Abstract
Background and purpose
Studies have shown that some cytokines in COVID‐19 patients were elevated. This study aims to assess whether IL‐10, IL‐1β, IL‐6, MCP‐1, TNF‐α, IP‐10 and IL‐4 serve as potential diagnostic biomarkers of COVID‐19.
Methods
The above serum cytokines in COVID‐19 patients and non‐COVID‐19 patients were detected by ELISA and SARS‐CoV‐2 IgM and IgG were detected by the chemiluminescence method. The independent‐sample Mann‐Whitney U test was utilised to compare cytokine levels in different groups and courses, the Levene T‐test and T’‐test were utilised to compare they in different genders and the Spearman correlation test was utilised to analyse the correlation between the cytokine levels with ages and SARS‐CoV‐2 IgG and IgM.
Results
Serum levels of IL‐10, IL‐1β, MCP‐1, TNF‐α and IL‐4 in COVID‐19 patients were significantly higher than those in non‐COVID‐19 patients, while IL‐6 were only significantly higher than in healthy people, IP‐10 were significantly lower than in other diseases patients. AUCs of COVID‐19 diagnosed by IL‐10, IL‐1β, IL‐6, MCP‐1, TNF‐α, IP‐10 and IL‐4 were 0.735, 0.775, 0.595, 0.821, 0.848, 0.38 and 0.682, respectively. In the COVID‐19 patients’ serum, the levels of IL‐10 and MCP‐1 of male were noticeably higher than those of female, and all cytokines were significantly positively correlated with age, IL‐1β and IL‐4 were significantly negatively correlated with SARS‐CoV‐2 IgM, while IL‐10, IL‐1β, IL‐6, TNF‐ and IP‐10 were significantly negatively correlated with SARS‐CoV‐2 IgG. IL‐10 on 43‐56 days was significantly lower than at 29‐42 days, TNF‐α at 15‐42 days was significantly higher than at 0‐14 days, IP‐10 at 0‐14 days was the highest and IL‐4 at 29‐42 days was significantly higher than at 0‐14 days.
Conclusions
The detection of IL‐10, IL‐1 β, IL‐6, MCP‐1, TNF‐α and IL‐4 would assist the clinical study of COVID‐19, and IP‐10 may be the cytokine of early elevation in COVID‐19 patients.
What's known
Symptoms of COVID‐19 patients, infection pathways of SARS‐CoV‐2 and the COVID‐19 patients have higher cytokines.
What's new
What kinds of cytokines in COVID‐19 patients were increased, whether they were potential diagnostic biomarkers of COVID‐19 and offer prognostic insight upon initial presentation to help guide treatment, their relationship with age, gender, antibody concentration and course of the disease were also discussed.
1. INTRODUCTION
The novel coronavirus (SARS‐CoV‐2) has high infectivity, 1 , 2 the main methods for diagnosing it were nucleic acid detection and serological antibody detection. 3 Inflammatory factors are often increased in severe and critical patients, 4 including interleukin (IL), colony‐stimulating factor (CSF), chemokine, interferon (IFN), tumour necrosis factor (TNF), chemokine and growth factor (GF). 5 Most of cytokines are produced by T lymphocytes, fibroblasts and mononuclear macrophages, and can in turn act on these cells, and these cytokines could promote each other and jointly mediate inflammation; however, IL‐10 can inhibit the inflammatory process. The early symptoms of coronavirus disease 19 (COVID‐19) patients were fever, dry cough and fatigue, 6 but severe patients may develop even multiple organ failure, 4 which may be related to the levels of cytokines. 7 , 8 , 9 The production of cytokines is related to the individual immune function, so we assumed that the severity of COVID‐19 can be predicted according to their levels. Our study aims to reveal the changes of serum levels in IL‐10, IL‐1β, IL‐6, monocyte chemoattractant protein (MCP)‐1, TNF‐α, interferon‐inducible protein (IP)‐10 and IL‐4 in COVID‐19 patients and assist clinical treatment of COVID‐19.
