Abstract
Purpose
Dysregulation of the alternative complement pathway is a major pathogenic mechanism in age-related macular degeneration. We investigated whether locally synthesized complement components contribute to AMD by profiling complement expression in postmortem eyes with and without AMD.
Methods
AMD severity grade 1 to 4 was determined by analysis of postmortem acquired fundus images and hematoxylin and eosin stained histological sections. TaqMan (donor eyes n = 39) and RNAscope/in situ hybridization (n = 10) were performed to detect complement mRNA. Meso scale discovery assay and Western blot (n = 31) were used to measure complement protein levels.
Results
The levels of complement mRNA and protein expression were approximately 15- to 100-fold (P < 0.0001–0.001) higher in macular retinal pigment epithelium (RPE)/choroid tissue than in neural retina, regardless of AMD grade status. Complement mRNA and protein levels were modestly elevated in vitreous and the macular neural retina in eyes with geographic atrophy (GA), but not in eyes with early or intermediate AMD, compared to normal eyes. Alternative and classical pathway complement mRNAs (C3, CFB, CFH, CFI, C1QA) identified by RNAscope were conspicuous in areas of atrophy; in those areas C3 mRNA was observed in a subset of IBA1+ microglia or macrophages.
Conclusions
We verified that RPE/choroid contains most ocular complement; thus RPE/choroid rather than the neural retina or vitreous is likely to be the key site for complement inhibition to treat GA or earlier stage of the disease. Outer retinal local production of complement mRNAs along with evidence of increased complement activation is a feature of GA.
Keywords: age-related macular degeneration, complement expression, geographic atrophy, RNAscope
There is substantial evidence that dysregulation of the complement system plays an important role in the pathogenesis of age-related macular degeneration.1–3 Complement proteins are components of drusen, a clinical hallmark of AMD.1,4–6 Single-nucleotide polymorphisms in multiple complement genes are associated with the risk for AMD.4,7–23 AMD high-risk alleles encode variant proteins with increased ability to activate the alternative complement pathway (AP).2 Complement activation products are moderately elevated in the plasma and eyes from AMD patients.24–33 Some (but not all) complement inhibitors delivered intravitreally in early clinical trials modestly reduced the rate of geographic atrophy growth.34–38
The complement cascade is a critical part of the innate immune response to pathogens and tissue damage. Excessive complement activation is a feature of many diseases, including C3 glomerulopathy and paroxysmal nocturnal hemoglobinuria.39,40 In AMD, local tissues including the choroidal vasculature, the retinal pigment epithelium (RPE), and photoreceptors are dysfunctional. The rich vascularity of the choroid exposes that tissue to systemic complement components, whereas the neural retina and photoreceptors exposure to blood-borne complement proteins is prevented by a functional outer blood-retinal barrier, formed by RPE/Bruch's membrane. The eye is an immune privileged site partly because of the presence of surface complement regulators preventing or rapidly shutting down complement activation.41–44 Recent single-cell and single nuclear RNAseq studies of normal healthy human eyes showed specific ocular cell types expressing certain complement genes,45–50 although complement expression and its cellular distribution in AMD eyes are still not well understood.
In this study, we compared complement mRNA and protein levels in isolated neural retina versus RPE/choroid tissues and analyzed the expression of complement genes in normal donor eyes and those with early, intermediate, and late stages of AMD. We identified that the RPE/choroid tissue is the major source of ocular complement components, by mRNA, as well as protein analysis. In addition, we found the normal expression pattern of complement mRNA and alterations in its spatial distribution in GA eyes.
Methods
Postmortem Donor Eye Collection and AMD Severity Grading
Postmortem human eyes were procured by the Lions Eye Institute for Transplant & Research (Tampa, FL, USA) with consent of donors or donors’ next of kin and in accordance with the Eye Bank Association of America medical standards, US/Florida law for human tissue donation, the Declaration of Helsinki and Food and Drug Administration regulations, and Novartis human tissue registration working practice guidelines regarding research using human tissues. Postmortem eye collection methods and reagents are in Supplementary Materials. AMD grading on hematoxylin and eosin sections was determined following criteria from the Sarks, Age-Related Eye Disease Study, and The Beckman Group clinical grading systems.51–53 The postmortem eye's AMD status for TaqMan and protein assays was determined by analyzing the fundus images of posterior eyes for criteria from the Minnesota Grading System for assignment into four levels (normal/unaffected controls = AMD1, early = AMD2, intermediate = AMD3, and late AMD = AMD4) as described.51
RNA and Protein Assays
Sample preparation methods and materials for TaqMan, Meso scale discovery (MSD) assay, and Western blot are in Supplementary Materials. TaqMan method was described previously.54 The ΔCt value was calculated by subtracting the GAPDH Ct from the gene of interest Ct. The relative expression of each gene of interest was calculated as 2−ΔCt. Certain complement mRNA values were absent in some samples because of a lack of detectable amplification (Ct = 40) whereas all samples had normal GAPDH values. Gel electrophoresis and Western blot methods were as described by Crowley et al.54 MSD-based assays (MSD, Rockville, MD, USA) were run on a Sector S 600 imager, using capture/detection antibody pairs to obtain molar concentration for each complement protein, which was then converted to nanograms or milligrams per milliliter on the basis of individual molecular weight. The concentration for each complement protein in tissue lysates was normalized by the total protein concentration in milligrams per milliliter measured by BCA (Bicinchoninic Acid) Protein Assay (Thermo Fisher Scientific, Waltham, MA, USA) to generate nanograms or micrograms of each complement component per milligram of total protein lysate. C3b MSD captures C3 alpha chain and C3(H2O). CFH MSD captures both full-length FH and truncated FHL-1 proteins and was expressed as picomoles per milligram lysate.
RNAScope and Immunohistochemistry
Methods and materials for RNAScope and immunohistochemistry are in Supplementary Materials.
Data Analysis
Prism software was used for statistics. Data are presented as mean ± standard error of mean (SEM). In heat maps, mRNA or protein expression values were converted to Z-score (= (X − mean)/standard deviation). Comparisons of means for complement mRNA or protein levels between neural retina and RPE/choroid tissue were generated using unpaired two-tailed t-tests, adjusting for overall alpha level by number of genes tested (n = 5, P < 0.01 significance). For comparisons of means between normal control/AMD1 and different AMD grades (early/AMD2, intermediate/AMD3, or late/AMD4), one-way ANOVA with Dunnett's test for multiple comparison was used (P < 0.05 significance).
