Abstract
Many drug therapies are associated with prolongation of the QT interval. This may increase the risk of Torsades de Pointes (TdP), a potentially life-threatening cardiac arrhythmia. As the QT interval varies with a change in heart rate, various formulae can adjust for this, producing a ‘corrected QT’ (QTc) value. Normal QTc intervals are typically <450 ms for men and <460 ms for women. For every 10 ms increase, there is a ~5% increase in the risk of arrhythmic events. When prescribing drugs associated with QT prolongation, three key factors should be considered: patient-related risk factors (eg, female sex, age >65 years, uncorrected electrolyte disturbances); the potential risk and degree of QT prolongation associated with the proposed drug; and co-prescribed medicines that could increase the risk of QT prolongation. To support clinicians, who are likely to prescribe such medicines in their daily practice, we developed a simple algorithm to help guide clinical management in patients who are at risk of QT prolongation/TdP, those exposed to QT-prolonging medication or have QT prolongation.
Keywords: Cardiology, Pacing & electrophysiology, Clinical pharmacology, Therapeutics, Adverse events
INTRODUCTION
The QT interval is the time from the onset of ventricular depolarisation to the end of ventricular repolarisation; the latter accounting for the majority of the interval as depolarisation in the absence of conduction disease is rapid (figure 1). 1 The underlying physiological processes are a result of movement of sodium, potassium and calcium via specific receptors located in the cell membrane and endoplasmic reticulum. Sodium channels are mainly responsible for depolarisation; however, the late sodium current contributes to repolarisation. Calcium channels are important in maintaining the plateau phase of the action potential, and potassium channels play a major role in repolarisation. Abnormalities of these receptors can have profound effects on the cardiac action potential, for example, abnormalities in the potassium channels can prolong repolarisation. 1 The latter can result in QT prolongation on the ECG.
Figure 1.
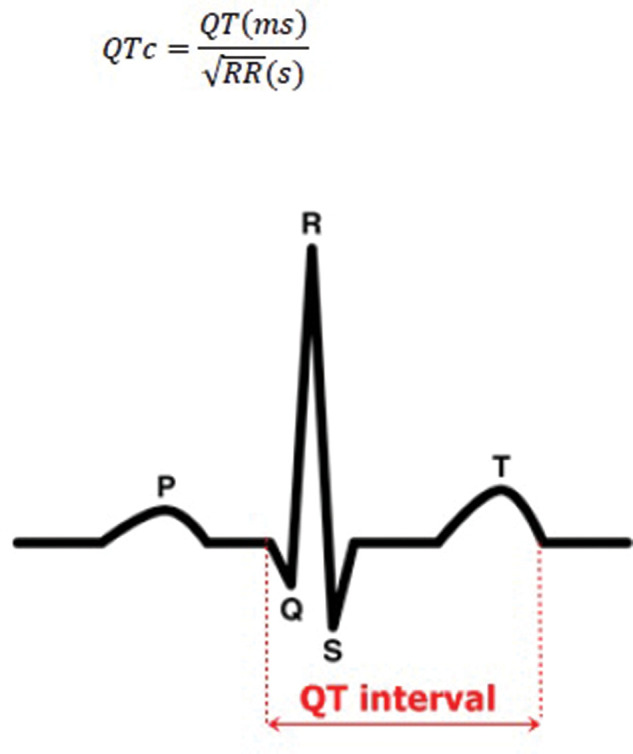
Measurement of the QT interval.
Causes of QT prolongation can be divided into two categories: congenital or acquired. Congenital long QT syndrome (LQTS) is an inherited disease, it has several forms, and is caused by mutations in the genes encoding specific ion-channel subunits or regulatory proteins. 2 Polymorphisms in various other genes—for example that encoding the nitric oxide synthase 1 adaptor protein (NOS1AP)—have also been associated with increases in the QT interval. 2 3 Nonetheless, LQTS is typically rare, with an estimated prevalence of approximately 1 in 2000–2500 live births. 4 Many patients are asymptomatic and thus congenital LQTS is often discovered incidentally on an ECG, by family history, or after eventually experiencing symptoms such as syncope. 2 Acquired QT prolongation is more prevalent than the congenital form and is often a result of structural heart disease (eg, myocardial infarction, heart failure, left ventricular hypertrophy) and drugs that prolong the QT interval. 5 Many other individual risk factors, detailed later, may also predispose to the prolongation of QT interval. 6
Whatever the underlying cause, prolongation of the QT interval is clinically significant. Syncope, cardiac arrest and sudden death typically increase with increasing QT although assessment is complicated by the relationship being non-linear (figure 2). 7 8 QT prolongation may be accompanied by arrhythmogenic after-depolarisations, and predisposes patients to a condition known as Torsades de Pointes (TdP), a potentially life-threatening form of polymorphic ventricular tachycardia. 9 10 For some individuals, TdP may occur with modest QT prolongation, whereas others may experience no effects even with markedly prolonged QT. 1
Figure 2.
