, CR, and PR associated with triple therapy and control therapy were showed in Figures 3C–E . The overall ORR was 67.1% (n=425/633 responses; CR 112; PR 313) in triple therapy and 62.7% (n=397/633 responses; CR 101; PR 296) in control group. However, triple therapy had no significant improvement compared with control and the risk ratio (RR) for ORR was 1.09 (95% CI = 0.91 to 1.30; P = 0.37; I2 = 68%). In addition, there was no clear benefit for triple therapy in CR (RR = 1.08; 95% CI = 0.84 to 1.38; P= 0.54; I2 = 0%) and PR (RR = 1.05; 95% CI = 0.84 to 1.31; P= 0.69; I2 = 61%).
Adverse Events
Despite the improved survival benefit associated with triple therapy, concerns of AEs, especially the immune-related adverse events (irAEs), occurring during triple combination treatments are growing because of their functional mechanisms (27). In this study, we analyzed the immune-related adverse events and other AEs for all grades. We classified a total of 18 different types of adverse events that were mentioned in the included studies and the statistical details were listed in Table 2 . There was no significant difference in the incidence of adverse events for all grades (any events, RR = 1.01; 95% CI = 0.99 to 1.04; P= 0.39; I2 = 47%) and grade≥3 (RR = 1.21; 95% CI = 0.99 to 1.49; P= 0.07; I2 = 74%) between the triple therapy and control group. For dermatologic irAEs, similar incidence of rash, pruritus, and dermatitis acne occurred in both triple therapy and control group. For gastrointestinal irAEs, triple therapy also showed similar risk of nausea, diarrhea, and vomiting in both triple and control groups. For endocrine irAEs, triple therapy had the higher risk of hypothyroidism than control. For musculoskeletal AEs, triple therapy was associated with more frequent incidences of arthralgia and myalgia between triple therapy and control. Analysis results of hepatic irAEs, such as ALT increased and AST increased in triple therapy showed significant toxicity incidences than control, while blood ALP increased showed no significant toxicity event difference. For general disorders, no significant toxicity event difference was shown in chills, fatigue, headache, and decreased appetite between triple therapy and control group. Moreover, the number of patients experiencing asthenia and pyrexia were greater in triple therapy. In conclusion, our random-effects model analysis revealed that triple therapy could significantly increase the incidence of hypothyroidism, arthralgia, myalgia, ALT increased, AST increased, asthenia and pyrexia (P < 0.05).
Table 2.
Outcomes of all-grade adverse events (AEs) and grade ≥ 3 adverse events for triple combination therapy versus control therapy.
| Adverse events | No. studies | RR, 95%CI | P value | Heterogeneity | |
|---|---|---|---|---|---|
| I2 | P value | ||||
| Any events | 4 | 1.01 [0.99, 1.04] | 0.39 | 47% | 0.13 |
| Grade ≥3 | 5 | 1.21 [0.99, 1.49] | 0.07 | 74% | 0.004 |
| Rash | 5 | 1.04 [0.89, 1.22] | 0.58 | 0% | 0.72 |
| Dermatitis acne | 3 | 1.04 [0.71, 1.52] | 0.84 | 5% | 0.35 |
| Pruritus | 4 | 1.20 [0.83, 1.75] | 0.33 | 8% | 0.35 |
| Nausea | 5 | 1.14 [0.82, 1.58] | 0.43 | 52% | 0.08 |
| Diarrhea | 5 | 1.20 [0.86, 1.69] | 0.28 | 66% | 0.02 |
| Vomiting | 4 | 1.20 [0.75, 1.93] | 0.45 | 35% | 0.2 |
| Hypothyroidism | 3 | 2.74 [1.64, 4.56] | 0.0001 | 0% | 0.76 |
| Arthralgia | 5 | 1.57 [1.04, 2.37] | 0.03 | 67% | 0.02 |
| Myalgia | 3 | 1.57 [1.10, 2.24] | 0.01 | 0% | 0.63 |
