Abstract
Background
The prognostic value of within-day sCr variation serum creatinine variation is unknown in the setting of the novel coronavirus disease 2019 (COVID-19). We evaluated the prognostic significance of 24-hour serum creatinine variation in COVID-19 patients.
Methods
A monocentric retrospective analysis was conducted in COVID-19 patients not admitted to the intensive care unit. Three groups were subdivided based on 24 hours serum creatinine variation from admission. In the stable kidney function group, 24-hour serum creatinine variation ranged from +0.05 to –0.05 mg/dL; in the decreased kidney function group, 24-hour serum creatinine variation was >0.05 mg/dL; in the improved kidney function group, 24-hour serum creatinine variation was <–0.05 mg/dL.
Results
The study population included 224 patients with a median age of 66.5 years and a predominance of males (72.3%). Within 24 hours of admission, renal function remained stable in 37.1% of the subjects, whereas it displayed improved and deteriorated patterns in 45.5% and 17.4%, respectively. Patients with decreased kidney function were older and had more severe COVID-19 symptoms than patients with stable or improved kidney function. About half of patients with decreased kidney function developed an episode of acute kidney injury (AKI) during hospitalization. Decreased kidney function was significantly associated with AKI during hospitalization (hazard ratio [HR], 4.6; 95% confidence interval [CI], 1.9–10.8; p < 0.001) and was an independent risk factor for 30-day in-hospital mortality (HR, 5.5; 95% CI, 1.1–28; p = 0.037).
Conclusion
COVID-19 patients with decreased kidney function within 24 hours of admission were at high risk of AKI and 30-day in-hospital mortality.
Keywords: Acute kidney injury, Biomarkers, Coronavirus, Kidney, Creatinine, Mortality
Introduction
The novel coronavirus disease 2019 (COVID-19) is a complex disease presenting principally with pneumonia and multiorgan disease in aged and highly comorbid patients [1]. Acute decline of kidney function is a relatively frequent complication of COVID-19, especially in patients with severe lung involvement [2]. The etiological mechanisms causing acute kidney injury (AKI) are still unknown. The interplay between hyperactive immune response, cytopathic effects of the virus, and homeostatic reactions to balance pulmonary and hemodynamic responses have been postulated to be the main causes of kidney damage [3]. The rate of this complication ranges from 0.5% to 36.6% [2,4–9]. In intensive care unit (ICU), the prevalence of this disease was higher, accounting for about 80% of patients [10].
The diagnosis of AKI relies on the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines and is defined as the combination of a peak increase in serum creatinine (sCr) level and abrupt reduction of urinary output [11]. In the real world, small sCr variations are frequent and often overlooked. The prognostic value of sCr within-day variation has been poorly investigated because fluctuations of sCr can be linked to nonpathological issues including analytical, biological, and dietary factors [12,13]. A study of 14,912 adults who underwent two sCr measurements within 24 hours showed that monitoring 24-hour sCr variation was useful to identify AKI earlier than with the conventional criteria. A 24-hour sCr variation greater than 0.1 mg/dL (or an increase of 5%) was associated with increased all-cause mortality, especially in inpatient settings [14].
As a predictor of poor outcomes, change in sCr can be a useful risk-stratification tool to quickly identify high-risk COVID-19 patients requiring intensive management. Comprehensive risk assessment assumes paramount importance in the management of these vulnerable subjects. Identification of high-risk patients soon after admission allows timely supportive treatment and limits further insult to the kidneys. Based on this background, we evaluated 24-hour sCr differences (ΔsCr) after admission in a cohort of hospitalized patients with COVID-19 to assess the prognostic effect of early sCr variation.
Methods
We retrospectively reviewed the electronic charts of all non-ICU hospitalized patients with COVID-19 at the University Hospital of Modena, Italy from March 4 to June 20, 2020. The study was approved by the regional ethical committee of Emilia Romagna (No. 0013376/20). Written informed consent was waived due to containment restrictions between COVID-19 patients and healthcare workers.
Population
Demographic, clinical, and laboratory data of 432 consecutive adult patients (≥18 years) admitted with COVID-19 were collected. According to the international guidelines, diagnosis of COVID-19 was defined as a positive real-time reverse transcriptase-polymerase chain reaction assay of nasopharyngeal swabs or lower respiratory tract specimens [15]. Study inclusion criteria comprised patients older than 18 years with a second measurement of sCr within 24 hours from admission. Patients on renal replacement therapy were excluded from the analysis.
