Abstract
Background and Aims:
Whether non-alcoholic fatty liver disease (NAFLD) is associated with an increased risk of incident chronic kidney disease (CKD) independent of established cardio-renal risk factors remains controversial. We aimed to provide a quantitative estimate of the association and strength between NAFLD and risk of CKD after adjustment for multiple cardio-renal risk factors.
Methods:
We searched electronic databases (PubMed, Embase, and Google Scholar) for studies published from database inception until 30 November 2020. Analysis included cohort studies that reported multivariable-adjusted risk ratios [including odds ratios, relative risks (RRs), or hazard ratios] and 95% confidence intervals (CIs) for CKD of NAFLD compared with individuals without NAFLD.
Results:
A total of 11 cohort studies were included comprising 1,198,242 participants (46.3% women) for analysis. The median follow-up duration was 3.7 years, with 31,922 cases of incident CKD. Compared with individuals without NAFLD, unadjusted models showed that NAFLD was associated with a higher risk of CKD (RR 1.54, 95% CI 1.38–1.71). After adjusting for multiple cardio-renal risk factors, the CKD risk was still significantly increased in patients with NAFLD (RR 1.39, 95% CI 1.27–1.52). Compared with individuals without NAFLD, the adjusted absolute risk increase in NAFLD for CKD was 5.1 (95% CI 3.5–6.8) per 1000 person-years.
Conclusion:
NAFLD is associated with an increased risk of incident CKD independent of established cardio-renal risk factors.
Keywords: cardio-renal risk factors, chronic kidney disease, non-alcoholic fatty liver disease, risk
Introduction
The definition of non-alcoholic fatty liver disease (NAFLD) is the accumulation of fat (>5%) in liver cells in the absence of excessive alcohol intake or other causes of liver disease. 1 NAFLD has become the most common chronic liver disease worldwide, with a prevalence ranging from 25% to 45%. 2 The disease spectrum of NAFLD ranges from simple steatosis progressing through non-alcoholic steatohepatitis (NASH) with liver fibrosis. 3 Epidemiological evidence has shown that NAFLD not only affects the liver but also increases the risk of developing multiple extrahepatic diseases, including atherosclerotic cardiovascular disease, cardiac arrhythmia, chronic kidney disease (CKD), type 2 diabetes, and extrahepatic cancers.4–8
CKD is a complex, progressive chronic condition defined by either abnormalities of kidney structure or function present for ⩾3 months. 9 CKD has a major effect on global health, both as a direct cause of global mortality and as an important risk factor for cardiovascular disease. Globally, 697.5 million cases of all-stage CKD were recorded, with a global prevalence of 9.1%, and 1.2 million deaths from CKD in 2017. 10 Identifying novel modifiable risk factors for CKD is of paramount importance to reduce the disease burden of CKD.
Cross-sectional studies have shown robust and consistent evidence that NAFLD is associated with an increased prevalence of CKD. However, whether NAFLD is also a ‘driving force’ for the development of CKD remains unclear.11,12 Longitudinal studies on this topic have shown inconsistent results for the association between NAFLD and the incident of CKD and premature mortality.13–17 The inconsistent results may be caused by differences in study characteristics, inclusion criteria, and endpoints assessment. Furthermore, NAFLD and CKD share many common cardio-renal risk factors, including diabetes mellitus, obesity, dyslipidemia, and hypertension. It remains unclear as to whether NAFLD is an independent risk factor of CKD or whether NAFLD is just an epiphenomenon of other cardio-renal risk factors causally related to CKD.
Therefore, we performed an updated meta-analysis of longitudinal cohort studies to determine the association and strength of NAFLD with the risk of CKD after adjustment for other cardio-renal risk factors.
Materials and methods
Search strategy and selection criteria
The study was performed according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline. 18 The electronic databases (PubMed, Embase, and Google Scholar) were searched for studies until 31 November 2020, using a combined medical subject headings and text search strategy with multiple terms associated with ‘non-alcoholic fatty liver disease’ and ‘chronic kidney disease’. The search strategy for PubMed is presented in Supplemental File 1 and the strategies for other databases were similar. We also reviewed reference lists of included studies to identify other potential articles.
