Abstract
Triple-negative breast cancer (TNBC) is characterized by a high degree of immune infiltrate in the tumour microenvironment, which may influence the fate of TNBC cells. We reveal that loss of the tumour suppressive transcription factor Elf5 in TNBC cells activates intrinsic interferon-γ (IFN-γ) signalling, promoting tumour progression and metastasis. Mechanistically, we find that loss of the Elf5-regulated ubiquitin ligase FBXW7 ensures stabilization of its putative protein substrate IFN-γ receptor 1 (IFNGR1) at the protein level in TNBC. Elf5low tumours show enhanced IFN-γ signalling accompanied by an increase of immunosuppressive neutrophils within the tumour microenvironment and increased programmed death ligand 1 expression. Inactivation of either programmed death ligand 1 or IFNGR1 elicited a robust anti-tumour and/or anti-metastatic effect. A positive correlation between ELF5 and FBXW7 expression and a negative correlation between ELF5, FBXW7 and IFNGR1 expression in the tumours of patients with TNBC strongly suggest that this signalling axis could be exploited for patient stratification and immunotherapeutic treatment strategies for Elf5low patients with TNBC.
Triple-negative breast cancers (TNBCs) are strongly immunogenic1-4, and immune cells present in the breast cancer tumour microenvironment (TME) contribute to cancer progression and metastasis5-8. Specific subsets of infiltrating immune cells in TNBCs have prognostic significance9-11, suggesting that understanding the mechanisms that dictate the immune landscape and their role in tumour progression will provide valuable insight for future immunotherapies.
E74-like transcription factor (Elf5)—a member of the E26 transformation-specific (ETS) family of transcription factors—is expressed predominantly in epithelial cells12-14 and functions as a suppressor of epithelial-to-mesenchymal transition (EMT) and a crucial regulator of mammary gland development13-16. Here we demonstrate that Elf5 loss activates constitutive interferon-γ (IFN-γ) signalling in cancer cells, creating an immunosuppressive environment within the TME with an increased number of neutrophils. Although IFN-γ is considered anti-tumorigenic17,18, recent studies have shown that IFN-γ signalling maybe pro-tumorigenic in melanoma and gastric cancer19,20. Our findings that enhanced IFN-γ signalling downstream of Elf5 is correlated with increased neutrophils in the TME of TNBC and promotes tumour progression and metastasis provides critical insight into this process.
We demonstrate that heterozygous deletion of Elf5 downregulates FBXW7—a ubiquitin ligase involved in proteasomal degradation of IFN-γ receptor 1 (IFNGR1). In our preclinical TNBC mouse model, the absence of ELF5 led to reduced expression of FBXW7, stabilizing the IFNGR1 protein and sustaining IFN-γ signalling and programmed death ligand 1 (PD-L1), which could potentially contribute to the observed immune suppressive alterations in the TME and increased tumorigenesis and metastasis. Our spontaneous and orthotropic systems show that sustained IFN-γ signalling due to IFNGR1 stabilization following loss of the EFF5–FBXW7 axis contributes to the highly aggressive nature of TNBCs.
Finally, our clinical dataset analysis indicates that patients expressing high levels of ELF5 and FBXW7 expression show better prognosis than patients with high IFNGR1 expression in multiple TNBC subsets. Human breast cancer samples and patient-derived xenograft (PDX) models show a positive correlation between EFF5 and FBXW7 expression and an inverse correlation between EFF5 and IFNGR1 expression, corroborating data from our mouse model. Together, our data show that constitutive IFN-γ signalling induced by Elf5 loss causes an increase in the number of highly immune suppressive neutrophils in the TME, increasing tumour growth and metastasis and worsening the prognosis of TNBC.
Results
Heterozygous loss of Elf5 increases tumour burden and metastasis in a basal TNBC tumour model.
Elf5 is a critical regulator of EMT—a characteristic that imparts tumours’ invasive and metastatic properties21-24. As TNBC is highly heterogeneous, invasive and metastatic25-29, we sought to determine whether Elf5 expression has prognostic significance in TNBC subtypes. Kaplan–Meier plots showed a direct correlation between high Elf5 expression and increased distant metastasis-free survival (DMFS) in basal, immunomodulatory and mesenchymal TNBCs (Fig. 1a), suggesting that Elf5 could play a protective (tumour suppressive) role in multiple subsets of TNBC.
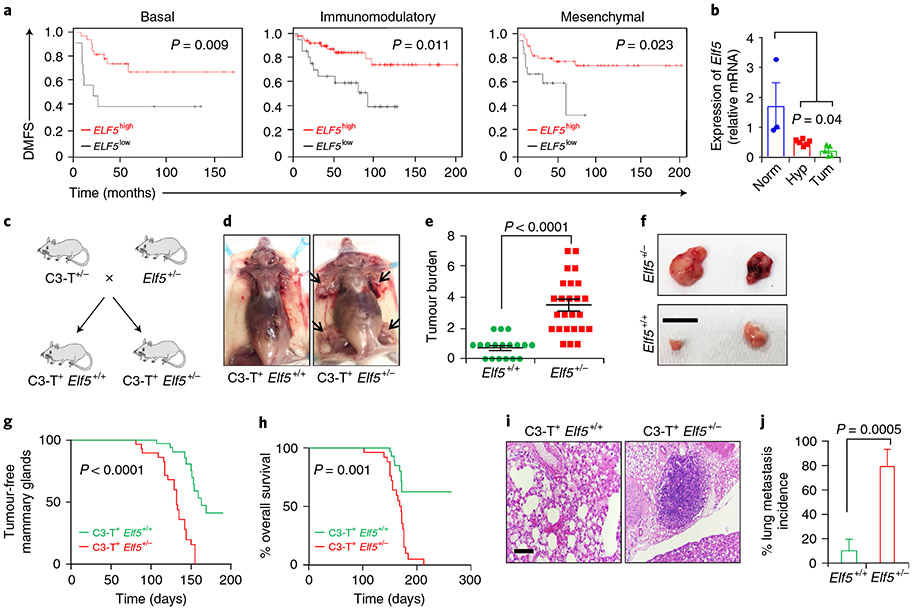
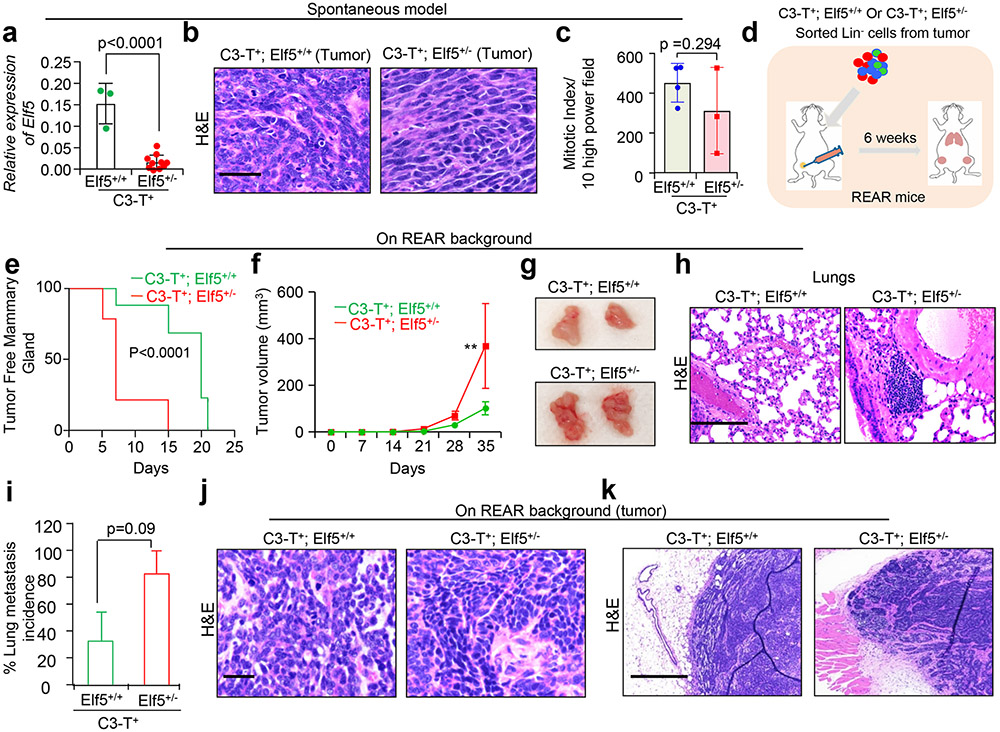
Fig. 1 ∣. Loss of Elf5 increases tumour initiation, progression and metastasis in a basal/mesenchymal C3-T+ mouse model.
a, Kaplan–Meier plots for patients with basal (n = 39), immunomodulatory (n = 96) and mesenchymal TNBCs (n = 65) indicate that high ELF5 correlates with better DMFS. Statistical significance was determined by log-rank test and P values are indicated. b, qPCR for Elf5 in C3-T+ mice showed sequential reduction in Elf5 expression upon progression from mammary gland (norm; 5weeks; n = 3) to hyperplasia (hyp; 8–10 weeks; n = 6) to tumour (tum; 5 months; n = 5). qPCR values were normalized to Gapdh. The experiments were performed three times, each with qPCR in technical duplicate. The data presented as means ± s.e.m. Statistical significance was determined by two-sided Student’s t-test. c, Schematic showing the generation of C3-T+ Elf5+/− mice. d, Images showing C3-T+ Elf5+/− mice bearing a greater number of tumours (black arrows). e, Scatter plot showing a higher tumour burden in C3-T+ Elf5+/− mice. The data are presented as means ± s.e.m. Statistical significance was determined by two-tailed Mann–Whitney U-test (n = 18 for C3-T+ Elf5+/+; n = 25 for C3-T+ Elf5+/−). f, Age-matched tumours excised from C3-T+ Elf5+/− mice were larger compared with those excised from C3-T+ Elf5+/+ mice. g,h, Graphs showing a lower number of tumour-free mammary glands (g) and decreased survival (h) in C3-T+ Elf5+/− mice compared with C3-T+ Elf5+/+ mice (n = 19 for C3-T+ Elf5+/+ and n = 20 for C3-T+ Elf5+/− in g; n = 16 for C3-T+ Elf5+/+ and n = 22 for C3-T+ Elf5+/− in h). Statistical significance was determined by log-rank test. i,j, Haematoxylin and eosin stains of lung sections (i) and quantification of these results (j) showed an increase in the incidence of lung metastatic nodules in lungs harvested from C3-T+ Elf5+/− mice (n = 10 for C3-T+ Elf5+/+; n = 10 for C3-T+ Elf5+/−). Statistical significance was determined by two-tailed Student’s t-test. The data are presented as means ± s.e.m. Scale bars: 2 mm (f) and 40 μm (i). For the Kaplan–Meier plots in a, the KM Plotter database was used to obtain the data57. Mice with high and low expression of Elf5 were stratified based on the best cut-off of values between the lower and upper quartile in the dataset57 (*P < 0.05).
To determine the role of Elf5 in TNBCs, we utilized a C3-T antigen (C3-T+) murine model that closely resembled basal and mesenchymal TNBC phenotypes30-32. Elf5 decreased during C3-T+ tumour progression, supporting its potential role as a tumour suppressor in TNBC (Fig. 1b). Since complete knockout of Elf5 is embryonic lethal33, we mated C3-T+ mice with mice having heterozygous loss of Elf5 (Elf5+/−) (Fig. 1c). Quantitative PCR (qPCR) confirmed the loss of Elf5 in C3-T+ Elf5+/− tumours compared with wild-type (C3-T+ Elf5+/+) tumours (Extended Data Fig. 1a). Compared with C3-T+ Elf5+/+ mice, we observed an increased tumour burden and tumour size, a lower number of tumour-free mammary glands, lower overall survival and increased lung metastasis in age-matched C3-T+ Elf5+/− mice (Fig. 1d-j). Furthermore, C3-T+ Elf5+/− tumours exhibited a strong invasive phenotype characterized by the presence of long mesenchymal cells, indicating increased aggressiveness of the tumour (Extended Data Fig. 1b). Despite increased tumour size in C3-T+ Elf5+/− mice, tumour sections showed no difference in mitotic potential (Extended Data Fig. 1c), indicating similar proliferative potential to C3-T+ Elf5+/+ tumours.
To understand whether Elf5 loss in tumour epithelial cells leads to increased tumour burden, growth and metastasis in C3-T+ Elf5+/−, we sorted cells into C3-T+ Elf5+/− and C3-T+ Elf5+/+ and injected them into REAR recipient mice (Extended Data Fig. 1d) receptive to C3-T+ tumour implant30. In REAR recipient mice, C3-T+ Elf5+/− tumour cells grew faster, along with increased metastatic nodules in the lungs, compared with C3-T+ Elf5+/+ tumour cells (Extended Data Fig. 1e-i). Tumours that formed from this orthotropic model were morphologically similar to primary spontaneous tumours from C3-T+ Elf5+/− and C3-T+ Elf5+/+ mice, showing invasive mesenchymal tumour cells with a darker nucleus (Extended Data Fig. 1j,k). Collectively, these results suggest that Elf5 loss in tumour cells is associated with increased TNBC tumour initiation, progression and metastasis, which imparts poor survival.
Loss of Elf5 increases IFN-γ signalling in C3-T+ Elf5+/− tumours through elevated IFNGR1 protein expression.
Unbiased RNA sequencing (RNA-Seq) on sorted enriched tumour cells from C3-T+ Elf5+/+ and C3-T+ Elf5+/− mice revealed that tumour cells from C3-T+ Elf5+/− tumours exhibited several increased IFN signalling pathways (Fig. 2a). Most substantially altered datasets corresponded to IFN-γ signalling in gene set enrichment analysis (GSEA) (Fig. 2a). qPCR analysis established increased expression of IFN-γ-stimulated genes (ISGs)34, such as Ccl3, Cxcl10, Ccl4, Pd-l1, Ctla4 and Cxcl9 (Fig. 2b), indicating a possible role for Elf5-suppressed IFN-γ signalling in creating an immune suppressive TME by modulating signalling by PD-L1 and other ISGs in C3-T+ Elf5+/− mice.
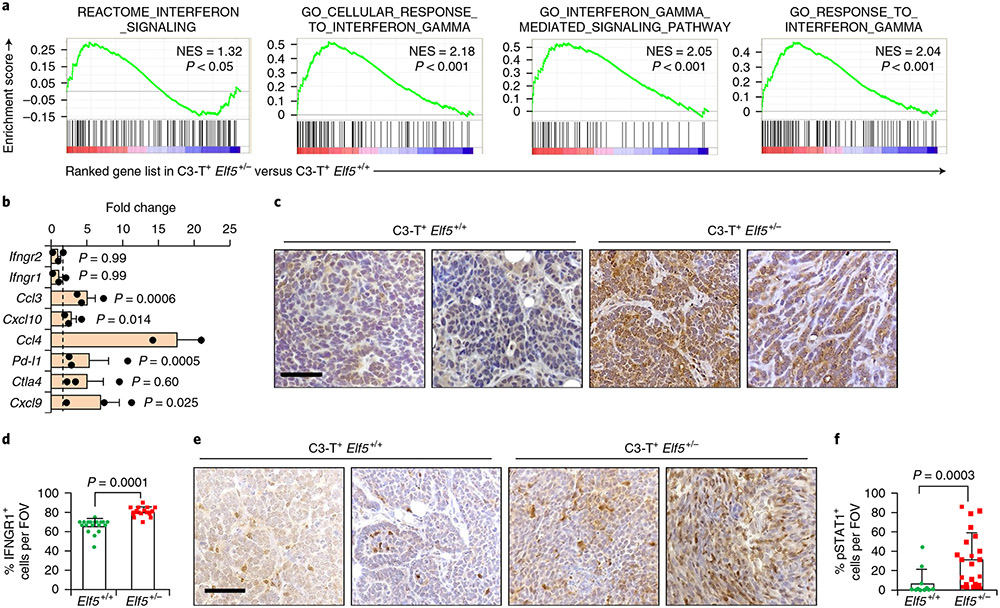
Fig. 2 ∣. Loss of Elf5 promotes IFN-γ signalling in C3-T+ Elf5+/− tumours.