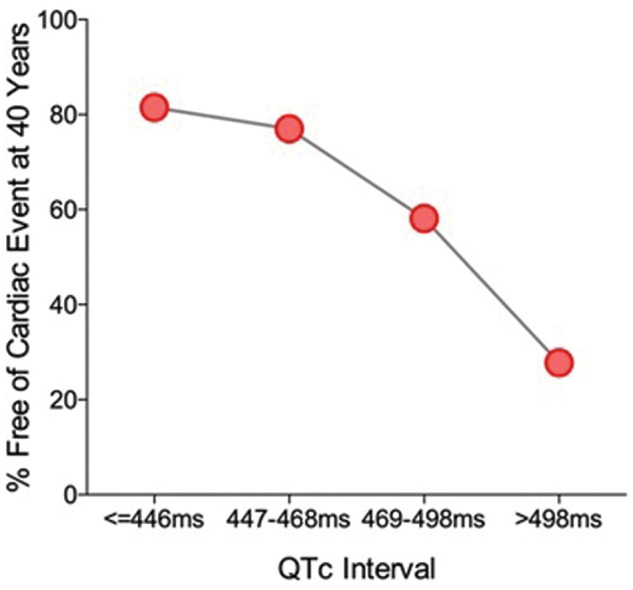
Non-linear relationship in the estimated rate of patients being free of cardiac events at 40 years of age and QT interval corrected for heart rate (QTc). The study included 647 patients from families with long-QT syndrome divided into four quartiles based on QTc interval. ‘Cardiac events’ were defined as the occurrence of syncope, cardiac arrest or sudden death, and these increased non-linearly with increasing QTc. Data were extracted from a Kaplan–Meier survival graph presented in Priori et al (2003) 8 using WebPlotDigitizer v3.9.
There are multiple factors that can increase the risk of TdP (table 1). 11 Many are not modifiable and hence not amenable to specific medical intervention. However, the most common environmental stressor resulting in acquired LQTS is the use of particular drug therapies, 12 which can often be modified to reduce the risk of QT interval prolongation and hence of TdP.
Table 1.
Patient risk factors for Torsades de pointes with drug-induced QT prolongation. 10 14 15
| Non-modifiable | Potentially modifiable |
|---|---|
|
|
*Congenital long QT syndrome is rare (estimated prevalence: ~1 in 2000–2500 infants) but is associated with a risk of arrhythmia and premature sudden death. 4
The mechanisms that underlie the impact of certain drugs on the QT interval have not been fully elucidated. However, in most instances, it is thought to relate to effects on specific potassium channels known as rapid delayed rectifiers (IKr). 9 These play a crucial role in cardiac repolarisation and their inhibition extends individual action potentials and hence prolongs the QT interval. 9 For example, the antipsychotic medication, haloperidol, is a more specific blocker of the IKr potassium channel and prolongs QT (by 15–30 ms) in a dose-dependent manner; and it is known to cause TdP. 13 Antiarrhythmic agents such as quinidine and disopyramide are known to block both the sodium and potassium channels, and are associated with QT interval prolongation. TdP was reported with these drugs at therapeutic or subtherapeutic doses. 13 Antiarrhythmics-induced TdP is often precipitated in the presence of hypokalaemia or hypomagnesaemia. 13 Tricyclic antidepressants can prolong the QT interval primarily by blocking sodium channels, although this effect is increased if a potassium channel blocker is co-administered. 13
To reduce the risk of TdP, healthcare professionals should be aware of drugs that can prolong the QT interval. However, few recommendations exist for managing the risk of drug-induced QT prolongation in clinical practice. Many studies have introduced risk models for predicting prolongation of the QT interval/TdP, 16–18 but these tools are often not generalisable across all populations. They also do not provide guidance on how to manage high-risk cases once identified. In this review, we provide guidance on what should be considered when prescribing QT-prolonging medications in any speciality, in order to rationalise the associated risks and benefits and support clinicians in making clinical judgements in a holistic manner. We also describe the recommended steps in managing QT prolongation if it arises.
WHAT IS A NORMAL QT INTERVAL AND WHAT LEVEL OF INCREASE IS SIGNIFICANT?
The QT interval should be assessed from a good-quality ECG readout that is free from noise artefacts and has a stable baseline. It should be determined as a mean value based on at least 3–5 cardiac cycles. 19
It is important to review the entire ECG before specifically assessing the QT interval, to ensure that other conditions such as bradycardia, myocardial infarction or cardiomyopathy are not responsible for any changes in QT. In addition, the QT interval cannot be assessed in the usual way in patients whose QRS duration is abnormal (eg, those with bundle branch block). 19
Even in patients with an otherwise normal ECG readout, analysis of the QT interval is made more difficult by substantial natural variability. 1 For example, it is typically prolonged at slower heart rates and shortened when heart rate is increased. However, various formulae have been proposed to adjust for these variations, thus producing a ‘corrected QT’ (QTc) value. 1 The most frequently used formula in clinical practice is the version proposed by Bazett, 20 although this is associated with potential overcorrection at high heart rates and undercorrection at lower heart rates. 21 Other formulae, such as those of Fridericia or Framingham, may perform better. 21
Many physicians—including cardiologists—are not always able to accurately identify a long QT interval, 22 and this can lead to ‘false alarms’ that cause unnecessary concern to patients. The following three-step approach may be an easy-to-use standardised method for measuring QT 23 :
Measure QT from the initial inflection of the Q wave to the end of the T wave, defined as the intersection of a tangent to the steepest slope of the last limb of the T wave and the baseline.
