Abstract
Animal-borne tags (biologgers) have now become extremely sophisticated, recording data from multiple sensors at high frequencies for long periods and, as such, have become a powerful tool for behavioural ecologists and physiologists studying wild animals. But the design and implementation of these tags is not trivial because engineers have to maximize performance and ability to function under onerous conditions while minimizing tag mass and volume (footprint) to maximize the wellbeing of the animal carriers. We present some of the major issues faced by tag engineers and show how tag designers must accept compromises while maintaining systems that can answer the questions being posed. We also argue that basic understanding of engineering issues in tag design by biologists will help feedback to engineers to better tag construction but also reduce the likelihood that tag-deploying biologists will misunderstand their own results. Finally, we suggest that proper consideration of conventional technology together with new approaches will lead to further step changes in our understanding of wild-animal biology using smart tags.
This article is part of the theme issue ‘Measuring physiology in free-living animals (Part II)’.
Keywords: tagging, behaviour, technology, movement, logger, biologging
1. Introduction
Data logging technology these days is ubiquitous due to current vehicle and mobile phone technology, logging data either within localized memory storage or transmitting-on-request, location (GPS) and movement (accelerometer) information to a distant server for processing.
Animal-borne tags recording information have, however, been around for over 4 decades now, collecting many different types of information, not unlike mobile phone technology, including, but not limited to, GPS location [1], acceleration [2], magnetic field strength [3], temperature [4], pressure [5], heart rate [6], ambient light levels [7], conductivity [8] etc., and have transformed our understanding of wild-animal physiology [9–11], ecology [12] and behaviour [13] and, as such, play a major role in informing conservation [14]. By combining some, or all, of these sensors, one can ‘see’ what an animal is doing, where it is doing it and potentially, what the environmental (and terrain) conditions are, and so generate a second-by-second diary of the animal's behaviour in three-dimensional space, and subsequently, potentially determine if the behaviour is borne from environmental effects or something else.
The development of these tags has been extraordinary, primarily as a result of developments in the solid-state industry, driven by consumers. Alone within the data logger (aka biologgers) community, data storage is now as high as 64 GB [15], sensor count may exceed 9 (e.g. [16]) with deployment periods ranging to years [17] and recording frequencies of now up to greater than 180 kHz [18] on animals as diverse as small bats [18] and 100 ton blue whales [19,20]. As a result of recent advances in semiconductor technology, storage capacity is no longer an issue; microSD cards are now available with 1 TB of data storage. This is an extraordinary amount of storage space. For example, a data logger storing: time (at 40 B s−1), tri-axial acceleration (6 B at 40 Hz), tri-axial magnetometry (6 B at 13 Hz), temperature and pressure (both using 3 B at 4 Hz), equates to just under 512 B s−1. At that rate, it would take about 7 years to fill a 64 GB card and over a hundred years to fill a single 1 TB card.
The changing range of animals used as a function of the time over which the technology has been developed reflects perhaps the most fundamental element of all. That tag ‘size’ affects animals, and what this means to the engineer [21]. Animal-associated tags affect animals in a suite of ways ranging from increased energy expenditure [22] to increased mortality [23], and it is clear that larger tags are expected to lead to greater detriment (but see [22]), so biologists should be striving to reduce the size of the tags, almost whatever the size of the carrier. Coupled with this, though, is the reasonable expectation that ever smaller tags can be deployed on ever smaller animals [24], which opens up the potential of tag technology insights on an increasing range of species because most animals are small.
This is the major challenge for the tag engineer because high performance measuring systems that operate under challenging conditions can be constructed relatively easily if they are not size limited. But beyond that, these tags, and their deployment protocols, need to be fool-proof so that when tags are being deployed under the onerous conditions that typify much fieldwork, no pilot errors [25] are made. Finally, assuming the tag, or at least the data, can be recovered, it is useful if there is software that allows the biologist to see what she/he has got. Indeed, rapid repeat deployments in the field should rely on confirmation of quality before tags are redeployed.
This article takes an engineering perspective to detail some of the important consideration in the design of biologgers, rather than systems that also, or uniquely, incorporate transmission telemetry. Cognizance of this is not just for the engineers because if the design and decision-making process is understood by biologists, judgements can be made in the field about how to programme the tags, for example, to get the best (duration, resolution, sensor activation, duty-cycling etc.) from the data. Finally, this paper will muse over where the future might take animal tagging, which is rapidly becoming a mainstream discipline in its own right.
2. Animals first
There is an increasing number of publications which show the detrimental effect of tags on their carriers [22,26,27] such as changes in behaviour and increased energy uses, and, in the interests of ethically, morally and otherwise good science, we need to be working to minimize these effects [28]. This not only requires that workers consider tag placement on their study animals [29] but also that they minimize tag mass [30], because this creates forces that can lead to sores and even death [31], minimize the tag-animal footprint for inter alia heat loss reasons [32], maximize streamlining [33] and even consider tag colour [34] and electronic blinking lights that may cause behavioural changes of predator, prey or conspecifics. Most of these issues are the province of engineers. This is because, on the one hand, it is linked to the physicality of tag effects [35] where techniques like flow visualization [36] and computer fluid dynamics can help minimize detriment [33], but, on the other, the miniaturization and precise layout of the tag components define the physical form of the tags. Tag design engineers, therefore, have to work under severe constraints, which are further complicated since an optimized tag design for one species will not necessarily be right for another. The niceties of this are not covered here but will be reflected in an important drive for engineers to reduce tag size in general, so minimization of tag size underpins the rest of this paper.
