Abstract
Background and Aims
The therapeutic effect of tenofovir alafenamide fumarate (TAF), tenofovir disoproxil fumarate (TDF) and entecavir (ETV) on chronic hepatitis B (CHB) patients remains inconsistent. The aim of this study was to explore the differences in virological responses to TAF, TDF and ETV in patients with CHB.
Methods
Literature searches were conducted of the PubMed, EMBASE, and the Cochrane Library databases to identify randomized controlled trials and observational studies published up to July 21, 2020. Statistical comparisons of virological response between TDF, ETV, and TAF were carried out with pooled odds ratio (OR) values.
Results
The virological response in TDF-treated CHB patients was notably superior to that of the ETV-treated CHB patients after 12-weeks [OR=1.12, 95% confidence interval (CI): 0.89–1.41], 24-weeks (OR=1.33, 95% CI: 1.11–1.61), 48-weeks (OR=1.62, 95% CI: 1.16–2.25), 72-weeks (OR=1.43, 95% CI: 0.78–2.62), and 96-weeks (OR=1.56, 95% CI: 0.87–2.81) treatment. No significant difference was observed for the virological responses in CHB patients after 48-weeks treatment with TAF or TDF. The virological response in TDF+ETV-treated CHB patients was superior to that of TDF-treated CHB patients after 24-weeks, 48-weeks (OR=1.54, 95% CI: 1.17–2.02), 96-weeks, and 144-weeks.
Conclusions
The virological response in TDF-treated CHB patients was superior to that in ETV-treated CHB patients, but there was no significant difference between TAF and TDF. In addition, the therapeutic effect of TDF+ETV was superior to TDF alone.
Keywords: Tenofovir alafenamide, Entecavir, Tenofovir disoprox, Chronic hepatitis B, Virological response
Introduction
Hepatitis B virus (HBV) infection is a serious global health problem and patients are considered to be at high risk of developing hepatic cirrhosis and hepatocellular carcinoma (referred to herein as HCC).1 A report from the World Health Organization indicated that 292 million individuals are positive for the hepatitis B surface antigen across the globe, and the distribution of chronic hepatitis B (CHB) patients is region-dependent.2,3 HBV infection has become one of the principal causes of liver-related mortality globally, and approximately 700,000 HBV-related deaths occurred in 2013 alone.3
Tenofovir alafenamide fumarate (TAF), tenofovir disoproxil fumarate (TDF) and entecavir (ETV) are recommended as the first-line oral drugs for patients with CHB.4,5 Furthermore, these drugs exert good effect on the suppression of HBV replication, provide histologic improvement, and reduce the incidence of HCC after the long-term nucleos(t)ide analogue therapy.6,7 TAF is a bioavailable prodrug of tenofovir (TFV), which is regarded as an effective therapeutic drug for both HBV and human immunodeficiency virus (i.e. HIV-1) infection.8,9 A previous study found that TAF possesses greater plasma stability, safety and toleration than TDF.10 According to the clinical trials, TAF was more likely to be safe compared to TDF, most notably for patients with bone and renal dysfunction.11,12 Ridruejo et al.13 reported that ETV had long-term effectiveness and safety for HBV patients, while some other studies have demonstrated that the rate of HBV DNA suppression achieved was less than that with TDF or TAF within 3 years.
In consideration of the inconsistency of the therapeutic effects of TAF, TDF, and ETV for patients with CHB, and whether the combination of TAF and ETV possesses a better effect than single-agent TAF treatment in CHB patients, this study was designed to explore the difference of virological response with TAF, TDF, and ETV, and the combination of TAF and ETV in the patients with CHB.
Methods
Study selection
A literature search was performed in the PubMed, Cochrane Library, and Embase databases according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (commonly known as PRISMA) process. Studies published up to July 21, 2020 and in the English language were considered. Various combinations of the following keywords were applied in the search strategy: tenofovir alafenamide, TAF, emlidy, ETV, entecavir, ECV, Enti, En, Viread, tenofovir disoprox, TDF.
Inclusion and exclusion criteria
The inclusive criteria were as follows: 1) research type: randomized controlled trial (RCT) and observational study; 2) research subjects: patients with chronic hepatitis B and only those patients diagnosed by HBV DNA test; 3) data on virological response, defined as undetectable HBV DNA level in serum and the lower limit for undetectable HBV DNA having been determined; and 4) receipt of treatment with TDF, TAF, or ENT or combination of these drugs. The exclusion criteria were as follows: 1) research type: review; 2) data unable to be extracted or utilized; 3) data based upon animal experiments; or 4) patients co-infected with HIV or other hepato-tropic viruses.
Data extraction and quality assessment
Information including the first author, publication date, country, sample size, study type, intervention mode, and undetectable HBV DNA level were extracted from each study. The virological response rate of the intervention group and control group were pooled for the meta-analysis. The article list and extracted data were checked by a third researcher, to ensure no patient overlap was present among the different included studies. Quality of the included studies was assessed independently by two authors (Xuefeng Ma and Shousheng Liu). Any discrepancies were resolved through consensus decision upon discussion with a third author. The cohort studies were evaluated according to the Newcastle-Ottawa scale (commonly known as the NOS).14 The NOS is comprised of the following three sections: selection (up to 4 points); comparability (up to 2 points); and, outcome (up to 3 points). The maximum score is 9 points. Study quality was classified as poor (score, 0–3), fair (score, 4–6), or good (score, 7–9). For RCTs, the updated Cochrane tool (https://www.riskofbias.info/) was used to assess the risk of bias. The updated Cochrane tool was made up of the following domains: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selective reporting bias; and other sources of bias. The high, low, or unclear risks of bias of each study were determined in those domains.
