Abstract
Corona virus disease (COVID)-19 is caused by the novel severe acute respiratory syndrome coronavirus-2 (commonly referred to as SARS-CoV-2). In March 2020, the World Health Organization declared the COVID-19 outbreak a pandemic. Though the target organ for the virus is primarily the lungs, with the recent understanding of the pathobiology of this disease and the immune dysregulation associated with it, it is now clear that COVID-19 affects multiple organ systems. Several drugs and therapies have been tried or repurposed to combat the wrath posed by this disease. On October 22, 2020, the USA Food and Drug Administration approved remdesivir for use in adults and pediatric patients (12 years of age and older). Several of the drugs being tried against COVID-19 have hepatotoxicity as their potential side effect. This review aims to provide the latest insights on various drugs being used in the treatment of COVID-19 and their effects on the liver.
Keywords: COVID-19, Drugs, Liver, Drug-induced liver injury
Introduction
Coronavirus disease (COVID)-19 caused by the novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has affected millions worldwide and the numbers of cases are consistently rising.1 The ongoing COVID-19 caused by SARS-CoV-2 poses a serious threat to healthcare systems globally. As the virus continues to create havoc across the globe, it is eminent that the knowledge about the impact of this virus and its potential impact on different organs will evolve. Pulmonary and extra-pulmonary manifestations of COVID-19 are increasingly being recognized.2
Information on how COVID-19 affects the liver and how the drugs used for its treatment can affect the liver are slowly emerging. Although the real burden of this is currently unknown, as our understanding of the disease is constantly evolving, hepatic manifestations are being increasingly recognized. Various management strategies and research on drugs for COVID-19 are currently under study, many of which may have significant impact on liver.3 In the present review we aim to provide updated information regarding interplay of liver and COVID-19 in the face of this pandemic and to promote understanding of the role of drugs used for COVID-19 treatment and their effects on the liver.
Virology: key aspects
SARS-CoV-2 is an enveloped, positive-sense, single-stranded RNA virus classified as the newest member of the family of β coronaviruses.4 The life cycle of this spiked virus typically involves attachment, penetration, biosynthesis, maturation, and release. Angiotensin converting enzyme 2 (ACE2) has been identified as an important functional receptor, to which the virus attaches and continues its lifecycle. The spike protein of the virus binds to the ACE2 receptors of the cell, which enables the virus to enter and subsequently replicate within the cells.5 The receptor is not only present in the lungs but is also present in many extra-pulmonary sites like the kidney and gastrointestinal tract.6 This may explain the extra-pulmonary symptoms associated with COVID-19. The virus, after its entry, induces an inflammatory response and virus-specific T cells are attracted to the site of infection.7 The disease manifestations are primarily the result of direct viral-mediated damage and immune-mediated injury.8
Clinical manifestations
The symptoms of patients infected with SARS-CoV-2 can range from none or minimal to severe respiratory failure with multiple organ involvement.9 The majority of patients experience mild symptoms, like fever, cough, myalgia, fatigue and less commonly headache, hemoptysis and diarrhea. The clinical severity in the largest published registry to date (i.e. Chinese Center for Disease Control and Prevention) reported disease being mild in 81.4%, severe in 13.9%, and critical in 4.7%.10 The severe clinical manifestations have typically been described as severe pneumonia, acute respiratory distress syndrome and respiratory failure.11 However, the recent literature has shed light on the extra-pulmonary manifestations of the virus. Although the major manifestations involve the respiratory system, owing to its attachment to the ACE2 receptors, the cardiac, vascular, neurological, renal, and hepatic manifestations have also been described.12
Liver involvement in COVID-19
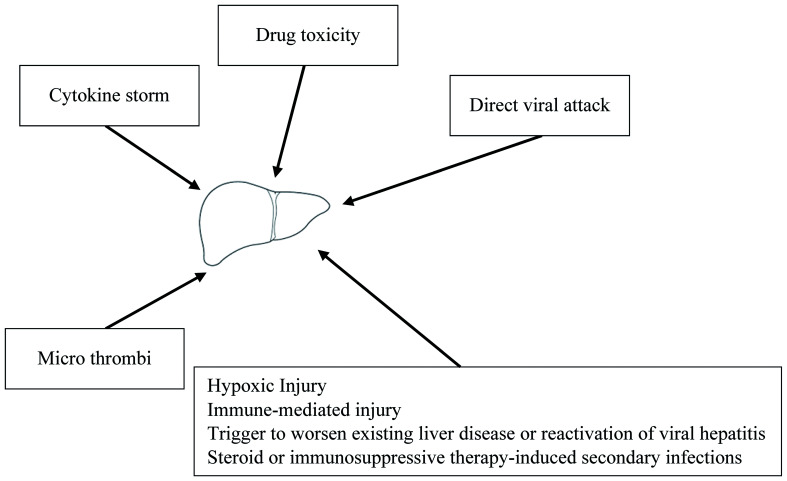
Liver injury in patients with COVID-19 might be due to a direct viral infection of the liver cells or due to multiple indirect pathways (Fig. 1). Given the higher expression of ACE2 receptors in cholangiocytes, the liver forms a potential target for SARS-CoV-2. Studies from biopsy of liver tissues of SARS-CoV-2-infected patients have shown liver cell apoptosis, which supports the direct hit hypothesis for this virus.13 In an elegant study by Lagana SM et al,14 histopathologic analysis of liver sections in a cohort of 40 COVID-19 autopsies was performed. Histologically, the most frequently encountered findings were macrovesicular steatosis, minimal-to-mild portal inflammation, and mild acute hepatitis. Thirty eight percent of cases had lobular cholestasis. Two cases had pale ovoid sinusoidal inclusions, which at low power resembled apoptotic hepatocytes. Vascular findings were focal in nature, with sinusoidal microthrombi being present in six cases. Polymerase chain reaction (commonly known as PCR) was performed on 20 autopsied livers and was positive in eleven (55%); however, there were no significant correlations between PCR positivity and any histologic findings.14 Other reports have described a significant cluster or scattered apoptotic hepatocytes, which are characterized by condensed nuclear or formed apoptotic bodies. There was no eosinophil infiltration, granuloma formation, centrilobular necrosis, or evidence of interface hepatitis.15 The virus may bind to cholangiocytes and cause bile duct dysfunction, thereby impairing liver regeneration and immune responses.16
Fig. 1. Possible mechanisms of effects of SARS-CoV-2 on liver.
SARS-CoV-2 can affect the liver directly but also indirectly, via several mechanisms.17 Indirect effects may be multifactorial, as depicted in Figure 1. Liver injury in patients with COVID-19 may be accounted for by a systemic inflammation induced by the cytokine storm or secondary to hypoxia or acute respiratory distress syndrome. The cytokine storm secondary to the virus infection can trigger extra-pulmonary systemic hyperinflammation syndrome. The cytokine surge (including interleukin (IL)-1, IL-6, and IL-10), inflammation and sepsis-related factors can damage the liver directly or indirectly.18 The possibility of hypoxia-induced damage, microthrombi, immune dysfunction or drug toxicities are other important mechanisms which can impact the liver.19
To battle this new enigmatic virus, a plethora of drugs and therapies have been tried or repurposed. Newer drugs or drug combinations may have concerns of exacerbating liver diseases or causing drug-induced hepatotoxicity, or can interact with other drugs to exacerbate their hepatotoxic potential. Some drugs may also reactivate a latent virus, which might lead to liver damage. In patients with pre-existing liver disease, COVID-19 infection could trigger a potentially fatal acute-on chronic liver failure.20
Several case reports, series and studies have shed light on the hepatobiliary manifestations of the disease. Transaminitis has been found to be associated in up to 14% to 53% of COVID-19 cases.21,22 Recent studies have reported abnormal liver function tests in as many as 76.3% of patients admitted with COVID-19. The authors also noted that liver test abnormalities became more pronounced during hospitalization. This can be explained in part by disease progression and super-added drug-induced liver injury (DILI). Hyperbilirubinemia has been documented in 11–18% of cases in some series.23 One series from New York, USA showed 46.5% of the patients had aspartate aminotransferase (AST) >40 U/L, 32% had alanine aminotransferase (ALT) >40 U/L, and 9.1% had total bilirubin >17.1 μmol/L.24 Cases of acute liver injury (ALI) have been reported and are associated with higher mortality.22 Most of the transaminitis may be self-resolving; however, more studies are needed to determine the significance of mildly deranged liver enzymes with the outcome of the disease. An extensive meta-analysis including 21 studies concluded that altered liver and kidney function and increased coagulation parameters are seen in severe and fatal cases of COVID-19.25
Patients with chronic medical comorbidities have been clearly shown to have severe COVID-19 disease and worse outcomes. A systematic review including 1,527 patients reported the prevalence of hypertension, cardiac and cerebrovascular disease, and diabetes to be 17.1%, 16.4%, and 9.7%, respectively.26 However, there is growing evidence to predict worse outcomes in patients with underlying liver disease.27 Literature has suggested patients who have a second ‘hit’, that is liver injury on the background of underlying liver disease, have poor outcomes.27 The first author of the manuscript has also noted a higher mortality in patients with acute-on chronic liver failure, in whom the acute precipitant was linked to COVID-19 infection.
