Abstract
The goal of achieving enhanced diagnosis and continuous monitoring of human health has led to a vibrant, dynamic and well-funded field of research in medical sensing and biosensor technologies. The field has many sub-disciplines which focus on different aspects of sensor science; engaging engineers, chemists, biochemists and clinicians, often in interdisciplinary teams. The trends which dominate include the efforts to develop effective point of care tests and implantable/wearable technologies for early diagnosis and continuous monitoring. This review will outline the current state of the art in a number of relevant fields, including device engineering, chemistry, nanoscience and biomolecular detection, and suggest how these advances might be employed to develop effective systems for measuring physiology, detecting infection and monitoring biomarker status in wild animals. Special consideration is also given to the emerging threat of antimicrobial resistance and in the light of the current SARS-CoV-2 outbreak, zoonotic infections. Both of these areas involve significant crossover between animal and human health and are therefore well placed to seed technological developments with applicability to both human and animal health and, more generally, the reviewed technologies have significant potential to find use in the measurement of physiology in wild animals.
This article is part of the theme issue ‘Measuring physiology in free-living animals (Part II)’.
Keywords: biosensors, MEMs, neuroprosthetic devices, antimicrobial resistance, zoonoses
1. Background
Sensor technologies have been an active area of research for a great many years with examples of the oxygen monitoring electrode for surgery and first-, second- and third-generation glucose biosensors finding widespread adoption and now routine use [1]. Sensor technologies have enabled environmental monitoring [2], improved control of industrial processes [3], given rise to purer drug formulations [4,5] and found numerous medical and biological applications, including the measurement of glucose for diabetes [6], lactate for a range of conditions [7] and the routine determination of blood clotting [8]. Sensor technologies have also been used in wild animal research since the first application of an intravascular pO2 electrode in a diving penguin in 2005 [9], but the field of wild animal sensing lags far behind the biomedical industry. The development and implementation of smart sensing technologies is a growing area and builds on these advances in sensor science. For humans, there is now a strong focus on converting existing and established sensor formats into ‘wearable devices’ in order to achieve real time surveillance of physiological status and biomarker levels [10–12]. The ability to miniaturize and implant sensor systems is driven by fundamental advances in a range of disciplines, including microsystems engineering, chemistry (enhanced bio-recognition elements, hydrogel research and new nanoscale technologies) and biomarker discovery.
Biosensor technologies can take many forms but a convenient and generic way to think about a biosensor is presented in figure 1. The sensor must be brought into contact with the sample. The sample can undergo a pre-processing step such as the conversion of blood to serum via centrifugation if required, or unprocessed samples can either be placed on the sensor (in vitro diagnostics) or allowed to come directly into contact with the sensor in the natural physiological state as in the case of wearable or implantable devices. Selectivity for the analyte of interest is achieved by employing a ‘bio-recognition element’ to selectively capture the analyte of interest from the sample. Binding and de-binding of the analyte gives rise to signal changes which can be measured through the sensor's transducer element. There are many governing principles for the method of signal transduction, including, optical, electrochemical, piezoelectric, thermal, magnetic and electrical. Once the signal has been generated it must be turned into an electrical format for display and interpretation.
Figure 1.
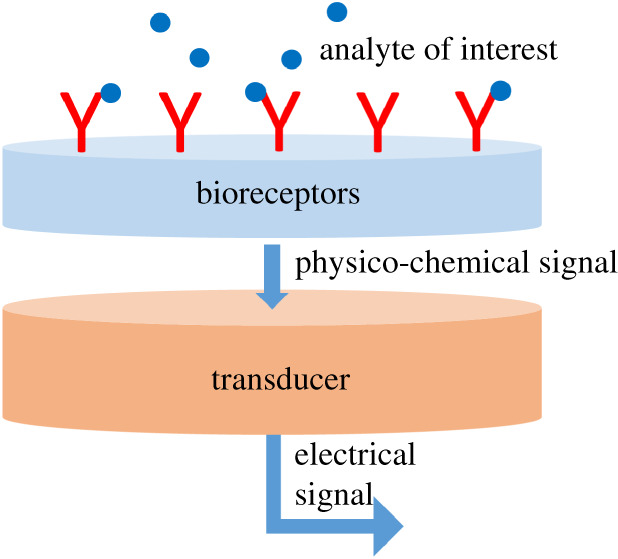
Conceptual scheme for a generic biosensor. The receptor layer can use many biological receptors (enzymes, antibodies, oligonucleotide sequences, aptamers, etc.) or non-biological entities such as molecularly imprinted polymers (MIPs) or various chemical functionalities to increase selectivity. Detectable analytes include: chemical species, nucleic acid sequences and proteins. The transducer element refers to the underlying sensor principle, of which there are many, including: optical, electrochemical, piezoelectric, thermal and electrical sensors. (Online version in colour.)
The biomedical sensing field is a hotbed of innovation with numerous academic and industrial groups working directly on the development of new technologies. The relatively high levels of competition in the field along with the fact that consumers are embracing smart healthcare technologies allied with the economic pressures on healthcare providers and governments are in combination leading to new technologies which are by design low cost, mass manufacturable, durable, ‘connected’ and simple to use. At the same time, physiological research over the last several decades has showed that wild animals have developed physiological mechanisms with which to cope with some of the greatest problems facing society at present, including obesity [13], hyperglycaemia [14], hypoxia/hypoxaemia [15,16] and viral infection [17], but these have been relatively poorly studied to date owing to a paucity of available technologies. Many new biosensor systems have applicability to the study of physiological status in free-living animals where constant manipulation of the device is not possible and also where long-term power budget, connectivity and durability are important considerations. The fact that devices are becoming more lightweight, less invasive and easier to place is also an important trend. After giving some consideration to existing systems for recording physiological measurements in wild animals, this article will explore recent innovations in human biomedical sensing on a sub-disciplinary basis, highlighting high quality articles which report useful developments of potential relevance to studying animal physiology and more broadly discusses the prospects for deploying such technologies in wild-animal monitoring. The article has chosen to focus on developments in disciplines which focus on detection of diseased states in human health. This is because these technologies face the most stringent demands in terms of sensitivity, implantability and power budget and therefore have the most to offer in terms of measuring ill health and disease among animal populations as well as monitoring animals in normal health. Developments in human wearable devices are well covered elsewhere and are signposted in the section below which makes a brief commentary on wearable devices and smart textiles. Finally, the article introduces the idea of extrapolating the measurement of human biomarkers into animal populations using cutting-edge technologies. This is important because many of the current wearable technologies do not have the ability to measure beyond simple biomarkers (blood glucose, lactate, O2) meaning that some of the technologies highlighted raise the potential for monitoring real time biomarker changes in animals, in vivo, at ng ml−1 and pg ml−1 levels—something outside the limits of current wearable solutions.
2. Examples of current technologies used in wild-animal monitoring
The range of measurements available and the underlying principles of animal monitoring technology have been well reviewed recently [18]. Wild-animal tracking is a vibrant and mature field and it is clear that established technologies allow the measurement of several important parameters, including many characteristics of animal performance such as speed [19], acceleration and motion [20], stroke rate/venous oxygenation during diving [21] and soaring height [22], which are measured with ease. Physiological, biochemical and environmental parameters such as dissolved oxygen [23], heart rate [24], electroencephalography (EEG) [25] and gastric pH [26] are also measurable with current devices and provide valuable information on important aspects of animal physiology and the nature of the environment in which they live. Consideration has also been given to effective blood glucose monitoring in populations of grey seals [27]. These are a very small subset of published studies with similar works existing for a wide range of parameters and sensor types [18]. As with humans the main challenge for next generation sensing is reliably accessing the biochemical realm with easily deployed, ‘smart’ devices with useful measurement lifetimes. In the face of climate change, increased interactions with human societies and disease outbreaks, it will be ever more important to be able to measure the biochemical, physiological and pathological status of wild and captive animals. One very pertinent example is the outbreak of a mutated strain of SARS-CoV-2 in mink in Denmark1 and the requirement to track pathogen spread quickly within an animal population. A highly intriguing possibility is the development of such ‘smart’, low cost, easy to deploy and robust sensors to allow animals living freely in the environment to be used as a network of sensors for environmental change, pathogen emergence and spread and as a proxy for certain aspects of human activity—this area has been recently well reviewed [28]. The following sections of this review will outline recent advances in engineering, chemistry and biomolecular science specifically for medical devices and suggest how and where these can potentially be applied to measurements in wild animals. This article is not intended to be an exhaustive review of established monitoring technologies in wild animals; its aim is to highlight cutting-edge advances in chemistry, microsystems engineering and nanoscience, particularly those arising from human medical applications and point out how in the future they might be developed for use in wild animals.