Statistical analysis
STATA 14 software (Stata Corporation, College Station, TX, USA) was used for data analysis in this study. Dichotomous variables were expressed as odds ratio (OR; as an effective indicator) and the estimated value and 95% confidence interval (CI) were included as effect analysis statistics. For the Q statistic, heterogeneity was considered present when p was <0.1 or I2 was >50%. A fixed-effect model was used when literature heterogeneity did not exist; otherwise, a random-effect model was used. Publication bias was calculated visually with funnel plots. Publication bias was considered significant when p was <0.05 in Begg’s test. Subgroup analyses were performed according to undetectable HBV DNA level and design of the study.
Results
Literature search results and study characteristics
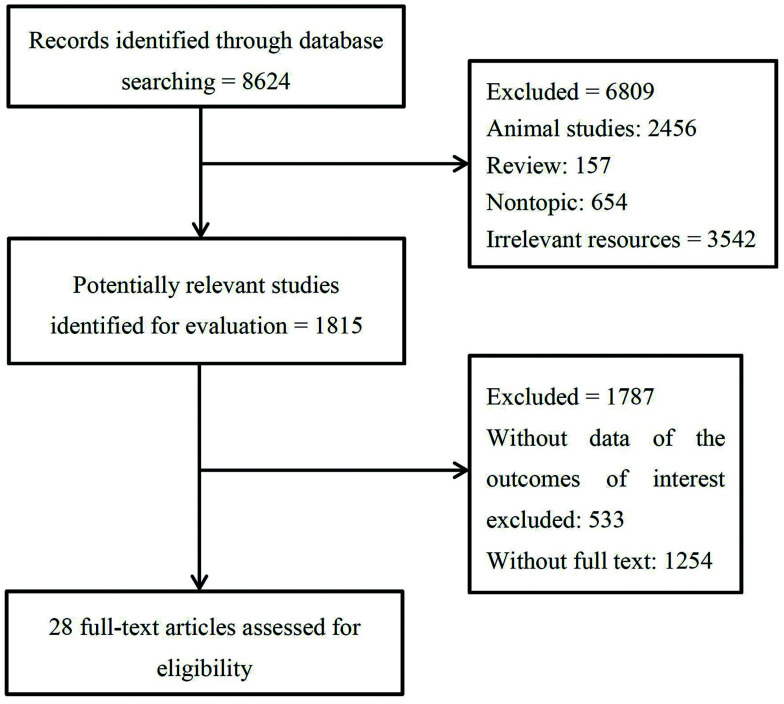
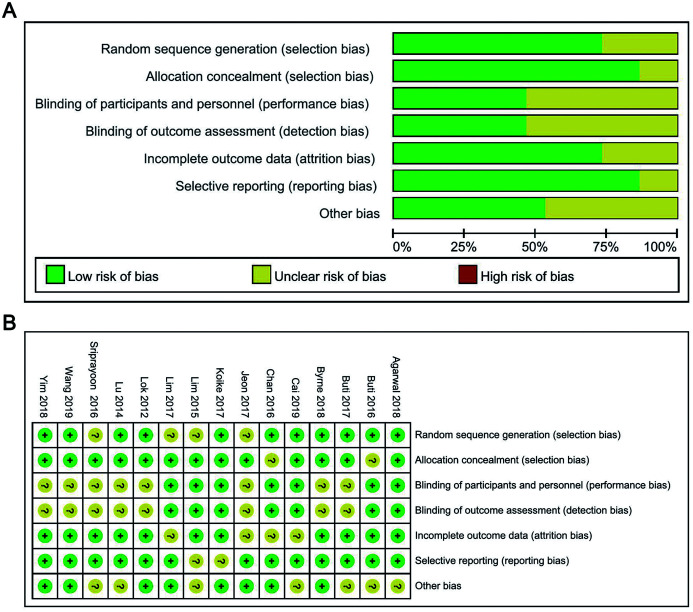
A total of 8,624 studies were identified as potentially relevant studies from the databases. After removing animal studies, reviews, non-topical studies and irrelevant resources, 1,815 studies were retrieved for further evaluation. After excluding studies which did not provide detailed information of virologic response and those without full-text, 28 studies were included for this meta-analysis (Fig. 1). Among these selected studies, 17 focused on the comparison of TDF vs. ETV, 5 focused on the comparison of TAF vs. TDF, and 6 focused on the comparison of TDF+ETV vs. TDF. Among these selected studies, 13 were RCTs,15–27 14 were cohort studies,28–41 and only 1 was a cross-sectional study42 (Table 1). Quality assessment suggested that all the cohort studies and RCTs possessed high quality (Table 1 and Fig. 2). Other characteristics of included studies are presented in Supplementary Table 1.
Fig. 1. Flow chart of the literature search process.
Table 1. Characteristics of studies that met our inclusion criteria.