Results
AMD Eye Selection and Classification
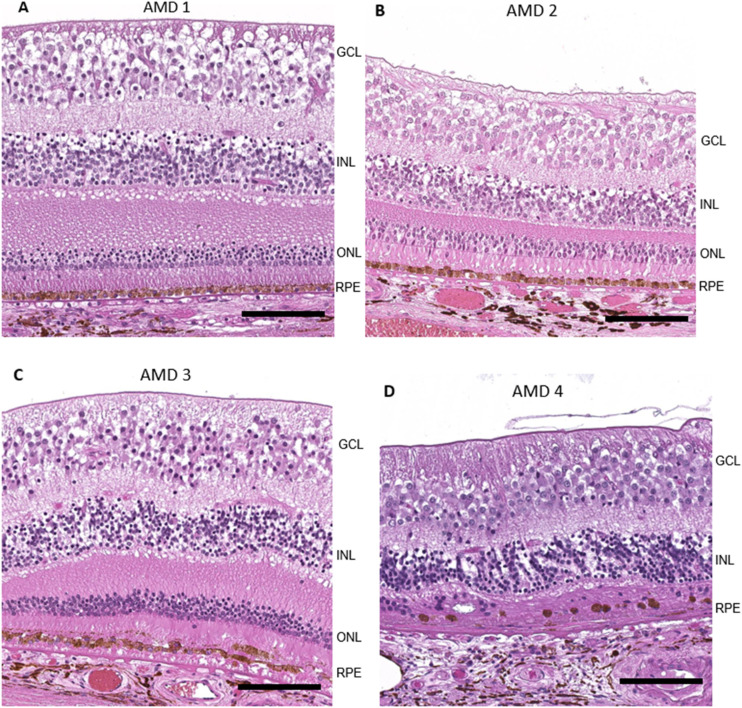
Stratification of donor eyes (normal control/AMD1, early/AMD2, intermediate/AMD3, late AMD/AMD4) selected for TaqMan and protein studies was based on the Minnesota Eye grading system of postmortem fundus images.51 Donor eye sections for RNAscope were selected on the basis of histopathological features (Supplementary Table S1).52,53 AMD1 eyes had normal retinal morphology and were considered healthy controls (Fig. 1A). AMD2 (early-stage AMD) eyes had small drusen and subtle morphological changes in the RPE such as a cobble stone appearance and hypertrophy (Fig. 1B). AMD3 (intermediate AMD) eyes displayed partial loss of photoreceptor outer segments, extensive sub-RPE deposits, and abnormal morphology such as focal RPE loss, pigmentary changes, and hypertrophy (Fig. 1C). AMD4 (advanced AMD) eyes either had geographic atrophy (Fig. 1D, extensive atrophic lesions such as loss of RPE cells, degeneration of inner and outer segment [IS/OS], and severe thinning or absence of the outer nuclear layer), or had choroidal neovascularization (CNV) in the macula; some eyes had both GA and CNV. For the present study, eyes with CNV were excluded from analysis. AMD stage determined by histology was generally consistent with fellow eye AMD disease stage determined by the Minnesota Eye postmortem fundus image analysis.
Figure 1.
Representative hematoxylin and eosin (H&E) images of macular area AMD1–4. Donor eyes with AMD grading based on the Age-Related Eye Disease Study grading system52 were evaluated histologically with standard H&E staining on paraffin sections. (A) Macular region of an AMD1 donor with normal retinal morphology. (B) Sub-RPE deposits and subtle morphological changes in RPE layer in an AMD2/early AMD macula. The section in B is thinner than section in A because it is further from the fovea. (C) Extensive sub-RPE deposits, abnormal RPE morphology, and partial loss of photoreceptor outer segments in an AMD3/intermediate macula. (D) Severe degeneration of RPE cells, IS/OS, and ONL in an AMD4/geographic atrophy macula. Scale bar: 100 µm.
High Complement Gene Expression in Macular RPE/Choroid Regardless of AMD Grade and in the Macular Neural Retina of GA eyes
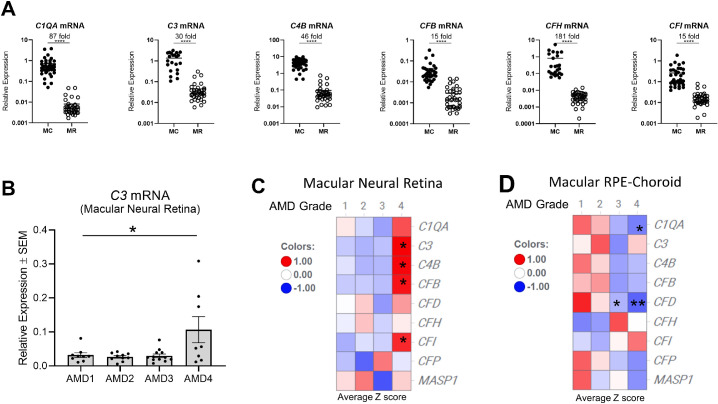
Complement gene expression of nine complement components (C1QA, C3, C9, CFB, CFD, CFH, CFI, CFP, and MASP1) was assessed by Taqman analysis of macular tissues from donor eyes with AMD (Supplementary Table S2). We compared the average level of each specific mRNA in both the neural retina and RPE/choroid across all AMD grades. Regardless of AMD status, the levels of mRNAs (C1QA, C3, CFB, CFH, CFI) in the macular RPE/choroid were 15- to 180-fold higher than in macular neural retina of the eye (P < 0.001–0.0001) (Fig. 2A), suggesting that cells within RPE/choroid produce much more complement mRNA compared to neural retinal cells. CFH, one of the strongest AMD associated genes in GWAS studies,8 is particular interesting because its mRNA had the greatest difference between RPE/choroid and neural retina (∼180-fold, P < 0.0001). C9 mRNA levels were nondetectable (Ct value = 40) in both tissues using our analytical methods. The analysis of mRNA for CFP, CFD, and MASP1 gave Ct values mostly in the range of 32–40 in macular neural retina, close to the level of sensitivity for the assay. This suggests that these factors are either not expressed or expressed at very low levels in the macular neural retina tissues. The low levels of expression meant that comparisons of these complement factors between retina and RPE/choroid would have low statistical power, and thus comparisons were not carried out.
Figure 2.
Complement mRNA level is higher in RPE/choroid than in neural retina and is increased in macular neural retina of AMD4 eyes. TaqMan analysis of selected complement genes was performed with both macular RPE/choroid (MC) and macular neural retina (MR) tissue from AMD1 to AMD4 eyes. N = 8–12 donors per AMD grade group. Levels of complement gene expression were normalized with GAPDH and expressed as relative expression. (A) Comparison of relative complement mRNA levels (C1QA, C3, CFB, CFH, and CFI) between macular RPE/choroid (filled circle) and macular neural retina (open circle) regardless of AMD grades. Donor eye number for each gene: N = 39 macular neural retinas and N = 21–39 macular RPE/choroid tissues. Average fold changes between macular RPE/choroid and neural retina for each complement mRNA are indicated. ***P < 0.001, ****P < 0.0001 unpaired two-sided t test. (B) A dot plot of C3 mRNA levels versus AMD grades in macular neural retina. *P < 0.05, one-way ANOVA Dunnett posttests relative to AMD1 normal control. Heatmaps of C1QA, C3, CFB, CFD, CFH, CFI, CFP, and MASP1 gene expression in macular neural retina (C) and macular RPE/choroid (D) of AMD grade 1 to 4 eyes were expressed as average z-score and colored in gradients. Donor eye number for each AMD grade: AMD1 N = 9, AMD2 N = 10, AMD3 N = 12, AMD4 N = 8. Red values are above mean value and blue below mean value. *P < 0.05, **P < 0.01, one-way ANOVA Dunnett posttests relative to AMD1 normal control.
The second analysis examined if complement gene expression in the eye correlates with AMD disease severity. In the macular neural retina, C3 mRNA levels in eyes with AMD grades AMD2 and AMD3 were comparable to normal eyes (AMD1). In comparison, eyes with AMD grade AMD4 had levels about threefold higher (P < 0.05) (Fig. 2B). Levels of CFB and CFI expression in macular neutral retinas from AMD1 to AMD3 were also comparable to AMD1 and on average two- to threefold higher in AMD4 GA eyes (P < 0.05) (Fig. 2C). The higher expression in AMD4 GA eyes suggests that complement synthesis by the neural retina may contribute to disease at this late stage (AMD4) but is unlikely to have a role in the early (AMD2) or intermediate (AMD3) stages of the disease.
