Abstract
Background and Aims
Post-hepatectomy liver failure (PHLF) is a severe complication and main cause of death in patients undergoing hepatectomy. The aim of this study was to build a predictive model of PHLF in patients undergoing hepatectomy.
Methods
We retrospectively analyzed patients undergoing hepatectomy at Zhongshan Hospital, Fudan University from July 2015 to June 2018, and randomly divided them into development and internal validation cohorts. External validation was performed in an independent cohort. Least absolute shrinkage and selection operator (commonly referred to as LASSO) logistic regression was applied to identify predictors of PHLF, and multivariate binary logistic regression analysis was performed to establish the predictive model, which was visualized with a nomogram.
Results
A total of 492 eligible patients were analyzed. LASSO and multivariate analysis identified three preoperative variables, total bilirubin (p=0.001), international normalized ratio (p<0.001) and platelet count (p=0.004), and two intraoperative variables, extent of resection (p=0.002) and blood loss (p=0.004), as independent predictors of PHLF. The area under receiver operating characteristic curve (referred to as AUROC) of the predictive model was 0.838 and outperformed the model for end-stage liver disease score, albumin-bilirubin score and platelet-albumin-bilirubin score (AUROCs: 0.723, 0.695 and 0.663, respectively; p<0.001 for all). The optimal cut-off value of the predictive model was 14.7. External validation showed the model could predict PHLF accurately and distinguish high-risk patients.
Conclusions
PHLF can be accurately predicted by this model in patients undergoing hepatectomy, which may significantly contribute to the postoperative care of these patients.
Keywords: Hepatectomy, Post-hepatectomy liver failure, LASSO, Nomogram
Introduction
Hepatectomy is the main treatment for patients with benign or malignant liver lesions. However, patients undergoing liver resection are at increased risk for peri- and postoperative complications. Among these, post-hepatectomy liver failure (PHLF), defined as the impaired ability of the liver to maintain its synthetic, excretory and detoxifying functions, is one of the worst complications after hepatectomy and one of the major causes of perioperative mortality.1,2 Despite improvements in operative techniques, perioperative management and understanding of liver regeneration have improved the safety of liver resection over years, PHLF remains a challenge for patients undergoing hepatectomy and a concern of hepatic surgeons.3
Various assessment tools for liver function assessment and prediction of PHLF prior to surgery have been developed to reduce the incidence of PHLF and postoperative mortality. Indocyanine green retention rate at 15 min (ICG-R15) can measure the global liver function, and has been widely adopted in Eastern centers, whereas it is rarely used in Western countries due to its expensive cost and time-consuming requirement for performance.4 Clinic-biological scores like the model for end-stage liver disease (MELD) score, albumin-bilirubin (ALBI) score and platelet-albumin-bilirubin (PALBI) score are also adopted to evaluate the functional liver reserve,5–7 and are reported to accurately predict PHLF following hepatectomy.8–10 Volume and function of the future liver remnant (FLR), as accessed by different imaging modalities, also have a superior ability to predict PHLF, but they could delay the time to surgery and also have financial constraints.11,12
Intraoperative events can also influence the risk of PHLF.13 However, none of the models mentioned above include surgery-related factors, such as blood loss, extent of hepatectomy and intraoperative transfusions, to predict the probability of PHLF immediately after surgery.
The aim of this study was, therefore, to determine predictors of PHLF, including preoperative and intraoperative variables, and to build predictive models of PHLF in patients undergoing hepatectomy.
Methods
Study population
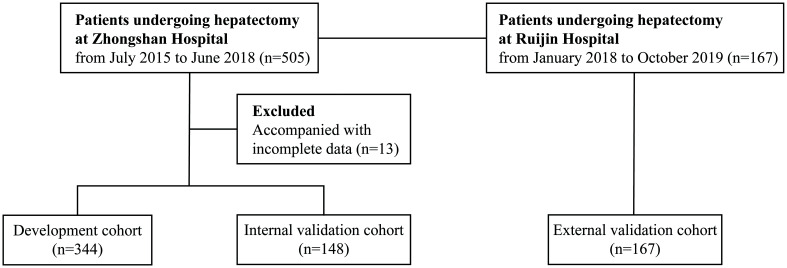
Five hundred and five consecutive patients who underwent hepatectomy at Zhongshan Hospital, Fudan University (Zhongshan cohort, from July 2015 to June 2018), and 167 consecutive patients at Ruijin Hospital, Shanghai Jiao Tong University School of Medicine (Ruijin cohort, from January 2018 to October 2019) were included in this study. Thirteen (2.6%) of the total patients in the Zhongshan cohort were excluded because of incomplete data. The remaining 492 patients in the Zhongshan cohort were randomly divided into a development cohort (n=344) and an internal validation cohort (n=148) using simple random sampling, with a random number seed of 2,017,0307. All patients in the Ruijin cohort were used as an external validation cohort (n=167) (Fig. 1).
Fig. 1. Flowchart of this study’s design.
The inclusion criteria were as follows: (i) patients who received hepatectomy; (ii) patients who received contrast-enhanced computed tomography (CT) scans or magnetic resonance imaging (MRI) conducted 1 week before resection; and (iii) patients who received blood routine test, biochemical test, coagulation function test, hepatitis B serologic test, liver fibrosis test14 and liver stiffness (LS)15 assessed by shear wave elastography conducted within 1 week before surgery.
This study was approved by the Institutional Ethics Committee of the two hospitals and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from all patients.
Data collection and definition
Clinical characteristics, including 22 preoperative variables, 3 intraoperative variables and 2 clinical outcomes, were recorded (Table 1). In addition, the MELD, ALBI and PALBI scores were calculated as reported,5–7 to compare with the model established in this study. No missing data were found for any patient in any of the study cohorts.