Apply Bazett’s formula to obtain QTc (defined as QT/√RR from the RR interval between the measured and the preceding complex on the ECG; figure 1).
Apply an appropriate QTc threshold to differentiate normal from prolonged QT intervals.
Normal QTc intervals are typically considered to be <450 ms for men and <460 ms for women. 24 It should be noted that the predisposition to prolonged QT interval in women diminishes with increasing age 6 ; it has been suggested that cardiac ion channel activity is altered by sex hormones, which in turn affects the QT interval. 25 Thus, differences in cut-off points between men and women are not as relevant among older people. 26 Irrespective of this, there is no established threshold below which QTc prolongation is free from proarrhythmic risk. 27 A QTc interval between the sex-specific ‘normal’ threshold and 500 ms is generally considered to be prolonged; a QTc interval >500 ms may be associated with a substantially elevated risk of TdP. 12 In general, for every 10 ms increase in the QTc interval, there is around a 5–7% increase in the risk of developing TdP. 28
WHAT SHOULD BE CONSIDERED WHEN ASSESSING QT PROLONGATION RISK?
When prescribing a drug that may be associated with QT prolongation, the following three factors should be considered to better understand the risk and decide on appropriate actions.
Individual patient risk factors that increase the risk of TdP with QT-prolonging drugs
Key patient-related risk factors are summarised in table 1. Some are very common, such as female sex and age >65 years; others are rare, such as congenital long QT syndrome. Most clinical cases of drug-induced QT prolongation occur in the presence of at least one of these risk factors, and >70% occur in the presence of two or more. 14 For individuals with an elevated risk of TdP, the decision to commence a QT-prolonging drug should be made collaboratively with the patient, and the potential impact should be clearly communicated.
Potential risk and degree of QT prolongation associated with the drug
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has released warnings relating to drug-induced QT prolongation for many commonly used drugs, such as citalopram, domperidone, ondansetron and quinine. 29–32 From a practical perspective, data are inconclusive with regard to arrhythmic risk for drugs that increase the QTc interval by <20 ms; drugs associated with a change in baseline QTc of >20 ms should raise concern. 33 For example, haloperidol prolongs the QT interval by 15–30 ms, and hence may be considered high risk. 13 Moxifloxacin 400 mg has been associated with QTc interval increases of 7.5–12.5 ms, indicative of some potential risk. 13 By contrast, clarithromycin is associated with increases in QTc interval of <5 ms and has been categorised as ‘uncertain’ risk, but in view of rare reports of TdP, some caution may be advisable, particularly when using it with other QT-prolonging drugs. 33 The extent and the associated risk of developing QT prolongation when combining drugs with QT-prolonging effects are still unknown, 34 but such combinations do not necessarily have an additive effect. 16 Hence, a pragmatic approach should be employed.
The link between changes in QT and TdP is highly variable. For example, amiodarone can markedly increase QT, but typically has a homogenous effect on the ventricular myocardium and rarely causes TdP 35 ; by contrast, sotalol is associated with a greater incidence of TdP, particularly at higher doses. The effect of sotalol on QTc prolongation varies from 10 to 40 ms at doses of 160–640 mg/day. 12 It should also be noted that sotalol is associated with increased dispersion of ventricular repolarisation and can cause TdP in up to 5% of exposed patients. 9 Furthermore, the extent of QT prolongation can vary between individuals taking the same drug, and can often be dose dependent. 12 36
Nonetheless, comprehensive lists of drugs that are linked to QT prolongation are available online, 15 33 and individual MHRA Drug Safety Updates may provide further information. Specific drugs can be classified as having ‘high risk’, ‘some risk’ or potential risk that is currently ‘not categorised’. Selected drugs from each category are provided in table 2, although this list is certainly not exhaustive.
Table 2.
Selected drugs that can cause QT prolongation 15 33
| High risk | Some risk | Risk not categorised* |
|---|---|---|
|
|
|
*Drugs with less clear evidence of the risk of QT prolongation.
These lists are not exhaustive. Furthermore, other drugs (not included here) do not themselves prolong the QT interval but potentiate the effect of drugs that do. More information can be found online. 15 33.