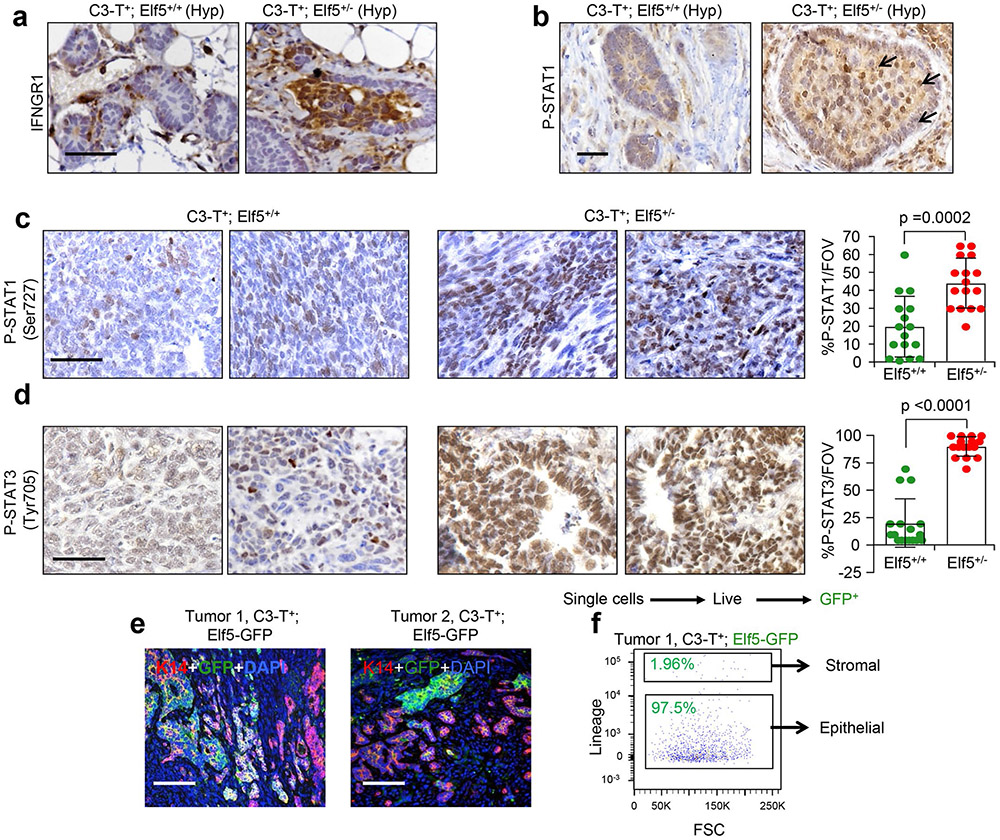
a, GSEA analysis showing increased IFN-γ signalling in tumour cells sorted from C3-T+ Elf5+/− tumours compared with those from C3-T+ Elf5+/+ tumours (n = 2 individual tumours per group). b, qPCR analysis suggesting increased expression of IFN-γ-associated signatures in a tumour cell-enriched population (lineage negative) sorted from C3-T+ Elf5+/− tumours compared with those from C3-T+ Elf5+/+ tumours (n = 2 tumours for C3-T+ Elf5+/+; n = 3 tumours for C3-T+ Elf5+/−; in technical duplicates). Lineage-negative tumour cells were sorted based on CD31, Ter119 and CD45 antibodies37. The relative expression of C3-T+ Elf5+/+ was considered to be a fold change of one, which is depicted by the dashed line. qPCR values were normalized to the housekeeping gene Gapdh. The experiments were performed three times, each with qPCR in technical duplicate. The data are presented as means ± s.e.m. Statistical significance was determined by two-tailed Student’s t-test. c,d, IHC staining (c) and quantification (d) of IFNGR1 in C3-T+ Elf5+/− and C3-T+ Elf5+/+ tumours, showing increased expression of cytoplasmic and membrane IFNGR1 protein in C3-T+ Elf5+/− tumours (n = 3 samples were used, with analysis of n = 14 random fields from C3-T+ Elf5+/+ tumours and n = 16 random fields from C3-T+ Elf5+/− tumours for quantification). The data are presented as means ± s.e.m. e,f, IHC staining (e) and quantification (f) of pSTAT1 in C3-T+ Elf5+/− and C3-T+ Elf5+/+ tumours, showing increased expression of pSTAT1 protein (nuclear) in C3-T+ Elf5+/− tumours (n = 3 individual tumours were used, with analysis of n = 11 random fields from C3-T+ Elf5+/+ tumours and n = 21 random fields from C3-T+ Elf5+/− tumours for quantification). Images in c,e represent IHC staining in two different tumours/group. The data are presented as means ± s.d. Statistical significance in d and f was determined by two-tailed Mann–Whitney U-test. Scale bars in c and e: 40 μm. FOV, field of view; NES, normalized enrichment score.
qPCR and immunohistochemistry (IHC) revealed that protein (not messenger RNA (mRNA)) levels of IFNGR1 were significantly higher in C3-T+ Elf5+/− tumours compared with C3-T+ Elf5+/+ tumours (Fig. 2b-d). An increased nuclear signal of phospho-STAT1 (pSTATl) in C3-T+ Elf5+/− tumours compared with C3-T+ Elf5+/+ tumours (Fig. 2e,f) suggested active IFNGR1 signalling35,36. Hyperplastic (pre-neoplasia) mammary glands from C3-T+ Elf5+/− mice showed increased numbers of IFNGR1 and pSTAT1+ cells (Extended Data Fig. 2a,b). Additionally, phosphorylation of STAT1 at Ser727 and of STAT3 at Tyr705 was elevated in C3-T+ Elf5+/− tumours, suggesting that additional elements important for type II IFN signalling were impacted by Elf5 signalling in TNBC (Extended Data Fig. 2c,d). These data collectively suggest that IFN-γ signaling is increased in C3-T+ Elf5+/− tumours, probably due to increased IFNGR1 protein.
Elf5 represses IFN-γ signalling.
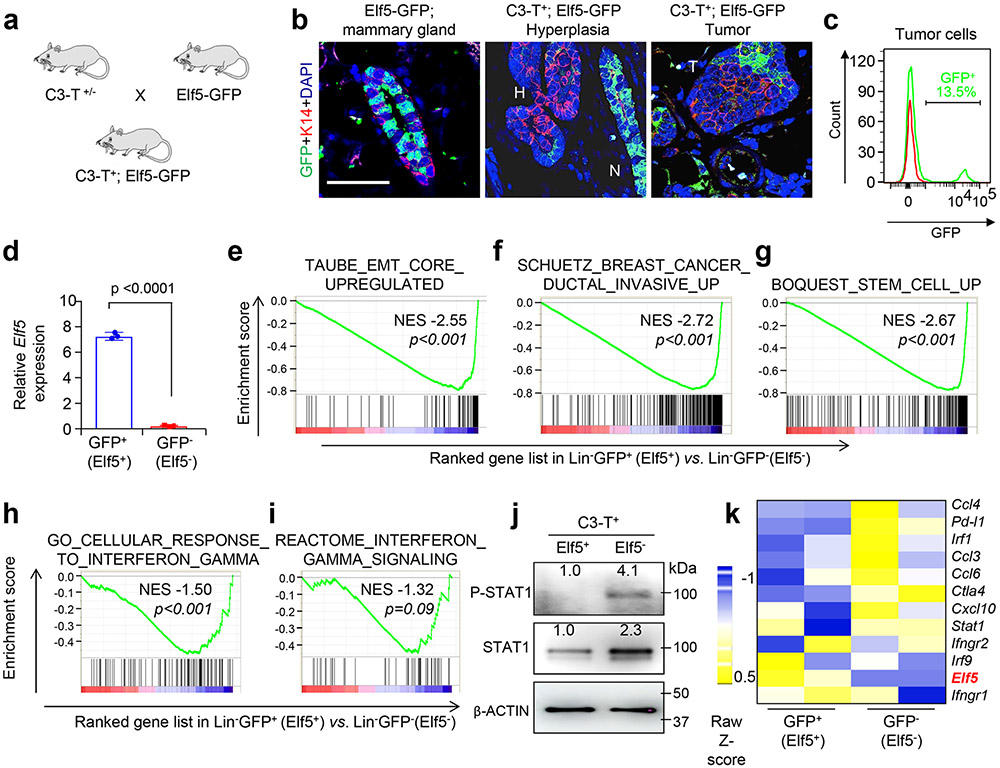
Elf5 expression in the Elf5 reporter model (C3-T+ Elf5-GFP, where Elf5 was tagged with green fluorescent protein (GFP)) (Extended Data Fig. 3a) showed that Elf5-driven GFP expression decreased from normal mammary gland tissue to hyperplasia to tumour cells, supporting Elf5 as a tumour suppressor in TNBC (Extended Data Fig. 3b). The GFP+ population in enriched tumour cells (stromal cells were removed by cell sorting37) in these mice varied from 5–16% (Extended Data Fig. 3c) and showed high Elf5, indicating the faithfulness of the model (Extended Data Fig. 3d). Elf5 expression was minimal in stromal cells (Extended Data Fig. 2e,f), strengthening our hypothesis that Elf5 loss in tumour epithelial cells is largely responsible for increased tumour burden, growth and metastasis in C3-T+ Elf5+/− tumours.
An unbiased RNA-Seq analysis of Elf5+GFP+ and Elf5−GFP− tumour cells followed by GSEA indicated that, along with IFN-γ signalling, several signatures for invasiveness, stemness pathways and EMT signatures were upregulated in the GFP−Elf5− population (Extended Data Fig. 3e-i), supporting earlier published roles of Elf5 as an EMT and sternness suppressor15,16. Furthermore, Elf5− tumour cells showed higher pSTAT1 (Tyr701) (Extended Data Fig. 3j). Core gene analysis of IFN-γ signatures showed increased expression of ISGs in Elf5− tumour cells (Extended Data Fig. 3k), suggesting that Elf5 may repress IFN-γ signalling in C3-T+ tumours.
ELF5-regulated FBXW7 modulates IFNGR1 protein levels in TNBC cells.
RNA-Seq from Elf5+ and Elf5− cells from Elf5 reporter mice suggested that Elf5+ cells have upregulated expression of genes involved in proteasomal and ubiquitin-mediated degradation (Extended Data Fig. 4a,b). Detailed analysis of core enriched genes in GSEA of the proteasome pathway from the Elf5+ population revealed upregulation of 15 ubiquitin ligases, two of which (Fbxw7 and Vhl) are known tumour suppressors in several cancers, including breast cancer38,39 (Extended Data Fig. 4c). Re-expression of Elf5 in LM2 cells (Extended Data Fig. 4d)—lung metastatic derivatives of MDA-MB-231 cells (human TNBC cells with low ELF5 expression)—increased Fbxw7 and Vhl mRNA levels (Extended Data Fig. 4e,f) but had no effect on Ifngr1 mRNA levels (Extended Data Fig. 4g). Data mining of our published microarray data (GSE32144)16 showed that in parent MDA-MB-231 TNBC cells, expression of a DNA-binding mutant form of ELF5 (ref. 16) failed to increase FBXW7 levels, whereas VHL remained unaltered (Extended Data Fig. 4h,i), suggesting that FBXW7 is a direct transcriptional target of ELF5. Increased FBXW7 expression in ELF5-expressing LM2 cells was further confirmed at the protein level (Extended Data Fig. 4j). Chromatin immunoprecipitation sequencing data obtained in normal mammary epithelial cells from pregnant and lactating mice40 revealed that Elf5 binds to several Fbxw7 genomic regions, suggesting that Elf5 may promote expression by binding to the enhancer region of Fbxw7 (Extended Data Fig. 5a). A strong positive correlation between ELF5 and FBXW7 and a negative correlation of both proteins with IFNGR1 was found in multiple TNBC cells, such as MX1 (high ELF5), LM2 (low ELF5), HCC1806 (low ELF5) and BT549 cells (low ELF5) (Extended Data Fig. 4k). In addition, we observed a direct correlation between ELF5 and FBXW7 mRNA and protein levels in EpRas murine TNBC cells, which express low ELF5–FBXW7 and high IFNGR1 protein (Extended Data Fig. 4l-o).
ELF5-driven FBXW7 mediates proteasomal degradation of IFNGR1.
To determine whether FBXW7 affects IFNGR1 protein levels in an ELF5-dependent manner, we made stable knockdowns of ELF5 in MX1 cells. ELF5 knockdown (KD1, KD2 and KD3) reduced FBXW7 and increased IFNGR1 protein (Fig. 3a), which was further confirmed by flow cytometry (Extended Data Fig. 5b). The difference in IFNGR1 expression upon ELF5 knockdown was more prominent in western blots compared with flow cytometry analysis, suggesting that IFNGR1 may be internalized upon binding to its ligand41.
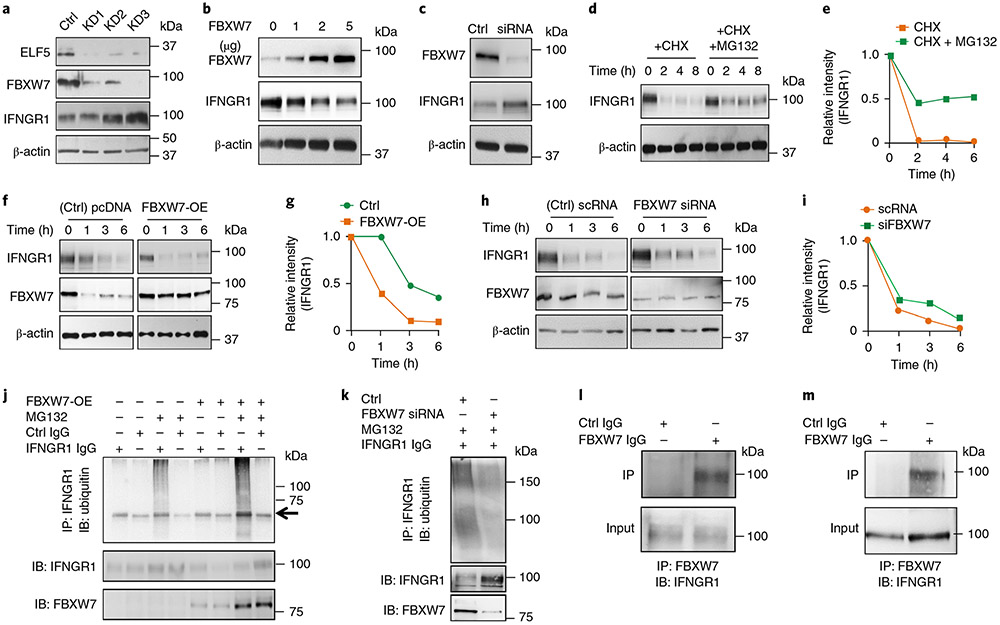
Fig. 3 ∣. ELF5 regulates FBXW7-mediated destabilization of IFNGR1 in TNBC.
a, Stable knockdown of ELF5 in MX1 cells decreases FBXW7 levels and increases IFNGR1 protein expression. Three independent shRNA sequences were used to knockdown ELF5 (KD1, KD2 and KD3). b, Overexpression of FBXW7 in LM2 cells decreases IFNGR1 protein expression in a dose-dependent manner. c, Silencing FBXW7 in MX1 cells by siRNA58 causes an increase in IFNGR1 levels. d,e, Western blot (d) and quantification of the results (e), showing degradation of IFNGR1 in LM2 cells upon treatment with CHX and rescue upon co-treatment with MG132, which prevents proteasomal degradation. f,g, Overexpression of FBXW7 in LM2 cells enhances degradation of IFNGR1, as shown by western blot (f) and quantification of the results (g). h,i, Knockdown of FBXW7 in MX1 cells delays CHX-mediated degradation of IFNGR1 protein, as shown by western blot (h) and quantification of the results (i). j, LM2 cells were transfected with FBXW7 overexpressing plasmid followed by treatment with MG132 after 48 h. Immunoprecipitation was performed with anti-IFNGR1 antibody followed by western blot with ubiquitin antibody. The results show that polyubiquitination of IFNGR1 was increased upon overexpression of FBXW7. The arrow shows a heavy chain. k, MX1 cells were transfected with FBXW7 siRNA followed by treatment with MG132 after 48 h. Immunoprecipitation was performed with anti-IFNGR1 antibody followed by western blot with ubiquitin antibody. The results show that polyubiquitination of IFNGR1 was decreased upon knockdown of FBXW7. l,m, LM2 (l) and MX1 (m) cells were subjected to endogenous immunoprecipitation using FBXW7 antibody followed by probing with IFNGR1 antibody. Direct interaction between FBXW7 and IFNGR1 was observed in both sets of TNBC cells. All experiments in a-m were repeated three times. Uncropped western blot images are provided as source data. Ctrl, control; IB, immunoblotting; IP, immunoprecipitation; pcDNA, plasmid cloning DNA; scRNA, small conditional RNA.