| Author | Year | Country | Sample size | Study type | Follow-up in weeks | Intervention | Undetectable HBV DNA level | Study quality | |
|---|---|---|---|---|---|---|---|---|---|
| TDF vs. ENT | Batirel et al.28 | 2014 | Turkey | 195 | Retrospective study | 48 | Received TDF (245 mg/day) or ETV (0.5 mg/day) | 20 IU/mL | Good |
| Cai et al.19 | 2019 | China | 315 | RCT | 144 | All the drugs, either TDF capsule or ETV capsule, were provided by Cosunter Pharmaceutical (Ningde, Fujian, China) | 20 IU/mL | NA | |
| Centeno et al.34 | 2016 | Spain | 64 | Retrospective study | 48 | Initiated treatment with TDF or ETV between January 1998 and 2013 | 20 IU/mL | Good | |
| Ceylan et al.29 | 2013 | Turkey | 117 | Retrospective study | 96 | Treatment with TDF or ETV | 20 IU/mL | Good | |
| Ha et al.30 | 2016 | USA | 556 | Retrospective study | 96 | Either ETV 0.5 mg daily or TDF 300 mg daily | 40 IU/mL | Good | |
| Jayakumar et al.31 | 2012 | India | 39 | Prospective study | 24 | Treated with lamivudine (100 mg/day) plus adefovir (10 mg/day) combination ETV monotherapy (0.5 mg/day) and tenofovir monotherapy (300 mg/day) | 400 copies/mL | Good | |
| Kayaaslan et al.32 | 2017 | Turkey | 252 | Retrospective study | 96 | Therapy with ETV 0.5–1 mg/day or TDF 245 mg/day | 20 IU/mL | Good | |
| Koike et al.22 | 2017 | Japan | 166 | RCT | 24 | TDF 300 mg QD and ETV 0.5 mg QD | NA | NA | |
| Kwon et al.33 | 2015 | Korea | 79 | Retrospective study | 48 | Treatment with TDF or ETV | 20 IU/mL | Good | |
| Ozaras et al.36 | 2014 | Turkey | 251 | Cohort study | 72 | Treatment with TDF or ETV | 2*106 IU/mL | Good | |
| Park et al.37 | 2017 | Korea | 210 | Cohort study | 48 | Treatment with TDF or ETV | 20 IU/mL | Good | |
| Riveiro-Barciela et al.38 | 2017 | Spain | 611 | Cohort study | 60 | ETV at dose of 0.5 or 1 mg per day, and TDF at dose of 245 mg per day | 69 IU/mL | Good | |
| Pereira et al.42 | 2016 | Brazil | 294 | Cross-sectional study | 48 | Treatment with TDF or ETV | NA | NA | |
| Shi et al.39 | 2016 | China | 96 | Retrospective study | 96 | Treated orally with ETV at 0.5 mg/day (ETV group) and tenofovir at 300 mg/day (TDF group) | 100 IU/mL | Good | |
| Sriprayoon et al.26 | 2016 | Thailand | 400 | RCT | 144 | Receive either ETV 0.5 mg or TDF 300 mg daily | 20 IU/mL | NA | |
| Yim et al.27 | 2018 | Korea | 40 | RCT | 48 | One group received 300 mg of TDF once daily and the other received 0.5 mg of ETV once daily | 120 copies/mL | NA | |
| Yu et al.41 | 2014 | Korea | 107 | Retrospective study | 48 | Treated with TDF 300 mg daily, and the ETV group treated with ETV 0.5 mg daily | 50 IU/mL | Good | |
| TAF vs. TDF | Agarwal et al.15 | 2018 | United Kingdom | 1,298 | RCT | 48 | Receive 25 mg TAF or 300 mg TDF | 29 IU/mL | NA |
| Buti et al.16 | 2016 | Spain | 425 | RCT | 96 | Receive once-daily oral doses of TAF 25 mg or TDF 300 mg, each with matching placebo | 29 IU/mL | NA | |
| Buti et al.17 | 2017 | Spain | 1,298 | RCT | 48 | Receive oral tablets containing 150 mg elvitegravir, 150 mg cobicistat, 200 mg emtricitabine, and 10 mg TAF (elvitegravir/cobicistat/emtricitabine/TAF) or 300 mg TDF (elvitegravir/cobicistat/emtricitabine /TDF) | 29 IU/mL | NA | |
| Byrne et al.18 | 2018 | Spain | 1,298 | RCT | 96 | Different doses of TAF (8, 25, 40 or 120 mg) or to TDF 300 mg | 29 IU/mL | NA | |
| Chan et al.20 | 2016 | China | 873 | RCT | 96 | Received TAF 25 mg orally once daily or TDF 300 mg orally once daily. | 29 IU/mL | NA | |
| TDF+ETV vs. TDF | Jeon et al.21 | 2017 | Korea | 54 | RCT | 96 | Related with TDF monotherapy (n=12), TDF+LAM (n=19), or TDF+ETV (n=42) | 12 IU/mL | NA |
| Lim et al.23 | 2015 | Korea | 90 | RCT | 48 | Receive TDF (300 mg/day) monotherapy or TDF and ETV (1 mg/day) combination therapy | 15 IU/mL and 60 IU/mL | NA | |
| Lim et al.24 | 2017 | Korea | 192 | RCT | 144 | After completing 48 weeks of randomized, parallel comparisons of TDF monotherapy (300 mg once daily) vs. TDF/ETV combination therapy, patients were eligible to continue TDF monotherapy (TDF-TDF group) or switch to TDF monotherapy (TDF/ETV-TDF group) | 15 IU/mL and 60 IU/mL | NA | |
| Lok et al.25 | 2012 | Turkey | 379 | RCT | 96 | Receive ETV 0.5 mg plus TDF 300mg once daily or ETV 0.5 mg once daily for 100 weeks | 50 IU/mL | NA | |
| Lu et al.35 | 2014 | USA | 68 | Retrospective cohort study | 68 | At least 12 months of ETV, and were switched to TDF monotherapy (n=25) or ETV+TDF (n=43) | 60 IU/mL | Good | |
| Wang et al.40 | 2019 | China | 143 | Retrospective study | 143 | CHB patients with PVR to ETV were switched to TDF monotherapy or TDF+ETV combination therapy | 100IU/mL | Good |
Fig. 2. Risk-of-bias summary for the included randomized controlled trials (RCTs).
(A) Overall risk of bias of the included RCTs. (B) Performance of bias in each study. RCT, randomized controlled trial.
Comparison of the virological response in TDF-treated vs. ETV-treated CHB patients
A total of 17 studies investigated the difference of virological response in patients with CHB after treatment with TDF and ETV19,22,26–34,36–39,41,42 (Table 1). Among these studies, four were conducted in South Korea, four in Turkey, two in China, one in Japan, one in India, one in Brazil, one in Thailand, and one in the USA. Furthermore, 13 were observational studies, which included 12 cohort studies and 1 cross-sectional study, and 4 were RCTs. A total of 3,792 patients were involved in the 17 total studies.
