Abstract
Objective
To describe the characteristics, management and outcomes of hospitalised patients with Clostridioides difficile infection (CDI) treated with and without fidaxomicin.
Methods
This prospective, multicentre, observational study (DAFNE) enrolled hospitalised patients with CDI, including 294 patients treated with fidaxomicin (outcomes recorded over a 3-month period) and 150 patients treated with other CDI therapies during three 1-month periods. The primary endpoint was baseline and CDI characteristics of fidaxomicin-treated patients.
Results
At baseline, the fidaxomicin-treated population included immunocompromised patients (39.1%) and patients with severe (59.2%) and recurrent (36.4%) CDI. Fidaxomicin was associated with a high rate of clinical cure (92.2%) and low CDI recurrence (16.3% within 3 months). Clinical cure rates were ≥90% in patients aged ≥65 years, those receiving concomitant antibiotics and those with prior or severe CDI. There were 121/296 (40.9%) patients with adverse events (AEs), 5.4% with fidaxomicin-related AEs and 1.0% with serious fidaxomicin-related AEs. No fidaxomicin-related deaths were reported.
Conclusions
Fidaxomicin is an effective and well-tolerated CDI treatment in a real-world setting in France, which included patients at high risk of adverse outcomes.
Trial registration: Description of the use of fidaxomicin in hospitalised patients with documented Clostridium difficile infection and the management of these patients (DAFNE), NCT02214771, www.ClinicalTrials.gov.
Keywords: Clostridioides (Clostridium) difficile, clinical, antibiotic usage, fidaxomicin, infection, real-world setting
Introduction
Clostridioides (Clostridium) difficile infection (CDI) results from the use of broad-spectrum antibiotics, which disrupts the gut microbiota, allowing the overgrowth of C. difficile. 1 The illness ranges from mild, self-limiting diarrhoea to severe, potentially fatal outcomes. 2 C. difficile is the most common cause of healthcare-associated infectious diarrhoea. 2
In a multicentre, prospective, biannual, point-prevalence study of CDI in hospitalised patients with diarrhoea across 20 European countries, the reported mean CDI rate between 2012 and 2013 was 7.3 per 10,000 patient bed days; in France, the mean rate was 3.3 per 10,000 patient bed days. 3 A wide variation in the mean CDI rate was noted between countries (range: 0.7–28.7 per 10,000 patient bed days). Importantly, this study revealed the underdiagnosis of CDI across Europe due to a lack of clinical suspicion and suboptimal diagnostic methods, potentially resulting in a large number of undetected CDI cases. 3 Studies show that CDI imposes a considerable burden on healthcare systems and society. 4
For most patients with CDI, including initial (both non-severe and severe) and recurrent infections, the European and US guidelines recommend oral administration of vancomycin or fidaxomicin.5,6 Although the 2014 European guidelines, which are currently under review, recommend metronidazole as a first-line treatment for initial non-severe CDI, 5 the 2018 US guidelines only recommend metronidazole for initial non-severe CDI if vancomycin and fidaxomicin are unavailable. 6 Faecal microbiota transplantation is recommended in patients with multiple recurrences of CDI.5,6
In a network meta-analysis of 24 randomised controlled trials with 13 different treatments for CDI, fidaxomicin was associated with a higher rate of sustained symptomatic cure than vancomycin in all patient subgroups assessed (initial vs recurrent CDI and age <65 years vs ≥65 years) except those with severe (vs mild-to-moderate) CDI. 7 Both fidaxomicin and vancomycin were superior to metronidazole in terms of sustained symptomatic cure, and fidaxomicin was associated with significantly fewer recurrences than vancomycin and metronidazole. Recurrence is a major issue in the management of CDI, resulting in prolonged lengths of hospital stay, considerable impacts on quality of life and additional costs.6,8 Data from a randomised clinical trial showed that in contrast to vancomycin, fidaxomicin preserved the intestinal microbiota, thereby providing a selective therapy for CDI with reduced disruption of the microbiome and a decreased risk of CDI recurrence. 9 Thus, fidaxomicin may be an option for the treatment of patients with CDI, especially those at risk of recurrence. 10
In addition to randomised controlled clinical trials, it is important to evaluate the performance of a treatment under real-world conditions with heterogeneous patient populations and less stringent treatment and delivery protocols. 11 In observational studies, fidaxomicin has shown efficacy in real-world clinical practice in France, Spain and the US,12–14 but limited data are available on the use of fidaxomicin in routine clinical practice in French hospitals. 12 Therefore, the present study (DAFNE) was conducted in France to describe the characteristics, management and outcomes of hospitalised patients with CDI who were treated with fidaxomicin. The study also describes the management of patients with CDI who did not receive fidaxomicin.
Patients and methods
Study design and patients
This was a prospective, multicentre, observational, longitudinal study conducted in France between September 2014 and November 2017. The study was proposed to all healthcare facilities in which fidaxomicin was included in their formulary at the time of study initiation. In total, 120 sites were invited to participate, including mainly general hospitals (61 sites) and university hospitals (37 sites). Of these, 28 sites accepted, including mostly university hospitals (17 sites) and general hospitals (8 sites). Eligible patients were adults (≥18 years) who were hospitalised and diagnosed with CDI. All data used in this study were collected from patients’ medical records.
