Abstract
Background:
The potential adverse effects of exposures to general anesthesia on the developing human brain remain controversial. It has been hypothesized that hypotension accompanying anesthesia could be contributory. We hypothesized that among children exposed to multiple anesthetics prior to age 3, children developing adverse neurodevelopmental outcomes would be more likely to have intraoperative hypotension.
Methods:
Two previously published study cohorts were utilized for analysis: the retrospective and prospective Mayo Anesthesia Safety in Kids cohorts. The two lowest consecutive systolic blood pressure measurements were abstracted and standardized by calculating a z-score for noninvasive blood pressure reference ranges for children. The lowest systolic blood pressure z-score (continuous variable) and intraoperative hypotension (lowest systolic blood pressure z-score <−1.0) were used to assess the association of intraoperative hypotension with the incidence of learning disabilities or attention-deficit/hyperactivity disorder(retrospective cohort) and factor scores/cluster membership (prospective cohort).
Results:
One hunderd and sixteen and 206 children with multiple exposures to general anesthesia were analyzed in the retrospective and prospective cohorts with mean lowest systolic blood pressure z-scores −0.26 (SD 1.02) and −0.62 (SD 1.10), respectively. There was no overall association of the lowest z-score or hypotension with learning disabilities or attention-deficit/hyperactivity disorder in the retrospective cohort. In the prospective cohort, there was no overall association of the lowest systolic blood pressure or hypotension with factor scores or cluster membership.
Conclusions:
We did not find evidence to support the hypothesis that, among children exposed to multiple anesthetics prior to age 3, children developing adverse neurodevelopmental outcomes would be more likely to have intraoperative hypotension compared with those who did not.
Keywords: anesthetic neurotoxicity, children, general anesthesia, hypotension, neurodevelopment
1 |. INTRODUCTION
The potential adverse effects of exposures to general anesthesia (GA) on the developing human brain continue to be controversial. Although studies performed on young animals consistently demonstrate neurodegeneration and long-term deficits in learning and behavior after exposure to GA,1 human studies show mixed results.2–5 Several recent studies, including the only randomized trial, have not found an association between a single exposure to GA in young children and the primary neurodevelopmental outcomes studied.6–10 However, multiple anesthetic exposures to GA before the 3rd birthday are associated with an increased risk of learning disabilities (LDs) and attention-deficit/hyperactivity disorder (ADHD), as well as decreased academic achievement.2,11,12 Further, the Mayo Anesthesia Safety in Kids (MASK) study found that multiple exposures were associated with deficits in processing speed and fine motor abilities, but not general intelligence.10,13
Several mechanisms by which anesthetics could produce neurotoxicity via direct effects on neuronal structures have been proposed, including the induction of neuronal apoptosis.1,14 It has also been hypothesized that physiological changes accompanying GA could also be contributory.15–17 Specifically, alterations in the factors that reduce cerebral perfusion, including hypotension, are postulated to contribute to adverse neurodevelopmental outcomes following exposure to GA. As part of two prior studies, the retrospective11 and prospective10 MASK studies from our group, several aspects of anesthetic management for each procedure were recorded, including the extremes of systolic blood pressure (SBP), making it possible to study the association between this parameter and outcomes.
The overall goal of this secondary analysis of the MASK data is to examine the relationship between these SBP measurements and neurodevelopment outcomes measured in these two studies. We reasoned that if reduced cerebral perfusion contributed to adverse outcomes, these outcomes would be more common in children with lower blood pressures. We thus tested the hypothesis that, among children exposed to multiple anesthetics prior to age 3, children developing adverse neurodevelopmental outcomes would be more likely to have intraoperative hypotension compared with those who did not.
2 |. METHODS
This study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards, and written informed consent/ assent was obtained. Study methods have been published previously.10,11,13,18 Two previously published study cohorts were utilized for analysis: the retrospective and prospective MASK study cohorts. Here, we provide a summary of these cohorts.
