Abstract
Aims
Aim of this study is to report severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection, responsible for coronavirus disease 2019 (COVID‐19), as a possible cause for type 1 diabetes by providing an illustrative clinical case of a man aged 45 years presenting with antibody‐negative diabetic ketoacidosis post‐recovery from COVID‐19 pneumonia and to explore the potential for SARS‐CoV‐2 to adhere to human islet cells.
Methods
Explanted human islet cells from three independent solid organ donors were incubated with the SARS‐CoV‐2 spike protein receptor biding domain (RBD) fused to a green fluorescent protein (GFP) or a control‐GFP, with differential adherence established by flow cytometry.
Results
Flow cytometry revealed dose‐dependent specific binding of RBD‐GFP to islet cells when compared to control‐GFP.
Conclusions
Although a causal basis remains to be established, our case and in vitro data highlight a potential mechanism by which SARS‐CoV‐2 infection may result in antibody‐negative type 1 diabetes.
Keywords: COVID‐19, critical care, diabetic ketoacidosis, islets of Langerhans, pneumonia, SARS‐CoV‐2, type 1 diabetes
What's new.
There is evidence to suggest that severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) can affect insulin sensitivity and pancreatic β‐cell function.
Our clinical case of new‐onset type 1 diabetes after recovery from coronavirus disease 2019 and our in vitro data suggest SARS‐CoV‐2 may infect islet cells, followed by potentially virus mediated toxicity or immune autoreactivity against the virus within the pancreas.
Together, these findings support a causal link between SARS‐CoV‐2 and type 1 diabetes.
1. INTRODUCTION
Observations during the coronavirus disease 2019 (COVID‐19) pandemic, due to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection, suggest a rise in the number of cases of ketosis presenting type 1 diabetes. 1 , 2 Furthermore, SARS‐CoV‐2 appears to affect insulin sensitivity and β‐cell function as infected individuals presenting with diabetic ketoacidosis (DKA), both with and without an antecedent diagnosis of diabetes, have higher insulin requirements and more prolonged ketonemia compared with DKA precipitated by other causes. 3 We describe an illustrative case of acute‐onset type 1 diabetes post‐recovery from SARS‐CoV‐2 pneumonia.
2. CASE DESCRIPTION
A 45‐year‐old man of Somalian origin was admitted to hospital with severe hyperosmolar DKA 6 weeks post‐discharge from hospital with SARS‐CoV‐2 pneumonia.
His past medical history includes hypertension, chronic kidney disease (unclear aetiology; creatinine 180 μmol/L and estimated glomerular filtration rate 39 ml/min in 2017) and gout. His medications included irbesartan and colchicine. There was no family history of diabetes. A fasting blood glucose level in July 2017 was 5.4 mmol/L.
He was admitted (8/July/2020–20/July/2020) with SARS‐CoV‐2 pneumonia. His body mass index at the time was 30.3 kg/m2 (height 1.79 m, weight 97.6 kg). His nonfasting blood glucose level at presentation was 9.4 mmol/L. Dexamethasone was administered between Day 4 and Day 10 (6 mg/day) for mild hypoxemia, resulting in glucocorticoid‐induced hyperglycemia without ketosis. This was treated with intermittent doses of subcutaneous insulin aspart without basal insulin and gliclazide. The final dose of gliclazide (40mg) was administered 48 h prior to discharge. Insulin aspart (eight units) was given at 13:10 on the day before discharge. On the day of discharge, a fasting blood glucose level was 7.6 mmol/L at 09:15. Laboratory results predischarge are in Table 1. An HbA1c level was not measured during or after this hospital admission.
TABLE 1.