Transient overexpression of FBXW7 increased FBXW7 in a dose-dependent manner that directly correlated with decreased IFNGR1 in LM2 cells (Fig. 3b). Knockdown of FBXW7 in MX1 cells caused upregulation of IFNGR1 protein (Fig. 3c). To determine whether the IFNGR1 increase upon inactivation of the ELF5–FBXW7 pathway in TNBCs was due to stabilization, we first assessed proteolytic turnover of IFNGR1. The time-dependent degradation of IFNGR1 upon treatment with CHX in LM2 cells was attenuated upon treatment of cells with MG132 (Fig. 3d,e), supporting the involvement of proteasomal machinery in the maintenance of IFNGR1 protein levels.
Next, overexpression of FBXW7 in LM2 (IFNGR1high/FBXW7low) rapidly degraded IFNGR1 (Fig. 3f,g), whereas knocking down FBXW7 in MX1 cells (IFNGR1low/FBXW7high) resulted in a slower turnover of IFNGR1 (Fig. 3h,i). To further determine whether FBXW7 ubiquitinates IFNGR1, we performed immunoprecipitation of endogenous IFNGR1 in LM2 cells with and without FBXW7 overexpression. The results showed that FBXW7 overexpression in ELF5low LM2 cells increases polyubiquitination of IFNGR1 compared with controls (Fig. 3j). Complementarity, immunoprecipitation of IFNGR1 in Elf5high MX1 cells showed that loss of FBXW7 reduces polyubiquitination of IFNGR1 (Fig. 3k). Immunoprecipitation showed physical interaction between FBXW7 and IFNGR1 in LM2 and MX1 cells (Fig. 3l,m). Together, these results suggest that endogenous IFNGR1 physically interacts with FBXW7 and undergoes FBXW7-dependent ubiquitination followed by degradation in ELF5high TNBC cells. Our data indicate that the loss of FBXW7 in TNBCs results in a failure to ubiquitinate IFNGR1, thereby stabilizing this receptor.
Loss of Elf5 increases IFN-γ signalling, promotes the accumulation of neutrophils and augments the tumorigenic and metastatic potential of TNBC.
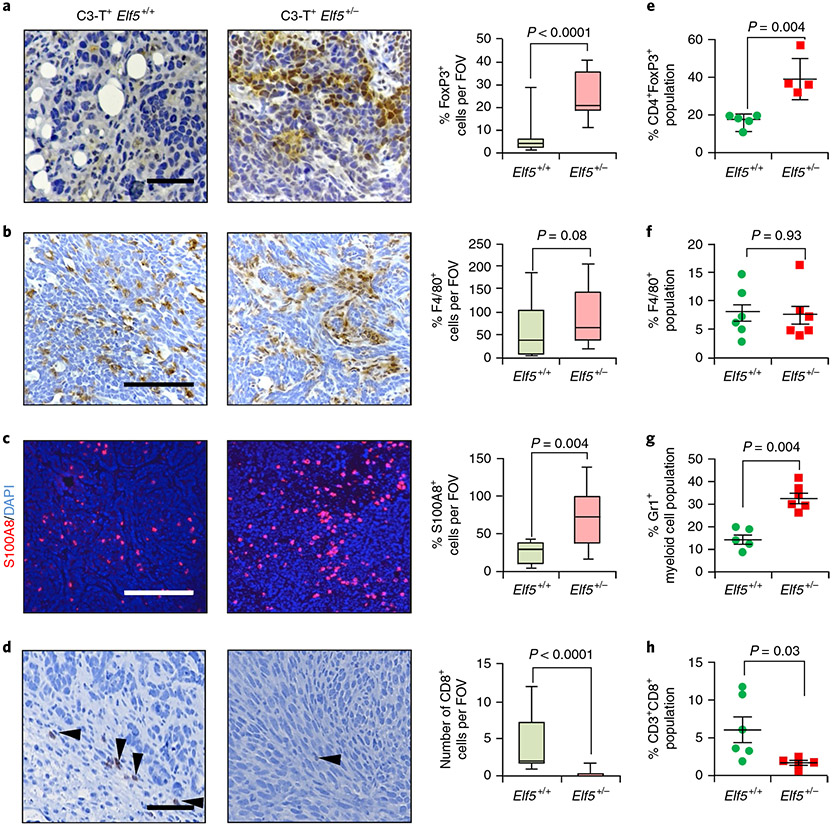
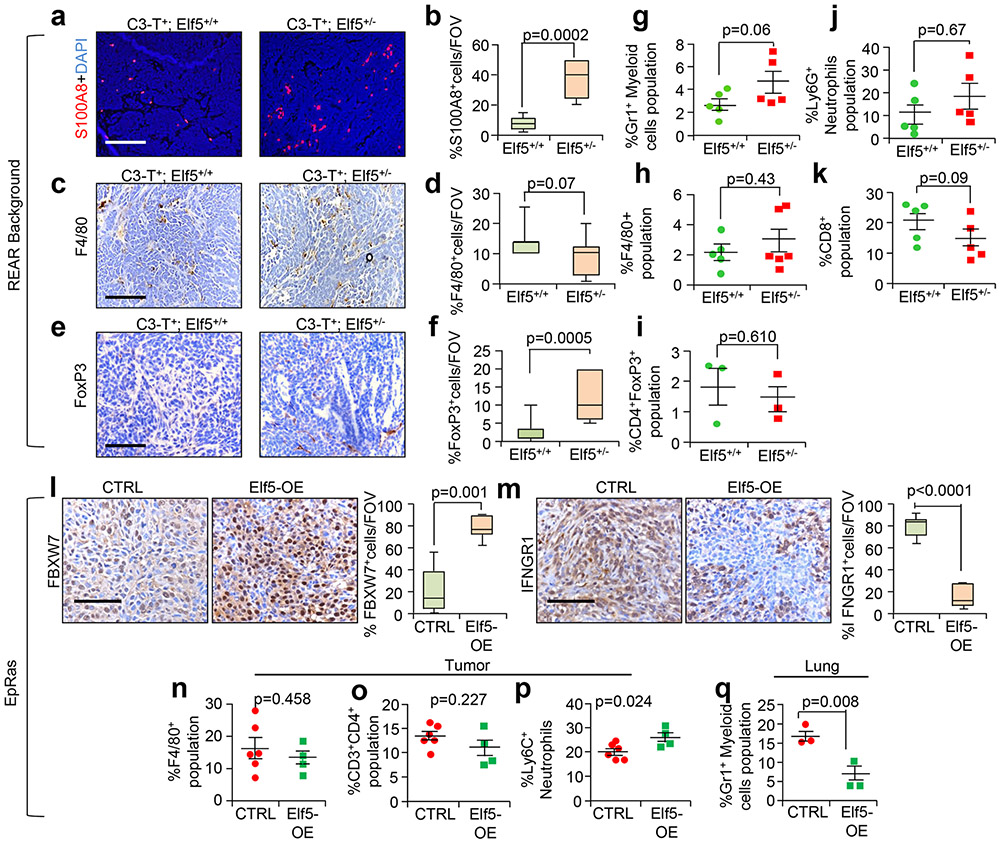
To understand whether enhanced IFN-γ signalling is accompanied by alterations in the immune landscape of the TME, we compared C3-T+ Elf5+/+ and C3-T+ Elf5+/− tumours, focusing on regulatory T cells (Treg cells) and myeloid cells such as neutrophils and macrophages that promote immune evasion42-44. Numbers of Foxp3+ Treg cells and S100A8+ myeloid cells were significantly higher in C3-T+ Elf5+/− tumours (Fig. 4a-c and Extended Data Fig. 5c,d). Increased neutrophils in C3-T+ Elf5+/− tumours were predominantly Ly6Ghigh neutrophils (Extended Data Fig. 5e-g) and were capable of suppressing T cell proliferation in vitro, verifying their immune suppressive behaviour and identifying them as Gr1+ myeloid cells (Extended Data Fig. 5h). A decrease in CD8+ T cells in C3-T+ Elf5+/− tumours was observed (Fig. 4d), suggesting a more immune suppressive environment. Furthermore, high numbers of Treg cells and myeloid cells, and lower numbers of CD8+ T cells, were observed in the TME of C3-T+ Elf5+/− tumours, while macrophage numbers did not differ significantly (Fig. 4e-h). In REAR recipients injected with either C3-T+ Elf5+/− or C3-T+ Elf5+/+ tumour cells, we found a strong trend only towards an increased neutrophil population in C3-T+ Elf5+/− tumours compared with C3-T+ Elf5+/+ tumours. Gates are shown in Extended Data Fig. 6. The Treg cell population and macrophages were unaltered between tumours from the two groups (Extended Data Fig. 7a-k), suggesting that Elf5 loss in tumour cells is associated with potentially altered numbers of neutrophils, which may contribute to immune evasion.
Fig. 4 ∣. Loss of Elf5 alters the immune landscape in C3-T+ tumours.
a, FoxP3 staining shows an increased number of Treg cells in C3-T+ Elf5+/− tumours compared with C3-T+ Elf5+/+ tumours. b, F4/80 staining shows an increased number of macrophages in C3-T+ Elf5+/− tumours compared with C3-T+ Elf5+/+ tumours. c, S100A8 analysis shows an increased number of myeloid cells in tumours from C3-T+ Elf5+/− mice, d, CD8 staining shows decreased numbers of cytotoxic T cells in tumours from C3-T+ Elf5+/− mice (arrowheads). In a-d, images of the stains are shown to the left, with quantification of the results to the right, and n = 3 individual tumours were used with a minimum of ten random fields per genotype for IHC quantification. The boxplot data represent medians, interquartile ranges and spikes to upper and lower adjacent values. Scale bars: 40 μm. e-h, Flow cytometry analyses (FACS) showing increased Treg cells (CD45+CD3+CD4+FoxP3+; e) with no major change in F4/80+ (CD45+G4/80+; f), increased Gr1+ myeloid cells (CD45+CD11b+Gr1+; g) and decreased cytotoxic T cells (CD45+CD3+CD8+; h) in C3-T+ Elf5+/− tumours compared with C3-T+ Elf5+/+ tumours. All of the gates were drawn according to a published protocol59. The scatter plots show the number per group of samples from flow analysis and the data are presented as medians ± s.e.m. Statistical significance was determined by two-tailed Mann–Whitney U-test (a-d and f-h) and two-tailed Student’s t-test (e).
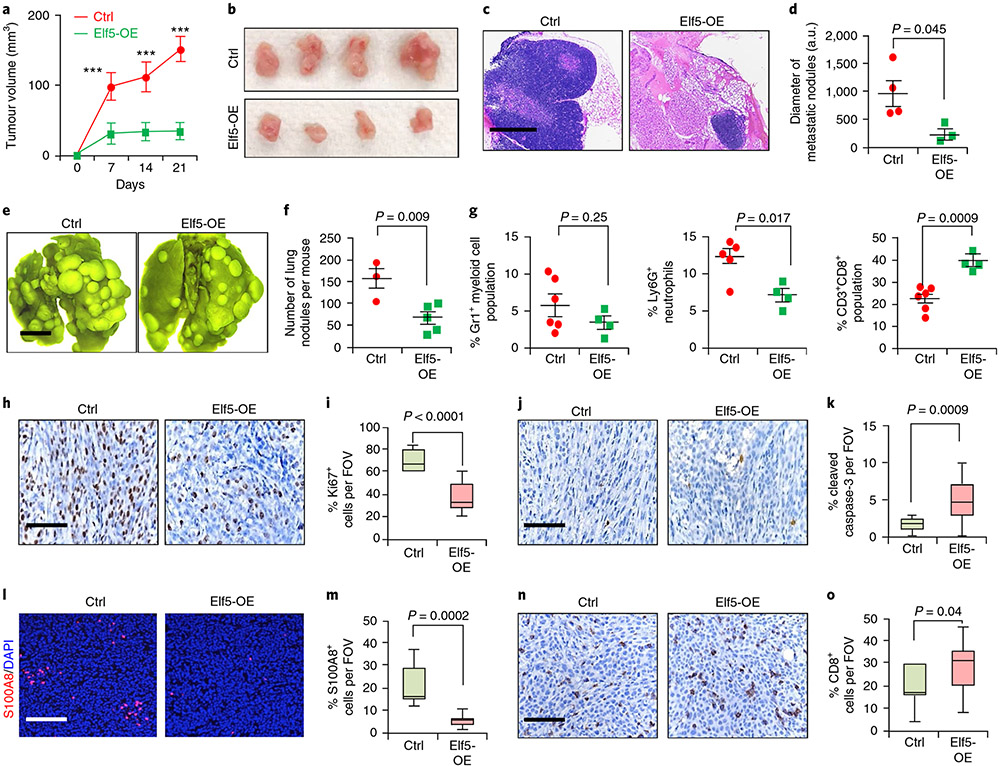
In gain-of-function studies (Extended Data Fig. 4l-o), tumour cells re-expressing Elf5 showed higher expression of FBXW7 and lower expression of IFNGR1 protein. Elf5-expressing tumours grew slower (Fig. 5a,b) and yielded smaller metastatic nodules (Fig. 5c,d). Tail vein injection of EpRas cells overexpressing Elf5 (Elf5-OE cells) yielded reduced numbers of metastatic nodules (Fig. 5e,f). These results are consistent with a cell-autonomous tumour suppressive role of Elf5.
Fig. 5 ∣. Re-expression of Elf5 in EpRas TNBC cells decreases tumour growth and metastasis.
a, Tumour growth curve showing slower tumour progression in Elf5-OE cells compared with control cells (n = 7 tumours for the control; n = 8 tumours for Elf5-OE). A total of 50,000 EpRas cells per MFP were injected. Statistical significance was determined by two-way ANOVA with Bonferroni correction (***P < 0.001). b, Image showing bigger tumours formed from control cells compared with Elf5-OE cells. c,d, Haematoxylin and eosin stains (c) and graphical representation, in arbitary units (a.u.) used by ImageJ (d), showing the presence of larger metastatic lung nodules in control tumours compared with lungs in mice injected with Elf5-OE tumour cells (n = 4 mice for the control; n = 3 mice for Elf5-OE). The images are representative of two independent experiments. e,f, Lung images (e) and graphical representation (f) showing a lower number of metastatic nodules in lungs upon tail vein injection of EpRas Elf5-expressing cells (n = 3 mice for the control; n = 5 mice for Elf5-OE). A total of 150,000 EpRas cells per tail vein were injected. The images are representative of two independent experiments. g, Flow cytometry graphs showing decreased Gr1+ myeloid cells (n = 6 for the control; n = 4 for Elf5-OE tumours), decreased Ly6G+ neutrophils (n = 5 for the control; n = 4 for Elf5-OE tumours) and increased CD8+ T cells (n = 6 for the control; n = 4 for Elf5-OE tumours) in primary mammary tumours formed from Elf5-OE cells. h,i, IHC images (h) and graphical representation (i) showing decreased Ki67+ cells in Elf5-OE tumour cells, j,k, IHC images (j) and graphical representation (k) showing Increased cleaved caspase-3 in Elf5-OE tumour cells. l,m, Immunofluorescence image (l) and graphical representation (m) showing decreased neutrophils in Elf5-OE tumours, n,o, IHC images (n) and graphical representation (o) showing increased CD8+ T cells in Elf5-OE tumour cells. In d, f, g and h-o, statistical significance was determined by two-tailed Student’s t-test. In a, d, f and g, the data are presented as means ± s.e.m. The boxplot data represent medians, interquartile ranges and spikes to upper and lower adjacent values. Scale bars: 100 μm (c), 2 mm (e) and 40 μm (h, j, l and n).