3. Developments in microsystems engineering and implantable devices
Modern biosensor technologies are often underpinned by cutting-edge engineering. To understand how such devices operate and might be applied to the measurement of physiological status in wild animals it is necessary to understand the production techniques employed, key design features and the relative advantages these approaches confer upon the final device and its performance. Due to the fact that our focus is on measurement in wild animals, engineering themes which are applicable to this area have been selected for further elaboration.
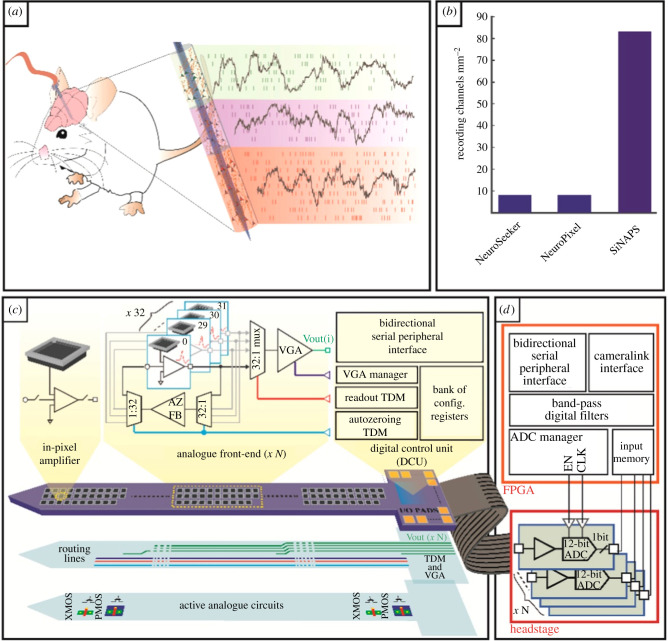
Microsystems engineering is a key area which underpins many modern biosensor technologies. The area is more often referred to as ‘microelectromechanical systems' (MEMS) and commonly refers to devices on the scale of 1–100 µm with smaller devices referred to as ‘nanoelectromechanical systems’ (NEMS). Effective biosensing strategies and investigations into the enhanced analytical performance of ‘small’ electrode sensors have been demonstrated with micro- [29] and nanoscale electrodes [30]. These studies have often focussed on ex vivo measurement of a particular gene sequence or protein and so do not shed much insight into the prospects for long-term implantation or wearability. A current area in clinical/biomedical engineering where MEMS devices play a key role is neuroscience and there is a whole sub-discipline dedicated to the design, fabrication and deployment of neuroprosthetic devices for real time recording of brain activity [31]. Indeed perhaps the most sophisticated physiological devices in use in wild animals so far is also in this discipline, with small loggers recording brain activity in several species of free-flying birds [32], tackling research questions concerning navigation [33] and sleep [34] A strong theme at present driving much innovation is the field of ‘optogenetics’ where optical stimulations in combination with genetic manipulation of neural circuits are used to modulate neuronal activity, which is then subsequently recorded using microelectrodes or microelectrode arrays [35]. The ability to specifically modify neuronal circuits and modulate their activity has provided great insight into brain structure and function. Additionally, the area is seen as a promising route towards the treatment of conditions such as epilepsy and Parkison's disease. Recent work in this area has seen the combination of optogenetic modulation of neuronal circuitry coupled with dopamine detection using a microfabricated device bearing a micro light-emitting diode (LED) and a thin film PEDOT working electrode [36]. The reported device was implanted into free-living mice and used to modulate and record neurotransmitter release. An elegant aspect of this study is the selective measurement of a neurotransmitter in combination with neuronal activity and optogenetic manipulation. The use of MEMS-based systems to achieve neuro-optogenetic modulation along with targeted refillable drug release has also been demonstrated in an impressive study showing realization of an injectable device with on-board drug delivery for optogenetic stimulation and combined drug release [37], which opens up potential for simultaneous stimulation and release in wild-animal populations. A recently reported system called ‘SiNAPS’ [38] is featured in figure 2 and is a multi-channel single shaft neuroprosthetic device capable of recording neuronal activity in highly localized regions of the brain with exceptionally high spatial and temporal resolution. Through a new and innovative design, the technology overcomes previous challenges of making simultaneous multi-channel neuronal recordings without expanding the size of the chip. Neuroprosthetic devices such as those mentioned above have tremendous applicability to the measurement of physiology in wild animals; the ability to track neuronal activity in brain circuits has largely been restricted to laboratory-based investigations but with developments such as these the possibility of long-term neurological monitoring and even neurological manipulation in wild animals is coming closer. Indeed such technology would not just allow description of the neuronal activity of wild animals at liberty (e.g. during flying and diving [39,40]) but also reveal and even mechanistically isolate the neural factors that pre-dispose wild animals to stressors such as capture and handling for study, and anthropogenic light [32].
Figure 2.
Architecture of the SiNAPS probe and of the recording system. (a) Implantable CMOS (complementary metal-oxide semiconductor) probes with dense electrode arrays can record broad-band bioelectrical signals across brain circuits with sub-millisecond and single-neuron resolutions. (b) Comparison of the integration potential of simultaneously recording electrodes (i.e. channels) per total silicon area (i.e. shaft and base of the probe) for different architectures proposed in the literature. SiNAPS probes achieve a number of effectively recording channels per unit of silicon area that is one order of magnitude larger than other presently available CMOS architectures and the NeuroPixels. (c,d) Schematics of the circuit architecture for the SiNAPS probe (c) and its acquisition system providing simultaneous neural recordings from the entire electrode array (d). Each electrode-pixel features an electrode and a small area DC-coupled in-pixel circuit for local amplification and low-pass filtering. A probe integrates multiple instances of the same low-area and low-power analogue front-end module of 32 electrode-pixels that are read out in a time-division multiplexed fashion. The on-probe digital control unit (DCU) provides the timing signals required for correct circuit operation and implements a bidirectional serial peripheral interface (SPI) for device configuration. A field-programmable gate array (FPGA)-based acquisition unit generates timing signals for the analog to digital converters (ADCs) and provides a camera link standard connection with a PC for data storage and online visualization [38]. TDM, time division multiplexing; VGA, video graphics array. (Online version in colour.)
Electrochemical biosensors rely on the use of a potentiostat to measure currents and voltages at the biosensor electrodes under test. Traditionally, the potentiostat has tended to be a laboratory-based instrument, often large in size and inflexible in terms of its measurement options. In recent years, there has been a large amount of research on portable, low cost instruments with a strong emphasis on instruments which can be assembled for less than $100 for use in field testing and also on the development of potentiostats using integrated circuits for implantable detection purposes. The DSTaT [41] and PSoC-Stat [42] instruments were both developed in an open source manner using off-the-shelf components and bespoke graphical user interfaces and, using both voltammetric and amperometric measurements, were employed to detect the potassium ferri-ferro cyanide redox couple and citric acid, glucose and lead, respectively. Another device, the SimpleStat was developed at a cost of approximately £5 per unit and was used to detect the oxacillin resistance gene from bacterial samples [43]. Low cost potentiostatic systems are likely to drive the adoption of simple in-the-field measurements which, for example, might allow the detection of drug resistant bacteria or potentially zoonotic infections among groups of wild animals. With this in mind, consideration has been given specifically to the requirements of any potentiostat dedicated to probing the properties of bacterial samples [44]. Finally, on the topic of low cost electrochemical platforms, the iMED device shows promise as a combined potentiostat with microfluidic and electrochemical components, already achieving reliable glucose detection [45]. To progress beyond low cost external circuitry and realize implanted electrochemical measurements it is necessary to develop potentiostatic circuits which can be miniaturised onto a MEMS device. Research groups have addressed this area of need and on-board potentiostats have been developed. One example involves design, simulation and example measurements from a low power implantable potentiostat system [46]. Other implantable potentisotat systems are featured in the literature, very often as part of a fully implanted probe system and so other examples will be featured in later sections.
