SUMMARY
Severe allergic reactions (anaphylaxis) are unpredictable, and initial signs of what could be fatal anaphylaxis can be mild
Adrenaline (epinephrine) remains the first-line drug of choice for the acute management of anaphylaxis and should be administered early
There are no contraindications to intramuscular adrenaline in the treatment of anaphylaxis
Correct positioning of the patient is vital as death can occur within minutes if a patient stands, walks or sits up suddenly. Position the patient correctly first and then promptly administer intramuscular adrenaline
Updated guidelines by the Australasian Society of Clinical Immunology and Allergy now recommend that the 0.15 mg adrenaline injector device may be prescribed for infants and children weighing 7.5–10 kg. The recommendation to use the 0.3 mg adrenaline injector device for those over 20 kg remains unchanged
The adrenaline doses in Australian Prescriber’s anaphylaxis wallchart remain valid
Keywords: adrenaline (epinephrine), allergy, anaphylaxis, adrenaline (epinephrine) injector device
Introduction
Anaphylaxis is a potentially life-threatening severe allergic reaction and adrenaline (epinephrine) remains the first-line treatment. The Australasian Society of Clinical Immunology and Allergy (ASCIA) has recently updated its dose recommendations for adrenaline injectors in children.1
Rates of hospital admission for anaphylaxis in Australia are increasing, particularly for food-related anaphylaxis in older children and adolescents.2 There has been a similar increase in all-cause fatal anaphylaxis in Australia in the last two decades. In keeping with the increasing incidence in children and adolescents, these age groups have the highest risk of fatality from food-related anaphylaxis.3
Anaphylaxis – presenting symptoms
ASCIA defines anaphylaxis as:
any acute onset illness with typical skin features (e.g. urticarial rash or erythema or flushing with or without angioedema), plus involvement of respiratory or cardiovascular symptoms with or without persistent severe gastrointestinal symptoms, OR
any acute onset of hypotension or bronchospasm or upper airway obstruction where anaphylaxis is considered possible, even if typical skin features are not present.1
Gastrointestinal symptoms of any severity including abdominal pain or vomiting may be signs of anaphylaxis from an insect sting or injected drug allergy. However, severe, persistent gastrointestinal symptoms may be a feature of anaphylaxis from any cause (see Box 1).1,4-6
Box 1. Signs and symptoms of allergic reactions in infants6.
|
Mild to moderate reaction Cutaneous • urticaria • angioedema Gastrointestinal • tingling mouth • abdominal pain • vomiting* Other signs • face (eye, ear, nose) rubbing • sneezing • sudden onset of clear nasal discharge • conjunctival redness • irritability • clinging to caregiver |
Severe reaction (anaphylaxis) Respiratory • swelling of tongue • swelling in throat (e.g. hoarseness, croakiness or difficulty vocalising) • wheeze, stridor or persistent cough • laboured or noisy breathing • low oxygen saturation • rapid respiratory rate for age • low respiratory rate may indicate impending respiratory arrest Cardiovascular • hypotension is a late sign in infants due to high peripheral vascular resistance and can represent a pre-arrest sign • collapse • pallor and floppiness • tachycardia – rapid resting heart rate for age may signal hypotension Behavioural changes • sudden drowsiness • unresponsiveness • loss of consciousness |
| * abdominal pain and vomiting are signs of anaphylaxis in insect sting or injected drug allergy | |
Anaphylaxis is unpredictable and signs of a potentially fatal anaphylaxis can initially appear mild.3,7 However, mild or moderate allergic symptoms may not always precede anaphylaxis.4,5,7 Risk factors for fatal anaphylaxis include upright posture during and after anaphylaxis, delayed administration of adrenaline, concomitant asthma and delayed initiation of CPR after collapse.3,8
Management
Adrenaline is the first-line drug for anaphylaxis.5,9-11 It works by reducing airway mucosal oedema, inducing bronchodilation and vasoconstriction and increasing cardiac contraction strength. Intramuscular adrenaline should be administered without delay into the outer mid-thigh if features of anaphylaxis are present.1,5,9 There are no contraindications to intramuscular adrenaline in the treatment of anaphylaxis.11-13
Given the rapid onset of anaphylaxis, its potential severity and ethical issues, there are no published pharmacodynamic dose response studies of adrenaline in anaphylaxis. Adrenaline dosage is based on common practice and limited studies in healthy volunteers.12,14
Intramuscular adrenaline is first line
In patients of all ages using adrenaline 1:1000, 0.01 mL/kg up to 0.5 mL (0.5 mg) per dose is recommended using an adrenaline ampoule and syringe or an adrenaline injector device (Table).1,5,9,10,13 Repeat doses are recommended at five minute intervals if there are ongoing symptoms. An adrenaline infusion should be considered if signs persist despite administration of two or more doses, if skills and equipment are available.1,5,9,11-13
Table. Adrenaline (epinephrine) dosing for the treatment of anaphylaxis.