The study population comprised 224 patients. The ΔsCr was calculated by subtracting the first sCr value from the second value collected within 24 hours of admission (ΔsCr = sCr24h – sCrbaseline). The entire cohort was subdivided according to 24-hour ΔsCr. In the stable kidney function group, the 24-hour ΔsCr ranged from +0.05 to –0.05 mg/dL: in the decreased kidney function group, the 24-hour ΔsCr was >0.05 mg/dL; in the improved kidney function group, the 24-hour ΔsCr was <–0.05 mg/dL.
Baseline clinical characteristics
All measurable comorbidities were quantified in our study population at admission. Chronic kidney disease (CKD) was defined as a chronic (>3 months) reduction of glomerular filtration rate (GFR) < 60 mL/min; cardiovascular disease (CVD) included a wide array of diseases affecting the cardiac muscle and the vascular system supplying the heart, brain, and other vital organs; chronic obstructive pulmonary disease was defined as a previous diagnosis of airflow obstruction with reduction of forced expiratory volume in 1 second; hypertension referred to high blood pressure (≥140/90 mmHg) requiring at least one antihypertensive medication; diabetes included altered glucose metabolism requiring treatment; AKI has been defined according to 2012 KDIGO guidelines without urine output criteria [11].
All patients were treated in agreement with the Regional Guidelines of Emilia Romagna [12] regarding the treatment of COVID-19; these were continuously updated during the period of the study. The treatments consisted of (1) hydroxychloroquine (400 mg twice a day [BID] on day 1 followed by 200 mg BID on days 2 to 5, eventually adjusted for creatinine clearance estimated by a CKD algorithm); (2) azithromycin (500 mg once a day [QD] for 5 days) at physician discretion when suspecting a bacterial respiratory superinfection; (3) low molecular weight heparin for prophylaxis of deep vein thrombosis according to body weight and kidney function; (4) darunavir/cobicistat (800/150 mg QD) or lopinavir/ritonavir (400/100 mg BID) for 14 days were used until March 18, when a clinical trial on the former did not show any benefit of protease inhibitors against the standard of care. Other antiviral agents showing promising results, such as remdesivir, were not used in our patients due to lack of availability in the market.
Serum creatinine measurement
The sCr level was measured in a single laboratory at the University Hospital of Modena using the Jaffe (CREJ2, Roche/Hitachi Cobas Systems; Roche Diagnostics GmbH, Mannheim, Germany) or the enzymatic method (Ortho Clinical Diagnostics, Rochester, NY, USA). The latter has been used to measure sCr in urgent blood tests. Precision in sCr measurement ranged from 1.2% to 5.0% for the Jaffe method and from 1.3% to 2.6% for the enzymatic method. The estimated GFR was calculated using the Chronic Kidney Disease-Epidemiology Collaboration equation [16]. Peak sCr value was selected among patients with multiple sCr measurements (>2 times) performed on the same day. A cut-off of ±0.05 mg/dL was chosen to overcome interlaboratory variability [17].
Outcome
The primary outcome measure was 30-day in-hospital mortality among patients stratified according to 24-hour ΔsCr.
Statistical analysis
Comparisons of population characteristics were performed using the paired Student t-test or Wilcoxon signed-rank test, as appropriate, and the chi-square test was used for categorical variables. One-way analysis of variance evaluated differences in continuous variables between groups, and Tukey test was used for post hoc analysis.
Kaplan-Meier analysis assessed the survival of patients. Cox regression was used to estimate time-to-event data; the hazard ratio (HR) for death was adjusted for age, sex, PaO2/FiO2 ratio, CKD, AKI, CVD, hypertension, diabetes, C-reactive protein, and GFR. The stable kidney function group was considered as a reference. All independent variables satisfied the proportional hazard assumption. A two-sided p-value less than 0.05 was considered statistically significant. Statistical analysis was performed using IBM SPSS version 27 (IBM Corp., Armonk, NY, USA).
Results
The median patient age was 66.5 years (interquartile range, 55.5–77.7 years), with a predominance of males (72.3%). Clinical and laboratory examinations of all enrolled patients are reported in Table 1.
Table 1.
