Abstract
Background and Aims
Hepatic sinusoidal obstruction syndrome (HSOS) is caused by toxic injury to sinusoidal endothelial cells in the liver. The intake of pyrrolizidine alkaloids (PAs) in some Chinese herbal remedies/plants remains the major etiology for HSOS in China. Recently, new diagnostic criteria for PA-induced HSOS (i.e. PA-HSOS) have been developed; however, the efficacy has not been clinically validated. This study aimed to assess the performance of the Nanjing criteria for PA-HSOS.
Methods
Data obtained from consecutive patients in multiple hospitals, which included 86 PA-HSOS patients and 327 patients with other liver diseases, were retrospectively analyzed. Then, the diagnostic performance of the Nanjing criteria and simplified Nanjing criteria were evaluated and validated. The study is registered in www.chictr.org.cn (ID: ChiCTR1900020784).
Results
The Nanjing criteria have a sensitivity and specificity of 95.35% and 100%, respectively, while the simplified Nanjing criteria have a sensitivity and specificity of 96.51% and 96.33%, respectively, for the diagnosis of PA-HSOS. Notably, a proportion of patients with Budd-Chiari syndrome (11/49) was misdiagnosed as PA-HSOS on the basis of the simplified Nanjing criteria, and this was mainly due to the overlapping features in the enhanced computed tomography/magnetic resonance imaging examinations. Furthermore, most of these patients (10/11) had occlusion or thrombosis of the hepatic vein, and communicating vessels in the liver were found in 8/11 patients, which were absent in PA-HSOS patients.
Conclusions
The Nanjing criteria and simplified Nanjing criteria exhibit excellent performance in diagnosing PA-HSOS. Thus, both could be valuable diagnostic tools in clinical practice.
Keywords: Drug-induced liver injury, Hepatic sinusoidal obstruction syndrome, Pyrrolizidine alkaloids, Sensitivity, Specificity
Introduction
Hepatic sinusoidal obstruction syndrome (HSOS), which is also referred to as hepatic veno-occlusive disease (or simply HVOD), is a hepatic vascular disease featured by edema, detachment of endothelial cells in the small sinusoidal hepatic and interlobular veins, necrosis, portal hypertension, intrahepatic congestion, and liver dysfunction.1–6 It has been demonstrated that HSOS is caused by toxic injury to sinusoidal endothelial cells in the liver, and that this injury can be induced by different etiological factors. In Western countries, HSOS usually occurs as a consequence of bone marrow hematopoietic stem cell transplantation (HSCT) treatment for hematological malignancies.7 In China, the intake of pyrrolizidine alkaloids (PAs) in certain Chinese herbal remedies/plants (e.g., Senecio, Crotalaria, heliotropes, and Tusanqi) remains the major etiology for HSOS.8 The manifestations in HSOS patients include jaundice, abdominal distention, hepatomegaly and ascites, which overlap with other liver diseases, such as Budd-Chiari syndrome (BCS), acute liver failure, or decompensated cirrhosis of various causes.9,10 This has posed challenges in the diagnosis of HSOS. In recent years, the incidence of PA-induced HSOS (i.e. PA-HSOS) in China has been increasing. However, PA-HSOS has been frequently misdiagnosed as the following diseases: acute/subacute severe hepatitis, decompensated cirrhosis, BCS, etc. This misdiagnosis delays the proper treatment of patients with PA-HSOS, in which a proportion of severe cases can progress into severe dysfunction, and even multiple organ failure. Therefore, there is an urgent need to develop diagnostic criteria specific to PA-HSOS.4
For HSOS caused by bone marrow HSCT (HSCT-HSOS), the diagnosis is made according to the improved Seattle and Baltimore criteria.11 However, it has been noted that there are significant differences, in terms of epidemiology, ethnicity, etiology and underlying diseases, between PA-HSOS and HSCT-HSOS, and that patients with PA-HSOS have specific clinical characteristics. For instance, previous study2 revealed that PA-HSOS patients have a relatively longer incubation period from the initial exposure to PA, which ranges from 3 days to 3 months, and that serum total bilirubin (TBil) <34.2 µmol/L can be found in approximately 40% of PA-HSOS patients. In addition, as high as 90% of PA-HSOS patients presented with distinctive imaging findings, such as hepatomegaly, uneven liver perfusion in the balance phase, compressive stenosis of the hepatic segmental inferior vena cava in the imaging findings of the enhanced computed tomography (CT) scan or magnetic resonance imaging (MRI), and decreased peak velocity of portal vein blood flow in the Doppler ultrasonography. On the other hand, a history of chemotherapeutic exposure for 20 or 21 days before onset and 2%–5% weight gain at onset are required in the improved Seattle and Baltimore criteria. However, PA-HSOS patients do not often have the experience of using chemotherapy. Doctors do not usually have accurate weight-change data due to the sporadic nature of the disease. Based on these reasons, the improved Seattle and Baltimore criteria are not suitable for the diagnosis of PA-HSOS. Therefore, in 2017, the Chinese Society of Gastroenterology Committee of Hepatobiliary Disease reached a consensus, and proposed the Nanjing criteria for the diagnosis of PA-HSOS (Table 1).6 Nevertheless, to date, the clinical efficacy of the Nanjing criteria has not been validated.