We included studies for analysis if they met the following criteria: (a) a cohort study involving adult individuals (aged ⩾18 years) and with a follow-up duration of at least 1 year; (b) NAFLD and other potential risk factors associated with CKD were evaluated at baseline; (c) multivariable-adjusted risk ratios [including odds ratios (ORs), relative risks (RRs), or hazard ratios (HRs)] and their 95% confidence intervals (CIs) for CKD associated with NAFLD versus those without NAFLD were reported. Studies were excluded: (a) if they were case-control or cross-sectional studies or cohort studies with a follow-up evaluation <1 year; (b) studies defined NAFLD based only on liver enzymes, including gamma-glutamyltransferase (GGT), aspartate transaminase, or alanine transaminase; (c) only unadjusted risks were reported; (d) interested data were derived from the same cohort.
Data extraction and quality assessment
Two investigators (XC and XL) independently conducted literature searches, reviewed potentially suitable studies, and extracted data for analysis. We evaluated the quality of the included studies based on the Newcastle–Ottawa Quality Assessment Scale for cohort studies. 19 Studies were classified as good, fair, or poor quality, if they met ⩾7 points, 4–6 points, or <4 points, respectively.20,21 We also evaluated whether the included studies were adequately adjusted for potential confounders of at least six of the following eight factors: age, sex, smoking status, body mass index or overweight/obesity, baseline estimated glomerular filtration rate (eGFR), hypertension or blood pressure, fasting plasma glucose or hemoglobin A1c or diabetes mellitus, and serum cholesterol levels or hypercholesterolemia.
Data analysis
To evaluate the strength of coexistent risk factors on the estimated risk of CKD, we pooled unadjusted outcome data (univariate analysis), as well as those adjusted for the maximal number of potential confounders, respectively. We combined the log RRs and corresponding standard errors by the inverse variance approach. HRs were regarded as approximate to RRs. 22 ORs were converted to RRs by the formula [RR = OR/(1 − pRef) + (pRef × OR)], where pRef is the incidence of the outcome (CKD) in the reference group (individuals without NAFLD).23,24 We used the I 2 statistics to test heterogeneity among the included studies, and a random-effects model was used for meta-analysis if there was significant heterogeneity (I 2 ⩾50%). Otherwise, a fixed-effects model was used.
We calculated the absolute risk difference (expressed as events per 1000 person-years) for the incidence of CKD associated with NAFLD by multiplying the assumed referent risk by the pooled RR-1. 25 The median risk of CKD in individuals without NAFLD across included studies was regarded as the assumed referent risk. Subgroup analyses were conducted according to ethnicity (Asian versus non-Asian), sex (men versus women), study design (prospective versus retrospective), age (average <60 years versus ⩾60 years), methods for defining NAFLD [ultrasonography versus other methods (e.g. database record, computed tomography, transient elastography, or fatty liver index)], population characteristics (general population versus diabetes), follow-up duration (<10 years versus ⩾10 years), sample size (<10,000 versus ⩾10,000), adjustment of risk factors (adequate versus inadequate), and severity of NAFLD. More ‘severe’ NAFLD was defined as patients with ‘severe steatosis’ (detected by ultrasonography or fatty liver index), or ‘advanced fibrosis’ (with higher NAFLD fibrosis score, significant liver fibrosis by ultrasound elastography, or elevated GGT level in the presence of NAFLD).6,26 To further explore the effect of different stage NAFLD on the incidence of CKD, we also performed subgroup analysis according to ‘severe steatosis’ or ‘advanced fibrosis’ separately. Publication bias was evaluated by inspecting funnel plots, as well as Begg’s test and Egger’s test. Sensitivity analyses were conducted whereby the use of random-effects models was changed to fixed-effects models for the meta-analysis, or the RRs were recalculated by omitting one study at a time to evaluate the impact of individual studies on the estimated risk. Meta-regression analysis was used to determine the impact of study characteristics, including sample size, participants’ age, sex, the prevalence of NAFLD, follow-up duration, and study quality score upon the outcome. 27
Analyses were performed using RevMan 5.3 (Cochrane Collaboration, Copenhagen, Denmark). All p values were two-tailed, and statistical significance was set at less than 0.05.