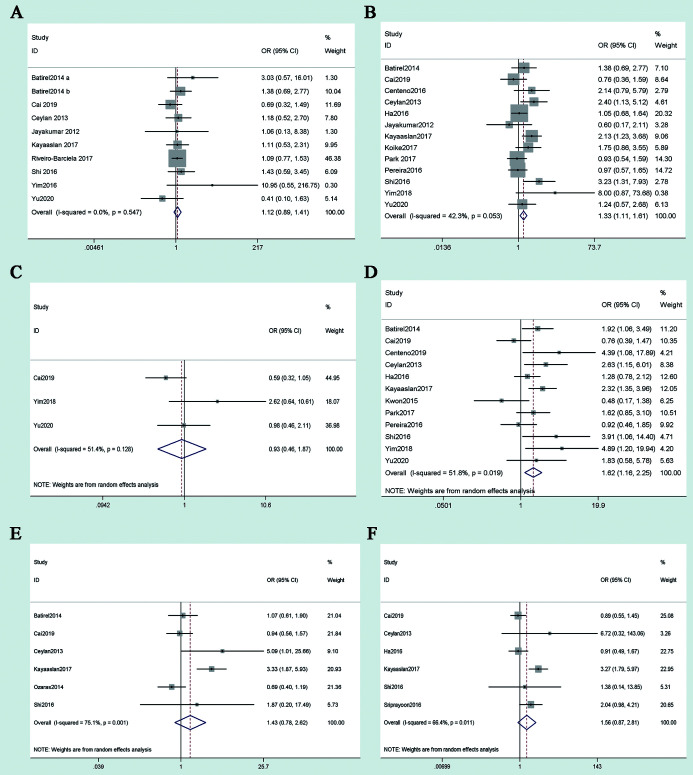
Nine studies reported the virological response of patients with CHB after 12 weeks of treatment with TDF and ETV. The pooled effects of TDF and ETV on virological response were analyzed by using the fixed-effects model (p=0.547, I2=0.0%). The results showed that the virological response of TDF was superior to that of ETV (OR=1.12, 95% CI: 0.89–1.41), but the difference was not statistically significant (p>0.05) (Fig. 3A). Thirteen studies reported the virological response of patients with CHB after 24 weeks of treatment with TDF and ETV. The outcome was demonstrated by a fixed fixed-effects model (p=0.053, I2=42.3%), and the pooled OR was 1.33 (95% CI: 1.11–1.61, p<0.05) (Fig. 3B). Three studies reported the virological response of patients with CHB after 36 weeks of treatment with TDF and ETV. The outcome was demonstrated by the random-effects model (p=0.128, I2=51.4%), and the pooled OR was 0.93 (95% CI: 0.46–1.87, p>0.05) (Fig. 3C). Twelve studies reported the virological response of patients with CHB after 48 weeks of treatment with TDF and ETV. The outcome was demonstrated by the random-effects model (p=0.007, I2=51.8%), and the pooled OR was 1.62 (95% CI: 1.16–2.25, p<0.05) (Fig. 3D). Six studies reported the virological response of patients with CHB after 72 weeks of treatment with TDF and ETV. The outcome was demonstrated by the random-effects model (p=0.001, I2=75.1%), and the pooled OR was 1.43 (95% CI: 0.78–2.62, p>0.05) (Fig. 3E). Six studies reported the virological response of patients with CHB after 96 weeks of treatment with TDF and ETV. The outcome was demonstrated by the random-effects model (p<0.001, I2=88.0%), and the pooled OR was 1.56 (95% CI: 0.87–2.81, p>0.05) (Fig. 3F). The virological response of patients with CHB after 120 weeks of treatment with TDF and ETV was reported in one study and after 144 weeks treatment was reported in two studies; the results suggested that there was no strong difference in the virological response after treatment with TDF or ETV.
Fig. 3. Pooled odds ratios (ORs) of virological response in tenofovir disoproxil fumarate (TDF)-treated vs. entecavir (ETV)-treated chronic hepatitis B (CHB) patients.
After (A) 12 weeks, (B) 24 weeks, (C) 36 weeks, (D) 48 weeks, (E) 72 weeks and (F) 96 weeks of treatment. CHB, chronic hepatitis B; ETV, entecavir; OR, odds ratio; TDF, tenofovir disoproxil fumarate.
Comparison of the virological response in TAF-treated vs. TDF-treated CHB patients
Five of the studies investigated the difference of virological response in patients with CHB after treatment with TAF and TDF15–18,20 (Table 1). Among those studies, three were conducted in Spain, one was conducted in the UK and 1 was conducted in China. All were RCTs, and a total of 5,192 patients were included in these studies.