| ALT increased | 4 | 1.54 [1.12, 2.13] | 0.009 | 8% | 0.35 |
| AST increased | 4 | 1.43 [1.03, 1.98] | 0.03 | 7% | 0.36 |
| Blood ALP increased | 2 | 0.98 [0.67, 1.44] | 0.92 | 0% | 0.43 |
| Chills | 3 | 1.74 [0.81, 3.75] | 0.15 | 85% | 0.001 |
| Fatigue | 5 | 1.13 [0.84, 1.51] | 0.42 | 57% | 0.05 |
| Asthenia | 4 | 1.32 [1.05, 1.67] | 0.02 | 0% | 0.63 |
| Pyrexia | 3 | 1.86 [1.17, 2.95] | 0.009 | 86% | 0.001 |
| Headache | 3 | 2.16 [0.69, 6.76] | 0.18 | 63% | 0.07 |
| Decreased appetite | 2 | 0.95 [0.61, 1.47] | 0.82 | 0% | 0.39 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Sensitivity Analysis
To ensure the robustness of the findings, we further conducted the sensitivity analysis of outcomes to evaluate the influence of every single study on overall results and detect the source of heterogeneity. The sensitivity analyses revealed stable results for PFS, ORR, CR, and PR, with results relatively consistent to those of the pooled outcomes in all included studies ( Table 3 and Figures S1 – S7 ). However, omitting Gutzmer et al. study showed impact on the 2-year OS rate (P value for significance changed from 0.01 to 0.06) and AE-all grade (P value changed from 0.39 to 0.04), which indicated that this study might influence the pooled results of these two outcomes mentioned above. When omitting Ribas et al. study in AE-grade≥3 results, P value decreased from 0.07 to 0.03 with significance.
Table 3.
Sensitivity analysis of efficiency outcomes (PFS, OS, ORR, CR, PR) and adverse events (AEs-all grade, AEs-grade ≥ 3).
| Outcomes, heterogeneity | |||||||
|---|---|---|---|---|---|---|---|
| PFS, I2 | OS, I2 | ORR, I2 | CR, I2 | PR, I2 | AE-all grade, I2 | AE-grade≥3, I2 | |
| All studies | 0.71 [0.59, 0.86], 28% | 1.12 [1.03, 1.23], 0% | 1.09 [0.91, 1.30], 68% | 1.08 [0.84, 1.38], 0% | 1.05 [0.84, 1.31], 61% | 1.01 [0.99, 1.04], 47% | 1.21 [0.99, 1.49], 74% |
| Study omitted | |||||||
| Ferrucci et al. (24) | 0.77 [0.66, 0.90], 3% | 1.11 [1.01, 1.23], 0% | 1.16 [0.93, 1.43], 71% | 1.06 [0.81, 1.38], 2% | 1.12 [0.89, 1.42], 58% | 1.01 [0.99, 1.04], 64% | 1.12 [0.98, 1.27], 48% |
| Gutzmer et al. (25) | 0.63 [0.46, 0.88], 42% | 1.11 [1.00, 1.25], 0% | 1.17 [0.86, 1.61], 75% | 1.20 [0.88, 1.65], 0% | 1.07 [0.73, 1.56], 71% | 1.02 [1.00, 1.05], 0% | 1.35 [0.93, 1.96], 75% |
| Nathan et al. (21) | 0.64 [0.48, 0.84], 30% | 1.15 [1.01, 1.31], 0% | 1.16 [0.84, 1.58], 76% | 1.09 [0.72, 1.64], 9% | 1.07 [0.73, 1.56], 71% | 1.00 [0.98, 1.01], 20% | 1.28 [0.88, 1.86], 78% |
| Ribas et al. (26) | 0.74 [0.62, 0.89], 25% | 1.13 [1.03, 1.23], 0% | 1.03 [0.95, 1.11], 0% | 1.06 [0.83, 1.36], 0% | 1.01 [0.89, 1.14], 4% | – | 1.30 [1.04, 1.62], 77% |
| Rozeman et al. (20) | 0.72 [0.59, 0.88], 35% | – | 1.09 [0.90, 1.33], 76% | 1.07 [0.84, 1.37], 0% | 1.08 [0.84, 1.37], 69% | 1.01 [0.99, 1.03], 46% | 1.19 [0.98, 1.45], 78% |
Discussion
This meta-analysis combines all currently available data from previous trials to compare a triple combination of BRAF inhibition, MEK inhibition, and PD-1/PD-L1 inhibition with the two-drug combination regimen or monotherapy alone in patients with metastatic melanoma, thereby providing a reliable assessment of the role of triple therapy in advanced melanoma disease. Meta-analysis has been recognized as an effective method to assess the totality of the available data, thus avoiding selective emphasis and providing answers to controversial or unresolved clinical questions.