The RPE/choroid had no significant differences in the mRNA expression of complement components between AMD eyes and healthy controls (Fig. 2D) except for C1QA and CFD in AMD4 eyes, which were reduced to 21% and 37% of control AMD1 values (P < 0.05 and 0.01, respectively). Possibly, this is due to reduced numbers of choroidal macrophages or other resident cell types associated with choriocapillaris loss in advanced AMD.55–57 A single cell RNA-seq study of human RPE/choroid indicated that both C1Q and CFD were expressed by choroidal macrophages.48 CFD was also expressed in choroidal fibroblasts and Schwann cells.48
Alternative Complement Pathway Gene Expression Within Geographic Atrophy Lesions
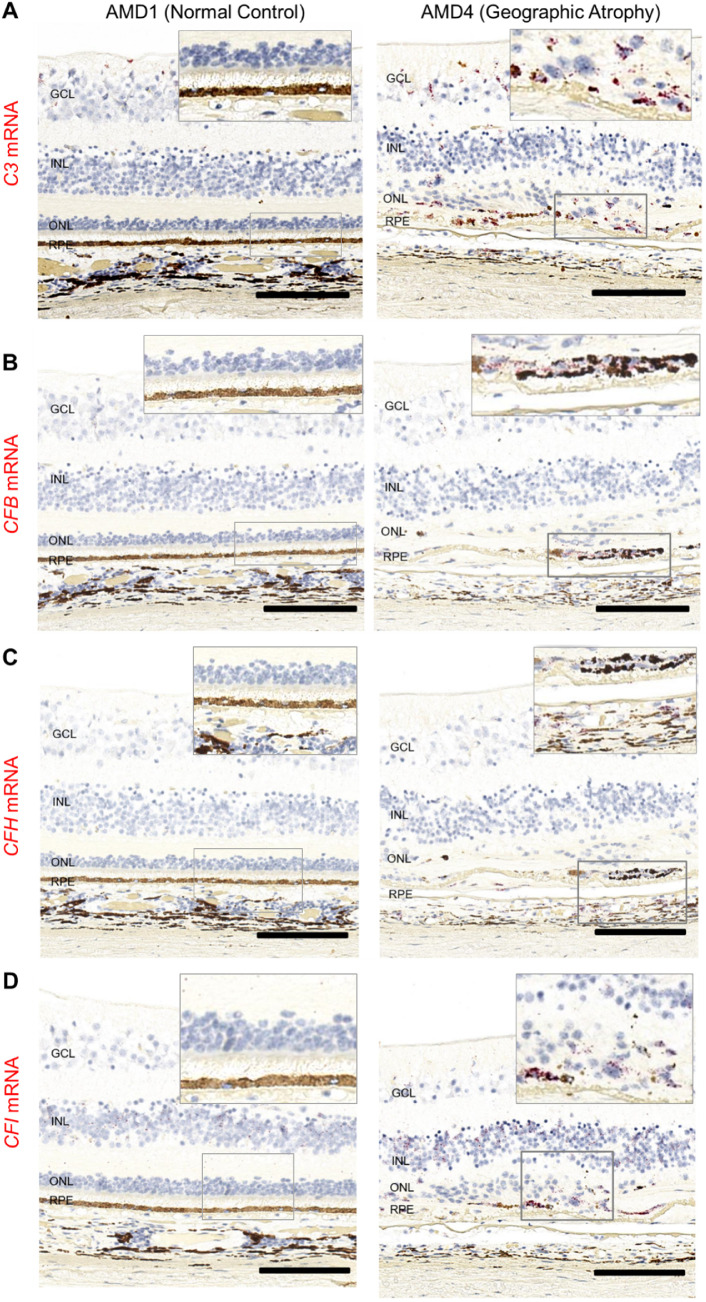
The higher complement gene expression (TaqMan) in some macular neural retinas with GA prompted us to examine eye sections by RNAscope to localize the cellular source of complement mRNAs. Genes in alternative and downstream common complement pathways (C3, C5, C9, CFB, CFD, CFH, CFI, CFP) were evaluated. Only a qualitative analysis was possible due to the lack of RNAscope controls to normalize the variability between postmortem eyes. However, several AP mRNAs, C3, CFB, CFH, and CFI, were noticeably more frequently seen in GA eyes compared to normal controls (AMD1 donors) (Fig. 3). Details for the detection and localization of each mRNA probe in eyes with each AMD grade are summarized in Supplementary Table S3.
Figure 3.
Spatial distribution of complement C3, CFB, CFH, and CFI mRNAs in macular area AMD1 and AMD4 macula. Donor eyes with AMD1–4 were evaluated for complement gene expression using RNAscope technology on paraffin sections. In representative examples of an AMD1 and an AMD4 macula, we identify changes in mRNA distribution (red): (A) C3 mRNA expression present in the GCL and INL of AMD1 macula but not in outer retina (left image). In addition to inner retina C3 expression, there is a strong C3 positive signal in the atrophic area of the AMD4 donor (right image). (B) CFB mRNA positive signal is present in the GCL and INL of AMD1 macula, but not in outer retina (left). In addition to staining of the GCL and INL, in the GA lesion area there is a strong CFB positive signal (right). (C) CFH mRNA expression is present in RPE, retinal and choroidal vessels of both AMD1 and AMD4 macula. In the atrophic lesion, CFH positive signal is prominent in a patch of abnormal-appearing RPE. (D) CFI mRNA expression is present in GCL, INL, and also in RPE and choroid of AMD1 and AMD4 macula. In the atrophic area of AMD4, there is readily detectable CFI expression in the subretinal space. Total number of eyes examined: AMD1 N = 3, AMD4 N = 4. Scale bar: 100 µm.
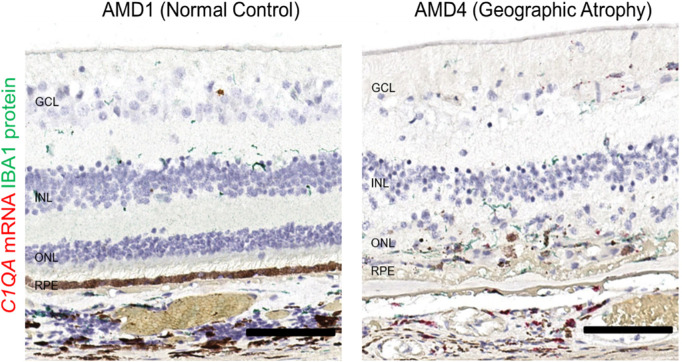
Serum C3 concentrations are the highest of all complement proteins.58 Similarly, the C3 transcript is relatively abundant in many cells in the back of the eye and is confined to the inner neural retina and choroid in normal AMD1 eyes (Fig. 3A). C3 mRNA-positive cells were often observed in the inner nuclear layer (INL), in the transition zone of AMD4 GA eyes between the edge of geographic atrophy and the surrounding histologically normal neural retina,59 They also appeared in the outer retina or in the subretinal space in areas of photoreceptor attenuation near GA lesions. Within the atrophic area of the same eye, there were many C3-positive cells. The localization of C3 mRNA in AMD2 or AMD3 eyes was similar to AMD1 (data not shown) and is confined to the inner retina. Low abundance CFB mRNA was present in retinal vessels and in the choroid in all eyes; however, in AMD4 eyes it can be seen within GA lesions (Fig. 3B). CFH mRNA levels are low in the neural retina, and the signal is associated with retinal vessels, choroid, and RPE (Fig. 3C) in all eyes. CFH mRNA also appeared in atrophic areas of AMD4 macula. CFI mRNA is notably localized to the INL of the retina in all AMD grades including normal eyes (Fig. 3D), consistent with single-cell RNASeq finding of CFI expression in Müller glia cells.45,49,50 CFI mRNA is apparent in RPE and choroid in all eyes, but like C3, CFB, and CFH, CFI also can be seen in the atrophic area in GA eyes. The increase of C3, CFB, and CFI mRNA signals in AMD4 atrophic areas compared to normal controls (AMD 1) is consistent with the modest increase of their mRNA determined by TaqMan analysis from neural retina tissue of AMD4 eyes with a different set of donor eyes (Figs. 2B, 2C). By RNAscope, C9, C5, CFD, and CFP mRNAs were either undetectable or inconsistently expressed at low levels in postmortem eyes (data not shown).
