Abstract
Thus far, ecophysiology research has predominantly been conducted within controlled laboratory-based environments, owing to a mismatch between the recording technologies available for physiological monitoring in wild animals and the suite of behaviours and environments they need to withstand, without unduly affecting subjects. While it is possible to record some physiological variables for free-living animals using animal-attached logging devices, including inertial-measurement, heart-rate and temperature loggers, the field is still in its infancy. In this opinion piece, we review the most important future research directions for advancing the field of ‘physiologging’ in wild animals, including the technological development that we anticipate will be required, and the fiscal and ethical challenges that must be overcome. Non-invasive, multi-sensor miniature devices are ubiquitous in the world of human health and fitness monitoring, creating invaluable opportunities for animal and human physiologging to drive synergistic advances. We argue that by capitalizing on the research efforts and advancements made in the development of human wearables, it will be possible to design the non-invasive loggers needed by ecophysiologists to collect accurate physiological data from free-ranging animals ethically and with an absolute minimum of impact. In turn, findings have the capacity to foster transformative advances in human health monitoring. Thus, we invite biomedical engineers and researchers to collaborate with the animal-tagging community to drive forward the advancements necessary to realize the full potential of both fields.
This article is part of the theme issue ‘Measuring physiology in free-living animals (Part II)’.
Keywords: artificial intelligence, photoplethysmography, sensing technology, health management, wearable devices
1. Introduction
Studies in ecophysiology have provided astonishing insights into how animals respond to extreme environments and manage athletic feats such as deep breath-hold diving and endurance migration [1–6]. However, the field of ‘physiologging’ (defined here as the use of archival devices attached to, or implanted in, animals to record their physiology) significantly lags behind advances in the development of wearables for monitoring of human health and fitness, owing to significant practical, technological and fiscal challenges. Real-time recording can often be achieved with much smaller and simpler electronic devices such as continuous radio transmitters [7], but is only possible within the reception range of a radio receiver that is then coupled to a data-logging device. Using this approach, the heart rate of very small animals such as free-living songbirds and bats could be recorded during their regular daily routines [8,9], describing energetic changes [10–12] or migrations [13–16]. However, when animals move outside of radio reception, or where there is a wish to log data over the longer term, these devices do not suffice. Portable, animal-borne devices have therefore been developed to record key variables of interest, such as ECG, EEG, blood pressure, body temperature and indwelling compounds (including lactate, table 1), but there is significant scope for further development. Many physiological variables, such as changes in blood glucose, circulating hormones or energetic expenditure, are still only monitored in controlled laboratory settings (e.g. [20–23]), limited by either the invasiveness or size of available measuring instruments. Unlike health and sports practitioners, ecophysiologists require devices that are unobtrusive enough to allow for uninhibited natural behaviour, while robust enough to withstand particularly harsh environments to log data continuously over extended periods of time, ranging from weeks, to months, to years.
Table 1.
Availability of portable devices to measure physiological variables—this table is not an exhaustive list of all suppliers.
| physiological variable | animals | humans |
|---|---|---|
| respiratory gases | (−) | COSMEDa
Metamax 3Bb [17] VO2 Masterc |
| respiratory rate and/or depth | (−) | Astroskind
Hexoskind Current Healthe Equivitalf |
| blood gases (pO2, pCO2, pH, etc.) | (−) | Astroskind
Sentecg |
| blood glucose | Dexcom G6h
Freestyle Libre 14i |
Dexcomh
Freestyle Librei Guardianj Kenzen Echok Medtronic MiniMedl Omnipodm Sanon Sweatio |
| other compounds in the blood | (−) | Sempionatto et al. [18] |
| blood pressure | Transonic Endogearp | Astroskind
Omron Heart Guideq |
| heart rate (inc. ECG) | StarOddi DSTr
Transonic Endogearp |
Astroskind
Current Healthe Hexoskind AmbioTexs AIOsleevet OmSignalu VivaLnk ECG patchv |
| muscle contraction (EMG) | (−) | LiveAthosw |
| muscle gases (pO2, pCO2, pH, etc.) | (−) | Humonx
Moxyy |
| body temperature | StarOddi DSTr
Transonic Endogearp |
Current Healthe
COREz VivaLnk Fever Scoutaa TempTRAQab Equivitalf |
| brain activity (EEG) | Neurologgerac | BitBrain Diademad
Unicorn BIae |
| visual function | (−) | TobiiPro glasses Xaf |
| lactate | Payne et al. [19] | BSX Insighty
Sweatio |
In this opinion piece, we will review the current state of physiologging, as well as the technical challenges that require future advancement critical for ethical and measurement performance. Specifically, we explore the opportunity to foster collaborative exchange between the fields of animal physiologging and human biomedical and sports monitoring. We highlight how ecophysiologists may benefit from impressive advances made by the wearables industry [24,25] and how work on wild animals can, in turn, become a major engine of innovation for human applications and research.
2. Current state of physiologging
Since the first pioneering studies in the late 1930s [7], the field of physiologging has been transformed by significant advances in the miniaturization of sensor technology [26], in on-board memory capacity, and in power demand, management and supply [27,28]. The earliest biologging devices [29,30] were large film time-depth recorders (TDR) deployed on seals. For example, the Kooyman-Billups TDR was approximately 20 × 8 cm long, weighed more than 1 kg, and could record for up to an hour [31]. Nowadays, it is possible to record several orders of magnitude more dive data on tiny high-resolution bio-logging devices (e.g. Cefas G5 TDR is 36.5 × 12 mm, weighs 6.5 g, and records at 10 Hz for more than 730 days).
A diverse array of devices is now available for recording information about the three-dimensional movements and behaviour of wild animals [32–35]. For example, the last 15 years have seen the development of loggers that can record sub-second movement data via inertial measurement units (IMUs) that are sensitive to micromovements and magnetic fields (e.g. [36–39]), or even obtain high-resolution video recordings of an animal's activities, including breathing and prey-capture rates [40–42]. Information on subjects' physiological responses to stressors can be gathered through measurements of heart rate, blood flow and pressure (e.g. [43]), blood and tissue oxygenation [44,45] as well as electroencephalogram (EEG) data [46,47]. Physiologging technology is now advancing our understanding of the ecophysiology of flight [48–50], terrestrial locomotion [51,52], swimming [53] and diving [26,45,54].
Ecophysiology research, however, is restricted to ‘working within the limits’ of the technology available. We asked 20 leading biologging scientists (from biology and engineering backgrounds, appendix A) to help us chart the state-of-the-art of the field as well as future opportunities, using an anonymous online questionnaire. Temperature was the most commonly measured physiological variable, not least because temperature sensors are easily integrated into devices and are straightforward to calibrate. Interestingly, however, many respondents suggested that temperature was not particularly useful for their research compared with other available physiological variables, probably because it is often only the output of an underlying physiological alteration such as the heat increment of feeding, or changes in heart rate, but does not in itself reveal the physiological cause of state alterations. Heart rate was the next most commonly recorded variable and was considered more helpful than temperature, but heart-rate logging devices for wild animals are only available from a very small number of manufacturers. Respondents said that recording durations achievable currently (generally up to a week or a month per deployment) were satisfactory, noting that longer recordings would be limited by battery size, where devices that record for longer may be too large or heavy to be ethically acceptable. Longer deployment durations also increase the risk of device loss, as the likelihood of device retrieval decreases with deployment time (e.g. the subject migrates away). However, in response to open horizon-scanning questions, several colleagues highlighted that physiologging research requires longer datasets of higher recording frequencies, and devices that return data in their raw form (i.e. not summarized or binned).
Thus, research in ecophysiology has naturally focused on variables that pose fewer challenges, on physiological responses during relatively short observation windows, and in species that are accessible to study and large enough to carry available devices. However, the discipline arguably requires longer-term datasets, from a broader range of animals and a greater scope of physiological variables to explore the range of physiological responses of wild animals to the abiotic and biotic environment. Our expert respondents indicated a particular desire to gather reliable data on respiration and blood gases, muscle glycogen and lactate, and visual function. Such information would not only provide a greater appreciation of the physiological strategies wild animals employ to cope with demanding and rapidly changing environmental conditions but may also offer new perspectives for those tackling major public health challenges, including hyperglycaemia and obesity. In the following section, we review what we, and our questionnaire respondents, perceive as major barriers to the field's advancement, and sketch possible solutions.
3. Major barriers to physiologging research
(a) . Internal logger placement
The predominant barrier to recording the internal physiological state of organisms is the impervious nature of the skin. The majority of geolocating biologging devices (such as GPS, Argos, or ICARUS tags) are externally attached, permitting long-distance data transmission and solar recharging, do not require veterinary expertise to implant them and circumvent the need to recapture subjects for data retrieval. However, by efficiently isolating the animal from the harshness of their external environment, the skin layer restricts direct measurement of many aspects of internal state, such as blood chemistry and muscular physiology. The majority of physiologging devices to date have therefore been surgically implanted (e.g. [1,7,43,55–60]). Some devices may be mounted externally but are still physically connected to surgically placed indwelling electrodes or cannulas (reviewed in Williams et al. [26]). Importantly, most physiologgers do not remotely transmit data, either because biological tissue is relatively opaque to radio wave transmission, and/ or because devices collect huge volumes of raw data during a typical deployment (e.g. for ECG and EEG), which would require excessive power for transmission. Subsequent removal of the device or electrode usually necessitates recapture and repeat surgery, with important ethical implications (reviewed in [61]).