In addition to the on-board circuitry, it is important to provide power to the biomedical sensor and if necessary the option to recharge implantable devices, particularly those intended for long-term use. Powering of medical devices is a subfield of its own with consideration given to overall power budgets of devices across their useful lifetime and the development of novel batteries and powering schemes for specific use in medical technologies. An important consideration in this area are the regulations around powering of medical devices and in particular, safety limits which are often established by national regulators. Examples of studies which give consideration to power include: design and testing of a far-field based radio frequency (RF) powering system for implantable devices where wireless power transfer was shown as feasible using a frequency of 403 Hz at a working distance of 17 cm; the development of an algorithm to ensure optimum power consumption and effective recharging for a network of body area sensors [47]; and finally, a study which elegantly shows development of a battery- and lead-less pacemaker where the energy harvested from contraction and relaxation of heart muscle is used to harvest the electrical energy necessary to drive a commercial pacemaker circuit [48]. To realize long-term implantation and therefore devices which can give insight into wild animal behaviour, the question of power supply and power budget is critical. To track migration events or free-living animals on a long-term basis, perpetual power with the need for minimal interference from the scientist is most desirable and the application of power sources which harvest energy from movement or blood chemistry (e.g. glucose) stand to contribute significantly to research efforts in this area. Such efforts would help to minimize the size and mass of physiological recording devices on wild animals, which may not only impact movement and survival [49] but also data integrity [50]. Alternatively, RF powering systems could be used to investigate the physiology of wild animals that return to specific locations, such as nests (e.g. seabirds), burrows (e.g. badgers), territories (e.g. lions) or stop-over sites during migration (e.g. migrating birds).
The combination of sensor chemistry, measurement circuitry and power options when successfully achieved can give rise to surprisingly effective devices, with recent examples including the measurement of oxygen tension in the gastrointestinal tract of rats [51] via the miniaturization of the Clark electrode into an implantable device which can be administered via a needle, followed by a demonstration of successful mapping of tumour hypoxia in sheep using the same device [52]. Both of these studies showcase sensitive and responsive measurements of oxygen tension in animals and raise the prospect of monitoring physiological status independently and in an intervention-free manner (post implantation). Currently, intravascular oxygen electrodes have been used in penguins, seals and turtles to measure oxygen during diving but are generally limited to several days to a week in recording duration [21]. A critical performance characteristic for implantable devices is efficacy over time. This includes biocompatibility and sensor longevity and there are examples of recent studies which attempt to investigate these phenomena. For example, the PRECISE study [53] studied the behaviour of an implanted glucose monitoring system designed for use over a period of 90 days, finding satisfactory accuracy, no adverse safety events and development of a new algorithm to help adjust for changes in sensor performance over time. The biocompatability and electrical activity of parylene-platinum-based microelectrode arrays were also evaluated following a twelve week implantation into rats [54]. Again, a good safety profile was observed with very little signature of inflammation or rejection of the implant in combination with satisfactory electrical performance. Studies like these, which show good biocompatibility, an absence of adverse events and good measurement reliability, are demonstrating development of technologies with strong prospects for implantation into wild animals.
4. Developments in wearable devices
Wearable devices are a popular area of research with technologies in this area featuring strongly in the literature. The definition of a wearable device is incredibly wide and so the discipline spans many technology forms, disease areas and use cases. The diversity of the field means there are a wealth of technologies which could potentially be applied to the challenge of measuring physiological status in animals. A strong area of interest within wearables is the diabetes market with several commercially available systems currently available for wearable and continuous monitoring of blood glucose levels. This is a well-researched and burgeoning area of literature with comprehensive and relatively recent review articles for the interested reader [10]. Recent examples of innovative new wearable glucose technologies include a fully autonomous wearable droplet microfluidic device which samples glucose and lactate in dermal tissue using a microdialysis probe in combination with a microfluidic chip [55]. In addition, is a highly stretchable and strain insensitive glucose sensor, produced by employing gold fibres to generate a three electrode cell in a woven fabric [56]. The sensor was found to be strain insensitive and following functionalization with Prussian blue and glucose oxidase was able to successfully record glucose levels in sweat. Finally, a nanoporous gold electrode functionalised for detection of glucose was created and deployed on a stretchable three-dimensional micro-patterned polydimethylsiloxane (PDMS) membrane to achieve wearable detection of glucose in sweat [57]. The continuous measurement of blood glucose in wild animals could be extremely informative for understanding fuelling for peak and endurance exercise (e.g. predation events versus migration [58]), as well as how animals manage limited resources in heterogeneous landscapes [59].
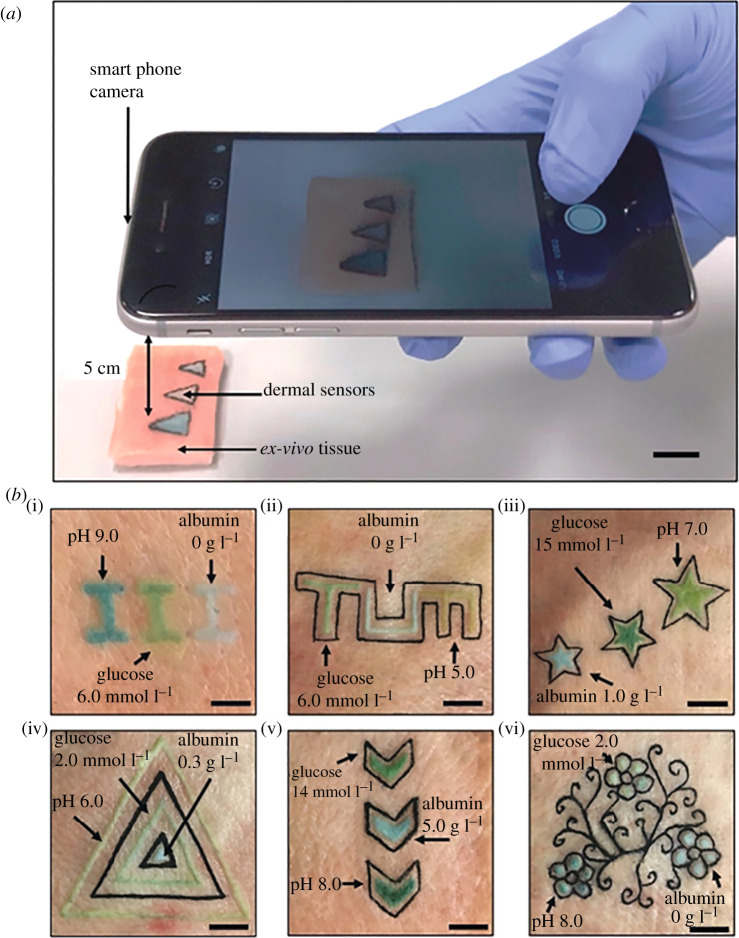
Aside from glucose, other wearable sensor systems are becoming more commonly reported in the literature. For example, lactate is an important biomarker molecule, the levels of which can give important information on physiological status. Lactate is an important biomarker of sepsis [60]. A recent study showed the development of a thin, flexible wearable system which could be controlled by near-field communication (NFC) for lactate detection in sweat [12]. The measurement of lactate is of particular interest in understanding the physiology of diving animals, as the accumulation of lactate marks the point at which the ‘aerobic dive limit’ has been exceeded [61]. Nevertheless, it has important implications for understanding the effects of fisheries by-catch of air-breathing species, for understanding how they may be impacted by overfishing of prey stocks and by climate change. Another example of a flexible, and in this case stretchable, system was the design of a soft, conformable, biocompatible strain sensor based on ultra-thin stretchable electronics for the measurement of bladder wall stretch [62]. Microneedles are a popular area of research. Their placement onto the skin can allow sampling from interstitial fluid with many glucose and lactate systems proving popular in the literature. Other microneedle type devices include a microneedle patch for the measurement of ‘levodopa’ which is an important drug used to treat Parkinson's disease [63]. The ability to measure the concentration of the drug in the blood allows consistent dosing to be realized. Wearability also extends to the concept of ‘tattoo’ biosensors where conductive inks are deployed onto skin to sense biochemical changes [64]. Figure 3 gives an example of tattoo-based sensor systems for a range of biochemicals, achieved by placement of colorimetric ink on the skin. The tattoos probe interstitial fluid with pH, glucose and albumin measured at physiologically relevant concentrations and using quantification provided by smart-phone imaging. This technique may be useful in wild animals that are either regularly sighted (e.g. on nests, in territories, etc.) or that can be remotely observed (e.g. from a bird hide). It may also be a useful technique in managed animals, for example, farmed pigs. For the interested reader, the area of wearables and in particular tattoos and microneedles is well reviewed elsewhere [65]. The concept of wearable extends beyond placement on the skin to areas such as smart clothing and devices woven into fabric. For example, recent study in this area reports near-field-enabled clothing capable of real time networked multi-mode measurement of spinal posture and continuous monitoring of body temperature and gait during exercise [37]. This is achieved through use of woven conductive fibres, rather than silicon components, which can be fragile. Currently, tracking devices are often attached to migrating birds using harnesses that pass between the wings or birds legs [66], and it would be easy to envisage such textile technology integrated to gather physiological measurements that could be relayed to the tracking device, and onwards remotely to researchers via the global system for mobile communications (GSM).