This study assessed two groups of patients with CDI: those treated with fidaxomicin (main analysis population) and those who did not receive fidaxomicin (registry population). The latter also comprised patients who did not receive CDI-specific treatment. All consecutive, eligible patients with CDI treated with fidaxomicin between 3 September 2014 and 27 March 2017 were included in the study and followed up for 3 months after the fidaxomicin treatment period. Data for these patients were collected at inclusion, during their hospital stay and for 3 months after treatment with fidaxomicin. Because this was an observational study, exact timeframes for evaluating outcomes were not always possible, and times were divided into 1-, 2-, 3- and >3-month periods after fidaxomicin treatment. All consecutive, eligible patients with CDI who did not receive fidaxomicin were assessed over three 1-month inclusion periods: March 2015, March 2016 and March 2017. The separate inclusion periods enabled the evaluation of the characteristics of non-fidaxomicin-treated patients and the assessment of potential changes in treatment patterns over the inclusion periods. For all patients, the CDI therapy was selected by the treating physician and was independent of study participation. Patient-reported health-related quality of life (HRQOL) was assessed using the EQ-5D-5L questionnaire, which included five domains (mobility, self-care, usual activities, pain/discomfort, anxiety/depression), and the 0 to 100 visual analogue score (EQ-VAS) for self-rated overall health, 15 which was administered at the time of CDI diagnosis and the 3-month follow-up visit.
Ethics
This observational study did not require submission to the Ethics Committee because it did not modify usual medical care, cause physical or psychological harm or require patients to attend special follow-up visits. Approval for this observational study was obtained from the Advisory Committee for Data Processing in Health Research. The purpose of the Committee was to validate the following: the scientific objective of the study; the correlation between the scientific objectives and the data collected with respect to patient rights; and the patient information leaflet given at the beginning of the study. Authorisation for the study was also received from the French Data Protection Authority. All included patients provided verbal informed consent to participate in the study.
Definitions
CDI was defined as either: (1) a clinical presentation compatible with CDI, with microbiological evidence of free toxins or the presence of toxigenic C. difficile in stools without reasonable evidence of another cause of diarrhoea or (2) pseudomembranous colitis diagnosed during endoscopy, after colectomy or on autopsy. Healthcare-associated CDI was defined as CDI with the onset of symptoms >48 hours after admission to a healthcare facility or in the community ≤4 weeks after discharge from a healthcare facility. 16 Community-associated CDI was defined as CDI with the onset of symptoms while outside a healthcare facility and without discharge from a healthcare facility within the previous 12 weeks or with the onset of symptoms ≤48 hours following admission to a healthcare facility and not resident in a healthcare facility within the previous 12 weeks. 16
Patients with CDI symptoms 4 to 12 weeks after hospital discharge were included in this study but considered to have CDI of an unknown origin and therefore not counted as healthcare- or community-associated CDI. 16 Clinical cure was defined as no further requirement for CDI treatment at Day 12 (2 days after treatment discontinuation) and either: (1) a maximum of three unformed bowel movements over 2 consecutive days and healthy until treatment was discontinued or (2) a marked reduction in the number of unformed bowel movements at the end of treatment but with residual mild abdominal discomfort that the investigator considered to be healing and signs/symptoms of CDI that did not worsen in the 2 to 3 days following treatment discontinuation. Sustained clinical cure was defined as clinical cure with no recurrence during the 3-month follow-up period. Recurrence was defined as a new episode of microbiologically documented CDI diarrhoea (according to the tests used in each participating hospital) requiring anti-infective therapy in a cured patient during the 3-month follow-up period; this definition excluded cases of refractory CDI with no diarrhoea improvement following treatment of the primary episode. Patients were classed as immunocompromised if they were a) receiving treatment that reduces resistance to infection, including immunosuppressants, chemotherapy, radiotherapy, corticosteroids for ≥30 days or recent high-dose corticosteroid therapy (>5 mg/kg prednisolone or equivalent for >5 days) and/or b) had progressive disease, such as haemopathy, metastatic cancer or human immunodeficiency virus with CD4 counts <500 cells/mm3.
Endpoints
The primary endpoint was baseline and CDI characteristics of the patient population treated with fidaxomicin. Secondary endpoints in patients treated with fidaxomicin (during a 3-month follow up) included therapeutic management of CDI, clinical outcomes and HRQOL. Clinical outcomes included clinical cure, sustained clinical cure and CDI recurrence and were assessed in both the overall main analysis population and the following subgroups within this population: age ≥65 years; concomitant antibiotic therapy; 0, 1 or ≥2 recurrences at inclusion; and severe CDI according to the 2014 European Society of Clinical Microbiology and Infectious Diseases (ESCMID) definition of an episode with one or more signs and symptoms of severe colitis. 5 Severe CDI was defined as CDI without signs of severe colitis in patients with advanced age (≥65 years), serious comorbidity, intensive care unit admission or immunodeficiency. Secondary endpoints in patients who did not receive fidaxomicin (over three 1-month periods) were baseline and CDI characteristics and therapeutic management of CDI. Safety endpoints in all patients were adverse events (AEs).
Statistical analyses
Regardless of the binomial criterion studied or the observed percentage (10%–50%), it was determined that 300 fidaxomicin-treated patients would be sufficient to describe each characteristic or outcome measurement with a two-sided 95% confidence interval width ±5.7%. All data were analysed descriptively; no statistical hypothesis was tested, and there was no control of multiplicity. Missing data were presented for each endpoint. Statistical analysis was performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). The statistical analysis plan was finalised on 16 October 2017, prior to database lock.
The main analysis population included patients with CDI treated with fidaxomicin. The registry population included patients who did not receive fidaxomicin, regardless of what other prescribed treatment they received.