The need for surgical removal of some implanted device types could be avoided if, in principle, it was possible to transmit data from the implant over short distances (e.g. via low-energy Bluetooth connection) to externally mounted logging devices (e.g. [43]), such as those developed for laboratory studies (e.g. for neuroscience research in mice, [62]), or by first reducing data volume (see below) and then transmitting the condensed data to autonomous receivers placed strategically in the environment (near nests, inside burrows, at haul outs, or near natural or experimental feeding sites; e.g. [63,64]). Promising future solutions also include physiologically sensitive skin ‘tattoo’ biosensors (e.g. [27,65,66]), the colour of which reflects biochemical changes (e.g. in pH or glucose) and can be recorded via imaging devices. In biomedical applications, this is achieved with an app on a smartphone, but for wild animals, such an imaging device could be placed at sites that are routinely revisited by tagged subjects.
However, considerable advances have been made within the biomedical industry to measure various parameters non-invasively with externally mounted devices, such as ECG, respiratory rate, blood oxygen and blood pressure, or with minimal implantation (e.g. for circulating compounds such as glucose, table 1), suggesting that many of these technologies may soon be within reach for use on free-living animals. In biomedicine, these measurements are generally made in one of four ways: (i) photoplethysmography (PPG, e.g. heart rate and oxygen saturation estimation by smart watches, [67]); (ii) sampling interstitial fluids via microneedle (e.g. continuous glucose monitors, [68]); (iii) motion sensors (e.g. ventilation, [69]); or (iv) sampling of eccrine sweat (e.g. lactate sensing skin patch, [70], figure 1). These technologies are by no means perfected for human deployment, and their utility for non-human application remains to be explored. PPG, for example, is vulnerable to interference from light and the effects of variable perfusion from changing blood-flow dynamics and temperatures [71]. A particular challenge for PPG is ‘signal crossover’ where the periodic signal from repetitive motion is mistaken for the cardiovascular cycle [71]. Respiratory rate can be estimated by spirometry, capnography, impedance pneumography or accelerometry but these approaches still require considerable refinement in the context of commercial wearables [72]. Non-invasive measures of temperature are relatively poor at estimating deep body temperature [73], even though they are in widespread use, and because the skin is the major organ for heat dissipation and conservation, its temperature may lag behind or trend in the opposite direction to deep body temperature. There is still no satisfactory means of non-invasive continuous blood pressure measurement in biomedicine [74]. Unfortunately, no non-human animal is known to produce eccrine sweat, so sports wearable technologies that sample sweat are not transferable.
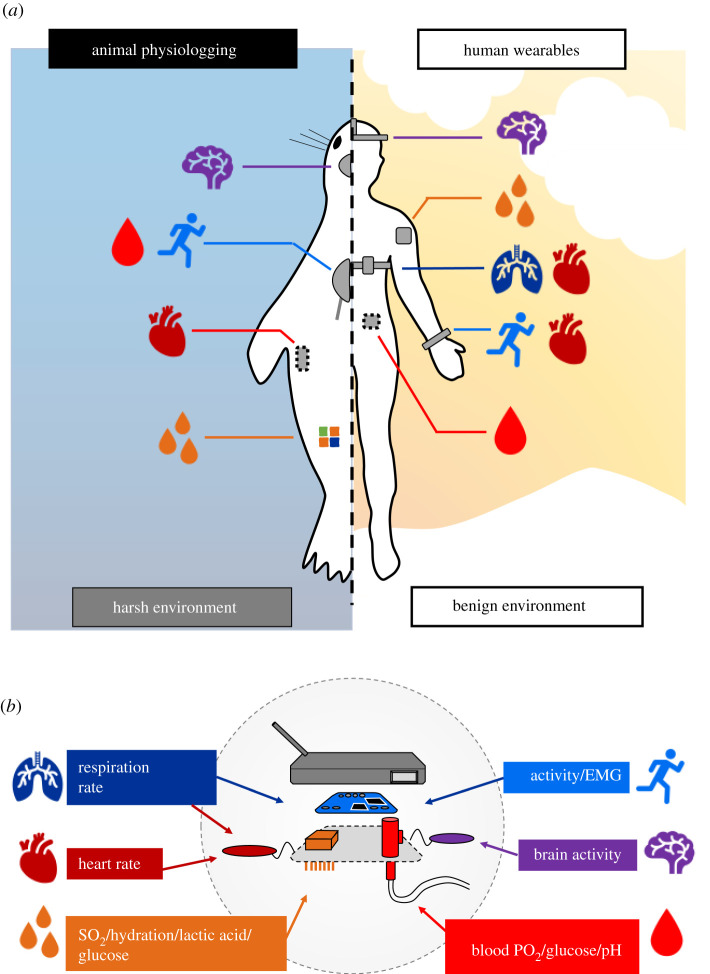
Figure 1.
Schematic for recording of physiological variables in wild-animal physiologging and human health monitoring technologies. (a) Typical device attachments include external devices recording motion, PPG and sampling fluids with microneedles, internal devices (dashed outline) recording blood chemistry and heart rate, and physiologically sensitive tattoos. (b) Basic hardware housed in externally attached devices records the suite of physiological variables in both systems.
Because these non-invasive approaches are designed for use on humans, they usually have short battery life (i.e. days to weeks), but to our knowledge none of these devices have yet been experimentally deployed on animals. It is clear that there is abundant potential to refine these technologies for use on wild animals. This will no doubt pose significant engineering challenges, but successful innovation would be rewarded with unprecedented insight into ecophysiological processes.
(b) . Power requirements
Ethical guidelines have been proposed, such as a rule that biologging devices should not exceed 3% of an animal's body mass [6,75–79], but it is notable that most current physiologgers would exceed such limits for most study species of interest, in part due to the significant mass of on-board power storage required for high-frequency and long-duration sampling protocols. For example, the smallest available heart rate logger to our knowledge weighs 3.3 g (Star Oddi DST micro-HRT), which means that 74% of extant bird species are too light to carry them according to the 3% rule [80]. Consequently, there has been a considerable drive to create lightweight devices to study smaller animals.
The advances made have been possible, primarily, due to increased efficiency of microcontrollers and usage of low power states [81], rather than increased battery energy density. For example, over the last decade, lithium ion batteries have nearly tripled in energy density from about 100 to 300 W kg−1, yet during the same period microprocessor computing efficiency has increased approximately 16-fold [82]. In addition, as batteries become physically smaller, they tend to be less efficient in terms of energy per unit of mass, as the external casing is a larger percentage of total battery mass. Thus, future advances in increasing the quantity of data, the lifetime of devices and reducing detrimental effects on tagged animals is likely to be driven by increases in power use efficiency, rather than jumps in emerging battery technology [83]. Generally, physiologging devices record huge volumes of original sensor data and use fairly unsophisticated first-level evaluations of the logged data to calculate, for example, heart rate, which is then stored. Quite often such a system results in either memory overrun within a short amount of time, or erroneous data because of noise in the sensor system or unanticipated physiological data ranges. Perhaps one of the biggest improvements in physiologgers would be if the on-board electronic processors were powerful enough to run the computational algorithms now used on post-processing computers after the return of the physiologger to the laboratory. The best available ASICs (application-specific integrated circuits) are now capable of running machine learning algorithms ‘on-board’, with minimal energy requirements, thus allowing for long-term deployments and mass-recordings of multi-sensor original data and intelligently evaluated and combined physiological data.
An exciting prospect to reduce battery mass further is the design of physiologging devices that can harness power from the animals to which they are attached [84,85]. There have been several advances in energy harvesting recently, with the three primary approaches being: (i) piezoelectric, which involves bending of a membrane, best suited to dynamically moving, flying or running animals [84]; (ii) hydrostatic, which is ideal for diving animals [85]; and (iii) exploitation of temperature differentials [86] using the Peltier-Seebeck effect (although it is worth noting the inefficiency of this method). In the case of birds, for example, estimates of harvestable power during flight show that it would be possible to power physiologgers solely by kinetic energy harvesting methods [84], with data resolution dependent on device components and the specific device-to-animal-mass ratio. The maximum power that individual species are capable of generating depends on wingbeat frequency and amplitude, and the time spent in different flight modes (i.e. powered versus soaring flight), but such devices may even be suitable for small-bodied passerines [84]. Experiment-specific tailoring of sampling rates and duty cycles (the pre-programmed schedule that determines when a system is active and inactive) means that even peripheral sensors with relatively high power consumption, such as ECG, are potential future candidates for coupling with energy-harvesting technologies.