Figure 3.
Overview of a dermal tattoo sensor produced using colorimetric inks for measurement of pH, glucose and albumin in interstitial fluid [64]. (Online version in colour.)
5. Developments in sensor chemistry
In order for a biosensor system to achieve the desired selectivity, it is necessary to develop enhanced chemical/biological receptors, new surface functionalization chemistries (particularly ones which are resistant to bio-fouling) and finally, exploit the enhanced sensitivity offered through adoption of nanoscale sensing elements. Again, there have been developments in these fields specifically directed at solving human medical challenges which may find use in new technologies for wild-animal monitoring. The first and most obvious thing to consider is the bio-recognition element itself and the advances made to improve performance of biosensing technology through this route. Traditionally, biosensor systems have involved the use of enzymes, antibodies, oligonucleotide probe sequences, etc., to achieve the desired specificity for the analyte of interest. While effective, these bio-recognition elements are subject to the disadvantage of relative instability (tendency of antibodies and enzymes to denature over time) which leads to storage problems and lack of long-term functionality. New developments are augmenting existing approaches and include the maturation of the field of ‘molecularly imprinted polymers (MIPs)’, giving rise to selective polymer films and nanoparticles with high affinities for their analytes and critically high stability, meaning long-term storage and use of MIP functionalised sensors is becoming highly feasible. An example of an interesting advance in this field is the use of the nano-MIP, which is a nanoparticle bearing high affinity binding sites for the analyte of interest, to detect epidermal growth factor receptor EGFR using a thermistor [67]. Another promising development in improving the durability and longevity of bioreceptors is the ensilication process. Studies have been published showing the possibility of stabilizing protein structures such as vaccines and antibodies by ensilication, a method of silica-based cross linking, with one study of note using the process to stabilize the tetanus antigen in a vaccine preparation [68] giving the vaccine enhanced thermal storage and reduced tendency to degrade. This type of approach has immense promise to lengthen the active life of biological receptors and improve usability and shelf life in a biosensor product. It is evident that this will make the monitoring of wild animals much more feasible because sensor units will not have to be changed as often or, from one sensor, a much longer measurement window will be possible. Although it varies by taxa, in many studies, there is extremely limited opportunity to recapture study animals, and sometimes researchers must wait a year to recapture an animal when it returns to breed. Another approach, which is proving increasingly popular in detection science and which is beginning to enable live monitoring, is the use of aptamers. Aptamers are DNA sequences which under specific conditions take on three-dimensional structures giving rise to specific binding motifs, not unlike antibodies and with similar KD values. It is possible through a directed evolution process known as SELEX to generate aptamers with high specificity for individual analytes. The advantages of these agents as the binding element in a sensor is the ability to tailor binding kinetics, the ability to produce sequences quickly with a range of standard and non-standard chemical modifications (surface attachment groups, florescent labels, redox labels, etc.) and the increase in longevity which is gained when aptamers replace antibodies as the bio-recognition element. A relatively recent and elegant study shows the use of an implanted electrochemical aptamer-based sensor to measure drug concentrations in the blood of ambulatory rats with high sensitivity and good temporal resolution (3 s) [69]. Another impressive study shows the use of an aptamer-functionalised array in combination with a robotically controlled sample handler to measure luteinizing hormone and therefore hormone ‘pulsatility’ in patients with reproductive conditions [70]. These are interesting studies because they highlight routes, either through direct sampling of animal populations or through use of wearable devices, to the possibility of sampling physiological pulsing, hormones, antimicrobials or pollutants in the circulatory systems of wild animals. Indeed, the measurement of adrenocorticotropic hormones, glucocorticoid stress hormones (e.g. cortisol), thyroid hormones (such as leptin, insulin and glucagon), melatonin (regulating the ‘biological clock’) over long periods (months) would shed light on some of the most dramatic periods in wild animals lives, such as breeding, moulting and migration [58].
Following on from developments which prolong the lifetime of the bio-recognition element through synthetic alternatives or stabilization chemistry, attempts are being made to replace the biological element of a biosensor altogether. One very pertinent example is the ‘enzyme-free’ glucose biosensor. In these systems, nanomaterials are employed because of their high catalytic activity and ability to selectively oxidise glucose at moderate voltages (meaning there are low background interference currents from other common analytes). The benefit of enzyme-free glucose biosensors again relates to the gains offered in the final sensor system's working life. Enzymes lose activity over time and this is a key limitation on wearable glucose monitors (to circumvent this issue, replacement sensors are used every few months). Recent examples of non-enzymatic glucose biosensors include: a system on a glass substrate composed of hydrothermally grown CuO nanorods decorated with Au nanoparticles and used to detect glucose levels in saliva [71]; the development of a metal oxide sensor induced by an electrical potential to non-enzymatically detect glucose in artificial tears via wireless connection [72]; and use of a Ni60Nb40 amorphous nanoglass composite to non-enzymatically detect glucose with 100 nM sensitivies, opening up the potential of using nickel-based nanoglasses in wearable sensors [73]. All of these systems are offering the sensitivity and selectivity required to measure glucose in blood, sweat and interstitial fluid and so it seems inevitable that products based on this approach will reach the blood glucose monitoring market.
A crucial issue in implantable sensing is the tendency of proteins and cells from biofluids to adhere to the transducer element of a sensor and complicate the measurement by affecting the analytical response of the device. This phenomenon is often referred to as ‘biofouling’, but is different from the biofouling which affects marine sensors and is a key reason why implanted detection of blood-based biomarkers in human subjects has not yet reached maturity. Such biofouling has been a persistent issue in the physiological telemetry of wild animals, particularly in extreme habitats such as the frozen Antarctic seas. The issue of biofouling is a key area of biosensor research with many groups working on improved surface chemistries or gel-based systems which reduce biofouling and improve the reporting of a device's true analytical response. The ability to precisely control the formation of hydrogels was shown in a study that used nanoelectrode structures to monitor the formation of gels in real time and then used the underlying electrode as a sensor for specific drug resistance DNA gene sequences with the formed hydrogel behaving as a filter, effectively screening out background interferents to improve the analytical response of the device [74].
6. Synthetic biology approaches to biomedical sensing
Synthetic biology as referred to in this article means the use of cells and biochemical machinery to engineer novel biosensors. This technique has many advantages and these include: the use of already existing enzymes, proteins and nucleic acid sequences; the molecular biology field has matured allowing easy modification and characterization of synthetic systems; high biocompatibility; and the existence of manufacturing techniques and production processes for high volumes of sensor reagents. A recent and popular development has been to engineer biosensors which employ the CRISPR-Cas gene systems for the purposes of detection of specific nucleic acid sequences. For example, the CRISPS-Cas9 system has been recently used to develop biosensors for tumour microRNAs (miR-19b and miR-20a) with times to result of less than 4 h and sensitivities as low as 10 ppm [75]. In addition to this, the CRISPR-Cas12a system was developed for detection of the Epstein-Bar virus in a lateral flow/point of care format [76]. Other approaches involve genetic engineering and the use of genetic circuits in combination with microelectronics to detect analytes of interest, for example, the detection of heavy metals present in bacteria through use of engineered genetic circuits [77].