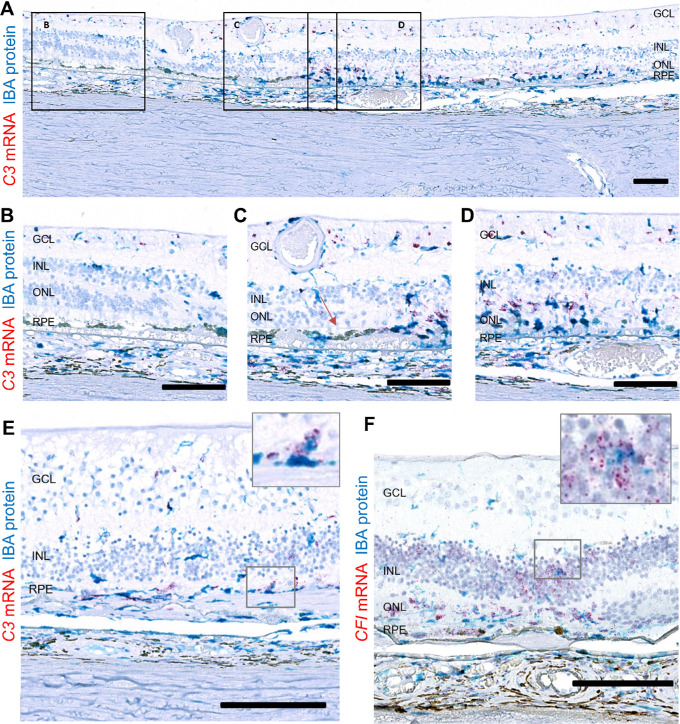
Some C3 mRNA–producing Cells in GA Lesions are Microglia/Macrophages
Microglia/macrophage cells expressing IBA1 protein are typically found in the inner retina and choroid but not in the outer retina, containing photoreceptors and RPE, of healthy eyes. In GA eyes, many IBA1+ cells are present near and in the outer retinal atrophic lesions.60 Because IBA1 expression and C3 mRNA seem to follow a similar pattern, we conducted colocalization studies. C3 RNAscope followed by IBA1 immunohistochemical staining demonstrated partially overlapping expression of C3 mRNA and IBA1 (Fig. 4A). In nonadvanced AMD eyes or in nonatrophic areas of GA eyes, only a small fraction of IBA1+ cells produced C3 mRNA at a low copy number (Fig. 4B). In contrast, almost all IBA1+ cells around GA lesions and in the transition zone had multiple copies of C3 mRNA molecules (Figs. 4C–4E), suggesting that a subset of the C3-synthesizing cells belong to the microglial or macrophage lineage and that upregulation of C3 mRNA in IBA1+ cells is associated with the severity of AMD. The identity of C3 producing cells other than microglia or macrophages remains unknown. The majority of CFI mRNA signals did not colocalize with IBA1+ cells (Fig. 4F). CFB and CFH mRNA appeared to colocalize with very few IBA1+ cells in the atrophic area of AMD4 eyes (not shown).
Figure 4.
Spatial distribution of complement C3 and CFI mRNAs relative to IBA1+ cells in atrophic lesion of AMD4 eyes. An atrophic region of an AMD4 eye labeled with C3 RNAScope (red) and IBA1 antibody (blue). (A) A low-magnification image of a donor with GA including atrophic, transition zone, and nonatrophic area. Higher magnification images of (B) Nonatrophic area with intact outer nuclear layer (ONL) and subtle RPE morphological changes. C3 mRNA+ label is in the inner plexiform layer (IPL), RPE, and choroid. Some C3 mRNA+ cells were also IBA1+. (C) Transition zone with partial loss of ONL and more extensive RPE changes. Many C3 mRNA-expressing IBA1+ cells are in subretinal space. Red arrow = ELM (external limiting membrane) loss indication the edge of the transition zone (D) In the atrophic area the ONL has collapsed, there are few remaining cones and scattered hypertrophic RPE. Numerous C3 mRNA expressing amoeboid-shaped IBA1+ cells are in the GA lesion with some IBA1-, C3 mRNA+ cells. (E) Another AMD4 macula with C3 mRNA+ IBA1 cell in the atrophic area. (F) An atrophic region of an AMD4 eye staining with CFI RNAscope and IBA1 antibody. Fewer CFI mRNA signals colocalized with IBA1+ cells. Total number of eyes examined: AMD1 N = 3, AMD4 N = 4. Scale bar: 100 µm.
Recent reports of cell-type–specific expression of AMD-associated complement genes in single-cell RNASeq studies with normal eyes suggests that C3 appears to be mainly produced in microglia/macrophages, CFI primarily in Müller glia, and CFH in vascular endothelium and the RPE.45,47–50 Our localizations of C3, CFH, and CFI mRNA signals in non-AMD eyes are consistent with these specific cell-type assignments (Table). In eyes with GA, these mRNA signals were additionally detected in the region of the outer retina associated with photoreceptor and RPE dysmorphism and death. In the case of C3, at least some of the signal is derived from IBA1+ microglia or macrophages entering the outer retina. The cellular source of the lesion-associated CFH and CFI signals is yet to be determined.
Table.
Summary of Key AMD-Associated Complement Genes Expressed in Specific Ocular Cell Types
| AMD1 (Control Eyes) | AMD4 (GA Eyes) | |||||||
|---|---|---|---|---|---|---|---|---|
| Ocular Cell Type | C3 | CFB | CFH | CFI | C3 | CFB | CFH | CFI |
| Microglia macrophage myeloid cell | 45,47–50* | * | * | * | * | |||
| Fibroblast | 48,49 | 48,49 | 48,49 | 48 | ||||
| Muller glia/astrocyte | 49,50 | 49,50 | 45,48–50* | ** | ||||
| RPE | 47–50 * | 47,48,50* | * | * | ||||
| Vascular endothelium/pericyte | 50 | 48 * | 45,48–50* | 48,49 | ||||
Numbers are reference numbers for published single cell or single nuclear RNASeq studies where indicated complement genes were reported in each specific ocular cell type.
Indicated findings from the current study.
The pattern of CFI mRNA distribution in INL is consistent with its expression in Müller glia cells.