Laboratory investigation results prior to discharge during the first admission with COVID‐19 pneumonia
| Investigation | Result | Reference range |
|---|---|---|
| Sodium (mmol/L) | 130 | 135–45 |
| Chloride (mmol/L) | 94 | 95–110 |
| Potassium (mmol/L) | 4.8 | 3.3–4.9 |
| Creatinine (µmol/L) | 188 | 60–110 |
| Urea (mmol/L) | 14.5 | 2.3–7.6 |
| Estimated glomerular filtration rate (ml/min) | 36 | >60 |
| C‐reactive protein (mg/L) | 5 | 0–10 |
| Haemoglobin (g/L) | 140 | 130–180 |
Five weeks post‐discharge the individual developed symptoms of polydipsia and polyuria. One week later he was found at home unconscious requiring readmission to hospital (31/Aug/2020–8/Sept/2020). At presentation to the Emergency Department his temperature was 31.0℃; heart rate 84 bpm (sinus rhythm); blood pressure 84/33 mmHg; respiratory rate 27 min−1 (oxygen saturation 100% on 5 L via nasal prongs). Laboratory results confirmed hyperosmolar DKA with a pH of 6.86; bicarbonate of 5 mmol/L; blood glucose of 77.3 mmol/L; β‐hydroxybutyrate of 14.2 mmol/L. Serum lipase was 1776 U/L (reference range 0–60) on admission without clinical or radiological evidence of acute pancreatitis on computed tomography. By Day 2, the serum lipase level was 882 U/L. Further investigations results are detailed in Tables 2 and 3.
TABLE 2.
Laboratory investigations performed during the admission with hyperosmolar diabetic ketoacidosis
| Investigation | Result | Reference range |
|---|---|---|
| Day 0 | ||
| Venous glucose (mmol/L) | 77.3 | 4.0–7.8 |
| Arterial blood gas | ||
| pH | 6.86 | 7.35–7.45 |
| Bicarbonate (mmol/L) | 5 | 22–28 |
| pO2 (mmHg) | 259 (34.5 kPa) | 83–108 |
| pCO2 (mmHg) | 20 (2.7 kPa) | 35–45 |
| Lactate (mmol/L) | 3.5 | <2.2 |
| Venous ketones (mmol/L) | 14.2 | ≤0.5 |
| Sodium (mmol/L) | 117 | 135–45 |
| Chloride (mmol/L) | 73 | 95–110 |
| Potassium (mmol/L) | 6.7 | 3.3–4.9 |
| Creatinine (µmol/L) | 394 | 60–110 |
| C‐reactive protein (mg/L) | 27 | 0–10 |
| Lipase (U/L) | 1776 | 0–60 |
| Haemoglobin (g/L) | 124 | 130–180 |
| Day 1 | ||
| Cholesterol (mmol/L) a | 5.6 | 0.0–5.6 |
| Triglycerides (mmol/L) a | 3.2 | <2.0 |
| Day 4 | ||
| Random serum Insulin (pmol/L) b | 187.5 |
Fasting: 20.8–173.6 Post 75 g oral glucose load: 30 min: 208–1597.2 60 min: 125–1916.7 120 min: 111–1152.8 |
| Day 5 | ||
| Random serum C‐Peptide (nmol/L) c | 0.60 | 0.30–2.30 |
Checked approximately 14 h after the insulin infusion was started.
Checked whilst on an insulin infusion.
Checked whilst on an insulin infusion, blood glucose level 23.1 mmol/L.
TABLE 3.
Radiological investigations performed on the Day 1 of the admission to hospital with hyperosmolar diabetic ketoacidosis
| Imaging modality | Findings |
|---|---|
| Computed tomography (Brain) | Normal |
| Computed tomography (chest, abdomen, pelvis) |
Lung:
Nodular surface of both kidneys Normal pancreas |
| Abdominal ultrasound |
Bilateral scarred kidneys Other organs normal |
Intravenous hydration and insulin were administered, and he was transferred to the intensive care unit where he required a period of vasopressor support. The individual's conscious state and metabolic parameters improved over the first 24 h, and he was transferred to the general ward on Day 3.
The HbA1c was 154 mmol/mol (16.2%). A nonfasting C‐peptide level on Day 5 was 0.6 nmol/L (reference range 0.3–2.30) with a capillary blood glucose level of 23.1 mmol/L. Islet cell antibodies (glutamic acid decarboxylase [anti‐GAD65], tyrosine phosphatase [anti‐IA2] and zinc transporter 8 [anti‐ZnT8]) were not detected.
He commenced subcutaneous insulin and was receiving insulin aspart 14 units thrice daily with insulin glargine 25 units at night on discharge.
3. RESEARCH DESIGN AND METHODS
3.1. Human islet isolation
Human pancreata were obtained, with informed consent from next of kin, from three heart‐beating, brain‐dead donors, following research approval from the Human Research Ethics Committee at St. Vincent's Hospital Melbourne.