Table 1. Nanjing criteria for the diagnosis of PA-HSOS.
| No. | Diagnostic criteria |
|---|---|
| i | A definite history of PA-containing plants |
| ii | Abdominal distention and/or pain in the hepatic region, hepatomegaly and ascites |
| iii | Elevation of serum TBil or abnormal liver function |
| iv | Typical features in the enhanced CT or MRI |
Patients with i, ii, iii, iv or i, and pathological evidence, and the ruling out the other known causes of liver injury can be diagnosed as PA-HSOS.
The present multi-center retrospective study intends to validate the diagnostic performance of the Nanjing criteria for Chinese patients with PA-HSOS.
Methods
Study population
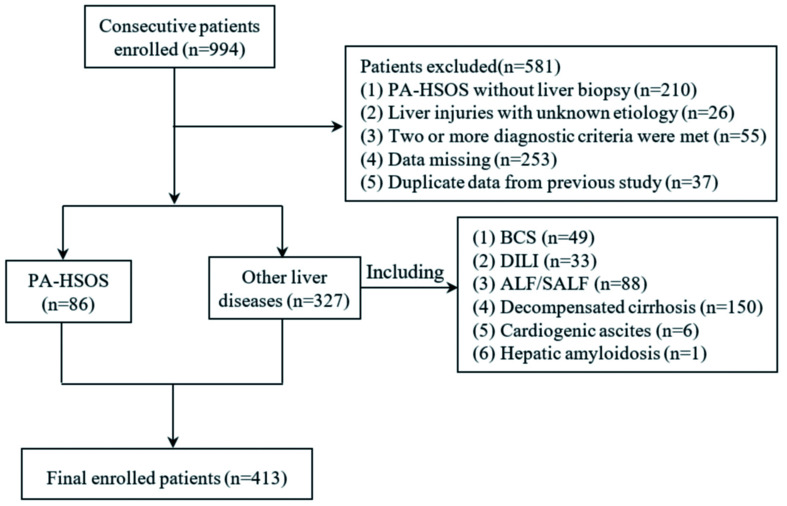
During the period between November 2011 and December 2018, a total of 994 consecutive patients in multiple centers were retrospectively evaluated for eligibility in the present study. The participating hospitals were, as follows: (1) Department of Gastroenterology, The Affiliated Drum Tower Hospital of Nanjing University Medical School; (2) Department of Hepatology, Nanjing Second Hospital, Nanjing University of Chinese Medicine; and (3) Liver Diseases Center of PLA and Department of Infectious Diseases, General Hospital of Eastern Theater Command, and Bayi Hospital Affiliated to Nanjing University of Chinese Medicine. The inclusion criteria used for the enrollment of patients were, as follows: (1) presentation of clinical symptoms at ≤6 months; (2) abnormal liver function [elevated alanine transaminase (ALT), aspartate aminotransferase (AST), or total bilirubin (Tbil)]; (3) ascites and/or abdominal distension; or (4) anorexia. Patients with the following criteria were excluded from the present study: (1) suspected, but not pathologically diagnosed, with PA-HSOS; (2) two or more liver diseases, or PA-HSOS in combination with biliary diseases; (3) unknown cause of liver disease; (4) missing clinical data; and (5) duplicate data from the previous study of the Nanjing Criteria.
Finally, 86 patients with PA-HSOS and 327 patients with other liver diseases of various etiologies were retrospectively investigated (Fig. 1). All patients with PA-HSOS underwent transjugular liver biopsy (TJLB).
Fig. 1. Flow diagram for the patient enrollment.
The present study was reviewed and approved by the Ethics Committee of each participating hospital. The requirement for written informed consent was waived due to the retrospective nature of the study.
Diagnostic criteria
The reference standards of the diagnostic criteria for PA-HSOS were, as follows: (1) pathologically proven edema, necrosis, detachment of hepatic sinusoidal endothelial cells in the hepatic acinus zone III, and significant dilation and congestion of hepatic sinusoids; and (2) confirmed history of intake of PA-containing herbs/plants, or the detection of blood pyrrole-protein adducts (PPAs). All liver tissue specimens were independently examined by two experienced hepatopathologists.
The clinical components of the Nanjing criteria for PA-HSOS were, as follows: (1) definite history of intake of PA-containing plants; (2) abdominal distention and/or pain in the hepatic region, hepatomegaly and ascites; (3) elevated serum TBil or abnormal serum markers for liver function; and (4) typical features in the enhanced CT or MRI examinations (e.g., diffuse hepatomegaly, ascites, and plain scans revealing a heterogeneous decreased density of the hepatic parenchyma; enhancement characterized by a map-like or mottle-like nonhomogeneous appearance in the venous phase and equilibrium phase; hepatic vein lumen being stenotic or obscured; hepatic segment of the inferior vena cava being compressed and thinner; and MRI findings being similar to CT findings).
The diagnostic criteria for other liver diseases were determined upon referring to established guidelines or text books. The diagnosis was independently performed by two experienced hepatologists (Wei Zhang and Yuzheng Zhuge), according to the diagnostic criteria. Simply, patients with a consistent diagnosis were included in the study, and those with inconsistent diagnosis were excluded. The diagnosis of BCS was made in accordance with the 2016 European Association for the Study of the Liver (EASL) Clinical Practice Guidelines.12
The diagnosis of decompensated cirrhosis was made according to the 2018 EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis, which was based on the characteristic clinical manifestation, laboratory tests, imaging performance, and histological data.13 In addition, the cause of chronic liver damage was determined by medical history and laboratory examination.14–18
The diagnostic criteria for acute liver failure (ALF) or subacute liver failure (SALF) were determined by reference to the 2017 EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure.19
The diagnosis for drug-induced liver injury (DILI) was made according to the American College of Gastroenterology Clinical Guidelines on the diagnosis and management of idiosyncratic DILI.20 PA-HSOS is a unique sub-group of DILI, and has its own diagnostic criteria. Thus, DILI cases in the present study were defined as DILI except when HSOS was induced by PA or other drugs.