| Approximate age (years) | Weight (kg) | Vol. adrenaline 1:1000 | Adrenaline injector |
|---|---|---|---|
| <1 | <7.5 | 0.1 mL | 0.1 mg device (not currently available in Australia or New Zealand) |
| 1–2 | 10 | 0.1 mL | 7.5-20 kg (~<5yrs) 0.15mg device |
| 2–3 | 15 | 0.15 mL | |
| 4–6 | 20 | 0.2 mL | |
| 7–10 | 30 | 0.3 mL | >20kg (~>5yrs) 0.3mg device |
| 10–12 | 40 | 0.4 mL | |
| >12 and adults | >50 | 0.5 mL | >50kg (~>12 years) 0.3mg or 0.5mg devices |
Reproduced with permission from the Australasian Society of clinical Immunology and Allergy (ASCIA)
Boluses of intravenous adrenaline are associated with a significantly increased risk of adverse effects and overdose, and should be avoided.15,16 Subcutaneous adrenaline is not as reliably absorbed as intramuscular and should also be avoided.17
A person with both known asthma and an allergy to food, insects or a medicine who has a sudden onset of difficulty breathing (including wheeze, persistent cough or hoarse voice) should always be given adrenaline first, then a bronchodilator, even in the absence of cutaneous symptoms.
Adrenaline injectors
Adrenaline injectors allow rapid, reliable delivery of intramuscular adrenaline. They were designed to make it easier for non-medical people to administer adrenaline in an emergency. Adrenaline injectors also reduce the risk of dosing errors associated with adrenaline ampoules and syringes, especially in the community.14,18
Up to two adrenaline injectors can be prescribed on the Pharmaceutical Benefits Scheme (PBS) for patients at risk of anaphylaxis. The initial PBS authority requires either discharge from the emergency department or hospital after treatment with adrenaline for anaphylaxis or consultation with a clinical immunologist or allergy specialist, paediatrician or respiratory physician. Additional devices can be purchased over-the-counter at full cost.
Updated guidelines for infants and small children
From 1 September 2021, injector devices will be available in three dose sizes containing 0.15 mg, 0.3 mg and 0.5 mg adrenaline. In infants and young children weighing less than 10 kg, this poses a challenge for prescribing an adrenaline injector. ASCIA recently updated the weight recommendations for the use of an adrenaline injector in children.1 A 0.15 mg device may now be prescribed for an infant weighing 7.5–10 kg, following a considered assessment. Previously this device was only recommended for children weighing 10–20 kg. This update is based on the safety of intramuscular adrenaline in children at the recommended doses and is supported by international professional consensus.5,9,11,13,14
The use of a 0.15 mg adrenaline injector device for infants weighing 7.5 kg will deliver up to 200% of the recommended 0.01 mg/kg adrenaline dose. However, delivering it via an injector poses less risk than using an adrenaline ampoule and syringe where dosing errors and delays in administration increase the risk of harm, particularly when used without medical training.14,18
There are no published cases of bone injury or adrenaline delivery failure from an adrenaline injector needle tip striking the femur in children weighing less than 10 kg, despite theoretical risks. Bunching the skin and muscle of the mid-thigh may help to reduce this risk.14,19 The ASCIA recommendation to prescribe a 0.3 mg adrenaline injector to individuals weighing at least 20 kg to reduce the risk of under-dosing adrenaline remains unchanged.1,11,14
Infants with anaphylaxis may remain pale despite 2–3 doses of adrenaline. This can resolve without further doses6 so persistent pallor alone is not an indication for more adrenaline. In addition, more than 2–3 doses of adrenaline in infants may cause hypertension and tachycardia, and the tachycardia is often misinterpreted as an ongoing cardiovascular compromise or anaphylaxis.6 To check if additional doses of adrenaline are required, measuring blood pressure can provide a guide to the effectiveness of treatment.6,16
Positioning of the patient
Correct positioning of a patient being treated for anaphylaxis is vital (see Fig.) as death can occur within minutes if a patient stands, walks or sits up suddenly.3 Laying the patient flat improves venous blood return to the heart. A patient must not walk or stand, even if they appear to have recovered. A wheelchair, stretcher or trolley should always be used to transfer the patient to and from the ambulance, treatment room bed and toilet.1 The left lateral (recovery) position is recommended if someone with anaphylaxis is vomiting.
