Abstract
Objective
To determine whether restricting the use of inotrope after diagnosis of low blood pressure (BP) in the first 72 hours of life affects survival without significant brain injury at 36 weeks of postmenstrual age (PMA) in infants born before 28 weeks of gestation.
Design
Double-blind, placebo-controlled randomised trial. Caregivers were masked to group assignment.
Setting
10 sites across Europe and Canada.
Participants
Infants born before 28 weeks of gestation were eligible if they had an invasive mean BP less than their gestational age that persisted for ≥15 min in the first 72 hours of life and a cerebral ultrasound free of significant (≥ grade 3) intraventricular haemorrhage.
Intervention
Participants were randomly assigned to saline bolus followed by either a dopamine infusion (standard management) or placebo (5% dextrose) infusion (restrictive management).
Primary outcome
Survival to 36 weeks of PMA without severe brain injury.
Results
The trial terminated early due to significant enrolment issues (7.7% of planned recruitment). 58 infants were enrolled between February 2015 and September 2017. The two groups were well matched for baseline variables. In the standard group, 18/29 (62%) achieved the primary outcome compared with 20/29 (69%) in the restrictive group (p=0.58). Additional treatments for low BP were used less frequently in the standard arm (11/29 (38%) vs 19/29 (66%), p=0.038).
Conclusion
Though this study lacked power, we did not detect major differences in clinical outcomes between standard or restrictive approach to treatment. These results will inform future studies in this area.
Trial registration number
NCT01482559, EudraCT 2010-023988-17.
Keywords: cardiology, neonatology, pharmacology
What is already known on this topic?
The most frequently used indication for treatment is a mean blood pressure (BP) below a predetermined threshold, typically defined as mean BP (measured in mm Hg) less than the infant’s gestational age in completed weeks.
Many preterm infants continue to receive intervention for low BP with little evidence to support such practices and volume followed by dopamine is the most common approach.
What this study adds?
The overall incidence of low blood pressure is less than previously determined with less than one quarter of infants having at least one numerically low BP in the first days of life. The mean time to presentation with low BP is early, less than 6 hours.
Conducting studies of cardiovascular instability in extreme preterm infants is challenging, and alternative trial designs and consent strategies need to be considered.
Introduction
The definition and management of low blood pressure (BP) in preterm infants, particularly those born before 28 weeks of gestation, are controversial.1 2 Many preterm infants continue to receive intervention for low BP with little evidence to support such practices.3 In a recent multisite observational study, over 50% of extremely preterm infants received an intervention for low BP, and almost 30% received an inotrope.4 Surveys from Europe,5 Canada6 and Australia7 identified variations in the definition and treatment of low BP. The most frequently used indication for treatment was a mean BP below a predetermined threshold, typically defined as mean BP (measured in mm Hg) less than the infant’s gestational age (GA), in completed weeks.
Preterm infants are usually treated for low BP in the first 24 hours of life; the more immature they are, the more likely they are to be treated.8 9 The most commonly used treatment is a volume bolus followed by dopamine infusion.10 Dopamine usually increases BP, but its short-term and long-term effects are unclear.11 As there are concerns that inotrope therapy may be associated with an increase in the prevalence of intraventricular haemorrhage (IVH)12 and adverse long-term outcome,13 a more restrictive approach has been suggested. However, no adequately powered trial that measures both the short-term and long-term outcomes of inotrope therapy in hypotensive infants has yet been performed.14
The Hypotension In Preterm Infants (HIP) trial was designed to compare the standard management approach—saline bolus and dopamine infusion based on a GA threshold—to a more restrictive approach—saline bolus and placebo infusion, with further intervention dictated by assessment of the infant’s overall haemodynamic status15 within the first 72 hours after birth. We hypothesised that a more restrictive approach would improve survival without significant brain injury at 36 weeks of postmenstrual age (PMA) in infants born before 28 weeks of GA.
Methods
Study design
The HIP trial was an international, multicentre, parallel design randomised trial that compared two strategies for the management of hypotension in infants born before 28 weeks of GA. Infants were enrolled at 10 sites across Europe and Canada between February 2015 and September 2017.
Participants
Infants were eligible for inclusion if they had all of the following: (1) a GA of <28 weeks at birth; (ii) an indwelling arterial line to monitor BP, suitably calibrated and zeroed with the measuring dome at the level of the infants mid-axillary line when supine; (3) a cranial ultrasound scan demonstrating no significant IVH (grade 3 or 4); and (4) low BP, defined as a mean BP of 1 mm Hg or more below a mean BP value equivalent to the GA in completed weeks, which persisted over at least a 15 min period within the first 72 hours of birth. Patients were ineligible if they were considered unlikely to survive by attending clinicians, had significant congenital anomalies or if there was evidence of frank hypovolaemia.
