Abstract
The physiological mechanisms by which animals regulate energy expenditure, respond to stimuli and stressors, and maintain homeostasis at the tissue, organ and whole organism levels can be described by ‘physiologging’—that is, the use of onboard miniature electronic devices to record physiological metrics of animals in captivity or free-living in the wild. Despite its origins in the 1960s, physiologging has evolved more slowly than its umbrella field of biologging. However, the recording of physiological metrics in free-living animals will be key to solving some of the greatest challenges in biodiversity conservation, issues pertaining to animal health and welfare, and for inspiring future therapeutic strategies for human health. Current physiologging technologies encompass the measurement of physiological variables such as heart rate, brain activity, body temperature, muscle stimulation and dynamic movement, yet future developments will allow for onboard logging of metrics relating to organelle, molecular and genetic function.
This article is part of the theme issue ‘Measuring physiology in free-living animals (Part II)’.
Keywords: bioengineering, medical technology, wildlife surveillance, telemetry
1. Introduction
Physiology is the science of life, and can be broadly defined as a branch of biology that aims to understand the internal mechanisms that allow living things to stay healthy and respond to the challenges of everyday life. Physiological research spans a wide range of variables from the basis of cell function at the ionic and molecular level, to the integrated response of the whole organism and the influence of the external environment. In the context of ‘biologging’ research [1–4], the sub-discipline of ‘physiologging’ is defined here as ‘the recording of physiological metrics (e.g. metrics that describe causality, homeostasis and energy expenditure) onboard miniature electronic devices carried by animals both in captivity and at liberty in the wild’ (figure 1). Physiologging does not include analyses of physiological variables ex situ (e.g. processing a blood sample in the field with a hand-held instrument, or bringing an animal to a measurement device not mounted on or in the animal), but instead the data are logged onboard the animal (although the animal need not be in the wild, see [5]). In this context, it is important to define which variables can be considered as ‘physiological’ so that developments and evolution in this field can be measured. Generally speaking, physiologging is not considered to include metrics that describe the three-dimensional location or orientation of an animal (e.g. GPS or satellite location, dive depth or flight height, magnetic direction etc.), but rather includes a suite of sensors or metrics that measure cellular, chemical and systemic (e.g. cardiovascular and respiratory) changes that describe how an animal functions in response to environmental and/or anthropogenic stimuli (table 1, see [37,38]). Often, physiologging is combined with behavioural information, such as location tracking, and in these cases offers some of the strongest insights into the coupling between physiological mechanisms and resulting behaviours (see [22,39–41]).
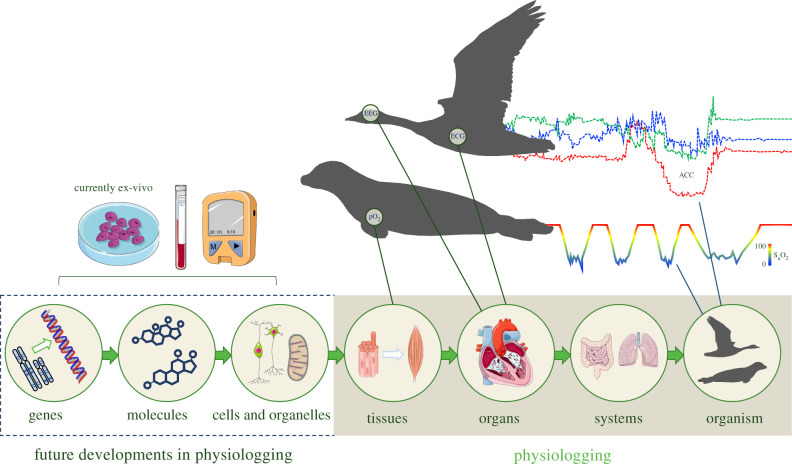
Figure 1.