Fig.
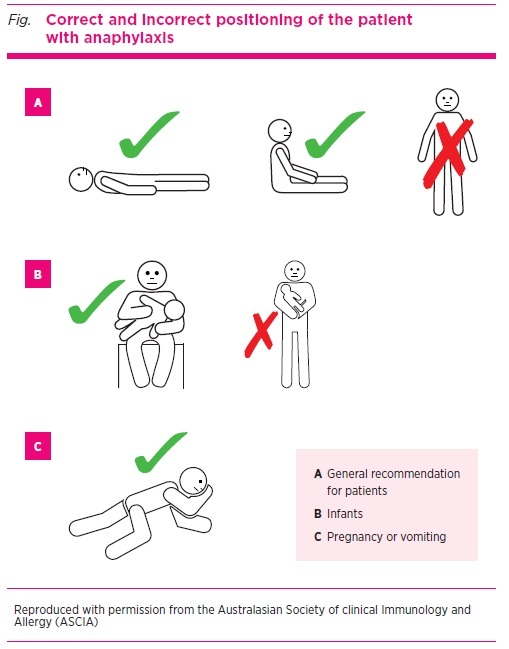
Correct and incorrect positioning of the patient with anaphylaxis
Patients with respiratory symptoms, which are the most common feature of anaphylaxis in children, may prefer to sit to help with breathing and improve ventilation. They should sit with their legs outstretched in front of them, not in a chair, and must be monitored closely as even sitting may trigger hypotension.
Infants with anaphylaxis may appear pale and floppy and should be held horizontally in the carer’s arms and not upright over the shoulder. The left lateral (recovery) position is recommended for pregnant patients as this reduces the risk of compression of the inferior vena cava by the uterus and improves venous return to the heart.20
Other drug options after adrenaline
Bronchodilators such as salbutamol may be given for persistent wheeze after adrenaline. Antihistamines and corticosteroids should not be given before or in place of adrenaline in the treatment of anaphylaxis. Antihistamines do not treat or prevent anaphylaxis or biphasic reactions but may reduce pruritis and can be given after adrenaline.1,5,9 The benefit of corticosteroids is unproven in anaphylaxis, but they are sometimes given after adrenaline in people with a history of reactive airways or to help prevent biphasic reactions. However, the evidence of benefit is scant.1,2,9 Intravenous promethazine should not be used in anaphylaxis because it can cause hypotension and muscle necrosis.
For hypotensive patients, give intravenous fluids (normal saline 20 mL/kg to a maximum of 1 L in the first 30 minutes) and consider an adrenaline infusion. In patients with cardiogenic shock despite these measures (especially if taking beta blockers) glucagon may improve cardiac output. Consider an intravenous glucagon bolus of 1–2 mg in adults or 20–30 microgram/kg up to 1 mg in children and seek specialist advice.