Drugs used in the management of COVID-19
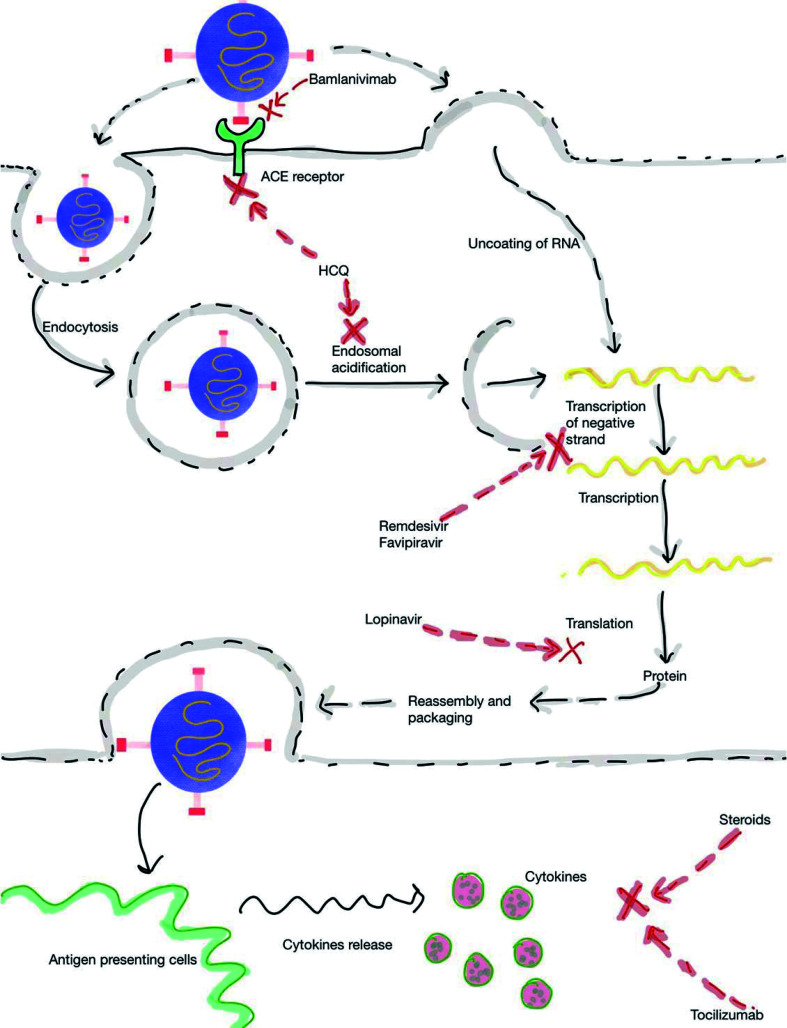
Several drugs have been tried in the prophylaxis and treatment of COVID-19 (Fig. 2); however, only one drug (remdesivir, RDV) has recently been approved by the USA FDA. Supplementary Table 1 depicts the current treatment protocol for COVID-19. The following section describes the various drugs used for COVID-19 and their implications on liver.
Fig. 2. Mechanism of action of drugs used in the treatment for COVID-19.
Antivirals
Hydroxychloroquine (HCQ)
HCQ is an oral drug which has both antimalarial and anti-inflammatory properties. It is commonly used in the management of rheumatological diseases. It is increasingly being used for management of COVID-19 based on in vitro data and initial reports.28 HCQ is supposed to act by preventing ACE2-mediated or endosomal-mediated viral entry (Fig. 2).29 Although it is considered as a relatively safe drug, reports of adverse cardiac effects in patients with COVID-19 is concerning (Supplementary Table 1).30 Despite retrospective observational studies showing mixed results, randomized controlled trials have not shown any benefit in COVID-19 patients irrespective of severity (Supplementary Table 2).31 HCQ has also been tried for the prophylaxis to prevent COVID-19. In an elegant randomized, double-blind, placebo-controlled trial, HCQ was used in people within 4 days of exposure to someone with confirmed COVID-19. After a high-risk or moderate-risk exposure to COVID-19, HCQ was not found to be effective in preventing illness compatible with COVID-19 (Supplementary Table 2).32 In a study by Cavalcanti et al.,33 the use of HCQ with or without azithromycin (AZT) in patients with mild to moderate COVID-19 did not improve clinical status at 15 days, as compared with standard care. In another randomized, controlled, open-label platform trial, the investigators noted that among patients hospitalized with COVID-19, HCQ usage did not result in a lower incidence of death at 28 days than those who received the usual care.34
HCQ is metabolized in the liver and may alter the metabolism of other drugs. HCQ has not been associated with significant elevations of liver enzymes and is not usually incriminated as a cause of DILI. ALI with jaundice due to usage of HCQ is very rare, with only few reports in the literature.35 An exception to this is when HCQ is used in patients with porphyria cutanea tarda. Its usage in high doses can trigger ALI, which is associated with sudden onset of fever and marked serum enzyme elevations. This reaction appears to be caused by the sudden mobilization of porphyrins and can be avoided when HCQ is started at lower doses.36 There have been scattered case reports in literature regarding DILI with the usage of HCQ in patients with COVID-19; however, it is to be noted that this is extremely rare.37
With recent data pointing at ineffectiveness of HCQ in altering the course of COVID-19 infection, the authors of this manuscript are not in favor of its clinical usage in patients with COVID-19.
Azithromycin (AZT)
AZT is commonly used in the treatment of bacterial infections and might also have antiviral activity against certain RNA viruses.38 AZT has also been shown to be effective in vitro against viruses such as Zika and rhinovirus, in addition to SARS-CoV-2,39 depicts immunomodulatory properties and can reduce exacerbations in chronic airway diseases.40 The COALITION II is an open-label randomized trial evaluating AZT in addition to standard of care (SOC), which included HCQ, compared with SOC alone in patients admitted to hospital with severe COVID-19. The investigators however found no benefit of AZT on clinical outcomes, including clinical status or mortality when added to SOC (odds ratio 1.36 [95% confidence interval: 0.94–1.97]; p=0.11).41
AZT may lead to idiosyncratic ALI. The clinical presentation of AZT-related DILI is usually of a cholestatic hepatitis arising within 1–3 weeks after start of treatment. It occasionally arises after AZT is stopped, and can occur even after a short 2 to 3 day course. This form of DILI due to AZT usually follows a benign course, but in some instances is associated with a prolonged jaundice and persistence of liver test abnormalities for 6 months or more.42 Case reports of vanishing bile duct syndrome with AZT usage have also been reported.43 AZT can occasionally be associated with hepatocellular injury as well. In these instances, the period of latency is typically short. Serum aminotransferase levels are markedly elevated and alkaline phosphatase (ALP) and gamma glutamyl transpeptidase is usually less than twice the upper limit of normal (ULN). The hepatocellular forms of DILI can be severe and lead to acute liver failure, mandating the need for an urgent liver transplant (LT) in certain patients. AZT has also been linked to the development of cutaneous reactions, such as erythema multiforme, Stevens Johnson syndrome and toxic epidermal necrosis. These cutaneous reactions are often associated with a certain degree of liver injury.44 HCQ and AZT are known to induce QT prolongation via a human Ether-à-go-go–related gene potassium channel blockade.45 In certain instances, this can trigger ventricular arrhythmias.