7. Future perspectives (antimicrobial resistance and zoonotic infections)
Antimicrobial resistance (AMR) is typically seen as a problem affecting only humans with its emergence and spread having potentially dire consequences for the global population. To provide further context, if left unaddressed, by 2050 AMR is expected to account for more deaths per year than cancer and diabetes combined. The phenomenon of AMR is driven by many factors which include: inappropriate prescriptions, the fact that a proportion of countries allow over the counter purchasing in pharmacies, shortages of new antimicrobials, the overuse of antibiotics in agriculture and ineffective environmental controls (particularly in manufacture and disposal) which facilitate accumulation in the environment. To try and alleviate some of the present and predicted problems, governments are trying to stimulate innovation through encouraging the development of new antimicrobial compounds, encouraging good antibiotic stewardship and stimulating the development of new diagnostic tests in the hope that the technology will reach a performance level where antibiotic prescriptions can be made contingent on a confirmatory test. Technological advances in the area of AMR diagnostics have the general aim of producing low cost, sub half-hour testing technologies. Since AMR is in fact not just a human problem (due to the widespread prevalence of residual antibiotics in the environment) drug resistant infections will become an increasing problem for free-living and farm animals and the new diagnostic tests which are designed to be low cost and simple to use will enhance the study of AMR in populations of wild animals and improve antibiotic stewardship in the veterinary sector. Biosensor technology could also be used in the future in managed (agriculture and aquaculture) systems as a surveillance technology to proactively detect infections before antibiotic treatment is given, which may help to reduce the total use of antibiotics.
Recent developments in the field of diagnostic tests for AMR can be divided into two major groups: phenotypic and genotypic tests. In a broad sense, phenotypic tests generally attempt to recreate classical microbiological strategies of growing bacteria and assessing their antibiotic susceptibility profiles. A number of innovative platforms have been developed which include gel modified electrode sensors capable of discriminating between Staphylococcus aureus and MRSA in under 1 h [78], the use of the redox agent potassium ferricyanide to report bacterial respiration in antibiotic-infused cultures [79], a capillary-based optical platform capable of aspartate aminotransferase (AST) results within 4–8 h [80], and the use of microfluidic platforms in combination with the commonly employed respiration rate sensitive compound resazurin to quickly determine antibiotic susceptibility from a sample [81,82]. Genotypic sensors have been developed for detection of AMR which directly probe for the presence of specific gene sequences indicative of bacterial species and any resistance genes [29,83]. These tests give more information because they probe the genetic basis of the resistance, but still require careful interpretation because the presence of a particular drug resistance gene does not always confer resistance upon a pathogen; sometimes additional transcription factors and other genes are necessary to produce a drug resistant phenotype.
Given the situation the world currently finds itself in—in the midst of a pandemic caused by the SARS-CoV-2 virus which is thought to have originated in bats, potentially crossing to humans via an intermediary mammalian species—it is important to raise the use of biosensor technologies for monitoring virus transfer and mutation between wild animals. Monitoring will be particularly important in animals that are responsible for a large number of zoonotic infections (e.g. birds and bats) with technologies which can fill this space offering the ability to quickly pinpoint and hopefully supress the development of viral strains with pandemic-causing potential. Indeed, the power and scale of existing structured sampling of zoonoses in wild animals (e.g. from faeces from bat roosts) would benefit enormously, and rapidly, from such technology [84].
Work dedicated to the detection of viral infections of the human population includes detection of Ebola virus [85] using an array of nano-antennae. Ebola is a serious and recurring threat to human health with regular outbreaks necessitating the development of low cost, simple to use and innovative diagnostic technologies. Another viral infection which gained large amounts of attention in recent years is Zika, the enzoonotic virus with a life cycle involving monkeys and mosquitoes. Here, sensor technologies have been developed to detect live virus using an imprinted sensor surface [86], but also crucially to profile blood for the presence of Zika antibodies [87] in order to map spread within communities and regions. The prospect of pandemics, particularly those of an avian origin and especially respiratory pathogens, has long concerned biosensor scientists [88] and the response to the need for diagnostic devices for COVID-19 has seen, alongside widespread adoption and upscaling of traditional methods, the emergence of novel approaches such as a field-effect transistor-based system [89] for COVID detection in nasopharyngeal swabs. As the COVID-19 pandemic continues, innovative biosensor solutions are emerging, for example application of the synthetic biology CRISPR-Cas system to SARS-CoV-2 detection [90] and single tube reverse transcription loop mediated isothermal amplification (RT-LAMP) techniques [91]. The emergence of new diagnostic solutions which the COVID-19 outbreak is accelerating means that identifying and tracking animal populations harbouring pathogenic strains will become easier and hopefully more widespread in use, perhaps increasing the opportunity to track and eliminate potential outbreaks of viruses with pandemic-causing potential.
8. Potential for cutting-edge technology to cross over into animal monitoring
The review has highlighted a number of papers which showcase state of the art advances in micro systems engineering and chemical science which have given rise to new approaches to monitoring human health. These advances all have the potential to contribute to monitoring of physiological status in wild animals. Implantable and wearable devices show the most promise for long-term monitoring of animals and developments which prolong the lifetime of the biosensing element are crucial because, at present, denaturation of antibodies or enzymes over time and the need to either swap or recalibrate the sensor are a major limitation on prolonged and unsupervised use. Advances in human monitoring that sample less invasively by taking measurements from interstitial fluid, sweat or tears are of interest to animal monitoring because they negate the requirement for an invasive test. Patch-type wearable physiological devices [92] would also provide data in a less invasive manner than is used at present (physiological devices are often surgically implanted in wild animal research). As has been discussed in the review, sampling of analytes such as glucose and lactate from biofluids is now being achieved using a whole range of innovative technologies intended for human use. Adapting these devices for animal use will involve making sure the instrumentation can access the correct fluid sample and this will involve giving consideration to the differences in the animal of interest's own physiology and the accessibility and availability of the biofluids needing to be sampled. A theme which will emerge in human medical technologies in the near future will be routine monitoring of more complex biomarkers (e.g. other than glucose, lactate and pH) and this includes biomarkers diagnostic of inflammatory changes (sepsis, bacterial versus non bacterial infection), neurological status (e.g. blood-based biomarkers for dementia) and cancer (circulating tumour DNA and circulating tumour cells). Following maturation of these technologies in humans, it will be possible to deploy them in animals in order to track the effects of pollution (e.g. antimicrobials, micro-plastics) and the effects of climate change (through e.g. spread of parasitic diseases). In addition, the increasing simplicity of these technologies, the potential to interface with smart phones and the lack of requirements for sample handling will hopefully assist with design and realization of less invasive and more easily deployed devices (still with potential to cause distress during application to animals) which give genuine insight into the physiological status of wild animals. Such insights would not only revolutionize the study of wild animal physiology, but would also reveal the mechanisms with which wild animals have mastered many of the future challenges to human society (obesity, diabetes, zoonoses and AMR).
Acknowledgements
A.M.'s EngD studentship is funded by the EPSRC CDT in Biomedical Devices and Health Technologies (EP/L015595/1).
Footnotes
Data accessibility
This article has no additional data.
