Abstract
Aims
To identify, systematically evaluate and summarise the best available evidence on the frequency of long COVID‐19 (post‐acute COVID‐19 syndrome), its clinical manifestations, and the criteria used for diagnosis.
Methods
Systematic review conducted with a comprehensive search including formal databases, COVID‐19 or SARS‐CoV‐2 data sources, grey literature, and manual search. We considered for inclusion clinical trials, observational longitudinal comparative and non‐comparative studies, cross‐sectional, before‐and‐after, and case series. We assessed the methodological quality by specific tools based on the study designs. We presented the results as a narrative synthesis regarding the frequency and duration of long COVID‐19, signs and symptoms, criteria used for diagnosis, and potential risk factors.
Results
We included 25 observational studies with moderate to high methodological quality, considering 5440 participants. The frequency of long COVID‐19 ranged from 4.7% to 80%, and the most prevalent signs/symptoms were chest pain (up to 89%), fatigue (up to 65%), dyspnea (up to 61%), and cough and sputum production (up to 59%). Temporal criteria used to define long COVID‐19 varied from 3 to 24 weeks after acute phase or hospital discharge. Potentially associated risk factors were old age, female sex, severe clinical status, a high number of comorbidities, hospital admission, and oxygen supplementation at the acute phase. However, limitations related to study designs added uncertainty to this finding. None of the studies assessed the duration of signs/symptoms.
Conclusion
The frequency of long COVID‐19 reached up to 80% over the studies included and occurred between 3 and 24 weeks after acute phase or hospital discharge. Chest pain, fatigue, dyspnea, and cough were the most reported clinical manifestations attributed to the condition. Based on these systematic review findings, there is an urgent need to understand this emerging, complex and challenging medical condition. Proposals for diagnostic criteria and standard terminology are welcome.
Review criteria: how did you gather, select and analyze the information you considered in your review?
Systematic review conducted with a comprehensive search including formal databases, COVID‐19 or SARS‐CoV‐2 data sources, grey literature, and manual search.
We considered for inclusion clinical trials, observational longitudinal comparative and non‐comparative studies, cross‐sectional, before‐and‐after and case series.
We presented the results as a narrative synthesis regarding frequency and duration of long COVID, signs and symptoms, criteria used for diagnosis, and potential risk factors.
Message for the clinic: what is the ‘take‐home’ message for the clinician?
The frequency of long COVID reached up to 80% over the studies included and occurred between 3 to 24 weeks after acute phase or hospital discharge. Chest pain, fatigue, dyspnea and cough were the most reported clinical manifestations attributed to the condition.
Temporal criteria used to define long COVID varied from 3 to 24 weeks after acute phase or hospital discharge.
Based on this systematic review findings, there is an urgent need to understand this emerging, complex and challenging medical condition. Proposals for diagnostic criteria and standard terminology are welcome.
1. INTRODUCTION
Long COVID‐19 is a term used to describe the condition presented by individuals who have recovered from the acute phase of COVID‐19 but are still reporting lasting effects of the infection or having had the usual clinical picture for much longer than expected, or have new symptoms and signs. Two presentations of this condition have been observed: (a) a severe form, with the occurrence of thromboembolic complications, for example: and (b) a nonspecific form, usually emphasised by fatigue and dyspnea. 1 The clinical management of cases requires an approach that considers the severity and its prognosis and impact on affected individuals' quality of life.
Researchers, healthcare managers, and health professionals have now discussed the challenge of rehabilitating patients facing a disease with a plethora of clinical presentation. 2 , 3 Studies have sought to understand the convalescent phase of the COVID‐19 better. 4 , 5 One of the critical issues for these achievements is identifying the frequency, defining criteria for diagnosis, and understanding the spectrum and severity of clinical manifestations observed in long COVID‐19. From this knowledge, preventive and therapeutic measures can be investigated and implemented, either at the individual or population level.
To scientifically and impartially inform healthcare decision making, we developed a systematic review to map and critically evaluate the available information on the frequency of long COVID‐19 and the characteristics of its clinical manifestations.
2. OBJECTIVES
To identify, systematically evaluate and summarise the best available evidence on the frequency of long COVID‐19, the characteristics of its clinical manifestations, and the adopted criteria for its diagnosis.
3. METHODS
3.1. Design and setting
A systematic review conducted in the Health Technology Assessment Center at Hospital Sírio‐Libanês, São Paulo, SP, Brazil. The protocol was prospectively registered in the PROSPERO database (CRD42020214587, available from https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020214587). The review's planning and conduct were carried out following the Cochrane Handbook for Systematic Reviews of Interventions, 6 considering those sections relevant for a systematic review of frequency. The report of the review was prepared following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) Statement. 7
3.2. Criteria for including studies
Considering the research question and the outcomes of interest, we searched for the following study designs:
Experimental studies: randomised, quasi‐randomised or non‐randomised trials.
Observational longitudinal comparative studies (cohort or case‐control).
Observational non‐comparative studies (case series, case studies reporting the experience of a specific service).
Cross‐sectional studies (prevalence, survey, or analytical cross‐sectional).
Uncontrolled before‐and‐after studies (including interrupted time series with two or more measures before and after the event of interest).
Controlled before‐and‐after studies.
Reports of a single individual case were excluded. Studies available only as preprint versions and without a peer‐review process were not considered for this systematic review.
For those studies comparing participants with vs without COVID‐19, we restricted our analyses for the COVID‐19 participants (population of interest for this review), and they were treated as single‐arm cohort studies.
3.3. Outcomes of interest
3.3.1. Primary outcomes
Frequency of long COVID‐19, or post‐acute COVID‐19 or persistence of clinical manifestations after the acute phase (defined by the authors of primary studies).
Frequency of signs and symptoms experienced by patients after COVID‐19 acute phase.
3.3.2. Secondary outcomes
Frequency of different criteria used for the definition of long COVID‐19 or post‐acute COVID‐19.
Mean duration of the long COVID‐19 or post‐acute COVID‐19 reported by the primary studies included.
Risk factors associated with the occurrence of long COVID‐19 or post‐acute COVID‐19.
Given the lack of a worldwide accepted definition and to avoid redundancy, we adopted the term “long COVID” to refer to long COVID‐19, post‐acute COVID‐19 or persistence of signs and symptoms after the COVID‐19 acute phase. This assumption will be addressed henceforth under the discussion section.