Lopinavir/ritonavir (LPV/r)
LPV/r is a protease inhibitor used in the treatment of human immunodeficiency virus (commonly known as HIV) infection. In the initial part of the COVID-19 pandemic, LPV/r was one of the first antivirals to be used in an attempt to improve clinical outcomes. Except for minor gastrointestinal disturbances and potential for drug interactions, the short-term use of this drug is not associated with major side effects (Supplementary Table 1).46 Although retrospective observational studies showed faster clearance with LPV/r, it was not associated with significantly better outcomes in randomized trials. In one of the pivotal randomized, controlled, open-label, platform trials of LPV/r conducted in patients admitted to the hospital with COVID-19, the investigators noted LPV/r not to be associated with reductions in 28-day mortality, duration of hospital stay, or risk of progressing to invasive mechanical ventilation or death.47 In another key study in patients with severe COVID-19, no benefit was noted with LPV/r treatment beyond standard care.48
LPV/r is primarily metabolized in the liver, largely via the cytochrome (CYP) P450 pathway. This pathway can lead to the formation of a toxic intermediate, which can cause DILI.49 Though mild elevation of liver enzymes can happen with LPV/r therapy, clinically apparent hepatotoxicities appear to be rare. The rate of DILI is higher in patients having underlying hepatitis B virus (HBV) and hepatitis C virus (HCV) infection.50 The latency to the onset of symptoms is usually 1 to 8 weeks, and the pattern of serum enzyme elevations varies from cholestatic to hepatocellular or mixed.51 The injury is usually self-limited; however, fatal cases have been reported. Using LPV/r in patients with underlying HBV and HCV infection can also lead to exacerbation of underlying chronic liver disease, with associated rise in HBV DNA or HCV RNA levels.52 In the context of LPV/r, the Réseau d’Étude Francophone de l’Hépatotoxicité des Produits de Santé (also known as REFHEPS), which is a European French-speaking study network, reported that within 2 weeks, four cases of LPV/r combination discontinuation occurred in patients with COVID-19 who were being treated with this drug.53 As the LPV/r combination is falling out of practice to treat COVID-19, its potential to cause DILI in patients with COVID remains more of a theoretical problem.
Remdesivir (RDV)
RDV is an adenosine analogue that is an RNA-dependent RNA polymerase (RdRp) inhibitor. It was developed by Gilead Sciences and was initially used for the treatment of Ebola virus disease. It is a broad spectrum antiviral drug that has shown to inhibit SARS-CoV-2, in vitro and in vivo.54,55 RDV has recently been approved by the USA FDA for use in patients who are older than 12 years of age and weighing at least 40 kg for the treatment of COVID-19 requiring hospitalization. In a recent double-blind, randomized, placebo-controlled trial of using intravenous RDV in patients who were hospitalized with COVID-19 and had evidence of lower respiratory tract infection; the investigators noted that RDV was superior to placebo in shortening the time to recovery in adults who were hospitalized with COVID-19 (Supplementary Table 2).56 There have also been reports suggesting the use of RDV to not be associated with a difference in time to clinical improvement; however, it has been suggested that RDV is to be used early in the clinical course of COVID-19 infection, before the peak viral replication occurs.57,58 The duration of therapy in most cases is 5 days.59
RDV is a prodrug and is metabolized in the cells into an alanine metabolite which is processed further into the monophosphate derivative and ultimately into the active nucleoside triphosphate.60 Studies have shown RDV usage to be associated with elevations of AST and ALT.61 In most instances, the enzyme elevations did not progress to severe liver damage, but cases of acute liver failure suspected as due to RDV usage have been reported.62 In the report describing two patients with RDV-induced acute liver failure, significant increases in transaminases occurred between day 3 and day 10 of RDV usage. This was also associated with coagulopathy and hepatic encephalopathy. The authors utilized the Naranjo algorithm to determine the possibility of a drug-induced effect and both the cases scored as a ‘probable’ adverse drug reaction, with a score of 6 each. After discontinuing the drug and treatment with N-acetyl cysteine infusion, there was a marked improvement in transaminases and liver functions.62 RDV is suggested to be stopped if the ALT >5-times ULN or ALP >2-times ULN, and total bilirubin >2-times ULN or in the presence of coagulopathy or clinical decompensation.63 In view of its potential for hepatotoxicity, the authors of this manuscript have not used RDV in patients with decompensated cirrhosis who have COVID-19 infection.
Favipiravir (FPR)
FPR is a prodrug with excellent bioavailability and has been approved in Japan for the treatment of influenza. FPR undergoes phosphoribosylation to favipiravir-RTP, which is the active form of this drug. It acts via inhibition of RdRp and also gets incorporated into the viral RNA strand, preventing its further extension.64 As the SARS-CoV-2-RdRp complex is at least 10-fold more active than any other viral RdRp known, the adequate dose of FPR for COVID-19 needs to be ascertained.65 The dose usually used in clinical practice is 1,800 mg twice a day on day 1, followed by 800 mg twice a day on days 2–14. An open-label, nonrandomized study conducted in China compared the effect of FPR vs. LPV/r in the treatment of COVID-19. Both groups had also received interferon-alpha (5 million units twice daily) by nasal inhalation. Compared with the LPV/r arm, patients in the FPR arm showed a statistically significant shorter median length of time to viral clearance (4 days vs. 11 days, p<0.001), improvement in chest computed tomography findings at day 14 (91.4% vs. 62.2%, p=0.004) and lower incidence of adverse effects (11.43% vs. 55.56% p<0.001).66 A phase 3 Russian trial (COVIDFPR 01) using FPR (ClinicalTrials.gov Identifier: NCT04434248) is currently ongoing and includes 330 patients from 30 medical centers across 9 Russian regions. A randomized, multicenter, open-labeled clinical trial in Indian patients has just been completed and the results are expected to be published soon.64
Though FPR usage can lead to increases in AST, ALP, ALT and total bilirubin, clinically apparent DILI seems rare. Less than 10% of patients with COVID-19 might experience ALT elevation with the use of FPR.63 Patients with severe liver dysfunction (Child-Pugh C) showed an increase in area under curve (6.3-fold) and Cmax (2.1-fold). It is thus suggested that FPR dosage should be reduced in patients with COVID-19 who have severe liver function impairment.67
Ivermectin (IVN)
IVN is well known for its antiparasitic activity. This drug has shown an in vitro reduction of viral RNA in Vero-hSLAM cells at 2 h post-infection with the SARS-CoV-2 clinical isolate Australia/VIC01/2020.68 IVN has demonstrated a broad spectrum of antiviral properties and acts as an inhibitor of the nuclear transport, which is mediated by the importin α/β1 heterodimer, itself which is pivotal for the translocation of viral species proteins (i.e. HIV-1 and SV40).69 The Ivermectin in COVID-19 trial is a retrospective cohort study (n=280) which enrolled patients with COVID-19 infection admitted at four Florida hospitals. This study documented a significantly lower mortality rate in the IVN (n=173) arm compared with the usual care (n=107) arm (15% vs. 25.2%; p=0.03).70 More data are needed to assess pulmonary tissue levels in humans and to assess its efficacy in the prophylaxis and treatment of COVID-19.