Physiologgers could also be powered via external sources, which with the notable exception of solar cells remains to the best of our knowledge a completely unexplored approach to powering devices in the field. For example, devices could be powered externally by electromagnetic induction or magnetic resonant coupling. Wireless recharging of device batteries (e.g. with the same Qi technology as used in wireless smartphone charging) would permit battery recharge and data transfer, extending data collection periods and reducing the need for repeated capture and associated handling stress. This technology is already being used in laboratory-based studies (e.g. [62]) and could be adapted for field deployment. However, a major caveat is that this recharging method would be restricted to species with sufficiently high (and predictable) site fidelity to ensure very close proximity between the device and the power source for extended periods of time (e.g. inside nestboxes or burrows).
(c) . Environment and animal proofing
By far the largest component of early biologging devices, in terms of mass, was that of the material required to protect sensitive electronics from seawater ingress (e.g. [87]). Developments in commodity plastics [88] have dramatically reduced the mass of device housings over the years. But the need to fully water-, depth- and impact-proof devices, while (often) connecting to power and transmitter components, means that housings and their attachment materials generally still remain large and heavy. As a consequence, externally attached loggers, although not considered invasive, can still have measurable effects on the behaviour and energetics of the tagged animal (e.g. due to placement and media flow effects, [89]). The same is true of implanted physiologging devices, which in addition to the above proofing need to be ‘biocompatible’, that is, manufactured from material that minimizes tissue inflammation. Perhaps the greatest challenge to material development is that unlike in human applications, studies on wild animals need to accommodate an enormous range of species-specific habitats, and have dramatically different skin types (e.g. covered with fur, scales or denticles), locomotion and lifestyles.
The biggest change in the housing of physiologgers could come from the miniaturization or elimination of batteries as the main power source, as described above. In addition, smart materials borrowed from the biomedical and sports industries (e.g. stretch sensitive, conductive or biocompatible materials) could be integrated into harnesses or pads used to attach devices to animals and simultaneously provide sensor functionality. A cutting-edge innovation in this regard is the development of biodegradable electronic devices [90], which operate for a defined period of time before complete degradation into biocompatible products. A proof-of-concept, degradable device has been developed to electrically stimulate nerve regrowth in mice, and remains stable for several days before degradation begins [91].
(d) . Fiscal constraints
A major hurdle for physiologging engineering has been a lack of market opportunity to provide revenue for innovation, development and testing. In comparison, companies in the sports wearables industry developing non-invasive devices to detect biomarkers in sweat, for example, have collaborated with multi-billion dollar market partners such as Gatorade and the US Air Force, who have profit, data harvesting or other motives for fostering opportunities for real-time health and performance monitoring [69]. Uptake of products by large consumer markets dramatically reduces cost through scale production (e.g. ‘roll to roll printing’ [92]) and generates revenue that can be reinvested into further research and development. Notwithstanding that within ecophysiology sample sizes should be kept to the minimum required to robustly test hypotheses and minimize device effects, larger-scale deployments within and across study species will be unable to go ahead until physiologging devices move from being custom-built by individual research groups to being mass-produced at affordable prices.
Large-scale (e.g. philanthropic) investment in animal physiologging is needed to facilitate future physiologging development, particularly by enabling embedded systems engineers to work as an integral part of the design, prototyping, device testing and version development process. In addition, better (open source) sharing of device design and manufacture (following collaboration models such as Arduino and Raspberry Pi; http://thingiverse.com) would enable local and tailored production for individual projects (e.g. device shape and size changes depending on the host animal). At present, engineers at different organizations across the world may simultaneously develop devices for measuring the same variables (e.g. heart rate) in wild animals, without ever having the opportunity to benefit from each other's ideas, prototyping, successes and failures. We envisage a future, open source ‘modular nano-tag’ that can be adapted to host a variety of sensor types and power inputs and can be potted to suit a variety of applications via user-contributed three-dimensional printed designs, democratising production. Translating new developments in consumer technology, such as wireless Qi smartphone battery charging (see above), will require a close partnership between the wildlife science community and the consumer technology industry, perhaps via agriculture and domestic industries, something that does not yet exist to our knowledge. It seems unlikely that wild-animal physiologging will ever be particularly profitable: some well-established biologging device manufacturers for wild-animal research, farming and aquaculture devices have a relatively modest annual revenue of $2 M to $23 M (www.zoominfo.com) compared to several billion dollars for consumer electronic companies. This underscores the need for philanthropy in shaping the future of this important discipline, although we note that supporting animal ecophysiological research could generate significant indirect benefits for manufacturers of human wearables, and others. Alternatively, following radio-astronomy and other fields, the technology developments could be taken over by a ‘national biologging laboratory’. We suggest there is enough technological scope and need, as well as expected scientific transformations, that a centralized and long-term high-tech laboratory should be considered. A drive by the community to describe the translational benefits of physiologging to the study of human physiology and broader ecological concerns may help in this endeavour.
4. Important ethical considerations
The study of animal physiology currently necessitates, in many cases, invasive procedures such as the withdrawal of blood or surgical implantation of physiologging devices, electrodes or cannulas [77,93,94]. The data obtained by such studies are only accurate and useful if animals are not affected by the tagging procedure, devices and/or post-procedure recovery (i.e. have an ‘observer effect’), although this has rarely been assessed to date [6,75–79]. The design of non-invasive physiologgers, deployment procedures (e.g. [95–98]) and remote data download (e.g. using robotics of Remote-control Operated Vehicles [99]) is critical for minimizing tagging effects and must be the field's top research priority. But, until such minimum-impact techniques become widely available (some are currently at proof-of-concept stage), there remains the challenge of ensuring the highest ethical standards in any work using current physiologging technologies. The ‘3Rs’ (replacement, reduction and refinement) are well embedded in laboratory animal research [100], but have yet to be implemented as an obligatory standard for all work, internationally, on wild-animals [101].
First, we believe there is an unacceptable lack of well-developed good-practice guidelines (including evidence-based recommendations for capture, anaesthesia, analgesia, instrumentation and post-operative care) for all but a few wild-animal study systems (see [93,102–106]). Species exhibit different pharmacokinetic and pharmacodynamic responses to anaesthetic drugs, and the morbidity and mortality of veterinary anaesthesia remains orders of magnitude higher than in humans [107]. Animals may also have different responses to foreign-body insertion, which requires careful investigation and adequate mitigation [61]. There remains significant potential for sub-standard practice, despite researchers' best intentions and efforts, which the community must address head-on. A mechanism for sharing practical experience and flagging potential concerns, akin to the processes used for reporting surgical ‘near miss’ incidents [108,109], would help avoid unnecessary repetition of suboptimal protocols, minimize impact on animals and contribute to refining physiological research on wild-animals.
Second, researchers may face the challenge of deciding how to proceed with a study when the technology available is not yet well-tested, and the capture opportunities are few and far between. For example, they may find themselves under pressure to collect data (e.g. if there is only a narrow time window for tagging animals due to the study system's seasonality or logistical constraints) or dealing with novel technologies where the evidence on which to base judgements of risk and benefits may be slim. Our position here is an uncompromising ‘do not tag’, unless all devices and protocols have been thoroughly tested and are known to be safe for deployment on wild-animals (e.g. using preliminary short-term deployments on captive animals in controlled conditions). A clearly defined ‘threshold to proceed’ score, based on cost-benefit assessments of the conservation value of the work and to prevent incremental research (see [110]), or a set of ‘no go’ criteria, could be established. Developed by experts, such criteria can then be adopted by relevant bodies representing the physiologging/biologging community, and employed by researchers, their institutional ethical review boards, and journal editors and reviewers.
We consider the development of a robust framework for the evaluation of all physiologging work essential. In an ideal future, the field will have completely transitioned to using loggers that do not break skin yet are capable of collecting useful data at high resolution, and either transmit the data, remain attached until they can be safely recovered, or degrade. We acknowledge that it may be difficult to collect some desirable physiological variables (such as core body temperature or blood hormones) non-invasively, and that the field is still in a nascent state, but we feel encouraged by recent advances with non-invasive biomedical techniques and see considerable scope for cross-disciplinary exchange.
5. Collaboration between physiologging and biomedicine
Biologging has fast become its own discipline within animal biology, accelerated by advances in sensor technology, miniaturization and analytical tools. Each development step has followed major advancements in human-focused, consumer-driven technologies [111], such as advancements in the cellular phone industry driving those in solid-state technology and power efficiency [83] and the miniaturisation of sensor types (e.g. video cameras). We are now at a point where externally mounted devices are routinely used by biologists for studying wild-animals, and by consumers who want to know the movements and behaviour of their livestock [112–114] and pets [115–117]. However, physiological logging lags behind both biologging of the movement of animals, and human health monitoring by wearables, which have overcome many of the fundamental challenges inherent in physiologging research. Ultimately, the goal of the wearables industry is to develop a multimodal, non-intrusive device capable of continuous measurement of both physiological variables and biomarkers (table 1), calibrated to general and individual trends. The translation of advanced human wearable technology to physiologging will drive forward the next paradigm shift in animal-attached devices, overcoming our own challenges towards what is a shared goal of the two fields.