Increased Classical Pathway C1QA mRNA Expression in GA Eyes
TaqMan analysis showed that classical pathway C1Q mRNA expression is elevated in GA macular neural retina compared to normal controls (Fig. 2C). To investigate this further, we combined C1QA RNAscope analysis with immunostaining for IBA1+ cells. C1QA mRNA-positive cells were generally confined to the inner retina, the outer plexiform layer, and the choroid in normal eyes (Fig. 5A). Consistent with previous findings that microglia cells produce C1Q,61 we found that C1QA-positive cells were exclusively IBA1+, but not all IBA1+ cells express detectable levels of C1QA. In the subretinal space of GA eyes, numerous IBA1+ cells contained higher copies of C1QA mRNA molecules, indicating that C1QA upregulation in microglia/macrophages may also be associated with AMD disease severity.
Figure 5.
Spatial distribution of complement classical pathway gene expression C1QA relative to IBA1+ cells in AMD1 and atrophic lesion region of AMD4 macula. (A). In an AMD1 macula, very few C1QA mRNA positive signals (red) were present and were exclusively in IBA1+ cells (green) (left image). Numerous C1QA mRNA expressing amoeboid-shaped IBA1+ cells were observed in atrophic lesion of an AMD4 eye (right image). These cells contained several copies of C1QA mRNA molecules. Total number of eyes examined: AMD1 N = 3, AMD4 N = 4. Scale bar: 100 µm.
Local Complement Protein Activation in Macular RPE/Choroid and Neural Retina of GA Eyes
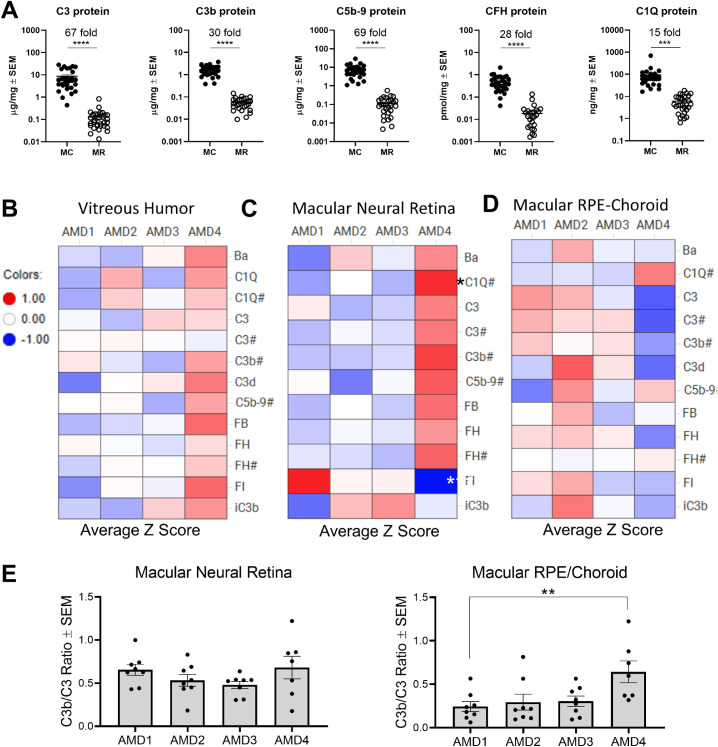
Complement activation involves a cascade of proteolytic enzymes and complement synthesis is known to be upregulated in inflammatory conditions.62 High levels of proteolytically cleaved products of complement proteins indicate complement activity. To determine whether the upregulation of complement synthesis in atrophic macular neural retina detected by TaqMan correlates with measurable complement activation, MSD assays and Western blotting (Supplementary Material, Supplementary Figs. S1 and S2) were performed on a third set of postmortem eyes (Supplementary Table S4). Levels of complement proteins and breakdown fragments were on average 15- to 65-fold higher in RPE/choroid than in the neural retina (P < 0.0001–0.001) (Fig. 6A), highlighting constant high levels of complement activity in RPE/choroid.
Figure 6.
Complement protein level is higher in RPE/choroid than in neural retina and is increased in macular neural retina of AMD4 GA eyes. (A) Comparison of complement protein levels of full-length (C1QA, C3, CFH) and activation products (C3b, C5b-9) between macular RPE/choroid and macular neural retina tissue regardless of AMD grades. These complement protein concentrations were determined by MSD assays. C3b assay measures the sum of three C3 breakdown products (C3b, iC3b, and C3c). Donor eye number for each gene: N = 31 for both macular neural retina and macular RPE/choroid. Average fold changes between macular RPE/choroid and neural retina for each complement protein are indicated. ***P < 0.001, ****P < 0.0001 unpaired two-sided t test. Heat maps for levels of full-length complement proteins and activation products are expressed in average z-score values at each AMD grade for vitreous humor (B), macular neural retina (C), and macular RPE/choroid tissue (D). Red values are above mean value and blue below mean value. # indicated the values that were determined by MSD and others that were assessed by Western blot. C3d signal in macular neural retina was too weak to quantify. (E) Ratio of C3b versus C3 were plotted against AMD grade for macular neural retina and macular RPE/choroid. Eight donor eyes were analyzed for each AMD grade except seven AMD4 eyes. P values were determined using one-way ANOVA Dunnett posttests relative to AMD1 normal control. *P < 0.05, ***P < 0.001.
To assess differential complement protein expression at various stages of AMD, protein data were transformed into Z-score values and presented in heat maps (Figs. 6B–6D). Levels of most complement full-length proteins were similar in eyes with AMD1 to AMD3; in GA eyes they were elevated in vitreous humor (Fig. 6B) and macular neural retina (Fig. 6C) but not in the macular RPE/choroid (Fig. 6D). Likewise, complement activation breakdown products C3b, iC3b, Ba, and C5b-9 were also higher in the vitreous humor and macular neural retina of AMD4 eyes (Figs. 6B, 6C) compared to non-advanced AMD eyes. The increase of complement activation in macular neural retina and vitreous was on average two- to threefold. These results demonstrate that complement activation increases in the macular neural retina at a late stage of AMD, AMD4. Levels of FI protein, the negative complement regulator, were reduced in macular neural retinas in AMD4 compared to AMD1 donors, in spite of the increased mRNA in the atrophic retina. Choriocapillaris dropout and choroid degeneration in the advanced stage of AMD is well documented.55–57 This could explain why the overall complement protein level in macular RPE/choroid tissue was not appreciably increased in AMD4 compared to AMD1. Besides direct quantitation of breakdown products, another approach to assess complement activation is based on determining the ratio between complement activation breakdown products and the full-length component.25,30 Using this type of analysis, we found that the average ratio of C3b/C3 in macular RPE/choroid was higher in AMD4 than in AMD1 (2.8-fold, P < 0.01), indicating increased proteolytic activity of complement in AMD4 RPE/choroid (Fig. 6E). In spite of the elevated complement components in the neural retina, the ratio of C3b/C3 did not increase.
Discussion
Understanding the roles of systemic and local complement activation in AMD may help guide the development of effective complement therapies. In this study, we characterized complement expression in donor eyes from early to late stages of AMD. Levels of complement mRNAs and proteins in both the AP and classical (CP) pathways were found to be at least 15-fold higher in RPE/choroid tissues than in neural retina in all eyes regardless of AMD status. Complement expression in the RPE/choroid, or perhaps the arrival of these components via the choroidal circulation, may be more important in the etiology of early stage AMD than expression within the neural retina. Local complement gene expression and complement activation protein products were two- to threefold higher in macular neural retinas from eyes with GA than in normal eyes, suggesting that local complement activation plays a role but perhaps only in the advanced stage of AMD. We identified activated microglia/macrophages as a key complement producing cell type in and around GA lesions.