The random-effects model of our meta-analysis demonstrated that the triple combination therapy resulted in an evident improvement in progression-free survival compared with control group. Also, overall survival rates were significantly higher in patients treated with triple therapy compared with those receiving control therapy. However, there was no difference between the triple combination therapy and control treatment regimen with regard to the overall response rate, and the results of ORR showed high heterogeneity. When the ORR was split into complete response and partial response, there were still no significant differences between these two treatments and the high heterogeneity of ORR was mainly caused by the partial response. Therefore, despite better PFS and OS outcomes in those patients receiving triple combination therapy, one thing needs to be noted that the overall response rate also served as an important therapeutic outcome which might be useful for better symptom control in certain clinical situations (28). In terms of safety, triple combination therapy did not increase the overall incidence of any AEs or grade ≥3 AEs, but the occurrence rates of hypothyroidism, arthralgia, myalgia, ALT increased, AST increased, asthenia, and pyrexia were significantly higher than in the control group.
BRAF and MEK inhibition generally resulted in higher ORR for patients, whereas PD-1/PD-L1 inhibition typically resulted in more durable responses. Because these treatments have distinct mechanisms of action in melanoma, triple therapy combining PD-1/PD-L1 inhibitors with BRAF and MEK inhibitors were proposed and gradually used in preclinical models and clinical trials (29, 30). However, meta-analysis from studies that directly compare the triple combination therapy with two-drug combination or monotherapy is lacking. Past studies mainly focused on examining the efficacy and adverse events of standard-of-care therapy such as BRAF inhibitor plus a MEK inhibitor (31, 32), anti-PD-1/PD-L1 monotherapy (33), and nivolumab (anti-PD-1) plus ipilimumab (anti-CTLA-4) combination (34). Although these treatments have improved patient survival, the problems of drug resistance to targeted therapies and low response rates to immunotherapies still need to be addressed. To our knowledge, this study is the first meta-analysis that systematically summarizes and analyzes the safety and efficacy of triple combination therapy as compared to two-drug combinations or monotherapy for advanced melanoma.
Triple combination therapy was associated with promising anti-tumor activity. When targeted therapy begins, a series of changes in the immune microenvironment will occur, which is conducive to subsequent immunotherapy. Previous mouse model of syngeneic BRAF V600E driven melanoma showed that combination of BRAF and MEK inhibitor (dabrafenib and trametinib) increased T cell infiltration into tumors, up-regulated PD-L1 expression and improved in vivo cytotoxicity. Triple combination of dabrafenib, trametinib with anti-PD-1 therapy offered superior anti-tumor effect in BRAF V600E murine melanoma (30). In a phase Ib study (35), patients treated with BRAF and MEK inhibitor (vemurafenib and cobimetinib) also induced changes in the tumor microenvironment, including the increased proportion of CD8+ T cells and increased proliferation of CD4+ T-helper cells, these favorable changes may enhance response to anti-PD-1/PD-L1 immunotherapy. Immunohistochemistry analysis and RNA sequencing data showed increased MHC class I expression and immune infiltration in triple therapy (17, 36). Therefore, these molecular mechanisms of triple combination therapy provided a feasible treatment approach for advanced melanoma.