IVN is usually considered a safe drug and reports of IVN-related DILI are rare. In a case report where IVN was used for the treatment of Loa loa, IVN resulted in DILI that manifested 1 month later with aminotransferase elevation, showing a hepatocellular type of DILI. Liver biopsy depicted acute hepatocellular necrosis, lymphocytic lobular infiltrates and no fibrosis. The patient improved clinically within days and serum aminotransferase levels fell rapidly, becoming normal 3 months later.71
Immunomodulators
Steroids
In patients with severe COVID-19, the pathogenesis has been described in two phases, namely the viremic phase and the hyper-inflammatory phase. The use of steroids has been proposed in the hyper-inflammatory phase based on the observations of trials, including the RECOVERY trial (Supplementary Table 2). In the UK-based RECOVERY trial, 6,425 patients [2,104 randomized to receive dexamethasone (DXA) and 4,321 randomized to receive SOC], treatment with DXA lead to a reduction in mortality by one-third in patients receiving mechanical ventilation and by one-fifth in patients receiving supplemental oxygen compared to usual care alone.72 The recommended dose of DXA was 6 mg for a duration of 10 days. The CoDEX multicenter, open-label trial enrolled 299 patients in Brazil with COVID-19 and moderate to severe acute respiratory distress syndrome to 20 mg DXA daily (intravenous) treatment for 5 days, then 10 mg daily for 5 days or until intensive care unit (ICU) discharge atop SOC, or to SOC alone. DXA increased days alive and days free from mechanical ventilation during the first 28 days to a mean of 6.6 vs. 4.0 among controls (p=0.04) and also reduced the acute morbidity of the disease, with lower mean sequential organ failure assessment (commonly referred to as SOFA) scores at day 7 than with usual care (6.1 vs. 7.5, p=0.004).73 In a recent meta-analysis by the World Health Organization’s Rapid Evidence Appraisal for COVID-19 Therapies (otherwise known as REACT) working group, a total of 1,703 critically ill patients with COVID-19 were analyzed. The studies analyzed in the meta-analysis enrolled patients who were randomized to receive systemic DXA, hydrocortisone, or methylprednisolone, or to receive usual care or placebo. The use of steroids reduced 28-day mortality by a relative 34% compared with controls and the mortality effect size appeared similar between drugs used.74
Studies have also evaluated pulse steroid therapy in the treatment of COVID-19. In a single-blind, randomized, controlled, clinical trial involving hospitalized patients with severe COVID-19 who were in the early pulmonary phase of the illness were enrolled. Patients were randomized to either the steroid arm or the SOC arm. Methylprednisolone pulse was given as an intravenous injection of 250 mg daily for 3 days in the steroid arm. Patients with clinical improvement were higher in the methylprednisolone group than in the SOC group (94.1% vs. 57.1%), and the mortality rate was numerically lower in the methylprednisolone group (5.9% vs. 42.9%; p<0.001).75
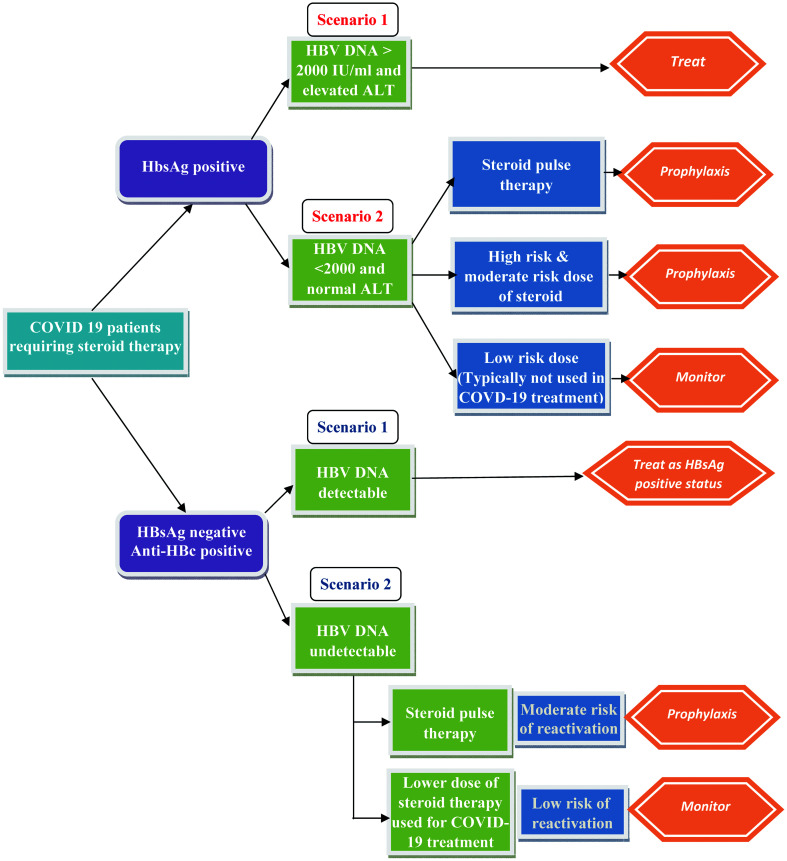
Though steroids are generally considered safe, they can lead to worsening of liver functions in certain specific clinical conditions. Of special interest is HBV reactivation and consequent liver involvement. It is well known that steroid treatment can lead to viral flare and HBV reactivation, and there exist specific guidelines to address this issue.76 In hepatitis B surface antigen (HBsAg)-positive patients, HBV reactivation is defined as a sudden and rapid increase in HBV DNA levels in patients with previously detectable DNA or reappearance of HBV DNA viremia in individuals who did not have viremia before the initiation of immunosuppressive therapy. In individuals who are initially negative for HBsAg and are hepatitis B core antibody (anti-HBc)-positive, HBV reactivation is defined by appearance of HBsAg and/or HBV DNA. In patients who are HBsAg-positive or patients who are HBsAg-negative but positive for anti-HBc, the doses of steroids which place the patient at risk of reactivation are reported as follows below.76
High risk (>10% risk)
Prednisone therapy at either medium dose (10–20 mg orally daily) or high dose (>20 mg orally daily) for more than 4-week duration increases the reactivation in patients who are HBsAg-positive.
Moderate risk (1–10% risk)
Low-dose steroid therapy equivalent to prednisone 10 mg administered orally daily over 4 weeks may increase the risk of reactivation up to 10% in HBsAg-positive individuals, and medium-dose steroids such as prednisone 10–20 mg administered orally (or equivalent) daily may increase the risk of seroconversion in HBsAg-negative and anti-HBc-positive individuals.
Low risk (<1% risk)
Patients are administered intra-articular steroid injections or a low dose of prednisone < 10 mg orally daily.
Recently, a pivotal study was published which analyzed the risk of HBsAg seroconversion in 12,997 patients exposed to at least one dose of systemic corticosteroids in the period between 2001 and 2010. Among the patients analyzed, 1,800 were positive for anti-HBc. Among those, 830 were positive for anti-HBs, which served as a protective factor. It was noted that in the remaining group of 970 anti-HBc-positive/anti-HBs-negative patients, the annual risk of presenting with a hepatitis flare was 16.2%, independent of the time of corticosteroid treatment. Patients who were anti-HBc-positive only had a higher risk of HBsAg Seroreversion (1-year incidence was 1.8%) as well.77
Drugs used to treat the disproportionate immune response after SARS-CoV-2 infection (mainly IL-6 receptor antagonists or high dose corticosteroids) were considered to be associated with moderate risk for HBV reactivation in HBsAg-negative/anti-HBc-positive individuals. However, a recent study enrolling 600 patients with severe COVID-19, who were treated with immune-modulator therapy, demonstrated that the risk of HBV reactivation while undergoing immunosuppressive treatment was low.78 This study was not randomized and had a small sample size, which is why more data is needed in this area to make strong recommendations.