Table 1. Comparison of clinical characteristics between development and internal validation cohorts.
| Variables | Development cohort, n=344 | Internal validation cohort, n=148 | p-value |
|---|---|---|---|
| Age in years | 56.4±11.2 | 57.0±10.9 | 0.625 |
| Sex | 0.714 | ||
| Male | 298 (86.6%) | 130 (87.8%) | |
| Female | 46 (13.4%) | 18 (12.2%) | |
| Diabetes | 0.878 | ||
| No | 291 (84.6%) | 126 (85.1%) | |
| Yes | 53 (15.4%) | 22 (14.9%) | |
| HBsAg | 0.078 | ||
| − | 56 (16.3%) | 34 (23.0%) | |
| + | 288 (83.7%) | 114 (77.0%) | |
| HBeAg | 0.763 | ||
| − | 282 (82.0%) | 123 (83.1%) | |
| + | 62 (18.0%) | 25 (16.9%) | |
| HBV DNA | 0.275 | ||
| ≤103/mL | 198 (57.6%) | 93 (62.8%) | |
| >103/mL | 146 (42.4%) | 55 (37.2%) | |
| Hb in g/L | 143.0 (127.0–153.0) | 142.0 (133.0–150.3) | 0.948 |
| WBC as ×109/L | 5.3 (4.2–6.5) | 5.3 (4.5–6.3) | 0.587 |
| PLT as ×109/L | 148.0 (106.0–207.0) | 162.5 (114–195.3) | 0.400 |
| TB in µmol/L | 11.9 (8.8–15.9) | 11.7 (9.2–16.5) | 0.886 |
| ALB in g/L | 42.0 (39.0–45.0) | 42.0 (39.0–45.0) | 0.708 |
| P-ALB in g/L | 0.22 (0.17–0.26) | 0.22 (0.18–0.26) | 0.590 |
| ALT in U/L | 29.0 (20.0–43.0) | 29.0 (20.8–42.3) | 0.717 |
| GGT in U/L | 56.5 (33.0–108.0) | 63.0 (34.8–115.5) | 0.734 |
| INR | 1.01 (0.96–1.07) | 1.03 (0.97–0.106) | 0.345 |
| HA in ng/mL | 87.3 (64.2–135.2) | 85.5 (60.0–135.4) | 0.486 |
| LN in ng/mL | 50.0 (50.0–67.0) | 50.0 (50.0–64.8) | 0.536 |
| PIIINP in ng/mL | 6.5 (5.3–8.4) | 6.7 (5.4–8.4) | 0.829 |
| IV-col in ng/mL | 51.8 (50.0–83.9) | 54.6 (50.0–79.6) | 0.807 |
| LS in kPa | 12.0 (9.2–15.2) | 11.4 (8.5–15.0) | 0.240 |
| Gastroesophageal varices | 0.634 | ||
| No | 309 (89.8%) | 135 (91.2%) | |
| Yes | 35 (10.2%) | 13 (8.8%) | |
| Splenomegaly | 0.285 | ||
| No | 90 (26.2%) | 32 (21.6%) | |
| Yes | 254 (73.8%) | 116 (78.4%) | |
| Extent of resection | 0.395 | ||
| Minor, <3 Couinaud’s segments | 250 (72.7%) | 113 (76.4%) | |
| Major, ≥3 Couinaud’s segments | 94 (27.3%) | 35 (23.6%) | |
| Hilar occlusion in min | 15.0 (0.0–18.0) | 14.5 (0.0–18.3) | 0.740 |
| Intraoperative blood loss in mL | 200.0 (100.0–300.0) | 200.0 (100.0–300.0) | 0.816 |
| Causes of hepatectomy | 1 | ||
| Malignant tumor | 343 (99.7%) | 148 (100%) | |
| Benign tumor | 1 (0.3%) | 0 (0%) | |
| Clinical outcomes | |||
| PHLF† | 0.330 | ||
| No | 253 (73.5%) | 115 (77.7%) | |
| Yes | 91 (26.5%) | 33 (22.3%) | |
| PHLF grade‡ | 0.300 | ||
| 0 | 253 (73.5%) | 115 (77.7%) | |
| A | 63 (18.3%) | 24 (16.2%) | |
| B | 19 (5.5%) | 8 (5.4%) | |
| C | 9 (2.6%) | 1 (0.7%) | |
| Hospital stay as median (IQR) in days | 8 (7–11) | 8.5 (7–11) | 0.863 |
†PHLF was defined as postoperative deterioration of liver function with an increase in the INR and concomitant hyperbilirubinemia on or after postoperative day 5, as proposed by the ISGLS. ‡Following the ISGLS definition of PHLF grade. ALT, alanine aminotransferase; Hb, hemoglobin; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV DNA, hepatitis B virus DNA; HA, hyaluronic acid; IV-col, type IV collagen; LN, laminin; LS, liver stiffness; P-ALB, pre-albumin; PIIINP, precollagen III N-terminal peptide; PLT, platelet count; WBC, white blood cell; GGT, γ-glutamyl transpeptidase.
PHLF was defined as postoperative deterioration of liver function with an increase in the international normalized ratio (INR) and concomitant hyperbilirubinemia on or after postoperative day 5, as proposed by the International Study Group of Liver Surgery (commonly known as the ISGLS).1
Presence of gastroesophageal varices and splenomegaly were confirmed by CT scans or MRI report.16–22 The extent of resection was defined by number of Couinaud’s segments. Extent of resection ≥3 Couinaud’s segments was defined as major resection, otherwise it was minor resection. The extent of resection was characterized as an intraoperative variable because the extent of resection planned preoperatively could differ from the actual extent during the surgery. Hospital stay was calculated from the date of surgery to date of discharge.