Elf5-expressing tumours showed a dramatic decrease in Ly6G+ neutrophils and an increase in Ly6C+ neutrophils (Fig. 5g and Extended Data Fig. 7p). Increased CD8+ T cells in tumours formed by Elf5-expressing cells (Fig. 5g) supported the role of Elf5 in controlling the number of intratumoral neutrophils. No differences in F4/80+ macrophages or the CD4+ T cell population were observed (Extended Data Fig. 7n,o). A similar decrease in Gr1+ myeloid cells was observed in lung metastatic sites of Elf5-OE tumour-bearing mice (Extended Data Fig. 7q). Elf5-OE tumours bore fewer neutrophils and more CD8+ T cells in the TME (Fig. 5l-o), consistent with fluorescence-activated cell sorting (FACS) data. Elf5-OE EpRas tumours showed fewer Ki67+ cells and increased cleaved caspase-3 staining (Fig. 5h-k). Together, our data suggest that re-expression of Elf5 in EpRas TNBC cells decreases their tumorigenic and metastatic potential by reducing the number of immune suppressive neutrophils.
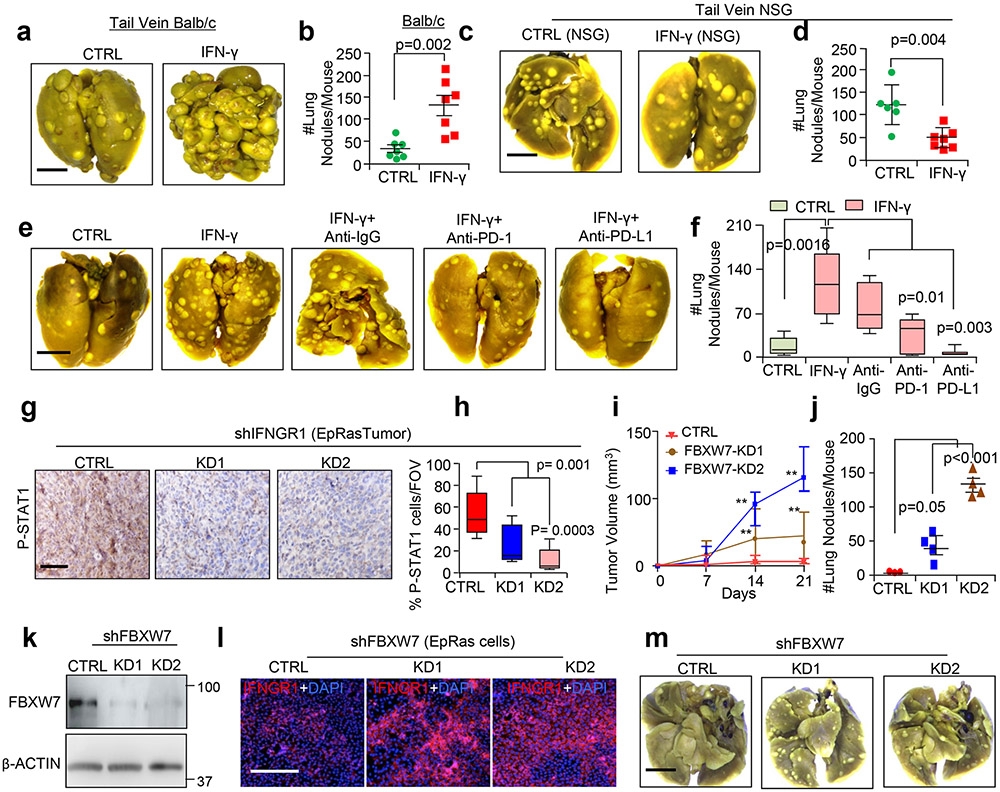
To study the functional significance of stabilized IFNGR1 in TNBC, we treated EpRas cells with IFN-γ. IFN-γ-dependent increases in both STAT1 and pSTAT1 protein were confirmed (Extended Data Fig. 8a), and this resulted in upregulation of several ISGs, including Pd-l1, Ctla4, Cxcl9 and Cxcl10 (Extended Data Fig. 8b). Notably, increases in PD-L1 but not CTLA4 were observed by FACS (Extended Data Fig. 8c). EpRas cells treated with IFN-γ formed tumours that progressed faster than tumours formed from untreated cells (Extended Data Fig. 8d) and showed increased mesenchymal/invasive morphology, as was evident from invasion into the surrounding muscle tissues and mammary fat pad (MFP) (Extended Data Fig. 8e). These mice had more metastatic nodules in the lungs (Extended Data Fig. 8f,g). Tumours formed from IFN-γ-treated EpRas cells showed a greater number of pSTAT1+ cells (Extended Data Fig. 8h) and S100A8+ myeloid cells (Extended Data Fig. 8i) and reduced numbers of CD8+ T cells (Extended Data Fig. 8j), further supporting the IFN-γ-dependent immune suppressive phenotype. Increased S100A8+ myeloid cells were observed in the lungs of mice bearing tumours formed from IFN-γ-treated cells, suggesting an immune suppressive environment at the metastatic site as well (Extended Data Fig. 8k,l). Tail vein injection of IFN-γ-treated EpRas cells resulted in increased lung metastasis (Extended Data Fig. 9a,b), showing that lung colonization is altered in IFN-γ-treated EpRas cells compared with controls. However, injection of IFN-γ-treated EpRas cells in immunocompromised NSG mice45,46 showed that this effect is dependent on immune cells (Extended Data Fig. 9c,d). To ascertain the PD-L1 role in metastasis, IFN-γ-treated EpRas cells were injected into the tail veins of mice, followed by treatment with either anti-programmed cell death protein 1 or anti-PD-L1 or corresponding control anti-immunoglobulin G (anti-IgG) antibodies. PD-L1 blocking completely reversed the increased metastasis in the lungs generated by IFN-γ-treated EpRas cells. Similarly, PD-1 blocking also reversed the metastasis phenotype significantly (Extended Data Fig. 9e,f). Taken together, our data strongly suggest that enhanced IFN-γ signalling in TNBC cells promotes multiple steps of tumour progression, including proliferation and metastasis, which are highly dependent on PD-L1.
Antibody-mediated blocking and genetic inhibition of IFNGR1 in vivo reverse tumour initiation and metastasis in TNBC.
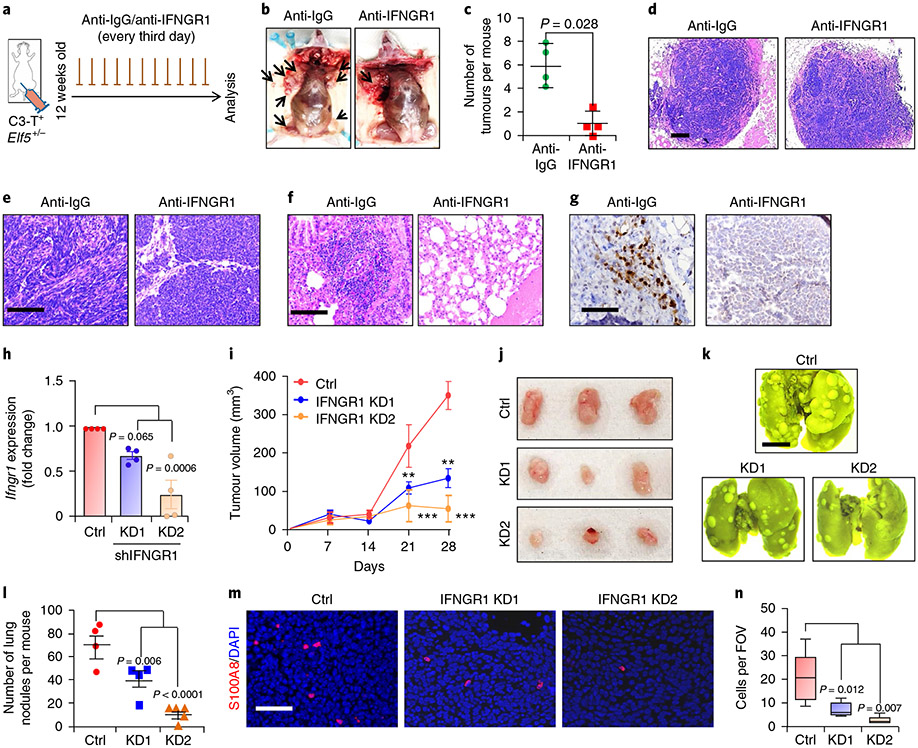
Next, we blocked IFN-γ signalling in C3-T+ Elf5+/− tumour cells in vivo using a neutralizing IFNGR1 antibody. Decreased tumour burden and tumour invasiveness, along with reversal of the mesenchymal phenotype, were observed in anti-IFNGR1 antibody-injected mice compared with mice injected with anti-IgG antibody (Fig. 6a-e). Additionally, injection of anti-IFNGRl antibody in C3-T+ Elf5+/− mice dramatically reduced lung metastasis (Fig. 6f). Reduced pSTAT1 levels confirmed the attenuation of IFNGR1 signalling in tumours of mice injected with anti-IFNGRl antibody (Fig. 6g). Decreased tumour burden, reversal of the mesenchymal phenotype of tumours and reduced lung metastasis upon inhibition of IFNGR1 in C3-T+ Elf5+/− mice strongly suggested a critical pro-tumorigenic and pro-metastatic role of IFN-γ signalling in the C3-T+ TNBC mouse model.
Fig. 6 ∣. Antibody-mediated and genetic inhibition of IFNGR1 in TNBC cells hampers tumour growth and metastasis.
a-c, Schematic of the experiment (a), mouse images (b) and a graph (c) showing the reduced tumour burden in C3-T+ Elf5+/− mice upon injection of anti-IFNGR1 (n = 4 mice per group for anti-IgG (500 μg per mouse per injection) or anti-IFNGR1 antibody (500 μg per mouse per injection). Statistical significance was determined by two-tailed Mann–Whitney U-test. The data are presented as means ± s.d. The arrows in b point to tumours. d,e, Haematoxylin and eosin analysis of tumours, showing the absence of invasive edges (d) and reversal of the mesenchymal phenotype (e) of C3-T+ Elf5+/− tumours upon neutralization of IFNGR1. f, Lung metastasis was reversed in C3-T+ Elf5+/− mice injected with anti-IFNGR1 antibody. g, Reduced pSTAT1 was observed in tumours harvested from the group injected with anti-IFNGR1 antibody. In d-g, the images are representative of two independent experiments. h, qPCR confirmed decreased mRNA of IFNGR1 in EpRas cells transduced with IFNGR1 shRNA. qPCR values were normalized to Gapdh (n = 4 biological replicates pooled from two independent experiments). The data are presented as means ± s.d. i,j, Graph (i) and images (j) showing decreased tumour growth in EpRas cells with IFNGR1 knockdown (KD1 and KD2) (n = 4 for the control and shIFNGR1 KD1; n = 5 for shIFNGR1 KD2). In i, statistical significance was determined by two-way ANOVA with Bonferroni post-test correction (**P < 0.01; ***P < 0.001). The data are presented as means ± s.e.m. k,l, Lung images (k) and graphical representation (l) showing fewer metastatic nodules upon tail vein injection of IFNGR1 knockdown cells (n = 4 for the control and shIFNGR1 KD1; n = 5 for shIFNGR1 KD2). The data are presented as means ± s.e.m. m,n, Immunofluorescence images (m) and graphical representation (n) showing the reduced number of S100A8+ myeloid cells from Ifngr1 knockdown tumours. The boxplot data represent medians, interquartile ranges and spikes to the upper and lower adjacent values (n = 5 fields of view from two samples per group). In h, l and n, statistical significance for multiple comparisons was determined by one-way ANOVA with Tukey’s post-hoc test. Scale bars: 100 μm (d), 40 μm (e, g and m), 2 mm (k) and 100 μm (f).
To rule out indirect involvement of stromal and immune cells on IFNGR1-regulated tumorigenesis, we generated EpRas TNBC cells with IFNGR1 knockdown (Fig. 6h). IFNGR1 knockdown (KD1 and KD2) TNBC cells formed tumours that grew slower than control tumours and had fewer pSTAT1+ cells (Fig. 6i,j and Extended Data Fig. 9g,h). Tail vein injection of IFNGR1 knockdown cells revealed reduced metastatic potential (Fig. 6k,l). We found decreased numbers of neutrophils in tumours formed from EpRas cells with IFNGR1 knockdown (Fig. 6m,n). Together, these studies suggested that active IFNGR1 signalling in TNBC tumour cells imparts tumorigenic and metastatic potential through immune suppressive neutrophils.
Genetic inhibition of FBXW7 signalling leads to enhanced IFNGR1 expression and TNBC tumour growth.
To understand whether FBXW7-mediated IFNGR1 regulation directly affects tumour growth and metastasis, FBXW7 knockdown EpRas cells were generated that showed increased IFNGR1 compared with control EpRas cells (Extended Data Fig. 9k,l). FBXW7 knockdown tumours grew faster than control tumours (Extended Data Fig. 9i). Tail vein injection showed that FBXW7 knockdown EpRas cells have higher metastatic potential (Extended Data Fig. 9j,m). These experiments suggest that genetic inhibition of FBXW7 increased IFNGR1 signalling, TNBC tumour growth and metastasis.
Depletion of neutrophils abrogates TNBC tumour growth and metastasis.
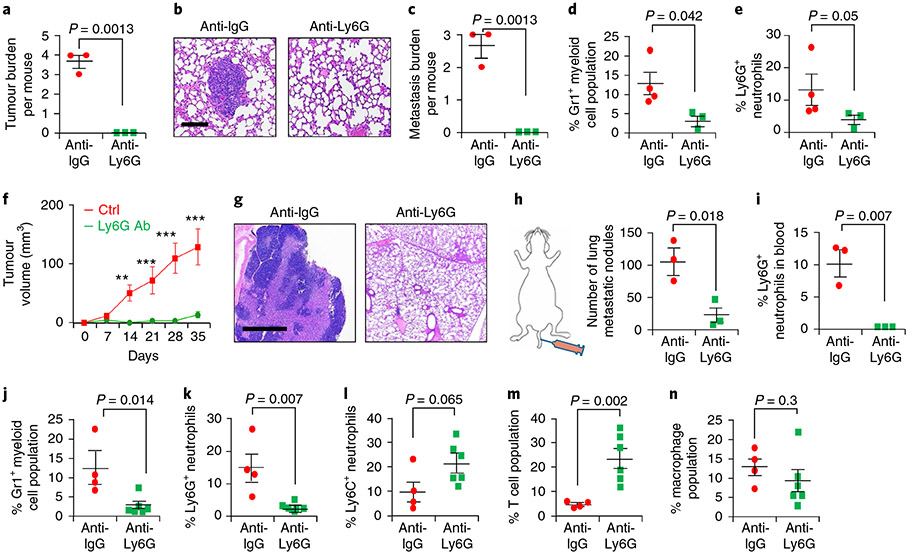
To show that Elf5–Fbxw7-dependent IFN-γ signalling increases neutrophil numbers promoting an immune suppressive environment, we depleted Ly6G+ neutrophils in vivo in C3-T+ Elf5+/− cells using anti-Ly6G antibody, which led to decreased tumour initiation/burden (Fig. 7a) and resulted in smaller lung metastatic nodules (Fig. 7b,c). Decreased numbers of Gr1+ myeloid cells and Ly6G+ neutrophils in the tumours were confirmed (Fig. 7d,e). The pro-tumour and pro-metastatic effect of Ly6G+ neutrophils was further confirmed by injecting IFN-γ-treated EpRas cells into the MFP and subsequently treating with anti-Ly6G antibody. Decreased tumour progression and lung metastasis were observed (Fig. 7f,g). Lung metastasis was reduced in both the orthotopic and tail vein model following Ly6G depletion (Fig. 7g,h). Decreases in the Ly6G+ neutrophil and Gr1+ myeloid populations were confirmed (Fig. 7i-k) and no non-specific effects were observed on either Ly6C+ neutrophils or macrophage populations (Fig. 7l,n). An increase in T cells was observed (Fig. 7m), suggesting that depletion of Ly6G+ cells may increase intratumoral T cells due to a loss of immune suppressive neutrophils.