Duration of monitoring
Patients with anaphylaxis can experience protracted or biphasic reactions7,9 and should be transported to hospital or other medical facility via ambulance (where possible) to allow management of these possibilities. Currently there is little evidence to guide the optimal duration of observation. True biphasic reactions are estimated to occur in 3–20% of patients at a median of 11 hours after the initial reaction (range 0.5–72 hours).9,21 ASCIA recommends clinical monitoring of a patient for a minimum of four hours after the last dose of adrenaline.1
Patients should strongly be considered for overnight hospital admission if they:
present with severe or protracted anaphylaxis (e.g. required repeated doses of adrenaline or intravenous fluid resuscitation)
have a history of severe or protracted anaphylaxis
have other concomitant illness (e.g. severe asthma, history of arrhythmia, systemic mastocytosis)
live alone or are remote from medical care
Follow-up
On discharge from hospital following anaphylaxis, patients at risk of re-exposure (e.g. to stings, foods, unknown cause) should be prescribed and ideally dispensed an adrenaline injector device pending specialist review.1,5,9,13 Patients should be provided with education about using the adrenaline injector and given an ASCIA Action Plan for Anaphylaxis so they can recognise and manage potential future reactions.
All patients who present with anaphylaxis should be referred for clinical immunology/allergy specialist review to confirm the cause of their reaction and discuss avoidance strategies and management of comorbidities. Patients should be advised to document episodes of anaphylaxis. This helps identify causes and co-factors like exercise in the 6–8 hours preceding the onset of symptoms. The ASCIA allergic reactions event record and clinical history forms can be used to collect and document this information. These and other anaphylaxis resources are available to download (see Box 2).
Box 2. Evidence-based anaphylaxis resources for clinicians and patients.
| • Australasian Society of Clinical Immunology and Allergy (ASCIA) resources: https://www.allergy.org.au/ hp/anaphylaxis • Nip Allergies in the Bub: https://preventallergies.org.au/ healthcare-professionals • Anaphylaxis: emergency management for health professionals. Aust Prescr 2018;41:54. https://doi.org/ 10.18773/austprescr.2018.014 Order a free Anaphylaxis Wallchart online. |
Conclusion
Anaphylaxis is a potentially life-threatening allergic reaction and adrenaline remains the first-line treatment. ASCIA recently updated guidelines to recommend 0.15 mg adrenaline injectors for infants and children weighing 7.5–10 kg. Correct positioning of a patient experiencing anaphylaxis is vital as death can occur within minutes if a patient stands, walks or sits up suddenly.
Footnotes
Conflicts of interest: none declared
REFERENCES
- 1.Australasian Society of Clinical Immunology and Allergy. ASCIA Guidelines – Acute management of anaphylaxis. Sydney: ASCIA; 2020. www.allergy.org.au/hp/papers/acute-management-of-anaphylaxis-guidelines [cited 2021 May 1]
- 2.Mullins RJ, Dear KB, Tang ML. Time trends in Australian hospital anaphylaxis admissions in 1998-1999 to 2011-2012. J Allergy Clin Immunol 2015;136:367-75. 10.1016/j.jaci.2015.05.009 [DOI] [PubMed] [Google Scholar]
- 3.Mullins RJ, Wainstein BK, Barnes EH, Liew WK, Campbell DE. Increases in anaphylaxis fatalities in Australia from 1997 to 2013. Clin Exp Allergy 2016;46:1099-110. 10.1111/cea.12748 [DOI] [PubMed] [Google Scholar]
- 4.Brown SG. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol 2004;114:371-6. 10.1016/j.jaci.2004.04.029 [DOI] [PubMed] [Google Scholar]
- 5.Muraro A, Roberts G, Worm M, Bilò MB, Brockow K, Fernández Rivas M, et al. EAACI Food Allergy and Anaphylaxis Guidelines Group . Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy 2014;69:1026-45. 10.1111/all.12437 [DOI] [PubMed] [Google Scholar]