Quantification of PPAs
Serum PPAs were examined using the pre-column derivatization liquid chromatography-tandem mass spectrometry method, as previously reported, with minor modifications.3
Statistical analysis
The statistical analyses were carried out using the SPSS version 22.0 software (IBM Corp., Armonk, NY, USA). Continuous data were expressed as mean ± standard deviation (SD), or median and interquartile range (25 and 75 percentiles), according to the data distribution. Baseline data were compared between PA-HSOS and other liver diseases (BCS, decompensated cirrhosis, DILI, ALF/SALF, and cardiogenic ascites), respectively. Continuous variables were compared using Student’s t-test or Manne-Whitney U-test, when an abnormal distribution was detected. In addition, Pearson’s chi-square test or Fisher’s exact test was used to compare the categorical variables among different groups. The sensitivity, specificity, overall accuracy, positive predictive value (PPV), negative predictive value (NPV), 95% confidence interval (CI), likelihood ratio, Kappa value, and area under the receiver operating characteristics curve (commonly referred to as AUC) were calculated to evaluate the diagnostic accuracy of the Nanjing criteria. A two-tailed p-value of <0.05 was considered statistically significant.
Results
Characteristics of the study subjects
A total of 413 patients, treated between November 2011 and December 2018, who fulfilled the eligibility criteria, were retrospectively enrolled in the present study (Fig. 1). Among these patients, 86 underwent TJLB and had ascites, and the PA of these patients met the criteria for the diagnosis of HSOS. The pathological findings confirmed edema, necrosis, detachment of hepatic sinusoidal endothelial cells in hepatic acinus zone III, and significant dilation and congestion of hepatic sinusoids (Fig. 2). Among the 86 PA-HSOS patients, 85 were found to have a clear history of intake of PA-containing herbal medicine/plants, and 1 was diagnosed with PA-HSOS through a blood test of PPAs that confirmed the history of PA intake. The remaining 327 patients were diagnosed with other various liver diseases, according to the diagnostic criteria described in the Materials and Methods section (BCS: n=49, DILI: n=33, ALF/SALF: n=88, decompensated cirrhosis: n=150, cardiogenic ascites: n=6, and liver amyloidosis: n=1).
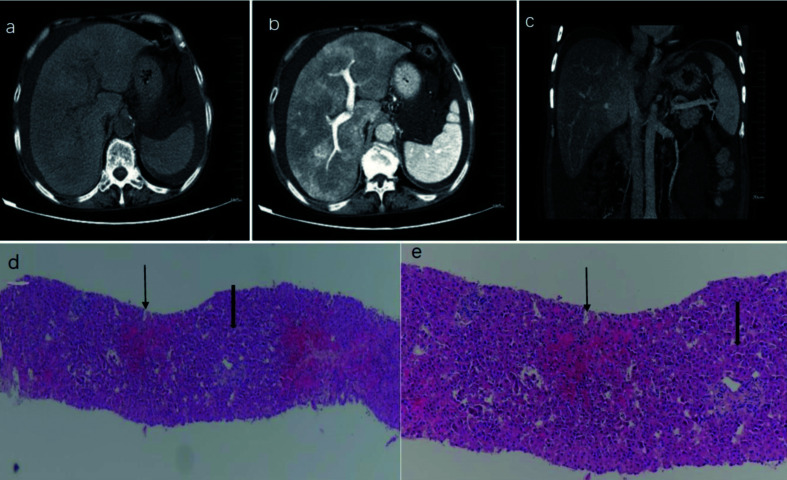
Fig. 2. Representative CT images and pathological findings for PA-HSOS patients.
(A) The CT imaging revealed diffuse hepatomegaly, ascites, and plain scans showing the heterogeneous decreased density of the hepatic parenchyma. (B) The CT enhancement characterized a map-like or mottle-like nonhomogeneous appearance in the equilibrium phase. (C) The CT images showed that the hepatic vein lumen was obscured, and the hepatic segment of the inferior vena cava was compressed and thinner. (D) hematoxylin-eosin (HE) ×40, Zone III, Zone I; (E) HE ×100, Zone III, Zone I. The pathological findings confirmed edema, necrosis, detachment of hepatic sinusoidal endothelial cells in hepatic acinus zone III, significant dilation and congestion of hepatic sinusoids, but showed no significant changes in zone I.
The demographic, laboratory and clinical characteristics of these patients are summarized in Table 2. Patients with PA-HSOS were older than patients with other liver diseases, and ascites was present in all PA-HSOS patients. The findings of the enhanced CT/MRI were significantly different between PA-HSOS and other liver injuries (p<0.001). Patchy enhancement and heterogeneous hypoattenuation were the typical features (Fig. 2). The majority of these PA-HSOS patients had mildly elevated TBil, and nearly normal transaminase and platelet levels. The laboratory characteristics were similar to those in BCS patients but were different from patients with decompensated cirrhosis, who had decreased platelet levels. For the Child-Turcotte-Pugh and Model for End-Stage Liver Disease (commonly known as MELD) scores, patients with ALF/SALF had the highest scores, followed by patients with DILI, PA-HSOS, decompensated cirrhosis, BCS, and cardiogenic ascites.