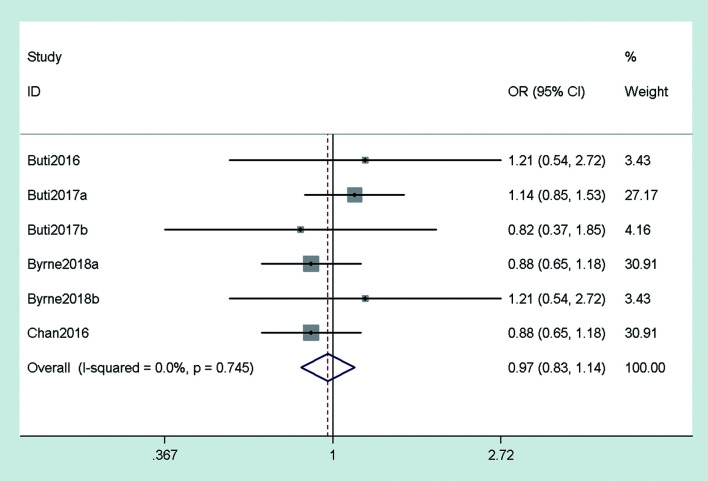
Four studies reported the virological response of patients with CHB after 48 weeks of treatment with TAF and TDF. The pooled effects of TAF and TDF on the virological response were analyzed by using the fixed-effects model (p=0.783, I2=0.0%). The results showed that the virological response of TAF was equivalent to that of TDF (OR=0.97, 95% CI: 0.83–1.14, p>0.05) (Fig. 4). The virological response of patients with CHB after 96 weeks of treatment with TAF and TDF was reported in two studies, the results suggested that there was no obvious differences in the virological response after treatment with TAF and TDF.
Fig. 4. Pooled OR of virological response in tenofovir alafenamide fumarate (TAF)-treated vs. TDF-treated CHB patients after 48 weeks of treatment.
CHB, chronic hepatitis B; OR, odds ratio; TAF, Tenofovir alafenamide fumarate; TDF, tenofovir disoproxil fumarate.
Comparison of the virological response in TDF+ETV-treated vs. TDF-treated CHB patients
Six studies investigated the difference of virological response in patients with CHB after treatment with TDF+ETV and TDF21,23–25,35,40 (Table 1). Among these, four were conducted in South Korea, one was conducted in China and one was conducted in the USA. Furthermore, two studies were cohort studies and one was an RCT; a total of 926 patients were included in these studies.
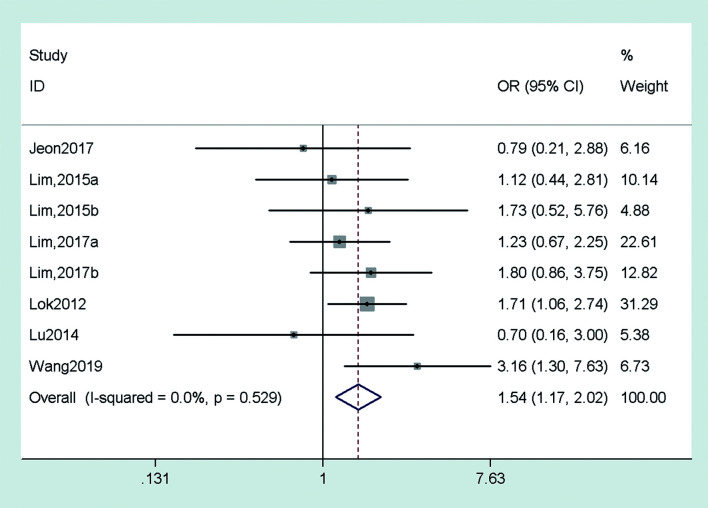
The virological response of patients with CHB after 24 weeks of treatment with TDF+ETV or TDF alone was reported in two studies. The results suggested that the therapeutic effect of TDF+ETV was significantly superior to that of TDF alone. Six studies reported the virological response of patients with CHB after 48 weeks of treatment with TDF+ETV or TDF alone. The outcome was demonstrated by the random-effects model (p=0.529, I2=0.0%), and the pooled OR was 1.54 (95% CI: 1.17–2.02, p<0.05) (Fig. 5). The virological response of patients with CHB after 96 weeks and 144 weeks of treatment with TDF+ETV or TDF alone was reported in two studies and one study, respectively. The results suggested that the therapeutic effect of TDF+ETV was significantly superior to that of TDF alone.
Fig. 5. Pooled OR of virological response in TDF+ETV-treated vs. TDF-treated CHB patients after 48 weeks of treatment.
CHB, chronic hepatitis B; ETV, entecavir; OR, odds ratio; TDF, tenofovir disoproxil fumarate.
Discussion
In this study, we systematically compared the therapeutic effect of TDF, TAF, ETV, and TDF+ETV on CHB patients. Our results suggest that in the TDF-treated CHB patients, the virological response was markedly superior to that of ETV-treated CHB patients after 12-, 24-, 48-, 72-, and 96-weeks treatment, which supports that TDF can be superior to ETV for the treatment of CHB patients. When compared to the therapeutic effect of TAF and TDF, no obvious difference was observed, which suggests that TAF is comparable to TDF for the treatment of CHB patients. In addition, we found that the virological response in TDF+ETV-treated CHB patients was superior to that of TDF-treated CHB patients after 24-, 48-, 96-, and 144-weeks treatment, which suggests that TDF combined with ETV exerts a better therapeutic effect for CHB patients than TDF alone.
TDF and ETV are two types of nucleos(t)ide analogues that can efficiently inhibit the replication of HBV via the blockade of DNA polymerase and reverse transcriptase, respectively.43 Nowadays, TDF and ETV are widely used for patients with CHB, due to their potent antiviral activities. The therapeutic effect of TDF and ETV in CHB patients has been investigated in some studies, but the conclusions have not been consistent. Yim et al.27 conducted a RCT to investigate the virological response in CHB patients upon treatment with TDF or ETV. They found that when patients were switched to TDF from ETV, the HBV DNA level was significantly lower than that detected in the ETV treatment group.
In this meta-analysis, we summarized all the relative studies to compare the value of TDF and ETV on the treatment of CHB. We found that TDF was superior to ETV for the treatment of CHB patients. However, there were some inconsistencies observed regarding safety. Cai et al.19 reported that both TDF and ETV were generally well tolerated, and the common adverse events were similar with no obvious fluctuation of the estimated glomerular filtration rate (commonly known as eGFR) found during the observational period between the TDF group and ETV group. Meanwhile, Centeno et al.34 reported that after 48 weeks of treatment, 19.4% of patients in the TDF group showed eGFR <60 mL/min/1.73m2 vs. 15.6% in the ETV group, which demonstrated the better effect achieved with TDF than ETV.