Drug interactions that can increase the risk of QT prolongation
In some instances, interactions between different drugs can increase the risk of QT prolongation. 37 38 There are three main mechanisms by which this occurs 1 37 38 :
Pharmacodynamic interactions, when two or more drugs that prolong QT interval are co-prescribed, which can lead to an additive or potentiating effect. for example, combining two or more drugs in table 2.
Pharmacokinetic interactions, when a drug that does not prolong the QT interval itself reduces the clearance or is metabolised by the same hepatic enzymes, resulting in increased concentrations of the QT-prolonging drug. for example, ritonavir increases the levels of quinidine by decreasing its metabolism (CYP3A4 inhibitor).
Drug-induced electrolyte imbalances, such as hypokalaemia and hypomagnesaemia, which can increase the risk of QT prolongation. for example, loop or thiazide diuretics causing hypokalaemia.
HOW SHOULD QT PROLONGATION BE MANAGED IN PRACTICE?
Various tools and models have been developed for predicting the risk of QT prolongation/TdP (table 3). Some were limited to specific settings (eg, the Tisdale tool included only patients admitted to cardiac care units), 16 and others were more general. Some did not account for drug interactions, and others detected high-risk patients when using two or more QTc-prolonging drugs. These tools typically aim to identify high-risk patients by generating a score. However, decision making based on these findings is largely left to clinicians, although some electronic versions also provide suggestions on what to do.
Table 3.
QT prolongation risk assessment tools
| Tool | Description |
|---|---|
| Tisdale risk score for QT prolongation (www.mdcalc.com/tisdale-risk-score-qt-prolongation) 16 | Predicts the risk of QT prolongation >500 ms in hospitalised patients. Uses risk factors that are weighted. Suitable for patients in CCCUs. A score ≥11 predicted development of a QT interval >500 ms. The tool was developed using patients admitted to CCCUs and hence generalisability to broader populations may be limited. |
| MedSafety Scan (MSS) QT prolongation risk score (https://medsafetyscan.org/) | A platform for therapeutic decision support that incorporates the QT drugs database from the CredibleMeds website with reliable drug–drug interaction predictions to identify patients at greatest risk of major adverse drug reactions. Built to deliver accurate therapeutic risk assessment without false positives or irrelevant information. Calculation of QT risk score for ICU patients based on Tisdale risk score (validated), or for non-ICU patients using the MSS QT prolongation risk score (non-validated). It includes the risk factors in the Tisdale tool and additional risk factors reported in the literature, such as drug interactions and other validated cardiac risk factors. It is more comprehensive than the Tisdale tool. Provides advice on drug interactions. |
| Risk of QT drug–drug interactions assessment tool. 34 | A tool enabling the identification of patients with an increased risk of QTc prolongation when using two or more QTc-prolonging drugs with a known risk of TdP. Includes seven risk factors that are predictors of QT prolongation. Development of the tool might have had selection bias as the prevalence of QT prolongation was quite high compared with the overall prevalence found in the literature review. Also, the tool does not take into account the variety of QT drug–drug interactions. This could be due to the fact that stratification of QT drug–drug interactions is extremely complex and will most likely require a clinical decision in the absence of clear studies in this area. |
| Sharma clinical decision support system. 39 | A clinical decision support system to prevent the use of QT-prolonging medications in the hospital setting. Detects patients at risk of significant QT prolongation (QTc >500 ms) and alerts providers ordering QT-prolonging drugs. ECGs are automatically screened and those with significant QT prolongation (QTc >500 ms for adults; >470 ms for paediatric patients) have “Prolonged QT” documented in their records. When QT-prolonging drugs are ordered in a patient previously identified as having significant QT prolongation, the prescriber is alerted. The alert presents the name of the drug, level of the risk (risk of or possible risk of TdP), any QT-prolonging drug already on the medication list, and a link to online educational resources with more information on how to manage QT prolongation. |
| Hincapie-Castillo predictive model for drug-associated QT prolongation. 40 | A model for predicting severe QT interval prolongation in hospitalised patients using inpatient electronic health record data. The model includes 26 factors for predicting the 24-hour risk of QT events on hospital day 1 and on hospital days 2–5. |
| Bindraban risk model for predicting QTc interval prolongation in patients using QTc-prolonging drugs. 41 | A risk model to predict QTc interval prolongation of eligible ECGs. The model was developed by examining ECGs recorded in patients using one or more QTc-prolonging drugs. Independent risk factors for QTc interval prolongation were determined and risk scores were assigned. The model predicts the risk of QTc interval prolongation. |
CCCU, cardiac critical care unit; ECG, electrocardiogram; ICU, intensive care unit; QTc, corrected QT; TdP, Torsades de Pointes.
