Abstract
Vascular occlusive crisis with a concurrent vision loss on both eyes is one of the most devastating disability for sickle cell disease patients. Reportedly occlusive crisis in the eyes is usually temporary whereas if not appropriately managed can result in permanent vision loss. A carefully managed sickle cell crisis could prevent multiple disabilities including blindness and stroke. We report a case of a 24-year-old female with a history of sickle cell disease who had acute bilateral vision loss during a sickle crisis and recovered significantly with a timely emergent erythrocytapheresis.
Keywords: central retinal artery occlusion, sickle cell disease, exchange transfusion
Introduction
During a crisis, sickled red blood cells (RBCs) can block blood vessels to one or both eyes and could result in severe grade vision loss. The approximate incidence of central retinal artery occlusion is estimated to be around 1 to 2/100 000, which includes sickle cell disease (SCD), atherosclerosis, and non-arteritic occlusions with predominance in older age groups.1,2 Clinical evaluation for central retinal artery occlusion is similar to cerebrovascular accident of the brain, but the blood vessel occlusion mechanisms are significantly different from each other. A timely emergent medical management could be vital to prevent a significant disability.
Case Presentation
A 24-year-old African American female with a history of SCD (homozygous SS type) came to the emergency department with acute onset of shortness of breath for the past 4 days. She developed bilateral intermittent vision loss at the hospital with each episode lasting for several minutes. On prior admissions she had acute chest syndrome and avascular necrosis of bilateral femoral heads.
The visual acuity was restricted to hand motions bilaterally at 4 feet distance with sluggish reacting pupils of 6 mm dilatation. Fundus examination showed flat tortuous vessels associated with cherry red macula suggestive of bilateral central retinal artery occlusion (CRAO) with the left eye worse than the right (Figure 1). There were decreased bilateral breath sounds on lung examination without any added sounds. Abnormal laboratory test results were found with positive serum anti-nuclear antibody (ANA) titer of 1:80 with speckled pattern (Table 1).
Figure 1.
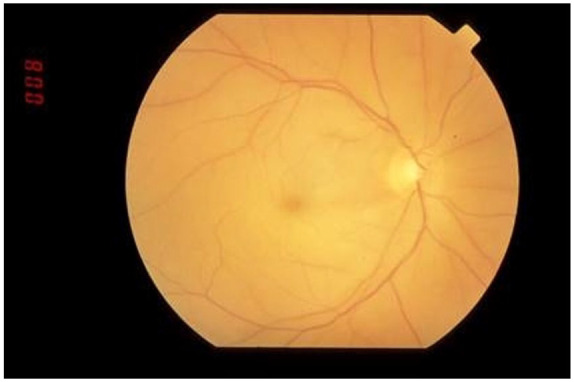
A central retinal artery occlusion of the right eye with inner retinal whitening from ischemia of the neural retina and a “cherry red spot” in the foveola due to absence of the nerve fiber and ganglion cell layers with an intact choroidal vasculature.
Table 1.
Laboratory Test Reports.
| Tests | Results | Reference values |
|---|---|---|
| Hemoglobin | 6.9 g/dL | 13.0 to 17.3 g/dL |
| Hematocrit | 19.60% | 38% to52% |
| Mean corpuscular volume | 111.0 fL/red cell | 80 to 98 fL/red cell |
| Red blood cells | 1.77 × 106/µL | 3.80 to 5.30 ×106/µL |
| Reticulocytes | 6.0% | 0.5% to 2.5% |
| Serum lactate | 2.4 mmol/L | 0.4 to 2.0 mmol/L |
| Total bilirubin | 1.6 mg/dL | 0 to 1.0 mg/dL |
| Conjugated bilirubin | 0.3 mg/dL | 0 to 0.2 mg/dL |
| Neutrophils | 8.3 × 103/µL | 1.6 to 7.7 ×103/µL |
| Antinuclear antibodies | Positive titer: 1:80; pattern: speckled | <1/40; pattern: no pattern |
| Dilute Russell’s viper venom screen time | 57 seconds | 23 to 27 seconds |
| Dilute Russell’s viper venom confirmation | Negative | Negative |
| PTT-lupus anticoagulant | 42 seconds | 24 to 36 seconds |
| Prothrombin time | 15 seconds | 12.5 to 14.2 seconds |
| Hexagonal (II) phase phospholipid neutralization assay | Negative | Negative |
| β-2 Glycoprotein IgG, IgM, and IgA | <9 U/mL | <9.4 to 20.0 U/mL |
| Anticardiolipin antibodies IgG | <12 GPL | Negative ≤GPL |
Abbreviations: PTT, partial thromboplastin time; Ig, immunoglobulin.