Results
Studies retrieved and characteristics
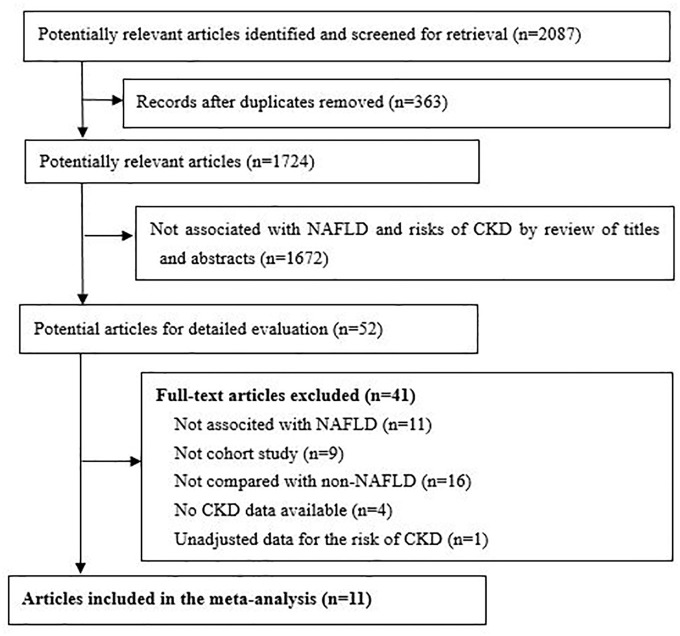
Our initial search returned 2087 articles. After screening the titles and abstracts, 52 qualified for a full-text review. Finally, 11 studies involving 1,198,242 participants (women 46.3%) were included in the analysis (Figure 1).13–17,28–33 The key characteristics of the included studies are presented in Table 1. Four studies were from Asia (Korea and China) and seven studies were from European and American countries. The median follow-up duration was 3.7 years (range 2.7–12.5 years) and 31,922 cases of incident CKD were recorded. The median prevalence of NAFLD in the studies was 38.1%. Different methods were used to define NAFLD (ultrasonography in six studies, fatty liver index, computed tomography, transient elastography in one study, and two studies defined NAFLD based on database record). The adjusted confounders in the estimated risk model are presented in Supplemental File 2, and seven studies met our criteria for adequate adjustment. According to the Newcastle–Ottawa quality assessment, three studies were graded as having fair quality, and eight studies were graded as having good quality (Supplemental File 3).
Figure 1.
The PRISMA flow diagram.
CKD, chronic kidney disease; NAFLD, non-alcoholic fatty liver disease; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1.
Characteristics of included studies in the meta-analysis.
| Study | Participants | Study design | Region | NAFLD definition and prevalence (%) | Sample size (% women) | CKD definition | Incident CKD (N) | Age (years), average | Follow up (years) |
|---|---|---|---|---|---|---|---|---|---|
| Kaps et al. 28 | General population | Retrospective cohort study | Germany | Database record (50%) | 96,114 (47.1%) | CKD stage 3–5, by ICD-10 | 13,796 | 58.8 | 10.0 |
| Krahn et al. 13 | HIV infected patients | Prospective cohort study | Canada | Transient elastography (38.1%) | 485 (24.3) | eGFR <60 ml/min/1.73 m2 or albuminuria | 84 | 49.5 | 3.3 |
| Wilechansky et al. 15 | General population | Prospective cohort study | USA | Computed tomography (19.1%) | 987 (56.2) | eGFR <60 ml/min/1.73 m2 | 99 | 60.0 | 12.5 |
| Park et al. 14 | General population | Retrospective cohort study | USA | Database record (25.4%) | 1,032,497 (47.0) | CKD stage 3–5, by ICD-9 | 14,421 | 51.0 | 2.7 |
| Sinn et al. 16 | General population | Retrospective cohort study | Korea | Ultrasonography (34.3%) | 41,430 (39.1) | eGFR <60 ml/min/1.73 m2 | 691 | 48.9 | 4.2 |
| Huh et al. 17 | General population | Prospective cohort study | Korea | Fatty liver index ⩾30 (39.3%) | 4761 (38.0) | eGFR <60 ml/min/1.73 m2 | 724 | 51.9 | 10.0 |
| Shen et al. 29 | General population | Retrospective cohort study | China | Ultrasonography (24.0%) | 10,775 (43.6) | eGFR <60 ml/min/1.73 m2 or albuminuria | 1068 | 46.2 | 3.2 |
| Targher et al. 30 | Type 1 diabetes | Retrospective cohort study | Italy | Ultrasonography (50.2%) | 261 (55.6) | eGFR <60 ml/min/1.73 m2 or albuminuria | 61 | 41.0 | 5.2 |
| Jenks et al. 31 | Type 2 diabetes | Prospective cohort study | UK | Ultrasonography (56.8%) | 933 (48.1) | eGFR <60 ml/min/1.73 m2 or albuminuria | 110 | 67.8 | 3.7 |
| Chang et al. 32 | General population | Prospective cohort study | Korea | Ultrasonography (31.1%) | 8239 (0) | eGFR <60 ml/min/1.73 m2 or albuminuria | 324 | 36.7 | 3.2 |
| Targher et al. 33 | Type 2 diabetes | Prospective cohort study | Italy | Ultrasonography (72.3%) | 1760 (39.1) | eGFR <60 ml/min/1.73 m2 or albuminuria | 547 | 57.9 | 6.5 |
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HIV, human immunodeficiency virus; ICD, International Classification of Diseases; NAFLD, non-alcoholic fatty liver disease.