2.1 |. Retrospective MASK cohort
Individuals born from January 1, 1996, to December 31, 2000, in Olmsted County, Minnesota, who lived within Olmsted County through the age of 3 years and who were enrolled in the local school district at the age of 5 years were identified. Anesthesia exposure status prior to 3 years of age was classified as unexposed, singly, or multiply exposed through a review of medical records, and a propensity-based strategy was used to select a matched sample of individuals for which LD and ADHD outcomes were obtained. After propensity matching, the study cohort consisted of 116 children who had two or more exposures to GA, 457 singly exposed and 463 unexposed subjects. Multiple, but not single, exposures were associated with an increased frequency of both LDs and ADHD.11
2.2 |. Prospective MASK cohort
Individuals born from January 1, 1994, to December 31, 2007, in Olmsted County, Minnesota, who lived within Olmsted County until age 3 years and who resided within 25 miles of Rochester, Minnesota, at the time of study according to available records at study onset were identified. Anesthesia exposure status at 3 years of age was classified as unexposed, singly, or multiply exposed through a review of medical records. Subjects were selected for recruitment using a frequency matched approach, with strata defined based on their propensity for receiving single- and multiple-exposure GA. Each subject was tested once, either when 8–12 or 15–20 years old, by a trained psychometrist. A total of 997 children completed testing (411, 380, and 206 unexposed, singly exposed, and multiply exposed, respectively). Among children with multiple exposures to GA, processing speed and fine motor abilities, but not general intelligence or other domains, were decreased.10
A subsequent secondary analysis13 performed factor and cluster analyses to examine associations between exposure and patterns of changes in multiple neuropsychological domains. The factor analysis demonstrated that the neuropsychological testing data were well fit to a five factor model. Multiple exposures to GA were associated with decrements in one of these factors (Factor 1), which reflected motor skills, visual-motor integration, and processing speed measures. The other four factors did not differ according to exposure status. The cluster analysis found that the pattern of neuropsychological testing results among the entire cohort fell into three groups (denoted A, B, and C), with group A having the lowest performance. Multiply-exposed children were more likely to belong to Group A.
2.3 |. Blood pressure data
In both studies, the two lowest consecutive SBP measurements were manually abstracted for each anesthetic exposure from the start of anesthetic exposure until emergence from anesthesia. To minimize artifact, the lowest two consecutive SBP measurements were abstracted and the lowest measurement selected for analysis. SBP measurements that were at extremes of measurement (z-scores < −2.0 and >2.0) were confirmed by a separate abstractor. The site of noninvasive measurement (e.g., arm vs. leg) was not recorded. Since intraoperative blood pressure is expected to differ according to age and sex, the lowest observed SBP for each anesthetic exposure was standardized by calculating a z-score using algorithms provided by the authors of a study, which determined reference values for noninvasive blood pressure in children during the “preparation phase” of GA.19 In order to reduce selection bias, comparison to the “preparation phase” was selected because we did not record the exact timing of lowest SBP values and this phase had lower blood pressure values overall.
2.4 |. Statistical analysis
All analyses were restricted to children who had multiple exposures to GA under the age of 3 years. Data from the retrospective MASK cohort were used to assess the association of intraoperative hypotension with the incidence of LD and ADHD, and data from the prospective MASK cohort were used to assess the association of intraoperative hypotension with factor scores and cluster membership determined using the results of neuropsychological testing data. In all cases, the lowest observed intraoperative SBP z-score from all exposures to general anesthesia under the age of 3 years was the explanatory variable of interest. The lowest SBP z-score was analyzed as a continuous variable and also using a binary indicator variable representing intraoperative hypotension defined as lowest SBP z-score <−1.0 (i.e., SBP more than 1 standard deviation below expected). Analyses were performed using multivariable proportional hazards regression for the LD and ADHD outcomes, multivariate linear regression for the neuropsychological factor scores, and multivariable multinomial logistic regression with generalized logit models for the cluster membership outcome. In all cases, models were adjusted for sex, birthweight, gestational age, mother’s education, and Housing-Based Socioeconomic Status (HOUSES) index,20 which were the variables used as adjustors in both the retrospective and prospective MASK primary analyses.10,11 Results are summarized using point-estimates and corresponding 95% confidence intervals (CI), interquartile ranges (IQR), or standard deviations (SD) as appropriate, with p-values <.05 considered statistically significant.