Primary and secondary outcome measures
The primary outcome was survival to 36 weeks of PMA free of severe brain injury on ultrasound. IVH was classified according to Volpe’s criteria and cystic periventricular leukomalacia according to de Vries criteria. We defined severe brain injury as IVH causing moderate or severe ventricular dilatation, intracerebral echodense lesions and cystic periventricular leucomalacia. Survival free of neurodisability at 2 years of corrected age was a coprimary outcome and will be reported at a later date. Prespecified secondary outcomes included all-cause mortality at 36 weeks of PMA, IVH grade 3 or 4 at any age, periventricular leucomalacia and/or ventriculomegaly on cranial ultrasound at 36 weeks of PMA, total duration of initial inotrope use and need for additional BP support.
Consent and randomisation
Verbal and written information was provided to parents antenatally where possible. When antenatal consent was impractical, the parents were informed of the study as early as possible following delivery, and eligible infants were enrolled only when written consent was obtained. No deferred or waiver of consent was permitted. Group allocation was generated using a web-based system. Infants were randomly assigned in a 1:1 ratio in blocks of four. Randomisation was stratified by centre and GA (23–25 weeks and 26–27 weeks). Infants from multiple births were randomised independently. Local principal investigators performed the randomisation and provided the code to the pharmacist or nurse preparing the infusion.
Trial interventions
Infants were assigned to one of two groups (standard vs restrictive). Infants received an infusion of 10mL/kg of 0.9% saline administered over 20 min before commencing their allocated study drug contained in 50 mL syringes identical in appearance. In the standard group, the syringe contained dopamine in 5% dextrose, commenced at a rate of 5 mcg/kg/min. In the restrictive group, the syringe contained 5% dextrose. The dose of study drug was not increased if the mean BP rose to greater than the GA threshold. If the mean BP remained below the GA, the study drug infusion was increased in mcg/kg/min dopamine or equivalent placebo increments every 30 min to a maximum dose of 20 mcg/kg/min or equivalent (figure 1). At the end of this 2-hour period, if the mean BP remained less than the GA threshold but with no signs of poor perfusion, ongoing clinical assessment was mandated every 6 hours or earlier if clinically indicated. Open-label use of dopamine was prohibited.
Figure 1.
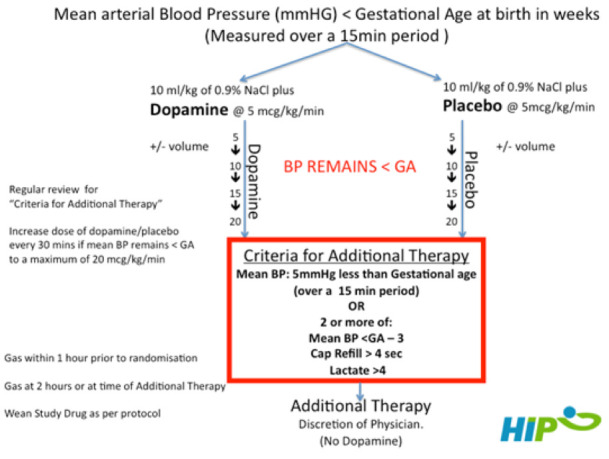
Trial design flow diagram. BP, blood pressure; GA, gestational age.
Rescue therapy
Additional therapy (typically epinephrine) was indicated if criteria for poor perfusion were met. A combination of BP values, clinical signs (skin colour, urine output and heart rate) and biochemical findings were considered. Additional therapy was indicated when the mean BP was more than 5 mm Hg below the threshold or when the infants had two or more of (1) mean BP of 3 mm Hg less than the threshold value, (2) lactate greater than 4 mmol/L and (3) prolonged capillary refill time (>4 s). Weaning of the study drug was as follows: if BP was 5 mm Hg greater than threshold, then study drug was reduced by 5 mcg/kg/min; if >10 mm Hg, it was reduced by 10 mcg/kg/min; and if 15 mm Hg greater, the study drug was stopped. If an infant developed hypotension at a later stage during their stay, dopamine administration was prohibited. Alternative interventions included volume and alternative inotropes/vasopressors (epinephrine, dobutamine, norepinephrine and milrinone).