Many of the greatest biomedical challenges faced by humanity today have been ‘evolutionarily solved’ in the animal kingdom, and there is rich potential for insights from animal ecophysiology research to inform healthcare solutions in the twenty-first century. Globally, the leading cause of human death is cardiovascular disease (including stroke and diabetes) and respiratory illness. Together these account for 29 million deaths annually, [118]), and the leading risk factors are high blood pressure, smoking, hyperglycaemia and obesity. Many species are known to experience chronic hypertension, hyperglycaemia and obesity. For example, birds have approximately double the circulating blood glucose of similarly sized mammals [119] and become seasonally morbidly obese, storing approximately 50–60% body fat, and as much as 150% body fat in some species [2]. The mechanisms by which birds can ameliorate chronic symptoms such as nerve damage, are not yet known, nor is the function of chronic hyperglycaemia across this taxon. Hypoxia is a major factor in conditions such as heart attacks and stroke, and is the final common pathway of many critical illnesses. Effective management of hypoxia in critical care medicine is still considered a challenge [120], and although current practice is generally to treat hypoxia by administering oxygen, hyperoxaemia may cause harm; indeed, ‘permissive hypoxaemia’ is now being used in critical care patients [121]. Diving animals like seals and whales have remarkable tolerance to hypoxia and regularly experience tissue ischemia that may last for tens of minutes during a dive [24,25]. The most remarkable example of this is probably the elephant seal, which spends more than 90% of its time at sea underwater, with mean surface intervals of just two minutes between dives [122]. While diving mammals have adaptations to protect against hypoxaemic damage at almost every step of the oxygen transport cascade [25], none have yet been adopted as therapeutic approaches for hypoxia treatment in human medicine. This may be because hypoxia from diving occurs in healthy subjects, whereas hypoxia in critical care is symptomatic of disease; but it seems possible that research on the former may inspire innovative strategies for tackling the latter.
Other major medical challenges arise from age- and immobilization-related sarcopenia [123,124], and countering muscle mass loss is a major hurdle for very long-duration spaceflight [125]. Of relevance are hibernating bears that can maintain lean body mass during this period of extreme inactivity and fasting, potentially using as-yet undescribed circulating serum compounds that have been shown to inhibit proteolysis in human muscle cells in vitro [126]. In addition, king penguins are able to preserve prey captured at sea to feed their chick after winter incubation (thus storing food for several weeks). An antimicrobial peptide that is efficient against Staphylococcus aureus was found in the stomachs of free-ranging penguins that likely helps to preserve undigested food during incubation [127]. The medical relevance of uncovering the strategies with which wild-animals can thrive in periods of extreme inactivity is clear, and data collection via physiologging provides a rich information source for medical innovation, not least because animals can be studied at liberty, under natural conditions. Presently, remote blood sampling devices (for example, that could collect circulating serum samples) have been developed for diving seals [94], and with further refinement could yield considerable gains for discovering the temporal variation in such compounds, as well as the promise of wearable analytical devices [27].
A striking recent example of potential for medical innovation via knowledge exchange is the emergence of the highly infectious novel coronavirus SARS-CoV-2 (COVID-19) in 2019 [128]. It has become clear that, in response to the virus, the host immune system can become dysregulated and release excess cytokines (small signalling proteins) in a ‘storm’, leading to overwhelming systemic inflammation, ultimately resulting in organ failure and death [129]. By contrast, birds and bats serve as viral reservoirs (and bats may be the original source of the SARS-CoV-2 virus; [130]), and yet bats rarely display clinical symptoms of infection (reviewed in [131]). Bats have many of the same immunological features as other mammals, but when infected, mount an immune response that restricts viral replication without triggering uncontrolled inflammatory gene expression, avoiding cytokine storms [132]. The ultimate driver for this adaptation may be to minimize oxidative stress produced during flight [133], and enables bats to live approximately three to four times longer than similar-sized non-flying mammals [134]. The physiological study of these highly adapted animals is not complete, and it remains limited by a lack of suitable tools as highlighted throughout this article. But the study of wild-animal physiology can clearly offer medicine insights into pathways and strategies to ameliorate the impact of many of the biggest public health challenges, perhaps even those we may face with rising CO2 levels and climate change (e.g. [135]).
6. The future of physiologging for ecophysiology
Over the past several decades, physiologists and ecologists alike have realized the inseparable linkages between processes that occur within an organism's internal and external environment. The local environment can not only influence the state of an animal's internal physiology but also in turn forms a feedback loop that determines how an organism responds to the environment in the future [136]. A range of physiological responses that result in increased fitness or survival have allowed animal life to conquer nearly all habitats on our planet, no matter how inhospitable [6,137–139]. However, in the face of threats such as climate change [140], the links between physiological responses and the environment may become mismatched, impacting effectiveness of future management. Thus, it is important to understand the linkage between physiology and space use, survival and fitness for conservation strategies to be successful [141]. Understanding physiological response within their environmental context is vital if we are to predict species response to the changing climate [136,142,143], quantify physiological stress caused by anthropogenic infrastructure (e.g. heart rate and roads, sleep and light pollution, and renewable energy structures [144,145]) or avoid direct negative impact in fishing and management policies with maximum net soak times to prevent drowning of bycatch species [146], to list but a few examples.
Success in biologging and data sharing has significantly enhanced our understanding of global trends, noteworthy meta-studies of the movement patterns and space-use of megafauna [147], of behavioural response to the anthropocene [148] and more recently the COVID-19 anthropause [149]. However, the addition of physiological data to the positional and behavioural data of biologging will increase our ability to predict behavioural response to external factors greatly. Underlying physiological processes are underappreciated in ecological studies, despite their known importance. In a rapidly changing world the development of physiologging will prove invaluable.
7. Concluding remarks
There exists unrealized potential for ecophysiologists to overcome the limitations of current physiologging technology by capitalising on the research efforts and developments of human wearables for biomedical science. Fostering collaboration between the two fields, it will be possible to design non-invasive devices needed to realize the full potential of physiologging, responsibly, while advancing our understanding of human health.
Appendix A
Data accessibility
This article has no additional data.
Authors' contributions
L.A.H. commissioned the original idea, all authors contributed to development of the ideas and drafting the manuscript, H.J.W. led the writing and figure development.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Bishop CM, et al. 2015. The roller coaster flight strategy of bar-headed geese conserves energy during Himalayan migrations. Science 647, 250-254. ( 10.1126/science.1258732) [DOI] [PubMed] [Google Scholar]
- 2.Guglielmo CG. 2018. Obese super athletes: fat-fueled migration in birds and bats. J. Exp. Biol. 221(Suppl. 1), 165753. ( 10.1242/jeb.165753) [DOI] [PubMed] [Google Scholar]
- 3.Dick MF, Guglielmo CG. 2019. Flight muscle protein damage during endurance flight is related to energy expenditure but not dietary polyunsaturated fatty acids in a migratory bird. J. Exp. Biol. 222, 187708. ( 10.1242/jeb.187708) [DOI] [PubMed] [Google Scholar]