3.4. Searching for studies
We conducted a sensitive search of the literature to identify any study that would meet the eligibility criteria. There were no reservations related to the status, language, or date of publication. We conducted electronic searches on February 01, 2021, in the following databases: CINAHL (Cumulative Index to Nursing and Allied Health Literature), CENTRAL (Cochrane Library Central Register of Controlled Trials, via Wiley), EMBASE (via Elsevier), Epistemonikos (https://www.epistemonikos.org/), Health Systems Evidence (https://www.healthsystemsevidence.org/), LILACS (Latin American and Caribbean Health Sciences Literature, via Biblioteca Virtual em Saúde), and MEDLINE (via PubMed). An additional search was conducted in the following COVID‐19 specialised sources: McMaster Daily News COVID‐19 (https://covid19.mcmaster.ca/), Oxford COVID‐19 Evidence Service, World Health Organization – Global Literature on Coronavirus Disease (https://search.bvsalud.org/global‐literature‐on‐novel‐coronavirus‐2019‐ncov/). A search for grey literature was conducted in the OpenGrey database (https://opengrey.eu). A manual search was performed in the reference lists of the relevant studies.
The strategies developed and used for each electronic database are presented in Table S1.
3.5. Study selection and data collection
The procedure for selecting studies was carried out in two phases within the Rayyan platform. 8 In the first phase, two reviewers independently evaluated all titles and abstracts retrieved by the searches. References deemed “potentially eligible” were then for the second phase (full‐text assessment) to confirm its eligibility. A third reviewer solved any dissension. Studies excluded during the second phase were listed in the “excluded studies table” along with the rationale for exclusions. The PRISMA flow diagram presented the selection process.
Two reviewers independently obtained data from the included studies on general information (authors, year, country, conflict of interest, funding), methods (design, inclusion criteria, follow‐up), and participant details (age, sex, diagnosis criteria for long COVID‐19, onset, and clinical manifestation of long COVID‐19).
3.6. Assessing the methodological quality or risk of bias of included studies
The risk of bias assessment was performed on the premises of the study design as presented below:
Randomised clinical trials (Cochrane Risk of Bias tool). 6
Cohort or case‐control, non‐randomised trials: ROBINS‐I. 9
Controlled before‐and‐after study: ROBINS‐I with additional issues for (controlled) before‐after studies. 9
Uncontrolled before and after the study (including interrupted time series): ROBINS‐I with additional issues for (uncontrolled) before‐after studies. 9
Analytical cross‐sectional study: the Joanna Briggs Institute checklist for analytical cross‐sectional studies. 10
Prevalence cross‐sectional study: the Joanna Briggs Institute checklist for prevalence studies. 11
Case series or single‐arm cohort: National Institute of Health. Quality Assessment Tool for Case Series Studies. 12 Considering the frequency of compliance with the relevant items, at the discretion of the review authors, the studies were categorised as presenting high quality (80% or more of accomplished items), moderate quality (≥50% to <70%) or low quality (<50%).
For cohort studies including a non‐COVID‐19 comparison group and those cohort studies that did not provide any comparable data from the group not experiencing long COVID‐19, we used the NIH tool for case series, assuming that this tool could be more suitable. Two independent reviewers carried the evaluation out and a third reviewer solved any dissension.
3.7. Analyses and results presentation
We created a table comprising the methodological characteristics, main findings, and funding sources from each included study. Whenever data were available, we presented the frequency of long COVID‐19, the criteria used for diagnosis, the description, frequency and duration of signs and symptoms, and risk factors suspected to be related to long COVID‐19 occurrence.
We presented the results using a narrative approach (qualitative synthesis) for the outcomes: frequency of long COVID‐19, frequency of signs and symptoms, frequency of different criteria used for the definition of long COVID‐19, and mean duration of the long COVID‐19 along the primary studies included.
For the outcome' risk factors for long COVID', depending on data availability of data and homogeneity of studies, we planned to pool results from similar studies by random‐effects meta‐analyses (software Review Manager 5.4). We planned to estimate the risk ratios (or odds ratios) for dichotomous data assuming a 95% confidence interval. As the meta‐analysis was not possible, we presented the results as qualitative synthesis (descriptive presentation). In this case, we planned to use tables comprising the main findings of the included studies and their methodological quality or risk of bias.
4. RESULTS
4.1. Search results
The search strategies retrieved 22 361 records and 1.680 identical duplicates were removed previously to the selection process. After the reading titles and abstracts (first phase), 20 641 references did not fit the inclusion criteria considering the review's PICO and were eliminated. The full text of the 40 remained records was read (second phase), 12 of which were excluded 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 (Table S2), and 2 were ongoing studies (NCT04632355, NCT04659889). Last, 25 studies (reported by 26 references) were included in this review. 4 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49
We presented the complete PRISMA flow diagram in Figure 1.
FIGURE 1.
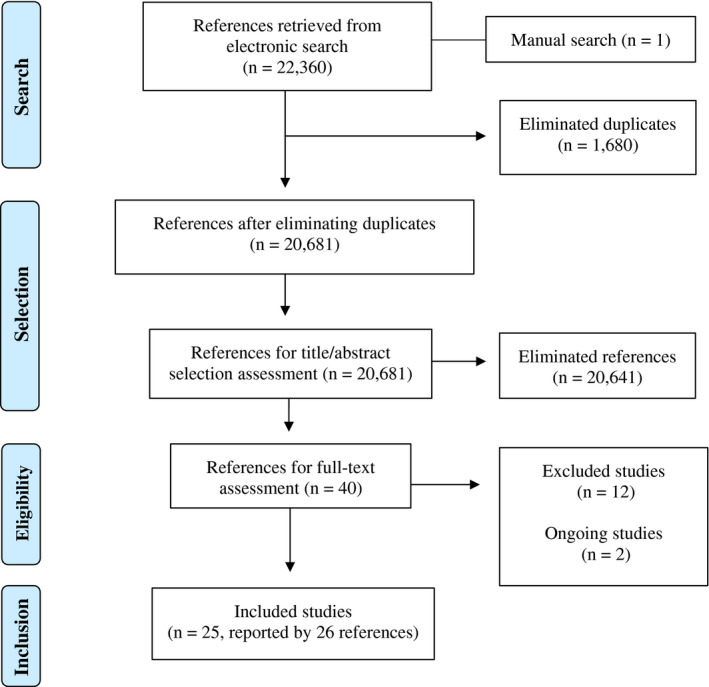
Flowchart of the study selection process
4.2. Characteristics of the included studies
The 25 included studies comprised a total sample of 5440 participants. We detailed the main characteristics of the studies in Table 1. From the 25 included studies, 10 were prospective cohort studies, 4 , 25 , 26 , 33 , 34 , 35 , 36 , 39 , 40 , 47 13 were retrospective cohort studies 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 42 , 44 , 45 , 46 , 48 , 49 and 2 were case series. 41 , 43 The sample size varied from 343 to 1733 participants. 31
TABLE 1.