Sample size and analysis
This was calculated assuming that the proportion of infants under standard intervention experiencing the primary outcome would be 50% and the desired difference to detect an improvement to 60%, using alpha of 0.05 and beta of 0.2. The required sample size was 385 infants per group. Assuming a loss to follow-up of no more than 10%, we planned enrolment of 830 subjects. Categorical variables were described using frequency and percentage (%), and continuous variables were described using mean and SD when the variable was normally distributed or the median and IQR when the variable was not normally distributed. Logistic random-effects regression was used for comparisons of binary outcomes between the groups, and linear random-effects regression was used for comparisons of continuous outcomes between the groups. For both regression models, group was a fixed effect and centre was a random effect. All statistical analysis was performed using STATA V.15.0.
Monitoring and regulatory issues
This study was part of a paediatric investigational plan (PIP) registered with the European Medicine Agency to develop a new paediatric dopamine formulation. The concentration of this ready-to-use product was 1.5 mg/mL of dopamine in a 30 mL vial. Drug production issues meant the final product was not available until the latter months of the project. However, drug preparation was standardised to reflect this concentration. Pharmacovigilance was conducted by the sponsor (BrePco Biopharma, Dublin). The Data Safety Monitoring Board comprised two independent neonatologists and a trial statistician. Clearly defined stopping rules were established based on safety and efficacy. After 2 years of enrolment, a decision was made by the trial steering committee to discontinue the study due to ongoing drug production issues, cessation of funding and the overall slow enrolment rate.
Results
There were 809 infants born before 28 weeks of GA at participating centres between February 2015 and September 2017. Overall, 197 infants (24%) had a low BP documented (invasively or non-invasively). Of the 809 patients, 307 had invasive BP monitoring. Thirty-six infants were ineligible due to an initial cranial ultrasound abnormality, while 9 were considered likely to die and 4 had congenital anomalies. Of the remaining 258 infants, 114 were hypotensive and met all inclusion criteria, and 58 (51%) consented. Reasons for non-inclusion are detailed in the Consolidated Standards of Reporting Trials diagram (figure 2). Baseline characteristics of enrolled infants are detailed in table 1 (maternal), table 2 (birth) and table 3 (enrolment) and show no major imbalances. The median age at enrolment was 5.28 hours (IQR 3.54–12.10 hours) in the standard care group and 6.12 hours (IQR 3.93–14.34 hours) in the restrictive management group. The mean BP at the time of enrolment was similar between the groups (21.4 mm Hg vs 21.5 mm Hg).
Figure 2.
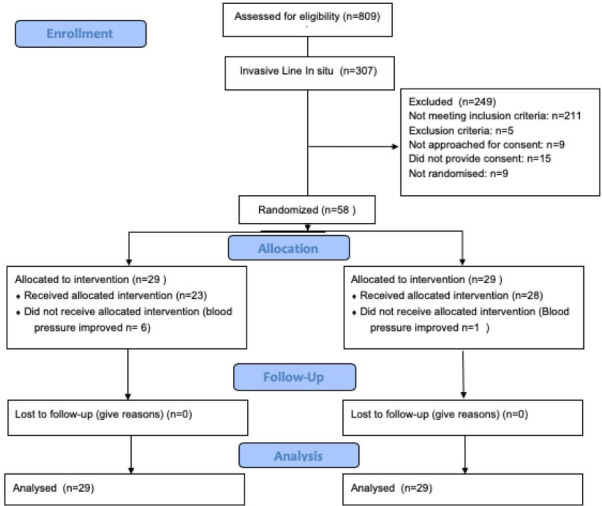
CONSORT diagram. CONSORT, Consolidated Standards of Reporting Trials.
Table 1.
Maternal characteristics
| Maternal characteristics | Standard (n=29) n (%)* |
Restrictive (n=29) n (%)* |
| Antenatal steroids (any) | 28 (97) | 26 (90) |
| Antenatal steroids (complete) | 23 (82) | 22 (85) |
| Maternal hypertension | 8 (28) | 7 (24) |
| Magnesium sulfate† | 18 (75) | 19 (83) |
| Placental abruption | 2 (7) | 3 (10) |
| PPROM | 9 (31) | 9 (31) |
| Chorioamnionitis‡ | ||
| Clinical | 6 (21) | 6 (21) |
| Histological | 3 (11) | 1 (3) |
| No | 19 (68 | 22 (76) |
| Presentation‡ | ||
| Breech | 9 (32) | 10 (35) |
| Cephalic | 19 (68) | 19 (66) |
| Cord PH obtained | 17 (59) | 20 (69) |
| Venous: median (IQR)§ | 7.34 (7.29–7.37) | 7.32 (7.30–7.38) |
| Arterial: median (IQR)¶ | 7.29 (7.23–7.35) | 7.28 (7.21–7.34) |
| Mode of delivery | ||
| Vaginal | 11 (38) | 8 (28) |
| Caesarean section | 18 (62) | 21 (72) |
| Cord clamping** | ||
| Immediate | 17 (61) | 15 (56) |
| Delayed cord clamping | 11 (39) | 12 (44) |
*Unless otherwise stated.