- 4.Meir JU, York JM, Chua BA, Jardine W, Hawkes LA, Milsom WK. 2019. Reduced metabolism supports hypoxic flight in the high-flying bar-headed goose (Anser indicus). Elife 8, e44986. ( 10.7554/eLife.44986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilmers CC, Nickel B, Bryce CM, Smith JA, Wheat RE, Yovovich V. 2015. The golden age of bio-logging: how animal-borne sensors are advancing the frontiers of ecology. Ecology 96, 1741-1753. ( 10.1890/14-1401.1) [DOI] [PubMed] [Google Scholar]
- 6.Andrews RD, Enstipp MR. 2016. Diving physiology of seabirds and marine mammals: relevance, challenges and some solutions for field studies. Comp. Biochem. Physiol. A 202, 38-52. ( 10.1016/j.cbpa.2016.07.004) [DOI] [PubMed] [Google Scholar]
- 7.Eliassen E. 1963. Preliminary results from new methods of investigating the physiology of birds during flight. Ibis 105, 234-237. ( 10.1111/j.1474-919X.1963.tb02497.x) [DOI] [Google Scholar]
- 8.Schaeffer PJ, Wikelski M. 2004. Daily energy use and oxidative capacity in tropical and temperate zone robins. Comp. Biochem. Physiol. A 148, S31. ( 10.1016/j.cbpa.2007.06.078) [DOI] [Google Scholar]
- 9.Steiger SS, Kelley JP, Cochran WW, Wikelski M. 2009. Low metabolism and inactive lifestyle of a tropical rain forest bird investigated via heart-rate telemetry. Physiol. Biochem. Zool. 82, 580-589. ( 10.1086/605336) [DOI] [PubMed] [Google Scholar]
- 10.Barske J, Fusani L, Wikelski M, Schlinger B. 2011. Heart rate as an index of increased metabolic output in a bird with a complex courtship display. Integr. Comp. Biol. 51(Suppl. 1), e1-e157. ( 10.1093/icb/icr008) [DOI] [Google Scholar]
- 11.Cornelius JM, Hahn TP, Hunt KE, Wikelski M. 2010. Energetic expenditure in free-living red crossbills, Loxia curvirostra, using heart rate telemetry. Integr. Comp. Biol. 50, E218. [Google Scholar]
- 12.O'Mara MT, Rikker S, Wikelski M, Ter Maat A, Pollock HS, Dechmann DKN. 2021. Heart rate reveals torpor at high body temperatures in lowland tropical free-tailed bats. R. Soc. Open Sci. 4, 171359. ( 10.1098/rsos.171359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowlin MS, Wikelski MC. 2006. Calibration of heart rate and energy expenditure during flight and at rest in a passerine. Integr. Comp. Biol. 46, E14. [Google Scholar]
- 14.Bowlin MS, Wikelski MC, Cochran WW. 2006. Heart rate and wingbeat frequency in naturally-migrating Swainson's thrushes. J. Ornithol. 147, 142. [Google Scholar]
- 15.Bowlin MS, Wikelski MC, Cochran WW. 2004. The relationship between individual morphology, atmospheric conditions, and inter-individual variation in heart rate and wingbeat frequency during natural migration in the Swainsons thrush (Catharus ustulatus). Integr. Comp. Biol. 44, 529. [Google Scholar]
- 16.Sapir N, Wikelski M, McCue MD, Pinshow B, Nathan R. 2010. Flight modes in migrating European bee-eaters: heart rate may indicate low metabolic rate during soaring and gliding. PLoS ONE 5, e13956. ( 10.1371/journal.pone.0013956) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levett DZH, Martin DS, Wilson MH, Mitchell K, Dhillon S, Rigat F, Montgomery HE, Mythen MG, Grocott MPW. 2010. Design and conduct of Caudwell Xtreme Everest: an observational cohort study of variation in human adaptation to progressive environmental hypoxia. BMC Med. Res. Methodol. 10, 98. ( 10.1186/1471-2288-10-98) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sempionatto JR, et al. 2021. An epidermal patch for the simultaneous monitoring of haemodynamic and metabolic biomarkers. Nat. Biomed. Eng. ( 10.1038/s41551-021-00685-1) [DOI] [PubMed] [Google Scholar]
- 19.Payne ME, Zamarayeva A, Pister VI, Yamamoto NAD, Printed AA. 2019. Flexible lactate sensors: design considerations before performing on-body measurements. Sci. Rep. 9, 13720. ( 10.1038/s41598-019-49689-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hedenström A, Lindström Å. 2017. Wind tunnel as a tool in bird migration research. J. Avian Biol. 48, 37-48. ( 10.1111/jav.01363) [DOI] [Google Scholar]
- 21.Jones PE, Svendsen JC, Börger L, Champneys T, Consuegra S, Jones JAH, Cooke S. 2020. One size does not fit all: inter- and intraspecific variation in the swimming performance of contrasting freshwater fish. Conserv. Physiol. 8, coaa126. ( 10.1093/conphys/coaa126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dailey RE, Smith K, Fontaine C, Jia Y, Avery JP. 2020. Response of metabolic hormones and blood metabolites to realimentation in rehabilitated harbor seal (Phoca vitulina) pups. J. Comp. Physiol. B 190, 629-640. ( 10.1007/s00360-020-01290-5) [DOI] [PubMed] [Google Scholar]
- 23.Ward S, Bishop CM, Woakes AJ, Butler PJ. 2002. Heart rate and the rate of oxygen consumption of flying and walking barnacle geese (Branta leucopsis) and bar-headed geese (Anser indicus). J. Exp. Biol. 205, 3347-3356. ( 10.1242/jeb.205.21.3347) [DOI] [PubMed] [Google Scholar]
- 24.Ponganis PJ. 2019. State of the art review: from the seaside to the bedside: insights from comparative diving physiology into respiratory, sleep and critical care. Thorax 74, 512-518. ( 10.1136/thoraxjnl-2018-212136) [DOI] [PubMed] [Google Scholar]
- 25.Williams TM, Davis RW. 2021. Physiological resiliency in diving mammals: insights on hypoxia protection using the Krogh principle to understand COVID-19 symptoms. Comp. Biochem. Physiol. A 253, 110849. ( 10.1016/j.cbpa.2020.110849) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams HJ, Shipley JR, Rutz C, Wikelski M, Wilkes M, Hawkes LA. 2021. Future trends in measuring physiology in free-living animals. Phil. Trans. R. Soc B 376, 20200230. ( 10.1098/rstb.2020.0230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macdonald A, Hawkes LA, Corrigan DK. 2021. Recent advances in biomedical, biosensor and clinical measurement devices for use in humans and the potential application of these technologies for the study of physiology and disease in wild animals. Phil. Trans. R. Soc. B 376, 20200228. ( 10.1098/rstb.2020.0228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawkes LA, Fahlman A, Sato K. 2021. What is physiologging? Introduction to the theme issue, part 2. Phil. Trans. R. Soc. B 376, 20210028. ( 10.1098/rstb.2021.0028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams C, Ponganis P. 2021. Diving physiology of marine mammals and birds: the development of biologging techniques. Phil. Trans. R. Soc. B 376, 20200211. ( 10.1098/rstb.2020.0211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kooyman GL, McDonald BI, Williams CL, Meir JU, Ponganis PJ. 2021. The aerobic dive limit: after 40 years, still rarely measured but commonly used. Comp. Biochem. Physiol. A 252, 110841. ( 10.1016/j.cbpa.2020.110841) [DOI] [PubMed] [Google Scholar]
- 31.Kooyman GL. 1965. Techniques used in measuring diving capacities of Weddell seals. Polar Rec. 12, 391-394. ( 10.1017/S003224740005484X) [DOI] [Google Scholar]
- 32.Rutz C, Hays GC. 2009. New frontiers in biologging science. Biol. Lett. 5, 289-292. ( 10.1098/rsbl.2009.0089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams HJ, et al. 2020. Optimizing the use of biologgers for movement ecology research. J. Anim. Ecol. 89, 186-206. ( 10.1111/1365-2656.13094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kays R, Crofoot MC, Jetz W, Wikelski M. 2015. Terrestrial animal tracking as an eye on life and planet. Science 348, aaa2478. ( 10.1126/science.aaa2478) [DOI] [PubMed] [Google Scholar]
- 35.Hussey NE, et al. 2015. Aquatic animal telemetry: a panoramic window into the underwater world. Science 348, 1255642. ( 10.1126/science.1255642) [DOI] [PubMed] [Google Scholar]
- 36.Wilson RP, Shepard ELC, Liebsch N. 2008. Prying into the intimate details of animal lives: use of a daily diary on animals. Endanger Species Res. 4, 123-137. ( 10.3354/esr00064) [DOI] [Google Scholar]
- 37.Noda T, Kawabata Y, Arai N, Mitamura H, Watanabe S. 2014. Animal-mounted gyroscope/accelerometer/magnetometer: in situ measurement of the movement performance of fast-start behaviour in fish. J. Exp. Mar. Biol. Ecol. 451, 55-68. ( 10.1016/j.jembe.2013.10.031) [DOI] [Google Scholar]
- 38.Wilson RP, et al. 2020. An ‘orientation sphere’ visualization for examining animal head movements. Ecol. Evol. 10, 4291-4302. ( 10.1002/ece3.6197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams HJ, et al. 2017. Identification of animal movement patterns using tri-axial magnetometry. Mov. Ecol. 5, 6. ( 10.1186/s40462-017-0097-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watanabe YY, Takahashi A. 2013. Linking animal-borne video to accelerometers reveals prey capture variability. Proc. Natl Acad. Sci. USA 110, 2199-2204. ( 10.1073/pnas.1216244110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rutz C, Bluff LA, Weir AAS, Kacelnik A. 2007. Video cameras on wild birds. Science 318, 765. ( 10.1126/science.1146788) [DOI] [PubMed] [Google Scholar]
- 42.Yoshino K, Takahashi A, Adachi T, Costa DP, Robinson PW, Peterson SH, Hückstädt LA, Holser RR, Naito Y. 2020. Acceleration-triggered animal-borne videos show a dominance of fish in the diet of female northern elephant seals. J. Exp. Biol. 223, jeb212936. ( 10.1242/jeb.212936) [DOI] [PubMed] [Google Scholar]