In patients who merit HBV prophylaxis (HBV is otherwise inactive, but antiviral therapy is started to prevent HBV reactivation), it should ideally be started 2–4 weeks before the initiation of immunosuppressive therapy and maintained for at least 6 months after the last dose of immunosuppressive therapy. In patients where a decision to monitor (antiviral therapy not being initiated) has been made, the strategy should be monitoring of viral reactivation with determination of aminotransferases and HBV DNA levels conducted every 3 months.76
Figure 3 describes an algorithm for COVID-19-positive patients who are candidates for treatment with steroid therapy according to serological findings of an HBV screening.76,79
Fig. 3. Antiviral treatment strategy in patients with COVID-19 at risk for HBV reactivation receiving steroid therapy.
IL-6 receptor antagonists
Tocilizumab (TCZ)
TCZ is a humanized IgG1 recombinant monoclonal antibody used for the treatment of cytokine release syndrome associated with rheumatological conditions. It inhibits the inflammatory action of IL-6 by inhibiting the IL-6 receptor (Fig. 2). Since IL-6 is one of the prominent cytokines responsible for the hyper-inflammatory phase of COVID-19, it was postulated to have some role in patients with a severe or life-threatening disease. In a recent randomized, double-blind, placebo-controlled trial involving patients with confirmed SARS-CoV-2 infection having features of hyperinflammation, patients were randomized to receive a single dose of either TCZ (8 mg/kg of body weight) or placebo. The hazard ratio for intubation or death in the TCZ group, as compared with the placebo group, was 0.83 (95% confidence interval: 0.38–1.81; p=0.64), and the hazard ratio for disease worsening was 1.11 (95% confidence interval: 0.59–2.10; p=0.73). It was thus inferred that TCZ was not effective for preventing intubation or death in moderately ill hospitalized patients with COVID-19.80 The EMPACTA trial is the first global phase III trial to demonstrate patients with COVID-19 pneumonia who received TCZ in the first 2 days of ICU admission to have a lower risk of in-hospital mortality compared with those not treated with TCZ. Patients randomized to the TCZ group were 44% less likely to progress to mechanical ventilation or death compared to patients who received placebo plus SOC.81
The most common side effects of TCZ are headache, upper respiratory symptoms and hypertension. TCZ has minimal hepatic metabolism, and early registration trials of the usage of TCZ in rheumatologic conditions have shown mild serum aminotransferase elevations to occur in a high proportion (10% to 50%) of patients receiving TCZ. In a minority of patients (1–2%) levels rose above 5-times the ULN, which triggered discontinuation of treatment. The liver injury with TCZ is predominantly hepatocellular in nature, with no immunoallergic or autoimmune features. While the liver injury was severe, it was usually self-limited, with complete recovery occurring in 2 to 3 months. The mechanism by which it causes DILI is unknown, but may be the result of its effects on the immune system or on the IL-6 pathway, which is important in liver regeneration. TCZ being an immunosuppressive medication might also cause liver injury indirectly by reactivation of HBV.
Data of TCZ-related DILI when being used in the management of COVID-19 is scarce and limited to case reports. Case reports also exist on the usage of TCZ in patients with elevated liver enzymes. In one of the case series using TCZ for patients with severe COVID-19 and having elevated liver enzymes (up to 5-times the ULN), it was noted that after TCZ administration, the clinical condition of patients rapidly improved and liver function test normalized within 3 weeks of treatment.82
TCZ, being an immunosuppressive agent, is associated with the risk of hepatitis B reactivation. There have been reports of HBV reactivation and flare in patients with rheumatoid arthritis who have chronic hepatitis B and receive a short course of TCZ therapy, which in severe cases has also led to liver failure.83
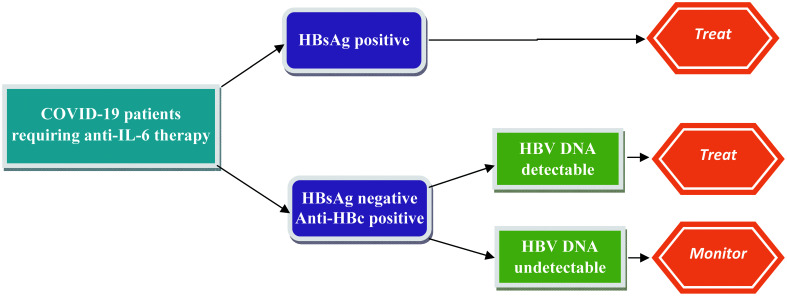
In patients with past resolved infection, the risk of HBV reactivation seems low with the use of TCZ therapy. In a key study, which enrolled 152 patients with resolved hepatitis B infection managed with disease-modifying anti-rheumatic drugs (including 25 patients with TCZ), the risk of HBV reactivation was very low (<5%).84 In a study enrolling patients with COVID-19 who received TCZ, the risk of HBV reactivation was reported to be low in patients with markers of past HBV infection.78 Though concrete guidelines on using antiviral prophylaxis to prevent HBV reactivation in patients with COVID-19 being treated with TCZ are lacking, we suggest using antiviral prophylaxis in patients with chronic hepatitis B infection and serial monitoring in patients with past HBV infection with undetectable HBV DNA levels (Fig. 4).
Fig. 4. Antiviral treatment strategy in patients with COVID-19 at risk for HBV reactivation receiving anti-IL-6 therapy.
Other IL-6 receptor antagonists being tried for COVID-19-related cytokine release syndrome are siltuximab and sarilumab.
IL-1 receptor antagonists
Endogenous IL-1 levels are elevated in patients with COVID-19 and high levels are associated with cytokine release syndrome.85 Anakinra (AKR) is the prototype drug being studied for COVID-19. A study conducted in Paris, France compared the outcomes of 52 patients with COVID-19 who were given AKR with 44 historical cohort patients. Admission to the ICU for invasive mechanical ventilation or death occurred for 13 (25%) patients in the AKR group and 32 (73%) patients in the historical group [hazard ratio of 0.22 (95% confidence interval: 0.11–0.41; p<0.0001).86 An increase in liver aminotransferases (>3-times the ULN) occurred in seven (13%) patients in the AKR group and four (9%) patients in the historical group.