Association between NAFLD and risk of CKD
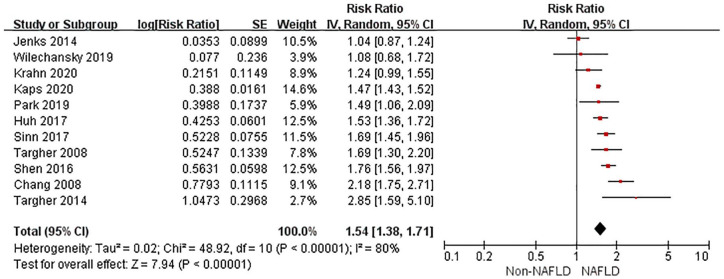
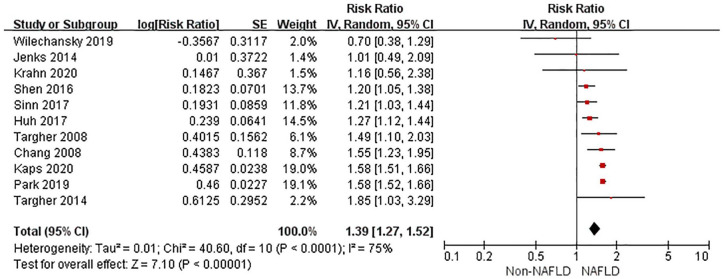
The random-effects model was used for analysis as significant heterogeneity was detected among studies. Compared with non-NAFLD, NAFLD was associated with an increased risk of incident CKD in unadjusted models (RR 1.54, 95% CI 1.38–1.71, p < 0.001, I 2 = 80%) (Figure 2). After multivariable adjustment, NAFLD was still associated with a higher risk of CKD (RR 1.39, 95% CI 1.27–1.52, p < 0.001, I 2 = 75%) (Figure 3). There was moderate heterogeneity for the risk of CKD between unadjusted and multivariable-adjusted models (I 2 = 50.9%, p for heterogeneity = 0.15). We identified no publication bias based on inspection of the funnel plot (Supplemental File 4) or by Begg’s test and Egger’s test (all p > 0.25).
Figure 2.
Forest plot of unadjusted risk of CKD associated with NAFLD.
CI, confidence interval; CKD, chronic kidney disease; df, degrees of freedom; NAFLD, non-alcoholic fatty liver disease; SE, standard error.
Figure 3.
Forest plot of multivariable-adjusted risk of CKD associated with NAFLD.
CI, confidence interval; CKD, chronic kidney disease; df, degrees of freedom; NAFLD, non-alcoholic fatty liver disease; SE, standard error.
The absolute risks of CKD in patients with and without NAFLD across studies are presented in Supplemental File 5. Compared with individuals without NAFLD, the adjusted absolute risk increase in NAFLD for CKD was 5.1 (95% CI 3.5–6.8) per 1000 person-years.