3. Hardware
(a) . Sensors
The number of sensors is extraordinary [37], with researchers now measuring everything from internal physiological parameters such as heart rate [38,39] and stomach pH [40] through sensors that work both internally and externally such as respiration rate sensors [41], accelerometers [42] and magnetometers [3] to transducers that interrogate the external environment for, e.g. pressure, salinity or temperature [43]. Generally, the quality of data retrieved from larger sensors is better than smaller sensors so that, other things being equal, we expect smaller animals to be served by lower quality sensor data. However, the exception to this is where sensors are required to react rapidly, for example, temperature sensors [44], because larger mass sensors have greater thermal inertia, which makes response times sluggish, a process exacerbated when sensors have to be covered (e.g. with resin) to protect them (cf. [45]). Users, therefore, have to decide on the trade-off between the value of rapid against accurate temperature measurement, something that will depend on the heterogeneity of the environment, the sampling rate (see below) and precise questions being asked. Within biologging, sensors are still remarkably small [16], and many are combined in single chips, such as the inertial measurement unit (IMU) chips used for dead-reckoning, which have tri-axial accelerometers, tri-axial gyro meters and tri-axial magnetic field intensity sensors and are a couple of millimetres in external dimensions. From a sensor size perspective, this tempts researchers into wanting increasing numbers of sensor types within one tag [16,46] and there is certainly a case for aspiring to have the ‘most complete monitoring possible’ of the study animal. Although this would seem to go against rigorous hypothesis-testing science [47], even hypothesis-testing science is formed on observations and biologgers do exactly that. But even small sensors require current to function, and so impose a cost on size that goes beyond their physical presence and is reflected in the battery size or deployment duration. An important determinant of power drain and, therefore, battery size, is the resolution at which the sensors are required to operate. To achieve higher resolutions, noise may make it difficult to directly measure small changes in signal, and so it may be necessary to oversample and average, i.e. collect more samples at a higher rate and then average, to improve the signal-to-noise ratio. All this assumes that biologists have unfettered choice about their ideal resolution for their study question, even though the ‘allowable’ questions also depend on what is possible. A good example of this is the measurement of depth, which has, over the last 4 decades, seen resolutions of pressure move between 8 and 16 bit [48]. This translates the sensor measurement range into between 256 and 65 536 steps, respectively. Thus, a depth sensor that operates over a range of 0–500 m, for example, will have depth resolution steps between 1.95 m and 7.6 mm, respectively. Assuming appropriate sampling frequency, this has a profound effect on the way results are interpreted, and even the extent to which we are presented information with serendipitous potential. For example, low depth resolution is the likely reason why many studies on penguin diving dismiss dives to less than 1–2 m [49], even though most commuting and some feeding behaviour occurs in this range for many species. Beyond that, as an example of high-resolution-enhanced serendipity, the use of high-resolution depth data indicates that porpoises Phocoena phocoena may use short, shallow dives to help them re-oxygenate their tissues by increasing the oxygen partial pressure difference between their lungs and their blood (figure 1), something that is otherwise unlikely to be considered.
Figure 1.
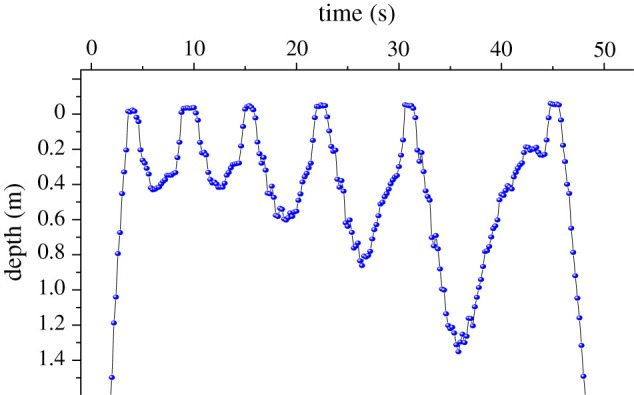
Sequential dives by a harbour porpoise P. phocoena, with pressure measured at 5 Hz and resolved at 16 bit, immediately following a single deep (22 m), long (165 s) dive. The short (3–13 s), shallow (0.21–1.2 m) dives with rapid surface periods (0.6 s) are easily discernible and may indicate how the animal could briefly flush the lungs [50] during the surface period and then use the water pressure to enhance the differential between lung and tissue oxygen partial pressures to facilitate the reoxygenation process.
We have used a variety of commercial depth sensors that specify their accuracy to be between 1 cm (Keller AG, www.keller-druck.com) and 50 cm (TE Connectivity Ltd, www.te.com) and so choice of which sensor to use as well as the temporal and bit resolution to apply obviously affects the ‘quality’ of data recorded. The use of the most accurate depth sensor sampled at 40 Hz has allowed us to determine, for example, that Magellanic penguins Spheniscus magellanicus swimming horizontally within the water column actually oscillate in depth around 23 mm due to the beating of their flippers which causes movement in the heave axis. This can help inform biomechanical studies but is unlikely to be ecologically relevant. Above all, it is important to choose the correct sensor for the likely quantity to be measured, i.e. for depth, a sensor that exceeds the range of the animal under study, but not overly so, as this will reduce the depth resolution and will impact on the ability to study fine movements in the water column. Similarly, selecting the likely g-range tri-axial accelerometers is very important as fine-scale movement at the milli-g range will be lost when the sensor itself is scaled to ±16 g, compared to ±2 g.
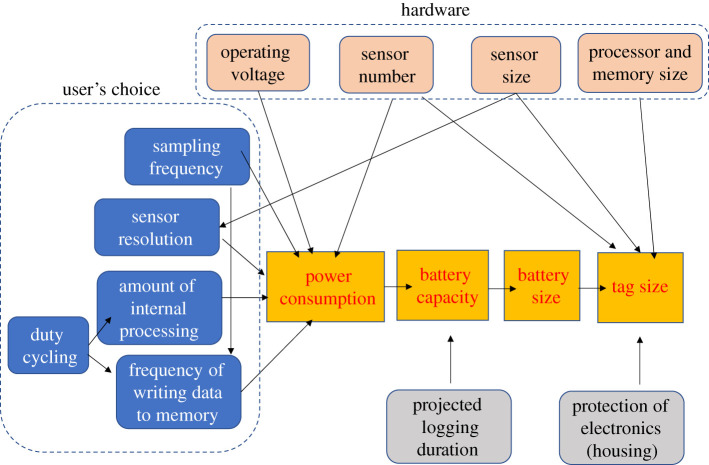
The frequency with which the sensors are interrogated also affects power consumption as an approximately linear function of sampling frequency and, therefore, battery capacity and ultimately size (figure 2). In a manner similar to that illustrated for the porpoise above, if sampling frequencies are to define particular waveforms, then data need to be sampled at a rate at least two times the highest frequency component in that waveform. Elephants are capable of generating infrasonic calls [51] with frequencies below 50 Hz being possible, and lasting for several seconds [52]. In this case, an accelerometer would need to record at twice the highest frequency of an elephant's vocalization range to detect/record the sound [53], assuming that the ensuing vibration can be sensed by accelerometers. In fact, by sampling acceleration at 320 Hz on African elephants Loxodonta africana, we have detected clear pulsed waveforms at 19 Hz, which we assumed were due to infrasound.
Figure 2.
Interaction of various parameters within animal tag construction and operation that ultimately affect tag size. (Online version in colour.)