In large registration trials enrolling patients with rheumatologic conditions, ALT elevations occurred in <1% of patients taking AKR, a rate not different from that in placebo recipients, and no cases of clinically apparent liver injury with jaundice were reported. AKR-related DILI usually follows a hepatocellular pattern. Liver biopsies have demonstrated an acute hepatocellular injury with prominence of eosinophils. Most patients with AKR-related DILI recovered within 2 to 8 weeks of stopping the drug, without evidence of residual injury. There have been cases reported where the DILI is severe, protracted and associated with transient features of hepatic failure.87
In patients with COVID-19 being treated with AKR, Cavalli et al.88 discuss the observations of elevated liver aminotransferases in some patients receiving the drug. Three of the 29 patients with COVID-19 who received AKR had derangement of liver enzymes, while 5 of 16 similar patients who did not receive AKR also showed increased enzymes. The authors, however, chose to taper AKR in those with elevated liver enzymes and observed that the LFTs did respond to the reduction in the dose of AKR. AKR has not been linked to reactivation of hepatitis B or exacerbation of chronic hepatitis C.89
Janus kinase (i.e. JAK) and numb associated kinase (i.e. NAK) inhibitors
ACE2 receptors are a point of cellular entry for the COVID-19 virus, which is expressed in lung alveolar epithelial type 2 cells. A known regulator of endocytosis is the adaptor-associated protein kinase 1 (i.e. AAK1). Disruption of AAK1 may interrupt intracellular entry of the virus. Baricitinib is a JAK inhibitor and has been identified as a NAK inhibitor, with a particularly high affinity for AAK1.90 Drugs which target NAK are likely mitigate alveolar and systemic inflammation in patients with COVID-19 pneumonia by inhibiting cytokine signaling.91 The National Institute of Allergy and Infectious Diseases (commonly referred to as the NIAID) Adaptive COVID-19 Treatment Trial evaluated the combination of RDV (100 mg administered intravenously daily, up to 10 days) along with baricitinib (4 mg per once daily, up to 14 days) compared with RDV alone. In September 2020, the investigators reported a 1-day reduction in the median time to recovery for the overall population treated with RDV plus baricitinib compared with RDV alone.92
In the large prelicensure clinical trials evaluating baricitinib in patients with rheumatoid arthritis, serum aminotransferase derangements occurred in up to 17% of subjects treated with baricitinib compared to 11% in placebo recipients. The aminotransferase elevations were typically mild and only in <1% of patients, and the values rose above 5-times the ULN. Less than 10% of the drug undergoes hepatic metabolism, which is primarily via the CYP 3A4 pathway. Serum aminotransferase elevations above 5-times the ULN should lead to temporary cessation of the drug. If liver enzyme elevations do not completely normalize or improve within a few weeks of drug cessation, or if symptoms of DILI worsen, baricitinib should be permanently discontinued.93
Convalescent plasma (CP)
Plasma from an individual who has recovered from COVID-19 with high titers of neutralizing antibodies has been proposed as a novel therapy for COVID-19. Although there is a theoretical risk of antibody enhancement and transfusion-related reactions, this therapy is otherwise considered safe. The FDA granted emergency use authorization on August 23, 2020 for use of CP in patients who are hospitalized with COVID-19. Early data indicated that the use of CP seemed to be effective for a better course of COVID-19 in critically ill patients.94 However, the multicenter, randomized, controlled PLACID trial, published recently and which enrolled 464 adults (≥18 years) admitted to the hospital with confirmed moderate COVID-19, demonstrated that the use of CP was not associated with a reduction in progression to severe COVID-19 or all-cause mortality.95 No serious adverse effects have been reported with use of CP; however, there exists a theoretical risk of transmission of infections like HBV and HCV.
Several other drugs being tried in the treatment of COVID-19 are mentioned in Supplementary Table 3.
Anticoagulants
The risk of venous thromboembolism (VTE) is increased in critically ill patients with COVID-19. A recent meta-analysis which analyzed 86 studies (33,970 patients) reported that VTE occurs in 22.7% of patients with COVID-19 who are admitted to the ICU. VTE risk was reported to be higher in non-ICU hospitalized patients as well.96 Reports have shown that there is a substantial microthrombosis, or immunothrombosis, related to hypoxemia, endothelial injury, and inflammation.97 Data is emerging on the discrepancy between the rate of pulmonary embolism and deep-vein thrombosis in patients with COVID-19 infection, and several cases are being reported where pulmonary embolism is occurring in the absence of deep-vein thrombosis and are located in the more peripheral pulmonary arteries. This leads to the hypothesis that immunothrombosis is probably much more prominent in patients with COVID-19 than originally recognized.97,98 Most current guidelines thus recommend that standard doses of anticoagulants be used for thromboprophylaxis in patients hospitalized with COVID-19. VTE prophylaxis is also to be administered post-discharge in patients with known additional risk factors for VTE, such as thrombophilia, obesity, advanced age, and a prior history of VTE.97
The potential effects of anticoagulants on liver are displayed in Supplementary Table 4.
Conclusions
COVID-19 is a disease which causes multisystem involvement. The immune dysregulation and the cytokine release syndrome associated with the disease are primarily responsible for the worse outcomes in those affected with it. Several drugs have been tried and several others remain in the pipeline to combat the deadly effects of this virus. Hepatotoxicity, reactivation of underlying viral hepatitis and potential to cause DILI remains with several of the drugs being used to treat COVID-19. However, as the involvement of liver can be the result of various pathobiologic pathways, in many instances it becomes difficult to discern the accurate etiology of the same.
Supporting information
Abbreviations
- AAK1
adaptor-associated protein kinase 1
- ACE2
angiotensin converting enzyme 2
- AKR
anakinra
- ALI
acute liver injury
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AZT
azithromycin
- COVID
coronavirus disease
- CP
convalescent plasma
- CYP
cytochrome
- DILI
drug-induced liver injury
- DXA
dexamethasone
- FDA
Food and Drug Administration
- FPR
favipiravir
- HBc
hepatitis B core
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HCQ
hydroxychloroquine
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- ICU
intensive care unit
- IL
interleukin
- IVN
ivermectin
- JAK
Janus kinase
- LPV/r
lopinavir/ritonavir
- NAK
numb associated kinase
- NIAID
National Institute of Allergy and Infectious Diseases
- PCR
polymerase chain reaction
- RdRp
RNA-dependent RNA polymerase inhibitor
- RDV
remdesivir
- REFHEPS
Réseau d’Étude Francophone de l’Hépatotoxicité des Produits de Santé
- SARS-CoV-2
severe acute respiratory syndrome coronavirus-2
- SOC
standard of care
- SOFA
sequential organ failure assessment
- TCZ
tocilizumab
- ULN
upper limit of normal
- VTE
venous thromboembolism
References
- 1.Ge H, Wang X, Yuan X, Xiao G, Wang C, Deng T, et al. The epidemiology and clinical information about COVID-19. Eur J Clin Microbiol Infect Dis. 2020;39(6):1011–1019. doi: 10.1007/s10096-020-03874-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu J, Song S, Cao HC, Li LJ. Liver diseases in COVID-19: Etiology, treatment and prognosis. World J Gastroenterol. 2020;26(19):2286–2293. doi: 10.3748/wjg.v26.i19.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng J. SARS-CoV-2: an Emerging Coronavirus that Causes a Global Threat. Int J Biol Sci. 2020;16(10):1678–1685. doi: 10.7150/ijbs.45053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ni W, Yang X, Yang D, Bao J, Li R, Xiao Y, et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020;24(1):422. doi: 10.1186/s13054-020-03120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grace JA, Casey S, Burrell LM, Angus PW. Proposed mechanism for increased COVID-19 mortality in patients with decompensated cirrhosis. Hepatol Int. 2020;14(5):884–885. doi: 10.1007/s12072-020-10084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parasher A. COVID-19: Current understanding of its pathophysiology, clinical presentation and treatment. Postgrad Med J. 2020. [DOI] [PMC free article] [PubMed]