Demographic, laboratory, and clinical characteristics of COVID-19 patients stratified according to 24-hour ΔsCr
| Variable | All patients (n = 224) | Decreased kidney function (ΔsCr > 0.05 mg/dL) (n = 39) | Stable kidney function (0.05 mg/dL ≤ ΔsCr ≥ –0.05 mg/dL) (n = 83) | Improved kidney function (ΔsCr < 0.05 mg/dL) (n = 102) | p-value |
|---|---|---|---|---|---|
| Basal characteristic | |||||
| Age (yr) | 66.5 (55.5–77.7) | 77.6 (71.4–83.7)a | 61.9 (53.07–72.1) | 66.2 (57.8–76.9) | <0.001* |
| Range of age (yr) | 28.8–97.3 | 39.9–94.5 | 29.1–94.4 | 28.9–97.3 | |
| Male sex | 162 (72.3) | 26 (66.7) | 61 (73.5) | 75 (73.5) | 0.22 |
| White blood cell (/mm3) | 6,525 (4,867–9,085) | 7,310 (5,675–8,935) | 6,805 (4,707–9,597) | 6,380 (4,720–8,990) | 0.43 |
| Hemoglobin (g/L) | 12.4 (10.9–13.5) | 11.6 (10.1–13.2) | 12.3 (11.3–13.5) | 12.8 (10.9–13.6) | 0.09 |
| Platelet (×109/L) | 213.0 (157.7–300.0) | 192.0 (137.5–256.5) | 230.5 (163.5–317.5) | 206.0 (156.0–294.0) | 0.11 |
| Glicemia | 102.5 (82.5–132.7) | 102.0 (81.7–133.2)b | 101.0 (86.0–128.5) | 81.0 (52.2–149.7) | 0.009* |
| Sodium (mmol/L) | 137.0 (135.0–140.0) | 143.0 (110.0–199.0) | 137.0 (135.7–140.0) | 137.0 (135.0–139.7) | 0.08 |
| Potassium (mmol/L) | 3.8 (3.5–4.2) | 3.9 (3.5–4.3) | 3.7 (3.4–4.1) | 3.8 (3.5–4.2) | 0.08 |
| Urea (mg/dL) | 45.0 (31.0–64.8) | 58.0 (47.7–105.2)a,b | 38.0 (30.2–56.5) | 42.0 (26.7–55.5) | <0.001* |
| Fibrinogen (mg/dL) | 626.0 (449.0–694.0) | 545.0 (327.0–646.0) | 656.0 (506.0–751.7) | 616.0 (470.0–684.0) | 0.42 |
| Albumin (g/dL) | 2.8 (2.6–3.3) | 2.6 (2.4–3.0) | 3 (2.6–3.3) | 2.9 (2.6–3.3) | 0.11 |
| D-dimer (mg/L) | 1,490.0 (820.0–2,680.0) | 1,725.0 (807.5–2,982.5) | 1,533.0 (800.0–2,795.0) | 2,380.0 (1,245.0–4,770.0) | 0.63 |
| Bilirubin (mg/dL) | 0.59 (0.47–0.83) | 0.70 (0.49–1.10)a, b | 0.57 (0.42–0.83) | 0.56 (0.41–0.68) | 0.05* |
| aPTT ratio | 1.15 (1.02–1.30) | 1.20 (1.14–1.60) | 1.08 (0.90–1.20) | 1.20 (1.10–1.90) | 0.08 |
| INR | 1.07 (1.02–1.14) | 1.10 (1.06–1.26) | 1.08 (1.03–1.20) | 1.06 (1.02–1.10) | 0.45 |
| Alanine amino-transferase (U/L) | 35.5 (23.0–58.0) | 31.0 (20.0–48.0) | 36.0 (23.0–61.0) | 38.0 (23.0–59.0) | 0.11 |
| CPK (U/L) | 111.5 (54.0–265.0) | 107.5 (35.0–341.0) | 111.0 (47.5–271.7) | 83.0 (37.0–185.0) | 0.07 |
| Lactate dehydrogenase (U/L) | 622.0 (475.2–812.0) | 660.0 (432.0–823.0) | 630.0 (486.0–781.0) | 595.0 (451.0–848.0) | 0.09 |
| PCR (mg/dL) | 6.2 (3.2–16.9) | 5.9 (3.5–19.8) | 5.9 (2.7–17.5) | 6.7 (3.4–16.4) | 0.677 |
| Admission sCr (mg/dL) | 0.86 (0.68–1.12) | 0.90 (0.71–1.39) | 0.76 (0.62–0.92) | 0.92 (0.79–1.21) | <0.001* |
| GFR (mL/min) | 83.5 (60.2–99.4) | 77.9 (45.9–91.0)a,b | 96.1 (76.3–111.9) | 76.6 (58.7–93.9) | <0.001* |
| 24-hour sCr (mg/dL) | 0.81 (0.66–1.08) | 1.23 (0.90–1.90)a,b | 0.75 (0.60–0.97) | 0.79 (0.66–1.04) | <0.001* |
| ΔsCr (mg/dL) | –0.04 (–0.13 to 0.03) | 0.17 (0.11–0.38)a,b | –0.01(–0.02 to 0.02) | –0.13 (–0.21 to –0.09) | <0.001 |
| ΔsCr range (mg/dL) | 1.24–2.78 | 0.05–2.78 | –0.04 to 0.04 | –1.24 to –0.05 | |
| Time elapsed from first sCr (hr) | 13 (13–19)a,b | 13 (10–13) | 16 (13–19) | 13 (13–19) | <0.001* |
| Systolic blood pressure (mmHg) | 120 (110–133) | 110 (100–130) | 120 (110–130) | 120 (110–140) | 0.32 |