2. MATERIALS AND METHODS
2.1. Specimens
77 serum samples from male patients and 43 serum samples from female patients were collected from 48 COVID‐19 patients in Loudi Center for Disease Control and Prevention, some of whom were repeatedly sampled two to four times, and their ages ranged from 8 to 78 years old. The demographic and characteristics of the study participants have been showed in Table 1. Throat swab samples of these patients, including 2 dead patients and 17 asymptomatic infected patients, were detected by real‐time PCR (test kits were purchased from Hunan Shengxiang Biotechnology Company), and the positive results of SARs‐CoV‐2 nucleic acid were confirmed. The diagnosis and treatment of COVID‐19 were according COVID‐19 diagnosis and treatment guideline (Seventh Edition). 4 Brief description is as follows: with epidemiological history and in line with the relevant clinical manifestations, as well as with new coronavirus aetiology or serological evidence can be diagnosed; most of COVID‐19 patients only received general treatment, including bed rest, timely effective oxygen therapy and antiviral treatment, severe and critical patients generally need to be transferred to ICU for treatment including above treatment and timely organ function support treatment. The patients in our study received antiviral drugs such as interferon alpha, ritonavir, ribavirin and some traditional Chinese medicine, such as Qingfei Paidu decoction.
TABLE 1.
The demographic and characteristics of the study participants
| Project | COVID‐19 patients | Healthy people | Other diseases patients |
|---|---|---|---|
| Gender | Male 77, female 43 | Male 14, female 21 | Male 25, female 28 |
| Age | 8‐78 | 778 | 28‐96 |
| IgM | 0.20‐115.59 | / | / |
| IgG | 1.39‐297.97 | / | / |
| IL‐10 | 0.01‐115.62 | 0.01‐2.52 | 0.37‐8.87 |
| IL‐1β | 0.02‐18.64 | 0.02‐3.29 | 0.02‐36.95 |
| IL‐6 | 0.03‐305.97 | 0.03‐1.33 | 0.03‐195.71 |
| MCP‐1 | 0.03‐1334.00 | 0.03‐73.18 | 0.03‐255.97 |
| TNF‐α | 0.02‐142.71 | 0.02‐40.69 | 0.02‐99.72 |
| IP‐10 | 0.01‐1267.20 | 12.61‐258.84 | 10.70‐650.32 |
| IL‐4 | 0.01‐240.72 | 0.03‐0.07 | 0.00‐3.63 |
In addition, we also collected the serum of non‐COVID‐19 patients from The First Hospital of Hunan University of Chinese medicine. Among these patients, 53 patients suffered from the malignant tumour, haematological disease, rheumatic immune system disease and other diseases that increase the level of inflammatory factors, and 35 healthy people. All patients and their families had informed consent to the inclusion of the study and authorised to use their test results for the study.
2.2. Methods
2.2.1. Cytokine detection
The 120 serum contents of IL‐10, IL‐1β, IL‐6, MCP‐1, TNF‐α, IP‐10 and IL‐4 were detected by ELISA reader at 450 nm, the test kits and their standard were purchased from Beijing Human Diagnostics Company (double‐antibody sandwich ELISA), TECAN 200‐8 were purchased from Shanghai TECAN Trading Company. Sensitivity: The minimal detectable concentrations for they were 0.0225, 0.0355, 0.0600, 0.0655, 0.0386, 0.0102 and 0.0180 pg/mL; and specificity: when 50 ng/mL (100 ng/mL for IL‐4) was used for specificity test, they did not react with common interfering cytokines and proteins. For statistical analysis: count the results which lower than the minimum detectable concentrations as half of the minimum detectable concentrations. A hole was added as a blank control. The samples whose OD value exceeds the linear range should be diluted before detection. The experiment was conducted in strict accordance with the instructions in the kit. All the reagents had their own standard. Both the repeatability (coefficient of variation between plates and within plates were less than 10%) and specificity were good. The kit has fixed value quality control and negative and positive control to ensure the accuracy of test results.
2.2.2. SAS‐CoV‐2 IgM and IgG antibody detection
The serum samples of all cases were venous blood of 12 hours fasting without haemolysis or hyperlipidaemia. The serum was obtained by centrifugation at 4000 RPM for 10 minutes, the contents of SARS‐CoV‐2 IgG and IgM in these serums were detected by magnetic particle chemiluminescence method, the SAS‐CoV‐2 IgM and IgG test kits were purchased from Shenzhen Yahuilong Biotechnology Company, and 10.00 AU/mL was taken as the positive judgment value, patients with results of 8‐10 AU/mL were retested 3‐5 days later. Sensitivity and specificity were 98.5% and 100% when the cut‐off value was 10.06 AU/mL; the HOOK effect would not appear when the antibody concentration was lower than 8000 AU/mL. The experiment was carried out in strict accordance with the instructions in the kits. All the kits were provided with calibration information and quality control materials, and the repeatability (intra assay coefficient of variation was no more than 8%, and the interassay coefficient of variation was no more than 15%) and specificity was good.