3 |. RESULTS
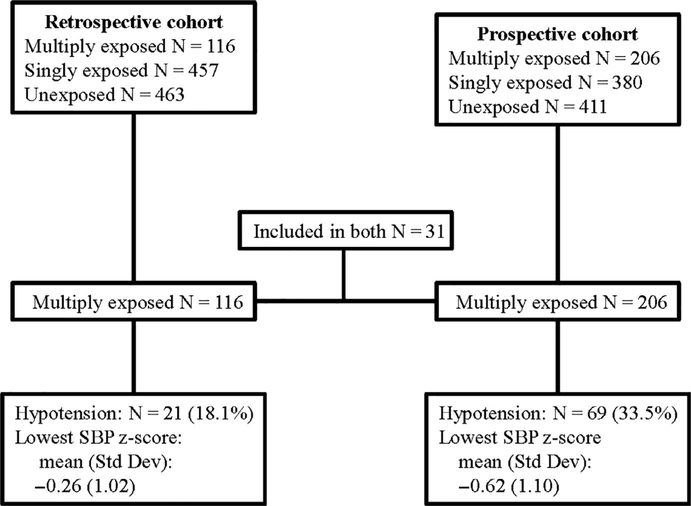
There were 116 (53% male) and 206 (60% male) children with multiple exposures to GA before the 3rd birthday analyzed in the retrospective and prospective MASK cohorts, respectively (Figure 1). Thirty-one (31) individuals were included in both study cohorts. Demographic and birth data are presented in Table 1. The median number of anesthetic exposures was 2 (IQR 2–3) for both cohorts. The median duration of anesthesia was 125 minutes (IQR 87–234) in the retrospective cohort and 187 minutes (IQR 99–326) in the prospective cohort.
FIGURE 1.
Flowchart. SBP, systolic blood pressure
TABLE 1.
Descriptive information for the two cohorts
| Retrospective cohort (N = 116) | Prospective cohort (N = 206) | |
|---|---|---|
| Mother of subject’s age (y) | ||
| Mean (SD) | 28.3 (5.6) | 30.0 (4.9) |
| Median (IQR) | 28 (24, 33) | 30 (27, 34) |
| Father of subject’s age (y) | ||
| Mean (SD) | 31.4 (6.7) | 31.9 (5.4) |
| Median (IQR) | 31 (27, 36) | 31 (28, 35) |
| Mother of subject’s education at birth, n (%) | ||
| <12 | 9 (8%) | |
| 12 | 21 (18%) | 35 (17%) |
| 13–15 | 36 (32%) | 62 (30%) |
| 16 | 28 (25%) | 69 (34%) |
| >16 | 20 (18%) | 39 (19%) |
| Missing | 2 | 1 |
| Father of subject’s education at birth, n (%) | ||
| <12 | 7 (7%) | 6 (3%) |
| 12 | 27 (26%) | 55 (28%) |
| 13–15 | 24 (24%) | 42 (21%) |
| 16 | 21 (21%) | 57 (29%) |
| >16 | 23 (23%) | 40 (20%) |
| Missing | 14 | 6 |
| HOUSES (cohort) | ||
| Mean (SD) | −0.39 (2.74) | −0.03 (3.24) |
| Median (IQR) | −0.6 (−2.4, 1.5) | −0.5 (−2.5, 1.8) |
| Gender, n (%) | ||
| Male | 61 (53%) | 124 (60%) |
| Female | 55 (47%) | 82 (40%) |
| Estimated gestational age | ||
| Mean (SD) | 38.7 (2.6) | 38.2 (2.8) |
| Median (IQR) | 39 (38, 40) | 39 (37, 40) |
| Apgar (1 min) | ||
| Mean (SD) | 7.7 (1.7) | 7.9 (1.6) |
| Median (IQR) | 8 (7, 9) | 8 (8, 9) |
| Apgar (5 min) | ||
| Mean (SD) | 9.1 (0.8) | 9.0 (0.9) |
| Median (IQR) | 9 (9, 10) | 9 (9, 9) |
| Count of anesthetics | ||
| Mean (SD) | 2.6 (1.4) | 3.3 (3.1) |
| Median (IQR) | 2 (2, 3) | 2 (2, 3) |
| Duration of anesthesia (m) | ||
| Mean (SD) | 209.2 (200.8) | 295.4 (354.3) |
| Median (IQR) | 125 (87, 234) | 187 (99, 326) |
| Maximum ASA PS, n (%) | ||
| 1 | 67 (58%) | 71 (34%) |
| 2 | 33 (28%) | 96 (47%) |
| 3 | 13 (11%) | 34 (17%) |
| 4 | 3 (3%) | 5 (2%) |
| Normalized SBP (z-score) | ||
| Mean (SD) | −0.26 (1.02) | −0.62 (1.10) |
| Median (IQR) | −0.2 (−0.8, 0.3) | −0.5 (−1.3, 0.2) |
| Normalized SBP—grouped, n (%) | ||
| <−2 | 6 (5%) | 17 (8%) |
| −2 to −1 | 15 (13%) | 52 (25%) |
| −1 to 0 | 48 (41%) | 74 (36%) |
| >0 | 47 (41%) | 63 (31%) |
Abbreviations: ASA PS, American Society of Anesthesiologists Physical Status score; HOUSES, Housing-Based Socioeconomic Status20; SBP, systolic blood pressure.