- 43.Brijs J, Sandblom E, Axelsson M, Sundell K, Sundh H, Kiessling A, Berg C, Gräns A. et al. 2019. Remote physiological monitoring provides unique insights on the cardiovascular performance and stress responses of freely swimming rainbow trout in aquaculture. Sci. Rep. 9, 9090. ( 10.1038/s41598-019-45657-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams CL, Czapanskiy MF, John JS, St. Leger J, Scadeng M, Ponganis PJ. 2020. Cervical air sac oxygen profiles in diving emperor penguins: parabronchial ventilation and the respiratory oxygen store. J. Exp. Biol. 224, 230219. ( 10.1242/jeb.230219) [DOI] [PubMed] [Google Scholar]
- 45.McKnight JC, et al. 2021. When the human brain goes diving: using near-infrared spectroscopy to measure cerebral and systemic cardiovascular responses to deep, breath-hold diving in elite freedivers. Phil. Trans. R. Soc. B 376, 20200349. ( 10.1098/rstb.2020.0349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vyssotski AL, Serkov AN, Itskov PM, Dell'Omo G, Latanov AV, Wolfer DP, Lipp H-P. 2006. Miniature neurologgers for flying pigeons: multichannel EEG and action and field potentials in combination with GPS recording. J. Neurophysiol. 95, 1263-1273. ( 10.1152/jn.00879.2005) [DOI] [PubMed] [Google Scholar]
- 47.Aulsebrook AE, Connelly F, Johnsson RD, Jones TM, Mulder RA, Hall ML, Vyssotski AL, Lesku JA. 2020. White and amber light at night disrupt sleep physiology in birds. Curr. Biol. 30, 3657-3663.e5. ( 10.1016/j.cub.2020.06.085) [DOI] [PubMed] [Google Scholar]
- 48.Rattenborg NC, Voirin B, Cruz SM, Tisdale R, Dell'Omo G, Lipp H-P, Wikelski M, Vyssotski AL. 2016. Evidence that birds sleep in mid-flight. Nat. Commun. 7, 12468. ( 10.1038/ncomms12468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Hasselt SJ, Rusche M, Vyssotski AL, Verhulst S, Rattenborg NC, Meerlo P. 2020. The European starling (Sturnus vulgaris) shows signs of NREM sleep homeostasis but has very little REM sleep and no REM sleep homeostasis. Sleep 43, zsz311. ( 10.1093/sleep/zsz311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guillemette M, Woakes AJ, Larochelle J, Polymeropoulos ET, Granbois J-M, Butler PJ, Pelletier D, Frappell PB, Portugal SJ. 2016. Does hyperthermia constrain flight duration in a short-distance migrant? Phil. Trans. R. Soc. B 371, 20150386. ( 10.1098/rstb.2015.0386) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perentos N, Nicol AU, Martins AQ, Stewart JE, Taylor P, Morton AJ. 2017. Techniques for chronic monitoring of brain activity in freely moving sheep using wireless EEG recording. J. Neurosci. Methods 279, 87-100. ( 10.1016/j.jneumeth.2016.11.010) [DOI] [PubMed] [Google Scholar]
- 52.Ditmer MA, Werden LK, Tanner JC, Vincent JB, Callahan P, Iaizzo PA, Laske TG, Garshelis DL. 2020. Bears habituate to the repeated exposure of a novel stimulus, unmanned aircraft systems. Conserv. Physiol. 7, coy067. ( 10.1093/conphys/coy067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brijs J, Føre M, Gräns A, Clark T, Axelsson M, Johansen J. 2021. Bio-sensing technologies in aquaculture: how remote monitoring can bring us closer to our farm animals. Phil. Trans. R. Soc. B 376, 20200219. ( 10.1098/rstb.2020.0219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McKnight JC, et al. 2019. Shining new light on mammalian diving physiology using wearable near-infrared spectroscopy. PLoS Biol. 17, e3000306. ( 10.1371/journal.pbio.3000306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Green JA, Boyd IL, Woakes AJ, Green CJ, Butler PJ. 2005. Do seasonal changes in metabolic rate facilitate changes in diving behaviour? J. Exp. Biol. 208, 2581-2593. ( 10.1242/jeb.01679) [DOI] [PubMed] [Google Scholar]
- 56.Green J, Boyd I, Woakes A, Warren N, Butler P. 2005. Behavioural flexibility during year-round foraging in macaroni penguins. Mar. Ecol. Prog. Ser. 296, 183-196. ( 10.3354/meps296183) [DOI] [Google Scholar]
- 57.Grémillet D, Kuntz G, Gilbert C, Woakes AJ, Butler PJ, Le Maho Y. 2005. Cormorants dive through the Polar night. Biol. Lett. 1, 469-471. ( 10.1098/rsbl.2005.0356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grémillet D, Kuntz G, Woakes AJ, Gilbert C, Robin J-P, Le Maho Y, Butler PJ. 2005. Year-round recordings of behavioural and physiological parameters reveal the survival strategy of a poorly insulated diving endotherm during the Arctic winter. J. Exp. Biol. 208, 4231-4241. ( 10.1242/jeb.01884) [DOI] [PubMed] [Google Scholar]
- 59.Evans AL, Singh NJ, Friebe A, Arnemo JM, Laske TG, Fröbert O, Swenson JE, Blanc S. 2016. Drivers of hibernation in the brown bear. Front. Zool. 13, 7. ( 10.1186/s12983-016-0140-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Streicher S, Lutermann H, Bennett NC, Bertelsen MF, Mohammed OB, Manger PR, Scantlebury M, Ismael K, Alagaili AN. 2017. Living on the edge: daily, seasonal and annual body temperature patterns of Arabian oryx in Saudi Arabia. PLoS ONE 12, e0180269. ( 10.1371/journal.pone.0180269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Forin-Wiart M-A, Enstipp MR, Le Maho Y, Handrich Y. 2019. Why implantation of bio-loggers may improve our understanding of how animals cope within their natural environment. Integr. Zool. 14, 48-64. ( 10.1111/1749-4877.12364) [DOI] [PubMed] [Google Scholar]
- 62.Zhang Y, et al. 2019. Battery-free, lightweight, injectable microsystem for in vivo wireless pharmacology and optogenetics. Proc. Natl Acad. Sci. USA 116, 21 427-21 437. ( 10.1073/pnas.1909850116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonter DN, Bridge ES. 2011. Applications of radio frequency identification (RFID) in ornithological research: a review. J. Field Ornithol. 82, 1-10. ( 10.1111/j.1557-9263.2010.00302.x) [DOI] [Google Scholar]
- 64.St. Clair JJ, Burns ZT, Bettaney EM, Morrissey MB, Otis B, Ryder TB, Fleischer RC, James R, Rutz C. 2015. Experimental resource pulses influence social-network dynamics and the potential for information flow in tool-using crows. Nat. Commun. 6, 7197. ( 10.1038/ncomms8197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bandodkar AJ, Jia W, Yardımcı C, Wang X, Ramirez J, Wang J. 2015. Tattoo-based noninvasive glucose monitoring: a proof-of-concept study. Anal. Chem. 87, 394-398. ( 10.1021/ac504300n) [DOI] [PubMed] [Google Scholar]
- 66.Yetisen AK, et al. 2019. Dermal tattoo biosensors for colorimetric metabolite detection. Angew. Chemie 131, 10 616-10 623. ( 10.1002/ange.201904416) [DOI] [PubMed] [Google Scholar]
- 67.Castaneda D, Esparza A, Ghamari M, Soltanpur C, Nazeran H. 2018. A review on wearable photoplethysmography sensors and their potential future applications in health care. Int. J. Biosens. Bioelectron. 4, 195-202. ( 10.15406/ijbsbe.2018.04.00125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ahmad Tarar A, Mohammad U, Srivastava SK. 2020. Wearable skin sensors and their challenges: a review of transdermal, optical, and mechanical sensors. Biosensors 10, 56. ( 10.3390/bios10060056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seshadri DR, Li RT, Voos JE, Rowbottom JR, Alfes CM, Zorman CA, Drummond CK. 2019. Wearable sensors for monitoring the physiological and biochemical profile of the athlete. npj Digit. Med. 2, 72. ( 10.1038/s41746-019-0150-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chung M, Fortunato G, Radacsi N. 2019. Wearable flexible sweat sensors for healthcare monitoring: a review. J. R Soc. Interface 16, 20190217. ( 10.1098/rsif.2019.0217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bent B, Goldstein BA, Kibbe WA, Dunn JP. 2020. Investigating sources of inaccuracy in wearable optical heart rate sensors. npj Digit. Med. 3, 18. ( 10.1038/s41746-020-0226-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu H, Allen J, Zheng D, Chen F. 2019. Recent development of respiratory rate measurement technologies. Physiol. Meas. 40, 07TR01. ( 10.1088/1361-6579/ab299e) [DOI] [PubMed] [Google Scholar]
- 73.Niven DJ, Gaudet JE, Laupland KB, Mrklas KJ, Roberts DJ, Stelfox HT. 2015. Accuracy of peripheral thermometers for estimating temperature. Ann. Intern. Med. 163, 768-777. ( 10.7326/M15-1150) [DOI] [PubMed] [Google Scholar]
- 74.Rastegar S, Gholam Hosseini H, Lowe A. 2020. Non-invasive continuous blood pressure monitoring systems: current and proposed technology issues and challenges. Phys. Eng. Sci. Med. 43, 11-28. ( 10.1007/s13246-019-00813-x) [DOI] [PubMed] [Google Scholar]
- 75.Barron DG, Brawn JD, Weatherhead PJ. 2010. Meta-analysis of transmitter effects on avian behaviour and ecology. Methods Ecol. Evol. 1, 180-187. ( 10.1111/j.2041-210X.2010.00013.x) [DOI] [Google Scholar]
- 76.Walker KA, Trites AW, Haulena M, Weary DM. 2012. A review of the effects of different marking and tagging techniques on marine mammals. Wildl. Res. 39, 15-30. ( 10.1071/WR10177) [DOI] [Google Scholar]