Characteristics of included studies
| Author, year | Country | Study design | Population included | Outcomes of interest for this review | Funding source | ||||
|---|---|---|---|---|---|---|---|---|---|
| Frequency of long COVID‐19 a | Main signs/symptoms of long COVID‐19 evaluated/observed | Temporal criteria used for long COVID‐19 or time point to measure signs/symptoms | Mean duration of long COVID‐19 | Main aspects investigated as a potential risk factor for long COVID‐19 | |||||
| Bellan, 2021 25 | Italy | Prospective cohort |
COVID‐19 participants Participants recovered from severe COVID‐19, requiring hospitalisation during the acute phase n = 256 |
No |
Ageusia Anosmia Arthralgia Chest pain Cough Diarrhoea Dyspnea Myalgia Pulmonary dysfunction Post‐traumatic stress |
16 weeks after hospital discharge | No |
Chronic Kidney Disease COPD Diabetes ICU admission during the hospital stay Modality of oxygen delivery Number of comorbidities Sex Smoking status |
AGEING Project, Department of Excellence, Department of Translational Medicine, Università del Piemonte Orientale and by the Azienda Ospedaliero–Universitaria Maggiore della Carità di Novara |
| Carfi, 2020 4 | Italy | Prospective cohort |
COVID‐19 participants Participants recovered from severe COVID‐19, requiring hospitalisation during the acute phase n = 143 |
Yes |
Anosmia Arthralgia Chest pain Cough Diarrhoea Dyspnea Dysgeusia Fatigue Headache Lack of appetite Myalgia Rhinitis Red eyes Sicca syndrome Sputum production Sore throat Vertigo |
5.1 weeks (mean) after hospital discharge | No | No | Not reported |
| Carvalho‐Schneider, 2020 26 | France | Prospective cohort |
COVID‐19 participants Participants recovered from COVID‐19, requiring or not hospitalisation during the acute phase n = 130 |
Yes |
Anosmia/ageusia Arthralgia Asthenia Chest pain Cutaneous signs Diarrhoea Dyspnoea Flulike symptoms (myalgia, headache) Palpitations |
8 weeks after acute phase onset | No |
Age Comorbidities Hospital admission at the acute phase Initial abnormal auscultation Signs and symptoms at the acute phase |
Not reported |
| El Sayed, 2020 27 | Saudi Arabia | Retrospective cohort |
Long COVID‐19 participants COVID‐19 after 2 consecutive negative PCR tests who attended for pulmonology clinic for follow‐up and psychiatric department for assessment. n = 200 |
No |
Fatigue Anhedonia |
11.8 weeks (mean) after 2 consecutive negative PCR tests | No |
Age Educational level Sex Smoking status |
Not reported |
| Garrigues, 2020 28 | France | Retrospective cohort |
COVID‐19 participants Participants recovered from COVID‐19, requiring hospitalisation during the acute phase. n = 120 |
No |
Anosmia Chest pain Concentration impairment Cough Dysgeusia Dyspnoea Fatigue Hair loss Memory impairment Sleep disorders |
14.2 weeks after hospital admission | No | ICU‐admission | Not reported |
| Guedj, 2020 29 | France | Retrospective cohort |
Long‐COVID‐19 participants Participants recovered from COVID‐19, requiring hospitalisation during the acute phase. n = 35 |
No |
Dyspnoea Hyposmia/anosmia Insomnia Memory/cognitive impairment Pain Post‐traumatic stress disorder |
3 weeks after acute phase onset | No | No | The local PET database of healthy controls was funded by Assistance Publique – Hopitaux de Marseille |
| Halpin, 2020 30 | United Kingdom | Retrospective cohort |
COVID‐19 participants COVID‐19 requiring hospitalisation during the acute phase n = 100 |
No |
Concentration impairment Continence impairment Dyspnoea Fatigue Faecal/Urinary incontinence Memory impairment Neuropsychological symptoms Pain Speech‐ and swallow‐ related symptoms |
At least 4 weeks after hospital discharge | No | No | National Institute for Health Research (NIH) infrastructure at Leeds and Sheffield |
| Huang, 2021 31 | China | Retrospective cohort |
COVID‐19 participants COVID‐19 requiring hospitalisation during the acute phase n = 1733 |
Yes |
Ageusia Anosmia Anxiety or depression Arthralgia Chest pain Dyspnoea Diarrhoea or vomiting Dizziness Fatigue or muscle weakness Fever Hair loss Headache Lack of appetite Mobility impairment Myalgia Palpitations Pain or discomfort Respiratory dysfunction Skin rash Sleep disorders Sore throat or difficult to swallow |
24 weeks after hospital discharge | No |
Age Severity of acute COVID‐19 infection Sex |
National Natural Science Foundation of China; Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences; the National Key Research and Development Programme of China; Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis; Peking Union Medical College Foundation |
| Jacobs, 2020 32 | United States of America | Retrospective cohort |
COVID‐19 participants COVID‐19 requiring hospitalisation during the acute phase n = 183 |
Yes |
Anosmia Arthralgia Confusion Cough Diarrhoea Dysgeusia Dyspnoea Eye irritation Fatigue Fever Headache Myalgia Sputum production Ulcer |
4 weeks after hospital discharge | No |
Age Comorbidities Oxygen requirements during and after hospitalisation Sex |
Not reported |
| Liang, 2020 33 | China | Prospective cohort |
COVID‐19 participants COVID‐19 requiring hospitalisation during the acute phase n = 76 |
No |
Chest pain on exertion Cough Diarrhoea Dyspnoea Fatigue Fever Sputum production Palpitations Respiratory dysfunction |
12 weeks after hospital discharge | No |
Age Comorbidities Hospital length of stay Severity of acute COVID‐19 infection Sex Treatment received in the acute phase |
Not reported |
| Lu, 2020 34 | China | Prospective cohort |
COVID‐19 participants COVID‐19 requiring hospitalisation during the acute phase n = 60 |
Yes |
Anosmia Dysgeusia Fatigue Headache Hearing loss Mobility impairment Limb numbness Memory impairment Myalgia Mood changes Vision changes Tremor |
12 weeks after hospital discharge | No | No | Shanghai Natural Science Foundation, Youth Programme of National Natural Science Foundation of China, Shanghai Sailing Programme, Shanghai Science and Technology Development, Shanghai Municipal Science and Technology Major Project and ZJ Lab |
| Moreno‐Perez, 2021 35 | Spain | Prospective cohort |
COVID‐19 participants COVID‐19 requiring or not hospitalisation during the acute phase n = 277 |
Yes |
Anosmia Arthralgia Cough Diahrrea Dysgeusia Dyspnoea Fatigue Myalgia Neurological symptoms (headache, memory or cognitive impairment) |
10‐14 weeks after discharge from hospital or ambulatory | No |
Age Comorbidities Sex Severity of acute COVID‐19 infection COVID‐19 GRAM score Inflammatory markers ICU‐admission in the acute phase ICU length of stay Treatment received in the acute phase |