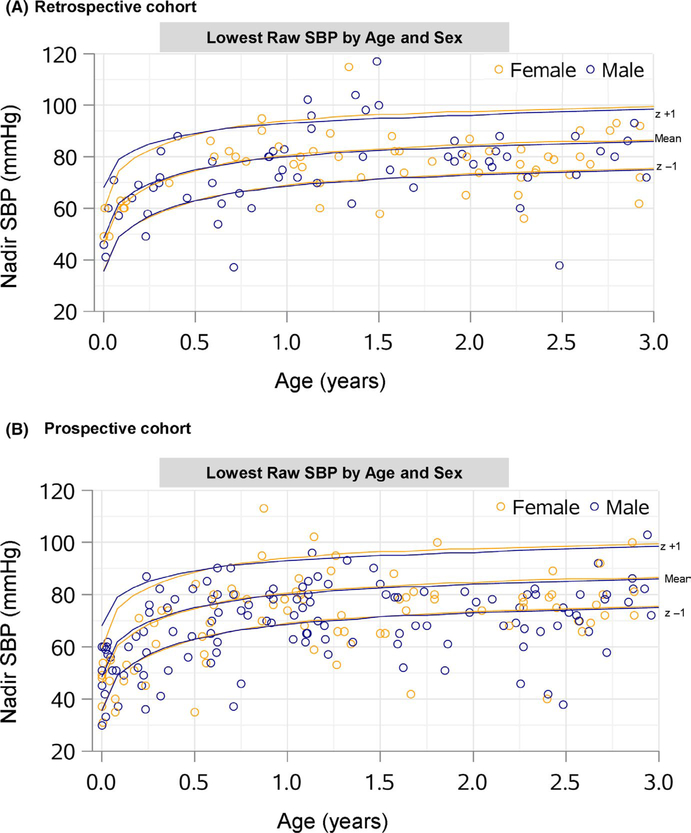
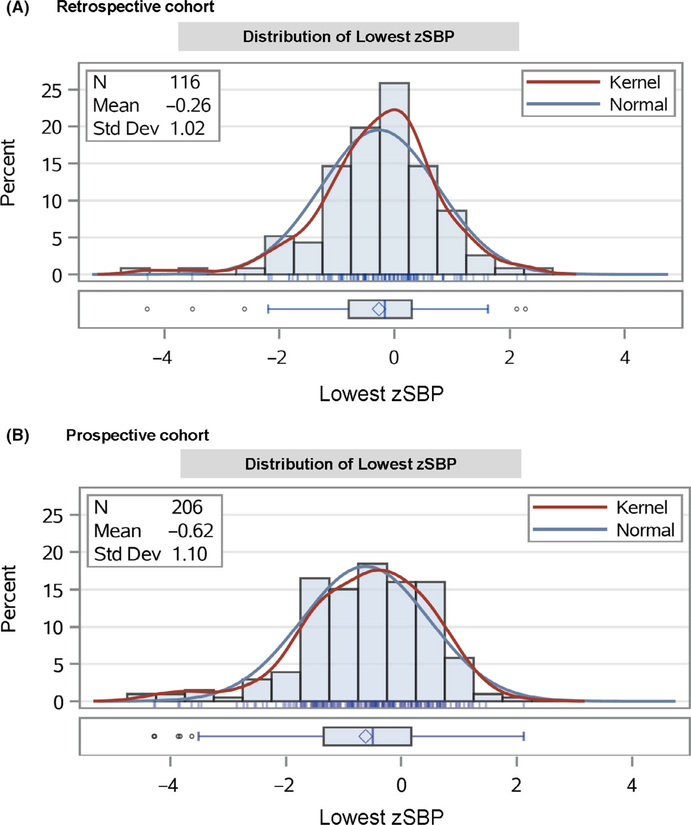
The lowest raw SBPs in mmHg are shown in Figure 2 by age and sex. The mean lowest SBP z-scores were −0.26 (SD 1.02) and −0.62 (SD 1.10) in the retrospective and prospective cohorts, respectively. The distribution of SBP z-scores are shown in Figure 3. There was no overall association of the lowest SBP with ADHD (hazard ratio (HR) 0.89, 95% CI. 0.68 to 1.17, p = .405) or LD (HR 1.16, 95% CI 0.79 to 1.72, p = .445) in the retrospective cohort. In the prospective cohort, there was no overall association of the lowest SBP with factor scores or cluster membership (Table 2).