- 77.White CR, Cassey P, Schimpf NG, Halsey LG, Green JA, Portugal SJ. 2013. Implantation reduces the negative effects of bio-logging devices on birds. J. Exp. Biol. 216, 537-542. ( 10.1242/jeb.076554) [DOI] [PubMed] [Google Scholar]
- 78.Bodey TW, Cleasby IR, Bell F, Parr N, Schultz A, Votier SC, Bearhop S. 2018. A phylogenetically controlled meta-analysis of biologging device effects on birds: deleterious effects and a call for more standardized reporting of study data. Methods Ecol. Evol. 9, 946-955. ( 10.1111/2041-210X.12934) [DOI] [Google Scholar]
- 79.Portugal SJ, White CR. 2018. Miniaturization of biologgers is not alleviating the 5% rule. Methods Ecol. Evol. 9, 1662-1666. ( 10.1111/2041-210X.13013) [DOI] [Google Scholar]
- 80.Blackburn TM, Gaston KJ. 1994. The distribution of body sizes of the World's bird species. Oikos 70, 127-130. ( 10.2307/3545707) [DOI] [Google Scholar]
- 81.Barroso LA, Hölzle U. 2007. The case for energy-proportional computing. IEEE Computer 40, 33-37. [Google Scholar]
- 82.Koomey JG. 2015. A primer on the energy efficiency of computing. In AIP Conf. Proc., vol. 1652, pp. 82-1659. [Google Scholar]
- 83.Holton MD, Wilson RP, Teilmann J, Siebert U. 2021. Animal tag technology keeps coming of age: an engineering perspective. Phil. Trans. R. Soc. B 376, 20200229. ( 10.1098/rstb.2020.0229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shafer MW, MacCurdy R, Shipley JR, Winkler D, Guglielmo CG, Garcia E. 2015. The case for energy harvesting on wildlife in flight. Smart Mater. Struct. 24, 025031. ( 10.1088/0964-1726/24/2/025031) [DOI] [Google Scholar]
- 85.Shafer MW, Hahn G, Morgan E. 2015. A hydrostatic pressure-cycle energy harvester. In Proc. SPIE Active and passive smart structures and integrated systems, 94310F. ( 10.1117/12.2084279) [DOI] [Google Scholar]
- 86.Bäumker E, Beck P, Woias P. 2020. Thermoelectric harvesting using warm-blooded animals in wildlife tracking applications. Energies 13, 2769. ( 10.3390/en13112769) [DOI] [Google Scholar]
- 87.Scholander PF. 1940. Experimental investigations on the respiratory function in diving mammals and birds. Hvalrådets Skr. 22, 1-131. [Google Scholar]
- 88.Andrady AL, Neal MA. 2009. Applications and societal benefits of plastics. Phil. Trans. R. Soc. B 364, 1977-1984. ( 10.1098/rstb.2008.0304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kay WP, et al. 2019. Minimizing the impact of biologging devices: using computational fluid dynamics for optimizing tag design and positioning. Methods Ecol. Evol. 10, 1222-1233. ( 10.1111/2041-210X.13216) [DOI] [Google Scholar]
- 90.Li C, et al. 2020. Design of biodegradable, implantable devices towards clinical translation. Nat. Rev. Mater. 5, 61-81. ( 10.1038/s41578-019-0150-z) [DOI] [Google Scholar]
- 91.Koo J, et al. 2018. Wireless bioresorbable electronic system enables sustained nonpharmacological neuroregenerative therapy. Nat. Med. 24, 1830-1836. ( 10.1038/s41591-018-0196-2) [DOI] [PubMed] [Google Scholar]
- 92.Zhou X, Xu H, Cheng J, Zhao N, Chen S-C. 2015. Flexure-based roll-to-roll platform: a practical solution for realizing large-area microcontact printing. Sci. Rep. 5, 10402. ( 10.1038/srep10402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cooke SJ, Wagner GN. 2004. Training, experience, and opinions of researchers who use surgical techniques to implant telemetry devices into fish. Fisheries 29, 10-18. ( 10.1577/1548-8446(2004)29[10:TEAOOR]2.0.CO;2) [DOI] [Google Scholar]
- 94.Takei Y, Suzuki I, Wong MKS, Milne R, Moss S, Sato K, Hall A. 2016. Development of an animal-borne blood sample collection device and its deployment for the determination of cardiovascular and stress hormones in phocid seals. Am. J. Physiol. Reg. Integr. Comp. Physiol. 311, R788-R796. ( 10.1152/ajpregu.00211.2016) [DOI] [PubMed] [Google Scholar]
- 95.Nassar JM, Khan SM, Velling SJ, Diaz-Gaxiola A, Shaikh SF, Geraldi NR, Torres Sevilla GA, Duarte CM, Hussain MM. 2018. Compliant lightweight non-invasive standalone ‘Marine Skin’ tagging system. npj Flex. Electron. 2, 13. ( 10.1038/s41528-018-0025-1) [DOI] [Google Scholar]
- 96.Lee MA, et al. 2019. Implanted nanosensors in marine organisms for physiological biologging: design, feasibility, and species variability. ACS Sensors 4, 32-43. ( 10.1021/acssensors.8b00538) [DOI] [PubMed] [Google Scholar]
- 97.Sakamoto K, Miyayama M, Kinoshita C, Fukuoka T, Ishihara T, Sato K. 2021. A non-invasive system to measure heart rate in hard-shelled sea turtles: potential for field applications. Phil. Trans. R. Soc. B 376, 20200222. ( 10.1098/rstb.2020.0222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aoki K, Watanabe Y, Inamori D, Funasaka N, Sakamoto KQ. 2021. Towards non-invasive heart rate monitoring in free-ranging cetaceans: a unipolar suction cup tag measured the heart rate of trained Risso's dolphins. Phil. Trans. R. Soc. B 376, 20200225. ( 10.1098/rstb.2020.0225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Le Maho Y, et al. 2014. Rovers minimize human disturbance in research on wild animals. Nat. Methods 11, 1242-1244. ( 10.1038/nmeth.3173) [DOI] [PubMed] [Google Scholar]
- 100.Sneddon LU, Halsey LG, Bury NR. 2017. Considering aspects of the 3Rs principles within experimental animal biology. J. Exp. Biol. 220, 3007-3016. ( 10.1242/jeb.147058) [DOI] [PubMed] [Google Scholar]
- 101.Lindsjö J, Fahlman Å, Törnqvist E. 2016. Animal welfare from mouse to moose: implementing the principles of the 3Rs in wildlife research. J. Wildl. Dis. 52, 65-77. ( 10.7589/52.2S.S65) [DOI] [PubMed] [Google Scholar]
- 102.Reese Robillard MM, Payne LM, Vega RR, Stunz GW. 2015. Best practices for surgically implanting acoustic transmitters in spotted seatrout. Trans. Am. Fish Soc. 144, 81-88. ( 10.1080/00028487.2014.965343) [DOI] [Google Scholar]
- 103.Horning M, et al. 2017. Best practice recommendations for the use of fully implanted telemetry devices in pinnipeds. Anim. Biotelemetry 5, 13. ( 10.1186/s40317-017-0128-9) [DOI] [Google Scholar]
- 104.Horning M, et al. 2019. Best practice recommendations for the use of external telemetry devices on pinnipeds. Anim. Biotelemetry 7, 20. ( 10.1186/s40317-019-0182-6) [DOI] [Google Scholar]
- 105.O'Mara MT, Wikelski M, Dechmann DKN. 2014. 50 years of bat tracking: device attachment and future directions. Methods Ecol. Evol. 5, 311-319. ( 10.1111/2041-210X.12172) [DOI] [Google Scholar]
- 106.Andrews RD, et al. 2019. Best practice guidelines for cetacean tagging. J. Cetacean Res. Manage. 20, 27-66. ( 10.47536/jcrm.v20i1.237) [DOI] [Google Scholar]
- 107.Carter J, Story DA. 2013. Veterinary and human anaesthesia: an overview of some parallels and contrasts. Anaesth. Intensive Care 41, 710-718. ( 10.1177/0310057X1304100605) [DOI] [PubMed] [Google Scholar]
- 108.Barach P, Small SD. 2000. Reporting and preventing medical mishaps: lessons from non-medical near miss reporting systems. BMJ 320, 759 LP-759763. ( 10.1136/bmj.320.7237.759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hossain T, Hossain N. 2015. Incident reporting in surgery: a review of the literature. Int. Surg J. 2, 157-160. ( 10.5455/2349-2902.ISJ20150506) [DOI] [Google Scholar]
- 110.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. 1985. APACHE II: a severity of disease classification system. Crit. Care Med. 13, 818-829. ( 10.1097/00003246-198510000-00009) [DOI] [PubMed] [Google Scholar]
- 111.Fehlmann G, King AJ. 2016. Bio-logging. Curr. Biol. 26, 830-831. ( 10.1016/j.cub.2016.05.033) [DOI] [PubMed] [Google Scholar]
- 112.Føre M, et al. 2018. Precision fish farming: a new framework to improve production in aquaculture. Biosyst. Eng. 173, 176-193. ( 10.1016/j.biosystemseng.2017.10.014) [DOI] [Google Scholar]
- 113.Vázquez Diosdado JA, Barker ZE, Hodges HR, Amory JR, Croft DP, Bell NJ, Codling EA. 2015. Classification of behaviour in housed dairy cows using an accelerometer-based activity monitoring system. Anim. Biotelemetry 3, 15. ( 10.1186/s40317-015-0045-8) [DOI] [Google Scholar]
- 114.Bailey DW, Trotter MG, Knight CW, Thomas MG. 2018. Use of GPS tracking collars and accelerometers for rangeland livestock production research. Transl. Anim. Sci. 2, 81-88. ( 10.1093/tas/txx006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zamansky A, van der Linden D, Hadar I, Bleuer-Elsner S. 2019. Log my dog: perceived impact of dog activity tracking. IEEE Computer 52, 35-43. ( 10.1109/mc.2018.2889637) [DOI] [Google Scholar]
- 116.Jones S, Dowling-Guyer S, Patronek GJ, Marder AR, Segurson D'Arpino S, McCobb E. 2014. Use of accelerometers to measure stress levels in shelter dogs. J. Appl. Anim. Welf. Sci. 17, 18-28. ( 10.1080/10888705.2014.856241) [DOI] [PubMed] [Google Scholar]
- 117.Admela J, Masip-Bruin X, Amla N. 2017. Smart computing and sensing technologies for animal welfare: a systematic review. Assoc. Comput. Mach. 50, 1-27. ( 10.1145/3041960) [DOI] [Google Scholar]
- 118.Institute for Health Metrics and Evaluation (IHME). 2018. Seattle US. Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2017 (GBD 2017) Results. http://ghdx.healthdata.org/gbd-results-tool.