(b) . Memory
A key element of biologgers is that they store, rather than transmit, data [54]. This has power and practicality advantages for many studies since signal transmission requires that the device use power to transmit. In any event, some studies require data from animals that operate in environments where signal attenuation prohibits useful transmission telemetry (e.g. [55]). There is also the large quantity of data that are collected with such devices, that would require a significant amount of power. As a partial solution to this, some hybrid devices may collect and store internally high-frequency data, and transmit either snippets, or summary information of the animal's recent, notable, behaviours. The disadvantage of biologging is, however, that the devices have to be recovered to access the data (but see [56]), which is almost impossible in marine species that cannot be relocated and where telemetry signals become essential for tag recovery. Unsurprisingly, writing sensor data to a memory requires power, with overall power requirement being proportional to the number of sensors, the sampling frequency of those sensors and the deployment duration. Additionally, the type of memory used is also important. Flash memory, of which there are several types, can be written to rapidly, but does require high peak current for short periods of time while the data are written into the store, with peak current reaching up to 50 mA or more. This can have a detrimental effect on the battery itself if it is not capable of delivering those currents and can result in diminished overall capacity. Moreover, the ambient temperature at which the batteries are both stored and used when deployed can more dramatically affect the resulting capacity, with recommended operating temperatures typically within the range −20 to +60°C. At the higher extreme, the loss of capacity occurs along with increased internal resistance [57], while at the lower extreme, chemical reactions slow to such an extent that in some applications, the battery becomes useless [58]. One of the most power-demanding memories is the microSD Flash cards which, for a single sized package, can have capacities from a few MB to 100s of GB. This is more than sufficient capacity for year-long deployments on animals with high sampling rates, provided that the animal can carry the necessarily larger battery to interrogate the sensors and power the processor (see below), in addition to writing the data to the card. Additionally, we have found that the longer the deployment period, the more likely the cards are to be corrupted, making data access difficult or impossible to recover. The precise reasons for this are unclear because large memory microSD cards can be used in cameras for many months without problem. However, we believe that this is likely down to one or more scenarios, such as power being constantly applied for several days/weeks/months, in addition to potentially large temperature swings due to local environmental conditions. Of the memory chips, volatile RAM, or dynamic RAM (DRAM), requires the least current, but these systems require more complex circuitry to refresh the stored data repeatedly, which either means an additional battery in the logger or the researcher taking a risk by using one battery both to power the tag and safeguard the data. Non-volatile static RAM (NVSRAM) is yet another storage medium that is typically faster than Flash due to not requiring data to be written back upon reading, as is the case with DRAM. NVSRAM, however, is more costly than Flash due to the high number of transistors required per data bit (typically six or more) and is, therefore, generally manufactured at lower capacity. It can be used in conjunction with other storage media as a temporary buffer in preparation for a burst-write of a more significant quantity of data to the primary store. These days, biologgers generally use Flash RAM for its capacity, where the data, once written, are stored without requiring additional power.
Flash storage mechanisms generally require data to be written into a ‘page buffer’ on the chip of a fixed size, perhaps 256 B. Once the processor has completed the transfer, this page of data is then copied into the main Flash storage array. The larger the overall Flash capacity, the larger this page capacity may be. Flash chip controllers normally allow any number of bytes within the page to be written to, i.e. a transfer can be any number of bytes up to the maximum size of the page. If the processor is only buffering/transferring 256 B of sensor data, and the Flash chip has a page size of 4096 B, then, once the 256 B have been written into the page (of 4096 size), all 4096 B are written back into the main Flash area. This is obviously a waste of power as 3840 extra, unused bytes are being stored back to the main array. Note that before data can be written to Flash memory, ‘blocks’ must first be erased, whereby all bits are set to the equivalent of a ‘1’. When writing to Flash, a ‘1’ can be set to a ‘0’, but not the other way round. If data have been written once, the only means of changing data in the main storage array is to first erase blocks, which are generally larger than a page size. Ideally, the processor's internal memory store should at least match that of the Flash page size or be able to use an external buffer and stream data to the Flash page in one storage session. If the latter, then the processor must communicate with the external buffer simultaneously, requiring separate communication lines, further complicating the design.
(c) . Processors
Everything costs power. Processors used in data loggers are typically either 8 or 16 bit for the low sensor sampling frequency (few 10s of Hz) devices. Overall, reduced bit resolution and reduced sampling frequency (assuming the biological questions being asked of the system can be answered with those resolutions—see above) can improve efficiency because there is less ‘work done’ by both the sensor and the processor in transferring data from the sensor to the memory store. This includes fitting more data points per ‘page’ of memory due to the reduced bit-count per sensor. Specifically, for a data logger repeatedly requesting data from a sensor, possibly 40 times a second, any reduction in bytes transferred is power saved as the processor can return to sleep mode quicker (40 times a second). Many operate at 3.3–5 V, while manufacturers also offer 1.8–2.0 V varieties, allowing for significant power savings. Lithium batteries, either non-rechargeable or rechargeable, having an on-load voltage of between 3.6 and 4.2 V at full charge, can be efficiently ‘chopped up’ with some judicious dc–dc converter techniques (buck converters) and dropped to the required 1.8–2.0 V range, almost halving the power consumption. The same can be said for a lot of sensors such as accelerometers, magnetometers, gyroscopes, etc. that have a wide operating voltage range, from 5.0 V down to as little as 1.8 V. Low voltage will equate to lower current draw and, therefore, lower power usage overall as power can be equated to voltage×current drawmean. If all components can operate at this lower voltage then this will be the optimal solution, while hybrid systems that use both lower and higher voltages can coexist at the expense of a potentially higher component count.
Ideally, processors should be active for the minimum amount of time, sleeping where possible to minimize current draw. Different processors have varying abilities to power down sufficiently, typically to nW levels, and quickly enough to warrant the transition. The time taken to wake the processor up and for a timing crystal or resonator to stabilize before computations can commence is important and can add to the energy budget. Thus, minimizing the number of times the processor wakes up can increase efficiency significantly. Processors typically have different ‘levels’ of sleep and can power down unused internal modules/circuits to reduce overall power consumption. Some can drop to nA current draw levels during deep-sleep modes, but these often have a significantly longer switch-on time. To mitigate this, many sensor chips such as accelerometers, magnetometers, etc. have buffers and internal clocking mechanisms enabling the automated timed collection and storage of multiple sensor readings. When its buffer is full, the sensor signals the processor to wake-up and transfer the sensor's data buffer. This allows the processor to minimize power, only waking to coordinate data transfers between sensors and storage.
The clocking speed of the processor can also be very important. Processor current draw will increase approximately linearly with its clocking frequency, equating to the number of instructions it can process per second. Minimizing clock speed to reduce current draw may result in longer times to complete data transfers, but with some protocols current consumption may also increase.
(d) . Logging duration and batteries
The length of time that a tag will log information depends on the power draw of the complete system for its logging protocol and the battery capacity. Nominally, if a tag draws an effective continuous current of Y mA and the battery has a capacity of Z mA hours, the system will work for Z/Y hours. This is a simplistic view, however, because the theoretical maximum capacity of the battery can be compromised if the pulsed current exceeds the battery's specified rating [59]. This can also be exacerbated by low temperatures. For example, we have found that a ‘Daily Diary’ tag [60] that draws a mean current of 1.5 mA (with much higher peak currents), deployed on penguins swimming in 14°C water, operates for between about 2 and 6 days even though it is powered by a 750 mAh lithium cell (Eve EF651625), which should last for approximately 21 days. Importantly, there is also appreciable variation even within specified cells produced by any one manufacturer, but also variation in the ability of the battery to provide the requisite current without breaking down. In any event, not all batteries are created equal and while bigger batteries in general have greater capacity, and can withstand higher currents without breaking down, the different battery types have different qualities. The two most commonly used in biologgers are non-rechargeable lithium thionyl chloride (LTC) and rechargeable lithium-ion polymer. LTC cells have a higher energy density and so can theoretically power devices for longer for a given volume. This would seem to make them better for animal tags, but that assumes that the cells are operating well within their specified current limits. In contrast with rechargeable batteries, LTC cells are, however, especially for the lower volume devices, less able to provide higher currents, either continuously or more importantly in pulses, and so may not be able to power the circuit at all or only do so for a fraction of the intended duration. This is particularly relevant for GPS systems, which may draw 30 mA or more continuously for a number of seconds to calculate every fix due to the highly complex calculations required to triangulate the logger's position in three-dimensional space [59,61].