TAF is a newly developed prodrug of TFV, which can facilitate better entry of TFV into hepatocytes than TDF. Agarwal et al.15 reported that after treatment with TAF, patients possessed higher intracellular concentrations of TFV and lower plasma concentrations of TFV compared to those who were on treatment with TDF. Our meta-analysis indicated that there was no significant difference in the virological responses of patients treated with TAF vs. TDF. Regarding safety, Chan et al.7,20 found that the unique pharmacokinetic profile of TAF had caused the declined rates of TFV-related major adverse events, kidney dysfunction, and bone mineralization, when compared with TDF. Furthermore, many studies have displayed that the levels of low-density lipoprotein, fasting total cholesterol, and high-density lipoprotein were all reduced in patients with HIV co-infection;44,45 although, the precise mechanism for these changes remains unclear. Besides the TAF vs. TDF comparison results, we also found the TDF+ETV combination can bring about an effective virological response compared to TDF alone in CHB patients. Whereas, Wang et al.40 found slightly increased serum creatinine level and decreased serum phosphorus level in TDF+ETV-treated CHB patients, but with both of which being within the normal range.
The therapeutic effect of TDF or ETV in CHB patients may be influenced by the genotype of HBV. Lok et al.25 demonstrated that loss of hepatitis B surface antigen and hepatitis B e antigen seroconversion signifies that patients with HBV genotype C infection enjoyed better performance of TDF+ETV combination than ETV alone; however, the performance of TDF+ETV combination was poorer than ETV alone for HBV genotypes A, B and D. In addition, the main indication and characteristics of patients treated by TDF+ETV combination was drug resistance. Except for patients in the study by Lok et al.,26 most of the patients in the studies using TDF+ETV combination have been reported to have resistance to ETV, lamivudine or adefovir dipivoxil.25
There are several inherent limitations to this study, which must be considered. First, only two studies were included to compare the therapeutic effect of TDF+ENT vs. TDF alone; therefore, more RCTs are needed to supported our conclusions. Second, almost all of the included studies were RCTs or interventional cohort studies, and a potential source of bias might have been introduced. Third, age, sex, hepatitis B e antigen status, cirrhosis stage, and HBV DNA level before therapy, duration of previous therapy, and baseline HBV DNA level may be factors associated with virologic response but which were not taken into account in our meta-analysis.
Conclusions
In summary, the therapeutic effect of TDF, ETV, TAF, and TDF+ETV in patients with CHB was investigated in this study. The virological response in TDF-treated CHB patients was superior to that achieved in the ETV-treated CHB patients, but no significant difference of virological response was found between TAF-treated and TDF-treated CHB patients. In addition, the therapeutic effect of TDF+ETV was superior to that of TDF. These conclusions were made from the available studies published to recent time, and more clinical RCTs or observational studies should be conducted to verify them. Also, the drug safety of TAF, TDF, and TDF+ETV should be investigated more systematically.
NA, not available or not applicable. CHB, chronic hepatitis B; ETV, entecavir; TAF, Tenofovir alafenamide fumarate; TDF, tenofovir disoproxil fumarate.
Supporting information
Acknowledgments
We thanks Jie Zhang for the assistance in this study.
Abbreviations
- CHB
chronic hepatitis B
- CI
confidence interval
- eGFR
estimated glomerular filtration rate
- ETV
entecavir
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HIV
human immunodeficiency virus
- NOS
Newcastle-Ottawa scale
- OR
odds ratio
- RCT
randomized controlled trial
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- TAF
Tenofovir alafenamide fumarate
- TDF
tenofovir disoproxil fumarate
- TFV
tenofovir
Data sharing statement
No additional data are available.
References
- 1.Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384(9959):2053–2063. doi: 10.1016/S0140-6736(14)60220-8. [DOI] [PubMed] [Google Scholar]
- 2.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48(2):335–352. doi: 10.1016/j.jhep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;385(9963):117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Clin Liver Dis (Hoboken) 2018;12(1):33–34. doi: 10.1002/cld.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Association for the Study of the Liver EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 6.Gordon SC, Lamerato LE, Rupp LB, Li J, Holmberg SD, Moorman AC, et al. Antiviral therapy for chronic hepatitis B virus infection and development of hepatocellular carcinoma in a US population. Clin Gastroenterol Hepatol. 2014;12(5):885–893. doi: 10.1016/j.cgh.2013.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Li JJ, Chen R, Li G, Ji J. Dynamics of HBV surface antigen related end points in chronic hepatitis B infection: a systematic review and meta-analysis. Antivir Ther. 2020;25:203–215. doi: 10.3851/IMP3366. [DOI] [PubMed] [Google Scholar]