FIGURE 2.
Scatter plot of lowest raw systolic blood pressure by age and sex. SBP, systolic blood pressure. Lines correspond to mean, +1 standard deviation (z + 1), and −1 standard deviation (z − 1)
FIGURE 3.
Distribution of lowest systolic blood pressure z-scores by cohort. zSBP, systolic blood pressure z-score. Kernel: kernel density estimation
TABLE 2.
Association between lowest systolic blood pressure (continuous variable) and outcomes by exposure category
| Outcome | Hazard ratio estimate (95% CI) | p |
|---|---|---|
| Retrospective cohorta | ||
| ADHD | 0.89 (0.68 to 1.17) | .405 |
| LD | 1.16 (0.79 to 1.72) | .445 |
| Prospective cohort | ||
| Factor scoresb | ||
| Factor 1 | 0.028 (−0.125 to 0.181) | .720 |
| Factor 2 | 0.027 (−0.104 to 0.159) | .685 |
| Factor 3 | 0.015 (−0.118 to 0.148) | .824 |
| Factor 4 | 0.039 (−0.072 to 0.150) | .491 |
| Factor 5 | −0.073 (−0.206 to 0.061) | .287 |
| Cluster membershipc | ||
| C | 0.79 (0.58 to 1.09) | .093 |
| B | Ref. | |
| A | 0.69 (0.49 to 0.98) |
Note: All models are adjusted for sex, birthweight, gestational age, mother’s education, and HOUSES index.20 In each analysis, the assumption of linearity for the effect of normalized systolic blood pressure was assessed and found to be valid.
Learning disability (LD) and attention-deficit/hyperactivity disorder (ADHD) endpoints were available in the retrospective cohort. Results are from multivariable proportional hazards models and estimates presented are hazard ratios associated with a 1 SD increase in normalized SBP.
Factor scores are derived from previously published factor analysis.13 Models are multivariable linear regression and estimates presented are for the change in the given factor score associated with a 1 SD increase in normalized SBP.
Cluster membership is derived from previously published cluster analysis.13 Models are multivariable generalized logit models. Children in group A in general had the lowest performance on neuropsychological testing. Estimates presented are for the increase in odds associated with a 1 SD increase in normalized SBP. In the generalized logit model, the odds ratios presented are for cluster membership relative to the reference cluster.
There were 21 (18.1%) and 69 (33.5%) subjects with hypotension in the retrospective and prospective cohorts, respectively. Hypotension was not associated with the development of ADHD (HR 1.88, 95% CI 0.81 to 4.34, p = .142) or LD (HR 1.02, 95% CI 0.34 to 3.07, p = .972). Hypotension was not associated with factor scores or cluster membership (Table 3).
TABLE 3.
Association between intraoperative hypotension (defined as −1.0 SD below expected SBP) and outcomes by exposure category
| Outcome | Hazard ratio estimate (95% CI) | p |
|---|---|---|
| Retrospective cohorta | 21/116 with zSBP<−1 | |
| ADHD | 1.88 (0.81 to 4.34) | .142 |
| LD | 1.02 (0.34 to 3.07) | .972 |
| Prospective cohort | 69/206 with zSBP<−1 | |
| Factor scoresb | ||
| Factor 1 | −0.066 (−0.426 to 0.295) | .720 |
| Factor 2 | −0.053 (−0.363 to 0.257) | .738 |
| Factor 3 | 0.074 (−0.239 to 0.387) | .643 |
| Factor 4 | −0.143 (−0.403 to 0.118) | .283 |
| Factor 5 | 0.008 (−0.308 to 0.323) | .963 |
| Cluster membershipc | ||
| C | 1.36 (0.64 to 2.89) | .093 |
| B | Ref. | |
| A | 2.44 (1.09 to 5.43) |
Note: All models are adjusted for sex, birthweight, gestational age, mother s education, and HOUSES index.20
Learning disability (LD) and attention-deficit/hyperactivity disorder (ADHD) endpoints were available in the retrospective cohort. Results are from multivariable proportional hazards models and estimates presented are hazard ratios associated with normalized lowest systolic blood pressure <−1.0.
Factor scores are derived from previously published factor analysis.13 Models are multivariable linear regression and estimates presented are for the change in the given factor score associated with normalized lowest systolic blood pressure <−1.0.