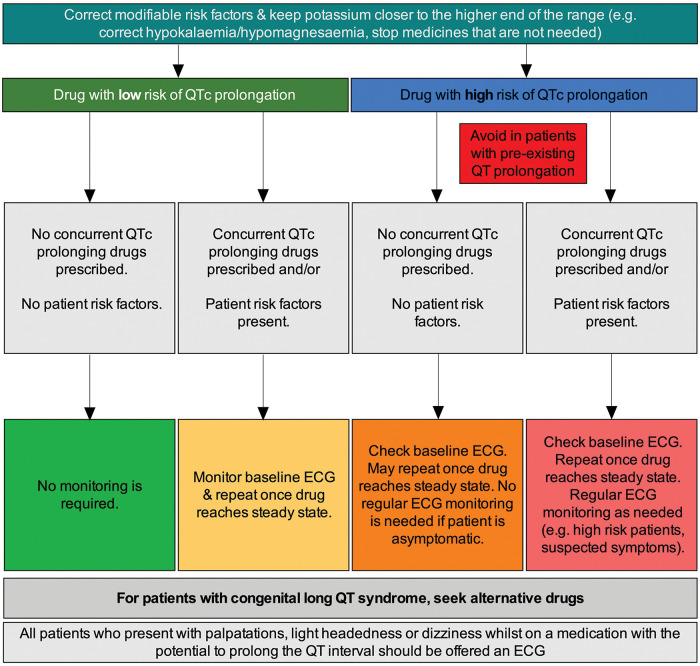
We have developed an algorithm that is more focused on what clinicians should do when using low- and high-risk QT-prolonging drugs (figure 3). Overall, it may be that risk assessment tools are useful supplements to better establish the degree of risk, while our simple algorithm can be used to help guide clinical management in patients who are at risk, exposed to QT prolonging medication or have QT prolongation. It provides guidance on what should be done to address many of these risk factors and for monitoring patients prescribed QT-prolonging drugs.
Figure 3.
Management of QT prolongation in practice.
Considerations before prescribing
Before starting a medicine that may prolong the QT interval, the prescriber should consider the degree of individual patient risk factors, the risk posed by the specific drug, and the potential impact of other medicines the patient is already receiving (table 4). Of course, these must then be balanced against the benefits of treatment with the proposed drug. Prescribers should also consider whether there are any alternative solutions that could reduce the risk of QT prolongation without compromising overall safety and efficacy.
Table 4.
How to assess the risk of drug-induced QT prolongation
| Key questions |
|---|
| Does the patient have any risk factors for QT prolongation? |
| Is the new medication associated with a risk of QT prolongation? |
| Are there any potential drug interactions that could increase the risk of QT prolongation? |
| Is the medication essential? Are there any alternatives? |
The QT-prolonging drugs management algorithm
If a QT-prolonging drug is considered appropriate, the prescriber may consider the management protocol shown in figure 3. First, they should correct modifiable risk factors as far as possible. In particular, electrolyte deficiencies such as hypokalaemia and hypomagnesaemia should be rectified, with the aim of increasing levels towards the higher end of the normal range. In addition, any other currently prescribed medicines that are not necessary should be stopped or changed, particularly if they are known to directly or indirectly increase the risk of QT prolongation.
If the new drug carries a low risk of QT prolongation (eg, listed as a potential side effect), no other drugs that prolong QT are co-prescribed, and the patient has no other associated risk factors, treatment should proceed and no additional monitoring is necessary. In the event that a drug with low QT prolongation risk is prescribed, but the patient has known risk factors and/or is prescribed another drug that is linked with QT prolongation, it may be necessary to take a baseline ECG and repeat once the drug reaches steady state (figure 3). Importantly, if the patient has congenital long QT syndrome, an alternative treatment should be sought even if the proposed drug has only a low risk of prolongation.
If the proposed drug carries a high risk of QT prolongation (eg, listed as a caution or contraindication in the product information), the management protocol should again be based on patient risk factors and/or concurrently prescribed QT-prolonging drugs (figure 3). In the absence of these additional concerns, treatment can proceed; however, a baseline ECG should be taken and may be repeated once the drug reaches steady state. No regular ECG monitoring is needed if the patient remains asymptomatic (although most patients will not have symptoms even if their QT interval is prolonged).
If any patient risk factors are present or another QT-prolonging drug is concurrently prescribed, treatment may still go ahead, but a baseline ECG is needed. Furthermore, the ECG should be repeated once the drug reaches steady state, and then repeated as appropriate (eg, in the event of symptoms). Alternatives should always be sought in patients with pre-existing QT prolongation or congenital long QT syndrome.
Where relevant, patients should be educated on the common symptoms of cardiac arrhythmias—such as dizziness, palpitations and syncope—and advised on when to seek medical attention. Any individual who presents with palpitations, light-headedness or dizziness while receiving a medicine associated with prolongation of the QT interval should be offered an ECG, regardless of other risk factors.
Monitoring
It is impractical to perform an ECG every time a QT-prolonging medicine is prescribed. Nonetheless, there are a number of situations in which ECG monitoring is recommended (eg, those listed in figure 3). In these instances, we advise the following:
Carry out a baseline ECG and repeat when drug levels are likely to be at steady state (generally after around 4–5 half-lives);
Consider repeating the ECG after dose changes;
If there is a significant change in QTc (eg, an increase of >50 ms or an absolute value >500 ms), the prescriber should check and correct any electrolyte imbalances; if QTc prolongation is not resolved, dose reduction or cessation should be considered.