Fig. 7 ∣. Antibody-mediated depletion of Ly6G+ neutrophils decreases tumour growth and metastasis in spontaneous and orthotopic TNBC models.
a-c, Tumour initiation (a) and metastatic burden (as shown by haematoxylin and eosin stains (b) and presented graphically (c)) were severely reduced in C3-T+ Elf5+/− mice injected with anti-Ly6G (250 μg per mouse per injection) compared with those injected with anti-IgG (250 μg per mouse per injection) (n = 3 mice per group). Anti-Ly6G antibody injection was given every third day after 12 weeks of age for the mice in each group. d,e, Anti-Ly6G injection decreased the Gr1+ myeloid population (d) and Ly6G+ neutrophil population (e) in tumours. f, Tumour growth was slower in mice injected with anti-Ly6G antibody (anti-Ly6G Ab; n = 8 individual tumours) compared with IgG control (n = 4 individual tumours) (**P < 0.01; ***P < 0.001). g, Haematoxylin and eosin stain images showing large metastatic nodules in the lungs of mice injected with anti-IgG antibody compared those treated with anti-Ly6G antibody. In f and g, a total of 70,000 EpRas cells were injected into the MFP. Anti-Ly6G treatment (250 μg per mouse per injection) started when tumours were 3 mm × 3 mm, and the drug was given every third day. h, A smaller number of metastatic nodules were obtained in mice injected with anti-Ly6G antibody compared with those receiving anti-IgG antibody. i, Systemic depletion of Ly6G was confirmed in the blood of injected mice (n = 3 mice per group by FACS). In h and i, 150,000 EpRas cells per mouse were injected into the tail vein, and anti-Ly6G antibody injection was given every third day (n = 3 mice per group). j-n, Results of flow cytometry of primary mammary tumours (n = 4 tumours for IgG; n = 6 tumours for anti-Ly6G antibody), showing a decreased Gr1+ myeloid cell population (j), decreased Ly6G+ neutrophils (k), a slight increase in Ly6C+ neutrophils (l), increased T cells (m) and no change in macrophages (n) in EpRas tumour-bearing mice injected via the MFP with anti-Ly6G antibody compared with those injected with IgG antibody. In j-n, each dot in the scatter plot represents an individual tumour. Statistical significance was determined by two-tailed Student’s t-test (a, c, d and h-n), two-tailed Mann–Whitney U-test (e) or two-way ANOVA with Bonferroni post-adjustment (to measure tumour progression; f). The data are presented as means ± s.e.m. Scale bars: 40 μm (b) and 100 μm (g).
Low ELF5–FBXW7 and high IFNGR1 correlate with worse DMFS in TNBC.
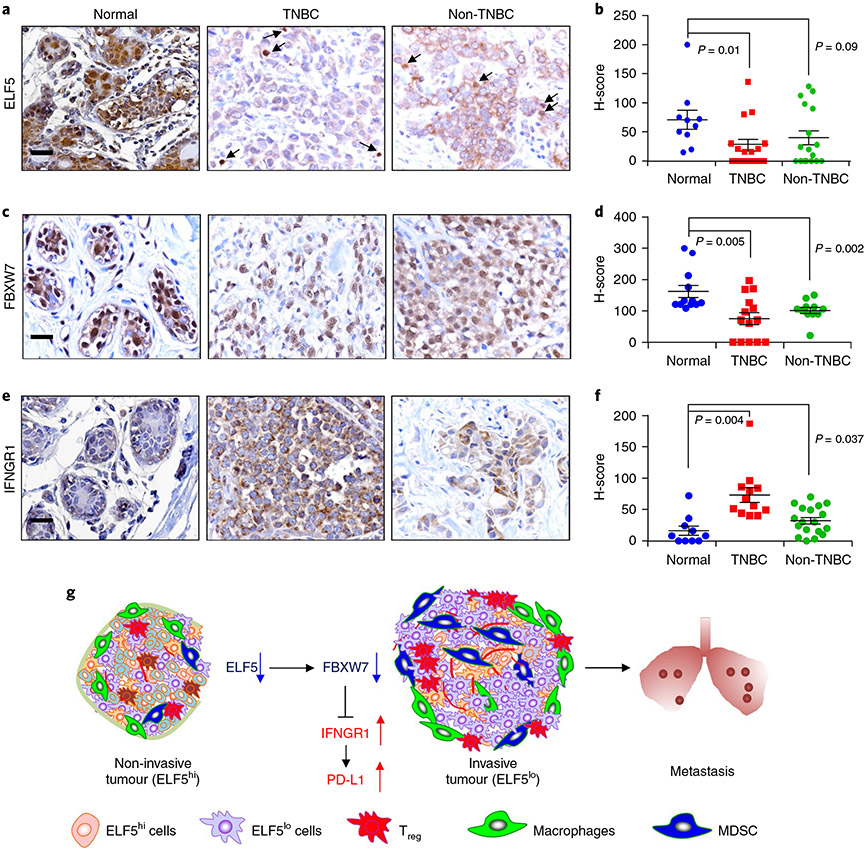
To determine the clinical significance of our studies, we assessed correlations of ELF5, FBXW7 and IFNGR1 with DMFS in patients with TNBC. Tumours with ELF5high/FBXW7high expression correlated with better DMFS, whereas patients with IFNGR1high expression had poor DMFS in basal, immunomodulatory and mesenchymal TNBCs (Extended Data Fig. 10a-c). In contrast, high IFNGR1 expression in patients with oestrogen receptor-positive breast cancer correlated with better DMFS (Extended Data Fig. 10d), suggesting a TNBC-specific effect. This inverse correlation was further confirmed by IHC on patient samples, where expression levels of both nuclear ELF5 and FBXW7 proteins were lower in breast tumours compared with normal breast tissues, supporting the tumour suppressive function of ELF5 and FBXW7 (Fig. 8a-d). Furthermore, breast tumours had higher IFNGR1 protein than normal adjacent breast tissue (Fig. 8e,f), supporting a pro-tumorigenic role of IFNGR1. Interestingly, TNBC tumours had higher IFNGR1 expression compared with non-TNBC tumours (Fig. 8e,f; P = 0.01). In contrast, tumours from patients with TNBC that expressed high IFNGR1 also expressed low levels of FBXW7 and ELF5 proteins (Fig. 8a-d). A strong positive correlation was observed between ELF5 and FBXW7 in the tumours of patients with TNBC (r = 0.43; P = 0.03; n = 13), but not in non-TNBC tumours, for which there was a modest trend, but the data did not reach statistical significance (r = 0.49; P = 0.07; n = 14). We also found a negative correlation between ELF5 and IFNGR1 proteins in TNBC (r = −0.50; P = 0.05; n = 15), which was not seen in non-TNBC tumours (r = 0.17; P = 0.28; n = 17). Patient details are provided in Supplementary Table 6. Similar findings were observed in PDXs derived from patients with TNBC47, wherein low ELF5 and FBXW7 levels were correlated with high IFNGR1 protein levels (Extended Data Fig. 10e-h). Overall, our clinical data fully support the data from experiments in mice with respect to the role of ELF5 as a tumour suppressor in TNBC that acts by repressing IFNGR1.
Fig. 8 ∣. Compared with normal breast cells, tumour cells have lower expression of the proteins ELF5 and FBXW7 and higher expression of IFNGR1.
a-f, IHC of TNBC and non-TNBC tumours along with adjacent normal tissue was performed using antibodies against ELF5 (a and b), FBXW7 (c and d) and IFNGR1 (e and f). Images (a, c and e) and graphical representations (b, d and f) show high ELF5 (n = 10 for normal; n = 16 for TNBC; n = 17 for non-TNBC) and high FBXW7 staining (n = 12 for normal; n = 14 for TNBC; n = 11 for non-TNBC) in normal tissues compared with TNBC and non-TNBC tissues, and higher expression of IFNGR1 in TNBC and non-TNBC tumours compared with adjacent normal tissues (n = 10 for normal; n = 12 for TNBC; n = 18 for non-TNBC tissue). In all instances, n denotes independent biological samples. Nuclear localization of ELF5 and FBXW7 was considered positive in tumour tissues. Quantification of H-scores (intensity × abundance) showed a significant difference in ELF5 and FBXW7 expression among normal and TNBC tissues and significantly higher expression of IFNGR1 in TNBC and non-TNBC compared with adjacent normal tissues. The arrows indicate nuclear ELF5 in tumour tissues. Cytoplasmic/membrane staining of IFNGR1 was scored as positive in tissues by IHC. Statistical significance was determined by Mann–Whitney U-test in b, d and f. The data are presented as means ± s.e.m. Scale bars: 40 μm (a, c and e). g, Schematic showing that low ELF5 and FBXW7 in TNBC tumour cells leads to stabilization of IFNGR1, resulting in increased PD-L1 expression. This increases invasive properties in TNBC tumour cells, followed by increased metastasis. MDSC, myeloid-derived suppressor cell.
Discussion
Despite an aggressive approach in the management of TNBCs with current therapy, the recurrence and 5-year survival rates for TNBC stand at 50 and 37%, respectively48,49. Our study shows that the transcription factor Elf5 functions as a tumour suppressor in basal and mesenchymal TNBC by preventing IFN-γ signalling-driven immunosuppressive alterations in the TME. We show that loss of Elf5 increases tumour burden, growth and metastasis in mice. This data are consistent with our observed association between ELF5 expression and disease outcome in humans (Figs. 1 and 8). Elf5 loss promotes an EMT-like phenotype in TNBC, and EMT can modulate the immune system50 and immunotherapy51, although, this aspect requires further exploration in the future.
Mechanistically, we find that Elf5 inhibits IFN-γ signalling in TNBC tumour cells by increasing FBXW7 expression, thereby promoting FBXW7-dependent IFNGR1 ubiquitination and degradation (Fig. 3 and Extended Data Fig. 4). We provide evidence that sustained IFN-γ signalling can be regulated at the receptor level by preventing FBXW7-mediated proteasomal degradation of IFNGR1. Our identification of the role of FBXW7 in proteasomal degradation of the IFNGR1 protein opens up an avenue for the modulation of IFN-γ signalling in tumours and various inflammatory diseases.
In our study, we demonstrate that Elf5 levels in tumour cells dictates neutrophil numbers in the TME of TNBCs. While future studies will determine how Elf5 loss is responsible for increased neutrophils, antibody-mediated and genetic inactivation of IFNGR1 in multiple TNBC models has been associated with reduced neutrophils, suggesting that ELF5, FBXW7 and IFNGR1 signalling is directly responsible for regulating this immune population. Our data show that pro-tumorigenic and pro-metastatic IFN-γ-IFNGR1 signalling upregulates PD-L1.
An inverse correlation between ELF5, FBXW7 and IFNGR1 proteins was observed in samples from patients with TNBC and TNBC PDXs. Similar to our experimental findings, Kaplan–Meier data suggest that high ELF5 and FBXW7 expression and low IFNGR1 expression is associated with better DMFS in patients with TNBC. In contrast, high IFNGR1 expression is associated with longer DMFS in patients without TNBC (that is, oestrogen receptor-positive breast cancer), highlighting a potential subset-specific role for IFNGR1 in TNBC.
Immunotherapies using anti-PD-L1 and anti-programmed cell death protein 1 antibodies are currently in clinical trials52-55. However, the response rate of patients with TNBC receiving anti-PD-L1 is very poor (~23%)56. We show that increased PD-L1 due to IFNGR1 stabilization may increase tumour evasion by promoting CD8+ T cell exhaustion in TNBC. Our studies unravel a pro-tumorigenic role of IFN-γ signalling, along with a putative biomarker, Elf5, in TNBCs that can predict patient response to immunotherapies such as anti-IFNGR1 and anti-PD-L1. Patients who are ELF5low and FBXW7low will express high levels of IFNGR1 and PD-L1, making them susceptible to immunotherapeutic drugs. The genetic and pharmacological studies here provide a proof-of-principle concept for the future use of antibodies targeting IFNGR1, along with checkpoint inhibitors, to improve the clinical outcome for patients with TNBC.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at https://doi.org/10.1038/s41556-020-0495-y.
Methods
Animal studies.
All animal procedures were conducted in compliance with the Institutional Animal Care and Use Committee of the University of Pennsylvania. To generate the C3-T+ Elf5+/− FVB mice, Elf5+/− mice (also known as Elf5+/− knock-in mice)33 were mated with C3-T+ antigen-bearing FVB mice (stock number 013591) procured from The Jackson Laboratory. Initially, Elf5+/− C57/B6 mice were bred with FVB mice for 10–12 generations to obtain Elf5+/− mice in FVB background. Genotyping with specific primers for LacZ (for the knock-in allele of Elf5+/−) and C3 was done to ascertain the phenotype. To generate C3-T+ Elf5–GFP FVB mice, Elf5–GFP mice60 were mated with C3-T+ antigen-bearing FVB mice (stock number 013591) procured from The Jackson Laboratory. Genotyping with specific primers for the Elf5–GFP DNA region and C3-T+ was done to ascertain the phenotype. For the MFP injections, female BALB/c mice aged 5–6 weeks were anaesthetized with ketamine/xylazine. EpRas cells were injected into the MFP of mice following an established protocol61,62. To study lung metastasis and lung colonization, EpRas cells were injected intravenously into the tail veins of BALB/c (stock number 00651) and NSG mice (stock number 005557) following an established protocol16. REAR mice (stock number 030386) were obtained from The Jackson Laboratory. Their genotype was confirmed using a protocol specified by The Jackson Laboratory. Five-week-old female REAR mice were injected with 225,000 cells per MFP of sorted epithelial cells from C3-T+ Elf5+/− and C3-T+ Elf5+/+ tumours. Tumour initiation, progression and metastatic nodules in the lungs were evaluated thereafter.
For the orthotopic tumour models, 70,000 EpRas cells per MFP of IFN-γ- and control-treated cells were injected into the MFP of 5-week-old BALB/c mice. In the experiments involving tumour generation from Elf5 re-expressing EpRas cells, 50,000 cells per MFP were injected into 5-week-old BALB/c mice. To study EpRas tumour progression upon IFNGR1 knockdown, EpRas cells with IFNGR1 knockdown (KD1 and KD2) and control cells were injected into the MFP of 5-week-old BALB/c mice. For this experiment, 70,000 cells per MFP were injected. To study tumour progression in the absence of Ly6G+ neutrophils, 70,000 EpRas cells per MFP were injected into 5-week-old BALB/c mice. Each mouse had contralateral mammary gland injection with the same number of tumour cells. Tumours were palpated once per week and the animals were terminated when the tumours reached a humane end point.