Table 2. Demographic, laboratory and clinical characteristics of the study patients.
| PA-HSOS, n=86 | BCS, n=49 | Decompensated cirrhosis, n=150 | DILI, n=33 | ALF/SALF, n=88 | Cardiogenic ascites, n=6 | |
|---|---|---|---|---|---|---|
| Age in years, median (range) | 65 (60.75, 70) | 40 (30, 52)*** | 57.5 (50, 68)*** | 59 (46.5, 68)* | 54 (42, 62.8)*** | 59 (31.25, 73.25) |
| Male, n (%) | 49 (56.98) | 20 (40.82) | 90(60) | 15 (45.45) | 67 (76.14)** | 3 (50) |
| Disease course in days, median (range) | 30 (20, 60) | 60 (30, 90)*** | 30 (20, 90) | 20 (9.5, 30)** | 20 (12.5, 30)** | 45 (13.75, 135) |
| History of ingesting PA-containing plants, n (%) | 85 (98.84) | 0 | 0 | 0 | 0 | 1 (16.67) |
| Ascites | ||||||
| No, n (%) | 0 (0) | 13 (26.53)*** | 10 (6.67)* | 3 (9.09) | 12 (13.64)** | 0 (0) |
| MI, n (%) | 5 (5.81) | 7 (14.29) | 49 (32.67)*** | 12 (36.36) | 22 (25)** | 1 (16.67) |
| MO, n (%) | 72 (83.72) | 23 (46.94)*** | 84 (56)*** | 17 (51.52)*** | 53 (60.23)** | 4 (66.66) |
| SE, n (%) | 9 (10.47) | 6 (12.24) | 7 (4.66) | 1 (3.03)*** | 1 (1.13)* | 1 (16.67) |
| ALT in U/L, median (range) | 40.9 (25.98, 70.88) | 23.6 (15.4, 34.45)*** | 34.3 (24.53, 64) | 201.5 (120.85, 505)*** | 238.7 (80.68, 894.35)*** | 18.45 (12.78, 25.85)** |
| Elevated ALT, n (%) | 43 (50) | 16 (32.65) | 73 (48.67) | 33 (100)*** | 84 (95.45)*** | 2 (33.33) |
| AST in U/L, median (range) | 56.7 (38.25, 91) | 29.1 (20.35, 43.8)*** | 50.55 (32.13, 95) | 186.5 (85.05, 444.15)*** | 242.95 (111.23, 635.13)*** | 27.75 (21.88, 38.7)** |
| Elevated AST, n (%) | 63 (73.26) | 44 (89.80)* | 97 (64.67) | 33 (100)** | 88 (100)*** | 4 (66.67) |
| TBil in µmol/L, median (range) | 33.7 (22.38, 46.2) | 26.6 (19.45, 47.33) | 29.25 (18.33, 56) | 164.2 (73.8, 308.6)*** | 273.05 (174.7, 419.83)*** | 20.9 (8.28, 36.7) |
| Elevated bilirubin, n (%) | 76 (88.37) | 8 (16.33)*** | 121 (80.67) | 32 (96.97) | 81 (92.05) | 2 (33.33)** |
| PT in s, median (range) | 14.55 (13, 16.93) | 13.8 (12.5, 15.6)* | 14.6 (13.13, 16.38) | 13.7 (11.85, 15.75) | 20.9 (17.95, 27)*** | 13.15 (12.3, 14.4) |
| PLT as ×109/L, median (range) | 99.5 (72.5, 133.25) | 106 (77.5, 190.5) | 71 (49, 112.25)*** | 158 (113, 238)*** | 109.5 (72.5, 144) | 144 (115.75, 215.75)* |
| Typical imaging features, n (%) | 83 (96.51) | 11 (22.45)*** | 0 (0)*** | 0 (0)*** | 0 (0)*** | 3 (50)*** |
| Child-Pugh stage, n | 86 | 49 | 150 | 29 | 87 | 6 |
| A, n (%) | 1 (1.16) | 14 (28.57)*** | 16 (10.67)* | 0 (0) | 0 (0) | 1 (16.67) |
| B, n (%) | 65 (75.58) | 28 (57.14) | 102 (68) | 20 (68.97) | 23 (26.44)*** | 5 (83.33) |
| C, n (%) | 20 (23.26) | 7 (14.29) | 32 (21.33) | 9 (31.03) | 64 (73.56)*** | 0 (0) |
| MELD, median (range) | 12 (10, 16.25) | 10 (8, 14.75) | 10 (6, 13)** | 17 (14, 20)*** | 24 (21, 28)*** | 8.5 (4.5, 12)* |
*p<0.05, **p<0.01, ***p<0.001, PA-HSOS vs. other liver diseases (BCS, decompensated cirrhosis, DILI, ALF/SALF, cardiogenic ascites). MI, mild; MO, moderate; SE, severe. Elevated bilirubin: serum total bilirubin >17.1 µmol/L; Elevated ALT: ALT >40 U/L; Elevated AST: AST >40 U/L.