Physiologging involves recording physiological metrics onboard miniature electronic devices carried by animals both in captivity and at liberty in the wild. Current physiologging technologies are capable of recording physiological metrics at the tissue, organ, system and whole organism levels (beige shaded area), but future developments will allow for onboard logging of metrics relating to organelle, molecular and genetic function (dashed box). ACC, acceleration.
Table 1.
Description of some physiologging metrics.
| variable | value of approach | examples |
|---|---|---|
| heart rate (ECG), variability and waveform characteristics | cannot expend energy without paying cardiovascular costs at some temporal scale | [6–10] |
| accelerometry (1-, 2- or 3-axes) | dynamic acceleration must be funded by muscular contraction with associated metabolic cost | [11–14] |
| body temperature | reveals patterns of diurnal behaviour, exercise thermogenesis and seasonal changes in homeotherms may be correlated with metabolic rate in heterotherms (following principles of Q10) |
[15–18] |
| partial pressure of O2 (pO2) | can predict blood O2 saturation as haemoglobin binds to O2 in relation to pO2 | [19–21] |
| brain activity (EEG) | directly measures neural transmission of stimuli perception and response | [22–24] |
| compounds in the blood (e.g. glucose, lactate, hormones) | provides insights into physiology, pharmacokinetics, toxicology of drugs and metabolites | [25–27] |
| ventilation (rate, tidal volume) | most air-breathing animals cannot acquire O2/release CO2 without ventilation | [28,29] |
| muscle movement (EMG) | measures motor unit action potentials of muscle groups, with associated metabolic costs | [30,31] |
| gastric pH | pH increases as prey is ingested, and decreases as gastric acids and enzymes are secreted for digestion | [32,33] |
| tissue blood flow and oxygenation | blood flow is managed to deliver O2 and remove CO2 depending on metabolic demand | [34–36] |
In this special issue, we feature some of the latest examples of physiologging in a range of animal systems to demonstrate the breadth of research questions and insights that this exciting field can produce. Arguably, the first and most fundamental physiologging metric is the recording of heart rate, which dates back as far as 1962 in birds [42], 1968 in fish [43] and 1972 for pinnipeds [44]. This metric has been successfully used to demonstrate some of the more astonishing accomplishments of free-living animals, for example, that diving whales can have heart rates as low as 2 beats per minute [45], whereas on the opposite end of the spectrum, flying birds can maintain heart rates of more than 500 beats per minute for many hours [46,47]. The rapid development and increasing availability of technologies capable of monitoring and analysing heart rate in recent times [6] have been used to gain unique insights into animal welfare (e.g. farmed terrestrial and aquatic species [48–51]), to assess social interactions in animals [52–55] and to comprehensively understand the cardiorespiratory adaptations of breath-hold diving species [7,8]. A more recent physiologging variable is accelerometry, where the two- or three-dimensional acceleration of an animal is recorded at a temporal resolution that is sufficiently high to reveal individual wing or tail beats during locomotion [56–59]. Although accelerometry does not directly measure energy expenditure, dynamic body acceleration has often been shown to be correlated with energy expenditure when the animal is in its primary mode of locomotion [60,61]. When used judiciously, accelerometry has revealed fascinating insights into the costs of movement in a range of species [62]. Physiologging of body temperature has also been widely used throughout the terrestrial and aquatic realms [63], and can yield broad insights into the activity patterns and behaviour of free-living animals [15–18,64]. Another commonly used physiologging metric is the intravascular partial pressure of O2 (pO2), which requires the use of highly specialized equipment and techniques (and has thus been restricted to just one extended laboratory of researchers). However, this metric has provided a foundation for much of what we know about diving physiology and oxygen management (see [65]). Finally, the recording of brain activity has revealed astonishing insights into cognition, navigation and sleep in both wild and captive animals [22,23,39], with advanced loggers currently as small as 1.92 g commercially available at the time of writing (see https://www.vyssotski.ch). Currently, there is comparatively little work in animal physiologging on circulating compounds in the blood, despite the fact that an automated blood sampling device was first envisaged and built in 1986 [66]. This device was further refined recently and a current version measures 18 × 8.6 cm, weighs 160 g in water and is capable of drawing two blood samples during deployment [67]. Furthermore, a technology from the biomedical field has been adapted to provide continuous, non-invasive measurements of blood flow and tissue oxygenation through Near Infrared Spectroscopy technology [34,35], and the future adaptation of medical technology will permit measurements of circulating chemicals in an animal's tissues. For example, the measurement of various hormones and metabolic substrates, without the need for sampling and storage of blood, is now commonplace in human wearable technologies [68,69].