†n=24 in the standard group and n=23 in the restrictive group.
‡n=28 in the standard group.
§n=14 in the standard group and n=13 in the placebo group.
¶n=14 in the standard group and n=9 in the restrictive group.
**n=28 in the standard group and n=27 in the restrictive group.
PPROM, preterm premature rupture of membranes.
Table 2.
Infant characteristics at delivery
| Standard (n=29) n (%)* |
Restrictive (n=29) n (%)* |
|
| GA (weeks), mean (SD) | 25.3 (1.5) | 25.4 (1.3) |
| Birth weight (g), mean (SD) | 683 (146) | 745 (171) |
| Male | 21 (72) | 20 (69) |
| Infant of multiple gestation | 9 (31) | 12 (41) |
| GA<26 weeks | 19 (66) | 19 (66) |
| Apgar at 1 min, median (IQR)† | 4 (2–6) | 4 (3.0–5.8) |
| Apgar at 5 min, median (IQR)† | 7 (5.5–8.0) | 7 (5.3–8.0) |
| Base excess on NICU admission blood gas, median (IQR) | −5.0 (−8.0 to −1.6) | −4.9 (−8.6 to −2.5) |
| Temperature on NICU admission (°C), median (IQR)‡ | 36.3 (36.0–37.0) | 36.4 (35.8–37.0) |
| Age at enrolment (hours), median (IQR) | 5.28 (3.54–12.10) | 6.12 (3.93–14.34) |
*Unless otherwise stated.
†n=28 in the restrictive group.
‡n=28 in the standard group.
GA, gestational age; NICU, neonatal intensive care unit.
Table 3.
Infant characteristics at enrolment
| Standard (n=29) n (%)* |
Restrictive (n=29) n (%)* |
|
| Received respiratory support | 29 (100) | 29 (100) |
| Supplemental oxygen | 25 (86) | 23 (79) |
| CPAP | 3 (10) | 5 (17) |
| Conventional ventilation | 24 (83) | 23 (79) |
| High-frequency ventilation | 2 (7) | 2 (7) |
| Respiratory distress syndrome | 25 (86) | 27 (93) |
| Surfactant first given in the delivery room | 17 (59) | 19 (66) |
| Surfactant first given in the NICU | 10 (34) | 9 (31) |
| Not given | 2 (7) | 1 (3) |
| Pneumothorax | 0 (0) | 2 (7) |
| Inhaled nitric oxide | 1 (3) | 2 (7) |
| Patien uctus rteriosu on echo | 5 (17) | 6 (21) |
| Indomethacin for prophylaxis | 0 (0) | 1 (3) |
| Indomethacin for PDA | 0 (0) | 0 (0) |
| Ibuprofen for PDA | 1 (3) | 0 (0) |
| Pulmonary haemorrhage | 1 (3) | 0 (0) |
| Lowest recorded MABP (mm Hg), median (IQR) | 21.0 (20.0–23.0) | 22.0 (19.5–23.5) |
| Mean (SD) mm Hg BP<GA | 3.6 (2.1) | 3.5 (1.9) |
| Lactate (mmol/L), median (IQR)† | 1.9 (1.2–3.1) | 2.2 (1.6–4.6) |
*Unless otherwise stated.
†n=27 in the standard group.
BP, blood pressure; CPAP, continuous positive airway pressure; GA, gestational age; MABP, mean arterial blood pressure; NICU, neonatal intensive care unit; PDA, patent ductus arteriosus.
The primary outcome of survival free of ultrasound abnormality at 36 weeks was reached by 18/29 (62%) in the standard group and by 20/29 (69%) in the restrictive management group (OR 0.74, 95% CI 0.25 to 2.18) (table 4). The frequency of the components of the primary outcome did not differ between groups. The frequency of the primary outcome or its components did not differ between groups in either GA stratum (ie, above or below 26 weeks of gestation). The frequency of each secondary outcome was similar by study group (table 5). Mean BP dynamics following commencement of investigational medicinal product are displayed in figure 3. Changes in mean BP from 0 to 2 hours differed between the placebo and dopamine groups (p=0.028 for group×time interaction). The largest difference between the two groups was at 30 min (difference in means 4.4, 95% CI 1.8 to 7.1, p=0.001).