Suspecting a vascular occlusion mediated by sickled RBCs, an immediate erythrocytapheresis was initiated and continued until her symptoms improved over the next 2 days. On computed thoracic angiogram of the chest, dilated tortuous blood vessels were seen. The supportive oxygen therapy and transfusions decreased the chest symptoms. The hemoglobin S type gradually reduced to 45% from 67% over the 2 days. Computed tomography of the brain, chest X-ray, and other laboratory tests were within normal limits. The visual symptoms partially recovered over several months.
Discussion
The atherosclerotic vessel occlusion in the eye can result in any acute loss of vision or visual acuity requiring evaluation for vascular involvement similar to a stroke. Commonly, the ophthalmic artery occlusion at the dural sheath of optic nerve due to atherosclerosis can result in unilateral CRAO. 3 The differential diagnosis for concurrent acute bilateral vision loss/acuity is SCD crisis, complex migraines, occipital epilepsy, and papilledema. 4 Appropriate neurological evaluation, visual acuity tests, and fundoscopy are necessary to differentiate the various disorders. Even though sickle cell–mediated acute vision loss/visual acuity in one/both eyes were previously reported, bilateral CRAO and severe symptoms of sickling are frequently noted in hemoglobin SS type. Clinically, different sickle cell genotypes present with various levels of vessel occlusions, the hemoglobin SS type present with most vascular occlusions and least association with retinal proliferative lesions. 5 The chronic proliferative changes are often associated with HbSC (39%) than with HbSS (8.4%) phenotype. 6 Endothelial tissues exposed to intermittent hypoxia mediated by vascular occlusion from sickling can result in localized chronic inflammatory vascular changes. 7 Unless a reversion occurs in the vascular bed, the clogged sickled cells could hemolyze and release hemoglobin. The released plasma hemoglobin scavenges the local nitric oxide (NO) by dioxygenation reaction to be converted to methemoglobin. 8 Even a small amount of plasma hemoglobin released due to any process can induce vasoconstriction by impairing the NO availability in the endothelium. NO has an additional role of inhibiting cellular activation of smooth muscle cell, fibroblast proliferation, and neo-vascularization. 9 Any decrease in NO may result in multitude of effects leading to endothelial dysfunction and activation of atherosclerotic process. Chronic mechanical injury to the endothelial cells by the sickled RBCs activate an immune attack on the endothelium resulting in further endothelial dysfunction. 10 SCD patients tend to have higher levels of plasma homocysteine levels and correspondingly are at a higher risk for stroke and vascular phenomenon.11,12 Elevated expression of platelet activation dependent antigens in SCD patients in periods of non-crisis and more at vaso-occlusive crisis promote aggressive atherosclerosis. 13
In our patient, considering the extent of bilateral retinal artery occlusion, it was reasonable to evaluate for a coexisting anti-phospholipid syndrome. The anti-phospholipid antibodies were inconclusive and unable to confirm the presence of anti-phospholipid syndrome. Our patient tested positive for serum ANA, which did not warrant further evaluation considering around 13% 14 of general population are ANA positive.