Subgroup analyses and sensitivity analyses
Subgroup analyses showed that the multivariable-adjusted risk of CKD was higher in patients with more ‘severe’ NAFLD than those with less ‘severe’ NAFLD, in non-Asians than in Asians, and in studies with inadequate adjustment of risk factors than those with adequate adjustment (all I 2 >75%, p for heterogeneity < 0.05). However, the risk of CKD was significantly increased across all these subgroups (Table 2). The risk of CKD was increased in patients with NAFLD, <60 years old (RR 1.39, 95% CI 1.26–1.53), but not in those aged ⩾60 years (RR 0.81, 95% CI 0.51–1.30) (subgroup heterogeneity: I 2 >79.1%, p for = 0.03). There was no significant heterogeneity in other subgroup analyses (Table 2). The sensitivity analyses confirmed that the association between CKD and NAFLD did not change with the use of random-effects models or fixed-effects models for the meta-analysis or with recalculation of the RRs by omitting one study at a time. Meta-regression analysis showed that no significant associations among study characteristics and the risk of CKD were observed (all p > 0.10) (Supplemental File 6).
Table 2.
Subgroup analyses of the association between NAFLD and risk of CKD.
| Subgroup | No. studies | RR (95% CI) | I2/p * |
|---|---|---|---|
| Ethnicity | |||
| Asians | 4 | 1.27 (1.16, 1.38) | 92.9%/<0.001 |
| Non-Asians | 7 | 1.56 (1.46, 1.66) | |
| Sex | |||
| Male | 4 | 1.55 (1.40, 1.71) | 0%/0.55 |
| Female | 3 | 1.48 (1.31, 1.67) | |
| Study design | |||
| Prospective cohort | 6 | 1.31 (1.11, 1.54) | 0%/0.33 |
| Retrospective cohort | 5 | 1.44 (1.30, 1.59) | |
| Participant’s average age | |||
| <60 years | 9 | 1.39 (1.26, 1.53) | 79.1%/0.03 |
| ⩾60 years | 2 | 0.81 (0.51, 1.30) | |
| Methods for defining NAFLD | |||
| Ultrasonography | 6 | 1.31 (1.17, 1.48) | 48.9%/0.1 |
| Other methods | 5 | 1.46 (1.33, 1.62) | |
| Community-based population | |||
| Yes | 7 | 1.38 (1.25, 1.53) | 0%/0.74 |
| No | 4 | 1.44 (1.14, 1.83) | |
| Presence of diabetes | |||
| Yes | 6 | 1.27 (1.07, 1.51) | 51.1%/0.15 |
| No | 2 | 1.60 (1.23, 2.07) | |
| Sample | |||
| <10,000 | 7 | 1.34 (1.14, 1.57) | 0%/0.50 |
| ⩾10,000 | 4 | 1.43 (1.28, 1.58) | |
| Follow-up duration | |||
| <10 years | 8 | 1.39 (1.21, 1.60) | 0%/0.68 |
| ⩾10 years | 3 | 1.31 (1.03, 1.67) | |
| Adjustment of confounders | |||
| Adequate $ | 7 | 1.32 (1.13, 1.54) | 78.8%/0.03 |
| Inadequate | 4 | 1.58 (1.51, 1.65) | |
| Severity of NAFLD | |||
| More severe | 4 | 1.57 (1.37, 1.78) | 82.5%/0.02 |
| Less severe | 4 | 1.22 (1.04, 1.43) | |
| Severe steatosis | |||
| Yes | 1 | 1.46 (1.19, 1.79) | 64.0%/0.10 |
| No | 1 | 1.17 (1.00, 1.37) | |
| Advanced fibrosis | |||
| Yes | 3 | 1.63 (1.36, 1.96) | 75.3%/0.04 |
| No | 3 | 1.23 (1.00, 1.51) | |
For heterogeneity among subgroups.
Adequate adjustment denoted adjustment of at least six of eight confounders including sex, age, smoking, hypertension or blood pressure or antihypertensive treatment, BMI or other measure of overweight/obesity, cholesterol, blood glucose or diabetes, and baseline eGFR.
BMI, body mass index; CI, confidence interval; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; NAFLD, non-alcoholic fatty liver disease; RR, relative risk.
Discussion
In this updated meta-analysis among 1.2 million participants with 31,922 cases of incident CKD, we demonstrated that compared with individuals without NAFLD, NAFLD was associated with a 39% higher risk of incident CKD, independent of multiple cardio-renal risk factors.