When to refer
A cardiologist should be consulted if there is uncertainty about the ECG (eg, patients with QT prolongation (eg, >480 ms) in the absence of QT prolonging drugs, or a persistent prolongation that is not resolved with the above measures) or in the presence of ventricular arrhythmia. It is important when referring to ensure that cardiac and family history is taken. A cardiologist can advise on the degree of QT prolongation and its implications, but may have limited knowledge on the individual agents used, for example in the case of psychotropic drugs. In many cases, a risk-benefit assessment will need to be undertaken on the implications of not taking the potential QT prolonging medication. Ultimately, the decision on whether or not to prescribe, and when to use a replacement agent, lies with the prescriber.
CONCLUSIONS
Prolongation of the QT interval can be associated with life-threatening cardiac arrhythmia. Although rare cases of inherited long QT syndrome may underlie this, QT prolongation is more commonly associated with particular drug therapies in the presence of modifiable and/or non-modifiable risk factors. Clinicians must be aware of drugs that can cause prolongation of the QT interval, their level of risk, associated risk factors, and how to manage patients taking these drugs.
Main messages.
Many drug therapies are associated with prolongation of the QT interval, and this may increase the risk a potentially life-threatening cardiac arrhythmia called Torsades de Pointes.
When prescribing drugs associated with QT prolongation, three key factors should be considered: patient-related risk factors; the potential risk and degree of QT prolongation associated with the proposed drug; and co-prescribed medicines that could increase the risk of QT prolongation.
If a QT-prolonging drug is prescribed, modifiable risk factors should be corrected as far as possible; appropriate use of ECG at baseline and again once the drug reaches steady state may be necessary in some cases.
Current research questions.
What are the predictors of Torsades de Pointes among patients who have QT prolongation?
Which patients who are prescribed medicines that could prolong the QT interval are likely to benefit from ECG monitoring?
What is the safe and ideal frequency for monitoring U&Es, magnesium and ECG among patients prescribed medicines that can prolong the QT interval.
Self-assessment questions.
-
True or false: The following patient risk factors for Torsades de Pointes with drug-induced QT prolongation are modifiable:
Congenital long QT syndrome
Female sex
Uncorrected electrolyte disturbances
-
Which of the following statements about QTc intervals are true?
The normal QTc intervals are typically considered to be <450 ms for men and <460 ms for women
In general, for every 20 ms increase in the QTc interval, there is a 5% increase in the risk of arrhythmic events
Drugs associated with changes of >20 ms should not typically raise concern
-
Which of the following mechanisms of interaction between different drugs can increase the risk of QT prolongation?
Pharmacodynamic interactions
Pharmacokinetic interactions
Drug-induced electrolyte imbalances
-
Which of the following should a prescriber consider when giving a drug with a high risk of QT prolongation to a patient with no additional risk factors?
Checking baseline ECG
Repeating the ECG once the drug reaches steady state
Regular ECG monitoring even if the patient is asymptomatic
-
A cardiologist should be consulted:
Whenever a drug with a high risk of QT prolongation is prescribed
If there is uncertainty about the ECG (eg, persistent prolongation that proves hard to resolve)
In the presence of ventricular arrhythmia.
Answers.
A (False), B (False), C (True)
A (True), B (False), C (False)
A (True), B (True), C (True)
A (True), B (True), C (False)
A (False), B (True), C (True).
Key References.
Trinkley KE, Lee Page R, Lien H, et al. QT interval prolongation and the risk of Torsades de Pointes: essentials for clinicians. Curr Med Res Opin 2013;29:1719–1726.
Baxter K, Preston CL (eds). Stockley’s drug interactions. 2019. https://about.medicinescomplete.com (accessed 6 September 2020).
Nachimuthu S, Assar MD, Schussler JM. Drug-induced QT interval prolongation: mechanisms and clinical management. Ther Adv Drug Saf 2012;3:241–53.
Acknowledgments
The authors thank Biological Communications Limited for medical writing support, funded by Leeds Teaching Hospitals NHS Trust.
Footnotes
Twitter: Rani Khatib @DrRaniKhatib.