No external funding was received |
| Nehme, 2020 36 | Switzerland | Prospective cohort |
COVID‐19 participants Participants recovered from severe COVID‐19, requiring hospitalisation during the acute phase n = 669 |
Yes |
Ageusia Anosmia Cough Digestive symptoms Dyspnea Fatigue Fever Headache |
4‐6 weeks after acute phase onset | No | No | Not reported |
| Otte, 2020a 37 /Otte, 2020b 38 | Germany | Retrospective cohort |
COVID‐19 participants Participants recovered from COVID‐19, not requiring hospitalisation during the acute phase n = 91 |
No | Olfactory dysfunction | At least 3 weeks after recovery | No | No | Not reported |
| Petersen, 2020 39 | Denmark | Prospective cohort |
COVID‐19 participants Participants recovered from COVID‐19, not requiring hospitalisation or not during the acute phase n = 187 |
Yes |
Anosmia Arthralgia Cough Diarrhoea Dysgeusia Dyspnea Fatigue Fever Headache Myalgia Nausea Rash Throat pain |
14.2‐17.8 weeks after acute phase onset | No |
Age Comorbidities Hospitalisation Self‐reported medication use Sex Smoking status |
Krúnborg and Bortartún |
| Puchner, 2021 40 | Austria | Prospective cohort |
COVID‐19 participants Participants recovered from severe COVID‐19, requiring hospitalisation during the acute phase n = 23 |
No |
Neuropsychological dysfunction Pulmonary dysfunction Sleep disorder |
3‐4 weeks after hospital discharge | No | No | No funding |
| Ramani, 2021 41 | United States of America | Case series |
COVID‐19 participants Participants recovered from COVID‐19, requiring hospitalisation during the acute phase n = 28 |
No |
Cognitive dysfunction Depression Insomnia Physical dysfunction Pulmonary dysfunction |
6 weeks after hospital discharge | No | No | No funding |
| Rosales‐Castillo, 2021 42 | Spain | Retrospective cohort |
COVID‐19 participants Participants recovered from COVID‐19, requiring hospitalisation during the acute phase n = 118 |
Yes |
Ageusia Anosmia Asthenia Cough Dyspnea Myalgia |
7 weeks (mean) after hospital discharge | No | No | Not reported |
| Roth, 2021 43 | United States of America | Case series |
Long‐COVID‐19 participants Participants recovered from severe COVID‐19, requiring hospitalisation during the acute phase n = 3 |
No | Cholestasis | Not specified | No | Ischemic hepatitis | No funding |
| Simani, 2021 44 | Iran | Retrospective cohort |
COVID‐19 participants Participants recovered from COVID‐19, requiring hospitalisation during the acute phase n = 120 |
No |
Chornic fatigue syndrome Post‐traumatic stress disorder |
24 weeks after disease onset | No |
Comorbidity Post‐traumatic stress disorder Sex |
Not reported |
| Suarez‐Robles, 2020 45 | France | Retrospective cohort |
COVID‐19 participants Participants recovered from COVID‐19, requiring hospitalisation during the acute phase n = 134 |
No |
Anosmia Arthralgia Cough Cutaneous manifestations Dyspnea Dysgeusia Dysphonia Fatigue Headache Palpitation Psychological symptoms Sputum production Walking disturbances |
12 weeks after hospital discharge | No | No | Not reported |
| Tarazona‐Fernandez, 2020 46 | Spain | Retrospective cohort |
COVID‐19 participants Participants recovered from COVID‐19, not requiring hospitalisation during the acute phase n = 37 |
Yes |
Anxiety Arthralgia Asthenia Chest pain Cough Depression Diahrrea Dyspnea Fever Headache Myalgia Red eyes Sore throat |
5.7 weeks (mean) after disease onset | No | No | No funding |
| Van den Borst, 2020 47 | Netherlands | Prospective cohort |
COVID‐19 participants Participants recovered from COVID‐19, requiring or not hospitalisation during the acute phase n = 124 |
No |
Anxiety/Depression Dyspnea Cognitive impairment Physical dysfunction Pulmonary dysfunction |
9.1 (1.6) weeks (mean/SD) after hospital discharge | No | COVID‐19 severity status in the acute phase | No funding |
| Xiong, 2021 48 | China | Retrospective cohort |
COVID‐19 participants Participants recovered from non‐critical COVID‐19, requiring hospitalisation during the acute phase n = 538 |
No |
Cardiovascular‐related symptoms General symptoms (arthralgia, chills, dizziness, fatigue, limb oedema, myalgia, sweating) Hair loss Psychosocial symptoms Pulmonary dysfunction |
More than 12 weeks after hospital discharge | No |
Age COVID‐19 severity status in the acute phase Sex Signs and symptoms at the acute phase |
Not reported |
| Zhao, 2020 49 | China | Retrospective cohort |
COVID‐19 participants Participants recovered from non‐critical COVID‐19, requiring hospitalisation during the acute phase n = 55 |
No |
Ageusia Anosmia Cough and sputum Dyspnea Gastrointestinal symptoms Fatigue Headache Pulmonary dysfunction |
12 weeks after hospital discharge | No |
Age Comorbidities Hospital length of stay Sex Signs and symptoms at the acute phase |
Key Scientific Research Projects of Henan Higher Education Institutions (under grant 20B320032) |
Abbreviations: COPD, chronic obstructive pulmonary disease; CT, computerised tomography; Dlco, diffusing lung capacity for carbon monoxide; HFNC, high‐flow nasal cannula; ICU, intensive care unit; IMV, invasive mechanical ventilation; NIV, non‐invasive mechanical ventilation; MRI, magnetic resonance imaging; SD, standard deviation; SPPB, short physical performance battery; US, ultrasonography; VHA, veteran health administration.
Frequency of long COVID‐19, or post‐acute COVID‐19 or persistence of clinical manifestations after the acute phase (as defined by the authors of primary studies).
4.3. Methodological quality or risk of bias of included studies
For all included studies, we used the National Institute of Health. Quality Assessment Tool for Case Series Studies, either because they were in fact case series or cohort studies, but the analyses/data of interest for this review were restricted to a single arm of the cohort developed. Five studies 4 , 36 , 41 , 46 , 49 were classified as presenting moderate quality, and the remaining 20, high quality. We presented the judgement for the methodological quality and the reason for each judgement in Table S3.
4.4. Results from the included studies
4.4.1. Frequency of long COVID‐19 (we considered the frequency of long COVID‐19 as defined by the authors of primary studies)
Overall, 10 studies 4 , 26 , 31 , 32 , 34 , 35 , 36 , 39 , 42 , 46 directly reported the frequency of long COVID‐19 (ranging from 4.7% to 80%). Additionally, for 13 studies, 25 , 28 , 29 , 30 , 33 , 34 , 35 , 40 , 41 , 44 , 45 , 48 , 49 it was possible to estimate the minimum frequency based on the most reported signs or symptoms (17.5%‐80%) (Table 2).
TABLE 2.