Results
Patient populations
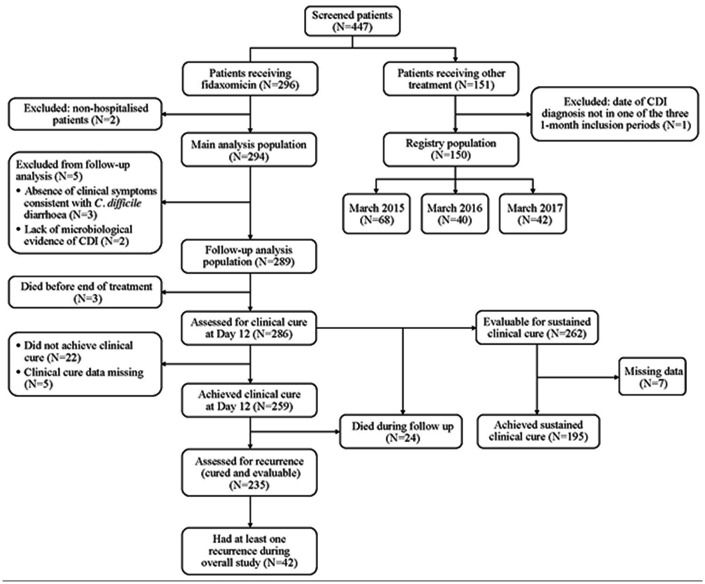
Across 25 sites, 447 patients with CDI were screened. Of 296 patients receiving fidaxomicin, two patients were excluded as they did not meet the inclusion criteria (both were non-hospitalised patients). Therefore, 294 fidaxomicin-treated patients were included in the main analysis population. Of 151 patients who did not receive fidaxomicin, one patient was excluded as he was not included in the three 1-month inclusion periods. Therefore, 150 patients comprised the registry population (Figure 1). The numbers of patients in the registry population across each of the inclusion periods were as follows: March 2015, n = 68; March 2016, n = 40; and March 2017, n = 42.
Figure 1.
Patient flow through the study.
C. difficile, Clostridioides difficile; CDI, Clostridioides difficile infection; N, number of patients.
Patient characteristics
In the main analysis population, 39.1% of patients were immunocompromised, and 59.2% had severe CDI according to 2014 ESCMID criteria 5 (Table 1 and Table S1). Nearly two-thirds (186/294, 63.3%) of patients in this population were receiving treatment for an initial CDI episode (Table 1). Treatment was administered for recurrent CDI in 107/294 (36.4%) patients (Table 1). Among patients with recurrent CDI, the number of recurrences was one in 61.3% of patients, two in 17.0% and three or more in 21.7% (Table 1).
Table 1.
Baseline patient demographics and clinical characteristics.
| Characteristic | Fidaxomicin-treated (main analysis) population, N = 294 | Non-fidaxomicin treated (registry) population,a N = 150 |
|---|---|---|
| Female, n (%) | 179 (60.9) | 80 (53.3) |
| Age in years, mean (SD) | 68.4 (17.3) | 67.8 (17.2) |
| Time between healthcare facility admission and CDI diagnosis, days (SD) | 15.4 (34.3) | 11.9 (17.8) |
| Immunocompromised, n (%) | 115 (39.1) | 39 (26.0) |
| Charlson Comorbidity Index (SD) | 5.9 (3.4) | N/A |
| Severe CDI according to 2014 ESCMID criteria, n (%)b | 174 (59.2) | 53 (35.8) |
| Prior treatment during previous 3 months, n (%) | ||
| Antibiotic therapyc | 248 (84.6) | N/A |
| PPI used | 166 (56.7) | N/A |
| Concomitant antibiotic therapy at the time of diagnosis, n (%)e | 132 (45.1) | 67 (45.0) |
| Presence of clinical symptoms consistent with C. difficile diarrhoea, n (%) | 291 (99.0) | 144 (96.0) |
| UBMs per day, mean (SD)f | 5.5 (3.2) | 5.1 (2.8) |
| CDI status, n (%) | ||
| Initial | 186 (63.3) | 132 (88.0) |
| Recurrence | 107 (36.4) | 14 (9.3) |
| Unknown | 1 (0.3) | 4 (2.7) |
| Number of recurrences, n (%)g | ||
| 1 | 65 (61.3) | 3 (23.1) |
| 2 | 18 (17.0) | 7 (53.8) |
| ≥3 | 23 (21.7) | 3 (23.1) |
| CDI origin, n (%) | ||
| Healthcare-associated | 219 (74.5) | 92 (61.3) |
| Community-associated | 55 (18.7) | 51 (34.0) |
| Unknown | 20 (6.8) | 7 (4.7) |
| Treatment of CDI, n (%)h,i | ||
| Metronidazole, oral | N/A | 80 (55.6) |
| Metronidazole, IV | N/A | 13 (9.0) |
| Vancomycin, oral | N/A | 60 (41.7) |
| Other | N/A | 6 (4.1) |
| No treatment | N/A | 5 (3.3) |
| Time between sample collection and confirmed CDI diagnosis, days, mean (SD) | 1.3 (1.6) | 1.0 (2.1) |
| PCR ribotyping done, n (%)j | 56 (19.2) | 13 (8.7) |
aThe questionnaire in relation to patients in the registry population did not include the same level of detail as that for the main analysis population, which accounts for the missing information.
Patient numbers in the main analysis and registry populations, respectively, were: b294 and 148; c293 and N/A; d293 and N/A; e293 and 149; f188 and 119; g106 and 13; hN/A and 144 (except 145 for Other); i292 and 150.
jFor the main analysis population, prior and concomitant treatments other than fidaxomicin are provided in Table 2.