The primary endpoint of investigator-assessed PFS in the IMSpire 150 study exhibited a significant improvement of 4.5 months difference (15.1 vs. 10.6 months) in the triple combination therapy, which led to FDA approval of this triple therapy (atezolizumab, vemurafenib and cobimetinib) for treatment of BRAF V600 mutation-positive melanoma. In terms of treatment therapies, IMSpire 150 study designed a run-in period of targeted therapy prior to the initiation of PD-L1 immunotherapy, which differed from the Keynote-022 and COMBI-I study that used three-drug combinations at the same time. The smart design resulted in better drug tolerance among patients in the triple therapy group of IMSpire 150 study. Therefore, we recommended introducing a run-in period of BRAF and MEK inhibitors before PD-1/PD-L1 immunotherapy to maximize the benefits of triple therapy. In addition, more clinical trials are required to find out the optimal triple combination regimen and administration time.
This study has several limitations that deserve to be mentioned. The first drawback is that the patients in the included studies were treated with different triple combinations and patients in the control arm also received different treatment regimens. Second, because the triple combination therapy is emerging and has been applied in clinical practice only in recent years, some studies such as Immu-Target (NCT02902042) (37) and NeoTrio (NCT02858921) (38) are still at the preliminary stage, therefore, the numbers of included studies were relatively small. The limited number of studies and patients for analysis could probably lead to decreased accuracy and reliability of our comparison results. To overcome this problem, we conducted sensitivity analysis to test the robustness of the results. Third, high heterogeneity existed in overall response rates, partial response and many adverse events. Our meta-analysis included phase 1, phase 2, and phase 3 clinical trials could introduce heterogeneity among the results. To overcome this issue, random-effects model was adopted in all analyses because unexplained heterogeneity was taken into account in this model.
Conclusion
In conclusion, triple combination therapy of PD-1/PD-L1, BRAF, and MEK inhibition had significant survival benefit over two-drug combination or monotherapy and should be a new preferred therapy for patients with stage III-IV advanced and metastatic melanoma, as confirmed by the present meta-analysis. Attention should be paid to some adverse events and physicians need to balance against the increased toxicity when using triple combination therapy. Utilizing the triple combination drugs sensitized the patients’ immune system to improve the effectiveness of immunotherapy and inhibited BRAF plus MEK to control tumor growth. From our results, we conclude that triple combination therapy has great benefits for patients harboring the BRAF V600 mutation-positive advanced melanoma, and designing a run-in period of BRAF and MEK targeted therapy prior to the initiation of PD-1/PD-L1 immunotherapy could maximize the benefits of triple therapy. Despite the promising results of triple combination therapy, longer follow-up is required to see the possible benefit from the triple therapy. Besides that, more clinical trials are required to determine which patients benefit the most from the triple therapy, and to find out the optimal sequence and dose of triple drug administration. We hope this current meta-analysis could provide a reference point for physicians in clinical treatment when considering the optimum combination regimen for advanced melanoma patients.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Author Contributions
Concept and design: YL, XC. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: YL, XC. Critical revision of the manuscript for important intellectual content: YL, XC. Statistical analysis: YL, GW, XZ. Administrative, technical, or material support: YL, XZ, GW. Supervision: GW, XC. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.693655/full#supplementary-material
Sensitivity analysis of progression free survival (PFS).
Sensitivity analysis of overall survival (OS).
Sensitivity analysis of overall response rate (ORR)
Sensitivity analysis of complete response (CR).
Sensitivity analysis of partial response (PR).
Sensitivity analysis of adverse events for all grades.
Sensitivity analysis of adverse events for grade≥ 3.
Search Strategies.