- 6.Simons FE. Anaphylaxis in infants: can recognition and management be improved? J Allergy Clin Immunol 2007;120:537-40. 10.1016/j.jaci.2007.06.025 [DOI] [PubMed] [Google Scholar]
- 7.Anagnostou K, Turner PJ. Myths, facts and controversies in the diagnosis and management of anaphylaxis. Arch Dis Child 2019;104:83-90. 10.1136/archdischild-2018-314867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liew WK, Williamson E, Tang ML. Anaphylaxis fatalities and admissions in Australia. J Allergy Clin Immunol 2009;123:434-42. 10.1016/j.jaci.2008.10.049 [DOI] [PubMed] [Google Scholar]
- 9.Shaker MS, Wallace DV, Golden DB, Oppenheimer J, Bernstein JA, Campbell RL, et al. Collaborators. Chief Editors. Workgroup Contributors. Joint Task Force on Practice Parameters Reviewers . Anaphylaxis-a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol 2020;145:1082-123. 10.1016/j.jaci.2020.01.017 [DOI] [PubMed] [Google Scholar]
- 10.Simons FE, Ebisawa M, Sanchez-Borges M, Thong BY, Worm M, Tanno LK, et al. 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Organ J 2015;8:32. 10.1186/s40413-015-0080-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenhawt M, Gupta RS, Meadows JA, Pistiner M, Spergel JM, Camargo CA, Jr, et al. Guiding principles for the recognition, diagnosis, and management of infants with anaphylaxis: an expert panel consensus. J Allergy Clin Immunol Pract 2019;7:1148-1156.e5. 10.1016/j.jaip.2018.10.052 [DOI] [PubMed] [Google Scholar]
- 12.Kemp SF, Lockey RF, Simons FE. World Allergy Organization ad hoc Committee on Epinephrine in Anaphylaxis. Epinephrine: the drug of choice for anaphylaxis – a statement of the World Allergy Organization. World Allergy Organ J 2008;1 Suppl:S18-26. 10.1097/WOX.0b013e31817c9338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sicherer SH, Simons FE, SECTION ON ALLERGY AND IMMUNOLOGY . Epinephrine for first-aid management of anaphylaxis. Pediatrics 2017;139:e20164006. 10.1542/peds.2016-4006 [DOI] [PubMed] [Google Scholar]
- 14.Brown JC. Epinephrine, auto-injectors, and anaphylaxis: Challenges of dose, depth, and device. Ann Allergy Asthma Immunol 2018;121:53-60. 10.1016/j.anai.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 15.Campbell RL, Bellolio MF, Knutson BD, Bellamkonda VR, Fedko MG, Nestler DM, et al. Epinephrine in anaphylaxis: higher risk of cardiovascular complications and overdose after administration of intravenous bolus epinephrine compared with intramuscular epinephrine. J Allergy Clin Immunol Pract 2015;3:76-80. 10.1016/j.jaip.2014.06.007 [DOI] [PubMed] [Google Scholar]
- 16.Liew PY, Craven JA. Adrenaline overdose in pediatric anaphylaxis: a case report. J Med Case Reports 2017;11:129. 10.1186/s13256-017-1290-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simons FE, Gu X, Simons KJ. Epinephrine absorption in adults: intramuscular versus subcutaneous injection. J Allergy Clin Immunol 2001;108:871-3. 10.1067/mai.2001.119409 [DOI] [PubMed] [Google Scholar]
- 18.Simons FE, Chan ES, Gu X, Simons KJ. Epinephrine for the out-of-hospital (first-aid) treatment of anaphylaxis in infants: is the ampule/syringe/needle method practical? J Allergy Clin Immunol 2001;108:1040-4. 10.1067/mai.2001.119916 [DOI] [PubMed] [Google Scholar]
- 19.Dreborg S, Tsai G, Kim H. Implications of variation of epinephrine auto-injector needle length. Ann Allergy Asthma Immunol 2019;123:89-94. 10.1016/j.anai.2019.04.027 [DOI] [PubMed] [Google Scholar]
- 20.Australasian Society of Clinical Immunology and Allergy. ASCIA Guidelines – Acute management of anaphylaxis in pregnancy. Sydney: ASCIA; 2020. www.allergy.org.au/hp/ papers/acute-management-of-anaphylaxis-in-pregnancy [cited 2021 May 1]
- 21.Lee S, Bellolio MF, Hess EP, Erwin P, Murad MH, Campbell RL. Time of onset and predictors of biphasic anaphylactic reactions: a systematic review and meta-analysis. J Allergy Clin Immunol Pract 2015;3:408-16.e1-2. 10.1016/j.jaip.2014.12.010 [DOI] [PubMed]