Contributors: RK developed the idea and structure of the paper based on multiple requests from clinicians for advice on this topic. The paper was drafted by RK and reviewed and enhanced by CP and MHT. Further reviews and suggestions were provided by FRNS, CO and MHT. FRNS also attended a Cardiovascular Patient and Public Involvement and Engagement group meeting held at Leeds General Infirmary. Here, patients were asked how clinicians could better involve patients in care management. A common theme emerged from this discussion, with the group expressing a need for clinicians to more clearly communicate the risk factors associated with a prolonged QT interval. As a result, we have articulated the need to inform and adopt a collaborative decision-making approach to prescribing drugs that prolong the QT interval. All authors approved the final version.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
REFERENCES
- 1. Al-Khatib SM, Lapointe NMA, Kramer JM, et al. What clinicians should know about the QT interval. JAMA 2003;289:2120–7. 10.1001/jama.289.16.2120 [DOI] [PubMed] [Google Scholar]
- 2. Ching CK, Tan EC. Congenital long QT syndromes: clinical features, molecular genetics and genetic testing. Expert Rev Mol Diagn 2006;6:365–74. 10.1586/14737159.6.3.365 [DOI] [PubMed] [Google Scholar]
- 3. Zang X, Li S, Zhao Y, et al. Systematic meta-analysis of the association between a common NOS1AP genetic polymorphism, the QTc interval, and sudden death. Int Heart J 2019;60:1083–90. 10.1536/ihj.19-024 [DOI] [PubMed] [Google Scholar]
- 4. Schwartz PJ, Stramba-Badiale M, Crotti L, et al. Prevalence of the congenital long-QT syndrome. Circulation 2009;120:1761–7. 10.1161/CIRCULATIONAHA.109.863209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. El-Sherif N, Turitto G, Boutjdir M. Acquired long QT syndrome and electrophysiology of torsade de pointes. Arrhythm Electrophysiol Rev 2019;8:122–30. 10.15420/aer.2019.8.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heemskerk CPM, Pereboom M, van Stralen K, et al. Risk factors for QTc interval prolongation. Eur J Clin Pharmacol 2018;74:183–91. 10.1007/s00228-017-2381-5 [DOI] [PubMed] [Google Scholar]
- 7. Higham PD, Campbell RW. QT dispersion. Br Heart J 1994;71:508–10. 10.1136/hrt.71.6.508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Priori SG, Schwartz PJ, Napolitano C, et al. Risk stratification in the long-QT syndrome. N Engl J Med 2003;348:1866–74. 10.1056/NEJMoa022147 [DOI] [PubMed] [Google Scholar]
- 9. Roden DM. Long-QT syndrome. N Engl J Med 2008;358:169–76. 10.1056/NEJMcp0706513 [DOI] [PubMed] [Google Scholar]
- 10. Roden DM. Predicting drug-induced QT prolongation and torsades de pointes. J Physiol 2016;594:2459–68. 10.1113/JP270526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cubeddu LX. QT prolongation and fatal arrhythmias: a review of clinical implications and effects of drugs. Am J Ther 2003;10:452–7. 10.1097/00045391-200311000-00013 [DOI] [PubMed] [Google Scholar]
- 12. Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart 2003;89:1363–72. 10.1136/heart.89.11.1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nachimuthu S, Assar MD, Schussler JM. Drug-induced QT interval prolongation: mechanisms and clinical management. Ther Adv Drug Safety 2012;3:241–53. 10.1177/2042098612454283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zeltser D, Justo D, Halkin A, et al. Torsade de pointes due to noncardiac drugs most patients have easily identifiable risk factors. Medicine (Baltimore) 2003;82:282–90. 10.1097/01.md.0000085057.63483.9b [DOI] [PubMed] [Google Scholar]
- 15. Azcert . Credible meds. 2019. Available http://www.crediblemeds.org (accessed 1 Jul 2020)
- 16. Tisdale JE, Jaynes HA, Kingery JR, et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes 2013;6:479–87. 10.1161/CIRCOUTCOMES.113.000152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haugaa KH, Bos JM, Tarrell RF, et al. Institution-wide QT alert system identifies patients with a high risk of mortality. Mayo Clin Proc 2013;88:315–25. 10.1016/j.mayocp.2013.01.013 [DOI] [PubMed] [Google Scholar]
- 18. Vandael E, Vandenberk B, Vandenberghe J, et al. Development of a risk score for QTc-prolongation: the RISQ-PATH study. Int J Clin Pharm 2017;39:424–32. 10.1007/s11096-017-0446-2 [DOI] [PubMed] [Google Scholar]
- 19. Goldenberg I, Moss AJ, Zareba W. QT interval: how to measure it and what is “normal”. J Cardiovasc Electrophysiol 2006;17:333–6. 10.1111/j.1540-8167.2006.00408.x [DOI] [PubMed] [Google Scholar]
- 20. Bazett HC. An analysis of the time‐relations of the electrocardiograms. Heart 1920;7:353–70. [Google Scholar]