Table 4.
Primary outcome survival without severe ultrasound abnormality at 36 weeks of PMA
| Standard (n=29) n (%) |
Restrictive (n=29) n (%) |
OR (95% CI)* | P value* | |
| All Infants | 18 (62) | 20 (69) | 0.74 (0.25 to 2.18) | 0.58 |
| <26 weeks (n=19 in each group) | 11 (58) | 10 (53) | 1.24 (0.34 to 4.45) | 0.74 |
| >26 weeks (n=10 in each group) | 7 (70) | 10 (100) | – | 0.21† |
*From logistic regression analysis.
†From Fisher’s exact test due to being unable to perform logistic regression analysis.
PMA, postmenstrual age.
Table 5.
Secondary outcomes
| Standard (n=29) n (%)* |
Restrictive (n=29) n (%)* |
Odds ratio (95% CI)† | P Value† | |
| Mortality | 6 (21) | 7 (24) | 0.82 (0.24 to 2.83) | 0.75 |
| Severe ultrasound abnormality | 5 (17) | 5 (17) | 1.00 (0.26 to 3.91) | 1 |
| Grade 3/4 IVH | 5 (17) | 2 (7) | 3.06 (0.51 to 18.41) | 0.22 |
| PVL | 2 (7) | 2 (7) | 1.04 (0.13 to 8.37) | 0.97 |
| Any ultrasound abnormality | 16 (55) | 13 (45) | 1.51 (0.54 to 4.26) | 0.43 |
| NEC | 1 (3) | 4 (14) | 0.22 (0.02 to 2.13) | 0.19 |
| SIP | 3 (10) | 3 (10) | 1.00 (0.18 to 5.42) | 1 |
| BPD‡ | 17 (74) | 14 (64) | 1.87 (0.45 to 7.68) | 0.39 |
| Duration of inotrope (hours) | 17.8 (7.5–30.6)§¶ | 13.7 (6.1–24.5)§** | 1.46 (0.84 to 2.56)†† | 0.18 |
| Any intervention | 11 (38) | 19 (66) | 0.32 (0.11 to 0.94) | 0.038 |
*Unless otherwise stated.
†From logistic regression analysis unless otherwise stated.
‡Of those who had survived to 36 weeks.
§Median (IQR).
¶n=21 in the standard group.
**n=22 in the restricted group.
††Ratio of geometric means as the duration variable was log-transformed.
BPD, bronchopulomary dysplasia; IVH, intraventricular haemorrhage; NEC, necrotising enetrocolitis; PVL, periventricular leucomalacia; SIP, spontaneous intestinal perforation.
Figure 3.
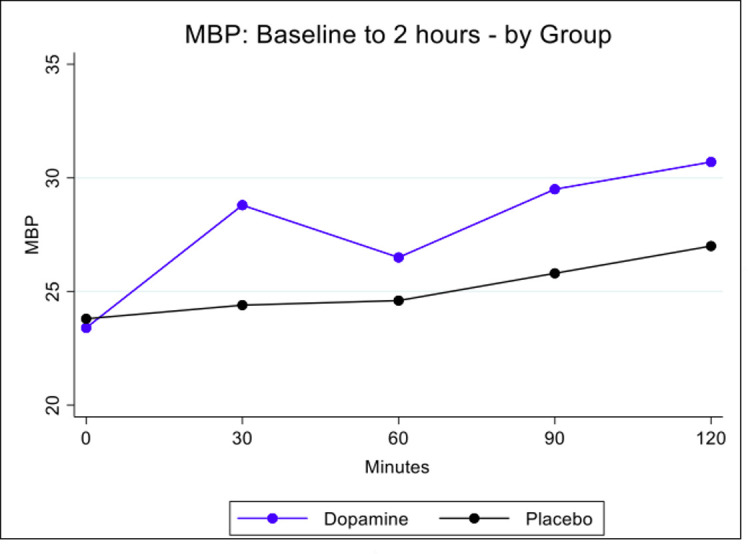
Changes in MBP over time by group. MBP, mean blood pressure.