Statistical analysis
Categorical variables were expressed as counts and percentages, and were compared using Pearson’s χ2 analysis, Fisher’s exact test or Mann-Whitney U test, as appropriate. Continuous variables were expressed as mean (± standard deviation) or median (interquartile range [IQR]) and were compared using Student’s t-test, Mann-Whitney U test or Kruskal-Wallis test, as appropriate. The p-values were adjusted by Holm’s method for multiple comparisons.
The least absolute shrinkage and selection operator (LASSO) logistic regression model with 10-fold cross-validation was performed to select perioperative variables associated with PHLF. As the group of variables selected by LASSO is not completely consistent every time due to randomness of cross-validation,23 we repeated the same LASSO algorithm with the same candidate variables 1,000 times, and the most frequent group of selected variables was accepted as significant variables.
A multivariate binary logistic regression model was then produced to identify significant independent predictors of PHLF, with a removal significance level of 0.05. No evidence of non-log-linear relationship was found for all continuous variables. All significant variables were reserved in the final model because multicollinearity was not found.
Predictive performance was assessed using the receiver operating characteristic (ROC) curve and compared by Delong’s test. The optimal cut-off value of the logistic model was determined using ROC by maximizing the Youden index (sensitivity plus specificity minus 1). Calibration curves were plotted to assess the calibration of the model. Decision curve analysis (DCA) was conducted to determine the clinical utility of the model.24 A nomogram was established based on the predictive model for the development cohort.
Statistical testing was carried out at the 2-sided tailed α level of 0.05. Data were analyzed using R version 3.6.2 (Vienna, Austria). Variable selection with LASSO was performed by the cv.glmnet function in the glmnet package. Binary logistic regression modeling was performed by the glm function. The nomogram was plotted by the nomogram function in the rms package. Delong’s test was produced by the roc.test function in the pROC package. Calibration curves and DCA were analyzed by the calibrate function in the rms package and the decision_curve function in the rmda package, respectively.
Results
Clinical characteristics
The clinical characteristics of patients in the Zhongshan cohort are listed in Table 1. The comparison of clinical characteristics between the Zhongshan cohort and the Ruijin cohort is shown in Supplementary Table 1. The clinical characteristics were similar between the development and internal validation cohorts.
In the Zhongshan cohort, hospital stay of patients without PHLF (median [IQR]: 8 [7–10] days) was shorter than that of grade A (10 [8–13] days), grade B (10 [8–13] days) and grade C (16.5 [10–29] days) PHLF patients (p<0.001 for all).
Establishment of the predictive model in the development cohort
All variables listed in Table 1 were analyzed. The result of variable selection by LASSO is shown in Supplementary Table 2, which identified type IV collagen, total bilirubin (referred to as TB), albumin (ALB), INR, platelet count, extent of resection and blood loss as the most significantly related factors to PHLF.
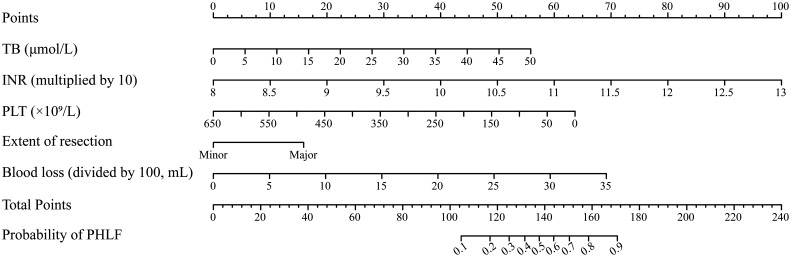
The result of multivariate logistic regression analysis is shown in Table 2. These independent predictors were used to establish a predictive model, which was designated as the PHLF score, and visualized with a nomogram (Fig. 2).
Table 2. Independent predictors of PHLF after multivariate logistic analysis.
| Variables | β | OR | 95% CI | p-value |
|---|---|---|---|---|
| Intercept | −15.585 | |||
| TB in µmol/L | 0.074 | 1.077 | 1.029–1.128 | 0.001 |
| INR†, per 0.1 increase | 1.332 | 3.788 | 2.531–5.867 | <0.001 |
| PLT, per 109/L increase | −0.007 | 0.993 | 0.989–0.998 | 0.004 |
| Extent of resection | ||||
| Minor, <3 segments | 1 | |||
| Major, ≥3 segments | 1.059 | 2.883 | 1.471–5.716 | 0.002 |
| Blood loss‡, per 100 mL increase | 0.132 | 1.141 | 1.043–1.251 | 0.004 |
†INR was multiplied by 10 and put into the multivariate binary logistic regression model. ‡Blood loss was divided by 100 and put into the multivariate binary logistic regression model. The score and predicted probability of PHLF can be calculated using the following formulas: PHLF score: 0.074 × TB + 1.332 × INR (multiplied by 10) − 0.007 × PLT (per 109/L) + 1.059 × extent of resection (major=1; minor=0) + 0.132 × blood loss (divided by 100). The predicted probability of PHLF=1/(1+exp (−PHLF score + 15.585)). CI, confidence interval; OR, odds ratio.
Fig. 2. Nomogram for the prediction of PHLF.
The nomogram was established based on the development cohort. PHLF, post-hepatectomy liver failure.