As the future brings about significant changes in climate and anthropogenic pressure on biodiversity [70–72], physiologging will fundamentally underpin our understanding of how to predict biodiversity responses and to set appropriate conservation policies [73,74]. Indeed, measuring physiology in free-living animals will underpin a huge range of future research priorities from understanding the potential of animals to tolerate and adapt to rapidly changing environments, to managing invasive species, to understanding the impact of threats such as pollution, for ensuring the success of restoration efforts and for managing human–wildlife interactions [75]. Physiologging will also be key for understanding the mechanisms with which vertebrate life copes with extremes of hypoxia, circulatory changes and infectious diseases, which will undoubtedly have important ramifications for future medical interventions in humans [76,77]. Many of these research questions will need to be tackled outside of the laboratory in wild animal study systems, and thus physiologging technologies perhaps provide the most critical tools for future biodiversity research.
Acknowledgements
This theme introduction was improved by the very constructive and helpful comments of two reviewers.
Data accessibility
This article has no additional data.
Authors' contributions
L.A.H. drafted the manuscript; A.F. and K.S. reviewed the final draft.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Hussey NE, et al. 2015. Aquatic animal telemetry: a panoramic window into the underwater world. Science 348, 1255642. ( 10.1126/science.1255642) [DOI] [PubMed] [Google Scholar]
- 2.Kays R, Crofoot MC, Jetz W, Wikelski M. 2015. Terrestrial animal tracking as an eye on life and planet. Science 348, aaa2478. ( 10.1126/science.aaa2478) [DOI] [PubMed] [Google Scholar]
- 3.Rutz C, Hays GC. 2009. New frontiers in biologging science. Biol. Lett. 5, 282-292. ( 10.1098/rsbl.2009.0089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fehlman G, King AJ. 2016. Bio-logging. Curr. Biol. 26, R823-RR37. ( 10.1016/j.cub.2016.05.033) [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, et al. 2019. Battery-free, lightweight, injectable microsystem for in vivo wireless pharmacology and optogenetics. Proc. Natl Acad. Sci. USA 116, 21427-21437. ( 10.1073/pnas.1909850116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakamoto KQ, Miyayama M, Kinoshita C, Fukuoka T, Ishihara T, Sato K. 2021. A non-invasive system to measure heart rate in hard-shelled sea turtles: potential for field applications. Phil. Trans. R. Soc. B 376, 20200222. ( 10.1098/rstb.2020.0222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blawas AM, Nowacek DP, Rocho-Levine J, Robeck TR, Fahlman A. 2021. Scaling of heart rate with breathing frequency and body mass in cetaceans. Phil. Trans. R. Soc. B 376, 20200223. ( 10.1098/rstb.2020.0223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aoki K, Watanabe Y, Inamori D, Funasaka N, Sakamoto KQ. 2021. Towards non-invasive heart rate monitoring in free-ranging cetaceans: a unipolar suction cup tag measured the heart rate of trained Risso's dolphins. Phil. Trans. R. Soc. B 376, 20200225. ( 10.1098/rstb.2020.0225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green JA. 2011. The heart rate method for estimating metabolic rate: review and recommendations. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 158, 287-304. ( 10.1016/j.cbpa.2010.09.011) [DOI] [PubMed] [Google Scholar]