In SCD crisis, the arteriolar involvement with RBCs stacked at the end arterioles than at the capillary bed without the activation of the clotting process enables the occlusion potentially reversible for a certain amount of time. 15 The bilateral occlusion due to blockade from the deformed red blood corpuscles in the retinal arteries lead to the current presentation. 16 While delivered through exchange transfusion, the normal RBCs being pliable squeeze through the partially blocked arterioles and deliver the oxygen to the distal tissues, thereby reverting the symptoms. 17
Conclusion
A timely emergent erythrocytapheresis is critical to reduce the number of sickled RBCs and in reversing the loss of vision. Early identification of microvascular complications is of primary importance to prevent end-organ damage and disability in SCD patients. SCD-related microvascular complications are often reversible to an extent and emergent intervention like erythrocytapheresis is advised for better outcomes in select patients.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Verbal informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
ORCID iD: Piruthiviraj Natarajan  https://orcid.org/0000-0003-2702-7476
https://orcid.org/0000-0003-2702-7476
References
- 1. Leavitt JA, Larson TA, Hodge DO, Gullerud RE. The incidence of central retinal artery occlusion in Olmsted County, Minnesota. Am J Ophthalmol. 2011;152:820-823.e2. doi: 10.1016/j.ajo.2011.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rumelt S, Dorenboim Y, Rehany U. Aggressive systematic treatment for central retinal artery occlusion. Am J Ophthalmol. 1999;128:733-738. doi: 10.1016/s0002-9394(99)00359-1 [DOI] [PubMed] [Google Scholar]
- 3. MacGrory B, Lavin P, Kirshner H, Schrag M. Thrombolytic therapy for acute central retinal artery occlusion. Stroke. 2020;51:687-695. doi: 10.1161/STROKEAHA.119.027478 [DOI] [PubMed] [Google Scholar]
- 4. Feroze KB, O’Rourke MC. Transient Loss of Vision. StatPearls Publishing. Published 2020. Accessed October 11, 2020. http://www.ncbi.nlm.nih.gov/books/NBK430845/ [PubMed] [Google Scholar]
- 5. Nagel RL, Fabry ME, Steinberg MH. The paradox of hemoglobin SC disease. Blood Rev. 2003;17:167-178. doi: 10.1016/S0268-960X(03)00003-1 [DOI] [PubMed] [Google Scholar]
- 6. Downes SM, Hambleton IR, Chuang EL, Lois N, Serjeant GR, Bird AC. Incidence and natural history of proliferative sickle cell retinopathy: observations from a cohort study. Ophthalmology. 2005;112:1869-1875. [DOI] [PubMed] [Google Scholar]
- 7. Stuart MJ, Setty BN. Sickle cell acute chest syndrome: pathogenesis and rationale for treatment. Blood. 1999;94:1555-1560. [PubMed] [Google Scholar]
- 8. Chenell D, Raat NJH, Kanias T, et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465-476. doi: 10.1161/CIRCULATIONAHA.110.008698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lau YT, Ma WC. Nitric oxide inhibits migration of cultured endothelial cells. Biochem Biophys Res Commun. 1996;221:670-674. doi: 10.1006/bbrc.1996.0654 [DOI] [PubMed] [Google Scholar]
- 10. de Chadarévian JP, Balarezo FS, Heggere M, Dampier C. Splenic arteries and veins in pediatric sickle cell disease. Pediatr Dev Pathol. 2001;4:538-544. doi: 10.1007/s10024001-0045-y [DOI] [PubMed] [Google Scholar]
- 11. Houston PE, Rana S, Sekhsaria S, Perlin E, Kim KS, Castro OL. Homocysteine in sickle cell disease: relationship to stroke. Am J Med. 1997;103:192-196. doi: 10.1016/s0002-9343(97)00129-0 [DOI] [PubMed] [Google Scholar]
- 12. Lowenthal EA, Mayo MS, Cornwell PE, Thornley-Brown D. Homocysteine elevation in sickle cell disease. J Am Coll Nutr. 2000;19:608-612. doi: 10.1080/07315724.2000.10718958 [DOI] [PubMed] [Google Scholar]
- 13. Wun T, Paglieroni T, Rangaswami A, et al. Platelet activation in patients with sickle cell disease. Br J Haematol. 1998;100:741-749. doi: 10.1046/j.1365-2141.1998.00627.x [DOI] [PubMed] [Google Scholar]
- 14. Satoh M, Chan EKL, Ho LA, et al. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum. 2012;64:2319-2327. doi: 10.1002/art.34380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Francis RB. Large-vessel occlusion in sickle cell disease: pathogenesis, clinical consequences, and therapeutic implications. Med Hypotheses. 1991;35:88-95. doi: 10.1016/0306-9877(91)90029-X [DOI] [PubMed] [Google Scholar]
- 16. Ahmed SG. The role of infection in the pathogenesis of vaso-occlusive crisis in patients with sickle cell disease. Mediterr J Hematol Infect Dis. 2011;3:e2011028. doi: 10.4084/mjhid.2011.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weissman H, Nadel AJ, Dunn M. Simultaneous bilateral retinal arterial occlusions treated by exchange transfusions. Arch Ophthalmol. 1979;97:2151-2153. doi: 10.1001/archopht.1979.01020020469013 [DOI] [PubMed] [Google Scholar]