References
- 1. Badea I. New Strategies in Melanoma Therapy: Can Nanoparticles Overcome Chemoresistance? Nanomedicine (Lond) (2017) 12(14):1623–6. 10.2217/nnm-2017-0145 [DOI] [PubMed] [Google Scholar]
- 2. Schadendorf D, van Akkooi ACJ, Berking C, Griewank KG, Gutzmer R, Hauschild A, et al. Melanoma. Lancet (2018) 392(10151):971–84. 10.1016/S0140-6736(18)31559-9 [DOI] [PubMed] [Google Scholar]
- 3. Li J, Wang Y, Liang R, An X, Wang K, Shen G, et al. Recent Advances in Targeted Nanoparticles Drug Delivery to Melanoma. Nanomedicine (2015) 11(3):769–94. 10.1016/j.nano.2014.11.006 [DOI] [PubMed] [Google Scholar]
- 4. Schadendorf D, Makki A, Stahr C, van Dyck A, Wanner R, Scheffer GL, et al. Membrane Transport Proteins Associated With Drug Resistance Expressed in Human Melanoma. Am J Pathol (1995) 147(6):1545–52. [PMC free article] [PubMed] [Google Scholar]
- 5. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in Previously Untreated Melanoma Without BRAF Mutation. N Engl J Med (2015) 372(4):320–30. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 6. Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Combined BRAF and MEK Inhibition Versus BRAF Inhibition Alone in Melanoma. N Engl J Med (2014) 371(20):1877–88. 10.1056/NEJMoa1406037 [DOI] [PubMed] [Google Scholar]
- 7. Gibney GT, Atkins MB. Choice of First-Line Therapy in Metastatic Melanoma. Cancer (2019) 125(5):666–9. 10.1002/cncr.31774 [DOI] [PubMed] [Google Scholar]
- 8. Smalley KS, Sondak VK. Inhibition of BRAF and MEK in BRAF-Mutant Melanoma. Lancet (2015) 386(9992):410–2. 10.1016/S0140-6736(15)60972-2 [DOI] [PubMed] [Google Scholar]
- 9. Constantinidou A, Alifieris C, Trafalis DT. Targeting Programmed Cell Death -1 (PD-1) and Ligand (PD-L1): A New Era in Cancer Active Immunotherapy. Pharmacol Ther (2019) 194:84–106. 10.1016/j.pharmthera.2018.09.008 [DOI] [PubMed] [Google Scholar]
- 10. Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and Ipilimumab Versus Ipilimumab in Untreated Melanoma. N Engl J Med (2015) 372(21):2006–17. 10.1056/NEJMoa1414428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kakadia S, Yarlagadda N, Awad R, Kundranda M, Niu J, Naraev B, et al. Mechanisms of Resistance to BRAF and MEK Inhibitors and Clinical Update of US Food and Drug Administration-Approved Targeted Therapy in Advanced Melanoma. Onco Targets Ther (2018) 11:7095–107. 10.2147/OTT.S182721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Trojaniello C, Vitale MG, Ascierto PA. Triplet Combination of BRAF, MEK and PD-1/PD-L1 Blockade in Melanoma: The More the Better? Curr Opin Oncol (2021) 33(2):133–8. 10.1097/CCO.0000000000000709 [DOI] [PubMed] [Google Scholar]
- 13. Khair DO, Bax HJ, Mele S, Crescioli S, Pellizzari G, Khiabany A, et al. Combining Immune Checkpoint Inhibitors: Established and Emerging Targets and Strategies to Improve Outcomes in Melanoma. Front Immunol (2019) 10:453. 10.3389/fimmu.2019.00453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vella LJ, Pasam A, Dimopoulos N, Andrews M, Knights A, Puaux AL, et al. MEK Inhibition, Alone or in Combination With BRAF Inhibition, Affects Multiple Functions of Isolated Normal Human Lymphocytes and Dendritic Cells. Cancer Immunol Res (2014) 2(4):351–60. 10.1158/2326-6066.CIR-13-0181 [DOI] [PubMed] [Google Scholar]