- 10.Trondrud LM, et al. 2021. Determinants of heart rate in Svalbard reindeer reveal mechanisms of seasonal energy management. Phil. Trans. R. Soc. B 376, 20200215. ( 10.1098/rstb.2020.0215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson RP, Quintana F, Hobson VJ. 2012. Construction of energy landscapes can clarify the movement and distribution of foraging animals. Proc. R. Soc. B 279, 975-980. ( 10.1098/rspb.2011.1544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Nickel B, Rutishauser M. 2015. Movement, resting, and attack behaviors of wild pumas are revealed by tri-axial accelerometer measurements. Movem. Ecol. 3, 1-2. ( 10.1186/s40462-015-0030-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosser AA, Avgar T, Brown GS, Walker CS, Fryxell JM. 2014. Towards an energetic landscape: broad-scale accelerometry in woodland caribou. J. Anim. Ecol. 83, 916-922. ( 10.1111/1365-2656.12187) [DOI] [PubMed] [Google Scholar]
- 14.Scharf AK, LaPoint S, Wikelski M, Safi K. 2016. Acceleration data reveal highly individually structured energetic landscapes in free-ranging fishers (Pekania pennanti). PLoS ONE 11, e0145732. ( 10.1371/journal.pone.0145732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donaldson MR, et al. 2009. Limited behavioural thermoregulation by adult upriver-migrating sockeye salmon (Oncorhynchus nerka) in the Lower Fraser River, British Columbia. Can. J. Zool. 87, 480-490. ( 10.1139/Z09-032) [DOI] [Google Scholar]
- 16.Jackson CR, Setsaas TH, Robertson MP, Scantlebury M, Bennett NC. 2009. Insights into torpor and behavioural thermoregulation of the endangered Juliana's golden mole. J. Zool. 278, 299-307. ( 10.1111/j.1469-7998.2009.00575.x) [DOI] [Google Scholar]
- 17.Hetem RS, Strauss WM, Fick LG, Maloney SK, Meyer LCR, Shobrak M, Fuller A, Mitchell D. 2012. Activity re-assignment and microclimate selection of free-living Arabian oryx: responses that could minimise the effects of climate change on homeostasis? Zoology 115, 411-416. ( 10.1016/j.zool.2012.04.005) [DOI] [PubMed] [Google Scholar]
- 18.Parr N, Bishop CM, Batbayar N, Butler PJ, Chua B, Milsom WK, Scott GR, Hawkes LA. 2019. Tackling the Tibetan Plateau in a down suit: insights into thermoregulation by bar-headed geese during migration. J. Exp. Biol. 222, jeb203695. ( 10.1242/jeb.203695) [DOI] [PubMed] [Google Scholar]
- 19.Mcdonald BI, Ponganis PJ. 2012. Lung collapse in the diving sea lion: hold the nitrogen and save the oxygen. Biol. Lett. 8, 1047-1049. ( 10.1098/rsbl.2012.0743) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mcdonald BI, Ponganis PJ. 2013. Insights from venous oxygen profiles: oxygen utilization and management in diving California sea lions. J. Exp. Biol. 216, 3332-3341. ( 10.1242/jeb.085985) [DOI] [PubMed] [Google Scholar]
- 21.Williams CL, Hicks JW. 2016. Continuous arterial PO2 profiles in unrestrained, undisturbed aquatic turtles during routine behaviours. J. Exp. Biol. 219, 3616-3625. ( 10.1242/jeb.141010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vyssotski AL, Dell'Omo G, Dell'Ariccia G, Abramchuk AN, Serkov AN, Latanov AV, Loizzo A, Wolfer DP, Lipp H-P. 2009. EEG responses to visual landmarks in flying pigeons. Curr. Biol. 19, 1159-1166. ( 10.1016/j.cub.2009.05.070) [DOI] [PubMed] [Google Scholar]