| Diastolic blood pressure (mmHg) | 70 (61–80) | 70 (60–75) | 70 (70–80) | 70 (60–80) | 0.33 |
| PaO2/FiO2 | 245.0 (147.0–291.0) | 167.0 (91.7–239.0)a,b | 264.0 (216.0–298.0) | 232.2 (149.0–303.0) | 0.002* |
| Temperature (°C) | 37.2 ± 1.0 | 37.6 ± 1.04 | 37.1 ± 1.0 | 37.1 ± 1.06 | 0.13 |
| Dyspneac | 96 (52.1) | 16 (66.7) | 34 (49.3) | 46 (50.5) | 0.31 |
| SOFA | 2.88 ± 1.98 | 4.00 ± 2.53a | 2.36 ± 1.30 | 2.96 ± 2.00 | 0.009* |
| CKD (<60 mL/min) | 12 (5.4) | 4 (10.3) | 2 (2.4) | 6 (5.9) | 0.18 |
| CVD | 123 (54.9) | 16 (41.0) | 51 (61.4) | 56 (54.9) | 0.11 |
| COPD | 20 (8.9) | 4 (10.3) | 6 (7.2) | 10 (9.8) | 0.79 |
| High blood pressure | 136 (60.7) | 20 (51.3) | 55 (66.3) | 61 (59.8) | 0.28 |
| Diabetes | 41 (18.3) | 10 (25.6) | 14 (16.9) | 17 (16.7) | 0.45 |
| First 24-hr treatment | |||||
| NaCl 0.9% infusion | 71 (31.7) | 14 (35.9) | 25 (30.1) | 32 (31.4) | 0.81 |
| NaCl 0.9% infusion (L)d | 62.0 (0.6) | 9.3 (0.5) | 21.8 (0.6) | 31 (0.6) | 0.73 |
| Dextrose 5% infusion | 5 (2.2) | 2 (5.1) | 0 (0) | 3 (2.9) | 0.16 |
| Dextrose 5% infusion (L)d | 5 (0.8) | 1 (0.5) | 0 (0) | 4 (1.0) | 0.40 |
| Danuravir/cobicistat | 56 (25.0) | 9 (23.1) | 27 (32.5) | 20 (19.6) | 0.12 |
| Lopinavir/ritonavir | 1 (0.4) | 1 (2.6) | 0 (0) | 0 (0) | 0.09 |
| RAAS-blocker | 14 (6.2) | 2 (5.1) | 5 (6.0) | 7 (6.9) | 0.92 |
| NSAID | 2 (0.9) | 0 (0) | 2 (2.4) | 0 (0) | 0.18 |
| Difference in outcomes | |||||
| AKI | 41 (18.3) | 17 (43.6)a,b | 8 (9.6) | 16 (15.7) | <0.001* |
| Hospitalization (day) | 11.1 (6.5–17.8) | 15.9 (8.3–21.1) | 13.9 (9.1–27.7) | 14.9 (7.8–23.4) | 0.997 |
| Death | 44 (19.6) | 17 (43.6)a,b | 6 (7.2) | 21 (20.6) | <0.001* |
| Still admitted | 8 (3.6) | 1 (2.6) | 1 (1.2) | 6 (5.9) | 0.12 |
Values are presented as median (interquartile range), number (%), or mean ± standard deviation unless otherwise specified.
AKI, acute kidney injury; aPTT, activated partial thromboplastin time; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; COVID-19, the novel coronavirus disease 2019; CVD, cardiovascular disease; CPK, creatine phosphokinase; GFR, glomerular filtration rate; INR, international normalized ratio; NSAID, nonsteroidal anti-inflammatory drugs; PCR, polymerase chain reaction; RAAS, renin-angiotensin-aldosterone system; sCr, serum creatinine; SOFA, sequential organ failure assessment score; ΔsCr, variation in serum creatinine.
Statistically significant difference between the decreased kidney function group and the stable kidney function group;
statistically significant difference between the decreased kidney function group and the improved kidney function group.
Data missing.
Total liter of intravenous infusion.
p < 0.05.
Within 24 hours from admission, kidney function decreased in 39 subjects (17.4%), improved in 102 (45.5%), and remained stable in 83 (37.1%). Patients with decreased kidney function were older and presented with more severe impairment of respiratory function than patients with stable kidney function or improved kidney function. No differences were observed in the administration of fluid or potentially nephrotoxic agents across groups. The fluctuations in sCr detected in the decreased kidney function group are detailed in Fig. 1. Notably, 69.2% of ΔsCr results were <0.3 mg/dL, a cut-off currently used to diagnose AKI [11].