Synthesis of results
| Main signs/symptoms of long COVID‐19 evaluated/observed | Frequency (% min‐max) | Studies/year |
|---|---|---|
| Long COVID‐19 or post‐acute COVID‐19 or persistent sign/symptom | 4.7‐80.0 | Carfi (2020), 4 Carvalho‐Scheneider (2020), 26 Huang (2020), 31 Jacobs (2020), 32 Lu (2020), 34 Moreno‐Perez (2021), 35 Nehme (2020), 36 Petersen (2020), 39 Rosales‐Castillo (2021), 42 Tarazona‐Fernandez (2020) 46 |
| Ageusia/dysgeusia | 1.0‐21.6 | Bellan (2021), 25 Carfi (2020), 4 Carvalho‐Scheneider (2020); 26 Garrigues (2020), 28 Huang (2021), 31 Jacobs (2020), 32 Liang (2020), 33 Lu (2020), 34 Moreno‐Perez (2021), 35 Nehme (2020), 36 Petersen (2020), 39 , Rosales‐Castillo (2021), 42 Suarez‐Robles (2020), 45 Zhao (2020) 49 |
| Anosmia | 0.0‐26.2 | Bellan (2021), 25 Carfi (2020), 4 Carvalho‐Scheneider (2020), 26 Garrigues (2020), 28 Guedj (2020), 29 Huang (2021), 31 Jacobs (2020), 32 Lu (2020), 34 Moreno‐Perez (2021), 35 Nehme (2020), 36 Otte (2020a, 37 Otte 2020b 38 ), Petersen (2020), 39 Rosales‐Castillo (2021), 42 ,Suarez‐Robles (2020), 45 Tarazona‐Fernandez (2020), 46 Van den Borst (2020), 47 Xiong (2021), 48 Zhao (2020) 49 |
| Arthralgia | 5.9‐54.7 | Bellan (2021), 25 Carfi (2020), 4 Carvalho‐Scheneider (2020), 26 Huang (2021), 31 Jacobs (2020), 32 Petersen (2020), 39 Suarez‐Robles (2020), 45 Tarazona‐Fernandez (2020), 46 Xiong (2021) 48 |
| Cardiovascular‐related symptoms | 13.0 | Xiong (2021) 48 |
| Chest pain | 0.4‐89.0 | Bellan (2021), 25 Carfi (2020), 4 Carvalho‐Scheneider (2020), 26 Huang (2021), 31 Liang (2020), 33 Tarazona‐Fernandez (2020), 46 Xiong (2021) 48 |
| Chills | 4.6 | Xiong (2021) 48 |
| Cognitive/memory/concentration impairment | 18.0‐57.1 | Garrigues (2020), 28 Guedj (2020), 29 Halpin (2020), 30 Jacobs (2020), 32 Lu (2020), 34 Moreno‐Perez (2021), 35 Ramani (2021), 41 Van den Borst (2020), 47 Xiong (2021) 48 |
| Communication difficulty | 6.0 | Halpin (2020) 30 |
| Cough/sputum production | 1.8‐59.0 | Bellan (2021), 25 Carfi (2020), 4 Garrigues (2020), 28 Halpin (2020), 30 Jacobs (2020), 32 Liang (2020), 33 Moreno‐Perez (2021), 35 Nehme (2020), 36 Rosales‐Castillo (2021), 42 Suarez‐Robles (2020), 45 Tarazona‐Fernandez (2020), 46 Xiong (2021), 48 Zhao (2020) 49 |
| Cutaneous signs | 1.5‐20.0 | Carvalho‐Scheneider (2020), 26 Huang (2021), 31 Jacobs (2020), 32 Suarez‐Robles (2020) 45 |
| Diarrhoea/vomiting/gastrointestinal symptoms | 1.3‐33.3 | Bellan (2021), 25 Carfi (2020), 4 Huang (2021), 31 Jacobs (2020), 32 Liang (2020), 33 Moreno‐Perez (2021), 35 Nehme (2020), 36 Tarazona‐Fernandez (2020), 46 Zhao (2020) 49 |
| Depression/anxiety | 3.0‐25.0 | El Sayed (2020), 27 Huang (2021), 31 Ramani (2021), 41 Tarazona‐Fernandez (2020), 46 Van den Borst (2020) 47 |
| Dyspnea/breathlessness | 5.5‐61.0 | Bellan (2021), 25 Carfi (2020), 4 Carvalho‐Scheneider (2020), 26 Garrigues (2020), 28 Guedj (2020), 29 Halpin (2020), 30 Huang (2021), 31 Jacobs (2020), 32 Liang (2020), 33 Moreno‐Perez (2021), 35 Nehme (2020), 36 Rosales‐Castillo (2021), 42 Suarez‐Robles (2020), 45 Tarazona‐Fernandez (2020), 46 Van den Borst (2020), 47 Zhao (2020) 49 |
| Fatigue/asthenia | 6.6‐64.0 | Carfi (2020), 4 Carvalho‐Scheneider (2020), 26 El Sayed (2020), 27 Garrigues (2020), 28 Halpin (2020), 30 Huang (2021), 31 Jacobs (2020), 32 Liang (2020), 33 Lu (2020), 34 Moreno‐Perez (2021), 35 Nehme (2020), 36 Petersen (2020), 39 Rosales‐Castillo (2021), 42 Simani (2021), 44 Suarez‐Robles (2020), 45 Tarazona‐Fernandez (2020), 46 Zhao (2020) 49 |
| Faecal incontinency | 3.0 | Halpin (2020) 30 |
| Fever | 0.0‐20.0 | Huang (2021), 31 Jacobs (2020), 32 Liang (2020), 33 Nehme (2020), 36 Petersen (2020), 39 Tarazona‐Fernandez (2020) 46 |
| Functional impairment | 5.7‐50.0 | Bellan (2021), 25 Suarez‐Robles (2020) 45 |
| Hair loss | 20.0‐28.6 | Garrigues (2020), 28 Huang (2021), 31 Xiong (2021) 48 |
| Headache | 2.0‐39.0 | Carfi (2020), 4 Carvalho‐Scheneider (2020), 26 Huang (2021), 31 Jacobs (2020), 32 Lu (2020), 34 Moreno‐Perez (2021), 35 Nehme (2020), 36 Petersen (2020), 39 Suarez‐Robles (2020), 45 Tarazona‐Fernandez (2020) 46 |
| Hearing loss | 1.6 | Lu (2020) 34 |
| Lack of appetite | 6.2‐8.0 | Carfi (2020), 4 Huang (2021) 31 |
| Laryngeal sensitivity | 17.0 | Halpin (2020) 30 |
| Limb numbness | 6.6 | Lu (2020) 34 |
| Limb oedema | 2.6 | Xiong (2021) 48 |
| Mobility dysfunction | 6.6‐7.0 | Huang (2021), 31 Lu (2020) 34 |
| Myalgia | 2.0‐50.6 | Bellan (2021), 25 Carfi (2020), 4 Carvalho‐Scheneider (2020), 26 Huang (2021), 31 Jacobs (2020), 32 Lu (2020), 34 Moreno‐Perez (2021), 35 Rosales‐Castillo (2021), 42 Tarazona‐Fernandez (2020), 46 Xiong (2021) 48 |
| Pain and discomfort | 19.0‐66.0 | Guedj (2020), 29 Halpin (2020), 30 Huang (2021) 31 |
| Palpitations | 9.0‐62.0 | Carvalho‐Scheneider (2020), 26 Huang (2021), 31 Liang (2020), 33 Suarez‐Robles (2020) 45 |
| Physical dysfunction | 4.0‐28.3 | Ramani (2021), 41 Suarez‐Robles (2020), 45 Van den Borst (2020), 47 Xiong (2021) 48 |
| Post‐traumatic stress/psychosocial symptoms/mood changes | 5.8‐ 57.1 | Bellan (2021), 25 Halpin (2020), 30 Lu (2020), 34 Puchner (2021), 40 Simani (2021) 44 |
| Rhinitis | 16.7 | Carfi (2020) 4 |
| Red eyes | 13.9 | Carfi (2020) 4 |
| Sicca syndrome | 17.4 | Carfi (2020) 4 |
| Sleep disorder/insomnia | 21.7‐53.0 | Garrigues (2020), 28 Guedji (2020), 29 Huang (2021), 31 Puchner (2021), 40 Ramani (2021) 41 |
| Sensitivity disorder | 7.5 | Suarez‐Robles (2020) 45 |
| Sore throat/throat pain | 3.2‐11.0 | Carfi (2020), 4 Halpin (2020), 30 Huang (2021), 31 Tarazona‐Fernandez (2020), 46 Xiong (2021) 48 |
| Swallow problem | 8.0 | Halpin (2020) 30 |
| Sweating | 23.6 | Xiong (2021) 48 |
| Tremor | 1.6 | Lu (2020) 34 |
| Urinary incontinency | 10.0 | Halpin (2020) 30 |