(i) . Topping batteries up
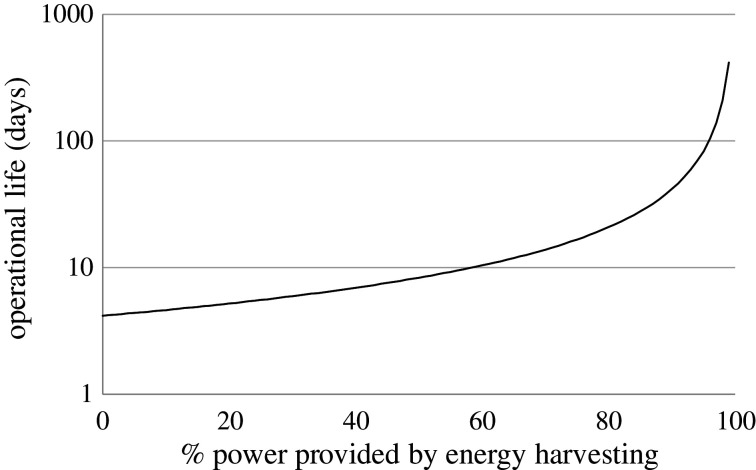
Any measure that can provide power to a rechargeable battery in a tag can increase operational life or allow a tag to have more sensors (see figure 2). Importantly, trickle-charging can be pivotal for study success, and not just because it may allow the unit to operate ‘indefinitely’, as is the aspiration of many users, such as those who transmit logger data rather than relying on tag recovery. Importantly, and perhaps more realistically, trickle-charging it can extend the operational life of the tag disproportionately in a predetermined manner that is analogous to the package actually having a markedly larger battery (figure 3).
Figure 3.
The theoretical operational life of a 100 mAh−1 battery required to provide a continuous current of 1 mA for a biologger while also being provided with power by an energy harvesting system. Note the nonlinear response (the y-axis is a log scale) and how small increases in inputted power produce a disproportionate extension of the operational life.
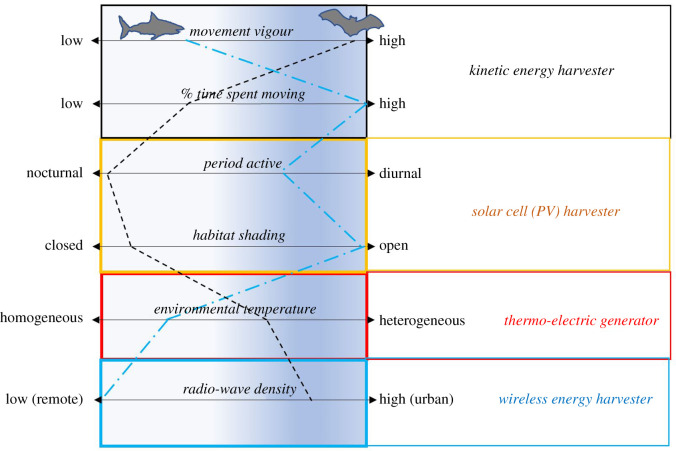
Trickle-charging is likely to become more topical, given the diversity of miniature energy harvesting systems available today, which range from mechano-harvesters [62] through thermo-electric generators [63] to radio-wave harvesters [64]. Wireless energy harvesting devices typically comprise a coil(s)/shaped antenna with dimensions typically designed for specific ranges of the radiofrequencies to be absorbed, to achieve maximum power transfer. However, the substantial attenuation of radio waves by saline environments (such as body tissues) and very reduced recovered energy anyway compared to the mean power usage of many data loggers storing to Flash memory makes this power source currently impractical. Solar cells (photovoltaics—PVs) absorb energy from the visible region of the electromagnetic spectrum, a different range to radio waves. Because of the wavelength of the visible spectrum, electron excitation through photon absorption generates free electrons, which can be stored either in a capacitor or rechargeable cell. It is surprising that biologgers today either have no topping-up system in place or those that do only use solar power [65]. Which energy harvesting system(s) is best suited for the study animal depends on the animal's lifestyle (figure 4) and also, critically, on whether the tag is implanted or external. Internal tags, especially in homeotherms, have little choice in energy harvesters since thermo-electric generators require a temperature difference to create a charge, while ambient energies such as electromagnetic radiation, including radio waves, cannot be perceived (see above) and so must operate with kinetic energy harvesters or none at all.
Figure 4.
Schematic diagram to show how animal behaviour and space/time use affects the viability of different energy harvesting mechanisms that might be employed to supply power to a biologger. The dashed lines crossing the left-hand panels show illustrative examples from a pelagic shark (blue) and an urban bat (black). (Online version in colour.)
Animal ecology and behaviour change, however, with season, so movement-based power harvesting systems which might work for a migrating bird may fail post-migration, so consideration also needs to be given to animal habits as well as the changes in available ambient energy (such as daylight length in solar cell systems). Finally, smart programming can also help extend the life of tags by duty-cycling or simply not recording data when the animal is quiescent [66].
As tags get smaller, and batteries become an increasingly important percentage of the overall tag volume and mass, we predict that interest in power generating systems for biologgers will increase. Smart phones will undoubtedly have a role in catalysing this process of technological advancement, as they have for sensors and memory.
(e) . Tag construction
Biologgers have to work in a diverse range of environments. The most accommodating are perhaps when they are implanted into the gentle, relatively stable insides of animals [67], although even there they may be subject to substantial changing pressure [68]. This contrasts to externally mounted options where the tags may have to operate in corrosive and conductive seawater environments (which can result in galvanic corrosion if there are dissimilar metals at the water interface), temperature extremes that may be below zero and/or be as high as 50°C, pressures as high as 200 bar [69] and have sensors that can be rapidly challenged by biofouling [70]. Sensors and data access ports that have to interact with the environment can, therefore, be problematic, not least because, even if the device is potted in resin, there is a line of weakness between them and the resin. This can lead to hairline cracks if the environment has large temperature fluctuations and there is differential thermal expansion between resin and them. Such large temperature fluctuations are prevalent in temperate penguins where, for example, in Magellanic penguins S. magellanicus, the temperature of the outside surface of the tag may range between 11 and 35°C with every dive cycle due to cold water and high insolation. Almost all marine tags that have to operate at depths exceeding 100 m are potted in resin, although at lesser depths, some companies use O-rings and air-filled systems. Any air in a tag (even as bubbles in resin) creates a weak spot since the pressure on it to compress (potentially causing cracks in the housing) goes up linearly with depth: at the surface, atmospheric pressure is about 68.8 kg m−2, but this goes up to 1.13 × 105 kg m−2 at 100 m depth, and 1.04 × 106 kg m−2 at 1 km. Thus, the design and construction of tags for deep-diving animals is critical.