2.2.3. Statistical analysis
All data were processed by IBM SPSS statistic 21 and were drawn by GraphPad Prism 7. According to the characteristics of data distribution, independent‐samples Mann‐Whitney U‐test was utilised to compare the serum levels of IL‐10, IL‐1 β, IL‐6, MCP‐1, TNF‐α, IP‐10 and IL‐4 in different groups (COVID‐19 patients, other diseases patients and healthy people) and course; Independent‐samples Levene T test or T’ test was utilised to compare cytokine levels of COVID‐19 patients in different genders; Spearman Correlation test was utilised to analyse the correlation between the levels of cytokines with ages, SARS‐CoV‐2 IgG and IgM. The difference was statistically significant with bilateral P‐value < .05.
3. RESULTS
3.1. Detection of IL‐10, IL‐1β, IL‐6, MCP‐1, TNF‐α, IP‐10 and IL‐4 in serum samples of COVID‐19 patients, other diseases patients, and healthy people by ELISA
After statistical analysis, as Figure 1 and Table 2 shows, it was found that the levels of IL‐10, IL‐1β, IL‐6, MCP‐1, TNF‐α and IL‐4 in the serum of COVID‐19 patients were significantly higher than that in the serum of healthy people (P IL‐10 = 0.000, P IL‐1β = 0.000, P IL‐6 = 0.000, P MCP‐1 = 0.000, P TNF‐α = 0.000 and P IL‐4 = 0.002, respectively), while the serum levels of IP‐10 between COVID‐19 patients and healthy people were not significantly different (P = .310); the serum levels of IL‐10, IL‐1β, MCP‐1, TNF‐α and IL‐4 in COVID‐19 patients were significantly higher than those in other diseases patients (PIL‐10 = 0.000, PIL‐1β = 0.000, PMCP‐1 = 0.000, PTNF‐α = 0.000 and PIL‐4 = 0.000, respectively), IP‐10 in COVID‐19 patients was significantly lower than that in other diseases patients (P = .004), while the serum levels of IL‐6 between COVID‐19 patients and other diseases patients were not significantly different (P = .078). In addition, IL‐10, IL‐6, MCP‐1 and IL‐4 in other diseases patients were significantly higher than those in healthy people (PIL‐10 = 0.000, PIL‐6 = 0.000, PMCP‐1 = 0.023 and PIL‐4 = 0.002, respectively), while the serum levels of IL‐1β, TNF‐α and IP‐10 between COVID‐19 patients and other diseases patients were not significantly different (PIL‐1β = 0.453, PTNF‐α = 0.147 and PIP‐10 = 0.073, respectively).
FIGURE 1.
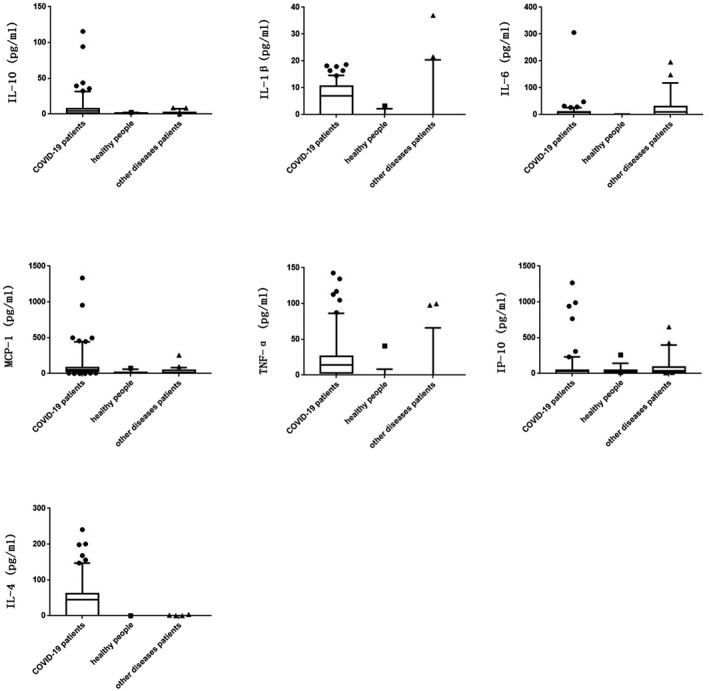
Serum levels of IL‐10, IL‐1β, IL‐6, MCP‐1, TNF‐α, IP‐10 and IL‐4 in COVID‐19 patients and non COVID‐19 patients by ELISA
TABLE 2.