| Vertigo/dizziness | 2.6‐6.0 | Carfi (2020), 4 Huang (2021), 31 Xiong (2021) 48 |
| Vision changes | 1.6 | Lu (2020) 34 |
| Voice change | 20.0 | Halpin (2020) 30 |
4.4.2. Frequency of signs and symptoms experienced by patients diagnosed with long COVID‐19
Table 2 details the frequency of the main signs and symptoms of long COVID‐19 observed in all included studies. The most prevalent were chest pain (up to 89%), fatigue (up to 65%), dyspnea (up to 61%), cough and sputum production (up to 59%), cognitive and memory impairment (up to 57.1%), arthralgia (up to 54.7%), sleep disorders (up to 53%), myalgia (up to 50.6%), and functional impairment (up to 50%).
4.4.3. Frequency of different criteria used for the definition of long COVID‐19
Temporal criteria used for considering long COVID‐19 or the timepoint assumed to measure signs and symptoms varied substantially amongst studies (3 weeks after acute phase onset to 24 weeks after hospital discharge) (Table 1).
4.4.4. Mean duration of long COVID‐19 reported by the primary studies included
None of the included studies assessed the duration of signs and symptoms attributed to long COVID‐19.
4.4.5. Risk factors associated with the occurrence of long COVID‐19
Fourteen studies 25 , 26 , 27 , 28 , 31 , 32 , 33 , 35 , 39 , 43 , 44 , 47 , 48 , 49 reported some aspects investigated as potential risk factor for long COVID‐19 (Table 1). The most prevalent aspects of persistent symptoms were old age, female sex, severe clinical status at acute phase, high number of comorbidities, hospital admission, and oxygen supplementation at the acute phase. Table S4 shows the detailed results from studies on the risk factors potentially associated with long COVID‐19.
5. DISCUSSION
This systematic review identified 25 observational studies reporting all but one of our outcomes of interest. The frequency of long COVID‐19 varied from 4.7% to 80% in the followed cohorts, and the most prevalent clinical manifestations were chest pain (reported by up to 89% of the participants), fatigue, dyspnea, cough, and sputum production. Temporal criteria used to define long COVID‐19 varied substantially from 3 to 24 weeks after the acute phase and/or hospital discharge. Advanced age, female sex, a high number of comorbidities and severe clinical status, hospital admission, and oxygen supplementation at acute phase were deemed to be related to long COVID‐19. None of the studies, however, assessed the duration of persistent signs/symptoms.
This review's main impact shies away from the idea of establishing a definitive diagnostic criterion but instead recognising that we are still one step behind in dealing with a new health condition. Does the misunderstanding already start with the different terms adopted in the literature to define the new condition: long COVID‐19? Post‐acute COVID‐19? Persistent COVID‐19? Furthermore, we cannot rule out the possibility that we face not one but two or more conditions: one post‐acute and one chronic, one with the persistence of the initial symptoms and another with emerging signs and symptoms, one with mild manifestations and another with severe ones.
In the absence of well‐established definitions, we included studies reporting persistence of symptoms or appearance of novel symptoms at least 3 weeks after the onset of the acute phase of COVID‐19, but we found studies reporting the condition up to 24 weeks after the acute phase or hospital discharge. The literature has been suggested a definition for post‐acute COVID‐19 as extending beyond 3 weeks from the onset of first symptoms and for chronic COVID‐19 as extending beyond 12 weeks. 1 Thereby, considering the lack of consensus, our review findings provide a robust basis for mathematical models for diagnostic and predictive aspects that could be addressed by further studies.
Most of the signs and symptoms attributed to long COVID‐19 could be found in the current pandemic scenario and non‐COVID‐19 individuals. Social isolation, modified lifestyle habits, reduced physical activity, social and economic insecurity may contribute to physical and psychological manifestations. This issue constitutes an important confounding factor that the included studies could not rule out because of the non‐comparative design and the lack of data for those studies with a non‐COVID‐19 comparator group.
The proper duration of long COVID‐19 is still uncertain, as, in most studies, there were still patients with attributable signs and symptoms at the time of the last assessment. Ongoing studies or extensions of the studies included in this review may follow the participants until the disappearance of signs and symptoms, and thus, we will have an idea of the maximum duration we can expect for this new medical condition.
This systematic review has some limitations. Many COVID‐19 studies are available by fast‐track procedure as a preprint, with or without peer review and even as part of regional databases, rather than in indexed journals. It increases the potential for missing studies that would otherwise be included. We mitigated this risk by searching multiple databases and overcomplimenting the search with a broad, unstructured search of relevant, and COVID‐19‐specific data sources.
We restricted our scope to clinical manifestations rather than covering laboratory, imaging or functional tests. Some might argue that considering these aspects adds objectivity to our results, but as we have observed a varied clinical‐laboratory relation, we could not say with certainty that this might reduce the impact of our results.
Additionally, the clinical heterogeneity amongst studies and the lack of data on the non‐long COVID‐19 groups did not allow us to perform meta‐analyses.