- 8.Molina JM, Ward D, Brar I, Mills A, Stellbrink HJ, López-Cortés L, et al. Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from dolutegravir plus abacavir and lamivudine in virologically suppressed adults with HIV-1: 48 week results of a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet HIV. 2018;5(7):e357–e365. doi: 10.1016/S2352-3018(18)30092-4. [DOI] [PubMed] [Google Scholar]
- 9.Sax PE, Wohl D, Yin MT, Post F, DeJesus E, Saag M, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385(9987):2606–2615. doi: 10.1016/S0140-6736(15)60616-X. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal K, Fung SK, Nguyen TT, Cheng W, Sicard E, Ryder SD, et al. Twenty-eight day safety, antiviral activity, and pharmacokinetics of tenofovir alafenamide for treatment of chronic hepatitis B infection. J Hepatol. 2015;62(3):533–540. doi: 10.1016/j.jhep.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 11.Fong TL, Lee BT, Tien A, Chang M, Lim C, Ahn A, et al. Improvement of bone mineral density and markers of proximal renal tubular function in chronic hepatitis B patients switched from tenofovir disoproxil fumarate to tenofovir alafenamide. J Viral Hepat. 2019;26(5):561–567. doi: 10.1111/jvh.13053. [DOI] [PubMed] [Google Scholar]
- 12.Lampertico P, Buti M, Fung S, Ahn SH, Chuang WL, Tak WY, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in virologically suppressed patients with chronic hepatitis B: a randomised, double-blind, phase 3, multicentre non-inferiority study. Lancet Gastroenterol Hepatol. 2020;5(5):441–453. doi: 10.1016/S2468-1253(19)30421-2. [DOI] [PubMed] [Google Scholar]
- 13.Ridruejo E. Treatment of chronic hepatitis B in clinical practice with entecavir or tenofovir. World J Gastroenterol. 2014;20(23):7169–7180. doi: 10.3748/wjg.v20.i23.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wells G, Shea JB, O’Connell J. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Health Research Institute Web site. 2014;7 [Google Scholar]
- 15.Agarwal K, Brunetto M, Seto WK, Lim YS, Fung S, Marcellin P, et al. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection. J Hepatol. 2018;68(4):672–681. doi: 10.1016/j.jhep.2017.11.039. [DOI] [PubMed] [Google Scholar]
- 16.Buti M, Gane E, Seto WK, Chan HL, Chuang WL, Stepanova T, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1(3):196–206. doi: 10.1016/S2468-1253(16)30107-8. [DOI] [PubMed] [Google Scholar]
- 17.Buti M, Riveiro-Barciela M, Esteban R. Tenofovir alafenamide fumarate: a new tenofovir prodrug for the treatment of chronic hepatitis B infection. J Infect Dis. 2017;216(suppl_8):S792–S796. doi: 10.1093/infdis/jix135. [DOI] [PubMed] [Google Scholar]
- 18.Byrne R, Carey I, Agarwal K. Tenofovir alafenamide in the treatment of chronic hepatitis B virus infection: rationale and clinical trial evidence. Therap Adv Gastroenterol. 2018;11:1756284818786108. doi: 10.1177/1756284818786108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai D, Pan C, Yu W, Dang S, Li J, Wu S, et al. Comparison of the long-term efficacy of tenofovir and entecavir in nucleos(t)ide analogue-naïve HBeAg-positive patients with chronic hepatitis B: a large, multicentre, randomized controlled trials. Medicine (Baltimore) 2019;98(1):e13983. doi: 10.1097/MD.0000000000013983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan HL, Fung S, Seto WK, Chuang WL, Chen CY, Kim HJ, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1(3):185–195. doi: 10.1016/S2468-1253(16)30024-3. [DOI] [PubMed] [Google Scholar]
- 21.Jeon HJ, Jung SW, Park NH, Yang Y, Noh JH, Ahn JS, et al. Efficacy of tenofovir-based rescue therapy for chronic hepatitis B patients with resistance to lamivudine and entecavir. Clin Mol Hepatol. 2017;23(3):230–238. doi: 10.3350/cmh.2017.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koike K, Suyama K, Ito H, Itoh H, Sugiura W. Randomized prospective study showing the non-inferiority of tenofovir to entecavir in treatment-naïve chronic hepatitis B patients. Hepatol Res. 2018;48(1):59–68. doi: 10.1111/hepr.12902. [DOI] [PubMed] [Google Scholar]
- 23.Lim YS, Byun KS, Yoo BC, Kwon SY, Kim YJ, An J, et al. Tenofovir monotherapy versus tenofovir and entecavir combination therapy in patients with entecavir-resistant chronic hepatitis B with multiple drug failure: results of a randomised trial. Gut. 2016;65(5):852–860. doi: 10.1136/gutjnl-2014-308353. [DOI] [PubMed] [Google Scholar]
- 24.Lim YS, Lee YS, Gwak GY, Byun KS, Kim YJ, Choi J, et al. Monotherapy with tenofovir disoproxil fumarate for multiple drug-resistant chronic hepatitis B: 3-year trial. Hepatology. 2017;66(3):772–783. doi: 10.1002/hep.29187. [DOI] [PubMed] [Google Scholar]
- 25.Lok AS, Trinh H, Carosi G, Akarca US, Gadano A, Habersetzer F, et al. Efficacy of entecavir with or without tenofovir disoproxil fumarate for nucleos(t)ide-naïve patients with chronic hepatitis B. Gastroenterology. 2012;143(3):619–628.e1. doi: 10.1053/j.gastro.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 26.Sriprayoon T, Mahidol C, Ungtrakul T, Chun-On P, Soonklang K, Pongpun W, et al. Efficacy and safety of entecavir versus tenofovir treatment in chronic hepatitis B patients: a randomized controlled trial. Hepatol Res. 2017;47(3):E161–E168. doi: 10.1111/hepr.12743. [DOI] [PubMed] [Google Scholar]
- 27.Yim HJ, Kim IH, Suh SJ, Jung YK, Kim JH, Seo YS, et al. Switching to tenofovir vs continuing entecavir for hepatitis B virus with partial virologic response to entecavir: a randomized controlled trial. J Viral Hepat. 2018;25(11):1321–1330. doi: 10.1111/jvh.12934. [DOI] [PubMed] [Google Scholar]