Additional BP support was used less frequently in the standard group (11/29, 38%) compared with the restrictive group (19/29, 66%) (p=0.038). Fewer infants in the standard group received additional inotropes (28% vs 48%, p=0.11). Among infants <26 weeks of gestation, this difference was most marked (11% vs 63%, p=0.002). Of 22 patients who received additional inotrope, the majority, 19, received epinephrine; 5 received dobutamine; and 4 received hydrocortisone. Additional therapy was commenced based on mean BP, which was >5 mm Hg less than the equivalent GA in seven cases, and in 12 cases, the mean BP was >3 mm Hg below but with additional clinical signs or abnormal lactate values. Two infants received open-label dopamine contrary to protocol during their neonatal intensive care unit stay beyond the first 72 hours.
Discussion
In this placebo-controlled trial, the frequency of survival free of cranial ultrasound abnormality at 36 weeks of PMA was similar between both groups. However, our ability to draw robust conclusions is limited as the overall number of included patients was low, rendering the study significantly underpowered. Similar studies of cardiovascular support in newborns have concluded early.16–19 A randomised trial of hydrocortisone for neonatal hypotension terminated early16 because a large number of patients did not meet eligibility criteria and there were difficulties obtaining timely informed consent. Physician unwillingness to enrol, a complex factorial trial design and low parental consent rate were identified as key factors in another trial that randomised only 10 patients from an eligible population of 126.17 Timely consent and a declining incidence were major factors highlighted in a recent trial of pulmonary hypertension management.20
Clinicians may be unwilling to enrol extremely preterm infants with very low BP values, or may have decided to include other parameters, such as echocardiography, to decide on inclusion. Future trials will need to consider alternative approaches to diagnosis. Relatively short time lines to obtain consent may also be a major factor in parental refusal of consent. In our study, the mean time patients were enrolled was approximately 6 hours.
The study was reviewed by the Paediatric Committee of the European Medicines Agency21 as part of a PIP, which mandated an invasive arterial catheter and pre-enrolment ultrasound free of significant IVH. Deferred consent was not permitted. These relatively strict inclusion criteria likely impacted recruitment. Significant challenges were met in the development of a paediatric formulation, a finding similar to the Neocirc study, which published a pilot trial comparing dobutamine to placebo in low flow states.22
There has been a move towards less intervention in the setting of low BP.12 15 However, data from a recent matched cohort study suggests that treating isolated low BP is associated with less severe brain injury.23 Therefore, whether or not to treat isolated low BP remains to be answered. We hope that our findings will help inform future studies in this area. The overall prevalence of low BP was 25% but varied from 9% at one site to 32% at another. Previous Canadian Neonatal Network data suggested a prevalence of over 50%,24 and the German Neonatal Network reported a frequency of 52%.12 The Australian Placental Transfusion Study identified a rate of approximately 32%.25 Our data represent the most recent data in a prospectively recorded group of extremely preterm infants delivered at tertiary centres across four countries. The wide variability may reflect different practices including timing of cord clamping,25–27 modes of mechanical ventilation28 (premedication and sedation) and presence of an invasive arterial line.
The need to enrol infants to studies shortly after birth places significant pressure on families to consent and alternative recruitment strategies need to be sought. In studies of therapeutic hypothermia,29 consent could only be obtained postnatally following the recognition of an encephalopathic infant. However, in a trial such as this, it could be anticipated from knowledge of the GA. A stepped and continuous consent process might be preferable with formal deferred consent. Antenatal consent is feasible but requires significant resources, as similar to the Surfactant Positive Airway Pressure and Pulse Oximetry Trial,30 many parents would need to be approached to enrol one infant. Our data suggest approaching a minimum of eight families antenatally to enrol one infant.
We identified some important patient characteristics. A disproportionate number were male (70%) and the majority (85%) were intubated. Mean BP for the group overall was low (21.3 mm Hg) at the time of randomisation, with a normal lactate value. This suggests that while the BP reading was low, perfusion was satisfactory, reflective of the problem being addressed. The overall mortality for the entire cohort was 22%. The overall rate of cranial ultrasound abnormality was 50%, but with severe cranial ultrasound abnormality present in only 15%, to give a combined overall primary outcome of 34%. Of those recruited, 11 (19%) had either necrotising enterocolitis or spontaneous intestinal perforation. Consideration should be given to other clinically relevant end points in future studies of cardiovascular support, in particular, the inclusion of gastrointestinal complications. Long-term outcome is ongoing for the enrolled infants.
In conclusion, conducting trials of haemodynamic support in extremely preterm infants is challenging. We did not detect differences in clinical outcomes between standard or restrictive approaches to management and so cannot comment on which treatment approach is superior. These results will inform future studies in this area.