(f) . Data recovery
Transmission of data is energetically costly but then so is recovery of the whole tag, especially if it is implanted. Today, a great many biologgers have to be recovered to acquire the data, a process which ranges from the trivial to extremely onerous [5]. In larger animals that can carry larger tags, a VHF or Argos location transmitter may be essential in tag recovery success [15]. Data transmission, where it can occur (because, for example, salt water does not allow the passage of radio waves), generally uses VHF systems (e.g. [71]), which is only viable for externally mounted tags. Transmission requires a link between the tag and a receiver that lasts long enough for a respectable amount of data to be transferred, and it requires that the tag have enough extra power to transmit. For both these reasons, most biologgers transmitting data cannot transmit continuous data recorded at high rates. Rather, they provide, for example, 3 s bursts of data for every minute of operation, which allows a reasonable snapshot of the animal's biology [72] while accepting the holes in information that this entails. Ultimately, the utility of this approach depends on the questions being asked.
(g) . Software
(i) . Tag programming
A logger with fixed logging frequency and sensor options will have a known power requirement and a reasonably well-defined projected run time. Against this, having smart logging protocols has a great potential for biologists to save power while acquiring the most useful data for their questions. For example, not powering up all sensors saves power, it gives the processor less data to collect, and subsequently less data to store, and so the primary data storage can be accessed less frequently. Additionally, not all sensors need to be sampled at the same frequency. For instance, acceleration might have sensors sampled at the highest frequency to enhance behaviour classification [73], while magnetometry may often be sampled at lower rates as it primarily provides heading metrics (e.g. [74]) and can easily be interpolated, while temperature measurements, for example, will likely have an inherent lag due to sensor encapsulation [44] so high-frequency measurements would be of little benefit. Overly complex programming options have the disadvantage of increasing the chances that the biologist in the field may make a mistake, something that is not trivial, given the difficulties of putting tags on animals in the first place.
(ii) . Quick inspection of tag data in the field
Many tag deployments are made after careful preparation in the laboratory and are ‘single shot’ events. Equally, many studies deploy tags repeatedly over time in the field, with researchers having to make tag-programming decisions based on the data they receive from deployments over time. This calls for proper inspection of recovered tag data to inform the next protocols. There is often poor appreciation of the importance of data inspection under such conditions. At its most base level, workers need to ascertain the correct functioning of all the sensors—without outliers and operating within the prescribed range—before redeployment. This requires that software be available in the field to allow the workers to inspect all of their recorded parameters as a function of time. Sadly, many do not do this, and may return from expeditions with poor, or no, data, which is a waste of time and resources and has important ethical implications [75]. We believe that tag manufacturers should provide such software, even if it is primitive, showing little more than sensor outputs over time graphically. Indeed, provision of this may have changed field studies considerably.
4. Common mistakes in data interpretation
Linked to the above point is more comprehensive understanding of sensor metrics, either direct or derived. The vast array of machine-learning programmes [76] makes it easy to collect data and, with little more than cursory inspection, analyse it within a programming ‘black box’. No biologist would take the mass of an animal without understanding what it means, but many today using accelerometry metrics, for example, are unclear what the values presented mean. This is particularly important when the behaviour-identifying programme relies on ‘validated’ data from an animal observed to behave in a particular manner while wearing a tag [77]. For instance, a study species ‘validated’ in level terrain will produce markedly different acceleration offsets if it normally lives in mountains because body pitch (manifest through the surge static acceleration values (cf. [73]) is rarely around zero. Similarly, proper understanding of the meaning of sensor data will help workers identify behaviours that are not observed in captivity and, therefore, cannot be ‘validated’ in the conventional sense. Some simple code can easily be constructed using either R or MatLab to allow the worker to visualize sample data while in the field and some tag manufacturers provide software that does this. There are numerous R packages available for deconstructing animal logger data, including using Boolean approaches for defining data [78], and visualizing this in two or more dimensions, including overlaying movement paths onto textured mapping technologies.
5. Conclusion
Although current biologgers are incredibly potent with respect to the information that they can gather, and thereby in their capacity to elucidate the behaviour, ecology and physiology of the study species, poor understanding of tag capacities and limitations can lead to misinterpretation. Basic knowledge of tag design and mode of operation, including issues such as resolution, sampling rate and power draw, should not be the province of the tag engineer alone. Indeed, proper understanding of such matters by scientists would, at once, ensure that the data taken represent what they are supposed to, and reduce the likelihood that the tag will underperform. Beyond this, the most comprehensive data on aspects of an animal's biology may be of little value if the tag itself causes aberrations in animal biology. We, therefore, need to be particularly vigilant with respect to potential tag detriment, understanding that good science and the wellbeing of the animals that carry the tags both depend on biological and engineering expertise operating together.
Contributor Information
Mark D. Holton, Email: m.d.holton@swansea.ac.uk.
Rory P. Wilson, Email: r.p.wilson@swansea.ac.uk.
Data accessibility
This article has no additional data.
Authors' contributions
M.D.H.: conception or design of the work; drafting the article; critical revision of the article throughout; final approval of the version. R.P.W.: conception or design of the work; drafting the article; critical revision of the article throughout; final approval of the version. J.T.: critical revision of the article throughout; final approval of the version. U.S.: critical revision of the article throughout; final approval of the version.
Competing interests
We declare we have no competing interests.
Funding
This research contributes to the CAASE project funded by King Abdullah University of Science and Technology (KAUST) under the KAUST Sensor Initiative.