Performance of the Nanjing criteria in the diagnosis of PA-HSOS
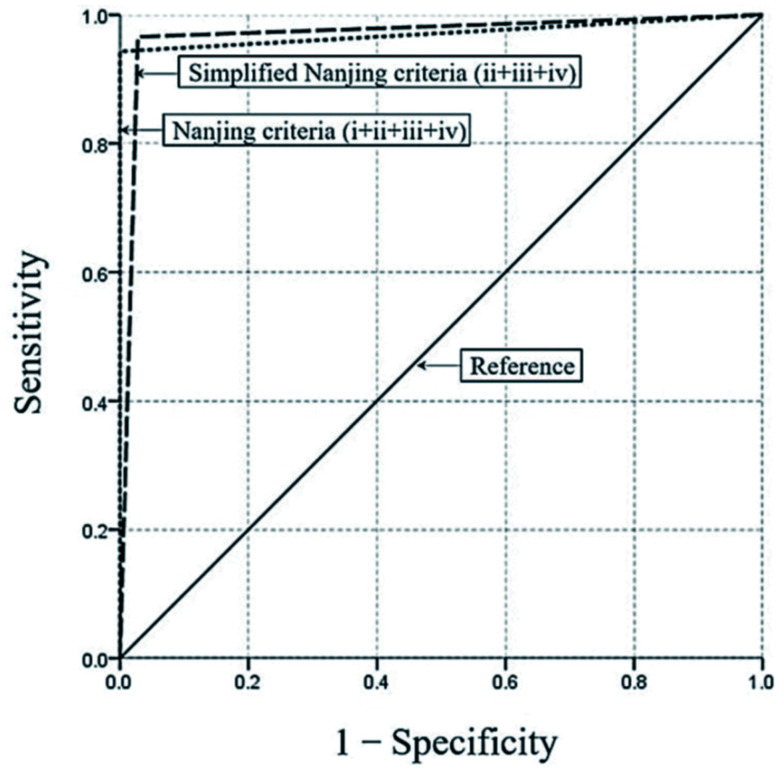
The diagnostic performance of the Nanjing criteria and simplified Nanjing criteria (ii, iii and iv in the Nanjing criteria) in diagnosing PA-HSOS was assessed. The results are presented in Table 3. The Nanjing criteria demonstrated a good ability in diagnosing PA-HSOS, with a sensitivity of 95.35% (95% CI: 90.81–99.89), a specificity of 100%, a PPV of 100%, a NPV of 98.79% (95% CI: 97.61–99.97), and an overall accuracy of as high as 99.03%. In contrast, the simplified Nanjing criteria had a sensitivity of 96.51% (95% CI: 92.55–100) and a specificity of 96.33% (95% CI: 94.28–98.38) for the diagnosis of PA-HSOS. It was noteworthy that the PPV and NPV of the simplified Nanjing criteria was 87.37% (95% CI: 80.57–94.17) and 99.06% (95% CI: 97.99–100), respectively, for the diagnosis of HSOS. The positive likelihood ratio for the simplified Nanjing criteria was 25.98. The negative likelihood ratios for the Nanjing criteria and simplified criteria were 0.046 and 0.036, respectively. The AUC for the Nanjing criteria in the diagnosis of PA-HSOS was 0.977 (95% CI: 0.951–1.000, p<0.01), while the AUC for the simplified Nanjing criteria was 0.964 (95% CI: 0.939–0.990, p<0.01) (Fig. 3). As shown in Table 4, the Kappa value between the Nanjing criteria and the liver pathology for the diagnosis of PA-HSOS was 0.970. In addition, the Kappa value for the simplified Nanjing criteria was 0.894, indicating a strong consistency with the gold standard.
Table 3. Performance of the Nanjing criteria and simplified Nanjing criteria for the diagnosis of PA-HSOS.
| Diagnostic criteria | Reference standard |
Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, %)(95% CI) | NPV, % (95% CI) | Positive likelihood ratio | Negative likelihood ratio | Overall accuracy | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | FP | TN | FN | ||||||||
| Nanjing criteria (i+ii+iii+iv) | 82 | 0 | 327 | 4 | 95.35 (90.81–99.89) | 100 | 100 | 98.79 (97.61–99.97) | — | 0.046 | 99.03 |
| Simplified Nanjing criteria (ii+iii+iv) | 83 | 12 | 315 | 3 | 96.51 (92.55–100) | 96.33 (94.28–98.38) | 87.37 (80.57–94.17) | 99.06 (97.99–100) | 25.98 | 0.036 | 96.37 |
i. History of ingesting PA-containing plants; ii. Clinical manifestation; iii. Abnormal liver function (abnormal liver function: ALT >40 U/L, AST >40 U/L or serum TBil >17.1 µmol/L); iv. Imaging performance. TP, true positive; FP, false positive; TN, true negative; FN, false negative.
Fig. 3. Receiver operating characteristic curves for the Nanjing criteria and simplified Nanjing criteria in the study patients.
The AUC for the Nanjing criteria in the diagnosis of PA-HSOS was 0.977 (95% CI: 0.951–1.000, p<0.01), while the AUC for the simplified Nanjing criteria was 0.964 (95% CI: 0.939–0.990, p<0.01).