Acknowledgments
The authors thank Ita Herlihy, Niamh O’Shea, Paula Hyland, Niamh Geaney, Jackie O’Leary, Barb Kamstrum and Sheryl George, and Data Monitoring Committee members, Henry Halliday, Al Ozonoff and Jean Christoph Mercier.
Footnotes
Twitter: @afif_elkhuffash
Contributors: EMD conceptualised, designed and coordinated the study, coordinated and supervised the data collection, analysed the data and drafted the initial manuscript, and revised the manuscript. JMi, GN, P-YC, JDC, AFE-K, GBB and ZS helped to conceptualise and design the study, coordinated and supervised the data collection, drafted the initial manuscript and revised the manuscript. KJB, NM, CPFO’D and DVL helped to conceptualise and design the study, drafted the initial manuscript and revised the manuscript. GP conceptualised and designed the study and revised the manuscript. VL made a substantial contribution to the analysis and interpretation of data and reviewed and revised the manuscript. JMa and HW coordinated and supervised the data collection, collected data, and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Funding: This work was supported by the EU FP7/2007-2013 under grant agreement number 260777 (the HIP Trial) and the EU Seventh Framework Programme (260777).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data may be obtained from a third party and are not publicly available. It is currently not possible to share the HIP Trial data set.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The research ethics committee at all participating sites approved this trial.
References
- 1. Rabe H, Rojas-Anaya H. Inotropes for preterm babies during the transition period after birth: friend or foe? Arch Dis Child Fetal Neonatal Ed 2017;102:F547–50. 10.1136/archdischild-2016-311709 [DOI] [PubMed] [Google Scholar]
- 2. Cox DJ, Groves AM. Inotropes in preterm infants--evidence for and against. Acta Paediatr 2012;101:17–23. 10.1111/j.1651-2227.2011.02545.x [DOI] [PubMed] [Google Scholar]
- 3. Dempsey EM, Barrington KJ. Treating hypotension in the preterm infant: when and with what: a critical and systematic review. J Perinatol 2007;27:469–78. 10.1038/sj.jp.7211774 [DOI] [PubMed] [Google Scholar]
- 4. Batton B, Li L, Newman NS, et al. Use of antihypotensive therapies in extremely preterm infants. Pediatrics 2013;131:e1865–73. 10.1542/peds.2012-2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dempsey EM, Barrington KJ, Marlow N, et al. Management of hypotension in preterm infants (the hip trial): a randomised controlled trial of hypotension management in extremely low gestational age newborns. Neonatology 2014;105:275–81. 10.1159/000357553 [DOI] [PubMed] [Google Scholar]
- 6. Dempsey EM, Barrington KJ. Diagnostic criteria and therapeutic interventions for the hypotensive very low birth weight infant. J Perinatol 2006;26:677–81. 10.1038/sj.jp.7211579 [DOI] [PubMed] [Google Scholar]
- 7. Sehgal A, Osborn D, McNamara PJ. Cardiovascular support in preterm infants: a survey of practices in Australia and New Zealand. J Paediatr Child Health 2012;48:317–23. 10.1111/j.1440-1754.2011.02246.x [DOI] [PubMed] [Google Scholar]
- 8. Batton B, Batton D, Riggs T. Blood pressure during the first 7 days in premature infants born at postmenstrual age 23 to 25 weeks. Am J Perinatol 2007;24:107–15. 10.1055/s-2007-970178 [DOI] [PubMed] [Google Scholar]
- 9. Laughon M, Bose C, Allred E, et al. Factors associated with treatment for hypotension in extremely low gestational age newborns during the first postnatal week. Pediatrics 2007;119:273–80. 10.1542/peds.2006-1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stranak Z, Semberova J, Barrington K, et al. International survey on diagnosis and management of hypotension in extremely preterm babies. Eur J Pediatr 2014;173:793–8. 10.1007/s00431-013-2251-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Subhedar NV, Shaw NJ. Dopamine versus dobutamine for hypotensive preterm infants. Cochrane Database Syst Rev 2000;2:CD001242. 10.1002/14651858.CD001242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Faust K, Härtel C, Preuß M, et al. Short-term outcome of very-low-birthweight infants with arterial hypotension in the first 24 h of life. Arch Dis Child Fetal Neonatal Ed 2015;100:F388–92. 10.1136/archdischild-2014-306483 [DOI] [PubMed] [Google Scholar]