Cluster membership is derived from previously published cluster analysis.13 Models are multivariable generalized logit models. Estimates presented are for the increase in odds associated with normalized lowest systolic blood pressure <−1.0. In the generalized logit model, the odds ratios presented are for cluster membership relative to the reference cluster.
4 |. DISCUSSION
In this secondary analysis of two studies examining the association between anesthesia exposure and neurodevelopmental outcomes, we found no evidence that intraoperative hypotension was associated with adverse neurodevelopmental outcomes among children with multiple anesthetic exposures.
The possible adverse neurodevelopmental effects of GA in early childhood continue to be controversial. Recent prospective studies have reassuringly demonstrated no detectable neurodevelopmental deficits with a single exposure to general anesthesia among the primary outcomes studied.8 One of these recent studies, the General Anesthesia compared to Spinal anesthesia (GAS), study demonstrated no detectable adverse neurodevelopmental outcomes in infants randomized to either GA or awake-regional anesthesia.6,7 However, concern remains regarding the effects of multiple exposures to GA.2,10,21 In 2 separate retrospective population-based birth cohorts, our group consistently found that children with multiple exposures were more likely to develop adverse outcomes related to learning and attention.2,11 Additionally, the prospective MASK study found that multiple exposures to GA were associated with a specific pattern of deficits in neuropsychological tests.10,13 Because children with multiple exposures had detectable neurodevelopmental deficits, we chose this group to study to determine possible differences in blood pressure.
In addition to the potential neurotoxic effects of anesthetics, as noted in animal studies,1,22–26 several other mechanisms could explain an association between exposure and outcomes, with confounding by indication perhaps the most important (i.e., children who require anesthesia differ in important ways from those who do not, and these underlying differences explain differential outcomes). Another suggested mechanism is that decreases in cerebral perfusion pressure accompanying anesthesia could contribute.15,27 Several factors that can occur during anesthesia can adversely affect cerebral perfusion pressure, including hypotension, hypocarbia, hypoxemia, hypoglycemia, and hypothermia.28 Decreases in cerebral perfusion pressure could have significant consequences, highlighted in a case series of six infants who developed postoperative encephalopathy, and presumed to be due to hypotension and hypocarbia.29 Blood pressure under anesthesia has been demonstrated to be directly correlated to cerebral blood flow, as measured by cerebral saturation.30 Moreover, baseline mean blood pressure may rest close to lower limits of cerebral autoregulation.31 Thus, the presence of hypotension under GA could be a plausible explanation for possible contribution to adverse neurodevelopmental outcomes after exposure to GA.
The definition of intraoperative hypotension, and the limit for blood pressure below which cerebral perfusion may be compromised, remains unclear. Clinically, many anesthesia providers utilize a 20%–30% reduction in baseline SBP to define hypotension in children under anesthesia, but there are notable regional differences in practices.32 In an attempt to clarify this issue, de Graaff and colleagues performed a retrospective observational cohort study to determine references ranges.19 The study analyzed noninvasive blood pressure measurements from 10 centers with more than 116,000 cases included and divided references ranges into “preparation phase” and “surgical phase,” with graphic and tabular sex-specific percentiles. We arbitrarily chose their “preparation phase” to normalize SBP measurements according to age and sex to 1) provide a consistent approach within anesthetic episodes that may be less sensitive to the type of surgical procedures, and 2) we were not confident that we could distinguish between the timing of these two phases in the anesthesia record. This approach may explain why mean z-score measurements were negative (albeit not significantly different from 0), as intraoperative SBP may be lower during the preparation phase.
We did not find evidence to support the hypothesis that intraoperative hypotension, either with SBP analyzed as a continuous variable or as an ordinal variable defined by 1 SD below the mean reference value, was associated with adverse neurodevelopmental outcomes. Analysis was performed only on children with multiple exposure, not single exposures, because only in these children was exposure status associated with adverse outcomes. Blood pressure data among subjects in the GAS study was also analyzed.33 The risk of hypotension was 4.5x higher in the GA group compared to the awake-regional group when analyzed per protocol, with mean arterial pressure approximately 10 mmHg lower in children receiving GA compared with those receiving awake-regional group. Nonetheless, outcomes were not different between the two groups.