Authors' contributions
The authors conceived, wrote and edited the manuscript together. A.M. and D.C. focused on engineering/chemistry developments and L.H. on major issues in animal monitoring.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Wang J. 2008. Electrochemical glucose biosensors. Chem. Rev. 108, 814-825. ( 10.1021/cr068123a) [DOI] [PubMed] [Google Scholar]
- 2.Sosna M, Denuault G, Pascal RW, Prien RD, Mowlem M. 2007. Development of a reliable microelectrode dissolved oxygen sensor. Sensors Actuators B 123, 344-351. ( 10.1016/j.snb.2006.08.033) [DOI] [Google Scholar]
- 3.Corrigan DK, Elliott JP, Blair EO, Reeves S, Schmüser I, Walton AJ, Mount AR. 2016. Advances in electroanalysis, sensing and monitoring in molten salts. Faraday Discuss. 190, 351-366. ( 10.7488/ds/1338) [DOI] [PubMed] [Google Scholar]
- 4.Corrigan DK, Whitcombe MJ, McCrossen S, Piletsky S. 2009. Reichardt's dye and its reactions with the alkylating agents 4-chloro-1-butanol, ethyl methanesulfonate, 1-bromobutane and Fast Red B - a potentially useful reagent for the detection of genotoxic impurities in pharmaceuticals. J. Pharm. Pharmacol. 61, 533-537. ( 10.1211/jpp.61.04.0017) [DOI] [PubMed] [Google Scholar]
- 5.Corrigan DK, Salton NA, Preston C, Piletsky S. 2010. Towards the development of a rapid, portable, surface enhanced Raman spectroscopy based cleaning verification system for the drug nelarabine. J. Pharm. Pharmacol. 62, 1195-1200. ( 10.1111/j.2042-7158.2010.01152.x) [DOI] [PubMed] [Google Scholar]
- 6.Scognamiglio V, Arduini F. 2019. The technology tree in the design of glucose biosensors. Trends Anal. Chem. 120, 115642. ( 10.1016/j.trac.2019.115642) [DOI] [Google Scholar]
- 7.Kucherenko IS, Topolnikova YV, Soldatkin OO. 2019. Advances in the biosensors for lactate and pyruvate detection for medical applications: a review. Trends Anal. Chem. 110, 160-172. ( 10.1016/j.trac.2018.11.004) [DOI] [Google Scholar]
- 8.Aria MM, Erten A, Yalcin O. 2019. Technology advancements in blood coagulation measurements for point-of-care diagnostic testing. Front. Biotechnol. Bioeng. 7, 1-18. ( 10.3389/fbioe.2019.00395) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stockard TK, Heil J, Meir JU, Sato K, Ponganis KV, Ponganis PJ. 2005. Air sac PO2 and oxygen depletion during dives of emperor penguins. J. Exp. Biol. 208, 2973-2980. ( 10.1242/jeb.01687) [DOI] [PubMed] [Google Scholar]
- 10.Kim J, Campbell AS, Wang J. 2018. Wearable non-invasive epidermal glucose sensors: a review. Talanta 177, 163-170. ( 10.1016/j.talanta.2017.08.077) [DOI] [PubMed] [Google Scholar]
- 11.Homayounfar SZ, Andrew TL. 2020. Wearable sensors for monitoring human motion: a review on mechanisms, materials, and challenges. SLAS Technol. 25, 9-24. ( 10.1177/2472630319891128) [DOI] [PubMed] [Google Scholar]
- 12.Currano LJ, Sage FC, Hagedon M, Hamilton L, Patrone J. 2018. Wearable sensor system for detection of lactate in sweat. Sci. Rep. 8, 1-11. ( 10.1038/s41598-018-33565-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guglielmo CG. 2018. Obese super athletes: fat-fueled migration in birds and bats. J. Exp. Biol. 221, 165753. ( 10.1242/jeb.165753) [DOI] [PubMed] [Google Scholar]
- 14.Braun EJ, Sweazea KL. 2008. Glucose regulation in birds. Comp. Biochem. Physiol. B 151, 1-9. ( 10.1016/j.cbpb.2008.05.007) [DOI] [PubMed] [Google Scholar]
- 15.Fago A, Jensen FB. 2015. Hypoxia tolerance, nitric oxide, and nitrite: lessons from extreme animals. Physiology 30, 116-126. ( 10.1152/physiol.00051.2014) [DOI] [PubMed] [Google Scholar]
- 16.Ponganis PJ. 2019. State of the art review: from the seaside to the bedside: insights from comparative diving physiology into respiratory, sleep and critical care. Thorax 74, 512-518. ( 10.1136/thoraxjnl-2018-212136) [DOI] [PubMed] [Google Scholar]
- 17.Nabi G, Wang Y, Lü L, Jiang C, Ahmad S, Wu Y, Li D. 2021. Bats and birds as viral reservoirs: a physiological and ecological perspective. Sci. Total Environ. 754, 142372. ( 10.1016/j.scitotenv.2020.142372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitford M, Klimley AP. 2019. An overview of behavioral, physiological and environmental sensors used in animal biotelemetry and biologging studies. Anim. Biotelem. 7, 1-24. ( 10.1186/s40317-019-0189-z) [DOI] [Google Scholar]
- 19.Kelly JT, Klimley AP. 2012. Relating the swimming movements of green sturgeon to the movement of water currents. Environ. Biol. Fishes 93, 151-167. ( 10.1007/s10641-011-9898-8) [DOI] [Google Scholar]
- 20.Brown DD, Kays R, Wikelski M, Wilson R, Klimley AP. 2013. Observing the unwatchable through acceleration logging of animal behavior. Anim. Biotelem. 1, 1-16. ( 10.1186/2050-3385-1-20) [DOI] [Google Scholar]
- 21.Tift MS, Hu LA, Mcdonald BI, Thorson PH, Ponganis PJ. 2017. Flipper stroke rate and venous oxygen levels in free-ranging California sea lions. J. Exp. Biol. 220, 1533-1540. ( 10.1242/jeb.152314) [DOI] [PubMed] [Google Scholar]
- 22.Murgatroyd M, Amar A, Africa S. 2018. Where eagles soar: fine-resolution tracking reveals the spatiotemporal use of differential soaring modes in a large raptor. Ecol. Evol. 8, 6788-6799. ( 10.1002/ece3.4189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coffey DM, Holland KN. 2015. First autonomous recording of in situ dissolved oxygen from free-ranging fish. Anim. Biotelem. 3, 1-9. ( 10.1186/s40317-015-0088-x) [DOI] [Google Scholar]
- 24.Mcdonald BI, Tift MS, Hu LA, Jeffko M, Ponganis PJ. 2020. Stroke effort and relative lung volume influence heart rate in diving sea lions. J. Exp. Biol. 223, 214163. ( 10.1242/jeb.214163) [DOI] [PubMed] [Google Scholar]
- 25.Scriba MF, Harmening WM, Vyssotski AL, Roulin A, Wagner H, Rattenborg NC. 2013. Evaluation of two minimally invasive techniques for electroencephalogram recording in wild or freely behaving animals. J. Comp. Physiol. A 3, 183-189. ( 10.1007/s00359-012-0779-1) [DOI] [PubMed] [Google Scholar]
- 26.Thouzeau C, Peters G, Le Bohec C, Le Maho Y. 2004. Adjustments of gastric pH, motility and temperature during long-term preservation of stomach contents in free-ranging incubating king penguins. J. Exp. Biol. 207, 2715-2724. ( 10.1242/jeb.01074) [DOI] [PubMed] [Google Scholar]
- 27.Bennett KA, Turner LM, Millward S, Moss SEW, Hall AJ. 2017. Obtaining accurate glucose measurements from wild animals under field conditions: comparing a hand held glucometer with a standard laboratory technique in grey seals. Conserv. Physiol. 5, 1-10. ( 10.1093/conphys/cox013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pei J, Neo S, Tan BH. 2017. The use of animals as a surveillance tool for monitoring environmental health hazards, human health hazards and bioterrorism. Vet. Microbiol. 203, 40-48. ( 10.1016/j.vetmic.2017.02.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blair EO, Hannah S, Vezza V, Avcı H, Kocagoz T, Hoskisson PA, Güzel FD, Corrigan DK. 2020. Biologically modified microelectrode sensors provide enhanced sensitivity for detection of nucleic acid sequences from Mycobacterium tuberculosis. Sensors Actuators Rep. 2, 100008. ( 10.1016/j.snr.2020.100008) [DOI] [Google Scholar]
- 30.Terry JG, Schmüser I, Underwood I, Corrigan DK, Freeman NJ, Bunting AS, Mount AR, Walton AJ. 2013. Nanoscale electrode arrays produced with microscale lithographic techniques for use in biomedical sensing applications. IET Nanobiotechnol. 7, 125-134. ( 10.1049/iet-nbt.2013.0049) [DOI] [PubMed] [Google Scholar]
- 31.Patil AC, Thakor NV. 2016. Implantable neurotechnologies: a review of micro and nanoelectrodes for neural recording. Med. Biol. Eng. Comput. 54, 23-44. ( 10.1007/s11517-015-1430-4) [DOI] [PubMed] [Google Scholar]