- 13. Batton B, Li L, Newman NS, et al. Early blood pressure, antihypotensive therapy and outcomes at 18-22 months' corrected age in extremely preterm infants. Arch Dis Child Fetal Neonatal Ed 2016;101:F201–6. 10.1136/archdischild-2015-308899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barrington KJ, Dempsey EM. Cardiovascular support in the preterm: treatments in search of indications. J Pediatr 2006;148:289–91. 10.1016/j.jpeds.2005.12.056 [DOI] [PubMed] [Google Scholar]
- 15. Dempsey EM, Al Hazzani F, Barrington KJ. Permissive hypotension in the extremely low birthweight infant with signs of good perfusion. Arch Dis Child Fetal Neonatal Ed 2009;94:F241–4. 10.1136/adc.2007.124263 [DOI] [PubMed] [Google Scholar]
- 16. Watterberg KL, Fernandez E, Walsh MC, et al. Barriers to enrollment in a randomized controlled trial of hydrocortisone for cardiovascular insufficiency in term and late preterm newborn infants. J Perinatol 2017;37:1220–3. 10.1038/jp.2017.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Batton BJ, Li L, Newman NS, et al. Feasibility study of early blood pressure management in extremely preterm infants. J Pediatr 2012;161:65–9. 10.1016/j.jpeds.2012.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Steinhorn RH, Fineman J, Kusic-Pajic A, et al. Bosentan as adjunctive therapy for persistent pulmonary hypertension of the newborn: results of the randomized multicenter placebo-controlled exploratory trial. J Pediatr 2016;177:90–6. 10.1016/j.jpeds.2016.06.078 [DOI] [PubMed] [Google Scholar]
- 19. Gagliardi L. Treatment of hypotension in preterm infants: pathophysiology versus evidence-based medicine. Acta Paediatr 2013;102:446–8. 10.1111/apa.12190 [DOI] [PubMed] [Google Scholar]
- 20. Padbury JF. Gasping for air. J Pediatr 2016;177:2–3. 10.1016/j.jpeds.2016.08.038 [DOI] [PubMed] [Google Scholar]
- 21. Dempsey EM, Connolly K. Who are the PDCO? Eur J Pediatr 2014;173:233–5. 10.1007/s00431-013-2096-2 [DOI] [PubMed] [Google Scholar]
- 22. Bravo MC, López-Ortego P, Sánchez L, et al. Randomized, placebo-controlled trial of dobutamine for low superior vena cava flow in infants. J Pediatr 2015;167:572–8. 10.1016/j.jpeds.2015.05.037 [DOI] [PubMed] [Google Scholar]
- 23. Durrmeyer X, Marchand-Martin L, Porcher R, et al. Abstention or intervention for isolated hypotension in the first 3 days of life in extremely preterm infants: association with short-term outcomes in the EPIPAGE 2 cohort study. Arch Dis Child Fetal Neonatal Ed 2017;102:490–6. 10.1136/archdischild-2016-312104 [DOI] [PubMed] [Google Scholar]
- 24. Barrington KJ, Stewart S, Lee S. Differing blood pressure thresholds in preterm infants, effects on frequency of diagnosis of hypotension and intraventricular haemorrhage. Pediatr Res 2002;51:455A. [Google Scholar]
- 25. Tarnow-Mordi W, Morris J, Kirby A, et al. Delayed versus immediate cord clamping in preterm infants. N Engl J Med 2017;377:2445–55. 10.1056/NEJMoa1711281 [DOI] [PubMed] [Google Scholar]
- 26. Katheria AC, Lakshminrusimha S, Rabe H, et al. Placental transfusion: a review. J Perinatol 2017;37:105-111. 10.1038/jp.2016.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Popat H, Robledo KP, Kirby A, et al. Associations of measures of systemic blood flow used in a randomized trial of delayed cord clamping in preterm infants. Pediatr Res 2019;86:71–6. 10.1038/s41390-019-0348-1 [DOI] [PubMed] [Google Scholar]
- 28. Göpel W, Kribs A, Ziegler A, et al. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open-label, randomised, controlled trial. Lancet 2011;378:1627–34. 10.1016/S0140-6736(11)60986-0 [DOI] [PubMed] [Google Scholar]
- 29. Azzopardi D, Brocklehurst P, Edwards D, et al. The TOBY study. whole body hypothermia for the treatment of perinatal asphyxial encephalopathy: a randomised controlled trial. BMC Pediatr 2008;8:17. 10.1186/1471-2431-8-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rich WD, Auten KJ, Gantz MG, et al. Antenatal consent in the support trial: challenges, costs, and representative enrollment. Pediatrics 2010;126:e215–21. 10.1542/peds.2009-3353 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data may be obtained from a third party and are not publicly available. It is currently not possible to share the HIP Trial data set.