- 23.Aulsebrook AE, Connelly F, Johnsson RD, Jones TM, Mulder RA, Hall ML, Vyssotski AL, Lesku JA. 2020. White and amber light at night disrupt sleep physiology in birds. Curr. Biol. 30, 3657-3663. ( 10.1016/j.cub.2020.06.085) [DOI] [PubMed] [Google Scholar]
- 24.Vyssotski AL, Serkov AN, Itskov PM, Dell'Omo G, Latanov AV, Wolfer DP, Lipp H-P. 2006. Miniature neurologgers for flying pigeons: multichannel EEG and action and field potentials in combination with GPS recording. J. Neurophysiol. 95, 1263-1273. ( 10.1152/jn.00879.2005) [DOI] [PubMed] [Google Scholar]
- 25.Rana R, Williams CL, Hicks J. 2014. Blood lactate accumulation in forced submerged turtles that can and cannot shunt. Physiology 879, 11. [Google Scholar]
- 26.Gough DA, Kumosa LS, Routh TL, Lin JT, Lucisano JY. 2010. Function of an implanted tissue glucose sensor for more than 1 year in animals. Sci. Transl. Med. 2, 42r.a53. ( 10.1126/scitranslmed.3001148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arroyo-Currás N, Somerson J, Vieira PA, Ploense KL, Kippin TE, Plaxco KW. 2017. Real-time measurement of small molecules directly in awake, ambulatory animals. Proc. Natl Acad. Sci. USA 114, 645-650. ( 10.1073/pnas.1613458114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sumich JL. 2021. Why Baja? A bioenergetic model for comparing metabolic rates and thermoregulatory costs of gray whale calves (Eschrichtius robustus). Mar. Mamm. Sci. 1. ( 10.1111/mms.12778) [DOI] [Google Scholar]
- 29.van der Hoop JM, Fahlman AF, Jensen FH, Beedholm K, Rocho-Levine JR, Wells RS, Madsen PT. 2021. Free-ranging bottlenose dolphins breathe with a low but variable tidal volume. Phil. Trans. R. Soc. B 376, 20200428. ( 10.1098/rstb.2020.0428) [DOI] [Google Scholar]
- 30.Quintella BR, Póvoa I, Almeida PR. 2009. Swimming behaviour of upriver migrating sea lamprey assessed by electromyogram telemetry. J. Appl. Ichthyol. 25, 46-54. ( 10.1111/j.1439-0426.2008.01200.x) [DOI] [Google Scholar]
- 31.Williams SH, Vinyard CJ, Glander KE, Deffenbaugh M, Teaford MF, Thompson CL. 2008. Telemetry system for assessing jaw-muscle function in free-ranging primates. Int. J. Primatol. 29, 1441-1453. ( 10.1007/s10764-008-9292-3) [DOI] [Google Scholar]
- 32.Papastamatiou YP, Purkis SJ, Holland KN. 2007. The response of gastric pH and motility to fasting and feeding in free swimming blacktip reef sharks, Carcharhinus melanopterus. J. Exp. Mar. Biol. Ecol. 345, 129-140. ( 10.1016/j.jembe.2007.02.006) [DOI] [Google Scholar]
- 33.Thouzeau C, Peters G, Le Bohec C, Le Maho Y. 2004. Adjustments of gastric pH, motility and temperature during long-term preservation of stomach contents in free-ranging incubating king penguins. J. Exp. Biol. 207, 2715-2724. ( 10.1242/jeb.01074) [DOI] [PubMed] [Google Scholar]
- 34.McKnight JC, et al. 2021. When the human brain goes diving: using near-infrared spectroscopy to measure cerebral and systemic cardiovascular responses to deep, breath-hold diving in elite freedivers. Phil. Trans. R. Soc. B 376, 20200349. ( 10.1098/rstb.2020.0349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKnight JC, et al. 2021. Shining new light on sensory brain activation and physiological measurement in seals using wearable optical technology. Phil. Trans. R. Soc. B 376, 20200224. ( 10.1098/rstb.2020.0224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKnight JC, et al. 2019. Shining new light on mammalian diving physiology using wearable near-infrared spectroscopy. PLoS Biol. 17, e3000306. ( 10.1371/journal.pbio.3000306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitford M, Klimley AP. 2019. An overview of behavioral, physiological, and environmental sensors used in animal biotelemetry and biologging studies. Anim. Biotelemetry 7, 1-24. ( 10.1186/s40317-019-0189-z) [DOI] [Google Scholar]