Human islets were isolated by intraductal perfusion and digestion of the pancreas with collagenase followed by purification using ficoll density gradients. 4 Islets were cultured in Connaught Medical Research Laboratories (CMRL) 1066 medium (Invitrogen) supplemented with 4% human serum albumin, 100 U/ml penicillin, 100 mg/ml streptomycin and 2 mM L‐glutamine (complete CMRL), in a 37°C, 5% CO2 humidified incubator.
3.2. Spike protein receptor binding domain‐GFP fusion
Residues 319–541 encoding the receptor biding domain (RBD) of the spike glycoprotein of SARS‐CoV‐2 (Jomar Life Research), a short linker (Gly‐Ser), super‐folder green fluorescent protein (GFP) and a C‐terminal deca‐His tag were subcloned into pCold‐IV (Takara). As a control, a plasmid containing only GFP and a C‐terminal deca‐His tag was also generated. After DNA sequencing, these plasmids were transformed into Escherichia coli C41 (DE3) cells, and one‐litre culture of 2YT grown at 37°C to OD600nm of ~0.8. Protein expression was induced by shifting cultures to 15°C and the addition of 0.5 mM isopropyl β‐D‐1‐thiogalactopyranoside. RBD‐GFP and GFP were purified using nickel affinity chromatography. Affinity pure RBD‐GFP and GFP were validated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis gels and confirmed by Western Blot.
3.3. Flow cytometry
Human islets were dispersed with accutase then rested in complete CMRL at 37℃ for 2 h. Cells (approximately 750 islet equivalents/tube) were stained at room temperature for 45 min with control‐GFP or RBD‐GFP serially diluted from 1:100 to 1:2000 in FACS buffer (PBS + 2% FBS). Propidium iodide (1 µg/ml) was added to exclude dead cells. Cells were analyzed on a BD Fortessa (Becton Dickinson) and data analyzed using FlowJo version 10 (TreeStar).
3.4. Statistics
Statistical analysis was done using GraphPad Prism version 9 (GraphPad). Multiple unpaired t tests were performed for n = 3, where each replicate is an independent islet donor. A p < 0.05 was considered significant.
4. RESULTS
The RBD of SARS‐CoV‐2 bound to cells within human islets, with high‐affinity adsorption compared to equimolar control‐GFP, which showed no binding similar to unstained islets (Figure 1a). The specificity of RBD binding is further illustrated by the dose‐dependent binding of the RBD‐GFP when compared to control‐GFP (Figure 1b).
FIGURE 1.
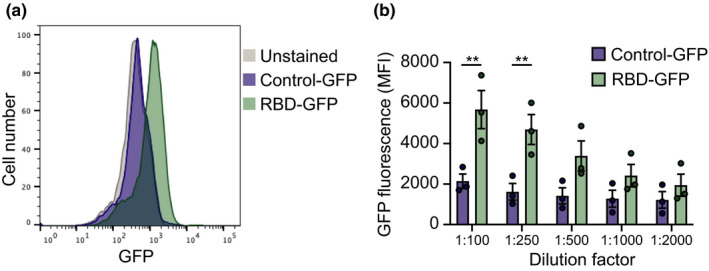
Flow cytometry of severe acute respiratory syndrome coronavirus 2 receptor binding domain binding to human islet cells. (a) Representative histogram plots of unstained, control‐green fluorescent protein (GFP) and receptor binding domain (RBD)‐GFP, both diluted 1:2000. (b) Mean fluorescence intensity (MFI) of GFP fluorescence of control‐GFP or RBD‐GFP binding to human islet cells at dilutions from 1:100 to 1:2000. Data show mean + SEM for n = 3 independent human islets donors. Individual data points for each donor are shown. ** p < 0.005, unpaired t test
5. CONCLUSIONS
While we are unable to definitively exclude antecedent type 2 diabetes in our individual prior to his admission with COVID‐19 pneumonia, the chronology suggests acute‐onset antibody negative type 1 diabetes associated with a recent SARS‐CoV‐2 infection. In addition, people with type 1 diabetes of African descent appear to have a lower prevalence of islet cell autoantibodies compared to data from European populations, although the data from regions across Africa vary. 5 In data from Tanzania, only 42.6% of individuals with type 1 diabetes had detectable levels of anti‐GAD65 and/or anti‐IA2. 6 A recent study of individuals from Ethiopia with newly diagnosed type 1 diabetes, revealed that only 60% had any detectable autoantibody (anti‐GAD‐65, anti‐IA2, anti‐ZnT8). 5