C. difficile, Clostridioides difficile; CDI, Clostridioides difficile infection; ESCMID, European Society of Clinical Microbiology and Infectious Diseases; IV, intravenous; n, number of patients with the characteristic; N, number of patients with information available; N/A, not applicable; PCR, polymerase chain reaction; PPI, proton pump inhibitor; SD, standard deviation, UBM, unformed bowel movement.
In the registry population of patients who did not receive fidaxomicin, 26.0% were immunocompromised, and 35.8% had severe CDI according to 2014 ESCMID criteria 5 (Table 1). In total, 132/150 (88.0%) registry population patients were receiving treatment for an initial CDI episode (Table 1). There were 14/150 (9.3%) patients receiving treatment for recurrent CDI; among these, the number of recurrences was one in 23.1% of patients, two in 53.8% and three or more in 23.1% (Table 1).
Baseline and CDI characteristics of the registry population were generally similar across the three 1-month periods (March 2015, March 2016 and March 2017). Age and the use of concomitant antibiotic therapy were the only exceptions, with a decrease in the mean age (71.2, 66.3 and 63.6 years in 2015, 2016 and 2017, respectively) and an increase in the use of concomitant antibiotic therapy (35.3%, 43.6% and 61.9%, respectively).
Therapeutic management of CDI
For the main analysis population, the mean (standard deviation [SD]) and median (interquartile range [IQR]) number of days for exposure to fidaxomicin were 10.9 (2.1) and 11.0 (10.0, 11.0), respectively. Fidaxomicin was administered for 10 days in 58/285 (20.4%) patients, >10 days in 206/285 (72.3%) patients and <10 days in 21/285 (7.4%) patients. The majority (n = 174; 65.9%) of patients received fidaxomicin for >11 days, but it was not possible to determine how many doses of the study drug were administered each day. Three patients received ≥20 days (maximum 32 days) of fidaxomicin treatment. The reason for fidaxomicin discontinuation was not collected in this study. The mean and median dosage of fidaxomicin was 400 mg/day (289 patients). Among the 289 patients included in the follow-up analysis population, fidaxomicin was the first-line treatment for CDI in 191 (66.1%) patients. In 98 patients, previous treatments included oral vancomycin (58.2%) and oral metronidazole (48.0%) (Table 2). Approximately 10% of patients received at least one concomitant medication, most commonly oral vancomycin (25.0%) and intravenous metronidazole (21.4%) (Table 2). In the follow-up analysis population, 22 (7.7%) patients were deceased at the end of hospitalisation. The mean (SD) duration of hospitalisation was 30.3 (35.4) days, and the median (IQR) was 19.0 (10, 37.0) days.
Table 2.
Other treatments prescribed in the main analysis (fidaxomicin-treated) population.
| Number of patients, N = 294 | ||
|---|---|---|
| Treatments prescribed for the CDI episode before fidaxomicin | ||
| Any treatment | Treated, n/N (%) | 98/289 (33.9) |
| Metronidazole (oral) | Treated, n/N (%) | 47/98 (48.0) |
| Dosage, Ng/day, mean (SD) | 461.5 (0.1) | |
| g/day, median (IQR) | 1.5 (1.5, 1.5) | |
| Vancomycin (oral) | Treated, n/N (%) | 57/98 (58.2) |
| Dosage, ng/day, mean (SD) | 560.7 (0.4) | |
| g/day, median (IQR) | 0.5 (0.5, 0.5) | |
| Metronidazole (IV) | Treated, n/N (%) | 9/98 (9.2) |
| Dosage, Ng/day, mean (SD) | 81.2 (0.5) | |
| g/day, median (IQR) | 1.5 (0.8, 1.5) | |
| Other treatmenta | Treated, n/N (%) | 5/98 (5.1) |
| Concomitant treatment | ||
| Any treatment | Treated, n/N (%) | 28/289 (9.7) |
| Metronidazole (oral) | Treated, n/N (%) | 4/28 (14.3) |
| Dosage, Ng/day, mean (SD) | 41.4 (0.3) | |
| g/day, median (IQR) | 1.5 (1.3, 1.5) | |
| Vancomycin (oral) | Treated, n/N (%) | 7/28 (25.0) |
| Dosage, Ng/day, mean (SD) | 71.2 (0.8) | |
| g/day, median (IQR) | 1.0 (0.5, 2.0) | |
| Metronidazole (IV) | Treated, n/N (%) | 6/28 (21.4) |
| Dosage, Ng/day, mean (SD) | 61.3 (0.4) | |
| g/day, median (IQR) | 1.5 (1.5, 1.5) | |
| Faecal microbiota transplantation | Treated, n/N (%) | 1/27 (3.7)b |
| Other | Treated, n/N (%) | 17/26 (65.4) |
aFive patients had previously received treatment with ceftriaxone (n = 1, one treatment), ofloxacin (n = 1, one treatment) and vancomycin (n = 5, seven treatments).
bA 93-year-old female patient with recurrent infection received both fidaxomicin and faecal microbiota transplantation (date of latter unknown). Clinical cure was reported at 2 days after the end of fidaxomicin treatment, and no recurrences of infection were reported for this patient.
CDI, Clostridioides difficile infection; IQR, interquartile range; IV, intravenous; n, number of patients treated; N, number of patients with information available; SD, standard deviation.