Predictive accuracy and calibration of the PHLF score compared to other scores in the development cohort
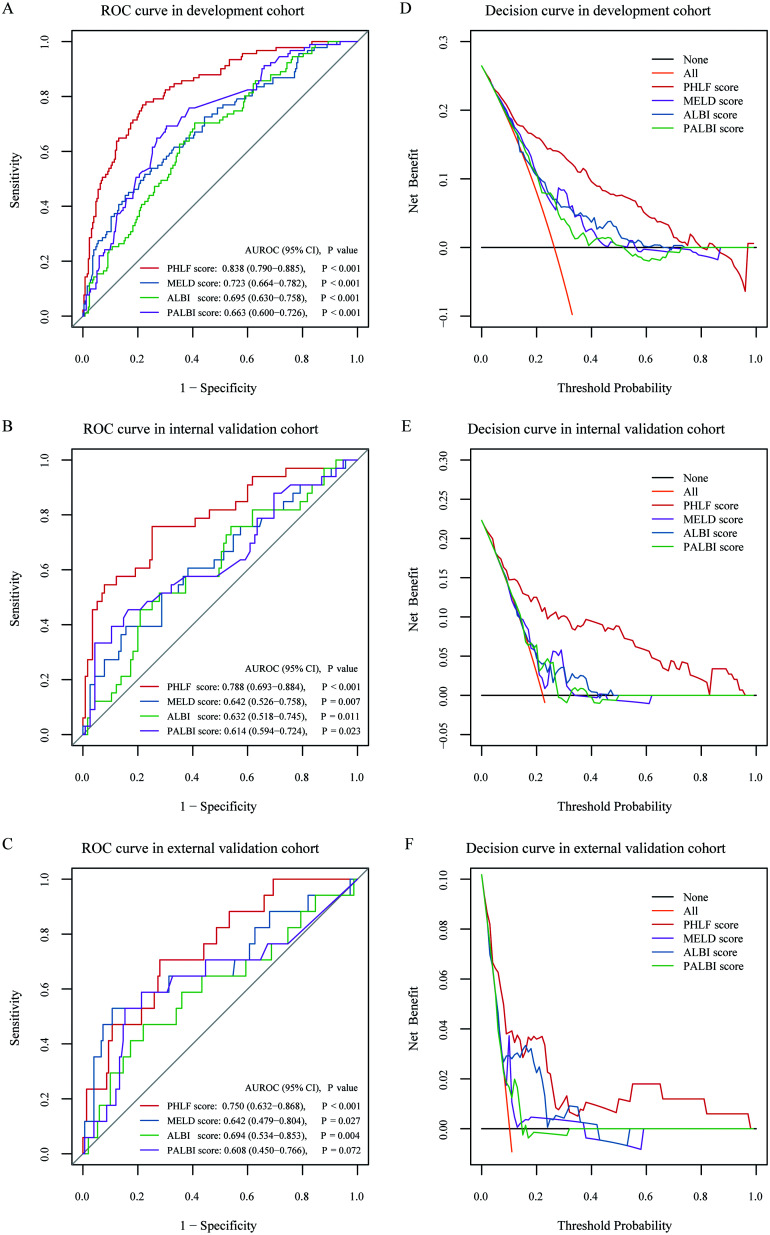
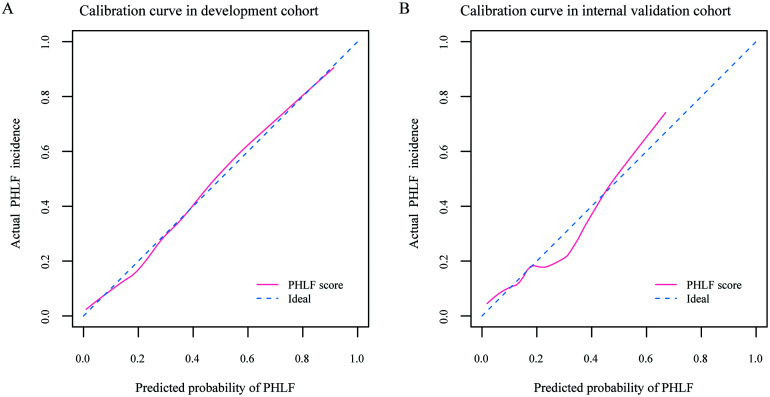
The area under the ROC curve (AUROC) [95% confidence interval (CI)] of the PHLF score was 0.838 (0.790–0.885), which has better accuracy in predicting PHLF than the other three scores (p<0.001 for all, compared by Delong’s test): MELD score, 0.723 (0.664–0.782); ALBI score, 0.695 (0.630–0.758) and PALBI score, 0.663 (0.600–0.726), respectively (Fig. 3A). Calibration curves showed good agreement between prediction and observation (Fig. 4A). DCA revealed that the PHLF score provided superior net benefit over the other three scores (Fig. 3D).
Fig. 3. ROC curves and decision curves for the prediction of PHLF.
ROC curves of PHLF score, MELD score, ALBI score and PALBI score in the (A) development cohort, (B) internal validation cohort and (C) external validation cohort. Decision curves of PHLF score, MELD score, ALBI score and PALBI score in the (D) development cohort, (E) internal validation cohort and (F) external validation cohort. The orange line indicates the net benefit of assuming that all patients have PHLF. The black line indicates the net benefit of assuming no patients have PHLF. ALBI, albumin-bilirubin; MELD, model for end-stage liver disease; PALBI, platelet-albumin-bilirubin; PHLF, post-hepatectomy liver failure; ROC, receiver operating characteristics curve.
Fig. 4. Calibration curves for the prediction of PHLF.
Calibration curves of the PHLF score in (A) development cohort and (B) internal validation cohort. The diagonal blue dashed line represents a perfect prediction by an ideal model. The pink solid line represents the performance of the predictive model, of which a closer fit to the diagonal blue dashed line represents a better prediction. PHLF, post-hepatectomy liver failure.
Risk stratification based on the PHLF score in the development cohort
The optimal cut-off value of the PHLF score was determined to be 14.7 using ROC by maximizing the Youden index. The sensitivity, specificity, positive predictive value (referred to as PPV) and negative predictive value in predicting PHLF were 76.9%, 78.3%, 56.0%, and 90.4%, respectively.