The clinical presentation and imaging did not support a diagnosis of acute inflammatory pancreatitis and elevated serum lipase levels have been previously described in severe DKA in the absence of pancreatitis. 7 Furthermore, our patient had an acute kidney injury on a background of underlying chronic kidney disease, both of which could have independently contributed to the elevated serum lipase level. 8
Our case has some features consistent with fulminant type 1 diabetes, which has been associated with viral infections. 9 In contrast with our case however, fulminant diabetes is characterized by normal to minimally elevated HbA1c. 9
There are other reports of new onset type 1 diabetes post‐recovery from SARS‐CoV‐2 infection, but with comparatively less severe metabolic derangement than in our case study. 10 , 11
The link between viral infections and the onset of type 1 diabetes has been previously studied. The TEDDY study revealed a temporal link between respiratory infections and islet cell autoantibody seroconversion in children at risk of developing type 1 diabetes. 12 Chronic enterovirus infection has been detected in human islets after type 1 diabetes diagnosis 13 and coxsackie viruses have also been shown to directly infect human pancreatic islets. 14
Angiotensin‐converting enzyme 2, the receptor for SARS‐CoV‐2, is expressed on β‐cells, 15 microvasculature pericytes and some ductal cells within the pancreas. 16 Our results indicate that SARS‐CoV‐2 can adhere to human islet cells. This is consistent with data indicating that primary human islet cells infected with SARS‐CoV‐2 stain positively for the viral spike protein on immunohistochemistry. 15 In a more recent paper, Müller et al. have demonstrated in vitro data revealing that SARS‐CoV‐2 can replicate in human pancreatic islets. They have further demonstrated that the pancreata of individuals who died from COVID‐19 infection positively stain for the SARS‐CoV‐2 nucleocapsid protein. 17 While a more detailed analysis is required to conclude whether the RBD binds to beta cells or other cells within the islet, collectively these results point to a mechanism by which SARS‐CoV‐2 may infect islet cells, followed by potentially virus mediated toxicity or immune autoreactivity against the virus within the pancreas.
Future directions include the collection of registry data (e.g. COVIDIAB Registry https://covidiab.e‐dendrite.com/) to further elucidate the temporal relationship between SARS‐CoV‐2 infection and the onset of type 1 diabetes.
In conclusion, we provide circumstantial clinical and in vitro evidence suggesting a causal link between type 1 diabetes and SARS‐CoV‐2 infection. Clinicians should remain mindful of this potential link when monitoring people both during and after SARS‐CoV‐2 infection for the onset of diabetes and ketosis.
CONFLICTS OF INTEREST
None
ACKNOWLEDGEMENTS
We thank the individual who provided consent for the publication of his clinical case. We thank all organ donors and their families, DonateLife and the staff of St. Vincent's Institute involved in the human islet isolation program. We thank Cameron Kos, Vincent Murphy and Andrew Deans (St. Vincent's Institute, VIC, Australia) for their technical advice, and Glenn Ward (University of Melbourne and St. Vincent's Hospital Melbourne, VIC, Australia) for advising on the case.
Venkatesh N, Astbury N, Thomas MC, et al. Severe acute respiratory syndrome coronavirus 2 as a potential cause of type 1 diabetes facilitated by spike protein receptor binding domain attachment to human islet cells: An illustrative case study and experimental data. Diabet Med. 2021;38:e14608. 10.1111/dme.14608
Helen Thomas and David N. O'Neal have equal senior authorship.
Funding information
This work was funded by the National Health and Medical Research Council of Australia (GNT1126237 and GNT1150425). The St. Vincent's Institute receives support from the Operational Infrastructure Support Scheme of the Government of Victoria, Australia.