References
- 1.Hebblewhite M, Haydon DT. 2010. Distinguishing technology from biology: a critical review of the use of GPS telemetry data in ecology. Phil. Trans. R. Soc. B 365, 2303-2312. ( 10.1098/rstb.2010.0087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown DD, Kays R, Wikelski M, Wilson R, Klimley AP. 2013. Observing the unwatchable through acceleration logging of animal behavior. Anim. Biotelemetry 1, 20. ( 10.1186/2050-3385-1-20) [DOI] [Google Scholar]
- 3.Williams HJ, et al. 2017. Identification of animal movement patterns using tri-axial magnetometry. Mov. Ecol. 5, 6. ( 10.1186/s40462-017-0097-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Godyń D, Herbut P, Angrecka S. 2019. Measurements of peripheral and deep body temperature in cattle—a review. J. Therm. Biol. 79, 42-49. ( 10.1016/j.jtherbio.2018.11.011) [DOI] [PubMed] [Google Scholar]
- 5.Williams H, Shepard E, Holton MD, Alarcón P, Wilson R, Lambertucci S. 2020. Physical limits of flight performance in the heaviest soaring bird. Proc. Natl Acad. Sci. USA 117, 17 884-17 890. ( 10.1073/pnas.1907360117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green JA. 2011. The heart rate method for estimating metabolic rate: review and recommendations. Comp. Biochem. Physiol. A 158, 287-304. ( 10.1016/j.cbpa.2010.09.011) [DOI] [PubMed] [Google Scholar]
- 7.Bridge ES, Kelly JF, Contina A, Gabrielson RM, MacCurdy RB, Winkler DW. 2013. Advances in tracking small migratory birds: a technical review of light-level geolocation. J. Field Ornithol. 84, 121-137. ( 10.1111/jofo.12011) [DOI] [Google Scholar]
- 8.Teilmann J, Agersted MD, Heide-Jørgensen MP. 2020. A comparison of CTD satellite-linked tags for large cetaceans—bowhead whales as real-time autonomous sampling platforms. Deep Sea Res. Part I 157, 103213. ( 10.1016/j.dsr.2020.103213) [DOI] [Google Scholar]
- 9.Block BA. 2005. Physiological ecology in the 21st century: advancements in biologging science. Integr. Comp. Biol. 45, 305-320. ( 10.1093/icb/45.2.305) [DOI] [PubMed] [Google Scholar]
- 10.Bishop CM, et al. 2015. The roller coaster flight strategy of bar-headed geese conserves energy during Himalayan migrations. Science 347, 250-254. ( 10.1126/science.1258732) [DOI] [PubMed] [Google Scholar]
- 11.Meir JU, York JM, Chua BA, Jardine W, Hawkes LA, Milsom WK. 2019. Reduced metabolism supports hypoxic flight in the high-flying bar-headed goose (Anser indicus). eLife 8, e44986. ( 10.7554/eLife.44986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Block BA, et al. 2001. Migratory movements, depth preferences, and thermal biology of Atlantic bluefin tuna. Science 293, 1310-1314. ( 10.1126/science.1061197) [DOI] [PubMed] [Google Scholar]
- 13.Toledo S, Shohami D, Schiffner I, Lourie E, Orchan Y, Bartan Y, Nathan R. 2020. Cognitive map-based navigation in wild bats revealed by a new high-throughput tracking system. Science 369, 188-193. ( 10.1126/science.aax6904) [DOI] [PubMed] [Google Scholar]
- 14.Bograd SJ, Block BA, Costa DP, Godley BJ. 2010. Biologging technologies: new tools for conservation. Endanger. Species Res. 10, 1-7. ( 10.3354/esr00269) [DOI] [Google Scholar]
- 15.Mikkelsen L, Johnson M, Wisniewska DM, van Neer A, Siebert U, Madsen PT, Teilmann J. 2019. Long-term sound and movement recording tags to study natural behavior and reaction to ship noise of seals. Ecol. Evol. 9, 2588-2601. ( 10.1002/ece3.4923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson MP, Tyack PL. 2003. A digital acoustic recording tag for measuring the response of wild marine mammals to sound. IEEE J. Oceanic Eng. 28, 3-12. ( 10.1109/JOE.2002.808212) [DOI] [Google Scholar]
- 17.Nielsen NH, Teilmann J, Sveegaard S, Hansen RG, Sinding M-HS, Dietz R, Heide-Jørgensen MP. 2018. Oceanic movements, site fidelity and deep diving in harbour porpoises from Greenland show limited similarities to animals from the North Sea. Mar. Ecol. Prog. Ser. 597, 259-272. ( 10.3354/meps12588) [DOI] [Google Scholar]
- 18.Stidsholt L, Johnson M, Beedholm K, Jakobsen L, Kugler K, Brinkløv S, Salles A, Moss CF, Madsen PT. 2019. A 2.6-g sound and movement tag for studying the acoustic scene and kinematics of echolocating bats. Methods Ecol. Evol. 10, 48-58. ( 10.1111/2041-210X.13108) [DOI] [Google Scholar]
- 19.DeRuiter SL, Langrock R, Skirbutas T, Goldbogen JA, Chalambokidis J, Friedlaender AS, Southall BL. 2016. A multivariate mixed hidden Markov model to analyze blue whale diving behaviour during controlled sound exposures. arXiv preprint. ( 10.1038/arXiv:1602.06570) [DOI]
- 20.Abrahms B, et al. 2019. Memory and resource tracking drive blue whale migrations. Proc. Natl Acad. Sci. USA 116, 5582-5587. ( 10.1073/pnas.1819031116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ripperger SP, et al. 2020. Thinking small: next-generation sensor networks close the size gap in vertebrate biologging. PLoS Biol. 18, e3000655. ( 10.1371/journal.pbio.3000655) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pennycuick C, Fast PL, Ballerstädt N, Rattenborg N. 2012. The effect of an external transmitter on the drag coefficient of a bird's body, and hence on migration range, and energy reserves after migration. J. Ornithol. 153, 633-644. ( 10.1007/s10336-011-0781-3) [DOI] [Google Scholar]
- 23.Saraux C, et al. 2011. Reliability of flipper-banded penguins as indicators of climate change. Nature 469, 203-206. ( 10.1038/nature09630) [DOI] [PubMed] [Google Scholar]
- 24.Portugal SJ, White CR. 2018. Miniaturization of biologgers is not alleviating the 5% rule. Methods Ecol. Evol. 9, 1662-1666. ( 10.1111/2041-210X.13013) [DOI] [Google Scholar]
- 25.Stanton NA, Salmon P, Harris D, Marshall A, Demagalski J, Young MS, Waldmann T, Dekker S. 2009. Predicting pilot error: testing a new methodology and a multi-methods and analysts approach. Appl. Ergon. 40, 464-471. ( 10.1016/j.apergo.2008.10.005) [DOI] [PubMed] [Google Scholar]
- 26.White CR, Cassey P, Schimpf NG, Halsey LG, Green JA, Portugal SJ. 2013. Implantation reduces the negative effects of bio-logging devices on birds. J. Exp. Biol. 216, 537-542. ( 10.1242/jeb.076554) [DOI] [PubMed] [Google Scholar]
- 27.Bodey TW, Cleasby IR, Bell F, Parr N, Schultz A, Votier SC, Bearhop S. 2018. A phylogenetically controlled meta-analysis of biologging device effects on birds: deleterious effects and a call for more standardized reporting of study data. Methods Ecol. Evol. 9, 946-955. ( 10.1111/2041-210X.12934) [DOI] [Google Scholar]
- 28.Hawkins P. 2004. Bio-logging and animal welfare: practical refinements. Mem. Natl Inst. Polar Res. 58, 58-68. [Google Scholar]
- 29.Vandenabeele SP, Grundy E, Friswell MI, Grogan A, Votier SC, Wilson RP. 2014. Excess baggage for birds: inappropriate placement of tags on gannets changes flight patterns. PLoS ONE 9, e92657. ( 10.1371/journal.pone.0092657) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gillies N, et al. 2020. Short-term behavioural impact contrasts with long-term fitness consequences of biologging in a long-lived seabird. Sci. Rep. 10, 1-10. ( 10.1038/s41598-020-72199-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasiulis AL, Festa-Bianchet M, Couturier S, Côté SD. 2014. The effect of radio-collar weight on survival of migratory caribou. J. Wildl. Manag. 78, 953-956. ( 10.1002/jwmg.722) [DOI] [Google Scholar]
- 32.McCafferty DJ, Currie J, Sparling CE. 2007. The effect of instrument attachment on the surface temperature of juvenile grey seals (Halichoerus grypus) as measured by infrared thermography. Deep Sea Res. Part II 54, 424-436. ( 10.1016/j.dsr2.2006.11.019) [DOI] [Google Scholar]
- 33.Kay WP, et al. 2019. Minimizing the impact of biologging devices: using computational fluid dynamics for optimizing tag design and positioning. Methods Ecol. Evol. 10, 1222-1233. ( 10.1111/2041-210X.13216) [DOI] [Google Scholar]
- 34.Wilson RP, Spairani HJ, Coria NR, Culik BM, Adelung D. 1990. Packages for attachment to seabirds: what color do Adelie penguins dislike least? J. Wildl. Manag. 54, 447-451. ( 10.2307/3809657) [DOI] [Google Scholar]
- 35.Jones TT, et al. 2011. Determining transmitter drag and best-practice attachment procedures for sea turtle biotelemetry. National Oceanographic and Atmospheric Administration Technical Memorandum 480, https://repository.library.noaa.gov/view/noaa/4512 .