Figure 1. Graphic representation of 24-hour ΔsCr in subjects with decreased kidney function.
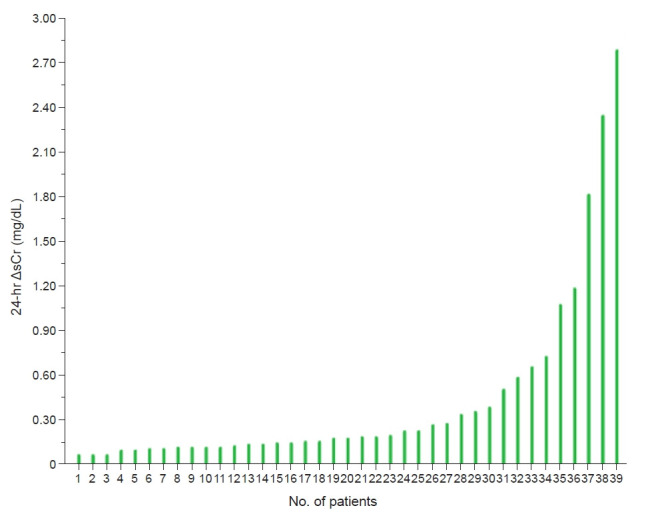
ΔsCr, variation in serum creatinine.
AKI was detected in 43.6%, 9.6%, and 15.7% of patients with decreased kidney function, stable kidney function, and improved kidney function, respectively. As expected, patients with decreased kidney function experienced more episodes of AKI than did the other patients (p < 0.001) (Table 1). Positive correlation was found between the continuous variable ΔsCr and AKI (r = 0.31; p = 0.001) and in-hospital mortality (r = 0.21; p = 0.002); univariate Cox regression analysis showed that ΔsCr was a predictor of AKI (HR, 7.9; 95% confidence interval [CI], 4.2–14.7; p < 0.001) and in-hospital mortality (HR, 4.0; 95% CI, 2.2–7.2; p < 0.001).
Cox regression analysis was performed to assess the association between groups and outcomes. Subjects with decreased kidney function had a 4.6-fold greater risk of developing episodes of AKI (95% CI, 1.9–10.8; p < 0.001); conversely, improvement of kidney function, was not associated with AKI (HR, 1.7; 95% CI, 0.6–3.7; p = 0.273).
Kaplan-Meier estimates revealed statistically significant differences in survival between the groups (log-rank test, p < 0.001). The chi-square test estimated that patients with decreased kidney function (p < 0.001) and improved kidney function (p = 0.02) had higher mortality compared to subjects with stable kidney function (Fig. 2). Cox hazards regression analysis showed that decreased kidney function was an independent risk factor for 30-day in-hospital mortality, with an adjusted HR of 5.5 (95% CI, 1.1–28.0; p = 0.04) (Table 2).
Figure 2. Kaplan-Meier curves describing the survival of patients with COVID-19 stratified according to the value of 24-hour ΔsCr.
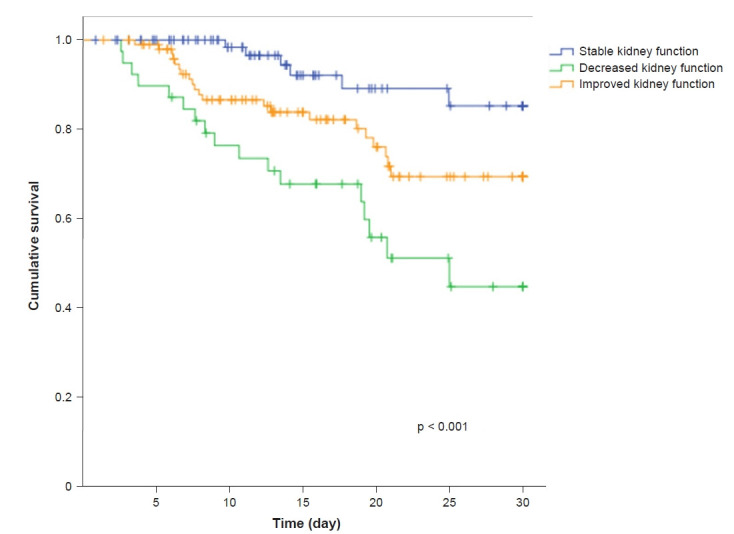
Thirty-day survival of patients with stable kidney function (-0.05 ≤ ΔsCr ≤ +0.05 mg/dL), decreased kidney function (ΔsCr > 0.05 mg/dL) and improved kidney function (ΔsCr < –0.05 mg/dL).