Of the 150 patients with CDI from the registry population, 145 (96.7%) received treatment for CDI, mainly oral metronidazole (80/144, 55.6%) and oral vancomycin (60/144, 41.7%). The reason for the lack of CDI-specific treatment was not collected. From 2015 to 2017, the use of oral metronidazole decreased from 63.1% (41/65) to 40.0% (16/40), and the use of oral vancomycin increased from 32.3% (21/65) to 55.0% (22/40).
Patient outcomes (main analysis population)
Of 281 patients with available clinical cure data, 259 (92.2%) achieved clinical cure at Day 12 (Figure 1 and Table 3). Clinical cure rates were also ≥90% in patients aged ≥65 years, those receiving concomitant antibiotic therapy, those with prior CDI and those with severe CDI (Table 3).
Table 3.
Subgroup analyses of clinical cure, sustained clinical cure and CDI recurrence in the main analysis (fidaxomicin-treated) population.
| Outcome, n (%) | All patients |
Age |
Concomitant antibiotic therapy |
Number of CDI recurrences at inclusion |
Severe CDI by 2014 ESCMID criteria 5 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≥65 years | <65 years | Yes | No | 0 (first episode) | 1 | ≥2 | Severe | Non-severe | ||
| Clinical cure (N = 286) | 259/281 (92.2) | 168/184 (91.3) | 91/97 (93.8) | 116/126 (92.1) | 143/155 (92.3) | 161/178 (90.4) | 61/62 (98.4) | 36/40 (90.0) | 155/168 (92.3) | 104/113 (92.0) |
| Sustained clinical cure (N = 262)a | 195/255 (76.5) | 121/163 (74.2) | 74/92 (80.4) | 90/114 (78.9) | 105/141 (74.5) | 124/161 (77.0) | 46/56 (82.1) | 24/37 (64.9) | 114/151 (75.5) | 81/104 (77.9) |
| At least one recurrenceb (N = 235) | 42/235 (17.9) | 29/149 (19.5) | 13/86 (15.1) | 16/105 (15.2) | 26/130 (20.0) | 22/145 (15.2) | 11/56 (19.6) | 9/33 (27.3) | 27/140 (19.3) | 15/95 (15.8) |
| Number of recurrences according to time (N = 233)c | ||||||||||
| Within 1 month | 21 (9.0) | N/A | N/A | N/A | N/A | |||||
| Within 2 months | 32 (13.7) | N/A | N/A | N/A | N/A | |||||
| Within 3 months | 38 (16.3) | N/A | N/A | N/A | N/A | |||||
| After 3 months | 2 (0.9) | N/A | N/A | N/A | N/A | |||||
aData for sustained clinical cure were missing for seven patients; bFor the overall study period; cPercentages are calculated as a proportion of cured patients.
CDI, Clostridioides difficile infection; ESCMID, European Society of Clinical Microbiology and Infectious Diseases; n, number of patients with the stated outcome; N, number of patients with information available; N/A, not available.
CDI recurred in 42/235 (17.9%) patients during the study period (Figure 1 and Table 3), including 41 with only one recurrence and one patient with two recurrences. Of those with evaluable data on the timing of outcomes (n = 233), CDI recurrence occurred in 13.7% of patients before 2 months, 16.3% before 3 months and 0.9% after 3 months (Table 3).
Sustained clinical cure was observed in 195/255 (76.5%) patients (evaluable, n = 262; missing data, n = 7) (Figure 1 and Table 3). Subgroup analyses showed similar sustained clinical cure rates regardless of age or concomitant antibiotic therapy (Table 3). Patients with two or more recurrences at study inclusion appeared to have a lower rate of sustained clinical cure and a corresponding higher rate of recurrence than the overall main analysis population. However, statistical tests were not performed on these data. Therefore, no formal conclusions were drawn from these data.
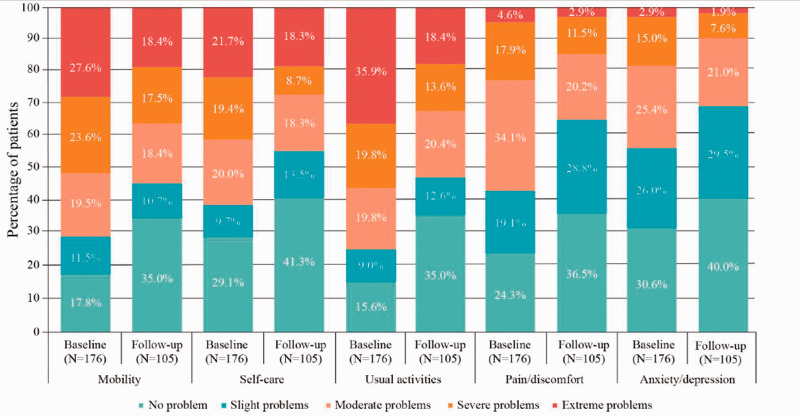
At the time of diagnosis, the mean health score (VAS) was 48.9 (SD 18.7), and the mean EQ-5D-5L score was 0.2 (SD 0.4), indicating substantially impaired HRQOL in patients with CDI. By 3 months of fidaxomicin treatment, the mean EQ-5D-5L score improved to 0.4 (SD 0.4). In all five EQ-5D-5L domains, the proportions of patients with severe-to-extreme complications were reduced from the time of diagnosis to the 3-month follow up (Figure 2). The mean health score (VAS) at the 3-month follow-up visit was 60.2 (SD 23.3).
Figure 2.
HRQOL (EQ-5D-5L) scores at baseline and the 3-month follow-up in the main analysis (fidaxomicin-treated) populationa.
aData from the EQ-5D-5L questionnaire were available for 176 patients at the time of CDI diagnosis and 105 patients at the 3-month follow-up.