To study lung metastasis upon IFN-γ treatment, 100,000 EpRas cells were injected into the tail vein of 5-week-old mice. To understand the implication of re-expression of Elf5 in EpRas cells and the metastatic potential of EpRas cells with IFNGR1 knockdown (KD1 and KD2), 150,000 cells were injected into the tail vein of 5-week-old BALB/c mice. The outcome of Ly6G depletion on lung metastasis of EpRas cells was studied by injecting 150,000 cells into the tail vein of 5-week-old BALB/c mice. The mice were sacrificed and their lungs were harvested approximately 14 d after injection. Metastatic nodules were counted and pictures were taken for documentation. This study was compliant with all of the relevant ethical regulations regarding animal research (as outlined by the Institutional Animal Care and Use Committee).
In vivo IFNGR1/Ly6G neutralization in the spontaneous model.
Twelve-week-old C3-T+ Elf5+/− mice (11–12 weeks of age) were treated with anti-IgG or anti-IFNGR1 (500 μg per mouse) or anti-Ly6G (250 μg per mouse) antibodies every third day. The antibodies were bought from Bio X Cell (see Supplementary Table 1). Treatment was continued until the tumour reached a size of 150–200 mm3 or became ulcerated (humane end point). Tumours were palpated weekly.
In vivo Ly6G/PD-L1/PD-1 neutralization in the orthotropic and tail vein models.
Female BALB/c mice (5–6 weeks) were injected with anti-PD-1 (BE0146-A025), anti-PD-L1 (BE0101-A025), anti-IFNGR1 (BE0029), anti-Ly6G (BE0075-1) and anti-IgG (BE0089-2A3) antibodies 2 d before tail vein injection of control and IFN-γ-treated EpRas cells. For further details and antibody doses per injection. see Supplementary Table 1. Treatment was given again on the third, sixth, ninth and twelfth days post-injection of cells. For the MFP assay, anti-IgG/Ly6G antibody was given every third day after the tumour size reached 3 mm × 3 mm. Tumours were palpated weekly.
Cell culture studies.
The HEK293T, LM2, EpRas and BT549 cell lines were kind gifts from the laboratory of Y. Kang at Princeton University. The HCC1806 cell line was a generous gift from S. Ran at Southern Illinois University. The MX1 cell line was shared by S.Sinha. LM2, EpRas, BT549, HCC1806 and HEK293T were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with high glucose (4.5 gm ml−1) (Invitrogen; catalogue number 11965084). The MX1 cell line was cultured in DMEM/F-12 medium with HEPES and glutamine (catalogue number D8062; Sigma–Aldrich), supplemented with 10% heat-inactivated foetal bovine serum (Gemini; catalogue number 900108), 100 U ml−1 penicillin and 100 U ml−1 streptomycin sulfate (Invitrogen; 15140122). The cell lines were maintained in a humidified incubator with 5% CO2 and maintained at 37 °C. The absence of Mycoplasma in all of the cells was confirmed by PCR. The Mycoplasma kit was purchased from the American Type Culture Collection (catalogue number 30-1012K). Short tandem repeat DNA profiling for the cell lines was performed to authenticate the cells. The DNA sequencing facility of the Perelman School of Medicine at the University of Pennsylvania was used for the short tandem repeat analysis.
Knockdown and overexpression studies.
We used a previously validated expression plasmid for the FBXW7 overexpression studies58. FBXW7-specific small interfering RNA (siRNA) was customized and purchased from Dharmacon. For the stable knockdown of ELF5 in MX1 cells, we used short hairpin RNA (shRNA) sequences from Dharmacon. Details of the shRNA and siRNA are provided in Supplementary Table 5. For the IFNGR1 and FBXW7 knockdown studies, pLKO.1 control or respective knockdown plasmids were packaged into virus using HEK293T cells as the packaging cell line. Then, 4 μg target DNA was used for packaging. The helper plasmids VSVG and R8.9 were used at 1 and 2.5 μg, respectively, to package the target DNA in the virus. Infectious supernatant was collected 48 and 72 h after transfection and filtered to remove cell debris. EpRas cells were exposed to virus-containing supernatant supplemented with 2 μg ml−1 polybrene for 48 h. Virally infected cells were selected with 2 μg ml−1 puromycin. Knockdown plasmid sequences are provided in Supplementary Table 2.
Recombinant IFN-γ treatment to cells.
To study the effect of IFN-γ on TNBC cells, EpRas cells were plated in 10-cm dishes. After 24 h, when the cells became 70–80% confluent, either a sterile water control or IFN-γ (10 ng ml−1; catalogue number 100-77; Shenandoah Biotechnology) was added directly to the plates with 10 ml fresh media. The cells were cultured further for 48 h and harvested for qPCR, western blotting, FACS or tumour cell injections. For injection into the mice, cells were trypsinized, washed with phosphate-buffered saline (PBS) and counted for MFP or intravenous injection into the mice.
Cycloheximide (CHX) chase experiment.
LM2 cells were treated with 20 μM MG132 post-CHX treatment, and MX1 cells were transiently transfected either with small conditional RNA or FBXW7 siRNA. Then, 48 h after transfection, CHX (Sigma–Aldrich; catalogue number 239764) was added to a final concentration of 50 μg ml−1 to inhibit new protein synthesis at the indicated time points, followed by whole-cell extract preparation in RIPA buffer and immunoblotting with anti-IFNGR1, anti-FBXW7 and anti β-actin antibodies.
T cell proliferation and suppression assay.
T cell proliferation and suppression assays were performed following the protocols published in the literature63. In brief, 10,000 T cells were used for the T cell suppression assay. The cells were labelled with 2 μm CFSE (Life Technologies). T cell activation was performed with anti-CD3 (eBioscience; clone-145-2c11; catalogue number 14-0031-82) and anti-CD23 (BD Pharmingen; catalogue number 553295) antibodies. After 3 d of culture, the cells were collected and flow cytometry analysis was performed. Samples were also stained for Gr1 and CD11b, to be able to exclude neutrophils from the mixed cells to obtain pure T cells for FACS.
Protein extraction, western blot analysis and immunoprecipitation.
Whole-cell extracts from cell lines and tumours were prepared in 1× RIPA buffer12,15 (20 mM Tris-HCl (pH 7.5), 150 mM sodium chloride, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate and 1 mM β-glycerophosphate) supplemented with protease (cOmplete Mini EDTA-free protease inhibitor; Roche; catalogue number 11836170001) and phosphatase inhibitors (PhosSTOP Roche tablets; 4906845001-SIG). Western blotting was performed using an established protocol. Briefly, equal amounts of total protein were separated on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis at 140 V for 1.5 h, followed by transfer to an Immobilon-P membrane (Millipore; IPVH00010) for 1 h at 140 V. Blocking of non-specific molecules was removed using 5% non-fat milk powder (Bio-Rad) dissolved in 1× TBST (10 mM Tris, 15 mM NaCl and 0.05% Tween 20 at pH 7.5) for 1 h at room temperature. Membranes were incubated with the primary antibodies anti-IFNGR1 (catalogue number PA5-27841; Thermo Fisher Scientific), anti-FBXW7 (catalogue number A301-7218; Bethyl Laboratories), anti-pSTAT1 (catalogue number 9167S; Cell Signaling) and anti-STAT1 (catalogue number 9172; Cell Signaling) overnight at 4 °C followed by washing with 1× TBST (three times for 10 min per wash) and incubation with the respective secondary antibodies for 1 h at room temperature. After secondary incubation, the membranes were again washed with 1× TBST four times (10 min per wash) and developed. Anti-actin antibody (A3854; Sigma–Aldrich) was probed as a loading control for 1 h at room temperature. Alternatively, anti-β-actin antibody (A5441; Sigma–Aldrich) was used. Chemiluminescence was detected with Immobilon Forte Western HRP Substrate (MilliporeSigma; catalogue number WBLUF0500) or Immobilon Crescendo Western HRP Substrate (MilliporeSigma; catalogue number WBLUF0500) using an Amersham Imager 680 (GE Healthcare).
For immunoprecipitation, cells were suspended in 1% Triton X-100 containing PBS and sodium dodecyl sulfate. Cell lysate (4 mg total protein) was made by boiling samples at 100 °C, followed by sonication. Lysate was pre-cleared by incubating with 50 μl of protein A/G agarose beads (Thermo Fischer Scientific) for 1 h at 4 °C. Anti-ubiquitin antibody, anti-IFNGR1 antibody or respective species-specific control antibodies were incubated overnight with cell lysates. At the time of antibody addition, complete protease inhibitor and phosphatase inhibitors (Roche) were added besides 1 mM dithiothreitol. The lysates were incubated overnight with the antibodies, followed by three washes with Triton X-100 containing PBS and one wash with 20% sucrose in PBS. Western blot analysis was performed using the standard protocol. Details of the antibodies are provided in Supplementary Table 3.
Histological analysis, IHC and immunofluorescence.
Tumours, lungs and PDXs from the mice were processed as described by Chakrabarti et al.15,64. In brief, slides were heated at 56 °C for 45 min before deparaffinization. The slides were then deparaffinized using a standard process (xylene for 5 min followed by a gradient of 100% ethanol for 5 min, 95% ethanol for 5 min, 80% ethanol for 5 min before the slides were brought to distilled water for 5 min). Deparaffinized slides were then treated with sodium citrate (10 mM sodium citrate and 0.05% Tween 20 (pH 6.0)) for antigen retrieval. Antigen retrieval was performed by heating the slides for 20 min in a standard microwave followed by 20 min of cooling at room temperature. For IHC with F480 antibody, proteinase K solution was used for antigen retrieval. Proteinase K antigen retrieval was performed using 20 μg ml−1 proteinase K for 3 min at room temperature. For IHC analysis, human and mice tumour sections were counterstained with haematoxylin, whereas for immunofluorescence, nuclei were stained with DAPI. For immunofluorescence, after deparaffinization using the abovementioned process, slides were treated with sodium citrate (pH 6.0) for antigen retrieval, similar to the IHC slides. Details on the immunofluorescence primary and secondary antibodies are provided in Supplementary Table 4. IHC and immunofluorescence images were acquired using a Nikon TiE microscope. A confocal microscope (Leica SP5) was used for the Elf5–GFP reporter normal mammary gland, hyperplasia and tumour sections. Scoring was performed in a blinded fashion, whereby the researcher did not have prior knowledge of the slide identity. Several fields of view were taken for each sample. Multiple numbers of samples were used for scoring per group or experiment.
For human patient tumour sections, slides were initially heated at 60 °C for 1 h to help with deparaffinization. Following a standard process of deparaffinization, antigen retrieval was performed by heating the slides for 20 min in a standard microwave. followed by 20 min of cooling at room temperature. H-scores were calculated by multiplying the percentage (or abundance) of cells (scale: 0–100) expressing specific protein by the intensity of protein expression (scale: 0–3). For the transcription factors ELF5 and FBXW7, nuclear expression of the protein was considered for scoring. For IFNGR1, cytoplasmic/membrane expression of the protein was considered for scoring. Details of the antibodies are provided in Supplementary Table 4.
Flow cytometry and FACS sorting.
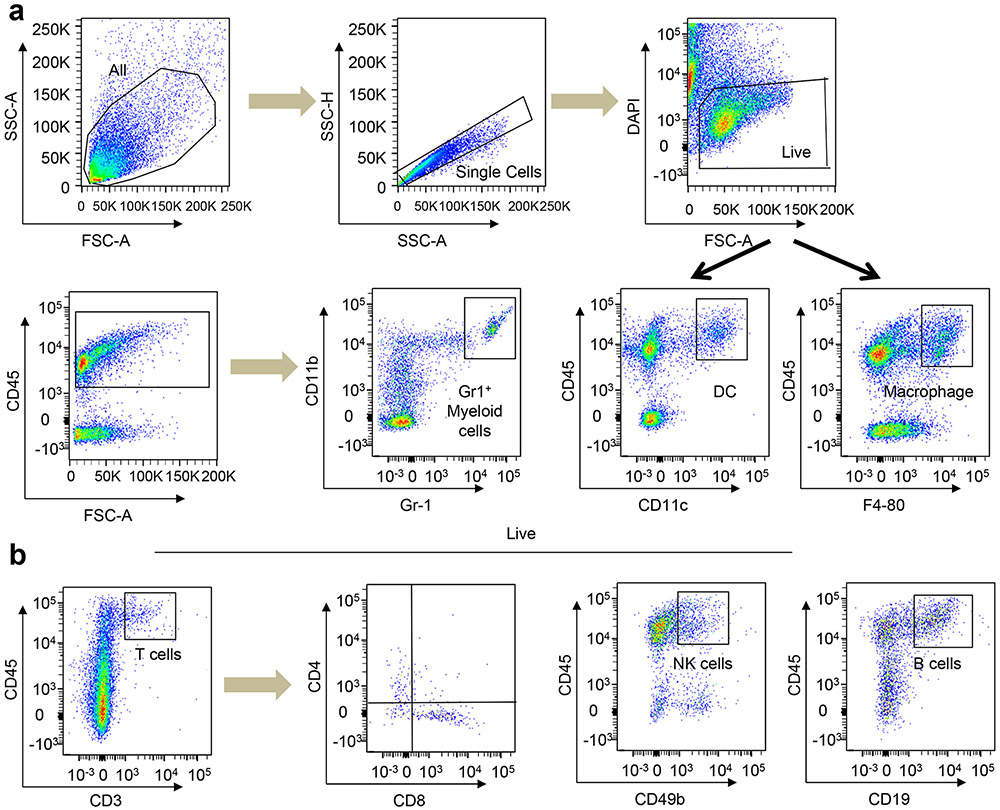
Tumours were processed to obtain single-cell suspension as described previously64. Tumour tissues were minced into small pieces followed by digestion using collagenase from Clostridium histolyticum (300 units ml−1; Sigma–Aldrich; catalogue number C2674) and hyaluronidase (100 units ml−1; Sigma–Aldrich; catalogue number H3884). The base medium used was DMEM/F-12 from Invitrogen (catalogue number 11330032) supplemented with epidermal growth factor (Novoprotein; catalogue number CO29), insulin (Life Sciences; catalogue number 51500056), (cholera toxin, Sigma–Aldrich; C8052) and hydrocortisone (Sigma–Aldrich; catalogue number h0888). Following digestions of whole tumour tissues, tumour cells were brought to single cells by the addition of 0.25% trypsin EDTA and dispase/DNAse solution (4 mg DNAse (Sigma–Aldrich) and 200 mg dispase (Invitrogen) in 40 ml PBS) sequentially. 10% heat-inactivated foetal bovine serum in PBS was used in between to stop the enzymatic reactions. Red blood cells were removed using 0.64% sterile ammonium chloride solution for 5 min at 37 °C. Tumour cells were further passed through a 0.45-μm strainer to obtain single cells. Single cells were then incubated with a combination of antibodies for 30 min in the dark. An LSRII/Fortessa Flow Cytometer (BD Biosciences) was used for FACS analysis, and FlowJo software (Tree Star) was used for analysis. Tumour and stromal cells were sorted using a FACSAria II flow cytometer. All antibodies were stained for 30 min in the dark at room temperature. For the lineage-negative epithelial-enriched tumour cells panel, we used the primary antibodies Biotin-CD45, Biotin-Ter119 and Biotin-CD31 to remove stromal cells along with red blood cells and leukocytes. The secondary antibodies used for this panel were streptavidin conjugated to PeCy7 (Invitrogen). For the myeloid immune cell panels, the direct fluorophore-conjugated antibodies used were CD45, CD11b, Gr1, CD11c and F4-80. For the lymphoid immune cell panels, the direct fluorophore-conjugated antibodies used were CD45, CD3, CD4, CD8, CD49b and CD19. Details of the antibodies are provided in Supplementary Table 5.
RNA and qPCR analysis.