Choroidal circulation provides 85% of total blood flow to the eye.63 One reason for the higher levels of complement in RPE/choroid than neural retina is due to heavily vascularized choroid where complement factors are major blood components. Our findings of high levels of choroid/RPE complement mRNA expression suggest that choroidal complement proteins are derived also from resident cellular sources. Local production confirmed by our RNAscope data showing complement mRNA in the choroid is consistent with abundant complement protein staining in Bruch's membrane and choroid in published immunohistology studies.5,59 High expression of FH in the RPE/choroid indicates it is likely a central complement negative regulator in this location.45,47,49,50,64 Tight regulation of systemic and choroidal complement activation at the interface between the neural retina and choroid (including the RPE and Bruch's membrane) is important to prevent damage to the local tissue, as well as the neural retina. This tight control is likely impaired gradually during AMD progression and is impaired in and around complement laden atrophic outer segments.59 FH is one of the major genetic risk factors for AMD, and loss of normal FH function likely enhances RPE-choroid complement activation.8
Within the retina, an immune-privileged site, complement is tightly controlled by complement regulators such as CD46, CD55, and CD59, in addition to the low expression of components of the activation cascade.43,65 Intrinsic Müller glia expression of complement negative regulator CFI adds another key negative regulator in neural retina to protect retina from complement-mediated damage.45,49,50 Genetic variants reducing CFI levels confer an increased risk of AMD, highlighting its protective role of dampening down complement.66–69 However, in advanced AMD, this protection may be overwhelmed by the robust complement activation at the atrophic lesion area where recruited microglia/macrophages supply high levels of C3 and C1Q.
Direct evidence of complement activation in eyes with AMD is limited. Two studies reported complement activation either in aqueous humor or in vitreous of AMD patients.70,71 Others have demonstrated C3 activation products by immunostaining of drusen and Bruch's membrane.72–74 Our data with quantitative complement mRNA and protein levels, spatial distribution, and cellular localization of complement gene expression not only strengthens the evidence of direct complement activation in eyes with advanced AMD but also highlights the compartmental difference in complement activation between neural retina and RPE-choroid. This information will help the development of future complement therapies for AMD by helping to select protein targets, the route of drug administration, and the stage of AMD most likely to respond.
There are many challenges to working with inherently heterogeneous postmortem donor eye samples. Because AMD is a complex multifactorial disease, each AMD eye may represent a different stage or subtype of the disease. The respective patients may carry different genetic AMD risk alleles and have had exposure to different environmental risk factors. Our results are thus limited in scope and should ideally be confirmed independently and, if possible, with larger sample sizes.
Our results have implications for the development of complement therapies for AMD. The inconsistent detection of C5 and very low levels of C9 mRNAs in donor eyes and the lack of efficacy with inhibitors of C5 delivered systemically (Eculizumab) or intravitreally (LFG316) in phase 2 GA trials (ClinicalTrials.gov Identifier: NCT00935883, NCT01527500) suggest that the terminal complement pathway may not be a driver of GA progression.75 However, the phase 2 and 3 clinical trial of Zimura, an RNA aptamer directed against C5, recently demonstrated modest efficacy slowing the growth rate of GA lesion (NCT02686658) and results of an ongoing second phase 3 trial should be informative (NCT04435366). C5a or the membrane attack complex (C5b-9) may play a role in early or intermediate AMD by damaging choroidal blood vessels.
We found that C3 mRNA expression was abundant relative to other complement genes in eyes. In advanced AMD, C3 is robustly expressed in cells within atrophic regions of macular neural retinas and could also be detected in isolated areas in choroid via RNAscope. These observations, in combination with positive phase 2 results for intravitreal APL-2 (an C3 inhibitor)76 and CLG561 (a properdin inhibitor, NCT02515942) slowing GA lesion growth by 28 and 18% respectively supports the idea that the alternative complement pathway in neural retina plays a modest role in advanced AMD. A role for C3 in the GA lesion growth waits to be confirmed in APL-2 phase 3 trials (NCT03525600, NCT03525613).
Many questions remain, including whether GA is too advanced for effective treatment with complement inhibitors. Most complement clinical trials for GA assessed intravitreal therapies, which is potentially limiting efficacy if systemic complement activation contributes to AMD. Dense deposit disease (membranoproliferative glomerulonephritis type II) features overactivation of systemic complement and AMD-like findings (drusen and progression to atrophy) but at a young age.77 This suggests systemic complement activation alone can cause AMD-like pathology. We have found that both alternative and classical pathway complement mRNAs and proteins are present at higher levels in the RPE/choroid than in the neural retina independent of AMD severity, suggesting the interface between neural retina and choroid (the outer blood-retina barrier), RPE/Bruch's membrane, is the site of complement action in AMD. Structurally, Bruch's membrane is similar to glomerular basement membrane and CFH mutations are associated with both AMD and dense deposit renal disease. Continuous exposure of complement factors coming from the bloodstream via the choroidal vasculature, along with continuous low grade complement activation might lead to choriocapillaris dropout and RPE dysfunction. Thus, systemic complement inhibition may be necessary for optimal treatment of early and intermediate AMD. Currently, a systemic antisense oligonucleotide targeting liver production of CFB is in a phase 2 trial for GA (Ionis/Roche, NCT03815825). The role and benefit of complement therapeutics in AMD is still an open question.
Supplementary Material
Acknowledgments
The authors thank Natasha Buchanan and Cynthia Grosskreutz for their support, comments, and suggestions and thank Nhi Vo and Barrett Leehy for technical contributions. We thank Timothy Olsen for postmortem AMD grading of donor eyes, Ashley Morganti, Nicholas Sprehe, and Lions Eye Institute For Transplant & Research for retrieval and dissection of human donor eyes.