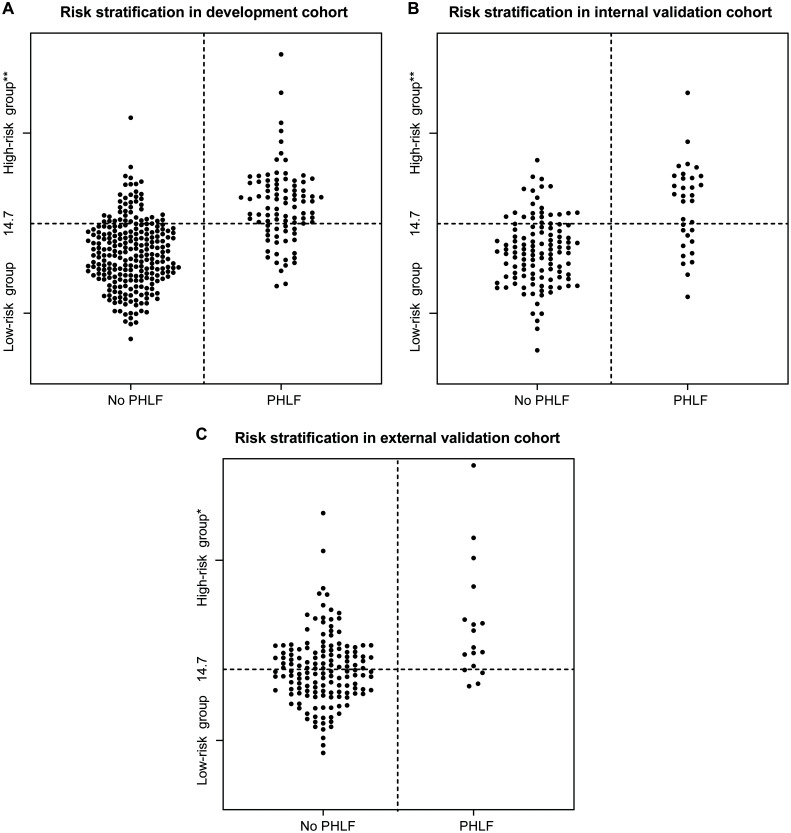
Patients with PHLF score ≥14.7 were defined as the high-risk group, otherwise the patients were classified as the low-risk group. The incidence (55.6% vs. 9.6%, p<0.001) and severity (p<0.001) of PHLF were significantly different between the two groups (Table 3 and Fig. 5A).
Table 3. Incidences of PHLF of high-risk and low-risk groups with a cut-off value of 14.7 by the PHLF score in development and two validation cohorts.
| Development cohort, n=344 |
Internal validation cohort, n=148 |
External validation cohort, n=167 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| High-risk group, n=126 | Low-risk group, n=218 | p-value | High-risk group, n=47 | Low-risk group, n=101 | p-value | High-risk group, n=71 | Low-risk group, n=96 | p-value | |
| PHLF† | <0.001 | <0.001 | 0.013 | ||||||
| No | 56 (44.4%) | 197 (90.4%) | 27 (57.4%) | 88 (87.1%) | 59 (83.1%) | 91 (94.8%) | |||
| Yes | 70 (55.6%) | 21 (9.6%) | 20 (42.6%) | 13 (12.9%) | 12 (16.9%) | 5 (5.2%) | |||
| PHLF grade† | <0.001 | <0.001 | 0.015 | ||||||
| 0 | 56 (44.4%) | 197 (90.4%) | 27 (57.4%) | 88 (87.1%) | 59 (83.1%) | 91 (94.8%) | |||
| A | 48 (38.1%) | 15 (6.9%) | 12 (25.5%) | 12 (11.9%) | 9 (12.7%) | 3 (3.1%) | |||
| B | 15 (11.9%) | 4 (1.8%) | 7 (14.9%) | 1 (1.0%) | 1 (1.4%) | 2 (2.1%) | |||
| C | 7 (5.6%) | 2 (0.9%) | 1 (2.1%) | 0 (0%) | 2 (2.8%) | 0 (0%) | |||
†PHLF was defined as postoperative deterioration of liver function with an increase in the INR and concomitant hyperbilirubinemia on or after postoperative day 5 as proposed by the ISGLS. ‡Following the ISGLS definition of PHLF grade.
Fig. 5. Relationship between the value of the PHLF score and occurrence of PHLF in (A) development cohort, (B) internal validation cohort and (C) external validation cohort.
The horizontal dotted line indicates the cut-off value of the PHLF score (14.7) for the prediction of PHLF. Patients with PHLF score ≥14.7 belong to the high-risk group, otherwise patients were classified in the low-risk group. *p<0.05, **p<0.001, in comparison with the low-risk group. PHLF, post-hepatectomy liver failure.
Validation of the PHLF score in two independent cohorts
In the internal validation cohort, the AUROC of the PHLF score was 0.788 (0.693–0.884), which outperformed the other three scores in predicting PHLF (compared by Delong’s test): MELD score (p=0.006), ALBI score (p=0.010) and PALBI score (p=0.002), respectively (Fig. 3B). PHLF score showed good agreement between prediction and observation in calibration curve (Fig. 4B) and provided superior net benefit over other scores in the DCA curve (Fig. 3E). The incidence (42.6% vs. 12.9%, p<0.001) and severity (p<0.001) of PHLF were significantly different between high-risk and low-risk groups (Table 3 and Fig. 5B).
In the external validation cohort, the AUROC of the PHLF score was 0.750 (0.632–0.868), which was marginally superior to other three scores in predicting PHLF (compared by Delong’s test): MELD score (p=0.103), ALBI score (p=0.535) and PALBI score (p=0.100), respectively (Fig. 3C). PHLF score also provided superior net benefit over other scores in DCA analysis (Fig. 3F). The incidence (16.9% vs. 5.2%, p=0.013) and severity (p=0.015) of PHLF were also significantly different between the high-risk and low-risk groups (Table 3 and Fig. 5C).
Discussion
In this study, PHLF in patients undergoing hepatectomy could be accurately predicted immediately after surgery using routinely available variables, including three preoperative (TB, INR and platelet count) and two intraoperative (extent of resection and blood loss) factors. In addition, patients could be properly stratified in terms of the risk of PHLF, with a cut-off value of 14.7.