CDI, Clostridioides difficile infection; EQ-5D-5L, HRQoL was assessed using the EQ-5D-5L questionnaire, which included five domains (mobility, self-care, usual activities, pain/discomfort, anxiety/depression); HRQOL, health-related quality of life; N, number of patients with information available.
Safety
The AE profile for all fidaxomicin-treated patients (N = 296) is summarised in Table 4. AEs were reported in 40.9% (121/296) of patients, including 69 (23.3%) who experienced severe AEs. Overall, the most common AEs were CDI (13.9%), ineffective drug treatment (4.4%) and general physical health deterioration (3.0%) (Table 5). Serious AEs were reported in 27.7% (82/296) of patients, and AEs related to fidaxomicin were observed in 5.4% (16/296) of patients. The most common fidaxomicin-related AE was ineffective drug treatment (11/296; 3.7%). Four serious AEs related to fidaxomicin were reported in three (1.0%) patients. These included severe lack of efficacy in two patients, which was subsequently reported as moderate lack of efficacy in one of these patients, and recurrence in one patient; all three patients subsequently recovered. Forty-eight (16.2%) patients had at least one AE leading to death (Table 4); most of these patients (18/48, 37.5%) had AEs within the System Organ Class General disorders and administration site conditions. There were no deaths related to fidaxomicin.
Table 4.
Adverse event profile for all fidaxomicin-treated patients.
| AE, n (%) | Number of AEs | Number of patients, N=296 |
|---|---|---|
| All AEs | 175 | 121 (40.9) |
| Severe AEs | 89 | 69 (23.3) |
| Serious AEs | 110 | 82 (27.7) |
| Severe serious AEs | 86 | 66 (22.3) |
| AEs leading to death | 52 | 48 (16.2) |
| AEs related to fidaxomicin | 18 | 16 (5.4) |
| Serious AEs related to fidaxomicin | 4 | 3 (1.0) |
| AEs related to underlying medical condition | 93 | 72 (24.3) |
| AEs related to concomitant medication | 17 | 16 (5.4) |
| AEs related to other condition | 47 | 40 (13.5) |
AE, adverse event; n, number of patients with the stated AE; N, number of fidaxomicin-treated patients.
Table 5.
Adverse events reported in at least two patients (among all fidaxomicin-treated patients).
| AE, n (%) | Number of AEs | Number of patients, N=296 |
|---|---|---|
| Clostridium difficile infectiona | 45 | 41 (13.9) |
| Drug ineffectiveb | 14 | 13 (4.4) |
| General physical health deterioration | 10 | 9 (3.0) |
| Death | 8 | 8 (2.7) |
| Diarrhoea | 4 | 4 (1.4) |
| Renal failure acute | 4 | 4 (1.4) |
| Cardiac failure | 3 | 3 (1.0) |
| Respiratory failure acute | 3 | 3 (1.0) |
| Septic shock | 3 | 3 (1.0) |
| Agranulocytosis | 2 | 2 (0.7) |
| Lung disorder | 3 | 2 (0.7) |
| Clostridium difficile colitis | 2 | 2 (0.7) |
| Colitis | 2 | 2 (0.7) |
| Intestinal obstruction | 2 | 2 (0.7) |
| Rash | 2 | 2 (0.7) |
| Respiratory distress | 2 | 2 (0.7) |
| Respiratory distress syndrome, acute | 2 | 2 (0.7) |
aReported at any point during the study, regardless of treatment timing. bNo improvement in diarrhoea symptoms. AE, adverse event; n, number of patients with stated AEs; N, number of fidaxomicin-treated patients.
Discussion
In this prospective, multicentre, observational, longitudinal study of hospitalised adults with CDI in France, treatment with fidaxomicin was effective. The clinical cure rate was 92.2% at Day 12 (2 days after the end of the standard treatment course), and the CDI recurrence rate was 16.3% during the 3-month follow-up period. Over 40% of fidaxomicin-treated patients experienced AEs, but the rates of fidaxomicin-related AEs and serious fidaxomicin-related AEs were low. There were no deaths related to fidaxomicin, and the incidence of AEs and serious AEs appeared similar to the incidences observed during phase 3 clinical trials. 17 , 18
The recommended dose of fidaxomicin in adults is 400 mg/day (one 200-mg tablet every 12 hours) for 10 days. 19 In the present study, the mean dosage of fidaxomicin was 400 mg/day, but a majority (66%) of patients received fidaxomicin for 11 days. However, this may have been in line with the recommended dose of 20 tablets if one tablet was administered on the first day and one on the last day of treatment (the study recorded only the duration of therapy and not the number of tablets administered per day). Almost two-thirds of fidaxomicin-treated patients were being treated for an initial episode of CDI. This is in accordance with European guidelines available at the time of the study and US standards, in which fidaxomicin is recommended for the treatment of both initial (non-severe and severe) and recurrent CDI episodes. 5 , 6
In patients who received antibiotic CDI treatment other than fidaxomicin, oral metronidazole and oral vancomycin were the main therapies administered. There was an observed decrease in metronidazole use and an increase in vancomycin use between the three 1-month periods (in 2015, 2016 and 2017). Interestingly, this trend appears to reflect a recent shift towards the 2018 version of the US guidelines (even though these had not been published at the time the study was conducted), which now recommend either vancomycin or fidaxomicin over metronidazole for the treatment of an initial non-severe episode of CDI, 6 in contrast to the 2010 US guidelines that recommended metronidazole for an initial mild-to-moderate CDI episode. 20 Furthermore, in a network meta-analysis, vancomycin showed greater efficacy than metronidazole. These antibiotics ranked seventh and eleventh of 13 treatments, respectively, in achieving a sustained clinical cure. 7 Similarly, in a pooled analysis of two comparative multinational, randomised, controlled trials, metronidazole was inferior to vancomycin with respect to clinical success (72.7% vs 81.1%), defined as the resolution of diarrhoea and absence of severe abdominal discomfort for more than 2 consecutive days including Day 10. 21