Table 4. Kappa analysis of the Nanjing criteria or simplified Nanjing criteria with the gold standard.
| Nanjing criteria (i+ii+iii+iv) | Simplified Nanjing criteria (ii+iii+iv) | |
|---|---|---|
| Gold standard | 0.970 | 0.894 |
Performance of the Nanjing criteria in differentiating PA-HSOS from BCS
Performance of the Nanjing criteria in distinguishing PA-HSOS from BCS was further evaluated. Both conditions share similar clinical characteristics and imaging features, which has posed difficulties to differential diagnosis. It was found that 11 of 49 (22.45%) BCS patients had typical features of patchy enhancement on the CT/MRI, and these patients were misdiagnosed as PA-HSOS according to simplified Nanjing Criteria. In order to differentiate BCS from PA-HSOS in patients with similar typical features of enhanced CT or MRI, the characteristics of the hepatic vein were further compared in the Doppler ultrasound and venography for the 44 PA-HSOS patients who underwent Doppler ultrasound examinations and the above mentioned 11 BCS patients. As shown in Table 5, among the 11 BCS patients with typical features of patchy enhancement on the CT/MRI, 5 had the hepatic vein type, 5 had the mixed type, and 1 had the inferior vena cava type. The Doppler ultrasound detected thrombosis or occlusion of the hepatic vein in 10 of 11 (90.9%) BCS patients and this was confirmed in the venography, while no thrombosis or occlusion was found in PA-HSOS patients. In addition, 8 of 11 (72.7%) BCS patients had a communicating branched vein within the liver detected by Doppler ultrasound, while none of the PA-HSOS patients had such feature. Furthermore, 35 of 44 (79.5%) PA-HSOS patients and 1 of 11 (9.1%) BCS patients had reductions in the diameter or velocity of the hepatic vein. However, none of the 11 BCS patients had a history of intake of PA. All differences between these two groups were statistically significant (p<0.001).
Table 5. Comparison of the characteristics of PA-HSOS and BCS.
| PA-HSOS, n=44 | BCS, n=11 | p-value | |
|---|---|---|---|
| Thrombosis or occlusion of hepatic vein by Doppler ultrasound, n (%) | 0 (0) | 10 (90.9) | <0.001 |
| Reduction of diameters or velocities of hepatic vein by Doppler ultrasound, n (%) | 35 (79.5) | 1 (9.1) | <0.001 |
| Communicating branched vessels found by Doppler ultrasound, n (%) | 0 (0) | 8 (72.7) | <0.001 |
| Thrombosis or occlusion of hepatic vein confirmed by venography, n (%) | 0 (0) | 10 (90.9) | <0.001 |
| History of PA intake, n | 44 | 0 | <0.001 |
Discussion
The Nanjing criteria has been recently proposed for the diagnosis of PA-HSOS. However, its efficacy and reliability has not been clinically validated. The present study demonstrated that the Nanjing criteria exhibits excellent performance in the diagnosis of PA-HSOS. This was demonstrated by multiple lines of evidence, including high sensitivity and specificity.
A variety of diagnostic criteria, such as the Baltimore criteria and Modified Seattle criteria, are available for the diagnosis of HSCT-HSOS.21–23 However, these criteria were specifically developed for the diagnosis of HSCT-HSOS and the clinical efficacy remains argumentative when applied for the diagnosis of PA-HSOS. For example, the first item in both the Baltimore criteria and Modified Seattle criteria is absent in PA-HSOS patients. Since PA-HSOS is a form of sporadic disease, it is difficult to obtain accurate data on the patient’s weight gain following the onset of the disease. In addition, more than 40% of PA-HSOS patients have mildly abnormal hepatic function at the time of the disease onset, with TBil <34.2 µmol/L, and this largely differs from that in HSCT-HSOS patients. Previously, the diagnosis of PA-HSOS has largely relied on the pathological examination of the liver biopsy specimen. Nevertheless, ascites has been detected in nearly all PA-HSOS patients, and percutaneous liver biopsy might be the cause of severe complications (e.g., intraperitoneal bleeding and liver rupture). TJLB has been considered to be better than percutaneous liver biopsy for PA-HSOS patients. However, TJLB has not become a routine procedure in most hospitals, mainly because it is relatively difficult to perform and costly. Recently, the Nanjing criteria was proposed and issued in the Expert consensus on the clinical management of PA-HSOS.6 To date, no clinical validation has been made by assessing the diagnostic performance of the criteria. In this context, the present study focused on the clinical items in the criteria to verify the diagnostic value of the Nanjing criteria for PA-HSOS.
Among the 86 PA-HSOS patients examined in this study, 82 were diagnosed with PA-HSOS on the basis of the Nanjing criteria, while 4 were determined to have been misdiagnosed, giving a false negative rate of 4.70% (4/86). Among these four patients, one had no history of PA-containing plant intake but showed positivity for PPA detection in the blood, while the remaining three patients had no typical imaging features in their enhanced CT/MRI scans. These results suggest that the intake of PA is subjective. Therefore, an objective proof of PA intake through the examination of serum PPAs might be necessary for the confirmation of the etiology. Previous studies have indicated that serum PPAs have a diagnostic sensitivity of 100%, a specificity of 94.1%, and a NPV of 100%.24 Thus, PPAs can be regarded as a specific biomarker of PA intake. In addition, not every PA-HSOS patient has typical features in the enhanced CT/MRI. Therefore, TJLB might be useful in the diagnosis of PA-HSOS.