- 38.Fahlman A, et al. 2021. The new era of physio-logging and their grand challenges. Front. Physiol. 12. ( 10.3389/fphys.2021.669158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rattenborg NC, Voirin B, Cruz SM, Tisdale R, Dell'Omo G, Lipp HP, Wikelski M, Vyssotski AL. 2016. Evidence that birds sleep in mid-flight. Nat. Commun. 7, 12468. ( 10.1038/ncomms12468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duriez O, Kato A, Tromp C, Dell'Omo G, Vyssotski AL, Sarrazin F, Ropert-Coudert Y. 2014. How cheap is soaring flight in raptors? A preliminary investigation in freely-flying vultures. PLoS ONE 9, e84887. ( 10.1371/journal.pone.0084887) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meir JU, Robinson PW, Vilchis LI, Kooyman GL, Costa DP, Ponganis PJ. 2013. Blood oxygen depletion is independent of dive function in a deep diving vertebrate, the Northern Elephant Seal. PLoS ONE 8, e83248. ( 10.1371/journal.pone.0083248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eliassen E. 1962. Preliminary results from new methods of investigating the physiology of birds during flight. Ibis 105, 234-237. ( 10.1111/j.1474-919X.1963.tb02497.x) [DOI] [Google Scholar]
- 43.Frank TH. 1968. Telemetering the electrocardiogram of free swimming Salmo Irideus. IEEE Trans. Biomed. Eng. 2, 111-114. ( 10.1109/TBME.1968.4502546) [DOI] [PubMed] [Google Scholar]
- 44.Kooyman GL, Campbell WB. 1972. Heart rates in freely diving weddell seals, Leptonychotes weddelli. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 43, 31-36. ( 10.1016/0300-9629(72)90465-3) [DOI] [PubMed] [Google Scholar]
- 45.Goldbogen JA, et al. 2019. Extreme bradycardia and tachycardia in the world's largest animal. Proc. Natl Acad. Sci. USA 116, 25 329-25 332. ( 10.1073/pnas.1914273116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sapir N, Wikelski M, McCue MD, Pinshow B, Nathan R. 2010. Flight modes in migrating European bee-eaters: heart rate may indicate low metabolic rate during soaring and gliding. PLoS ONE 5, e13956. ( 10.1371/journal.pone.0013956) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bowlin MS, Cochran WW, Wikelski M. 2005. Biotelemetry of New World thrushes during migration: physiology, energetics and orientation in the wild. Integr. Comp. Biol. 45, 295-304. ( 10.1093/icb/45.2.295) [DOI] [PubMed] [Google Scholar]
- 48.Hvas M, Folkedal O, Oppedal F. 2020. Heart rate bio-loggers as welfare indicators in Atlantic salmon (Salmo salar) aquaculture. Aquaculture 529, 735630. ( 10.1016/j.aquaculture.2020.735630) [DOI] [Google Scholar]
- 49.Andrewartha SJ, Elliott NG, McCulloch JW, Frappell PB. 2015. Aquaculture sentinels: smart-farming with biosensor equipped stock. J. Aquac. Res. Dev. 6, 393. ( 10.4172/2155-9546.1000393) [DOI] [Google Scholar]
- 50.Jukan A, Masip-Bruin X, Amla N. 2017. Smart computing and sensing technologies for animal welfare: a systematic review. ACM Comput. Surv. 50, Article 10. ( 10.1145/3041960) [DOI] [Google Scholar]