Two previous meta-analyses have reported significantly different strengths for the association between NAFLD and the risk of CKD. Musso et al. 34 included 33 studies (63,902 participants from 20 cross-sectional and 13 longitudinal studies) and reported that NAFLD was associated with a significantly increased risk of CKD compared with those without NAFLD (HR 1.79, 95% CI 1.65–1.95). However, the inclusion of studies without adjusted for cardio-renal risk factors, as well as those without a referent group without NAFLD, may overestimate the association between NAFLD and CKD. Mantovani et al. 6 included nine observational studies with 96,595 adult individuals of predominantly Asian descent for analysis, and found that patients with NAFLD had a higher risk of incident CKD than those without NAFLD (HR 1.37, 95% CI 1.20–1.53). However, the association between NAFLD and the risk of incident CKD was only observed in Asian populations (HR 1.40, 95% CI 1.22–1.58), but not in European populations (HR 1.29, 95% CI 0.82–1.76). Compared with those previous meta-analyses, our study has several strengths. First, we excluded studies that did not adjust for other cardio-renal risk factors, which mitigated the possibility of influencing the association between NAFLD and the risk of CKD by other confounding factors. Second, we did not include studies that defined NAFLD based only on the elevation of liver enzyme. Liver enzyme elevation is a nonspecific finding in many hepatobiliary disorders. Third, several included studies with a large sample size in this study were recently published and thus were not included in previous studies, which constituted the latest evidence in the field. We also documented that the risk of CKD associated with NAFLD was increased both in Asians and non-Asians.
It was documented that NAFLD and CKD share common cardio-renal risk factors, including diabetes mellitus, obesity, dyslipidemia, and hypertension. Recently, a cross-sectional study also showed that levels of triglycerides and very-low-density lipoprotein were higher in patients with CKD with NAFLD compared with those without NAFLD, and revealed that lipid profile may have an important impact on the interaction between CKD and NAFLD. 35 Interestingly, we found that there was only moderate heterogeneity for the risk of CKD in the unadjusted model compared with the maximal confounders adjusted model, which demonstrated that the coexisting risk factors could not explain the increased risk of CKD in patients with NAFLD. Other potential mechanisms may be involved in the link between NAFLD and the development of CKD. Although not yet completely understood, several plausible mechanisms have been suggested. First, lipotoxicity, hepatic/systemic insulin resistance, oxidative stress, increased activity of the renin-angiotensin-aldosterone system observed in NAFLD may also be involved in the kidney injury.36,37 Second, NAFLD is a status of metabolic inflammation and has been considered a low-grade inflammatory disorder, which is more obvious in patients with NASH and advanced hepatic fibrosis.38,39 Persistent, low-grade inflammation is now considered an important risk factor for the development and progression of CKD. 40 Third, other novel mechanisms, including altered level of hepatokines and perturbation of the gut microbiota (dysbiosis) in NAFLD may also play a role in the development of CKD.11,41,42 Considering the high prevalence of NAFLD worldwide and the significant association for CKD, effective intervention in populations with NAFLD could have important impacts on the global health burden of CKD. Nowadays, lifestyle intervention is the cornerstone of treatment for NAFLD. 43 A randomized controlled trial has shown that improved NAFLD histology associated with 1-year lifestyle modification was associated with improved kidney function. 44 Therefore, lifestyle intervention should also be advocated to prevent CKD in patients with NAFLD.
Risk stratification for CKD in patients with NAFLD is needed. Our results showed that in individuals with more ‘severe’ NAFLD, the risk of CKD is higher than those with less ‘severe’ NAFLD. Recently studies also showed that the patatin-like phospholipase domain-containing protein 3 (PNPLA3) rs738409 polymorphism was associated with the risk of renal damage in NAFLD. 45 Further studies are needed to explore the effect of screening and treatment for CKD in these high-risk patients with NAFLD.
We should note several limitations in the present study. First, NAFLD was defined with different methods in included studies. However, no significant heterogeneity was observed among subgroup analysis according to methods of defining NAFLD, and in studies that used the most common available method to define NAFLD, that is, ultrasonography, the risk of CKD was similar to the main analysis. Second, although all the included studies defined the outcome as CKD stage 3–5, the definition and methods for calculation eGFR varied in different studies, which may cause bias for comparison among studies. Third, biopsy is the gold standard for defining the histological findings of NAFLD. Whether patients with more severe NAFLD based on histological findings (e.g. NASH and hepatic fibrosis) carried a higher risk of CKD is not available.