In clinic, many PA-HSOS patients were not clear in their history of intake of PA-containing plants. Therefore, the diagnostic performance of the simplified Nanjing criteria (ii, iii and iv in the Nanjing criteria) was evaluated. Notably, 83 PA-HSOS patients were accurately diagnosed, while the remaining 3 patients were misdiagnosed due to the absence of typical features in the enhanced CT/MRI. Previous studies have demonstrated that the typical imaging features of PA-HSOS are critical for the diagnosis of PA-HSOS. In a single-center retrospective study, 293 patients, who were diagnosed with PA-HSOS (n=71), BCS (n=57) and liver cirrhosis (n=165), were enrolled, and the findings revealed that the radiologic finding of patchy liver enhancement yielded a sensitivity of 92.96%, a specificity of 92.79%, a PPV of 80.49%, a NPV of 97.63%, and an accuracy of 91.83%. The values for the heterogeneous hypoattenuation were 100%, 95.05%, 86.59%, 100% and 96.25%, respectively. A study indicated that contrast-enhanced CT (commonly referred to as CECT) is effective for diagnosing PA-HSOS.25 However, it was observed that 11 patients with BCS and 1 patient with liver amyloids were misdiagnosed as PA-HSOS in accordance with the simplified Nanjing criteria, and this was mainly due to the overlapping imaging findings with those in PA-HSOS. Thus, special caution should be taken in the differential diagnosis of PA-HSOS from BCS. Furthermore, it may merit attention in the present study that the communicating veins in the liver, thrombosis and occlusion of the hepatic vein were largely different between BCS and PA-HSOS cases. Furthermore, the history of ingesting PA-containing herbal medicine/plants and the detection of blood PPAs were critical for distinguishing between PA-HSOS and BCS cases, especially when the history of ingesting PA-containing plants was not clear and the simplified Nanjing criteria (ii, iii and iv in the Nanjing criteria) was used.24,26
The present study may have some limitations. First, the present study was retrospectively performed and not all patients had liver biopsy results. Hence, selection bias may exist in the retrospective design of the study. Second, a significant number of patients exposed to PA but who did not present with significant clinical symptoms might have been missed. In fact, people can be exposed to toxic PAs mainly through the consumption of PA-producing plants used as herbal medicines, teas, and dietary supplements and/or PA-contaminated staple foods, such as wheat and millets. In addition, the carry-over of PA through livestock into dietary foodstuffs (e.g., milk, eggs, honey and their downstream contamination in the food chain) significantly increases PA exposure in humans.27–29 Thus, these patients were not even aware that they had been exposed to PA. Blood PPAs might represent the specific biomarkers for determining exposure to PAs. Hence, a prospective study is needed to evaluate the diagnostic value of blood PPAs and the modified Nanjing criteria.
Conclusions
The present study suggests that the Nanjing criteria and simplified Nanjing criteria have excellent performance in diagnosing PA-HSOS, thereby representing a valuable diagnostic tool. It also merits attention that the differential diagnosis of PA-HSOS from BCS is highly recommended due to the shared imaging features.
Abbreviations
- ALF
acute liver failure
- ALT
alanine transaminase
- AST
aspartate aminotransferase
- AUC
area under the receiver operating characteristics curve
- BCS
Budd-Chiari syndrome
- CECT
contrast-enhanced computed tomography
- CI
confidence interval
- CT
computed tomography
- DILI
drug-induced liver injury
- EASL
European Association for the Study of the Liver
- FN
false negative
- FP
false positive
- HSCT
hematopoietic stem cell transplantation
- HSOS
hepatic sinusoidal obstruction syndrome
- HVOD
hepatic veno-occlusive disease
- MELD
model for end-stage liver disease
- MRI
magnetic resonance imaging
- NPV
negative predictive value
- PA
pyrrolizidine alkaloid
- PPA
pyrrole-protein adduct
- PPV
positive predictive value
- PT
prothrombin time
- SALF
subacute liver failure
- SD
standard deviation
- TBil
total bilirubin
- TJLB
transjugular liver biopsy
- TN
true negative
- TP
true positive
Data sharing statement
All data are available upon request.