Serum levels of IL‐10, IL‐1β, IL‐6, MCP‐1, TNF‐α, IP‐10 and IL‐4 in COVID‐19 patients and non‐COVID‐19 patients by ELISA
| Median (IQR) (pg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group | n | IL‐10 | IL‐1β | IL‐6 | MCP‐1 | TNF‐α | IP‐10 | IL‐4 |
| A | 120 | 4.17 (6.85) | 7.22 (10.39) | 7.56 (10.89) | 49.69 (55.19) | 14.21 (23.80) | 29.37 (44.70) | 44.43 (61.45) |
| B | 35 | 0.65 (1.10) | 0.02 (0.00) | 0.03 (0.00) | 8.83 (16.94) | 0.02 (0.00) | 35.32 (20.32) | 0.05 (0.01) |
| C | 53 | 1.28 (1.42) | 0.02 (0.00) | 9.12 (30.08) | 16.22 (37.68) | 0.02 (0.00) | 37.66 (54.44) | 0.06 (0.02) |
| PAB | / | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.310 | 0.002 |
| PAC | / | 0.000 | 0.000 | 0.078 | 0.000 | 0.000 | 0.004 | 0.000 |
| PBC | / | 0.000 | 0.453 | 0.000 | 0.023 | 0.147 | 0.073 | 0.002 |
A means COVID‐19 patients, B means healthy people and C means patients with other diseases.
3.2. Diagnostic efficacy of detection of the IL‐10, IL‐1β, IL‐6, MCP‐1, TNF‐α, IP‐10 and IL‐4 on COVID‐19 by ELISA
The abilities of prediction of IL‐10, IL‐1β, IL‐6, MCP‐1, TNF‐α, IP‐10 and IL‐4 on COVID‐19 were assessed by ROC curve, as Figure 2 shows, the COVID‐19 patients were positive, and the other diseases patients and healthy people were negative, and select the tangent point with the largest Youden's index as the cut‐off value. When choose 2.621, 4.898, 1.730, 19.948, 0.110, 7.083 and 4.015 pg/mL respectively as their cut‐off value, AUCs of COVID‐19 diagnosed by IL‐10, IL‐1β, IL‐6, MCP‐1, TNF‐α, IP‐10 and IL‐4 were 0.735, 0.775, 0.595, 0.821, 0.848, 0.387 and 0.682, respectively, which means that IL‐10, IL‐1β, IL‐6, MCP‐1, TNF‐α and IL‐4 have potential diagnostic value for COVID‐19, and TNF‐α and MCP‐1 have the best predictive effect. The sensitivities of diagnosis of TNF‐α, MCP‐1, IL‐1β, IL‐10, IL‐4 and IL‐6 were 81.2%, 84.6%, 63.2%, 65.8%, 67.5% and 72.6%, and the specificities of they were 93.0%, 69.8%, 95.3%, 89.5%, 100% and 64.0%; the sensitivity (parallel experiment) and specificity (series experiment) of combined diagnosis of TNF‐α and MCP‐1 were 97.1% and 97.9%, respectively.
FIGURE 2.