To extract RNA from sorted tumour cells and cell lines, we used an RNA extraction kit (Invitrogen). RNA was extracted per the manufacturer’s instructions. Typically, 1–2 μg RNA was used to make complementary DNA using a superscript kit from Life Technologies (catalogue number 18091050), following the manufacturers protocol. An Applied Biosystems QuantStudio 3 machine (Thermo Fisher Scientific) was used for qPCR, which was performed using Power SYBR Green (Life Science). Gene-specific primers were used at a final concentration of 0.2 μM. The primers were bought from Integrated DNA Technologies. All qPCR assays were performed in duplicate and were repeated at least three times. qPCR plates were purchased from Applied Biosystems. Details of the primers are provided in Supplementary Table 2.
RNA-Seq analysis and GSEA.
Raw sequence files (FASTQ) for the samples were mapped using Salmon (https://combine-lab.github.io/salmon/) against the mouse transcripts described in GENCODE (https://www.gencodegenes.org; version M16 (built on the mouse genome GRCm38.p5) for the Elf5 reporter mice and version M14 (built on the mouse genome GRCm38.p5) for wild-type/Het data), with an average of 39.4 million total input reads for each sample and an average mapping rate of 87.1%. Transcript counts were summarized to the gene level using tximport (https://bioconductor.org/packages/release/bioc/html/tximport.html) and normalized and tested for differential expression using DESeq2 (https://bioconductor.org/packages/release/bioc/html/DESeq2.html).
GSEA (http://software.broadinstitute.org/gsea/index.jsp) was run for contrasts of interest in pre-ranked mode using the DESeq2 statistic of the ranking metric. Mouse gene symbols were mapped to human gene orthologues using Ensembl’s BioMart (http://www.ensembl.org/biomart/martview/). Where there were redundant mappings, the statistic with the highest absolute value was chosen. GSEA was conducted using GSEA 2.2.4 software. Enrichment scores for gene sets were generated C2.all and C5.all with defaults settings. C2 stands for curated gene sets and C5 stands for GO gene sets in GSEA database. Gene sets were tested for enrichment in rank-ordered lists via GSEA using a weighted Kolmogorov–Smirnov-like statistic to calculate the enrichment score. Statistical significance was assessed by comparing the enrichment score with enrichment results generated from 1,000 random permutations of the gene set to obtain P values (nominal P values). Raw enrichment scores were converted to normalized enrichment scores using default GSEA parameters.
Statistics and reproducibility.
The results were quantified using GraphPad Prism (version 5 or 8). For normally distributed datasets, significance was calculated by two-tailed Students t-test. For non-parametric datasets, Mann–Whitney U-tests were performed. The tumour growth datasets were analysed using the Bonferroni-corrected two-way analysis of variance (ANOVA) to compute statistical significance. For parametric data with multiple comparisons, one-way ANOVA with post-hoc Tukey’s test was used. For all of the Kaplan–Meier plots, the KM Plotter ‘auto select best cut-off’ option was used for stratification. The differences between the survival groups were evaluated statistically using log-rank tests. A Mann–Whitney U-test was used to evaluate the significance of the correlation between ELF5, FBXW7 and IFNGR1 in human patients. Pearson’s correlation values were calculated to determine the correlation between the expression levels of different proteins in patient tumours. Each experiment was repeated independently with similar results.
Human study approval.
De-identified breast cancer specimens used in this study were obtained for a commercial fee and obtained from the Eastern Division of the Cooperative Human Tissue Network at the University of Pennsylvania. All samples were considered exempt by the Institutional Review Board of the University of Pennsylvania. We confirm that informed consent was obtained from all patients and that the study is compliant with all of the relevant ethical regulations regarding research involving human participants. Information on the human research participants (age, gender, genotypic information and other details) is provided in Supplementary Table 6. For the PDX samples, all information is available in a published report from the laboratory of A. Welm47.
Reporting Summary.
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The GSEA data can be accessed using the accession numbers GSE122090 and GSE122180. Previously published MDA-MB-231 GSEA data can be accessed at GSE32144. The data and/or reagents that support the findings of this study are available from the corresponding author upon reasonable request. Source data for Figs. 1-8 and Extended Data Figs. 1-5 and 7-10 are provided online. Each experiment was repeated independently with similar results.
Extended Data
Extended Data Fig. 1 ∣. C3-T+; Elf5+/− tumor cells show increased tumorigenesis in REAR mice.
a, mRNA expression of Elf5 in tumor cells from C3-T+; Elf5+/− (n = 10) and C3-T+; Elf5+/+ (n = 3) individual tumors. qPCR values were normalized to Gapdh. Experiments were performed thrice with qPCR in technical duplicate. b, H&E staining of tumors shows more mesenchymal cells in C3-T+; Elf5+/− tumors. Images are representative of two independent experiments. c, Quantification of mitotic index in tumor sections stained with H&E (right panel), n= 3, C3-T+; Elf5+/− and n=4, C3-T+; Elf5+/+ individual tumors were used. d, Schematic showing representation of experiment in REAR recipient mice. 225,000 cells/MFP of sorted tumor cells from C3-T+; Elf5+/+ and C3-T+; Elf5+/− tumors were injected into contralateral mammary fat pad of REAR mice REAR mice. C3-T+; Elf5+/− tumor cells formed e, tumors earlier, n=6 tumors/group f, g, with rapid growth and volume at sacrifice, n=5 tumors for C3-T+; Elf5+/− and n=4 tumors for C3-T+; Elf5+/+ group. Log-rank test was used for KM plots to calculate p-value, (C3-T+; Elf5+/+, n=6 tumors; C3-T+; Elf5+/−, n=6 tumors in (e). f, Two-way ANOVA test was used with Bonferroni post hoc test to calculate statistical significance. Data presented as mean ± SEM. h, i, Metastatic nodules visible in lungs of REAR mice injected with C3-T+; Elf5+/− tumor cells, n=6 mice/genotype, p=0.09. Experiment performed once. j, k, H&E images showed increased mesenchymal features and invasive structures in tumors formed from C3-T+; Elf5+/− tumor cells. Images are representative to two independent experiments. (a, c, i) Two-tailed student′s t test was used to calculate statistical significance. Data presented as mean ± SEM. Scale bars, (b) 40 μm, (h), 100 μm, (j) 60 μm, (k) 200 μm. **p < 0.01.
Extended Data Fig. 2 ∣. Hyperplastic mammary glands from C3-T+; Elf5+/− tumors show increased P-STAT1 and P-STAT3 protein levels and Elf5 is predominantly expressed in tumor cells.
a, b, IHC staining indicates increased staining of IFNGR1 and P-STAT1 protein in hyperplastic mammary glands harvested from C3-T+; Elf5+/− compared to C3-T+; Elf5+/+ mice. One representative image is shown from multiple experiments (n=5). Arrows in (b) indicate P-STAT1+ cells. c, d, IHC staining indicates increased staining of P-STAT1 (Ser727) and P-STAT3 (Tyr705) proteins in tumors harvested from C3-T+; Elf5+/− compared to C3-T+; Elf5+/+ mice. n=5 individual tumors were taken in each group and quantification was done using 16 random fields. Two-tailed Mann-Whitney U test was used to calculate statistical significance and data is represented as mean ± SEM in (c) and mean ± SD in (d). e, Immunofluorescence image showing expression of GFP (Elf5) in tumor cells and their absence in stromal cells in tumor tissues obtained from C3-T+; Elf5-GFP. Absence of GFP in stromal cells of C3-T+ was confirmed by immunofluorescence in n=5 tumors. f, Flow cytometry graph showing presence of Elf5 predominantly in epithelial cells in tumors obtained from C3-T+; Elf5-GFP, one representative image shown from multiple experiments (n=5). FOV; Field of view. Scale bar in (a, c, d, e) 40 μm, (b) 20 μm.
Extended Data Fig. 3 ∣. Elf5 negatively correlates with IFN-γ signaling in C3-T+ tumors.
a, Schematic diagram showing generation of C3-T+; Elf5-GFP mice. b, Immunofluorescence images showing Elf5 loss during progression from mammary gland (N) to hyperplasia (H) to tumors. K14 antibody was used to mark basal epithelial cells. Experiment was repeated twice with similar results. c, Histogram showing %GFP+ cells in epithelial enriched tumor cells in C3-T+; Elf5-GFP mice. Tumor cell enriched population was sorted using CD31, CD45 and Tert119 markers following published protocol37. Red line denotes WT tumor cells as control. Experiment was repeated thrice with similar results. d, qPCR analysis of Elf5 expression in GFP+ and GFP− tumor cells sorted from C3-T+; Elf5-GFP mice, n=3. qPCR values were normalized to the housekeeping gene Gapdh. Experiments were performed three times, each with qPCR in technical duplicate, and data presented as the mean ± SD. Two- tailed Student′s t test was used to calculate statistical significance. e-i, GSEA graphs showing high EMT signatures, (e); increased invasive signatures, (f); high expression of signatures associated with stemness, (g); and increased IFN-γ signaling in GFP− (Elf5−) tumor cells, (h-i). n=2 GFP+ and n=4 GFP− samples were used for RNA sequencing and GSEA analysis. Statistical significance was assessed by comparing the ES to enrichment results generated from 1000 random permutations of the gene set to obtain p values (nominal p value) j, Western blot showing high P-STAT1 and STAT1 in GFP− (Elf5−). Cell sorting was performed to isolate tumor cells and multiple independent tumors were pooled to make samples. This is a representative blot and experiment was repeated twice with n=9 tumors. k, Heat map showing increased ISG in GFP− (Elf5−) tumor cells. n=2 GFP+ and n=4 GFP− tumor cell population was used to generate heat map. Scale bar in (b), 20 μm.
Extended Data Fig. 4 ∣. FBXW7, a ubiquitin ligase is a direct target of ELF5.
a, b, GSEA of GFP+ (Elf5+) and GFP− (Elf5−) tumor cells show upregulation of genes involved in proteasomal and ubiquitin mediated degradation in GFP+ (Elf5+) tumor cells as compared to GFP− (Elf5−) tumor cells, n=2 GFP+ and n=4 GFP− individual samples. c, List of ubiquitin ligases differentially expressed in GFP+ (Elf5+) and GFP− (Elf5−) tumor cells. d-g, qPCR analysis shows (d) increased ELF5 (e) increased FBXW7 (f) increased VHL and (g) no change in IFNGR1 mRNA in ELF5 expressing LM2 (lung metastatic derivative of MDA-MB-231) cells, n= independent 4 samples. h, i, Data mining for our published microarray data16 shows (h) decreased FBXW7 (i) and no change in VHL in cells upon transduction of ELF5 plasmid encoding mutation in DNA binding domain of ELF5 in MDA-MB-231 cells16 (n=3 independent biological samples/group). j, Western blot showing high ELF5, high FBXW7 and low IFNGR1 proteins upon re-expression of ELF5 in LM2 cells. k, Western blot images showing positive correlation between FBXW7 and ELF5, and their negative correlation with IFNGR1 in TNBC cells (MX1, LM2, HCC1806, BT549). MCF7 cells was used as positive control. l-n, qPCR of EpRas cells (n=6 independent biological samples/group) with re-expression of ELF5 shows (l) high Elf5 (m) high Fbxw7 (n) and no change in Ifngr1 mRNA levels. o, Expression of ELF5 in EpRas cells increases FBXW7 and decreases IFNGR1 protein levels as shown by western blotting. qPCR values were normalized to the housekeeping gene Gapdh. Experiments were performed three times, each with qPCR in technical duplicate, and data presented as the mean ± SD. Two tailed Student′s t test was used to calculate statistical significance. Please see uncropped WB images in Source data file.
Extended Data Fig. 5 ∣. ELF5 binds to multiple genomic loci of FBXW7 in normal mammary epithelial cells and ELF5 KD MX1 cells show increased expression of IFNGR1.
a, In silico ChIP-seq analysis showing multiple binding sites of ELF5 on genomic loci of FBXW7. Lac1 denotes mammary gland obtained from lactating mouse and P13 denotes mammary gland obtained from pregnant mouse at day 13. Black vertical lines show binding sites. b, FACS analysis showing increase in IFNGR1 protein expression upon stable knockdown of ELF5 in MX1 TNBC cells using multiple shRNAs. Data is representative of two independent experiments. c, d IHC images showing FoxP3 staining in periphery and core of tumor sections obtained from C3-T+; ELF5+/+ and C3-T+; ELF5+/− mice. n=3 samples were used. e, Contour plots and f, g, scatter plots showing increased Ly6G+ neutrophil population in tumors from C3-T+; Elf5+/− mice as compared to C3-T+; Elf5+/+ tumors, n=3, C3-T+; Elf5+/+ tumors and n=5, C3-T+; Elf5+/− individual tumors. h, Suppression of T-cell proliferation confirms that increased myeloid cells in tumors are Gr1+ myeloid cells. Tumor CD45+CD11b+Gr1+ neutrophils were used. Tumor neutrophils (Gr1+ myeloid cells) were enriched using Gr1 enrichment kit following manufacture′s protocol. Data is representative of two independent experiments. (f, g) Mann-Whitney U test was used to calculate statistical significance. Data presented as mean ± SEM. Scale bar, 200 μM.
Extended Data Fig. 6 ∣. Flow cytometry gates showing different immune populations.
All the gates were drawn according to published protocol from Dr. Vonderheide group59. (a, b) Different myeloid and lymphoid populations are shown in a and b respectively.
Extended Data Fig. 7 ∣. Alteration of Elf5 in tumor cells alters TME in REAR background.
IHC analysis shows a, b, increased myeloid cells in C3-T+; Elf5+/− (n=8 FOV) tumors as compared to C3-T+; Elf5+/+ (n=6 FOV) c, d, decreased number of macrophages in C3-T+; Elf5+/− tumors (n=9 FOV) as compared to C3-T+; Elf5+/+ tumors (n=7 FOV), e, f, increased number of Foxp3+ cells in C3-T+; Elf5+/− tumors. (n=10 FOV for C3-T+; Elf5+/+ and n=11 FOV for C3-T+; Elf5+/−), n=3 individual tumors were used in a-f. g, j, increased Gr1+ myeloid cells (n=5 tumors/group) and Ly6G+ neutrophils (CD45+CD11b+Gr1+) (n=5 tumors/group) and decreased k, cytotoxic T-cells (CD45+CD8+) population in C3-T+; Elf5+/− tumors as compared to C3-T+; Elf5+/+ tumors (n=5 tumors/group) h, Macrophages (CD45+F4/80+) (n=5 C3-T+; Elf5+/+ tumors and n=6 C3-T+; Elf5+/− tumors) and i, Regulatory T-cells (CD45+CD3+FoxP3+) (n=3 tumors/group) were observed. IHC image showing increased FBXW7 (n=6 tumors for control and n=7 tumors for Elf5-OE from n=3 independent tumors). (l), decreased IFNGR1 (n=10 FOV for control and n=7 FOV for Elf5-OE from n=3 independent tumors) m, in Elf5-OE EpRas tumors. FACS plots showing no change in n, macrophages, o, CD4+ T-cells, p, increased Ly6C+ neutrophils in Elf5-OE tumors (n=6, control and n=4, Elf5-OE individual tumors). q FACS plots showing decreased number of Gr1+ myeloid cells in lungs of EpRas Elf5-OE tumor bearing mice (n=3 individual tumors/group). (a-j and n-q) Two-tailed student′s t test was used to compute p-value. (k, l, m) Mann-Whitney U test was used to calculate p value. Data are presented as the mean ± SEM. FOV; Field of view. (b, d, f, l, m) Boxplot data represent median, interquartile range, and spikes to upper and lower adjacent values. FOV; Field of view. (a, c, e, l, m) Images are representative of minimum three independent experiments, Scale bars, 40 μm.
Extended Data Fig. 8 ∣. IFN-γ signaling imparts more tumorigenic and metastatic potential.