A recent narrative review on long COVID‐19 addressed its pathophysiology, organ‐specific sequelae and proposed an interesting framework for the identification of patients at high risk for developing this condition. 50 However, there was no intention to systematically map and critically appraise the current literature on frequency or definition criteria for long COVID‐19. Then, our study is the first comprehensive systematic review that attempted to identify the frequency, characteristics, different definitions, and potential risk factors related to long COVID‐19. We anticipate that our findings would improve the current care settings for both the acute and the more long‐lasting phases of COVID‐19. From this information, it is possible to put forward preventive actions to control risk factors, clinical management, and rehabilitation.
The consumption of additional resources allocated to long COVID‐19 can be estimated from information about the disease's frequency, its main manifestations, and duration. Preventive strategies can also be adopted based on the recognition of associated risk factors.
Based on the findings that respiratory manifestations and fatigue were the most frequent clinical findings attributed to long COVID‐19 over the studies identified, rehabilitation services can be restructured, and health systems can be prepared to receive patients with these needs.
This review opens up several questions that need to be addressed by further studies. One of them refers to the underrepresentation of low‐ and middle‐income countries in the studies conducted so far, and it is precisely in those settings where the pandemic and the long COVID‐19 has had a more substantial impact on health resources and quality of life. Additionally, it is necessary to define the set of signs and symptoms that are most likely to be part of this new medical condition, its duration, and the acute phase's temporal criteria. It would allow new studies to estimate its prevalence and incidence in a more precise and more standardised way, minimising confounding factors.
As key messages from our findings, we emphasise that: (a) there is an urgent need to understand this emerging, complex, and challenging medical condition; (b) proposals for criteria that can define a standard terminology and classify the disease in terms of its severity, time of onset, and symptomatology are welcome; (c) the estimated frequency of long COVID‐19 may be under or overestimated in the literature because of a lack of well‐established criteria (in fact, a broad frequency variation was observed in this review); (d) from the data identified in the primary studies, this review can provide an initial basis to support health systems' decision making and to support the conduction of studies to assess the effects of preventive, therapeutical, and rehabilitation interventions for long COVID‐19.
6. CONCLUSION
This systematic review identified 25 observational studies with 5440 participants. The frequency of long COVID‐19 ranged from 4.7% to 80%, and the most prevalent signs and symptoms were chest pain (reported by up to 89% of the participants), fatigue, dyspnea, and cough. Temporal criteria used to define long COVID‐19 varied substantially (3‐24 weeks after acute phase or hospital discharge). Potentially associated risk factors were old age, female sex, severe clinical status, a high number of comorbidities, hospital admission, and oxygen supplementation at the acute phase. None of the studies assessed the duration of persistent symptoms.
DISCLOSURES
The authors declare no competing interests.
AUTHORS CONTRIBUTIONS
Conceptualisation: RR, RLP. Data acquisition: AMB, ALCM, RLP, RR. Writing – Original Draft: AMB, RR, RLP. Writing – Review & Editing: all authors. Final approval: all authors.
Supporting information
Table S1
Table S2
Table S3
Table S4
ACKNOWLEDGEMENTS
We would like to thank Michelle Quarti Rosa for her contribution during the protocol upload in the PROSPERO database.
Cabrera Martimbianco AL, Pacheco RL, Bagattini ÂM, Riera R. Frequency, signs and symptoms, and criteria adopted for long COVID‐19: A systematic review. Int J Clin Pract. 2021;75:e14357. 10.1111/ijcp.14357
PROSPERO: CRD42020214587
Funding information
This study was supported by Sociedade Beneficente de Senhoras Hospital Sírio‐Libanês.
REFERENCES
- 1. Greenhalgh T, Knight M, A'Court C, Buxton M, Husain L. Management of post‐acute covid‐19 in primary care. BMJ. 2020;370:m3026. [DOI] [PubMed] [Google Scholar]
- 2. Landi F, Carfì A, Benvenuto F, et al. Predictive factors for a new positive nasopharyngeal swab among patients recovered from covid‐19. Am J Prev Med. 2021;60(1):13‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gemelli Against COVID‐19 Post‐Acute Care Study Group . Post‐COVID‐19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. 2020;32(8):1613‐1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carfì A, Bernabei R, Landi F; Gemelli against COVID‐19 Post‐Acute Care Study Group . Persistent symptoms in patients after acute COVID‐19. JAMA. 2020;324(6):603‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tenforde MW, Kim SS, Lindsell CJ, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID‐19 in a multistate Health Care Systems Network – United States, March‐June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(30):993‐998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Higgins JPT, Thomas J, Chandler J, et al. ,editors. Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019). www.training.cochrane.org/handbook. Accessed January 29, 2021.
- 7. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan‐a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomized studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Joanna Briggs Institute . Checklist for cross sectional studies. https://jbi.global/sites/default/files/2019‐05/JBI_Critical_Appraisal‐Checklist_for_Analytical_Cross_Sectional_Studies2017_0.pdf. Accessed January 29, 2021.
- 11. Joanna Briggs Institute . Checklist for prevalence studies. 2017. https://joannabriggs.org/sites/default/files/2019‐05/JBI_Critical_Appraisal‐Checklist_for_Prevalence_Studies2017_0.pdf. Accessed January 29, 2021.
- 12. National Institute of Health . Quality assessment tool for case series studies. https://www.nhlbi.nih.gov/health‐topics/study‐quality‐assessment‐tools. Accessed June 30, 2020.