Disclosure: J.T. Demirs, Novartis (E); J. Yang, Novartis (E); M.A. Crowley, Novartis (E); M. Twarog, Novartis (E); O. Delgado, Novartis (E); Y. Qiu, Novartis (E); S. Poor, Novartis (E); D.S. Rice, Novartis (E); T.P. Dryja, Novartis (E); K. Anderson, Novartis (E); S-M. Liao, Novartis (E)
References
- 1. McHarg S, Clark SJ, Day AJ, Bishop PN.. Age-related macular degeneration and the role of the complement system. Mol Immunol. 2015; 67: 43–50. [DOI] [PubMed] [Google Scholar]
- 2. Tan PL, Bowes Rickman C, Katsanis N.. AMD and the alternative complement pathway: genetics and functional implications. Hum Genomics. 2016; 10: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wright AF, Barlow PN.. Genome-wide association studies identify disease mechanisms in age-related macular degeneration. Ophthalmology. 2018; 125: 962–964. [DOI] [PubMed] [Google Scholar]
- 4. Hageman GS, Anderson DH, Johnson LV, et al.. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci USA. 2005; 102: 7227–7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anderson DH, Radeke MJ, Gallo NB, et al.. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res. 2010; 29: 95–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baudouin C, Peyman GA, Fredj-Reygrobellet D, et al.. Immunohistological study of subretinal membranes in age-related macular degeneration. Jpn J Ophthalmol. 1992; 36: 443–451. [PubMed] [Google Scholar]
- 7. Black JR, Clark SJ.. Age-related macular degeneration: genome-wide association studies to translation. Genet Med. 2016; 18: 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fritsche LG, Igl W, Bailey JN, et al.. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016; 48: 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lu F, Liu S, Hao Q, et al.. Association between complement factor C2/C3/CFB/CFH polymorphisms and age-related macular degeneration: a meta-analysis. Genet Test Mol Biomarkers. 2018; 22: 526–540. [DOI] [PubMed] [Google Scholar]
- 10. Klein RJ, Zeiss C, Chew EY, et al.. Complement factor H polymorphism in age-related macular degeneration. Science. 2005; 308: 385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Edwards AO, Ritter R 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA.. Complement factor H polymorphism and age-related macular degeneration. Science. 2005; 308: 421–424. [DOI] [PubMed] [Google Scholar]
- 12. Haines JL, Hauser MA, Schmidt S, et al.. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005; 308: 419–421. [DOI] [PubMed] [Google Scholar]
- 13. Zareparsi S, Branham KE, Li M, et al.. Strong association of the Y402H variant in complement factor H at 1q32 with susceptibility to age-related macular degeneration. Am J Hum Genet. 2005; 77: 149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fagerness JA, Maller JB, Neale BM, Reynolds RC, Daly MJ, Seddon JM.. Variation near complement factor I is associated with risk of advanced AMD. Eur J Hum Genet 2009; 17: 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gold B, Merriam JE, Zernant J, et al.. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006; 38: 458–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spencer KL, Hauser MA, Olson LM, et al.. Protective effect of complement factor B and complement component 2 variants in age-related macular degeneration. Hum Mol Genet. 2007; 16: 1986–1992. [DOI] [PubMed] [Google Scholar]
- 17. Maller JB, Fagerness JA, Reynolds RC, Neale BM, Daly MJ, Seddon JM.. Variation in complement factor 3 is associated with risk of age-related macular degeneration. Nat Genet. 2007; 39: 1200–1201. [DOI] [PubMed] [Google Scholar]
- 18. Seddon JM, Yu Y, Miller EC, et al.. Rare variants in CFI, C3 and C9 are associated with high risk of advanced age-related macular degeneration. Nat Genet. 2013; 45: 1366–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nishiguchi KM, Yasuma TR, Tomida D, et al.. C9-R95X polymorphism in patients with neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012; 53: 508–512. [DOI] [PubMed] [Google Scholar]
- 20. Hughes AE, Orr N, Esfandiary H, Diaz-Torres M, Goodship T, Chakravarthy U.. A common CFH haplotype, with deletion of CFHR1 and CFHR3, is associated with lower risk of age-related macular degeneration. Nat Genet. 2006; 38: 1173–1177. [DOI] [PubMed] [Google Scholar]
- 21. Hageman GS, Hancox LS, Taiber AJ, et al.. Extended haplotypes in the complement factor H (CFH) and CFH-related (CFHR) family of genes protect against age-related macular degeneration: characterization, ethnic distribution and evolutionary implications. Ann Med. 2006; 38: 592–604. [PMC free article] [PubMed] [Google Scholar]
- 22. Majewski J, Schultz DW, Weleber RG, et al.. Age-related macular degeneration–a genome scan in extended families. Am J Hum Genet. 2003; 73: 540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weeks DE, Conley YP, Tsai HJ, et al.. Age-related maculopathy: a genomewide scan with continued evidence of susceptibility loci within the 1q31, 10q26, and 17q25 regions. Am J Hum Genet. 2004; 75: 174–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scholl HP, Charbel Issa P, Walier M, et al.. Systemic complement activation in age-related macular degeneration. PLoS One. 2008; 3: e2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smailhodzic D, Klaver CC, Klevering BJ, et al.. Risk alleles in CFH and ARMS2 are independently associated with systemic complement activation in age-related macular degeneration. Ophthalmology. 2012; 119: 339–346. [DOI] [PubMed] [Google Scholar]
- 26. Hecker LA, Edwards AO, Ryu E, et al.. Genetic control of the alternative pathway of complement in humans and age-related macular degeneration. Hum Mol Genet. 2010; 19: 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sivaprasad S, Adewoyin T, Bailey TA, et al.. Estimation of systemic complement C3 activity in age-related macular degeneration. Arch Ophthalmol. 2007; 125: 515–519. [DOI] [PubMed] [Google Scholar]
- 28. Reynolds R, Hartnett ME, Atkinson JP, Giclas PC, Rosner B, Seddon JM.. Plasma complement components and activation fragments: associations with age-related macular degeneration genotypes and phenotypes. Invest Ophthalmol Vis Sci. 2009; 50: 5818–5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stanton CM, Yates JR, den Hollander AI, et al.. Complement factor D in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011; 52: 8828–8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paun CC, Lechanteur YT, Groenewoud JM, et al.. A novel complotype combination associates with age-related macular degeneration and high complement activation levels in vivo. Sci Rep. 2016; 6: 26568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schick T, Steinhauer M, Aslanidis A, et al.. Local complement activation in aqueous humor in patients with age-related macular degeneration. Eye. 2017; 31: 810–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rohrer B, Frazer-Abel A, Leonard A, et al.. Association of age-related macular degeneration with complement activation products, smoking, and single nucleotide polymorphisms in South Carolinians of European and African descent. Mol Vis. 2019; 25: 79–92. [PMC free article] [PubMed] [Google Scholar]
- 33. Lynch AM, Mandava N, Patnaik JL, et al.. Systemic activation of the complement system in patients with advanced age-related macular degeneration. Eur J Ophthalmol. 2020; 30: 1061–1068. [DOI] [PubMed] [Google Scholar]
- 34. Troutbeck R, Al-Qureshi S, Guymer RH.. Therapeutic targeting of the complement system in age-related macular degeneration: a review. Clin Experiment Ophthalmol. 2012; 40: 18–26. [DOI] [PubMed] [Google Scholar]
- 35. Volz C, Pauly D.. Antibody therapies and their challenges in the treatment of age-related macular degeneration. Eur J Pharm Biopharm. 2015; 95: 158–172. [DOI] [PubMed] [Google Scholar]
- 36. Hanus J, Zhao F, Wang S.. Current therapeutic developments in atrophic age-related macular degeneration. Br J Ophthalmol. 2016; 100: 122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu H, Chen M.. Targeting the complement system for the management of retinal inflammatory and degenerative diseases. Eur J Pharmacol. 2016; 787: 94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Holz FG, Sadda SR, Busbee B, et al.. Efficacy and safety of lampalizumab for geographic atrophy due to age-related macular degeneration: Chroma and Spectri phase 3 randomized clinical trials. JAMA Ophthalmol. 2018; 136: 666–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McGeer PL, Lee M, McGeer EG.. A review of human diseases caused or exacerbated by aberrant complement activation. Neurobiol Aging. 2017; 52: 12–22. [DOI] [PubMed] [Google Scholar]