Conclusion
NAFLD is associated with an increased risk of CKD, especially in patients with ‘severe’ NAFLD or younger than 60 years of age. The increased CKD incidence could not be totally explained by coexisting cardio-renal risk factors. Proper screening, risk stratification, and management of NAFLD may reduce the risk of CKD.
Supplemental Material
Supplemental material, sj-pdf-1-taj-10.1177_20406223211024361 for Non-alcoholic fatty liver disease is associated with increased risk of chronic kidney disease by Xiaoyan Cai, Lichang Sun, Xiong Liu, Hailan Zhu, Yang Zhang, Sulin Zheng and Yuli Huang in Therapeutic Advances in Chronic Disease
Acknowledgments
We acknowledge all the contributing authors.
Footnotes
Author contributions: XC, YZ, and YH designed the study. XC and HZ designed the search strategy. XC and XL performed the search and the abstract screening, full-text screening, and data extraction. XC, LS, and SZ drafted the manuscript. All the authors revised the manuscript.
Availability of data and materials: The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by the Guangdong Basic and Applied Basic Research Fund (Key project of Guang-dong-Foshan Joint Fund) (2019B1515120044), Science and Technology Innovation Project from Foshan, Guangdong (FS0AA-KJ218-1301-0006), and the Clinical Research Startup Program of Shunde Hospital, Southern Medical University (CRSP2019001).
ORCID iD: Yuli Huang  https://orcid.org/0000-0001-5423-5487
https://orcid.org/0000-0001-5423-5487
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Xiaoyan Cai, Department of Scientific Research and Education, Shunde Hospital, Southern Medical University, Foshan, China.
Lichang Sun, Department of Cardiology, Shunde Hospital, Southern Medical University, Foshan, China.
Xiong Liu, Department of Cardiology, Shunde Hospital, Southern Medical University, Foshan, China.
Hailan Zhu, Department of Cardiology, Shunde Hospital, Southern Medical University, Foshan, China.
Yang Zhang, Department of Cardiology, Shunde Hospital, Southern Medical University, Foshan, China.
Sulin Zheng, Department of Cardiology, Shunde Hospital, Southern Medical University, Foshan, China.
Yuli Huang, Department of Cardiology, Shunde Hospital, Southern Medical University, Jiazhi Road, Lunjiao Town, Shunde District, Foshan, 528300, PR China.
References
- 1. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012; 55: 2005–2023. [DOI] [PubMed] [Google Scholar]
- 2. Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA 2015; 313: 2263–2273. [DOI] [PubMed] [Google Scholar]
- 3. Stefan N, Haring HU, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol 2019; 7: 313–324. [DOI] [PubMed] [Google Scholar]
- 4. Stahl EP, Dhindsa DS, Lee SK, et al. Nonalcoholic fatty liver disease and the heart: JACC state-of-the-art review. J Am Coll Cardiol 2019; 73: 948–963. [DOI] [PubMed] [Google Scholar]
- 5. Cai X, Zheng S, Liu Y, et al. Nonalcoholic fatty liver disease is associated with increased risk of atrial fibrillation. Liver Int 2020; 40: 1594–1600. [DOI] [PubMed] [Google Scholar]
- 6. Mantovani A, Zaza G, Byrne CD, et al. Nonalcoholic fatty liver disease increases risk of incident chronic kidney disease: a systematic review and meta-analysis. Metabolism 2018; 79: 64–76. [DOI] [PubMed] [Google Scholar]
- 7. Mantovani A, Byrne CD, Bonora E, et al. Nonalcoholic fatty liver disease and risk of incident type 2 diabetes: a meta-analysis. Diabetes Care 2018; 41: 372–382. [DOI] [PubMed] [Google Scholar]
- 8. Mantovani A, Petracca G, Beatrice G, et al. Non-alcoholic fatty liver disease and increased risk of incident extrahepatic cancers: a meta-analysis of observational cohort studies. Gut. Epub ahead of print 8 March 2021. DOI: 10.1136/gutjnl-2021-324191. [DOI] [PubMed] [Google Scholar]
- 9. Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158: 825–830. [DOI] [PubMed] [Google Scholar]
- 10. GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2020; 395: 709–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Byrne CD, Targher G. NAFLD as a driver of chronic kidney disease. J Hepatol 2020; 72: 785–801. [DOI] [PubMed] [Google Scholar]