REFERENCES
- 1. Unsworth R, Wallace S, Oliver NS, et al. New‐onset type 1 diabetes in children during COVID‐19: multicenter regional findings in the U.K. Diabetes Care. 2020;43(11):e170‐e171. 10.2337/dc20-1551 [DOI] [PubMed] [Google Scholar]
- 2. Kamrath C, Mönkemöller K, Biester T, et al. Ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes during the COVID‐19 pandemic in Germany. JAMA. 2020;324(8):801‐804. 10.1001/jama.2020.13445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Armeni E, Aziz U, Qamar S, et al. Protracted ketonaemia in hyperglycaemic emergencies in COVID‐19: a retrospective case series. Lancet Diabetes Endocrinol. 2020;8(8):660‐663. 10.1016/S2213-8587(20)30221-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barbaro B, Salehi P, Wang Y, et al. Improved human pancreatic islet purification with the refined UIC‐UB density gradient. Transplantation. 2007;84(9):1200‐1203. 10.1097/01.tp.0000287127.00377.6f [DOI] [PubMed] [Google Scholar]
- 5. Balcha SA, Demisse AG, Mishra R, et al. Type 1 diabetes in Africa: an immunogenetic study in the Amhara of North‐West Ethiopia. Diabetologia. 2020;63(10):2158‐2168. 10.1007/s00125-020-05229-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lutale JJK, Thordarson H, Holm PI, Eide GE, Vetvik K. Islet cell autoantibodies in African patients with Type 1 and Type 2 diabetes in Dar es Salaam Tanzania: a cross sectional study. J Autoimmune Dis. 2007;4(1):4. 10.1186/1740-2557-4-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yadav D, Nair S, Norkus EP, Pitchumoni CS. Nonspecific hyperamylasemia and hyperlipasemia in diabetic ketoacidosis: incidence and correlation with biochemical abnormalities. Am J Gastroenterol. 2000;95(11):3123‐3128. 10.1111/j.1572-0241.2000.03279.x [DOI] [PubMed] [Google Scholar]
- 8. Hameed AM, Lam VW, Pleass HC. Significant elevations of serum lipase not caused by pancreatitis: a systematic review. HPB (Oxford). 2015;17(2):99‐112. 10.1111/hpb.12277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Imagawa A, Hanafusa T, Uchigata Y, et al. Fulminant type 1 diabetes. Diabetes Care. 2003;26(8):2345. 10.2337/diacare.26.8.2345 [DOI] [PubMed] [Google Scholar]
- 10. Marchand L, Pecquet M, Luyton C. Type 1 diabetes onset triggered by COVID‐19. Acta Diabetol. 2020;57(10):1265‐1266. 10.1007/s00592-020-01570-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hollstein T, Schulte DM, Schulz J, et al. Autoantibody‐negative insulin‐dependent diabetes mellitus after SARS‐CoV‐2 infection: a case report. Nat Metab. 2020;2(10):1021‐1024. 10.1038/s42255-020-00281-8 [DOI] [PubMed] [Google Scholar]
- 12. Lönnrot M, Lynch KF, Elding Larsson H, et al. Respiratory infections are temporally associated with initiation of type 1 diabetes autoimmunity: the TEDDY study. Diabetologia. 2017;60(10):1931‐1940. 10.1007/s00125-017-4365-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krogvold L, Edwin B, Buanes T, et al. Detection of a low‐grade enteroviral infection in the islets of Langerhans of living patients newly diagnosed with type 1 diabetes. Diabetes. 2015;64(5):1682‐1687. 10.2337/db14-1370 [DOI] [PubMed] [Google Scholar]
- 14. Dotta F, Censini S, van Halteren AGS , et al. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent‐onset type 1 diabetic patients. Proc Natl Acad Sci USA. 2007;104(12):5115‐5120. 10.1073/pnas.0700442104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang L, Han Y, Nilsson‐Payant BE, et al. A human pluripotent stem cell‐based platform to study SARS‐CoV‐2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020;27(1):125‐136.e7. 10.1016/j.stem.2020.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fignani D, Licata G, Brusco N, et al. SARS‐CoV‐2 receptor Angiotensin I‐Converting Enzyme Type 2 (ACE2) is expressed in human pancreatic β‐cells and in the human pancreas microvasculature. Front Endocrinol (Lausanne). 2020;11(876). 10.3389/fendo.2020.596898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Müller JA, Groß R, Conzelmann C, et al. SARS‐CoV‐2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. 2021;3(2):149‐165. 10.1038/s42255-021-00347-1 [DOI] [PubMed] [Google Scholar]