This study suggested that hepatic surgeons can take the optimized measures to prevent or manage PHLF perioperatively. On the basis that patients reserve good liver function, surgeons can calculate the maximum of intraoperative blood loss they can tolerate to prevent PHLF, because the extent of resection can be estimated by preoperative imaging data, and blood loss was the only unknown variable. This could remind surgeons to be more careful during surgery to reduce blood loss in order to prevent PHLF. Furthermore, surgeons could better inform patients and their families of the risk of PHLF after surgery. When the risk of PHLF is highly predicted, surgeons may suggest patients take medications to improve liver function and/or take systemic therapy to shrink the tumor as the best choice at that time, rather than surgery. Then, when the liver function or the tumor regression reaches a certain extent, surgery can be performed. If patients insist on performance of the surgery, surgeons can determine the appropriate level of postoperative care and extend the length of hospital stay, in addition performing a more careful operation.
The aim of this study was to establish a model to predict PHLF in patients undergoing hepatectomy. Many useful criteria and scores were demonstrated to predict the incidence of PHLF. One of the most classic models was “Makuuchi’s criteria”, representing a decision tree for selection of operative procedures in patients with impaired liver function reserve, which included three determining factors: ascites, serum TB value, and ICG-R15.25 Imamura et al.26 reported zero-mortality after hepatectomy and only one patient developed PHLF from among nine hundred and fifteen consecutive patients within the criteria. However, within each category of Makuuchi’s criteria, there is a relatively wide range of hepatic function reserve and it does not take into account the individual variation in the FLR volume.27
MELD,8 ALBI10,28 and PALBI29 scores were previously reported to be accurate for the prediction of PHLF in patients with hepatocellular carcinoma. However, the ALBI score and PALBI score were based on a relatively low proportion of 727 (28.0%) patients undergoing hepatectomy.6,7 A study showed neither ALBI nor PALBI could predict survival of patients following transjugular intrahepatic portosystemic shunt creation,30 which may suggest that they were not the most suitable to predict PHLF for patients undergoing surgery. All patients in the current study underwent hepatectomy and their indications of surgery did not only include hepatocellular carcinoma, but all had reasoned hepatectomy. Our model may be more suitable for this target population and perform better in such.
Furthermore, ALB, which was included in both the ALBI and PALBI scores, was not included in our model. ALBI and PALBI scores were determined patients with data of ALB level, reported as median (IQR) of 35 (31–39) g/L, but the ALB level of patients in this study was 42 (39–45) g/L. Hence, the ability of ALB to predict PHLF was not as important as in patients with advanced diseases.
The indocyanine green clearance rate constant (referred to as ICG-K) and ICG-R15 have been widely adopted in Eastern centers to measure liver function, but neither of them is a routine test in our center. Hwang et al.31 established a quantified model combined with ICG-K and FLR to predict PHLF, which was similar to our model, both containing factors representing liver function and resected liver volume. However, surgery-related factors were not included in the model by Hwang et al.31 and in none of the models mentioned above. As PHLF could be influenced by surgery-related factors like blood loss, extent of hepatectomy,13,32 our model with intraoperative variables could predict PHLF more accurately.
This study included almost all indicators of laboratory tests, important clinical signs available before surgery, and three intraoperative factors. To develop a predictive model based on as many as 25 candidate variables, we employed LASSO, which has been developed to overcome the limitations when too many predictors are needing to be analyzed, to guarantee the objectivity of variables included in the model.33 In addition, DCA was performed to compare the clinical utility of different models, visualizing the clinical consequences of a diagnostic strategy.34 Traditional metrics of diagnostic performance, such as AUROC, sensitivity and specificity only measure the accuracy of one prediction model against another, but fail to consider whether patients will really benefit from a specific model with the high predictive accuracy.24
In addition, the PHLF score was validated externally and demonstrated satisfactory predictive accuracy and clinical utility. Furthermore, PHLF score can also stratify patients undergoing hepatectomy in terms of risk of PHLF in the external validation cohort.
The relatively low PPV indicates that patients who were actually at high risk of PHLF were not assigned to the high-risk group, and some factors that caused high risk of PHLF, such as repeated resection and tumor-related factors, were not included. Patients who underwent repeated resection were at higher risk of developing PHLF. In addition, this study enrolled patients either with benign or malignant lesions, so that tumor-related factors such as tumor number, size and biomarker were not included in the analysis.
This study has several limitations. First, we retrospectively investigated a group of patients with a relatively low proportion of grade B or C PHLF. Grade A PHLF represents a transient deterioration in liver function that does not require extra treatment. However, the hospital stay of grade A PHLF patients was longer than those without PHLF, indicating that this model is of clinical significance. Second, the predictive model cannot access the severity of PHLF and make a classification according to the ISGLS grade, where different grades of PHLF are subject to different treatments. Third, the extent of resection was defined as minor (<3 segments) or major (≥3 segments) in this study, which could not exactly reflect the FLR volume. Because the volume of segment II+III (left lateral section) is significantly smaller than that of segment VII+VIII (right lateral section), and the latter may exceed the volume of segment II+III+IV (left liver).35 In addition, inadequate FLR volume can lead to PHLF.36 The performance of the predictive model could be improved through measuring FLR volume and FLR function by three-dimensional CT reconstruction or other image fusion techniques preoperatively.12,37
In conclusion, this study showed that PHLF after hepatectomy can be accurately predicted by five simple and readily available perioperative variables, which may significantly contribute to the postoperative care of those patients and improving clinical outcomes.