COVID-19, the novel coronavirus disease 2019; ΔsCr, variation in serum creatinine.
Table 2.
Unadjusted and adjusted Cox regression analyses to predict 30-day in-hospital mortality
| Kidney function | Unadjusted analysis |
Adjusted analysis |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
||||||||
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Stable | (Reference) | (Reference) | (Reference) | ||||||
| Decreased | 5.8 | 2.3–14.9 | 0.0001* | 6.2 | 2.3–16.9 | 0.0001* | 5.5 | 1.1–28 | 0.04* |
| Improved | 2.8 | 1.1–6.9 | 0.04* | 2.8 | 1.1–7.1 | 0.23 | 2.1 | 0.6–7.2 | 0.24 |
HR has been adjusted for sex and age (model 1) and for PaO2/FiO2, acute kidney injury, diabetes, chronic kidney disease, cardiovascular disease, blood pressure, C-reactive protein, and glomerular filtration rate at admission (model 2).
CI, confidence interval; HR, hazard ratio.
p < 0.05.
Discussion
In clinical practice, sCr is the most common endogenous marker for assessing kidney function. Small increases in sCr have been recognized as a sign of impaired kidney function. According to the current guidelines, an increase in sCr ≥ 0.3 mg/day within 48 hours is widely recognized as the minimum criterion for the diagnosis of AKI [11]. In hospitalized patients, sCr variation is a frequent event, principally related to hemodynamic instability, systemic inflammatory response, dehydration, and use of nephrotoxic agents. Fluctuations in sCr generally occur in aged patients, especially if they are affected by systemic disease [18].
This study confirms that sCr elevation portends adverse prognostic significance in hospitalized patients with severe symptoms of COVID-19. We found that the decrease of kidney function whitin 24 hours from admission is an independent factor for poor outcomes in a group of non-ICU-admitted patients with symptoms of COVID-19.
In this study, the majority of patients (>80.0%) had stable (37.1%) or improved (45.5%) kidney function within 24 hours from admission. A small subset of the population (17.4%) experienced an increase in sCr over baseline. As the rates of supportive therapies and nephrotoxic agents were similar among the three groups, it is unclear why sCr changed in only some patients. Theoretically, supportive therapy including fluid administration, oxygen delivery, modulation of antihypertensive therapy, and withdrawal of offensive agents is expected to stabilize or even improve kidney function in ill and potentially dehydrated patients. Conversely, an increase in sCr, despite the best supportive care, is likely associated with a serious medical condition. Indeed, kidney function decline was documented in a subset of the population with different demographic and clinical characteristics compared to patients with stable or improved kidney function. Twenty-four-hour sCr elevation occurred in older subjects presenting with the higher sequential organ failure assessment score (SOFA score), including a lower mean PO2/FiO2 and a higher baseline sCr, compared to patients in the stable kidney function group. We suppose that baseline kidney function and severity of COVID-19 as key drivers of change in sCr within 24 hours from admission. As expected, the continuous variable ΔsCr was independently associated with AKI (HR, 7.9; 95% CI, 4.2–14.7; p < 0.001) and in-hospital mortality (HR, 4.0; 95% CI, 2.2–7.2; p < 0.001). Subsequent analysis showed that an increase in sCr > 0.05 mg/dL within 24 hours from admission occured in a high-risk cohort of patients who experienced poorer outcomes (Fig. 2). This subset of the population was associated with a 4.6-fold risk of developing AKI during hospitalization and with a 5.5-fold risk of 30-day in-hospital mortality. Similar results have been found in a previous study aimed to clarify the clinical meaning of within-day ΔsCr in adult hospitalized subjects. The results of that study reported that each 5% or 0.1 mg/dL elevation in ΔsCr was associated with increased 30-day all-cause mortality (adjusted HR, 1.08; 95% CI, 1.06–1.10) [14].
As previously mentioned, modest sCr variations are often overlooked in the real world because these changes are often linked to analytical (e.g., interindividual variability or analytic error) or biological (e.g., muscle mass, kidney tubular secretion, or protein-rich intake) variations. The cut-off of 0.05 mg/dL, indicating an increase of small magnitude, is theoretically greater than the “desirable” biologic variations of the laboratory methods used to measure sCr. The intralaboratory precision of sCr measurement is currently set at <4% to 5% below 1.13 mg/dL and at <2% above 1.13 mg/dL [17,19].