- 8.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manabe T, Akatsu H, Kotani K, Kudo K. Trends in clinical features of novel coronavirus disease (COVID-19): A systematic review and meta-analysis of studies published from December 2019 to February 2020. Respir Investig. 2020;58(5):409–418. doi: 10.1016/j.resinv.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 11.Tahvildari A, Arbabi M, Farsi Y, Jamshidi P, Hasanzadeh S, Calcagno TM, et al. Clinical features, diagnosis, and treatment of COVID-19 in hospitalized patients: A systematic review of case reports and case series. Front Med (Lausanne) 2020;7:231. doi: 10.3389/fmed.2020.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elhence A, Shalimar COVID-19: Beyond respiratory tract. J Digest Endosc. 2020;11(1):24–26. doi: 10.1055/s-0040-1712550. [DOI] [Google Scholar]
- 13.Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and the liver. J Hepatol. 2020;73(5):1231–1240. doi: 10.1016/j.jhep.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lagana SM, Kudose S, Iuga AC, Lee MJ, Fazlollahi L, Remotti HE, et al. Hepatic pathology in patients dying of COVID-19: a series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. 2020;33(11):2147–2155. doi: 10.1038/s41379-020-00649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73(4):807–816. doi: 10.1016/j.jhep.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130(5):2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine storm in COVID-19: The current evidence and treatment strategies. Front Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: A review. Clin Immunol. 2020;215:108427. doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji D, Zhang D, Yang T, Mu J, Zhao P, Xu J, et al. Effect of COVID-19 on patients with compensated chronic liver diseases. Hepatol Int. 2020;14(5):701–710. doi: 10.1007/s12072-020-10058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, et al. Acute liver injury in COVID-19: Prevalence and association with clinical outcomes in a large U.S. cohort. Hepatology. 2020;72(3):807–817. doi: 10.1002/hep.31404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paliogiannis P, Zinellu A. Bilirubin levels in patients with mild and severe Covid-19: A pooled analysis. Liver Int. 2020;40(7):1787–1788. doi: 10.1111/liv.14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical characteristics of Covid-19 in New York city. N Engl J Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58(7):1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 26.Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shalimar, Elhence A, Vaishnav M, Kumar R, Pathak P, Soni KD, et al. Poor outcomes in patients with cirrhosis and Corona Virus Disease-19. Indian J Gastroenterol. 2020;39(3):285–291. doi: 10.1007/s12664-020-01074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meo SA, Klonoff DC, Akram J. Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19. Eur Rev Med Pharmacol Sci. 2020;24(8):4539–4547. doi: 10.26355/eurrev_202004_21038. [DOI] [PubMed] [Google Scholar]
- 29.Satarker S, Ahuja T, Banerjee M, Vignesh Balaji E, Dogra S, Agarwal T, et al. Hydroxychloroquine in COVID-19: Potential Mechanism of Action Against SARS-CoV-2. Curr Pharmacol Rep. 2020;6:203–211. doi: 10.1007/s40495-020-00231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uzelac I, Iravanian S, Ashikaga H, Bhatia NK, Herndon C, Kaboudian A, et al. Fatal arrhythmias: Another reason why doctors remain cautious about chloroquine/hydroxychloroquine for treating COVID-19. Heart Rhythm. 2020;17(9):1445–1451. doi: 10.1016/j.hrthm.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pathak SK, Salunke AA, Thivari P, Pandey A, Nandy K, Ratna HVK, et al. No benefit of hydroxychloroquine in COVID-19: Results of Systematic Review and Meta-Analysis of Randomized Controlled Trials”. Diabetes Metab Syndr. 2020;14(6):1673–1680. doi: 10.1016/j.dsx.2020.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020;383(6):517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N Engl J Med. 2020;383(21):2041–2052. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horby P, Mafham M, Linsell L, Bell JL, Staplin N, Emberson JR, et al. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383(21):2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giner Galvañ V, Oltra MR, Rueda D, Esteban MJ, Redón J. Severe acute hepatitis related to hydroxychloroquine in a woman with mixed connective tissue disease. Clin Rheumatol. 2007;26(6):971–972. doi: 10.1007/s10067-006-0218-1. [DOI] [PubMed] [Google Scholar]
- 36. Hydroxychloroquine. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548738/ [PubMed]
- 37.Falcão MB, Pamplona de Góes Cavalcanti L, Filgueiras Filho NM, Antunes de Brito CA. Case report: Hepatotoxicity associated with the use of hydroxychloroquine in a patient with COVID-19. Am J Trop Med Hyg. 2020;102:1214–1216. doi: 10.4269/ajtmh.20-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schögler A, Kopf BS, Edwards MR, Johnston SL, Casaulta C, Kieninger E, et al. Novel antiviral properties of azithromycin in cystic fibrosis airway epithelial cells. Eur Respir J. 2015;45(2):428–439. doi: 10.1183/09031936.00102014. [DOI] [PubMed] [Google Scholar]
- 39.Oldenburg CE, Doan T. Azithromycin for severe COVID-19. Lancet. 2020;396(10256):936–937. doi: 10.1016/S0140-6736(20)31863-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gibson PG, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10095):659–668. doi: 10.1016/S0140-6736(17)31281-3. [DOI] [PubMed] [Google Scholar]
- 41.Furtado RHM, Berwanger O, Fonseca HA, Corrêa TD, Ferraz LR, Lapa MG, et al. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): a randomised clinical trial. Lancet. 2020;396(10256):959–967. doi: 10.1016/S0140-6736(20)31862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maggioli C, Santi L, Zaccherini G, Bevilacqua V, Giunchi F, Caraceni P. A case of prolonged cholestatic hepatitis induced by azithromycin in a young woman. Case Reports Hepatol. 2011;2011:314231. doi: 10.1155/2011/314231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Juricic D, Hrstic I, Radic D, Skegro M, Coric M, Vucelic B, et al. Vanishing bile duct syndrome associated with azithromycin in a 62-year-old man. Basic Clin Pharmacol Toxicol. 2010;106(1):62–65. doi: 10.1111/j.1742-7843.2009.00474.x. [DOI] [PubMed] [Google Scholar]
- 44.Brkljacić N, Gracin S, Prkacin I, Sabljar-Matovinović M, Mrzljak A, Nemet Z. Stevens-Johnson syndrome as an unusual adverse effect of azithromycin. Acta Dermatovenerol Croat. 2006;14(1):40–45. [PubMed] [Google Scholar]
- 45.Lopez-Medina AI, Campos-Staffico AM, Luzum JA. QT prolongation with hydroxychloroquine and azithromycin for the treatment of COVID-19: The need for pharmacogenetic insights. J Cardiovasc Electrophysiol. 2020;31(10):2793–2794. doi: 10.1111/jce.14722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sulkowski MS, Mehta SH, Chaisson RE, Thomas DL, Moore RD. Hepatotoxicity associated with protease inhibitor-based antiretroviral regimens with or without concurrent ritonavir. AIDS. 2004;18(17):2277–2284. doi: 10.1097/00002030-200411190-00008. [DOI] [PubMed] [Google Scholar]
- 47.Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial Lancet. 2020;396(10259):1345–1352. doi: 10.1016/S0140-6736(20)32013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yeh RF, Gaver VE, Patterson KB, Rezk NL, Baxter-Meheux F, Blake MJ, et al. Lopinavir/ritonavir induces the hepatic activity of cytochrome P450 enzymes CYP2C9, CYP2C19, and CYP1A2 but inhibits the hepatic and intestinal activity of CYP3A as measured by a phenotyping drug cocktail in healthy volunteers. J Acquir Immune Defic Syndr. 2006;42(1):52–60. doi: 10.1097/01.qai.0000219774.20174.64. [DOI] [PubMed] [Google Scholar]
- 50.Kottilil S, Polis MA, Kovacs JA. HIV Infection, hepatitis C infection, and HAART: hard clinical choices. JAMA. 2004;292(2):243–250. doi: 10.1001/jama.292.2.243. [DOI] [PubMed] [Google Scholar]
- 51.Zell SC. Clinical vignette in antiretroviral therapy: jaundice. J Int Assoc Physicians AIDS Care (Chic) 2003;2(4):133–139. doi: 10.1177/154510970300200402. [DOI] [PubMed] [Google Scholar]
- 52.Canta F, Marrone R, Bonora S, D’Avolio A, Sciandra M, Sinicco A, et al. Pharmacokinetics and hepatotoxicity of lopinavir/ritonavir in non-cirrhotic HIV and hepatitis C virus (HCV) co-infected patients. J Antimicrob Chemother. 2005;55(2):280–281. doi: 10.1093/jac/dkh516. [DOI] [PubMed] [Google Scholar]