Supporting information
Abbreviations
- ALB
albumin
- ALBI
albumin-bilirubin
- ALT
alanine aminotransferase
- AUROC
area under receiver operating characteristic curve
- CI
confidence interval
- CT
computed tomography
- DCA
decision curve analysis
- FLR
future liver remnant
- GGT
γ-glutamyl transpeptidase
- HA
hyaluronic acid
- Hb
hemoglobin
- HBeAg
hepatitis B e antigen
- HBsAg
hepatitis B surface antigen
- HBV DNA
HBV DNA copy number
- ICG-K
indocyanine green clearance rate constant
- ICG-R15
indocyanine green retention rate at 15 min
- INR
international normalized ratio
- IQR
interquartile range
- ISGLS
International Study Group of Liver Surgery
- IV-col
type IV collagen
- LASSO
least absolute shrinkage and selection operator
- LN
laminin
- LS
liver stiffness
- MELD
model for end-stage liver disease
- OR
odds ratio
- PIIINP
precollagen III N-terminal peptide
- P-ALB
pre-albumin
- PALBI
platelet-albumin-bilirubin
- PHLF
post-hepatectomy liver failure
- PLT
platelet count
- PPV
positive predictive value
- ROC
receiver operating characteristics curve
- TB
total bilirubin
- WBC
white blood cell
Data sharing statement
The data collected for this study will not be shared because the Institutional Ethics Committee of the two hospitals required that data involved in this study should be kept confidential. Other documents such as statistical analysis plan and informed consent form will be shared.
References
- 1.Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149(5):713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Citterio D, Facciorusso A, Sposito C, Rota R, Bhoori S, Mazzaferro V. Hierarchic interaction of factors associated with liver decompensation after resection for hepatocellular carcinoma. JAMA Surg. 2016;151(9):846–853. doi: 10.1001/jamasurg.2016.1121. [DOI] [PubMed] [Google Scholar]
- 3.Schreckenbach T, Liese J, Bechstein WO, Moench C. Posthepatectomy liver failure. Dig Surg. 2012;29(1):79–85. doi: 10.1159/000335741. [DOI] [PubMed] [Google Scholar]
- 4.Cescon M, Colecchia A, Cucchetti A, Peri E, Montrone L, Ercolani G, et al. Value of transient elastography measured with FibroScan in predicting the outcome of hepatic resection for hepatocellular carcinoma. Ann Surg. 2012;256(5):706–712. doi: 10.1097/SLA.0b013e3182724ce8. discussion 712-713. [DOI] [PubMed] [Google Scholar]
- 5.Kamath PS, Kim WR, Advanced Liver Disease Study Group The model for end-stage liver disease (MELD) Hepatology. 2007;45(3):797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 6.Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roayaie S, Jibara G, Berhane S, Tabrizian P, Park J-W, Yang J, et al. PALBI-an objective score based on platelets, albumin & bilirubin stratifies HCC patients undergoing resection & ablation better than child’s classification. Hepatology. 2015;62(1, SI):631A–632A. [Google Scholar]
- 8.Kong FH, Miao XY, Zou H, Xiong L, Wen Y, Chen B, et al. End-stage liver disease score and future liver remnant volume predict post-hepatectomy liver failure in hepatocellular carcinoma. World J Clin Cases. 2019;7(22):3734–3741. doi: 10.12998/wjcc.v7.i22.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andreatos N, Amini N, Gani F, Margonis GA, Sasaki K, Thompson VM, et al. Albumin-bilirubin score: predicting short-term outcomes including bile leak and post-hepatectomy liver failure following hepatic resection. J Gastrointest Surg. 2017;21(2):238–248. doi: 10.1007/s11605-016-3246-4. [DOI] [PubMed] [Google Scholar]
- 10.Zhang ZQ, Xiong L, Zhou JJ, Miao XY, Li QL, Wen Y, et al. Ability of the ALBI grade to predict posthepatectomy liver failure and long-term survival after liver resection for different BCLC stages of HCC. World J Surg Oncol. 2018;16(1):208. doi: 10.1186/s12957-018-1500-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Araki K, Harimoto N, Kubo N, Watanabe A, Igarashi T, Tsukagoshi M, et al. Functional remnant liver volumetry using Gd-EOB-DTPA-enhanced magnetic resonance imaging (MRI) predicts post-hepatectomy liver failure in resection of more than one segment. HPB (Oxford) 2020;22(2):318–327. doi: 10.1016/j.hpb.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Li C, Zhu A. Application of image fusion in diagnosis and treatment of liver cancer. Applied Sciences. 2020;10(3):1171–1171. doi: 10.3390/app10031171. [DOI] [Google Scholar]
- 13.Kauffmann R, Fong Y. Post-hepatectomy liver failure. Hepatobiliary Surg Nutr. 2014;3(5):238–246. doi: 10.3978/j.issn.2304-3881.2014.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen Y, Shi G, Huang C, Zhu X, Chen S, Sun H, et al. Prediction of post-operative liver dysfunction by serum markers of liver fibrosis in hepatocellular carcinoma. PLoS One. 2015;10(10):e0140932. doi: 10.1371/journal.pone.0140932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen Y, Zhou C, Zhu G, Shi G, Zhu X, Huang C, et al. Liver stiffness assessed by shear wave elastography predicts postoperative liver failure in patients with hepatocellular carcinoma. J Gastrointest Surg. 2017;21(9):1471–1479. doi: 10.1007/s11605-017-3443-9. [DOI] [PubMed] [Google Scholar]
- 16.Perri RE, Chiorean MV, Fidler JL, Fletcher JG, Talwalkar JA, Stadheim L, et al. A prospective evaluation of computerized tomographic (CT) scanning as a screening modality for esophageal varices. Hepatology. 2008;47(5):1587–1594. doi: 10.1002/hep.22219. [DOI] [PubMed] [Google Scholar]