Based on these results, decrease in kidney function might be clinically relevant because it can affect patient survival. The change in sCr can be a useful prognostic marker to stratify COVID-19 patients at high risk of AKI or poor outcomes. In the context of the emerging and rapidly evolving situation of the COVID-19 pandemic, timely identification of patients at high risk of AKI allows hospitals to prioritize supportive treatment in the most vulnerable subjects. In attempt to prevent and limit kidney injury, adequate care bundles for subjects with AKI should include adequate hydration, avoidance of nephrotoxic drugs, and withdrawal of potentially deleterious drugs such as renin-angiotensin system blockers and metformin [20].
In the absence of more detailed data about the pathogenicity of severe acute respiratory syndrome coronavirus (SARS-CoV-2) in the kidney parenchyma [21], we suppose that the results of this study are not exclusively related to COVID-19 and might be translated to other general systemic diseases including bloodstream infection or sepsis of other origins. In particular, sepsis is a unique milieu to evaluate the clinical significance of sCr fluctuations. In this setting, sCr elevation is associated with dramatic increases in morbidity and mortality because reduced creatinine production due to the proinflammatory state tends to magnify even small variations in sCr [22].
The retrospective nature of our study, the small sample size and the lack of intra-group stratified analysis are the main drawbacks and limit the generalizability of our results. Although all patients received the same treatment for COVID-19 at admission, we cannot estimate the effects of other medicaments administered before admission nor can we control for other potential confounding variables able to interact with the most relevant outcomes. Given the general interest in identification of an early marker of morbidity and mortality in the pathogenesis of COVID-19, confirmatory studies are needed before drawing firm conclusions. Furthermore, further investigations should confirm these findings in the general population.
In conclusion, sCr elevation (greater than 0.05 mg/dL) within 24 hours from admission selected a group of aged patients with severe manifestations of COVID-19 at high risk of 30-day in-hospital mortality.
Acknowledgments
We thank all colleagues involved in the care of COVID-19 patients for their intellectual generosity. Special thanks are due to all healthcare workers that have provided, and still provide support in caring for COVID-19 patients.
Footnotes
Conflict of interest
All authors have no conflicts of interest to declare.
Authors’ contributions
Conceptualization: GA, AF
Data curation, Formal analysis: GA, AF
Intellectual contributions: FF, JM, G Cappelli, GG, GL
Writing–original draft: GM, RM, M Meschiari, EF, M Menozzi, G Cuomo, GO, AS, MDG, CP, FC, AB, SG
Writing–review & editing: JM, CM, G Cappelli, GG
All authors read and approved the final manuscript.
Supplementary Material
Modena Covid-19 Working Group (MoCo19) includes: Cristina Mussini, Giovanni Guaraldi, Erica Bacca, Andrea Bedini, Vanni Borghi, Giulia Burastero, Federica Carli, Giacomo Ciusa, Luca Corradi, Gianluca Cuomo, Margherita Digaetano, Giovanni Dolci, Matteo Faltoni, Riccardo Fantini, Giacomo Franceschi, Erica Franceschini, Vittorio Iadisernia, Damiano Larné, Marianna Menozzi, Marianna Meschiari, Jovana Milic, Gabriella Orlando, Francesco Pellegrino, Alessandro Raimondi, Carlotta Rogati, Antonella Santoro, Roberto Tonelli, Marco Tutone, Sara Volpi and Dina Yaacoub (Infectious Diseases Clinics, University Hospital, via del Pozzo 71, 41124 Modena, Italy); Gianni Cappelli, Riccardo Magistroni, Gaetano Alfano, Francesco Fontana, Ballestri Marco, Giacomo Mori, Roberto Pulizzi, Elisabetta Ascione, Marco Leonelli, Francesca Facchini and Francesca Damiano (Nephrology Dialysis and Transplant Unit, University Hospital of Modena, Modena, Italy); Massimo Girardis, Alberto Andreotti, Emanuela Biagioni, Filippo Bondi, Stefano Busani, Giovanni Chierego, Marzia Scotti and Lucia Serio (Department of Anesthesia and Intensive Care, University Hospital, via del Pozzo 71, 41124 Modena, Italy); Andrea Cossarizza, Caterina Bellinazzi, Rebecca Borella, Sara De Biasi, Anna De Gaetano, Lucia Fidanza, Lara Gibellini, Anna Iannone, Domenico Lo Tartaro, Marco Mattioli, Milena Nasi, Annamaria Paolini and Marcello Pinti (Chair of Pathology and Immunology, University of Modena and Reggio Emilia, Via Campi, 287, 41125 Modena, Italy).