- 15. Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, et al. BRAF Inhibition Is Associated With Enhanced Melanoma Antigen Expression and a More Favorable Tumor Microenvironment in Patients With Metastatic Melanoma. Clin Cancer Res (2013) 19(5):1225–31. 10.1158/1078-0432.CCR-12-1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ascierto PA, Ferrucci PF, Fisher R, Del Vecchio M, Atkinson V, Schmidt H, et al. Dabrafenib, Trametinib and Pembrolizumab or Placebo in BRAF-Mutant Melanoma. Nat Med (2019) 25(6):941–6. 10.1038/s41591-019-0448-9 [DOI] [PubMed] [Google Scholar]
- 17. Ribas A, Lawrence D, Atkinson V, Agarwal S, Miller WH, Jr., Carlino MS, et al. Combined BRAF and MEK Inhibition With PD-1 Blockade Immunotherapy in BRAF-Mutant Melanoma. Nat Med (2019) 25(6):936–40. 10.1038/s41591-019-0476-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dummer R, Lebbe C, Atkinson V, Mandala M, Nathan PD, Arance A, et al. Combined PD-1, BRAF and MEK Inhibition in Advanced BRAF-Mutant Melanoma: Safety Run-in and Biomarker Cohorts of COMBI-I. Nat Med (2020) 26(10):1557–63. 10.1038/s41591-020-1082-2 [DOI] [PubMed] [Google Scholar]
- 19. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ (2009) 339:b2700. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rozeman EA, Sikorska K, Grijpink-Ongering L, Heeres B, Van De Wiel B, Sari A, et al. Updated Data From IMPemBra, a Phase 2 Study Comparing Pembrolizumab (PEM) With Intermittent/Short-Term Dual MAPK Pathway Inhibition (Mapki, Dabrafenib + Trametinib, D + T) Plus PEM in Patients Harboring the BRAFV600 Mutation. J Trans Med (2020) 18:20. 10.1186/s12967-020-02209-y [DOI] [Google Scholar]
- 21. Nathan P, Dummer R, Long GV, Ascierto PA, Tawbi HA, Robert C, et al. Spartalizumab Plus Dabrafenib and Trametinib (Sparta-DabTram) in Patients (pts) With Previously Untreated BRAF V600–Mutant Unresectable or Metastatic Melanoma: Results From the Randomized Part 3 of the Phase III COMBI-I Trial. Ann Oncol (2020) 31:S1172–. 10.1016/j.annonc.2020.08.2273 [DOI] [Google Scholar]
- 22. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated Guidance for Trusted Systematic Reviews: A New Edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev (2019) 10:ED000142. 10.1002/14651858.ED000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Higgins JP, Thompson SG. Quantifying Heterogeneity in a Meta-Analysis. Stat Med (2002) 21(11):1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 24. Ferrucci PF, Di Giacomo AM, Del Vecchio M, Atkinson V, Schmidt H, Schachter J, et al. KEYNOTE-022 Part 3: A Randomized, Double-Blind, Phase 2 Study of Pembrolizumab, Dabrafenib, and Trametinib in BRAF-Mutant Melanoma. J Immunother Cancer (2020) 8(2):e001806. 10.1136/jitc-2020-001806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gutzmer R, Stroyakovskiy D, Gogas H, Robert C, Lewis K, Protsenko S, et al. Atezolizumab, Vemurafenib, and Cobimetinib as First-Line Treatment for Unresectable Advanced BRAF(V600) Mutation-Positive Melanoma (Imspire150): Primary Analysis of the Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet (2020) 395(10240):1835–44. 10.1016/S0140-6736(20)30934-X [DOI] [PubMed] [Google Scholar]
- 26. Ribas A, Algazi A, Ascierto PA, Butler MO, Chandra S, Gordon M, et al. Pd-L1 Blockade in Combination With Inhibition of MAPK Oncogenic Signaling in Patients With Advanced Melanoma. Nat Commun (2020) 11(1):6262. 10.1038/s41467-020-19810-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol (2018) 36(17):1714–68. 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ives NJ, Stowe RL, Lorigan P, Wheatley K. Chemotherapy Compared With Biochemotherapy for the Treatment of Metastatic Melanoma: A Meta-Analysis of 18 Trials Involving 2,621 Patients. J Clin Oncol (2007) 25(34):5426–34. 10.1200/JCO.2007.12.0253 [DOI] [PubMed] [Google Scholar]