- 17.Kim SH, Kim YJ, Lee JM, Choi KD, Chung YJ, Han JK, et al. Esophageal varices in patients with cirrhosis: multidetector CT esophagography-comparison with endoscopy. Radiology. 2007;242(3):759–768. doi: 10.1148/radiol.2423050784. [DOI] [PubMed] [Google Scholar]
- 18.Lipp MJ, Broder A, Hudesman D, Suwandhi P, Okon SA, Horowitz M, et al. Detection of esophageal varices using CT and MRI. Dig Dis Sci. 2011;56(9):2696–2700. doi: 10.1007/s10620-011-1660-8. [DOI] [PubMed] [Google Scholar]
- 19.Shin SU, Lee JM, Yu MH, Yoon JH, Han JK, Choi BI, et al. Prediction of esophageal varices in patients with cirrhosis: usefulness of three-dimensional MR elastography with echo-planar imaging technique. Radiology. 2014;272(1):143–153. doi: 10.1148/radiol.14130916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ronot M, Lambert S, Elkrief L, Doblas S, Rautou PE, Castera L, et al. Assessment of portal hypertension and high-risk oesophageal varices with liver and spleen three-dimensional multifrequency MR elastography in liver cirrhosis. Eur Radiol. 2014;24(6):1394–1402. doi: 10.1007/s00330-014-3124-y. [DOI] [PubMed] [Google Scholar]
- 21.Bezerra AS, D’Ippolito G, Faintuch S, Szejnfeld J, Ahmed M. Determination of splenomegaly by CT: is there a place for a single measurement? AJR Am J Roentgenol. 2005;184(5):1510–1513. doi: 10.2214/ajr.184.5.01841510. [DOI] [PubMed] [Google Scholar]
- 22.Gunes SO, Akturk Y. Determination of splenomegaly by coronal oblique length on CT. Jpn J Radiol. 2018;36(2):142–150. doi: 10.1007/s11604-017-0704-1. [DOI] [PubMed] [Google Scholar]
- 23.Homrighausen D, McDonald DJ. Risk consistency of cross-validation with lasso-type procedures. Statistica Sinica. 2018;27(3):1017–1036. doi: 10.5705/ss.202015.0355. [DOI] [Google Scholar]
- 24.Fitzgerald M, Saville BR, Lewis RJ. Decision curve analysis. JAMA. 2015;313(4):409–410. doi: 10.1001/jama.2015.37. [DOI] [PubMed] [Google Scholar]
- 25.Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, et al. Surgery for small liver cancers. Semin Surg Oncol. 1993;9(4):298–304. doi: 10.1002/ssu.2980090404. [DOI] [PubMed] [Google Scholar]
- 26.Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138(11):1198–1206. doi: 10.1001/archsurg.138.11.1198. discussion 1206. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi Y, Kiya Y, Sugawara T, Nishioka Y, Hashimoto M, Shindoh J. Expanded Makuuchi’s criteria using estimated indocyanine green clearance rate of future liver remnant as a safety limit for maximum extent of liver resection. HPB (Oxford) 2019;21(8):990–997. doi: 10.1016/j.hpb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Fagenson AM, Gleeson EM, Pitt HA, Lau KN. Albumin-bilirubin score vs model for end-stage liver disease in predicting post-hepatectomy outcomes. J Am Coll Surg. 2020;230(4):637–645. doi: 10.1016/j.jamcollsurg.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Lu LH, Zhang YF, Mu-Yan C, Kan A, Zhong XP, Mei J, et al. Platelet-albumin-bilirubin grade: risk stratification of liver failure, prognosis after resection for hepatocellular carcinoma. Dig Liver Dis. 2019;51(10):1430–1437. doi: 10.1016/j.dld.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Khabbaz RC, Lokken RP, Chen YF, Lipnik AJ, Bui JT, Ray CE, Jr, et al. Albumin-bilirubin and platelet-albumin-bilirubin grades do not predict survival after transjugular intrahepatic portosystemic shunt creation. Cardiovasc Intervent Radiol. 2018;41(7):1029–1034. doi: 10.1007/s00270-018-1923-2. [DOI] [PubMed] [Google Scholar]
- 31.Hwang S, Ha TY, Song GW, Jung DH, Ahn CS, Moon DB, et al. Quantified risk assessment for major hepatectomy via the indocyanine green clearance rate and liver volumetry combined with standard liver volume. J Gastrointest Surg. 2015;19(7):1305–1314. doi: 10.1007/s11605-015-2846-8. [DOI] [PubMed] [Google Scholar]
- 32.Pruvot FR, Truant S. Major hepatic resection: from volumetry to liver scintigraphy. HPB (Oxford) 2016;18(9):707–708. doi: 10.1016/j.hpb.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo P, Zeng F, Hu X, Zhang D, Zhu S, Deng Y, et al. Improved variable selection algorithm using a LASSO-type penalty, with an application to assessing hepatitis B infection relevant factors in community residents. PLoS One. 2015;10(7):e0134151. doi: 10.1371/journal.pone.0134151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vickers AJ, Van Calster B, Steyerberg EW. Net benefit approaches to the evaluation of prediction models, molecular markers, and diagnostic tests. BMJ. 2016;352:i6. doi: 10.1136/bmj.i6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdalla EK, Denys A, Chevalier P, Nemr RA, Vauthey JN. Total and segmental liver volume variations: implications for liver surgery. Surgery. 2004;135(4):404–410. doi: 10.1016/j.surg.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 36.Kim HJ, Kim CY, Park EK, Hur YH, Koh YS, Kim HJ, et al. Volumetric analysis and indocyanine green retention rate at 15 min as predictors of post-hepatectomy liver failure. HPB (Oxford) 2015;17(2):159–167. doi: 10.1111/hpb.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Theilig D, Steffen I, Malinowski M, Stockmann M, Seehofer D, Pratschke J, et al. Predicting liver failure after extended right hepatectomy following right portal vein embolization with gadoxetic acid-enhanced MRI. Eur Radiol. 2019;29(11):5861–5872. doi: 10.1007/s00330-019-06101-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.