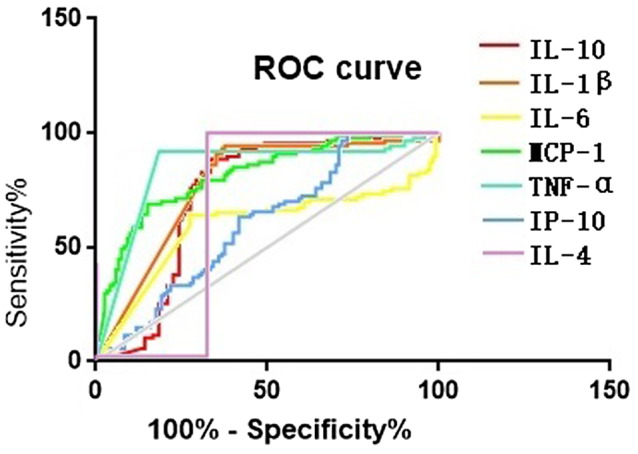
The ability of prediction of IL‐10, IL‐1β, IL‐6, MCP‐1, TNF‐α, IP‐10 and IL‐4 on COVID‐19 by ELISA
3.3. The relationship between serum levels of IL‐10, IL‐1β, IL‐6, MCP‐1, TNF‐α, IP‐10 and IL‐4 with gender and age in COVID‐19 patients
According to the statistical analysis, as Table 3 shows, it was found that the serum levels of IL‐10 and MCP‐1 in male COVID‐19 patients were markedly higher than those in female patients (PIL‐10 = 0.038 and PMCP‐1 = 0.031, respectively), while the differences of serum levels of IL‐1β, IL‐6, IP‐10, TNF‐α and IL‐4 between male COVID‐19 patients and female patients were not markedly significant (PIL‐1β = 0.611, PIL‐6 = 0.354, PTNF‐α = 0.152, PIP‐10 = 0.208 and PIL‐4 = 0.225, respectively).
TABLE 3.
Serum levels of IL‐10, IL‐1β, IL‐6, MCP‐1, TNF‐α, IP‐10 and IL‐4 of COVID‐19 patients in different genders
| ± SE (pg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group | n | IL‐10 | IL‐1β | IL‐6 | MCP‐1 | TNF‐α | IP‐10 | IL‐4 |
| Male | 77 | 9.15 ± 2.06 | 6.13 ± 0.61 | 12.60 ± 4.14 | 126.42 ± 24.06 | 22.11 ± 3.53 | 89.83 ± 23.87 | 47.34 ± 5.91 |
| Female | 43 | 4.63 ± 0.61 | 6.65 ± 0.81 | 7.48 ± 1.12 | 67.87 ± 11.88 | 16.17 ± 2.13 | 46.06 ± 17.65 | 38.41 ± 4.33 |
| P | / | .038 | .611 | .354 | .031 | .152 | .208 | .225 |
Ages of the owners of 7 serum samples were not clearly, the relationship between levels of cytokines and age in the other 113 serum samples of COVID‐19 patients were show in Figure 3. According to the statistical analysis, the levels of all cytokines in COVID‐19 patients were significantly positively correlated with their ages (rIL‐10 = 0.403, PIL‐10 = 0.000; rIL‐1β = 0.200, PIL‐1β = 0.034; rIL‐6 = 0.320, PIL‐6 = 0.001; rMCP‐1 = 0.431, PMCP‐1 = 0.000; rTNF‐α = 0.246, PTNF‐α = 0.009; rIP‐10 = 0.397, PIP‐10 = 0.000; and rIL‐4 = 0.283, PIL‐4 = 0.002, respectively).
FIGURE 3.
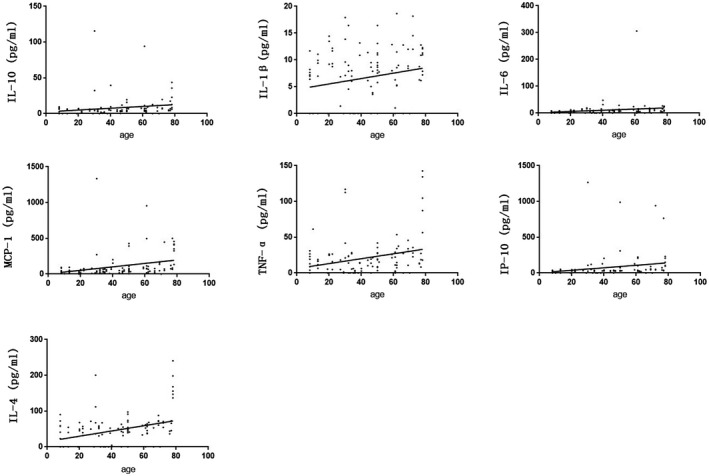
Correlation analysis of serum levels of IL‐10, IL‐1β, IL‐6, MCP‐1, TNF‐α, IP‐10 in COVID‐19 patients and their ages
3.4. The relationship between serum levels of IL‐10, IL‐1β, IL‐6, MCP‐1, TNF‐α, IP‐10 and IL‐4 and SAS‐CoV‐2 IgM and IgG concentrations in COVID‐19 patients