This study had several limitations. We relied on retrospective intraoperative blood pressure data spanning from 1994 to 2007. During this time, the method of anesthesia charting changed from manual to automated. Because some of the earlier anesthesia records were manual, we cannot exclude observer bias (likely toward higher values) and missed readings.34 In the retrospective cohort, the vast majority of charts were manual (94.7%) versus 66.2% in the prospective cohort, which may partially explain why hypotension was more common in the prospective than the retrospective cohort. It was not practically possible to calculate variables such as mean intraoperative SBP, which would have required the manual abstraction of many data. The abstraction of blood pressure data occurred manually, introducing the possibility of human error in the abstraction or errors in the automated reported values, which may bias the results toward a null effect.35 Also, the site of blood pressure measurement was not documented, which also may provide different measurements based on the anatomical measurement location.36
The lowest intraoperative SBP measure was used as the explanatory variable. This method does not provide information regarding the duration of any hypotensive episodes, which may be relevant, nor does it account for recurrent episodes. There are likely many factors other than blood pressure which may affect neurodevelopmental outcomes. Although we attempted to control for some of these factors in the multivariable analysis, not controlling for others would lessen the ability to detect an effect of hypotension on outcomes. Finally, we compared outcomes only within those children who received multiple anesthetics; these findings do not preclude the possibility that the hypotension accompanying any anesthetic could contribute to adverse outcomes as compared with unexposed children—although the lack of effects observed in singly exposed children makes this possibility unlikely.
In conclusion, we did not find evidence to support the hypothesis that, among children exposed to multiple anesthetics prior to age 3, children developing adverse neurodevelopmental outcomes would be more likely to have intraoperative hypotension compared with those who did not.
What is already known about the topic?
The potential adverse effects of exposures to general anesthesia on the developing human brain remain controversial and mechanisms by which anesthetics could produce neurotoxicity via direct effects on neuronal structures have been proposed. However, the contributions of physiologic factors that reduce cerebral perfusion, including hypotension, remain unknown.
What new information this study adds
While the presence of hypotension under general anesthesia could be a plausible explanation for possible contribution to adverse neurodevelopmental outcomes, we did not find any evidence of an increased risk of hypotension, suggesting that other mechanisms better explain an association between adverse neurodevelopmental outcomes after exposure to general anesthesia.
Acknowledgments
Funding information
Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (R01 HD071907); National Institute on Aging of the National Institutes of Health (R01 AG034676)
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilder RT, Flick RP, Sprung J, et al. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartels M, Althoff RR, Boomsma DI. Anesthesia and cognitive performance in children: no evidence for a causal relationship. Twin Res Hum Genet. 2009;12:246–253. [DOI] [PubMed] [Google Scholar]
- 4.DiMaggio C, Sun LS, Kakavouli A, Byrne MW, Li G. A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. J Neurosurg Anesthesiol. 2009;21:286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansen TG, Pedersen JK, Henneberg SW, Morton NS, Christensen K. Educational outcome in adolescence following pyloric stenosis repair before 3 months of age: a nationwide cohort study. Paediatr Anaesth. 2013;23:883–890. [DOI] [PubMed] [Google Scholar]
- 6.Davidson AJ, Disma N, de Graaff JC, et al. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet. 2016;387(10015):239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCann ME, de Graaff JC, Dorris L, et al. Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): an international, multicentre, randomised, controlled equivalence trial. Lancet. 2019;393:664–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun LS, Li G, Miller TL, et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016;315:2312–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vutskits L, Culley DJ. GAS, PANDA, and MASK: no evidence of clinical anesthetic neurotoxicity! Anesthesiology. 2019;131:762–764. [DOI] [PubMed] [Google Scholar]