- 51.Brijs J, Føre M, Gräns A, Clark TD, Axelsson M, Johansen JL. 2021. Bio-sensing technologies in aquaculture: how remote monitoring can bring us closer to our farm animals. Phil. Trans. R. Soc. B 376, 20200218. ( 10.1098/rstb.2020.0218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Viblanc VA, Smith AD, Gineste B, Groscolas R. 2012. Coping with continuous human disturbance in the wild: insights from penguin heart rate response to various stressors. BMC Ecol. 12, 10. ( 10.1186/1472-6785-12-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turbill C, Ruf T, Rothmann A, Arnold W. 2013. Social dominance is associated with individual differences in heart rate and energetic response to food restriction in female red deer. Physiol. Biochem. Zool. 86, 528-537. ( 10.1086/672372) [DOI] [PubMed] [Google Scholar]
- 54.Wascher CAF, Arnold W, Kotrschal K. 2008. Heart rate modulation by social contexts in greylag geese (Anser anser). J. Comp. Psychol. 122, 100-107. ( 10.1037/0735-7036.122.1.100) [DOI] [PubMed] [Google Scholar]
- 55.Wascher CAF. 2021. Heart rate as a measure of emotional arousal in evolutionary biology. Phil. Trans. R. Soc. B 376, 20200479. ( 10.1098/rstb.2020.0479) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taylor LA, Taylor GK, Lambert B, Walker JA, Biro D, Portugal SJ. 2019. Birds invest wingbeats to keep a steady head and reap the ultimate benefits of flying together. PLoS Biol. 17, e3000299. ( 10.1371/journal.pbio.3000299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams HJ, Shepard ELC, Holton MD, Alarcon PAE, Wilson RP, Lambertucci SA. 2020. Physical limits of flight performance in the heaviest soaring bird. Proc. Natl Acad. Sci. USA 117, 17 884-17 890. ( 10.1073/pnas.1907360117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kawabe R, Kawano T, Nakano N, Yamashita N, Hiraishi T, Naito Y. 2003. Simultaneous measurement of swimming speed and tail beat activity of free-swimming rainbow trout Oncorhynchus mykiss using an acceleration data-logger. Fish. Sci. 69, 959-965. ( 10.1046/j.1444-2906.2003.00713.x) [DOI] [Google Scholar]
- 59.Bouyoucos IA, Montgomery DW, Brown JW, Cooke SJ, Suski CD, Mandelman JW, Brooks EJ. 2017. Swimming speeds and metabolic rates of semi-captive juvenile lemon sharks (Negaprion brevirostris, Poey) estimated with acceleration biologgers. J. Exp. Mar. Biol. Ecol. 486, 245-254. ( 10.1016/j.jembe.2016.10.019) [DOI] [Google Scholar]
- 60.Halsey LG, Green JA, Wilson RP, Frappell PB. 2009. Accelerometry to estimate energy expenditure during activity: best practice with data loggers. Physiol. Biochem. Zool. 82, 396-404. ( 10.1086/589815) [DOI] [PubMed] [Google Scholar]
- 61.Wilson R, et al. 2020. Estimates for energy expenditure in free-living animals using acceleration proxies: a reappraisal. J. Anim. Ecol. 89, 161-172. ( 10.1111/1365-2656.13040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brown DD, Kays R, Wikelski M, Wilson R, Klimley AP. et al. 2013. Observing the unwatchable through acceleration logging of animal behavior. Anim. Biotelemetry 1, 20. ( 10.1186/2050-3385-1-20) [DOI] [Google Scholar]
- 63.Wilson A, Wikelski M, Wilson R, Cooke S. 2015. Utility of biological sensor tags in animal conservation. Conserv. Biol. 29, 1065-1075. ( 10.1111/cobi.12486) [DOI] [PubMed] [Google Scholar]
- 64.Botha A, Lease HM, Fuller A, Mitchell D, Hetem RS. 2019. Biologging subcutaneous temperatures to detect orientation to solar radiation remotely in savanna antelope. J. Exp. Zool. Part A Ecol. Integr. Physiol. 331, 267-279. ( 10.1002/jez.2267) [DOI] [PubMed] [Google Scholar]