- 40. Ricklin D, Reis ES, Lambris JD.. Complement in disease: a defence system turning offensive. Nat Rev Nephrol. 2016; 12: 383–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Streilein JW. Regulation of ocular immune responses. Eye (Lond). 1997; 11(Pt 2): 171–175. [DOI] [PubMed] [Google Scholar]
- 42. Sohn JH, Kaplan HJ, Suk HJ, Bora PS, Bora NS.. Complement regulatory activity of normal human intraocular fluid is mediated by MCP, DAF, and CD59. Invest Ophthalmol Vis Sci. 2000; 41: 4195–4202. [PMC free article] [PubMed] [Google Scholar]
- 43. Fett AL, Hermann MM, Muether PS, Kirchhof B, Fauser S.. Immunohistochemical localization of complement regulatory proteins in the human retina. Histol Histopathol. 2012; 27: 357–364. [DOI] [PubMed] [Google Scholar]
- 44. Ebrahimi KB, Fijalkowski N, Cano M, Handa JT.. Decreased membrane complement regulators in the retinal pigmented epithelium contributes to age-related macular degeneration. J Pathol. 2013; 229: 729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Menon M, Mohammadi S, Davila-Velderrain J, et al.. Single-cell transcriptomic atlas of the human retina identifies cell types associated with age-related macular degeneration. Nat Commun. 2019; 10: 4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pauly D, Agarwal D, Dana N, et al.. Cell-type-specific complement expression in the healthy and diseased retina. Cell Rep. 2019; 29: 2835–2848.e2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hu Y, Wang X, Hu B, et al.. Dissecting the transcriptome landscape of the human fetal neural retina and retinal pigment epithelium by single-cell RNA-seq analysis. PLoS Biol. 2019; 17: e3000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Voigt AP, Mulfaul K, Mullin NK, et al.. Single-cell transcriptomics of the human retinal pigment epithelium and choroid in health and macular degeneration. Proc Natl Acad Sci USA. 2019; 116: 24100–24107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cowan CS, Renner M, Gross-Scherf B, et al.. Cell types of the human retina and its organoids at single-cell resolution: developmental convergence, transcriptomic identity, and disease map. BioRxiv. 2019. [Google Scholar]
- 50. Orozco LD, Chen HH, Cox C, et al.. Integration of eQTL and a Single-Cell Atlas in the Human Eye Identifies Causal Genes for Age-Related Macular Degeneration. Cell Rep. 2020; 30: 1246–1259.e1246. [DOI] [PubMed] [Google Scholar]
- 51. Olsen TW, Feng X.. The Minnesota Grading System of eye bank eyes for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004; 45: 4484–4490. [DOI] [PubMed] [Google Scholar]
- 52. Ferris FL 3rd, Wilkinson CP, Bird A, et al.. Clinical classification of age-related macular degeneration. Ophthalmology. 2013; 120: 844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sarks SH. Ageing and degeneration in the macular region: a clinico-pathological study. Br J Ophthalmol. 1976; 60: 324–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Crowley MA, Delgado O, Will-Orrego A, et al.. Induction of ocular complement activation by inflammatory stimuli and intraocular inhibition of complement factor D in animal models. Invest Ophthalmol Vis Sci. 2018; 59: 940–951. [DOI] [PubMed] [Google Scholar]
- 55. Mullins RF, Johnson MN, Faidley EA, Skeie JM, Huang J.. Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011; 52: 1606–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McLeod DS, Taomoto M, Otsuji T, Green WR, Sunness JS, Lutty GA.. Quantifying changes in RPE and choroidal vasculature in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2002; 43: 1986–1993. [PubMed] [Google Scholar]
- 57. McLeod DS, Grebe R, Bhutto I, Merges C, Baba T, Lutty GA.. Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009; 50: 4982–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Morley BJW, Mark J.. The Complement FactsBook. Cambridge, MA: Academic Press; 1999: 240. [Google Scholar]
- 59. Katschke KJ Jr., Xi H, Cox C, et al.. Classical and alternative complement activation on photoreceptor outer segments drives monocyte-dependent retinal atrophy. Sci Rep. 2018; 8: 7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Guillonneau X, Eandi CM, Paques M, Sahel JA, Sapieha P, Sennlaub F.. On phagocytes and macular degeneration. Prog Retin Eye Res. 2017; 61: 98–128. [DOI] [PubMed] [Google Scholar]
- 61. Fonseca MI, Chu SH, Hernandez MX, et al.. Cell-specific deletion of C1qa identifies microglia as the dominant source of C1q in mouse brain. J Neuroinflammation. 2017; 14: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Luo C, Chen M, Xu H.. Complement gene expression and regulation in mouse retina and retinal pigment epithelium/choroid. Mol Vis. 2011; 17: 1588–1597. [PMC free article] [PubMed] [Google Scholar]
- 63. Ehrlich R, Harris A, Wentz SM, Moore NA, Siesky BA.. Anatomy and Regulation of the Optic Nerve Blood Flow. Reference Module in Neuroscience and Biobehavioral Psychology. Philadelphia: Elsevier; 2017. [Google Scholar]
- 64. Clark SJ, Perveen R, Hakobyan S, et al.. Impaired binding of the age-related macular degeneration-associated complement factor H 402H allotype to Bruch's membrane in human retina. J Biol Chem. 2010; 285: 30192–30202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yang P, Tyrrell J, Han I, Jaffe GJ.. Expression and modulation of RPE cell membrane complement regulatory proteins. Invest Ophthalmol Vis Sci. 2009; 50: 3473–3481. [DOI] [PubMed] [Google Scholar]
- 66. van de Ven JP, Nilsson SC, Tan PL, et al.. A functional variant in the CFI gene confers a high risk of age-related macular degeneration. Nat Genet. 2013; 45: 813–817. [DOI] [PubMed] [Google Scholar]
- 67. Kavanagh D, Yu Y, Schramm EC, et al.. Rare genetic variants in the CFI gene are associated with advanced age-related macular degeneration and commonly result in reduced serum factor I levels. Hum Mol Genet. 2015; 24: 3861–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. de Jong S, Volokhina EB, de Breuk A, et al.. Effect of rare coding variants in the CFI gene on Factor I expression levels. Hum Mol Genet. 2020; 29: 2313–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hallam TM, Marchbank KJ, Harris CL, et al.. Rare genetic variants in complement factor I lead to low FI plasma levels resulting in increased risk of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2020; 61: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Altay L, Sitnilska V, Schick T, et al.. Early local activation of complement in aqueous humour of patients with age-related macular degeneration. Eye (Lond). 2019; 33: 1859–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Loyet KM, Deforge LE, Katschke KJ Jr., et al.. Activation of the alternative complement pathway in vitreous is controlled by genetics in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012; 53: 6628–6637. [DOI] [PubMed] [Google Scholar]
- 72. Johnson LV, Leitner WP, Staples MK, Anderson DH.. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp Eye Res. 2001; 73: 887–896. [DOI] [PubMed] [Google Scholar]
- 73. Anderson DH, Mullins RF, Hageman GS, Johnson LV.. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002; 134: 411–431. [DOI] [PubMed] [Google Scholar]
- 74. Keenan TD, Toso M, Pappas C, Nichols L, Bishop PN, Hageman GS.. Assessment of proteins associated with complement activation and inflammation in maculae of human donors homozygous risk at chromosome 1 CFH-to-F13B. Invest Ophthalmol Vis Sci. 2015; 56: 4870–4879. [DOI] [PubMed] [Google Scholar]
- 75. Yehoshua Z, de Amorim Garcia Filho CA, Nunes RP, et al.. Systemic complement inhibition with eculizumab for geographic atrophy in age-related macular degeneration: the COMPLETE study. Ophthalmology. 2014; 121: 693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liao DS, Grossi FV, El Mehdi D, et al.. Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration: a randomized phase 2 trial. Ophthalmology. 2020; 127: 186–195. [DOI] [PubMed] [Google Scholar]
- 77. Fakhouri F, Fremeaux-Bacchi V, Noel LH, Cook HT, Pickering MC.. C3 glomerulopathy: a new classification. Nat Rev Nephrol. 2010; 6: 494–499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.