The present study findings are consistent with the two phase 3 randomised, double-blind clinical trials in which the rates of clinical cure with fidaxomicin were 91.7% 17 and 92.1% (per-protocol populations), 18 and the rates of recurrence over a 1-month follow-up period were 12.7% 17 and 15.4% (modified intent-to-treat population). 18 The present findings are also consistent with previous observational studies of fidaxomicin in clinical practice. In a prospective cohort study of 99 fidaxomicin-treated CDI episodes in a French University Hospital, a high proportion of these episodes were severe (42%) and/or recurrent (41%) CDI. 12 After fidaxomicin treatment, there was a high rate of clinical cure 2 days after the end of treatment (87%) and a low rate of recurrence over a 10-week follow-up period (15%). Similar results were observed in retrospective studies in Spain, in which fidaxomicin treatment of 72 patients with CDI was associated with clinical cure and 30-day recurrence rates of 90.3% and 16.7%, respectively, 13 and in the USA, in which fidaxomicin treatment of 81 patients with CDI was associated with a 90% complete response rate (defined as the resolution of diarrhoea during treatment) and a 19% recurrence rate within 8 weeks of treatment. 14
Despite the well-recognised negative effects of CDI on clinical parameters, few previous studies have assessed the impact of CDI on patients’ quality of life. 2 , 22 A large multinational survey showed that CDI is associated with impaired generic HRQOL (Short Form-36 survey) and a negative impact on work and activities (Work Productivity and Activity Impairment questionnaire). 23 In the present study, patients treated with fidaxomicin had substantially impaired HRQOL at baseline, but EQ-5D-5L scores in all five domains (mobility, self-care, usual activities, pain/discomfort, anxiety/depression) improved over 3 months of follow-up.
The strengths of the present study include the approximately 3-month follow-up period in fidaxomicin-treated patients; this is in contrast with the 1-month follow-up period in phase 3 clinical trials. 17 , 18 In addition, these real-world data provide an assessment of treatment with fidaxomicin in clinical practice in France. There are a number of potential limitations associated with this study. Observational studies may have methodological limitations, such as possible selection bias regarding the different time frames designated as inclusion periods for the main analysis and registry populations. The study was also performed only in French centres and may not be generalisable to other countries. However, the inclusion of a relatively large number of centres and the selection of different periods throughout the year may have minimised these biases. University hospitals were overrepresented regarding their acceptance rate to participate in the study. Thirty-seven were invited, and 17 (45.9%) accepted. In contrast, 61 general hospitals were invited, and only 8 (13.1%) accepted. Another limitation is that the number of patients in the registry group (not treated with fidaxomicin) was low; therefore, these results should be interpreted with caution. Last, PCR ribotyping of C. difficile isolates was conducted for only 56/292 (19.2%) of the main analysis population and 13/150 (8.7%) of the registry population; these proportions were too low to investigate associations between particular ribotypes and clinical outcomes.
In conclusion, the DAFNE study demonstrated that fidaxomicin is an effective and well-tolerated treatment in patients with CDI in a real-world setting in France, including patients with a high risk of adverse outcomes, such as those who are advanced in age or those with severe CDI.
Data sharing statement
Researchers may request access to anonymised participant level data, trial level data and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com.
For the Astellas criteria on data sharing, see https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
Acknowledgements
This study was initiated and supported by Astellas Pharma, S.A.S. Medical writing support was provided by Sharon Rayner, PhD, of Cello Health MedErgy, funded by Astellas Pharma, Inc.
We thank the DAFNE study collaborators: Dominique Descamps, Centre Hospitalier de Béthune, Béthune; Fabrice Camou and Emilie Bessède, Centre Hospitalier Universitaire de Bordeaux, Bordeaux; Anne-Laure Roux, Hôpital Ambroise Paré, Assistance Publique Hôpitaux de Paris, Boulogne-Billancourt; Olivier Rogeaux and Marion Levast, Centre Hospitalier Métropole Savoie, Chambéry; Véronique Leflon-Guibout, Hôpital Beaujon, Assistance Publique Hôpitaux de Paris, Clichy; Lionel Piroth, Centre Hospitalier Universitaire Dijon, Dijon; Aurélien Dinh, Hôpital Raymond-Poincaré, Assistance Publique Hôpitaux de Paris, Garches; Frédéric Wallet, Centre Hospitalier Universitaire de Lille, Lille; Joy Yoganaden Mootien, Hôpital Emile Muller, Mulhouse; Pascale Bémer, Centre Hospitalier Universitaire de Nantes, Nantes; Catherine Lechiche and Jean-Philippe Lavigne, Centre Hospitalier Universitaire Carémeau, Nîmes; Thierry Prazuck, Centre Hospitalier Régional Orléans La Source, Orléans; Carine Couzigou and Alban Le Monnier, Groupe Hospitalier Paris Saint Joseph, Paris; Yohan N’Guyen and Véronique Vernet Garnier, Centre Hospitalier Universitaire de Reims (Hôpital Robert Debré), Reims; Vincent Cattoir, Centre Hospitalier Universitaire de Rennes, Rennes; and Caroline Laurans and Anne Vachée, Centre Hospitalier de Roubaix, Roubaix.