- 36.Bannasch R, Wilson RP, Culik B. 1994. Hydrodynamic aspects of design and attachment of a back-mounted device in penguins. J. Exp. Biol. 194, 83-96. ( 10.1242/jeb.194.1.83) [DOI] [PubMed] [Google Scholar]
- 37.Whitford M, Klimley AP. 2019. An overview of behavioral, physiological, and environmental sensors used in animal biotelemetry and biologging studies. Anim. Biotelemetry 7, 1-24. ( 10.1186/s40317-019-0189-z) [DOI] [Google Scholar]
- 38.Clark TD, Sandblom E, Hinch S, Patterson D, Frappell P, Farrell A. 2010. Simultaneous biologging of heart rate and acceleration, and their relationships with energy expenditure in free-swimming sockeye salmon (Oncorhynchus nerka). J. Comp. Physiol. B 180, 673-684. ( 10.1007/s00360-009-0442-5) [DOI] [PubMed] [Google Scholar]
- 39.Williams TM, Blackwell SB, Richter B, Sinding M-HS, Heide-Jørgensen MP. 2017. Paradoxical escape responses by narwhals (Monodon monoceros). Science 358, 1328-1331. ( 10.1126/science.aao2740) [DOI] [PubMed] [Google Scholar]
- 40.Thouzeau C, Peters G, Le Bohec C, Le Maho Y. 2004. Adjustments of gastric pH, motility and temperature during long-term preservation of stomach contents in free-ranging incubating king penguins. J. Exp. Biol. 207, 2715-2724. ( 10.1242/jeb.01074) [DOI] [PubMed] [Google Scholar]
- 41.Wilson RP, Simeone A, Luna-Jorquera G, Steinfurth A, Jackson S, Fahlman A. 2003. Patterns of respiration in diving penguins: is the last gasp an inspired tactic? J. Exp. Biol. 206, 1751-1763. ( 10.1242/jeb.00341) [DOI] [PubMed] [Google Scholar]
- 42.Yoda K, Sato K, Niizuma Y, Kurita M, Bost C, Le Maho Y, Naito Y. 1999. Precise monitoring of porpoising behaviour of Adelie penguins determined using acceleration data loggers. J. Exp. Biol. 202, 3121-3126. ( 10.1242/jeb.202.22.3121) [DOI] [PubMed] [Google Scholar]
- 43.Wilmers CC, Nickel B, Bryce CM, Smith JA, Wheat RE, Yovovich V. 2015. The golden age of bio-logging: how animal-borne sensors are advancing the frontiers of ecology. Ecology 96, 1741-1753. ( 10.1890/14-1401.1) [DOI] [PubMed] [Google Scholar]
- 44.Wilson RP, et al. 2002. Remote-sensing systems and seabirds: their use, abuse and potential for measuring marine environmental variables. Mar. Ecol. Prog. Ser. 228, 241-261. ( 10.3354/meps228241) [DOI] [Google Scholar]
- 45.Weimerskirch H, Wilson RP, Guinet C, Koudil M. 1995. Use of seabirds to monitor sea-surface temperatures and to validate satellite remote-sensing measurements in the Southern Ocean. Mar. Ecol. Prog. Ser. 126, 299-303. ( 10.3354/meps126299) [DOI] [Google Scholar]
- 46.Ropert-Coudert Y, Wilson RP. 2005. Trends and perspectives in animal-attached remote sensing. Front. Ecol. Environ. 3, 437-444. ( 10.1890/1540-9295(2005)003[0437:TAPIAR]2.0.CO;2) [DOI] [Google Scholar]
- 47.Popper K. 1978. The myth of inductive hypothesis generation. Conjectures and refutations. London, UK: Routledge & Kegan Paul. [Google Scholar]
- 48.Wilson RP, Culik BM, Bannash R, Driesen H. 1992. Monitoring penguins at sea using data loggers. In Proc. Biotelemetry XII, 31 August–5 September, Ancona, Italy, pp. 205-214.