- 65.Williams CL, Ponganis PJ. 2021. Diving physiology of marine mammals and birds: the development of biologging techniques. Phil. Trans. R. Soc. B 376, 20200211. ( 10.1098/rstb.2020.0211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hill RD. 1986. Microcomputer monitor and blood sampler for free-diving Weddell seals. J. Appl. Physiol. 61, 1570-1576. ( 10.1152/jappl.1986.61.4.1570) [DOI] [PubMed] [Google Scholar]
- 67.Takei Y, Suzuki I, Wong MKS, Milne R, Moss S, Sato K, Hall A. 2016. Development of an animal-borne blood sample collection device and its deployment for the determination of cardiovascular and stress hormones in phocid seals. Reg. Integr. Comp. Physiol. 311, R788-R796. ( 10.1152/ajpregu.00211.2016) [DOI] [PubMed] [Google Scholar]
- 68.Macdonald A, Hawkes LA, Corrigan DK. 2021. Recent advances in biomedical, biosensor and clinical measurement devices for use in humans and the potential application of these technologies for the study of physiology and disease in wild animals. Phil. Trans. R. Soc. B 376, 20200228. ( 10.1098/rstb.2020.0228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Williams HJ, Shipley JR, Rutz C, Wikelski M, Wilkes M, Hawkes LA. 2021. Future trends in measuring physiology in free-living animals. Phil. Trans. R. Soc. B 376, 20200230. ( 10.1098/rstb.2020.0230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sippel S, Meinshausen N, Fischer EM, Székely E, Knutti R. 2020. Climate change now detectable from any single day of weather at global scale. Nat. Clim. Change 10, 35-41. ( 10.1038/s41558-019-0666-7) [DOI] [Google Scholar]
- 71.Trisos CH, Merow C, Pigot AL. 2020. The projected timing of abrupt ecological disruption from climate change. Nature 580, 496-501. ( 10.1038/s41586-020-2189-9) [DOI] [PubMed] [Google Scholar]
- 72.Jung M, Rowhani P, Scharlemann JPW. 2019. Impacts of past abrupt land change on local biodiversity globally. Nat. Commun. 10, 5474. ( 10.1038/s41467-019-13452-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seebacher F, Franklin CE. 2012. Determining environmental causes of biological effects: the need for a mechanistic physiological dimension in conservation biology. Phil. Trans. R. Soc. B 367, 1607-1614. ( 10.1098/rstb.2012.0036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cooke SJ, O'Connor CM. 2010. Making conservation physiology relevant to policy makers and conservation practitioners. Conserv. Lett. 3, 159-166. ( 10.1111/j.1755-263X.2010.00109.x) [DOI] [Google Scholar]
- 75.Cooke SJ, et al. 2021. One hundred research questions in conservation physiology for generating actionable evidence to inform conservation policy and practice. Conserv. Physiol. 9, coab009. ( 10.1093/conphys/coab009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ponganis PJ. 2019. State of the art review: from the seaside to the bedside: insights from comparative diving physiology into respiratory, sleep and critical care. Thorax 74, 512-518. ( 10.1136/thoraxjnl-2018-212136) [DOI] [PubMed] [Google Scholar]
- 77.Williams TM, Davis RW. 2021. Physiological resiliency in diving mammals: insights on hypoxia protection using the Krogh principle to understand COVID-19 symptoms. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 253, 110849. ( 10.1016/j.cbpa.2020.110849) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.