- 10.Warner DO, Zaccariello MJ, Katusic SK, et al. Neuropsychological and behavioral outcomes after exposure of young children to procedures requiring general anesthesia: the Mayo Anesthesia Safety in Kids (MASK) Study. Anesthesiology. 2018;129:89–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu D, Flick RP, Zaccariello MJ, et al. Association between exposure of young children to procedures requiring general anesthesia and learning and behavioral outcomes in a population-based birth cohort. Anesthesiology. 2017;127:227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sprung J, Flick RP, Katusic SK, et al. Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc. 2012;87:120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaccariello MJ, Frank RD, Lee M, et al. Patterns of neuropsychological changes after general anaesthesia in young children: secondary analysis of the Mayo Anesthesia Safety in Kids study. Br J Anaesth. 2019;122:671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCann ME, Soriano SG. Does general anesthesia affect neurodevelopment in infants and children? BMJ. 2019;367:l6459. [DOI] [PubMed] [Google Scholar]
- 15.Weiss M, Bissonnette B, Engelhardt T, Soriano S. Anesthetists rather than anesthetics are the threat to baby brains. Paediatr Anaesth. 2013;23:881–882. [DOI] [PubMed] [Google Scholar]
- 16.McCann ME, Lee JK, Inder T. Beyond anesthesia toxicity: anesthetic considerations to lessen the risk of neonatal neurological injury. Anesth Analg. 2019;129:1354–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCann ME, Schouten AN. Beyond survival; influences of blood pressure, cerebral perfusion and anesthesia on neurodevelopment. Paediatr Anaesth. 2014;24:68–73. [DOI] [PubMed] [Google Scholar]
- 18.Gleich SJ, Flick R, Hu D, et al. Neurodevelopment of children exposed to anesthesia: design of the Mayo Anesthesia Safety in Kids (MASK) study. Contemp Clin Trials. 2015;41:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Graaff JC, Pasma W, van Buuren S, et al. Reference values for noninvasive blood pressure in children during anesthesia: a multicentered retrospective observational cohort study. Anesthesiology. 2016;125:904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juhn YJ, Beebe TJ, Finnie DM, et al. Development and initial testing of a new socioeconomic status measure based on housing data. J Urban Health. 2011;88:933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ing C, Zaccariello MJ, Kirsch AC, Li G, Warner DO. GAS, PANDA, and MASK: comment. Anesthesiology. 2020;132(6):1587–1588. [DOI] [PubMed] [Google Scholar]
- 22.Istaphanous GK, Howard J, Nan X, et al. Comparison of the neuroapoptotic properties of equipotent anesthetic concentrations of desflurane, isoflurane, or sevoflurane in neonatal mice. Anesthesiology. 2011;114:578–587. [DOI] [PubMed] [Google Scholar]
- 23.Paule MG, Li M, Allen RR, et al. Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol. 2011;33:220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gleich S, Nemergut M, Flick R. Anesthetic-related neurotoxicity in young children: an update. Curr Opin Anaesthesiol. 2013;26:340–347. [DOI] [PubMed] [Google Scholar]
- 25.Creeley CE, Dikranian KT, Dissen GA, Back SA, Olney JW, Brambrink AM. Isoflurane-induced apoptosis of neurons and oligodendrocytes in the fetal rhesus macaque brain. Anesthesiology. 2014;120:626–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olney JW, Wozniak DF, Jevtovic-Todorovic V, Farber NB, Bittigau P, Ikonomidou C. Drug-induced apoptotic neurodegeneration in the developing brain. Brain Pathol. 2002;12:488–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen TG, Lonnqvist PA. The rise and fall of anaesthesia-related neurotoxicity and the immature developing human brain. Acta Anaesthesiol Scand. 2016;60:280–283. [DOI] [PubMed] [Google Scholar]
- 28.McCann ME, Soriano SG. Perioperative central nervous system injury in neonates. Br J Anaesth. 2012;109(Suppl 1):i60–i67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCann ME, Schouten AN, Dobija N, et al. Infantile postoperative encephalopathy: perioperative factors as a cause for concern. Pediatrics. 2014;133:e751–e757. [DOI] [PubMed] [Google Scholar]
- 30.Michelet D, Arslan O, Hilly J, et al. Intraoperative changes in blood pressure associated with cerebral desaturation in infants. Paediatr Anaesth. 2015;25:681–688. [DOI] [PubMed] [Google Scholar]
- 31.Vavilala MS, Lee LA, Lam AM. The lower limit of cerebral autoregulation in children during sevoflurane anesthesia. J Neurosurg Anesthesiol. 2003;15:307–312. [DOI] [PubMed] [Google Scholar]
- 32.Nafiu OO, Voepel-Lewis T, Morris M, et al. How do pediatric anesthesiologists define intraoperative hypotension? Paediatr Anaesth. 2009;19:1048–1053. [DOI] [PubMed] [Google Scholar]
- 33.McCann ME, Withington DE, Arnup SJ, et al. Differences in blood pressure in infants after general anesthesia compared to awake regional anesthesia (GAS Study-A Prospective Randomized Trial). Anesth Analg. 2017;125:837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thrush DN. Are automated anesthesia records better? J Clin Anesth. 1992;4:386–389. [DOI] [PubMed] [Google Scholar]
- 35.Hoorweg AJ, Pasma W, van Wolfswinkel L, de Graaff JC. Incidence of artifacts and deviating values in research data obtained from an anesthesia information management system in children. Anesthesiology. 2018;128:293–304. [DOI] [PubMed] [Google Scholar]
- 36.Short JA. Noninvasive blood pressure measurement in the upper and lower limbs of anaesthetized children. Paediatr Anaesth. 2000;10:591–593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.