- 49.Sutton G, Pichegru L, Botha JA, Kouzani AZ, Adams S, Bost CA, Arnould JP. 2020. Multi-predator assemblages, dive type, bathymetry and sex influence foraging success and efficiency in African penguins. PeerJ 8, e9380. ( 10.7717/peerj.9380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kooyman GL, Cornell LH. 1981. Physiological zoology. Chicago, IL: University of Chicago Press. [Google Scholar]
- 51.Herbst CT, Stoeger AS, Frey R, Lohscheller J, Titze IR, Gumpenberger M, Fitch WT. 2012. How low can you go? Physical production mechanism of elephant infrasonic vocalizations. Science 337, 595-599. ( 10.1126/science.1219712) [DOI] [PubMed] [Google Scholar]
- 52.Günther RH, O'Connell-Rodwell CE, Klemperer SL. 2004. Seismic waves from elephant vocalizations: a possible communication mode? Geophys. Res. Lett. 31, L11602. ( 10.1029/2004GL019671) [DOI] [Google Scholar]
- 53.Hedlin MA, Walker KT. 2013. A study of infrasonic anisotropy and multipathing in the atmosphere using seismic networks. Phil. Trans. R. Soc. A 371, 20110542. ( 10.1098/rsta.2011.0542) [DOI] [PubMed] [Google Scholar]
- 54.Naito Y. 2004. New steps in bio-logging science. Mem. Natl Inst. Polar Res. 58, 50-57. [Google Scholar]
- 55.Andrzejaczek S, Gleiss AC, Lear KO, Pattiaratchi CB, Chapple TK, Meekan MG. 2019. Biologging tags reveal links between fine-scale horizontal and vertical movement behaviors in tiger sharks (Galeocerdo cuvier). Front. Mar. Sci. 6, 229. ( 10.3389/fmars.2019.00229) [DOI] [Google Scholar]
- 56.Flack A, Nagy M, Fiedler W, Couzin ID, Wikelski M. 2018. From local collective behavior to global migratory patterns in white storks. Science 360, 911-914. ( 10.1126/science.aap7781) [DOI] [PubMed] [Google Scholar]
- 57.Ning G, Haran B, Popov BN. 2003. Capacity fade study of lithium-ion batteries cycled at high discharge rates. J. Power Sources 117, 160-169. ( 10.1016/S0378-7753(03)00029-6) [DOI] [Google Scholar]
- 58.Belt JR, Ho CD, Motloch CG, Miller TJ, Duong TQ. 2003. A capacity and power fade study of Li-ion cells during life cycle testing. J. Power Sources 123, 241-246. ( 10.1016/S0378-7753(03)00537-8) [DOI] [Google Scholar]
- 59.Savoye F, Venet P, Millet M, Groot J. 2011. Impact of periodic current pulses on Li-ion battery performance. IEEE Trans. Ind. Electron. 59, 3481-3488. ( 10.1109/TIE.2011.2172172) [DOI] [Google Scholar]
- 60.Wilson RP, Shepard E, Liebsch N. 2008. Prying into the intimate details of animal lives: use of a daily diary on animals. Endanger. Species Res. 4, 123-137. ( 10.3354/esr00064) [DOI] [Google Scholar]
- 61.El-naggar AM. 2012. New method of GPS orbit determination from GCPS network for the purpose of DOP calculations. Alexandria Eng. J. 51, 129-136. ( 10.1016/j.aej.2012.06.002) [DOI] [Google Scholar]
- 62.Niroomand M, Foroughi HR. 2016. A rotary electromagnetic microgenerator for energy harvesting from human motions. J. Appl. Res. Technol. 14, 259-267. ( 10.1016/j.jart.2016.06.002) [DOI] [Google Scholar]
- 63.Lund A, Tian Y, Darabi S, Müller C. 2020. A polymer-based textile thermoelectric generator for wearable energy harvesting. J. Power Sources 480, 228836. ( 10.1016/j.jpowsour.2020.228836) [DOI] [Google Scholar]
- 64.Song C, Huang Y, Zhou J, Carter P, Yuan S, Xu Q, Fei Z. 2016. Matching network elimination in broadband rectennas for high-efficiency wireless power transfer and energy harvesting. IEEE Trans. Ind. Electron. 64, 3950-3961. ( 10.1109/TIE.2016.2645505) [DOI] [Google Scholar]
- 65.Saha CR, Huda MN, Mumtaz A, Debnath A, Thomas S, Jinks R. 2020. Photovoltaic (PV) and thermo-electric energy harvesters for charging applications. Microelectron. J. 96, 104685. ( 10.1016/j.mejo.2019.104685) [DOI] [Google Scholar]
- 66.Korpela JM, Suzuki H, Matsumoto S, Mizutani Y, Samejima M, Maekawa T, Nakai J, Yoda K.. 2019. AI on animals: AI-assisted animal-borne logger never misses the moments that biologists want. bioRxiv 630053. ( 10.1038/bioRxiv:630053) [DOI] [PMC free article] [PubMed]
- 67.Grémillet D, Kuntz G, Gilbert C, Woakes AJ, Butler PJ, Yl M. 2005. Cormorants dive through the Polar night. Biol. Lett. 1, 469-471. ( 10.1098/rsbl.2005.0356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Green J, Butler P, Woakes A, Boyd I. 2003. Energetics of diving in macaroni penguins. J. Exp. Biol. 206, 43-57. ( 10.1242/jeb.00059) [DOI] [PubMed] [Google Scholar]
- 69.Tyack PL, Johnson M, Soto NA, Sturlese A, Madsen PT. 2006. Extreme diving of beaked whales. J. Exp. Biol. 209, 4238-4253. ( 10.1242/jeb.02505) [DOI] [PubMed] [Google Scholar]
- 70.Hammerschlag N, Cooke SJ, Gallagher AJ, Godley BJ. 2014. Considering the fate of electronic tags: interactions with stakeholders and user responsibility when encountering tagged aquatic animals. Methods Ecol. Evol. 5, 1147-1153. ( 10.1111/2041-210X.12248) [DOI] [Google Scholar]
- 71.Rock P, Camphuysen C, Shamoun-Baranes J, Ross-Smith VH, Vaughan IP. 2016. Results from the first GPS tracking of roof-nesting herring gulls Larus argentatus in the UK. Ringing Migr. 31, 47-62. ( 10.1080/03078698.2016.1197698) [DOI] [Google Scholar]
- 72.Mayer M, Fog Bjerre DH, Sunde P. 2020. Better safe than sorry: the response to a simulated predator and unfamiliar scent by the European hare. Ethology 126, 704-715. ( 10.1111/eth.13019) [DOI] [Google Scholar]
- 73.Shepard EL, et al. 2008. Identification of animal movement patterns using tri-axial accelerometry. Endanger. Species Res. 10, 47-60. ( 10.3354/esr00084) [DOI] [Google Scholar]
- 74.Walker JS, et al. 2015. Prying into the intimate secrets of animal lives; software beyond hardware for comprehensive annotation in ‘Daily Diary’ tags. Mov. Ecol. 3, 1-16. ( 10.1186/s40462-015-0056-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bidder O, Arandjelović O, Almutairi F, Shepard E, Lambertucci SA, Qasem L, Wilson R. 2014. A risky business or a safe BET? A Fuzzy Set Event Tree for estimating hazard in biotelemetry studies. Anim. Behav. 93, 143-150. ( 10.1016/j.anbehav.2014.04.025) [DOI] [Google Scholar]
- 76.Valletta JJ, Torney C, Kings M, Thornton A, Madden J. 2017. Applications of machine learning in animal behaviour studies. Anim. Behav. 124, 203-220. ( 10.1016/j.anbehav.2016.12.005) [DOI] [Google Scholar]
- 77.Campbell HA, Gao L, Bidder OR, Hunter J, Franklin CE. 2013. Creating a behavioural classification module for acceleration data: using a captive surrogate for difficult to observe species. J. Exp. Biol. 216, 4501-4506. ( 10.1242/jeb.089805) [DOI] [PubMed] [Google Scholar]
- 78.Wilson RP, et al. 2018. Give the machine a hand: a Boolean time-based decision-tree template for rapidly finding animal behaviours in multisensor data. Methods Ecol. Evol. 9, 2206-2215. ( 10.1111/2041-210X.13069) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.