- 13. Deisenhammer F, Borena W, Bauer A, et al. 6‐month SARS‐CoV‐2 antibody persistency in a Tyrolian COVID‐19 cohort. Wien Klin Wochenschr. 2020;9:1‐8. [Google Scholar]
- 14. Ladds E, Rushforth A, Wieringa S, et al. Developing services for long COVID: lessons from a study of wounded healers. Clin Med (Lond). 2021;21(1):59‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liao B, Liu Z, Tang L, et al. Longitudinal clinical and radiographic evaluation reveals interleukin‐6 as an indicator of persistent pulmonary injury in COVID‐19. Int J Med Sci. 2021;18(1):29‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dani M, Dirksen A, Taraborrelli P, et al. Autonomic dysfunction in ‘long COVID’: rationale, physiology and management strategies. Clin Med (Lond). 2021;21(1):e63‐e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu JWTW, de Luca RD, Mello Neto HO, Barcellos I. Post‐COVID‐19 syndrome? New daily persistent headache in the aftermath of COVID‐19. Arq Neuropsiquiatr. 2020;78(11):753‐754. [DOI] [PubMed] [Google Scholar]
- 18. Ladds E, Rushforth A, Wieringa S, et al. Persistent symptoms after Covid‐19: qualitative study of 114 “long Covid” patients and draft quality principles for services. BMC Health Serv Res. 2020;20(1):1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Z, Zheng C, Duan C, et al. Rehabilitation needs of the first cohort of post‐acute COVID‐19 patients in Hubei, China. Eur J Phys Rehabil Med. 2020;56(3):339‐344. [DOI] [PubMed] [Google Scholar]
- 20. Sofian M, Velayati AA, Banifazl M, et al. SARS‐CoV‐2, a virus with many faces: a series of cases with prolonged persistence of COVID‐19 symptoms. Wien Med Wochenschr. 2021;171(1–2):3‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rovere Querini P, De Lorenzo R, Conte C, et al. Post‐COVID‐19 follow‐up clinic: depicting chronicity of a new disease. Acta Biomed. 2020;91(9‐S):22‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Singh SJ, Barradell AC, Greening NJ, et al. British Thoracic Society survey of rehabilitation to support recovery of the post‐COVID‐19 population. BMJ Open. 2020;10(12):e040213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thai PQ, Toan DTT, Son DT, et al. Factors associated with the duration of hospitalization among COVID‐19 patients in Vietnam: a survival analysis. Epidemiol Infect. 2020;10(148):e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vink M, Vink‐Niese A. Could cognitive behavioural therapy be an effective treatment for long COVID and post COVID‐19 fatigue syndrome? Lessons from the Qure study for Q‐fever fatigue syndrome. Healthcare (Basel). 2020;8(4):552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bellan M, Soddu D, Balbo PE, et al. Respiratory and psychophysical sequelae among patients with COVID‐19 four months after hospital discharge. JAMA Netw Open. 2021;4(1):e2036142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carvalho‐Schneider C, Laurent E, Lemaignen A, et al. Follow‐up of adults with noncritical COVID‐19 two months after symptom onset. Clin Microbiol Infect. 2021;27(2):258‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. El Sayed S, Shokry D, Gomaa SM. Post‐COVID‐19 fatigue and anhedonia: a cross‐sectional study and their correlation to post‐recovery period. Neuropsychopharmacol Rep. 2021;41(1):50–55. 10.1002/npr2.12154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garrigues E, Janvier P, Kherabi Y, et al. Post‐discharge persistent symptoms and health‐related quality of life after hospitalization for COVID‐19. J Infect. 2020;81(6):e4‐e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guedj E, Campion JY, Dudouet P, et al. 18F‐FDG brain PET hypometabolism in patients with long. Eur J Nucl Med Mol Imaging. 2021;1‐11. 10.1007/s00259-021-05215-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID‐19 infection: a cross‐sectional evaluation. J Med Virol. 2021;93(2):1013‐1022. [DOI] [PubMed] [Google Scholar]
- 31. Huang C, Huang L, Wang Y, et al. 6‐month consequences of COVID‐19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jacobs LG, Gourna Paleoudis E, Lesky‐Di Bari D, et al. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID‐19 infection. PLoS One. 2020;15(12):e0243882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liang L, Yang B, Jiang N, et al. Three‐month follow‐up study of survivors of coronavirus disease 2019 after discharge. J Korean Med Sci. 2020;35(47):e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lu Y, Li X, Geng D, et al. Cerebral micro‐structural changes in COVID‐19 patients – an MRI‐based 3‐month follow‐up study. EClinicalMedicine. 2020;25:100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moreno‐Pérez O, Merino E, Leon‐Ramirez JM, et al. Post‐acute COVID‐19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect. 2021;S0163–4453(21):9‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nehme M, Braillard O, Alcoba G, et al. COVID‐19 symptoms: longitudinal evolution and persistence in outpatient settings. Ann Intern Med. 2020;174(5):723‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Otte MS, Eckel HNC, Poluschkin L, Klussmann JP, Luers JC. Olfactory dysfunction in patients after recovering from COVID‐19. Acta Otolaryngol. 2020;140(12):1032‐1035. [DOI] [PubMed] [Google Scholar]
- 38. Otte MS, Klussmann JP, Luers JC. Persisting olfactory dysfunction in patients after recovering from COVID‐19. J Infect. 2020;81(3):e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Petersen MS, Kristiansen MF, Hanusson KD, et al. Long COVID in the Faroe Islands: a longitudinal study among nonhospitalized patients. Clin Infect Dis. 2020;ciaa1792. 10.1093/cid/ciaa1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Puchner B, Sahanic S, Kirchmair R, et al. Beneficial effects of multi‐disciplinary rehabilitation in post‐acute COVID‐19 – an observational cohort study. Eur J Phys Rehabil Med. 2021;57(2):189‐198. [DOI] [PubMed] [Google Scholar]
- 41. Ramani C, Davis EM, Kim JS, Provencio JJ, Enfield KB, Kadl A. Post‐ICU COVID‐19 outcomes: a case series. Chest. 2021;159(1):215‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rosales‐Castillo A, de Los G, Ríos C, Mediavilla García JD. Persistent symptoms after acute COVID‐19 infection: importance of follow‐up. Persistencia de manifestaciones clínicas tras la infección COVID‐19: importancia del seguimiento. Med Clin (Barc). 2021;156(1):35‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roth NC, Kim A, Vitkovski T, et al. Post‐COVID‐19 Cholangiopathy: a novel entity. Am J Gastroenterol. 2021;116(5):1077–1082. [DOI] [PubMed] [Google Scholar]
- 44. Simani L, Ramezani M, Darazam IA, et al. Prevalence and correlates of chronic fatigue syndrome and post‐traumatic stress disorder after the outbreak of the COVID‐19. J Neurovirol. 2021;27(1):154‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Suárez‐Robles M, Iguaran‐Bermúdez MDR, García‐Klepizg JL, Lorenzo‐Villalba N, Méndez‐Bailón M. Ninety days post‐hospitalization evaluation of residual COVID‐19 symptoms through a phone call check list. Pan Afr Med J. 2020;37:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tarazona‐Fernández A, Rauch‐Sánchez E, Herrera‐Alanua O, Galán‐Rodas E. ¿Enfermedad prolongada o secuela pos‐COVID‐19? Prolonged disease or post‐COVID‐19 sequela? Acta Med Peru. 2020;37(4):565‐567. 10.35663/amp.2020.374.18669 [DOI] [Google Scholar]
- 47. van den Borst B, Peters JB, Brink M, et al. Comprehensive health assessment three months after recovery from acute COVID‐19. Clin Infect Dis. 2020. 10.1093/cid/ciaa1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xiong Q, Xu M, Li J, et al. Clinical sequelae of COVID‐19 survivors in Wuhan, China: a single‐centre longitudinal study. Clin Microbiol Infect. 2021;27(1):89‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhao YM, Shang YM, Song WB, et al. Follow‐up study of the pulmonary function and related physiological characteristics of COVID‐19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nalbandian A, Sehgal K, Gupta A, et al. Post‐acute COVID‐19 syndrome. Nat Med. 2021;27(4):601‐615. 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Table S4