References
- 1.Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol. 2020;215:108427. doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ronco C, Reis T, Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir Med. 2020;8:738–742. doi: 10.1016/S2213-2600(20)30229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pei G, Zhang Z, Peng J, et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. 2020;31:1157–1165. doi: 10.1681/ASN.2020030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubin S, Orieux A, Prevel R, et al. Characterization of acute kidney injury in critically ill patients with severe coronavirus disease 2019. Clin Kidney J. 2020;13:354–361. doi: 10.1093/ckj/sfaa099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 12.Joffe M, Hsu CY, Feldman HI, et al. Variability of creatinine measurements in clinical laboratories: results from the CRIC study. Am J Nephrol. 2010;31:426–434. doi: 10.1159/000296250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowe C, Sitch AJ, Barratt J, et al. Biological variation of measured and estimated glomerular filtration rate in patients with chronic kidney disease. Kidney Int. 2019;96:429–435. doi: 10.1016/j.kint.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 14.Yeh HC, Lo YC, Ting IW, et al. 24-hour serum creatinine variation associates with short- and long-term all-cause mortality: a real-world insight into early detection of acute kidney injury. Sci Rep. 2020;10:6552. doi: 10.1038/s41598-020-63315-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization (WHO) Team . Geneva: WHO; 2020. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases: interim guidance. WHO/COVID-19/laboratory/2020.5 [Internet] [cited 2020 Apr 12]. Available from: https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117. [Google Scholar]
- 16.Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55:622–627. doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee E, Collier CP, White CA. Interlaboratory variability in plasma creatinine measurement and the relation with estimated glomerular filtration rate and chronic kidney disease diagnosis. Clin J Am Soc Nephrol. 2017;12:29–37. doi: 10.2215/CJN.05400516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prowle JR, Kolic I, Purdell-Lewis J, Taylor R, Pearse RM, Kirwan CJ. Serum creatinine changes associated with critical illness and detection of persistent renal dysfunction after AKI. Clin J Am Soc Nephrol. 2014;9:1015–1023. doi: 10.2215/CJN.11141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reinhard M, Erlandsen EJ, Randers E. Biological variation of cystatin C and creatinine. Scand J Clin Lab Invest. 2009;69:831–836. doi: 10.3109/00365510903307947. [DOI] [PubMed] [Google Scholar]
- 20.Lameire N, Van Biesen W, Hoste E, Vanholder R. The prevention of acute kidney injury: an in-depth narrative review Part 1: volume resuscitation and avoidance of drug- and nephrotoxin-induced AKI. NDT Plus. 2008;1:392–402. doi: 10.1093/ndtplus/sfn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braun F, Lütgehetmann M, Pfefferle S, et al. SARS-CoV-2 kidney tropism associates with acute kidney injury. Lancet. 2020;396:597–598. doi: 10.1016/S0140-6736(20)31759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doi K, Yuen PS, Eisner C, et al. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J Am Soc Nephrol. 2009;20:1217–1221. doi: 10.1681/ASN.2008060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Modena Covid-19 Working Group (MoCo19) includes: Cristina Mussini, Giovanni Guaraldi, Erica Bacca, Andrea Bedini, Vanni Borghi, Giulia Burastero, Federica Carli, Giacomo Ciusa, Luca Corradi, Gianluca Cuomo, Margherita Digaetano, Giovanni Dolci, Matteo Faltoni, Riccardo Fantini, Giacomo Franceschi, Erica Franceschini, Vittorio Iadisernia, Damiano Larné, Marianna Menozzi, Marianna Meschiari, Jovana Milic, Gabriella Orlando, Francesco Pellegrino, Alessandro Raimondi, Carlotta Rogati, Antonella Santoro, Roberto Tonelli, Marco Tutone, Sara Volpi and Dina Yaacoub (Infectious Diseases Clinics, University Hospital, via del Pozzo 71, 41124 Modena, Italy); Gianni Cappelli, Riccardo Magistroni, Gaetano Alfano, Francesco Fontana, Ballestri Marco, Giacomo Mori, Roberto Pulizzi, Elisabetta Ascione, Marco Leonelli, Francesca Facchini and Francesca Damiano (Nephrology Dialysis and Transplant Unit, University Hospital of Modena, Modena, Italy); Massimo Girardis, Alberto Andreotti, Emanuela Biagioni, Filippo Bondi, Stefano Busani, Giovanni Chierego, Marzia Scotti and Lucia Serio (Department of Anesthesia and Intensive Care, University Hospital, via del Pozzo 71, 41124 Modena, Italy); Andrea Cossarizza, Caterina Bellinazzi, Rebecca Borella, Sara De Biasi, Anna De Gaetano, Lucia Fidanza, Lara Gibellini, Anna Iannone, Domenico Lo Tartaro, Marco Mattioli, Milena Nasi, Annamaria Paolini and Marcello Pinti (Chair of Pathology and Immunology, University of Modena and Reggio Emilia, Via Campi, 287, 41125 Modena, Italy).