- 32.Aulsebrook AE, Connelly F, Johnsson RD, Jones TM, Mulder RA, Hall ML, Vyssotski AL, Lesku JA. 2020. White and amber light at night disrupt sleep physiology in birds. Curr. Biol. 30, 3657-3663.e5. ( 10.1016/j.cub.2020.06.085) [DOI] [PubMed] [Google Scholar]
- 33.Vyssotski AL, Dell'Omo G, Dell'Ariccia G, Abramchuk AN, Serkov AN, Latanov AV, Loizzo A, Wolfer DP, Lipp H-P. 2009. EEG responses to visual landmarks in flying pigeons. Curr. Biol. 19, 1159-1166. ( 10.1016/j.cub.2009.05.070) [DOI] [PubMed] [Google Scholar]
- 34.van Hasselt SJ, Rusche M, Vyssotski AL, Verhulst S, Rattenborg NC, Meerlo P. 2020. Sleep time in the European starling is strongly affected by night length and moon phase. Curr. Biol. 30, 1664-1671.e2. ( 10.1016/j.cub.2020.02.052) [DOI] [PubMed] [Google Scholar]
- 35.Scharf R, Tsunematsu T, Mcalinden N, Dawson MD, Sakata S, Mathieson K. 2016. Depth-specific optogenetic control in vivo with a scalable, high-density μ LED neural probe. Sci. Rep. 6, 28381. ( 10.1038/srep28381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu C, Zhao Y, Cai X, Xie Y, Wang T, Cheng D, Li L, Li R, Deng Y. 2020. A wireless, implantable optoelectrochemical probe for optogenetic stimulation and dopamine detection. Microsyst. Nanoeng. 6, 1-2. ( 10.1038/s41378-020-0176-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, et al. 2019. Battery-free, lightweight, injectable microsystem for in vivo wireless pharmacology and optogenetics. Proc. Natl Acad. Sci. USA 116, 21 427-21 437. ( 10.1073/pnas.1909850116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Angotzi GN, Boi F, Lecomte A, Miele E, Malerba M, Zucca S, Casile A, Berdondini L. 2019. SiNAPS: an implantable active pixel sensor CMOS-probe for simultaneous large-scale neural recordings. Biosens. Bioelectron. 126, 355-364. ( 10.1016/j.bios.2018.10.032) [DOI] [PubMed] [Google Scholar]
- 39.Rattenborg NC, Voirin B, Cruz SM, Tisdale R, Dell'Omo G, Lipp H-P, Wikelski M, Vyssotski AL. 2016. Evidence that birds sleep in mid-flight. Nat. Commun. 7, 12468. ( 10.1038/ncomms12468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kendall-Bar JM, Vyssotski AL, Mukhametov LM, Siegel JM, Lyamin OI. 2019. Eye state asymmetry during aquatic unihemispheric slow wave sleep in northern fur seals (Callorhinus ursinus). PLoS ONE 14, e0217025. ( 10.1371/journal.pone.0217025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dryden MDM, Wheeler AR. 2015. DStat: a versatile, open-source potentiostat for electroanalysis and integration. PLoS ONE 10, e0140349. ( 10.1371/journal.pone.0140349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopin P, Lopin KV. 2018. PSoC-Stat: a single chip open source potentiostat based on a programmable system on a chip. PLoS ONE 13, e0201353. ( 10.1371/journal.pone.0201353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butterworth A, Corrigan DK, Ward AC. 2019. Electrochemical detection of oxacillin resistance with SimpleStat: a low cost integrated potentiostat and sensor platform. Anal. Methods 11, 1958-1965. ( 10.1039/c9ay00383e) [DOI] [Google Scholar]
- 44.Sánchez C, Dessì P, Duffy M, Lens PNL. 2020. Microbial electrochemical technologies: electronic circuitry and characterization tools. Biosens. Bioelectron. 150, 111884. ( 10.1016/j.bios.2019.111884) [DOI] [PubMed] [Google Scholar]
- 45.Mercer C, Bennett R, Conghaile PÓ, Rusling JF, Leech D. 2019. Glucose biosensor based on open-source wireless microfluidic potentiostat. Sensors Actuators B 290, 616-624. ( 10.1016/j.snb.2019.02.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roknsharifi M, Kamrul S, Kai I, Ifana Z. 2015. A low power, highly stabilized three electrode potentiostat for biomedical implantable systems. Analog Integr. Circuits Signal Process. 83, 217-229. ( 10.1007/s10470-015-0524-0) [DOI] [Google Scholar]
- 47.Rabby MK, Alam MS, Shawkat MS. 2019. A priority based energy harvesting scheme for charging embedded sensor nodes in wireless body area networks. PLoS ONE 14, 1-22. ( 10.1371/journal.pone.0214716) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yi Z, et al. 2020. A battery- and leadless heart-worn pacemaker strategy. Adv. Funct. Mater. 30, 2000477. ( 10.1002/adfm.202000477) [DOI] [Google Scholar]
- 49.Bodey TW, Cleasby IR, Bell F, Parr N, Schultz A, Votier SC, Bearhop S. 2018. A phylogenetically controlled meta-analysis of biologging device effects on birds: deleterious effects and a call for more standardized reporting of study data. Methods Ecol. Evol. 9, 946-955. ( 10.1111/2041-210X.12934) [DOI] [Google Scholar]
- 50.Authier M, Péron C, Mante A, Vidal P, Grémillet D. 2013. Designing observational biologging studies to assess the causal effect of instrumentation. Methods Ecol. Evol. 4, 802-810. ( 10.1111/2041-210X.12075) [DOI] [Google Scholar]
- 51.Gray ME, et al. 2019. In vivo validation of a miniaturized electrochemical oxygen sensor for measuring intestinal oxygen tension. Am. J. Physiol. Gastrointest. Liver Physiol. 317, 242-252. ( 10.1152/ajpgi.00050.2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marland JRK, et al. 2020. Real-time measurement of tumour hypoxia using an implantable microfabricated oxygen sensor. Sens. Bio-Sensing Res. 30, 100375. ( 10.1016/j.sbsr.2020.100375) [DOI] [Google Scholar]
- 53.Christiansen MP, Klaff LJ, Bailey TS, Brazg R, Carlson G, Tweden KS. 2019. A prospective multicenter evaluation of the accuracy and safety of an implanted continuous glucose sensor: the precision study. Diabetes Technol. Ther. 21, 231-237. ( 10.1089/dia.2019.0020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Torres-Martinez N, Ratel D, Crétallaz C, Gaude C, Maubert S, Divoux JL, Henry C, Guiraud D, Sauter-Starace F. 2019. Reliability of parylene-based multi-electrode arrays chronically implanted in adult rat brains, and evidence of electrical stimulation on contact impedance. J. Neural Eng. 16, 066047. ( 10.1088/1741-2552/ab3836) [DOI] [PubMed] [Google Scholar]
- 55.Nightingale AM, Leong CL, Burnish RA, Hassan S, Zhang Y, Clough GF, Boutelle MG, Voegeli D, Niu X. 2019. Monitoring biomolecule concentrations in tissue using a wearable droplet microfluidic-based sensor. Nat. Commun. 10, 1-12. ( 10.1038/s41467-019-10401-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao Y, Zhai Q, Dong D, An T, Gong S, Shi Q, Cheng W. 2019. Highly stretchable and strain-insensitive fiber-based wearable electrochemical biosensor to monitor glucose in the sweat. Anal. Chem. 91, 6569-6576. ( 10.1021/acs.analchem.9b00152) [DOI] [PubMed] [Google Scholar]
- 57.Bae CW, Toi PT, Kim BY, Lee WI II, Lee HB, Hanif A, Lee EH, Lee NE. 2019. Fully stretchable capillary micro fluidics-integrated nanoporous gold electrochemical sensor for wearable continuous glucose monitoring. ACS Appl. Mater. Interfaces 11, 14 567-14 575. ( 10.1021/acsami.9b00848) [DOI] [PubMed] [Google Scholar]
- 58.Jachowski DS, Singh NJ. 2015. Toward a mechanistic understanding of animal migration: incorporating physiological measurements in the study of animal movement. Conserv. Physiol. 3, cov035. ( 10.1093/conphys/cov035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shepard ELC, Wilson RP, Rees WG, Grundy E, Lambertucci SA, Vosper SB. 2013. Energy landscapes shape animal movement ecology. Am. Nat. 182, 298-312. ( 10.1086/671257) [DOI] [PubMed] [Google Scholar]
- 60.Biron BM, Ayala A, Lomas-Neira JL. 2015. Biomarkers for sepsis: what is and what might be? Biomark. Insights 10, 7-17. ( 10.4137/BMI.S29519) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kooyman GL, McDonald BI, Williams CL, Meir JU, Ponganis PJ. 2021. The aerobic dive limit: after 40 years, still rarely measured but commonly used. Comp. Biochem. Physiol. A 252, 110841. ( 10.1016/j.cbpa.2020.110841) [DOI] [PubMed] [Google Scholar]