- 119.Braun EJ, Sweazea KL. 2008. Glucose regulation in birds. Comp. Biochem. Physiol. B 151, 1-9. ( 10.1016/j.cbpb.2008.05.007) [DOI] [PubMed] [Google Scholar]
- 120.Martin DS, Grocott MPW. 2013. Oxygen therapy in critical illness: precise control of arterial oxygenation and permissive hypoxemia. Crit. Care Med. 41, 423-432. ( 10.1097/ccm.0b013e31826a44f6) [DOI] [PubMed] [Google Scholar]
- 121.McKenna HT, Murray AJ, Martin DS. 2020. Human adaptation to hypoxia in critical illness. J. Appl. Physiol. 129, 656-663. ( 10.1152/japplphysiol.00818.2019) [DOI] [PubMed] [Google Scholar]
- 122.Robinson PW, et al. 2012. Foraging behavior and success of a mesopelagic predator in the northeast Pacific Ocean: insights from a data-rich species, the northern elephant seal. PLoS ONE 7, e36728. ( 10.1371/journal.pone.0036728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, Kirkland JL, Sandri M. 2018. Sarcopenia: aging-related loss of muscle mass and function. Physiol. Rev. 99, 427-511. ( 10.1152/physrev.00061.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rundfeldt LC, Gunga HC, Steinach M. 2019. Anabolic signaling and response in sarcopenia as a model for microgravity induced muscle deconditioning: a systematic review. REACH 13, 100025. ( 10.1016/j.reach.2019.100025) [DOI] [Google Scholar]
- 125.Nordeen CA, Martin SL. 2019. Engineering human stasis for long-duration spaceflight. Physiology 34, 101-111. ( 10.1152/physiol.00046.2018) [DOI] [PubMed] [Google Scholar]
- 126.Chanon S, et al. 2018. Proteolysis inhibition by hibernating bear serum leads to increased protein content in human muscle cells. Sci. Rep. 8, 5525. ( 10.1038/s41598-018-23891-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Thouzeau C, Le Maho Y, Froget G, Sabatier L, Le Bohec C, Hoffmann JA, Bulet P. 2003. Spheniscins, avian β-defensins in preserved stomach contents of the king penguin, Aptenodytes patagonicus. J. Biol. Chem. 278, 51 053-51 058. ( 10.1074/jbc.M306839200) [DOI] [PubMed] [Google Scholar]
- 128.Zhu N, et al. 2020. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 382, 727-733. ( 10.1056/NEJMoa2001017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Machhi J, et al. 2020. The natural history, pathobiology, and clinical manifestations of SARS-CoV-2 infections. J. Neuroimmune Pharmacol. 15, 359-386. ( 10.1007/s11481-020-09944-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nabi G, Wang Y, Lü L, Jiang C, Ahmad S, Wu Y, Li D. 2021. Bats and birds as viral reservoirs: a physiological and ecological perspective. Sci. Total Environ. 754, 142372. ( 10.1016/j.scitotenv.2020.142372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Baker ML, Schountz T, Wang L-F. 2013. Antiviral immune responses of bats: a review. Zoonoses Public Health 60, 104-116. ( 10.1111/j.1863-2378.2012.01528.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Guito JC, et al. 2020. Asymptomatic infection of marburg virus reservoir bats is explained by a strategy of immunoprotective disease tolerance. Curr. Biol. 31, 257-270.e5. ( 10.1016/j.cub.2020.10.015) [DOI] [PubMed] [Google Scholar]
- 133.Brook CE, Dobson AP. 2015. Bats as ‘special’ reservoirs for emerging zoonotic pathogens. Trends Microbiol. 23, 172-180. ( 10.1016/j.tim.2014.12.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Munshi-South J, Wilkinson GS. 2010. Bats and birds: exceptional longevity despite high metabolic rates. Ageing Res. Rev. 9, 12-19. ( 10.1016/j.arr.2009.07.006) [DOI] [PubMed] [Google Scholar]
- 135.Montgomery DW, Simpson SD, Engelhard GH, Birchenough SNR, Wilson RW. 2019. Rising CO2 enhances hypoxia tolerance in a marine fish. Sci. Rep. 9, 15152. ( 10.1038/s41598-019-51572-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Huey RB, Kearney MR, Krockenberger A, Holtum JAM, Jess M, Williams SE. 2012. Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Phil. Trans. R. Soc. B 367, 1665-1679. ( 10.1098/rstb.2012.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Monge C, Leon-Velarde F. 1991. Physiological adaptation to high altitude: oxygen transport in mammals and birds. Physiol. Rev. 71, 1135-1172. ( 10.1152/physrev.1991.71.4.1135) [DOI] [PubMed] [Google Scholar]
- 138.Fago A, Jensen JB. 2015. Hypoxia tolerance, nitric oxide, and nitrite: lessons from extreme animals. Physiology 30, 116-126. ( 10.1152/physiol.00051.2014) [DOI] [PubMed] [Google Scholar]
- 139.Lindgren AR, Buckley BA, Eppley SM, Reysenbach A-L, Stedman KM, Wagner JT. 2016. Life on the edge—the biology of organisms inhabiting extreme environments: an introduction to the symposium. Integr. Comp. Biol. 56, 493-499. ( 10.1093/icb/icw094) [DOI] [PubMed] [Google Scholar]
- 140.Sippel S, Meinshausen N, Fischer EM, Székely E, Knutti R. 2020. Climate change now detectable from any single day of weather at global scale. Nat. Clim. Change 10, 35-41. ( 10.1038/s41558-019-0666-7) [DOI] [Google Scholar]
- 141.Cooke SJ, Hinch SG, Wikelski M, Andrews RD, Kuchel LJ, Wolcott TG, Butler PJ. 2004. Biotelemetry: a mechanistic approach to ecology. Trends Ecol. Evol. 19, 334-343. ( 10.1016/j.tree.2004.04.003) [DOI] [PubMed] [Google Scholar]
- 142.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637-669. ( 10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 143.Walther G-R, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin J-M, Hoegh-Guldberg O, Bairlein F. 2002. Ecological responses to recent climate change. Nature 416, 389-395. ( 10.1038/416389a) [DOI] [PubMed] [Google Scholar]
- 144.Ditmer MA, Vincent JB, Werden LK, Tanner JC, Laske TG, Iaizzo PA, Garshelis DL, Fieberg JR. et al. 2015. Bears show a physiological but limited behavioral response to unmanned aerial vehicles. Curr. Biol. 25, 2278-2283. ( 10.1016/j.cub.2015.07.024) [DOI] [PubMed] [Google Scholar]
- 145.Senzaki M, et al. 2020. Sensory pollutants alter bird phenology and fitness across a continent. Nature 587, 605-609. ( 10.1038/s41586-020-2903-7) [DOI] [PubMed] [Google Scholar]
- 146.Larocque SM, Cooke SJ, Blouin-Demers G. 2012. A breath of fresh air: avoiding anoxia and mortality of freshwater turtles in fyke nets by the use of floats. Aquat. Conserv. Mar. Freshw. Ecosyst. 22, 198-205. ( 10.1002/aqc.1247) [DOI] [Google Scholar]
- 147.Hindell MA, et al. 2020. Tracking of marine predators to protect Southern Ocean ecosystems. Nature 580, 87-92. ( 10.1038/s41586-020-2126-y) [DOI] [PubMed] [Google Scholar]
- 148.Tucker MA, et al. 2018. Moving in the Anthropocene: global reductions in terrestrial mammalian movements. Science 359, 466-469. ( 10.1126/science.aam9712) [DOI] [PubMed] [Google Scholar]
- 149.Rutz C, et al. 2020. COVID-19 lockdown allows researchers to quantify the effects of human activity on wildlife. Nat. Ecol. Evol. 4, 1156-1159. ( 10.1038/s41559-020-1237-z) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.