A full list of participating centres is given in the supplementary information.
Footnotes
Author contributions: Conceptualisation and methodology of the study: FB, PB, RG, BG.
Investigation of the data: FB, PB, BG.
Formal analysis of the data: FB, PB, RG, MG, BG.
Writing of the original draft and review and editing of the submitted article: FB, PB, RG, MG, BG.
Declaration of conflicting interest: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: FB reports personal fees from Pfizer, MSD and Sanofi Pasteur; non-financial support from Astellas Pharma Inc, Pfizer, Anios and MSD; and grants from Anios, MSD, Biomerieux, Quidel, Diasorin, Cubist, Biosynex, GenePOC, Cepheid and Sanofi Pasteur.
BG reports non-financial support from Astellas Pharma Inc and grants from Pfizer and MSD.
MG is a full-time employee of Astellas Pharma S.A.S.
PB received personal fees from Astellas Pharma Inc and Pfizer.
RG reports non-financial support from Astellas Pharma Inc and personal fees from Sanofi, Correvio, MSD, Pfizer, Eumedica and Frezenius.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study was initiated and supported by Astellas Pharma, S.A.S. Medical writing support was funded by Astellas Pharma Inc.
ORCID iD: Benoit Guery https://orcid.org/0000-0002-6526-0714
References
- 1.Buffie CG, Bucci V, Stein RR, et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 2015; 517: 205–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smits WK, Lyras D, Lacy DB, et al. Clostridium difficile infection. Nat Rev Dis Prim 2016; 2: 16020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies KA, Longshaw CM, Davis GL, et al. Underdiagnosis of Clostridium difficile across Europe: the European, multicentre, prospective, biannual, point-prevalence study of Clostridium difficile infection in hospitalised patients with diarrhoea (EUCLID). Lancet Infect Dis 2014; 14: 1208–1219. [DOI] [PubMed] [Google Scholar]
- 4.Bouza E. Consequences of Clostridium difficile infection: understanding the healthcare burden. Clin Microbiol Infect 2012; 18: 5–12. [DOI] [PubMed] [Google Scholar]
- 5.Debast SB, Bauer MP, Kuijper EJ. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect 2014; 20: 1–26. [DOI] [PubMed] [Google Scholar]
- 6.McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66: e1–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beinortas T, Burr NE, Wilcox MH, et al. Comparative efficacy of treatments for Clostridium difficile infection: a systematic review and network meta-analysis. Lancet Infect Dis 2018; 18: 1035–1044. [DOI] [PubMed] [Google Scholar]
- 8.Aguado JM, Anttila VJ, Galperine T, et al. Highlighting clinical needs in Clostridium difficile infection: the views of European healthcare professionals at the front line. J Hosp Infect 2015; 90: 117–125. [DOI] [PubMed] [Google Scholar]
- 9.Louie TJ, Cannon K, Byrne B, et al. Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin re-expression and recurrence of CDI. Clin Infect Dis 2012; 55: S132–S142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ooijevaar RE, Van Beurden YH, Terveer EM, et al. Update of treatment algorithms for Clostridium difficile infection. Clin Microbiol Infect 2018; 24: 452–462. [DOI] [PubMed] [Google Scholar]
- 11.Singal AG, Higgins PDR, Waljee AK. A primer on effectiveness and efficacy trials. Clin Transl Gastroenterol 2014; 5: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pichenot M, Héquette-Ruz R, Le Guern R, et al. Fidaxomicin for treatment of Clostridium difficile infection in clinical practice: a prospective cohort study in a French University Hospital. Infection 2017; 45: 425–431. [DOI] [PubMed] [Google Scholar]
- 13.Fehér C, Múñez Rubio E, Merino Amador P, et al. The efficacy of fidaxomicin in the treatment of Clostridium difficile infection in a real-world clinical setting: a Spanish multi-centre retrospective cohort. Eur J Clin Microbiol Infect Dis 2017; 36: 295–303. [DOI] [PubMed] [Google Scholar]
- 14.Spiceland CM, Khanna S, Pardi DS. Outcomes with fidaxomicin therapy in Clostridium difficile infection. J Clin Gastroenterol 2018; 52: 151–154. [DOI] [PubMed] [Google Scholar]
- 15.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011; 20: 1727–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuijper EJ, Coignard B, Tull P. Emergence of Clostridium difficile-associated disease in North America and Europe. Clin Microbiol Infect 2006; 12: 2–18. [DOI] [PubMed] [Google Scholar]
- 17.Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis 2012; 12: 281–289. [DOI] [PubMed] [Google Scholar]
- 18.Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364: 422–431. [DOI] [PubMed] [Google Scholar]
- 19.Astellas Pharma Europe B.V. Summary of Product Characteristics: DIFICLIR 200 mg film-coated tablets, https://www.ema.europa.eu/en/documents/product-information/dificlir-epar-product-information_en.pdf (2016).
- 20.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31: 431–455. [DOI] [PubMed] [Google Scholar]
- 21.Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis 2014; 59: 345–354. [DOI] [PubMed] [Google Scholar]
- 22.Barbut F, Galperine T, Vanhems P, et al. Quality of life and utility decrement associated with Clostridium difficile infection in a French hospital setting. Health Qual Life Outcomes 2019; 17: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heinrich K, Harnett J, Vietri J, et al. Impaired quality of life, work, and activities among adults with Clostridium difficile infection: a multinational survey. Dig Dis Sci 2018; 63: 2864–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]