- 28.Batirel A, Guclu E, Arslan F, Kocak F, Karabay O, Ozer S, et al. Comparable efficacy of tenofovir versus entecavir and predictors of response in treatment-naïve patients with chronic hepatitis B: a multicenter real-life study. Int J Infect Dis. 2014;28:153–159. doi: 10.1016/j.ijid.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Ceylan B, Yardimci C, Fincanci M, Eren G, Tozalgan U, Muderrisoglu C, et al. Comparison of tenofovir and entecavir in patients with chronic HBV infection. Eur Rev Med Pharmacol Sci. 2013;17(18):2467–2473. [PubMed] [Google Scholar]
- 30.Ha NB, Trinh HN, Rosenblatt L, Nghiem D, Nguyen MH. Treatment outcomes with first-line therapies with entecavir and tenofovir in treatment-naive chronic hepatitis B patients in a routine clinical practice. J Clin Gastroenterol. 2016;50(2):169–174. doi: 10.1097/MCG.0000000000000345. [DOI] [PubMed] [Google Scholar]
- 31.Jayakumar R, Joshi YK, Singh S. Laboratory evaluation of three regimens of treatment of chronic hepatitis B: tenofovir, entecavir and combination of lamivudine and adefovir. J Lab Physicians. 2012;4(1):10–16. doi: 10.4103/0974-2727.98664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kayaaslan B, Akinci E, Ari A, Tufan ZK, Alpat SN, Gunal O, et al. A long-term multicenter study: entecavir versus tenofovir in treatment of nucleos(t)ide analogue-naive chronic hepatitis B patients. Clin Res Hepatol Gastroenterol. 2018;42(1):40–47. doi: 10.1016/j.clinre.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Kwon YJ, Lee HS, Park MJ, Shim SG. Comparison of the efficacy of tenofovir and entecavir for the treatment of nucleos(t) ide-naïve patients with chronic hepatitis B. Niger J Clin Pract. 2015;18(6):796–801. doi: 10.4103/1119-3077.163296. [DOI] [PubMed] [Google Scholar]
- 34.López Centeno B, Collado Borrell R, Pérez Encinas M, Gutiérrez García ML, Sanmartin Fenollera P. Comparison of the effectiveness and renal safety of tenofovir versus entecavir in patients with chronic hepatitis B. Farm Hosp. 2016;40(4):279–286. doi: 10.7399/fh.2016.40.4.10492. English. [DOI] [PubMed] [Google Scholar]
- 35.Lu L, Yip B, Trinh H, Pan CQ, Han SH, Wong CC, et al. Tenofovir-based alternate therapies for chronic hepatitis B patients with partial virological response to entecavir. J Viral Hepat. 2015;22(8):675–681. doi: 10.1111/jvh.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ozaras R, Mete B, Ceylan B, Ozgunes N, Gunduz A, Karaosmanoglu H, et al. First-line monotherapies of tenofovir and entecavir have comparable efficacies in hepatitis B treatment. Eur J Gastroenterol Hepatol. 2014;26(7):774–780. doi: 10.1097/MEG.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 37.Park JW, Kwak KM, Kim SE, Jang MK, Suk KT, Kim DJ, et al. Comparison of the long-term efficacy between entecavir and tenofovir in treatment- naïve chronic hepatitis B patients. BMC Gastroenterol. 2017;17(1):39. doi: 10.1186/s12876-017-0596-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riveiro-Barciela M, Tabernero D, Calleja JL, Lens S, Manzano ML, Rodríguez FG, et al. Effectiveness and safety of entecavir or tenofovir in a spanish cohort of chronic hepatitis B patients: validation of the page-B score to predict hepatocellular carcinoma. Dig Dis Sci. 2017;62(3):784–793. doi: 10.1007/s10620-017-4448-7. [DOI] [PubMed] [Google Scholar]
- 39.Shi H, Huang M, Lin G, Li X, Wu Y, Jie Y, et al. Efficacy comparison of tenofovir and entecavir in HBeAg-positive chronic hepatitis B patients with high HBV DNA. Biomed Res Int. 2016;2016:6725073. doi: 10.1155/2016/6725073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang YH, Liao J, Zhang DM, Wu DB, Tao YC, Wang ML, et al. Tenofovir monotherapy versus tenofovir plus entecavir combination therapy in HBeAg-positive chronic hepatitis patients with partial virological response to entecavir. J Med Virol. 2020;92(3):302–308. doi: 10.1002/jmv.25608. [DOI] [PubMed] [Google Scholar]
- 41.Yu HM, Kwon SY, Kim J, Chung HA, Kwon SW, Jeong TG, et al. Virologic response and safety of tenofovir versus entecavir in treatment-naïve chronic hepatitis B patients. Saudi J Gastroenterol. 2015;21(3):146–151. doi: 10.4103/1319-3767.157558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pereira CV, Tovo CV, Grossmann TK, Mirenda H, Dal-Pupo BB, Almeida PR, et al. Efficacy of entecavir and tenofovir in chronic hepatitis B under treatment in the public health system in southern Brazil. Mem Inst Oswaldo Cruz. 2016;111(4):252–257. doi: 10.1590/0074-02760150390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scaglione SJ, Lok AS. Effectiveness of hepatitis B treatment in clinical practice. Gastroenterology. 2012;142(6):1360–1368.e1. doi: 10.1053/j.gastro.2012.01.044. [DOI] [PubMed] [Google Scholar]
- 44.Santos JR, Saumoy M, Curran A, Bravo I, Llibre JM, Navarro J, et al. The lipid-lowering effect of tenofovir/emtricitabine: a randomized, crossover, double-blind, placebo-controlled trial. Clin Infect Dis. 2015;61(3):403–408. doi: 10.1093/cid/civ296. [DOI] [PubMed] [Google Scholar]
- 45.Tungsiripat M, Kitch D, Glesby MJ, Gupta SK, Mellors JW, Moran L, et al. A pilot study to determine the impact on dyslipidemia of adding tenofovir to stable background antiretroviral therapy: ACTG 5206. AIDS. 2010;24(11):1781–1784. doi: 10.1097/QAD.0b013e32833ad8b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.