References
- 1.Fan CQ, Crawford JM. Sinusoidal obstruction syndrome (hepatic veno-occlusive disease) J Clin Exp Hepatol. 2014;4(4):332–346. doi: 10.1016/j.jceh.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhuge YZ, Wang Y, Zhang F, Zhu CK, Zhang W, Zhang M, et al. Clinical characteristics and treatment of pyrrolizidine alkaloid-related hepatic vein occlusive disease. Liver Int. 2018;38(10):1867–1874. doi: 10.1111/liv.13684. [DOI] [PubMed] [Google Scholar]
- 3.Lin G, Wang JY, Li N, Li M, Gao H, Ji Y, et al. Hepatic sinusoidal obstruction syndrome associated with consumption of Gynura segetum. J Hepatol. 2011;54(4):666–673. doi: 10.1016/j.jhep.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 4.Wang JY, Gao H. Tusanqi and hepatic sinusoidal obstruction syndrome. J Dig Dis. 2014;15(3):105–107. doi: 10.1111/1751-2980.12112. [DOI] [PubMed] [Google Scholar]
- 5.Xu Z, Zhuge Y, Xu T. Raise the recognition of hepatic veno-occlusive disease induced by chrysanthemum-like groundsel. Chin J Gastroen. 2009;14(10):577–579. [Google Scholar]
- 6.Zhuge Y, Liu Y, Xie W, Zou X, Xu J, Wang J. Expert consensus on the clinical management of pyrrolizidine alkaloid-induced hepatic sinusoidal obstruction syndrome. J Gastroenterol Hepatol. 2019;34(4):634–642. doi: 10.1111/jgh.14612. [DOI] [PubMed] [Google Scholar]
- 7.Dalle JH, Giralt SA. Hepatic veno-occlusive disease after hematopoietic stem cell transplantation: risk factors and stratification, prophylaxis, and treatment. Biol Blood Marrow Transplant. 2016;22(3):400–409. doi: 10.1016/j.bbmt.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Huo JR. Hepatic veno-occlusive disease associated with toxicity of pyrrolizidine alkaloids in herbal preparations. Neth J Med. 2010;68(6):252–260. [PubMed] [Google Scholar]
- 9.Zhu W, Chen S, Chen W, Li Y. Clinical analysis of 50 cases of hepatic veno-occlusive disease. Chin J Dig. 2012;32(9):620–624. [Google Scholar]
- 10.Gu C, Zou XP, Xu ZM, Zhuge Y. Clinical features of hepatic veno-occlusive disease caused by Gynura segetum (Lour.) Merr. Chin J Dig. 2010;30(10):771–772. [Google Scholar]
- 11.Dignan FL, Wynn RF, Hadzic N, Karani J, Quaglia A, Pagliuca A, et al. BCSH/BSBMT guideline: diagnosis and management of veno-occlusive disease (sinusoidal obstruction syndrome) following haematopoietic stem cell transplantation. Br J Haematol. 2013;163(4):444–457. doi: 10.1111/bjh.12558. [DOI] [PubMed] [Google Scholar]
- 12.EASL Clinical Practice Guidelines: Vascular diseases of the liver. J Hepatol. 2016;64(1):179–202. doi: 10.1016/j.jhep.2015.07.040. [DOI] [PubMed] [Google Scholar]
- 13.EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 14.EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. J Hepatol. 2018;69(1):154–181. doi: 10.1016/j.jhep.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 15.EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67(1):145–172. doi: 10.1016/j.jhep.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 16.EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 17.EASL Recommendations on Treatment of Hepatitis C 2016. J Hepatol. 2017;66(1):153–194. doi: 10.1016/j.jhep.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 18.EASL Clinical Practice Guidelines: Autoimmune hepatitis. J Hepatol. 2015;63(4):971–1004. doi: 10.1016/j.jhep.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 19.EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66(5):1047–1081. doi: 10.1016/j.jhep.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109(7):950–966. doi: 10.1038/ajg.2014.131. quiz 967. [DOI] [PubMed] [Google Scholar]
- 21.McDonald GB, Sharma P, Matthews DE, Shulman HM, Thomas ED. Venocclusive disease of the liver after bone marrow transplantation: diagnosis, incidence, and predisposing factors. Hepatology. 1984;4(1):116–122. doi: 10.1002/hep.1840040121. [DOI] [PubMed] [Google Scholar]
- 22.McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118(4):255–267. doi: 10.7326/0003-4819-118-4-199302150-00003. [DOI] [PubMed] [Google Scholar]
- 23.Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44(6):778–783. doi: 10.1097/00007890-198712000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Gao H, Ruan JQ, Chen J, Li N, Ke CQ, Ye Y, et al. Blood pyrrole-protein adducts as a diagnostic and prognostic index in pyrrolizidine alkaloid-hepatic sinusoidal obstruction syndrome. Drug Des Devel Ther. 2015;9:4861–4868. doi: 10.2147/DDDT.S87858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kan X, Ye J, Rong X, Lu Z, Li X, Wang Y, et al. Diagnostic performance of Contrast-enhanced CT in Pyrrolizidine Alkaloids-induced Hepatic Sinusoidal Obstructive Syndrome. Sci Rep. 2016;6:37998. doi: 10.1038/srep37998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao H, Li N, Wang JY, Zhang SC, Lin G. Definitive diagnosis of hepatic sinusoidal obstruction syndrome induced by pyrrolizidine alkaloids. J Dig Dis. 2012;13(1):33–39. doi: 10.1111/j.1751-2980.2011.00552.x. [DOI] [PubMed] [Google Scholar]
- 27.Ma C, Liu Y, Zhu L, Ji H, Song X, Guo H, et al. Determination and regulation of hepatotoxic pyrrolizidine alkaloids in food: A critical review of recent research. Food Chem Toxicol. 2018;119:50–60. doi: 10.1016/j.fct.2018.05.037. [DOI] [PubMed] [Google Scholar]
- 28.Xia Q, Zhao Y, Lin G, Beland FA, Cai L, Fu PP. Pyrrolizidine alkaloid-protein adducts: Potential non-invasive biomarkers of pyrrolizidine alkaloid-induced liver toxicity and exposure. Chem Res Toxicol. 2016;29(8):1282–1292. doi: 10.1021/acs.chemrestox.6b00120. [DOI] [PubMed] [Google Scholar]
- 29.Fu PP, Yang YC, Xia Q, Chou MW, Cui YY, Lin G. Pyrrolizidine Alkaloids-Tumorigenic Components in Chinese Herbal Medicines and Dietary Supplements. J Food Drug Anal. 2002;10(4):198–211. [Google Scholar]