- 29. Pelster MS, Amaria RN. Combined Targeted Therapy and Immunotherapy in Melanoma: A Review of the Impact on the Tumor Microenvironment and Outcomes of Early Clinical Trials. Ther Adv Med Oncol (2019) 11:1758835919830826. 10.1177/1758835919830826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hu-Lieskovan S, Mok S, Homet Moreno B, Tsoi J, Robert L, Goedert L, et al. Improved Antitumor Activity of Immunotherapy With BRAF and MEK Inhibitors in BRAF(V600E) Melanoma. Sci Transl Med (2015) 7(279):279ra41. 10.1126/scitranslmed.aaa4691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yu Q, Xie J, Li J, Lu Y, Liao L. Clinical Outcomes of BRAF Plus MEK Inhibition in Melanoma: A Meta-Analysis and Systematic Review. Cancer Med (2019) 8(12):5414–24. 10.1002/cam4.2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu M, Yang X, Liu J, Zhao B, Cai W, Li Y, et al. Efficacy and Safety of BRAF Inhibition Alone Versus Combined BRAF and MEK Inhibition in Melanoma: A Meta-Analysis of Randomized Controlled Trials. Oncotarget (2017) 8(19):32258–69. 10.18632/oncotarget.15632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hussaini S, Chehade R, Boldt RG, Raphael J, Blanchette P, Maleki Vareki S, et al. Association Between Immune-Related Side Effects and Efficacy and Benefit of Immune Checkpoint Inhibitors - A Systematic Review and Meta-Analysis. Cancer Treat Rev (2021) 92:102134. 10.1016/j.ctrv.2020.102134 [DOI] [PubMed] [Google Scholar]
- 34. Yun S, Vincelette ND, Green MR, Wahner Hendrickson AE, Abraham I. Targeting Immune Checkpoints in Unresectable Metastatic Cutaneous Melanoma: A Systematic Review and Meta-Analysis of Anti-CTLA-4 and Anti-PD-1 Agents Trials. Cancer Med (2016) 5(7):1481–91. 10.1002/cam4.732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sullivan RJ, Hamid O, Gonzalez R, Infante JR, Patel MR, Hodi FS, et al. Atezolizumab Plus Cobimetinib and Vemurafenib in BRAF-mutated Melanoma Patients. Nat Med (2019) 25(6):929–35. 10.1038/s41591-019-0474-7 [DOI] [PubMed] [Google Scholar]
- 36. Deken MA, Gadiot J, Jordanova ES, Lacroix R, van Gool M, Kroon P, et al. Targeting the MAPK and PI3K Pathways in Combination With PD1 Blockade in Melanoma. Oncoimmunology (2016) 5(12):e1238557. 10.1080/2162402X.2016.1238557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nct . Open Label, Multicentre Study of Encorafenib + Binimetinib + PD (Programmed Cell Death Protein) -1 Antibody Pembrolizumab. (2016). Available at: https://clinicaltrialsgov/show/NCT02902042.
- 38. Nct . Neoadjuvant Dabrafenib, Trametinib and/or Pembrolizumab in BRAF Mutant Resectable Stage III Melanoma. (2016). Available at: https://clinicaltrialsgov/show/NCT02858921.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sensitivity analysis of progression free survival (PFS).
Sensitivity analysis of overall survival (OS).
Sensitivity analysis of overall response rate (ORR)
Sensitivity analysis of complete response (CR).
Sensitivity analysis of partial response (PR).
Sensitivity analysis of adverse events for all grades.
Sensitivity analysis of adverse events for grade≥ 3.
Search Strategies.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.