The correlation analysis of levels of IL‐10, IL‐1β, IL‐6, MCP‐1, TNF‐α, IP‐10, IL‐4 and antibody concentration in serum samples of COVID‐19 patients were shown in Figure 4. It was found that the levels of IL‐1β and IL‐4 in COVID‐19 patients were negatively correlated with the level of SARS‐CoV‐2 IgM (rIL‐1β = −0.206, PIL‐1β = 0.024; and rIL‐4 = −0.208, PIL‐4 = 0.023, respectively), while there were no significant correlation between the levels of IL‐10, IL‐6, MCP‐1, TNF‐α and IP‐10 and the concentrations of SARS‐CoV‐2 IgM (PIL‐10 = 0.416, PIL‐6 = 0.056, PMCP‐1 = 0.675, PTNF‐α = 0.301 and PIP‐10 = 0.622, respectively); the levels of IL‐10, IL‐1β, IL‐6, TNF‐α and IP‐10 were negatively correlated with the level of SARS‐CoV‐2 IgG (rIL‐10 = −0.222, PIL‐10 = 0.015; rIL‐1β = −0.212, PIL‐1β = 0.020; rIL‐6 = −0.372, PIL‐6 = 0.000; rTNF‐α = −0.185, PTNF‐α = 0.043; and rIP‐10 = −0.273, PIP‐10 = 0.003, respectively), but there were no significant correlation between the levels of MCP‐1 and IL‐4 and the concentrations of SARS‐CoV‐2 IgG (PMCP‐1 = 0.082 and PIL‐4 = 0.067, respectively).
FIGURE 4.
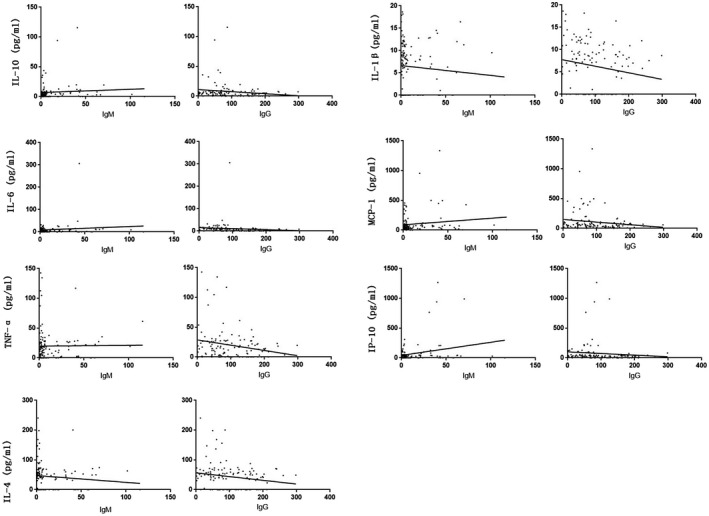
Correlation analysis of serum levels of IL‐10, IL‐1β, IL‐6, MCP‐1, TNF‐α, IP‐10 and SARS‐CoV‐2 IgM and IgG antibody in COVID‐19 patients
3.5. Changes in serum IL‐10, IL‐1β, IL‐6, MCP‐1, TNF‐α, IP‐10 and IL‐4 in COVID‐19 patients
47 sera are from patients who eventually died, or asymptomatic infected patients, 3 sera are from whose onset time are unknown, and the other 70 sera were divided into five groups according to the acquisition time from onset: (a) 0‐14 days, (b) 15‐28 days, (c) 29‐42 days, (d) 43‐56 days and (e) 57‐70 days. As it is shown in Table 4, IL‐10 on 43‐56 days was significantly lower than that on 29‐42 days (P = .049), TNF‐α on 15‐42 days was significantly higher than that on 0‐14 days (Pab = 0.030, Pac = 0.027), IP‐10 on 0‐14 days was highest and was significantly higher than that on 43‐56 days (P = .018) and IL‐4 on 29‐42 days was significantly higher than that on 0‐14 days (P = .018).
TABLE 4.