- 21. Vandenberk B, Vandael E, Robyns T, et al. Which QT correction formulae to use for QT monitoring? J Am Heart Assoc 2016;5:e003264. 10.1161/JAHA.116.003264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Viskin S, Rosovski U, Sands AJ, et al. Inaccurate electrocardiographic interpretation of long QT: the majority of physicians cannot recognize a long QT when they see one. Heart Rhythm 2005;2:569–74. 10.1016/j.hrthm.2005.02.011 [DOI] [PubMed] [Google Scholar]
- 23. Postema PG, De Jong JSSG, Van der Bilt IAC, et al. Accurate electrocardiographic assessment of the QT interval: teach the tangent. Heart Rhythm 2008;5:1015–18. 10.1016/j.hrthm.2008.03.037 [DOI] [PubMed] [Google Scholar]
- 24. Rautaharju PM, Surawicz B, Gettes LS, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram. Part iv: the ST segment, T and U waves, and the QT interval. A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Co. J Am Coll Cardiol 2009;53:982–91. 10.1016/j.jacc.2008.12.014 [DOI] [PubMed] [Google Scholar]
- 25. Sedlak T, Shufelt C, Iribarren C, et al. Sex hormones and the QT interval: a review. J Womens Health (Larchmt) 2012;21:933–41. 10.1089/jwh.2011.3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Straus SMJM, Kors JA, De Bruin ML, et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol 2006;47:362–7. 10.1016/j.jacc.2005.08.067 [DOI] [PubMed] [Google Scholar]
- 27. Bednar MM, Harrigan EP, Anziano RJ, et al. The QT interval. Prog Cardiovasc Dis 2001;43:1–45. 10.1053/pcad.2001.21469 [DOI] [PubMed] [Google Scholar]
- 28. Trinkley KE, Lee Page R, Lien H, et al. QT interval prolongation and the risk of torsades de pointes: essentials for clinicians. Curr Med Res Opin 2013;29:1719–26. 10.1185/03007995.2013.840568 [DOI] [PubMed] [Google Scholar]
- 29. Medicines and Healthcare products Regulatory Agency. Citalopram and escitalopram: QT interval prolongation: new maximum daily dose restrictions (including in elderly patients), contraindications and warnings. Drug Saf Update 2011;5:A1. [Google Scholar]
- 30. Medicines and Healthcare products Regulatory Agency. Domperidone: risks of cardiac side effects—indication restricted to nausea and vomiting, new contraindications, and reduced dose and duration of use. Drug Saf Update 2014;7:A1. [Google Scholar]
- 31. Medicines and Healthcare products Regulatory Agency . Ondansetron (Zofran): risk of QTc prolongation: important new intravenous dose restriction. Drug Saf Update 2012;6:A2. [Google Scholar]
- 32. Medicines and Healthcare products Regulatory Agency. Quinine: reminder of dose dependant QT prolonging effects; updated medicines interactions. Drug Saf Update 2017;11:2. [Google Scholar]
- 33. Baxter K, Preston CL. Stockley’s drug interactions . London: Pharmaceutical Press. Available http://www.new.medicinescomplete.com (accessed 3 Sep 2020). [Google Scholar]
- 34. Berger FA, van der Sijs H, Becker ML, et al. Development and validation of a tool to assess the risk of QT drug-drug interactions in clinical practice. BMC Med Inform Decis Mak 2020;20:171. 10.1186/s12911-020-01181-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Torres V, Tepper D, Flowers D, et al. QT prolongation and the antiarrhythmic efficacy of amiodarone. J Am Coll Cardiol 1986;7:142–7. 10.1016/S0735-1097(86)80272-8 [DOI] [PubMed] [Google Scholar]
- 36. Kannankeril PJ, Roden DM. Drug-induced long QT and torsade de pointes: recent advances. Curr Opin Cardiol 2007;22:39–43. 10.1097/HCO.0b013e32801129eb [DOI] [PubMed] [Google Scholar]
- 37. Wiśniowska B, Tylutki Z, Wyszogrodzka G, et al. Drug-drug interactions and QT prolongation as a commonly assessed cardiac effect—comprehensive overview of clinical trials. BMC Pharmacol Toxicol 2016;17:1–15. 10.1186/s40360-016-0053-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meid AD, Bighelli I, Mächler S, et al. Combinations of QTc-prolonging drugs: towards disentangling pharmacokinetic and pharmacodynamic effects in their potentially additive nature. Ther Adv Psychopharmacol 2017;7:251–644. 10.1177/2045125317721662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sharma S, Martijn Bos J, Tarrell RF, et al. Providers’ response to clinical decision support for QT prolonging drugs. J Med Syst 2017;41:161. 10.1007/s10916-017-0803-7 [DOI] [PubMed] [Google Scholar]
- 40. Hincapie-Castillo JM, Staley B, Henriksen C, et al. Development of a predictive model for drug-associated QT prolongation in the inpatient setting using electronic health record data. Am J Health Syst Pharm 2019;76:1059–70. 10.1093/ajhp/zxz100 [DOI] [PubMed] [Google Scholar]
- 41. Bindraban AN, Rolvink J, Berger FA, et al. Development of a risk model for predicting QTc interval prolongation in patients using QTc-prolonging drugs. Int J Clin Pharm 2018;40:1372–9. 10.1007/s11096-018-0692-y [DOI] [PubMed] [Google Scholar]