IFN-γ treatment upregulates a, STAT1 and P-STAT1 (Data representative of minimum of three independent experiments) b, mRNA expression of IFN-γ target genes. qPCR values were normalized to Gapdh. Experiments were performed twice in technical duplicate, and data presented as mean ± SD. Two-sided Student′s t test used to calculate p-value. c, FACS analysis show upregulation of PD-L1 but not CTLA4 in IFN-γ-treated EpRas cells. Data representative of two independent experiments. d, e, IFN-γ treated EpRas cells form larger and more invasive tumors, n=5 tumors/group. Inset shows higher magnification images. Data represented as mean ± SD. f, g, Small metastatic nodules (black arrows) in lungs of mice injected with IFN-γ treated EpRas cells. H&E staining shows presence of micro metastasis in lungs of indicated groups. (g). (e-g) Experiment was repeated twice with similar results. (h-l) IHC analysis shows high (h) P-STAT1+, (i) S100A8+ cells in IFN-γ treated EpRas tumors. (j) Decreased CD8+T-cells observed in IFN-γ treated tumors. (k, l) Increase in S100A8+ cells observed in IFN-γ treated EpRas lung sections (metastatic site) with quantification in the right panel. Boxplot data represent median, interquartile range, and spikes to upper and lower adjacent values. (h-l). Two-way ANOVA test was used with Bonferroni post hoc test to calculate p value in (d). Two-tailed Mann-Whitney U test was used to compute p-value for h, j, i, l and data is represented as mean ± SEM. n=10 random FOV were evaluated from 3 individual tumors (h, j) n=3 and 5 random FOV were evaluated (i), n=3 and 7 random FOV/group were used for quantification (l). (e-g) Representative images of minimum two independent experiments are presented. Scale bar, (e) 200μm, (f) 2mm, and (g-k) 40 μm respectively. ***p < 0.001. FOV; Field of view.
Extended Data Fig. 9 ∣. IFN-γ signaling renders more metastatic properties to EpRas cells via PD-1/PD-L1 signaling.
a, b, Tail vein (TV) injection of control and IFN-γ treated EpRas cells, n=7 mice/group. c, d, Lung images and metastatic nodule numbers in NSG mice injected with EpRas cells treated with IFN-γ via TV (n=6 mice, control and n=7 mice, IFN-γ treated). e, f, TV injection of control and IFN-γ treated EpRas cells in mice treated with anti-IgG (n=5 mice), anti-PD-1 (n=5 mice) and anti-PD-L1 (n=6 mice) antibodies. g, h, IHC in tumors from control cells compared to IFNGR1-KD tumor cells, n=3 individual tumors were used and 7 random FOV were used. i, FBXW7-KD tumors cells grew faster than control, n=5 tumors/group. k, Western blot showed decreased FBXW7 in EpRas cells transduced with FBXW7 shRNAs compared to control. (l) IF analysis shows increased expression of IFNGR1 in FBXW7-KD cells compared to control. Experiment was repeated twice (k, l). j, m, TV injection of control and FBXW7-KD EpRas cells shows increased number of lung metastasis in FBXW7-KD mice compared to control mice, n=3 CTRL, n=4 KD1, and n=4 KD2 mice were used. Two-tailed Mann-Whitney U test was used to compute p-value in (b, d) and data represented as mean ± SEM (b) or ±S.D (d). Boxplot represent median, interquartile range, and spikes to upper and lower adjacent values (f, h). One-way ANOVA test was used with Tukey post hoc test to compute p-values of multiple comparison data. Data is represented as mean ± SEM (f, h, j). Two-way ANOVA test was used with Bonferroni post hoc test to calculate p values, data is represented as mean ± SD (i). Scale bar (a, c, e, m) 2 mm, (g) 40 μm (l) 100 μm. **p < 0.01, ***p < 0.001. FOV; Field of view.
Extended Data Fig. 10 ∣. High expression of ELF5/FBXW7 and low expression of IFNGR1 is protective in Mesenchymal, basal and immunomodulatory TNBC.
a-d, High expression of ELF5/FBXW7 and low expression of IFNGR1 is correlated with better Distant metastasis free survival (DMFS) in (a) Basal (n=24 for Elf5/FBXW7 and (n=32 for IFNGR1) (b) Immunomodulatory (n=47 patient samples for Elf5/FBXW7 and (n=36 patient samples for IFNGR1) (c) Mesenchymal TNBC subsets (n=57 for Elf5/FBXW7 and (n=57 patient samples for IFNGR1). (d) High expression of ELF5/FBXW7 and low expression of IFNGR1 is correlated with worse Distant metastasis free survival (DMFS) in Non-TNBC ER+ patients (n=437 patient samples for Elf5/FBXW7 and (n=1391 patient samples for IFNGR1). P-value was computed by Log Rank test. KM plotter database was used57. e, TNBC PDXs showing direct correlation between ELF5 and FBXW7, and indirect correlation with IFNGR1. 5 TNBC PDXs were used for this experiment. Two-tailed Mann-Whitney U test was used for statistical analysis and data is represented as mean ± SEM. (f-h) IHC images of TNBC PDXs showing direct correlation between ELF5 and FBXW7, and indirect correlation with IFNGR1. Scale bar, 40 μm.
Supplementary Material
Acknowledgements
We thank L. King (University of Pennsylvania) for critical reading of the manuscript and helpful discussions. We thank A. Minn (University of Pennsylvania) for helpful discussions. We thank the Penn Vet Comparative Pathology Core for assistance with embedding, sectioning and consultation on tumour sample analysis. We thank Y. Kang (Princeton University) for the HEK293T, EpRas, 4T1, LM2 and BT549 cell lines. We thank S. Ran (Southern Illinois University) for the HCC1806 cell line. We thank the Eastern Division of the Cooperative Human Tissue Network at The University of Pennsylvania for providing human breast cancer fixed tissues from patients. We thank A. Welm (University of Utah) for the PDX tumour tissues. We thank the members of the Flow Cytometry Core at the Children’s Hospital of Philadelphia and University of Pennsylvania. We thank the Penn Vet Imaging Core for confocal microscopy. This work was supported by grants from the American Cancer Society, an NCI-K22 grant to R.C. (K22CA193661-01) and an NCI-R01 (R01 CA237243-01A1) grant to R.C.
Footnotes
Competing interests
The authors declare no competing interests.
Extended data is available for this paper at https://doi.org/10.1038/s41556-020-0495-y.
Supplementary information is available for this paper at https://doi.org/10.1038/s41556-020-0495-y.
Reprints and permissions information is available at www.nature.com/reprints.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kwa MJ & Adams S Checkpoint inhibitors in triple-negative breast cancer (TNBC): where to go from here. Cancer 124, 2086–2103 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Liu Z, Li M, Jiang Z & Wang X A comprehensive immunologic portrait of triple-negative breast cancer. Transl. Oncol 11, 311–329 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Teijido P, Cabal ML, Fernandez IP & Perez YF Tumor-infiltrating lymphocytes in triple negative breast cancer: the future of immune targeting. Clin. Med. Insights Oncol 10, 31–39 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar S et al. DeltaNp63-driven recruitment of myeloid-derived suppressor cells promotes metastasis in triple-negative breast cancer. J. Clin. Invest 128, 5095–5109 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quail DF & Joyce JA Microenvironmental regulation of tumor progression and metastasis. Nat. Med 19, 1423–1437 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Markowitz J, Wesolowski R, Papenfuss T, Brooks TR & Carson WE 3rd Myeloid-derived suppressor cells in breast cancer. Breast Cancer Res. Treat 140, 13–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanahan D & Coussens LM Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21, 309–322 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Zhou J et al. Cancer-associated fibroblasts correlate with tumor-associated macrophages infiltration and lymphatic metastasis in triple negative breast cancer patients. J. Cancer 9, 4635–4641 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu T & Di G Role of tumor microenvironment in triple-negative breast cancer and its prognostic significance. Chin. J. Cancer Res 29, 237–252 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng L et al. Immune profiles of tumor microenvironment and clinical prognosis among women with triple-negative breast cancer. Cancer Epidemiol. Biomark. Prev 28, 1977–1985 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Yuan ZY, Luo RZ, Peng RJ, Wang SS & Xue C High infiltration of tumor-associated macrophages in triple-negative breast cancer is associated with a higher risk of distant metastasis. Onco. Targets Ther 7, 1475–1480 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi YS, Chakrabarti R, Escamilla-Hernandez R & Sinha S Elf5 conditional knockout mice reveal its role as a master regulator in mammary alveolar development: failure of Stat5 activation and functional differentiation in the absence of Elf5. Dev. Biol 329, 227–241 (2009). [DOI] [PubMed] [Google Scholar]
- 13.Harris J et al. Socs2 and elf5 mediate prolactin-induced mammary gland development. Mol. Endocrinol 20, 1177–1187 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Zhou J et al. Elf5 is essential for early embryogenesis and mammary gland development during pregnancy and lactation. EMBO J. 24, 635–644 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakrabarti R et al. Elf5 regulates mammary gland stem/progenitor cell fate by influencing notch signaling. Stem Cells 30, 1496–1508 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakrabarti R et al. Elf5 inhibits the epithelial–mesenchymal transition in mammary gland development and breast cancer metastasis by transcriptionally repressing Snail2. Nat. Cell Biol 14, 1212–1222 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mojic M & Takeda K & Hayakawa Y The dark side of IFN-γ: its role in promoting cancer immunoevasion. Int. J. Mol. Sci 19, E89 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schreiber RD, Old LJ & Smyth MJ Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331, 1565–1570 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Mimura K et al. PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer. Cancer Sci. 109, 43–53 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaidi MR et al. Interferon-γ links ultraviolet radiation to melanomagenesis in mice. Nature 469, 548–553 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J & Weinberg RA Epithelial–mesenchymal transition: at the crossroads of development and tumor metastasis. Dev. Cell 14, 818–829 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Thiery JP Epithelial–mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2, 442–454 (2002). [DOI] [PubMed] [Google Scholar]
- 23.Polyak K & Weinberg RA Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat. Rev. Cancer 9, 265–273 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Thiery JP, Acloque H, Huang RY & Nieto MA Epithelial–mesenchymal transitions in development and disease. Cell 139, 871–890 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Lin NU et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 118, 5463–5472 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Ruijter TC, Veeck J, de Hoon JP, van Engeland M & Tjan-Heijnen VC Characteristics of triple-negative breast cancer. J. Cancer Res. Clin. Oncol 137, 183–192 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dent R et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin. Cancer Res 13, 4429–4434 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Sorlie T et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl Acad. Sci. USA 100, 8418–8423 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Echeverria GV et al. High-resolution clonal mapping of multi-organ metastasis in triple negative breast cancer. Nat. Commun 9, 5079 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aprelikova O et al. Development and preclinical application of an immunocompetent transplant model of basal breast cancer with lung, liver and brain metastases. PLoS ONE 11, e0155262 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katayama H et al. An autoimmune response signature associated with the development of triple-negative breast cancer reflects disease pathogenesis. Cancer Res. 75, 3246–3254 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jezequel P et al. Gene-expression molecular subtyping of triple-negative breast cancer tumours: importance of immune response. Breast Cancer Res. 17, 43 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi YS, Cheng J, Segre J & Sinha S Generation and analysis of Elf5-LacZ mouse: unique and dynamic expression of Elf5 (ESE-2) in the inner root sheath of cycling hair follicles. Histochem. Cell Biol 129, 85–94 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Ayers M et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Invest 127, 2930–2940 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu X et al. Activation of the JAK/STAT-1 signaling pathway by IFN-γ can down-regulate functional expression of the MHC class I-related neonatal Fc receptor for IgG. J. Immunol 181, 449–463 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villarino AV, Kanno Y, Ferdinand JR & O'Shea JJ Mechanisms of Jak/STAT signaling in immunity and disease. J. Immunol 194, 21–27 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shackleton M et al. Generation of a functional mammary gland from a single stem cell. Nature 439, 84–88 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Yeh CH, Bellon M & Nicot C FBXW7: a critical tumor suppressor of human cancers. Mol. Cancer 17, 115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gossage L, Eisen T & Maher ER VHL, the story of a tumour suppressor gene. Nat. Rev. Cancer 15, 55–64 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Shin HY et al. Hierarchy within the mammary STAT5-driven Wap super-enhancer. Nat. Genet 48, 904–911 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marchetti M et al. Stat-mediated signaling induced by type I and type II interferons (IFNs) is differentially controlled through lipid microdomain association and clathrin-dependent endocytosis of IFN receptors. Mol. Biol. Cell 17, 2896–2909 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takeuchi Y & Nishikawa H Roles of regulatory T cells in cancer immunity. Int. Immunol 28, 401–409 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vinay DS et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin. Cancer Biol 35, S185–S198 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Facciabene A, Motz GT & Coukos G T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 72, 2162–2171 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou Q, Facciponte J, Jin M, Shen Q & Lin Q Humanized NOD-SCID IL2rg−/− mice as a preclinical model for cancer research and its potential use for individualized cancer therapies. Cancer Lett. 344, 13–19 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Andre MC et al. Long-term human CD34+ stem cell-engrafted nonobese diabetic/SCID/IL-2Rγnull mice show impaired CD8+ T cell maintenance and a functional arrest of immature NK cells. J. Immunol 185, 2710–2720 (2010). [DOI] [PubMed] [Google Scholar]
- 47.DeRose YS et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat. Med 17, 1514–1520 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gradishar WJ et al. NCCN Guidelines Insights: Breast Cancer, Version 1.2017. J. Natl Compr. Cancer Netw 15, 433–451 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Blows FM et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 7, e1000279 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu W & Kang Y Epithelial–mesenchymal plasticity in cancer progression and metastasis. Dev. Cell 49, 361–374 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soundararajan R et al. Targeting the interplay between epithelial-to-mesenchymal-transition and the immune system for effective immunotherapy. Cancers (Basel) 11, E714 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McNutt M Cancer immunotherapy. Science 342, 1417 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Vikas P, Borcherding N & Zhang W The clinical promise of immunotherapy in triple-negative breast cancer. Cancer Manag. Res 10, 6823–6833 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dirix LY et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res. Treat 167, 671–686 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nanda R et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J. Clin. Oncol 34, 2460–2467 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li CH, Karantza V, Aktan G & Lala M Current treatment landscape for patients with locally recurrent inoperable or metastatic triple-negative breast cancer: a systematic literature review. Breast Cancer Res. 21, 143 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gyorffy B et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res. Treat 123, 725–731 (2010). [DOI] [PubMed] [Google Scholar]
- 58.Busino L et al. Fbxw7α- and GSK3-mediated degradation of p100 is a pro-survival mechanism in multiple myeloma. Nat. Cell Biol 14, 375–385 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bayne LJ & Vonderheide RH Multicolor flow cytometric analysis of immune cell subsets in tumor-bearing mice. Cold Spring Harb. Protoc 2013, 955–960 (2013). [DOI] [PubMed] [Google Scholar]
- 60.Pearton DJ, Broadhurst R, Donnison M & Pfeffer PL Elf5 regulation in the trophectoderm. Dev. Biol 360, 343–350 (2011). [DOI] [PubMed] [Google Scholar]
- 61.Deome KB, Faulkin LJ Jr, Bern HA & Blair PB Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 19, 515–520 (1959). [PubMed] [Google Scholar]
- 62.Chakrabarti R & Kang Y Transplantable mouse tumor models of breast cancer metastasis. Methods Mol. Biol 1267, 367–380 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Welte T et al. Oncogenic mTOR signalling recruits myeloid-derived suppressor cells to promote tumour initiation. Nat. Cell Biol 18, 632–644 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chakrabarti R et al. ΔNp63 promotes stem cell activity in mammary gland development and basal-like breast cancer by enhancing Fzd7 expression and Wnt signalling. Nat. Cell Biol 16, 1004–1015 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The GSEA data can be accessed using the accession numbers GSE122090 and GSE122180. Previously published MDA-MB-231 GSEA data can be accessed at GSE32144. The data and/or reagents that support the findings of this study are available from the corresponding author upon reasonable request. Source data for Figs. 1-8 and Extended Data Figs. 1-5 and 7-10 are provided online. Each experiment was repeated independently with similar results.