Changes of serum IL‐10, IL‐1β, IL‐6, MCP‐1, TNF‐α, IP‐10 and IL‐4 in COVID‐19 patients
| Median (IQR) (pg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Course | n | IL‐10 | IL‐1β | IL‐6 | MCP‐1 | TNF‐α | IP‐10 | IL‐4 |
| a | 3 | 3.15 | 8.77 | 6.17 | 49.69 | 4.77 | 120.66 | 31.30 |
| b | 7 | 7.60 (8.49) | 6.99 (4.89) | 7.95 (6.13) | 66.39 (82.28) | 22.18 (7.15)# | 32.62 (43.16) | 52.59 (32.80) |
| c | 31 | 5.53 (5.50) | 8.99 (3.70) | 8.85 (7.58) | 59.34 (96.05) | 19.48 (20.00)# | 30.60 (55.69) | 54.55 (24.74)# |
| d | 26 | 4.60 (5.21)* | 8.28 (6.83) | 7.42 (6.80) | 49.23 (41.98) | 18.57 (20.18) | 34.99 (24.99)# | 46.52 (41.95) |
| e | 3 | 2.64 | 9.94 | 8.62 | 52.04 | 16.28 | 28.12 | 55.39 |
| P | / | P > .05 | ||||||
a. 0‐14 days, b. 15‐28 days, c. 29‐42 days, d. 43‐56 days and e. 57‐70 days, #means compare with a, P < .05; *means compare with c, P < .05.
4. DISCUSSION
We measured the serum levels of these cytokines in COVID‐19 patients and non‐COVID‐19 patients by ELISA, and the results showed that the serum levels of IL‐10, IL‐1β, MCP‐1, TNF‐α and IL‐4 in COVID‐19 patients were significantly higher than those in non‐COVID‐19 patients, while IL‐6 were only significantly higher than in healthy people, IP‐10 in COVID‐19 patients were significantly lower than those in other diseases patients, which indicated that there was a cytokine storm in patients with COVID‐19. IL‐10, IL‐1β, IL‐6, MCP‐1, TNF‐α and IL‐4 have a potential study value for COVID‐19, and TNF‐α and MCP‐1 have the best predictive effect. It should be noted that the serum levels of IL‐10 and MCP‐1 in male COVID‐19 patients were significantly higher than those in the female patients, the serum levels of all cytokines in patients were significantly positively correlated with age, and these indicated that COVID‐19 prefers male and older. IL‐1β and IL‐4 were significantly negatively correlated with SARS‐CoV‐2 IgM, while IL‐10, IL‐1β, IL‐6, TNF‐α and IP‐10 were significantly negatively correlated with SARS‐CoV‐2 IgG, which were consistent with the rule of cytokine production. The continuous monitoring of the cured COVID‐19 patients showed that the levels of TNF‐α on 15‐42 days and IL‐4 on 29‐42 days were significantly higher than them on 0‐14 days, and the study shows that ICU patients had higher plasma levels of cytokines than non‐ICU patients, 1 which suggested that levels of cytokines may related to the severity of the disease, so we assume that the reason for this change is that the disease on 0‐14 days is light; IL‐10 on 43‐56 days were significantly lower than those on 29‐42 days, which may because on 43‐56 days, the immune system secreted more IL‐10 to induce excessive apoptosis of immune cells in order to avoid damaging its normal tissues in the late stage of inflammation; IP‐10 on 0‐14 days were highest and were significantly higher than those on 43‐56 days, and it may be the cytokine of early elevation in COVID‐19 patients.
5. CONCLUSIONS
To sum up, we found that the serum levels of IL‐1β, MCP‐1, IL‐6 and IP‐10 in patients with COVID‐19 were rise, which was same as severe acute respiratory syndrome (SARS), 10 , 11 , 12 this similarity may be related to the fact that both SARS‐CoV‐2 and SARS viruses attack angiotensin‐converting enzyme 2 (ACE2) 13 , 14 and produce similar inflammatory processes; TNF‐α in patients with COVID‐19 were also rise, which was same as Middle East Respiratory Syndrome Coronavirus (MERS) 11 , 15 , 16 ; what's interesting is that the level of IL‐4 and IL‐10 also rise in patients with COVID‐19, which was different with the another two high pathogenic coronavirus diseases. 10 , 11 , 12 , 15 , 16 IL‐10, IL‐1 β, IL‐6, MCP‐1, TNF‐α and IL‐4 would assist the clinical study of COVID‐19. In COVID‐19 patients, the serum levels of all cytokines were significantly positively correlated with age, the poor prognosis of the elderly may be related to this; some of they have relationships with the gender or antibody, the former may be related to the high level of ACE2 in male reproductive system 17 , 18 , 19 ; the levels of cytokines would change with the course of disease, and we assumed that IP‐10 may be the cytokine of early elevation in COVID‐19 patients. Due to the limited number of samples and enrolment, neither levels of cytokines between mild and severe patients were be compared. It has been reported that there are high levels of Pro‐inflammatory cytokines in severe COVID‐19 patients’ serum. 1 , 15 , 20 To make it clear that whether the cytokine level can predict the course of disease development of COVID‐19 patients, the follow‐up research will continue.