- 53.Olry A, Meunier L, Délire B, Larrey D, Horsmans Y, Le Louët H. Drug-induced liver injury and COVID-19 infection: The rules remain the same. Drug Saf. 2020;43(7):615–617. doi: 10.1007/s40264-020-00954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williamson BN, Feldmann F, Schwarz B, Meade-White K, Porter DP, Schulz J, et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature. 2020;585(7824):273–276. doi: 10.1038/s41586-020-2423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19 - Final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Glaus MJ, Von Ruden S. Remdesivir and COVID-19. Lancet. 2020;396(10256):952. doi: 10.1016/S0140-6736(20)32021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med. 2020;383(19):1827–1837. doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eastman RT, Roth JS, Brimacombe KR, Simeonov A, Shen M, Patnaik S, et al. Remdesivir: A review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent Sci. 2020;6(5):672–683. doi: 10.1021/acscentsci.0c00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zampino R, Mele F, Florio LL, Bertolino L, Andini R, Galdo M, et al. Liver injury in remdesivir-treated COVID-19 patients. Hepatol Int. 2020;14(5):881–883. doi: 10.1007/s12072-020-10077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carothers C, Birrer K, Vo M. Acetylcysteine for the treatment of suspected remdesivir-associated acute liver failure in COVID-19: A case series. Pharmacotherapy. 2020;40(11):1166–1171. doi: 10.1002/phar.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wong GL, Wong VW, Thompson A, Jia J, Hou J, Lesmana CRA, et al. Management of patients with liver derangement during the COVID-19 pandemic: an Asia-Pacific position statement. Lancet Gastroenterol Hepatol. 2020;5(8):776–787. doi: 10.1016/S2468-1253(20)30190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Agrawal U, Raju R, Udwadia ZF. Favipiravir: A new and emerging antiviral option in COVID-19. Med J Armed Forces India. 2020;76(4):370–376. doi: 10.1016/j.mjafi.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shannon A, Selisko B, Le N, Huchting J, Touret F, Piorkowski G, et al. Favipiravir strikes the SARS-CoV-2 at its Achilles heel, the RNA polymerase. bioRxiv. 2020:2020.05.15.098731. doi: 10.1101/2020.05.15.098731. [DOI] [Google Scholar]
- 66.Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, et al. Experimental treatment with favipiravir for COVID-19: An open-label control study. Engineering (Beijing) 2020;6(10):1192–1198. doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li L, Wang X, Wang R, Hu Y, Jiang S, Lu X. Antiviral agent therapy optimization in special populations of COVID-19 patients. Drug Des Devel Ther. 2020;14:3001–3013. doi: 10.2147/DDDT.S259058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rizzo E. Ivermectin, antiviral properties and COVID-19: a possible new mechanism of action. Naunyn Schmiedebergs Arch Pharmacol. 2020;393(7):1153–1156. doi: 10.1007/s00210-020-01902-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rajter JC, Sherman MS, Fatteh N, Vogel F, Sacks J, Rajter JJ. Use of ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019: The ivermectin in COVID nineteen study. Chest. 2021;159(1):85–92. doi: 10.1016/j.chest.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Veit O, Beck B, Steuerwald M, Hatz C. First case of ivermectin-induced severe hepatitis. Trans R Soc Trop Med Hyg. 2006;100(8):795–797. doi: 10.1016/j.trstmh.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 72.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: The CoDEX randomized clinical trial. JAMA. 2020;324(13):1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: A meta-analysis. JAMA. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Edalatifard M, Akhtari M, Salehi M, Naderi Z, Jamshidi A, Mostafaei S, et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial. Eur Respir J. 2020;56(6):2002808. doi: 10.1183/13993003.02808-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: Current concepts, management strategies, and future directions. Gastroenterology. 2017;152(6):1297–1309. doi: 10.1053/j.gastro.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wong GL, Wong VW, Yuen BW, Tse YK, Yip TC, Luk HW, et al. Risk of hepatitis B surface antigen seroreversion after corticosteroid treatment in patients with previous hepatitis B virus exposure. J Hepatol. 2020;72(1):57–66. doi: 10.1016/j.jhep.2019.08.023. [DOI] [PubMed] [Google Scholar]
- 78.Rodríguez-Tajes S, Miralpeix A, Costa J, López-Suñé E, Laguno M, Pocurull A, et al. Low risk of hepatitis B reactivation in patients with severe COVID-19 who receive immunosuppressive therapy. J Viral Hepat. 2021;28(1):89–94. doi: 10.1111/jvh.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Varona Pérez J, Rodriguez Chinesta JM. Risk of hepatitis B reactivation associated with treatment against SARS-CoV-2 (COVID-19) with corticosteroids. Rev Clin Esp. 2020;220(8):534–536. doi: 10.1016/j.rce.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Parr JB. Time to reassess tocilizumab’s role in COVID-19 pneumonia. JAMA Intern Med. 2021;181(1):12–15. doi: 10.1001/jamainternmed.2020.6557. [DOI] [PubMed] [Google Scholar]
- 82.Serviddio G, Villani R, Stallone G, Scioscia G, Foschino-Barbaro MP, Lacedonia D. Tocilizumab and liver injury in patients with COVID-19. Therap Adv Gastroenterol. 2020;13 doi: 10.1177/1756284820959183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sonneveld MJ, Murad SD, van der Eijk AA, de Man RA. Fulminant liver failure due to hepatitis B reactivation during treatment with tocilizumab. ACG Case Rep J. 2019;6(12):e00243. doi: 10.14309/crj.0000000000000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Watanabe T, Fukae J, Fukaya S, Sawamukai N, Isobe M, Matsuhashi M, et al. Incidence and risk factors for reactivation from resolved hepatitis B virus in rheumatoid arthritis patients treated with biological disease-modifying antirheumatic drugs. Int J Rheum Dis. 2019;22(4):574–582. doi: 10.1111/1756-185X.13401. [DOI] [PubMed] [Google Scholar]
- 85.Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huet T, Beaussier H, Voisin O, Jouveshomme S, Dauriat G, Lazareth I, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2(7):e393–e400. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Taylor SA, Vittorio JM, Martinez M, Fester KA, Lagana SM, Lobritto SJ, et al. Anakinra-induced acute liver failure in an adolescent patient with still’s disease. Pharmacotherapy. 2016;36(1):e1–e4. doi: 10.1002/phar.1677. [DOI] [PubMed] [Google Scholar]
- 88.Cavalli G, De Luca G, Campochiaro C, Della-Torre E, Ripa M, Canetti D, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2(6):e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Anakinra in: LiverTox: Clinical and research information on drug-induced liver injury. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548615/ [PubMed]
- 90.Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395(10223):e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stebbing J, Phelan A, Griffin I, Tucker C, Oechsle O, Smith D, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20(4):400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Adaptive COVID-19 treatment trial 2 (ACTT-2). Available from: https://clinicaltrials.gov/ct2/show/NCT04401579.
- 93.Jorgensen SCJ, Tse CLY, Burry L, Dresser LD. Baricitinib: A review of pharmacology, safety, and emerging clinical experience in COVID-19. Pharmacotherapy. 2020;40(8):843–856. doi: 10.1002/phar.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Altuntas F, Ata N, Yigenoglu TN, Bascı S, Dal MS, Korkmaz S, et al. Convalescent plasma therapy in patients with COVID-19. Transfus Apher Sci. 2021;60(1):102955. doi: 10.1016/j.transci.2020.102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ. 2020;371:m3939. doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nopp S, Moik F, Jilma B, Pabinger I, Ay C. Risk of venous thromboembolism in patients with COVID-19: A systematic review and meta-analysis. Res Pract Thromb Haemost. 2020;4(7):1178–1191. doi: 10.1002/rth2.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chowdhury JF, Moores LK, Connors JM. Anticoagulation in hospitalized patients with Covid-19. N Engl J Med. 2020;383(17):1675–1678. doi: 10.1056/NEJMclde2028217. [DOI] [PubMed] [Google Scholar]
- 98.Desborough MJR, Doyle AJ, Griffiths A, Retter A, Breen KA, Hunt BJ. Image-proven thromboembolism in patients with severe COVID-19 in a tertiary critical care unit in the United Kingdom. Thromb Res. 2020;193:1–4. doi: 10.1016/j.thromres.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.