- 62.Hannah S, Brige P, Ravichandran A, Ramuz M. 2019. Conformable, stretchable sensor to record bladder wall stretch. ACS Omega 4, 4-12. ( 10.1021/acsomega.8b02609) [DOI] [Google Scholar]
- 63.Goud KY, Moonla C, Mishra RK, Yu C, Narayan R, Litvan I, Wang J. 2019. Wearable electrochemical microneedle sensor for continuous monitoring of levodopa: toward Parkinson management. ACS Sens. 4, 2196-2204. ( 10.1021/acssensors.9b01127) [DOI] [PubMed] [Google Scholar]
- 64.Yetisen AK, et al. 2019. Dermal tattoo biosensors for colorimetric metabolite detection. Angew. Chemie 131, 10 616-10 623. ( 10.1002/ange.201904416) [DOI] [PubMed] [Google Scholar]
- 65.Hosu O, Mirel S, Săndulescu R, Cristea C. 2019. Minireview: smart tattoo, microneedle, point-of-care, and phone-based biosensors for medical screening, diagnosis, and monitoring. Anal. Lett. 52, 78-92. ( 10.1080/00032719.2017.1391826) [DOI] [Google Scholar]
- 66.Geen GR, Robinson RA, Baillie SR. 2019. Effects of tracking devices on individual birds—a review of the evidence. J. Avian Biol. 50, 01823. ( 10.1111/jav.01823) [DOI] [Google Scholar]
- 67.Betlem K, et al. 2020. Thermistors coated with molecularly imprinted nanoparticles for the electrical detection of peptides and proteins. Analyst 145, 5419-5424. ( 10.1039/d0an01046d) [DOI] [PubMed] [Google Scholar]
- 68.Doekhie A, et al. 2020. Ensilicated tetanus antigen retains immunogenicity: in vivo study and time-resolved SAXS characterization. Sci. Rep. 10, 1-9. ( 10.1038/s41598-020-65876-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arroyo-Currás N, Somerson J, Vieira PA, Ploense KL, Kippin TE, Plaxco KW. 2017. Real-time measurement of small molecules directly in awake, ambulatory animals. Proc. Natl Acad. Sci. USA 114, 645-650. ( 10.1073/pnas.1613458114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liang S, et al. 2019. Measuring luteinising hormone pulsatility with a robotic aptamer-enabled electrochemical reader. Nat. Commun. 10, 1-10. ( 10.1038/s41467-019-08799-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chakraborty P, Dhar S, Debnath K, Majumder T, Mondal SP. 2019. Non-enzymatic and non-invasive glucose detection using Au nanoparticle decorated CuO nanorods. Sensors Actuators. B 283, 776-785. ( 10.1016/j.snb.2018.12.086) [DOI] [Google Scholar]
- 72.Strakosas X, Selberg J, Pansodtee P, Yonas N, Manapongpun P, Teodorescu M, Rolandi M. 2019. A non-enzymatic glucose sensor enabled by bioelectronic pH control. Sci. Rep. 9, 1-7. ( 10.1038/s41598-019-46302-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bag S, Baksi A, Nandam SH, Wang D, Ye X, Ghosh J, Pradeep T, Hahn H. 2020. Nonenzymatic glucose sensing using Ni60Nb40 nanoglass. ACS Nano 14, 5543-5552. ( 10.1021/acsnano.9b09778) [DOI] [PubMed] [Google Scholar]
- 74.Piper A, Alston BM, Adams DJ, Mount AR. 2018. Functionalised microscale nanoband edge electrode (MNEE) arrays: the systematic quantitative study of hydrogels grown on nanoelectrode biosensor arrays for enhanced sensing in biological media. Faraday Discuss. 210, 201-217. ( 10.1039/C8FD00063H) [DOI] [PubMed] [Google Scholar]
- 75.Bruch R, Baaske J, Chatelle C, Meirich M, Madlener S, Weber W, Dincer C, Urban GA. 2019. CRISPR/Cas13a-powered electrochemical microfluidic biosensor for nucleic acid amplification-free miRNA diagnostics. Adv. Mat. 31, 1905311. ( 10.1002/adma.201905311) [DOI] [PubMed] [Google Scholar]
- 76.Yuan T, et al. 2020. A rapid and sensitive CRISPR/Cas12a based lateral flow biosensor for the detection of Epstein–Barr virus. Analyst 145, 6388-6394. ( 10.1039/d0an00663g) [DOI] [PubMed] [Google Scholar]
- 77.Din MO, Martin A, Razinkov I, Csicsery N, Hasty J. 2020. Interfacing gene circuits with microelectronics through engineered population dynamics. Sci. Adv. 6, eaaz8344. ( 10.1126/sciadv.aaz8344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hannah S, Addington E, Alcorn D, Shu W, Hoskisson PA, Corrigan DK. 2019. Rapid antibiotic susceptibility testing using low-cost, commercially available screen-printed electrodes. Biosens. Bioelectron. 145, 111696. ( 10.1016/j.bios.2019.111696) [DOI] [PubMed] [Google Scholar]
- 79.Chotinantakul K, Suginta W, Schulte A. 2014. Advanced amperometric respiration assay for antimicrobial susceptibility testing. Anal. Chem. 86, 10 315-10 322. ( 10.1021/ac502554s) [DOI] [PubMed] [Google Scholar]
- 80.Wang R, Vemulapati S, Westblade LF, Glesby MJ, Mehta S, Erickson D. 2020. cAST: capillary-based platform for real-time phenotypic antimicrobial susceptibility testing. Anal. Chem. 92, 2731-2738. ( 10.1021/acs.analchem.9b04991) [DOI] [PubMed] [Google Scholar]
- 81.Besant JD, Sargent EH, Kelley SO. 2015. Rapid electrochemical phenotypic profiling of antibiotic-resistant bacteria. Lab. Chip 15, 2799-2807. ( 10.1039/c5lc00375j) [DOI] [PubMed] [Google Scholar]
- 82.Yang Y, Gupta K, Ekinci KL. 2020. All-electrical monitoring of bacterial antibiotic susceptibility in a microfluidic device. Proc. Natl Acad. Sci. USA 117, 10 639-10 644. ( 10.1073/pnas.1922172117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Quan Li P, Piper A, Schmueser I, Mount AR, Corrigan DK. 2017. Impedimetric measurement of DNA-DNA hybridisation using microelectrodes with different radii for detection of methicillin resistant: Staphylococcus aureus (MRSA). Analyst 142, 1946-1952. ( 10.1039/c7an00436b) [DOI] [PubMed] [Google Scholar]
- 84.Watsa M. 2020. Rigorous wildlife disease surveillance. Science 369, 145-147. ( 10.1126/science.abc0017) [DOI] [PubMed] [Google Scholar]
- 85.Zang F, Su Z, Zhou L, Konduru K, Kaplan G, Chou SY. 2019. Ultrasensitive Ebola virus antigen sensing via 3D nanoantenna arrays. Adv. Mat. 30, 1902331. ( 10.1002/adma.201902331) [DOI] [PubMed] [Google Scholar]
- 86.Ricotta V, Yu Y, Clayton N, Chuang YC, Wang Y, Mueller S, Levon K, Simon M, Rafailovich M. 2019. A chip-based potentiometric sensor for a Zika virus diagnostic using 3D surface molecular imprinting. Analyst 144, 4266-4280. ( 10.1039/c9an00580c) [DOI] [PubMed] [Google Scholar]
- 87.Cabral-Miranda G, Cardoso AR, Ferreira LCS, Sales MGF, Bachmann MF. 2018. Biosensor-based selective detection of Zika virus specific antibodies in infected individuals. Biosens. Bioelectron. 113, 101-107. ( 10.1016/j.bios.2018.04.058) [DOI] [PubMed] [Google Scholar]
- 88.Astill J, Dara RA, Fraser EDG, Sharif S. 2018. Detecting and predicting emerging disease in poultry with the implementation of new technologies and big data: a focus on avian influenza virus. Front. Vet. Sci. 5, 1-12. ( 10.3389/fvets.2018.00263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Seo G, et al. 2020. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano 14, 5135-5142. ( 10.1021/acsnano.0c02823) [DOI] [PubMed] [Google Scholar]
- 90.Huang Z, et al. 2020. Ultra-sensitive and high-throughput CRISPR-powered COVID-19 diagnosis. Biosens. Bioelectron. 164, 112316. ( 10.1016/j.bios.2020.112316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang WE, et al. 2020. RT-LAMP for rapid diagnosis of coronavirus. Microb. Biotechnol. 3, 950-961. ( 10.1111/1751-7915.13586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nassar JM, Khan SM, Velling SJ, Diaz-Gaxiola A, Shaikh SF, Geraldi NR, Torres Sevilla GA, Duarte CM, Hussain MM. 2018. Compliant lightweight non-invasive standalone ‘Marine Skin’ tagging system. Flex. Electron. 2, 13. ( 10.1038/s41528-018-0025-1) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.