DISCLOSURES
The authors declare that they have no conflict of interest.
ACKNOWLEDGEMENT
The authors promise that all the data are from the clinical, and genuine and believable. The authors report no commercial associations that could be a conflict of interest. The Novel Coronavirus Pneumonia Emergency Project of Hunan Province Science and Technology Department (2020SK3009, 2020SK3018, 2020SK3042) and the First‐class Discipline Open Fund Project of Hunan University of Chinese Medicine (2018YXJS02) were received in support of this article.
Lu Q, Zhu Z, Tan C, et al. Changes of serum IL‐10, IL‐1β, IL‐6, MCP‐1, TNF‐α, IP‐10 and IL‐4 in COVID‐19 patients. Int J Clin Pract. 2021;75:e14462. 10.1111/ijcp.14462
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hongzhou LU, Stratton CW, Tang Y‐W. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92:401‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen YU, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The national health and Health Committee and the office of the State, Administration of traditional Chinese medicine (TCM). Diagnosis and Treatment Protocol for COVID‐19 (Trial version 7) (state health office Medical Letter No. 184) 2020. [EB/OL]. (2020‐03‐04). http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml
- 5. Chousterman Benjamin G, Swirski Filip K, Weber GF. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol. 2017;39:517‐528. [DOI] [PubMed] [Google Scholar]
- 6. Chan J‐W, Yuan S, Kok K‐H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2019;2020:514‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shimabukuro‐Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meng X‐Q, Chen X‐H, Sahebally Z, et al. Cytokines are early diagnostic biomarkers of graft‐versus‐host disease in liver recipients. Hepatobiliary Pancreat Dis Int. 2017;16:45‐51. [DOI] [PubMed] [Google Scholar]
- 9. Dekker A‐B, Krijnen P, Schipper IB. Predictive value of cytokines for developing complications after polytrauma. World J Crit Care Med. 2016;5:187‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rudragouda C, Stanley P. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kindler E, Thiel V, Weber F. Interaction of SARS and MERS coronaviruses with the antiviral interferon response. Adv Virus Res. 2016;96:219‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Channappanavar R, Fehr A, Vijay R, et al. Dysregulated type I interferon and inflammatory monocyte‐macrophage responses cause lethal pneumonia in SARS‐CoV‐infected mice. Cell Host Microbe. 2016;19:181‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao YU, Zhao Z, Wang Y, Zhou Y, Ma YU, Zuo W. Single‐cell RNA expression profiling of ACE2, the receptor of SARS‐CoV‐2. Am J Respir Crit Care Med. 2020;202:756‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade‐long structural studies of SARS coronavirus. J Virol. 2020;94:e00127‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim ES, Choe PG, Park WB, et al. Clinical progression and cytokine profiles of middle east respiratory syndrome coronavirus infection. J Korean Med Sci. 2016;31:1717‐1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ng DL, Hosani FA, Kelly Keating M, et al. Clinicopathologic, immunohistochemical, and ultrastructural findings of a fatal case of Middle East respiratory syndrome coronavirus infection in the United Arab Emirates. Am J Pathol. 2016;186:652‐658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fan Z, Chen L, Li J, et al. Clinical features of COVID‐19‐related liver functional abnormality. Clin Gastroenterol Hepatol. 2020;18:1561‐1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fan C, Li K, Ding Y, Lu WL, Wang J. ACE2 expression in kidney and testis may cause kidney and testis damage after 2019‐nCoV infection [J/OL]. https://www.medrxiv.org/content/10.1101/2020.02.12.20022418v1. Accessed February 13, 2020. [DOI] [PMC free article] [PubMed]
- 19. Sama IE, Ravera A, Santema BT, et al. Circulating plasma concentrations of angiotensin‐converting enzyme 2 in men and women with heart failure and effects of renin–angiotensin–aldosterone inhibitors. Eur Heart J. 2020;41:1810‐1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guo L, Wei D, Zhang X, et al. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA score. Front Microbiol. 2019;10. 10.3389/fmicb.2019.02752 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