- 12. Targher G, Francque SM. A fatty liver leads to decreased kidney function? J Hepatol 2017; 67: 1137–1139. [DOI] [PubMed] [Google Scholar]
- 13. Krahn T, Martel M, Sapir-Pichhadze R, et al. Non-alcoholic fatty liver disease predicts development of metabolic comorbidities in HIV-infected patients. J Infect Dis 2020; 222: 787–797. [DOI] [PubMed] [Google Scholar]
- 14. Park H, Dawwas GK, Liu X, et al. Nonalcoholic fatty liver disease increases risk of incident advanced chronic kidney disease: a propensity-matched cohort study. J Intern Med 2019; 286: 711–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilechansky RM, Pedley A, Massaro JM, et al. Relations of liver fat with prevalent and incident chronic kidney disease in the Framingham heart study: a secondary analysis. Liver Int 2019; 39: 1535–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sinn DH, Kang D, Jang HR, et al. Development of chronic kidney disease in patients with non-alcoholic fatty liver disease: a cohort study. J Hepatol 2017; 67: 1274–1280. [DOI] [PubMed] [Google Scholar]
- 17. Huh JH, Kim JY, Choi E, et al. The fatty liver index as a predictor of incident chronic kidney disease in a 10-year prospective cohort study. PLos One 2017; 12: e180951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009; 151: W65–W94. [DOI] [PubMed] [Google Scholar]
- 19. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 12 January 2015).
- 20. Cai X, Zhang Y, Li M, et al. Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis. BMJ 2020; 370: m2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li W, Huang A, Zhu H, et al. Gut microbiota-derived trimethylamine N-oxide is associated with poor prognosis in patients with heart failure. Med J Aust 2020; 213: 374–379. [DOI] [PubMed] [Google Scholar]
- 22. Cai X, Liu X, Sun L, et al. Prediabetes and the risk of heart failure: a meta-analysis. Diabetes Obes Metab. Epub ahead of print 26 March 2021. DOI: 10.1111/dom.14388. [DOI] [PubMed] [Google Scholar]
- 23. Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998; 280: 1690–1691. [DOI] [PubMed] [Google Scholar]
- 24. Yang Y, Li W, Zhu H, et al. Prognosis of unrecognised myocardial infarction determined by electrocardiography or cardiac magnetic resonance imaging: systematic review and meta-analysis. BMJ 2020; 369: m1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schünemann HJ, Higgins JPT, Vist GE, et al. Completing ‘summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, et al. (eds) Cochrane handbook for systematic reviews of interventions version 6.0, www.training.cochrane.org/handbook (2019, accessed 28 March 2020).
- 26. Tahan V, Canbakan B, Balci H, et al. Serum gamma-glutamyltranspeptidase distinguishes non-alcoholic fatty liver disease at high risk. Hepatogastroenterology 2008; 55: 1433–1438. [PubMed] [Google Scholar]
- 27. Deeks JJ, Higgins JPT, Altman DG. Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, et al. (eds) Cochrane handbook for systematic reviews of interventions version 6.0, www.training.cochrane.org/handbook (2019, accessed 28 March 2020).
- 28. Kaps L, Labenz C, Galle PR, et al. Non-alcoholic fatty liver disease increases the risk of incident chronic kidney disease. United European Gastroenterol J 2020; 8: 942–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shen Z, Ji X, Wang Q, et al. Association between nonalcoholic fatty liver disease and chronic kidney disease: a retrospective cohort study. J Shandong Univ (Health Sci) 2016, 54: 43–49. [Google Scholar]
- 30. Targher G, Mantovani A, Pichiri I, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of chronic kidney disease in patients with type 1 diabetes. Diabetes Care 2014; 37: 1729–1736. [DOI] [PubMed] [Google Scholar]
- 31. Jenks SJ, Conway BR, Hor TJ, et al. Hepatic steatosis and non-alcoholic fatty liver disease are not associated with decline in renal function in people with type 2 diabetes. Diabet Med 2014; 31: 1039–1046. [DOI] [PubMed] [Google Scholar]
- 32. Chang Y, Ryu S, Sung E, et al. Nonalcoholic fatty liver disease predicts chronic kidney disease in nonhypertensive and nondiabetic Korean men. Metabolism 2008; 57: 569–576. [DOI] [PubMed] [Google Scholar]
- 33. Targher G, Chonchol M, Bertolini L, et al. Increased risk of CKD among type 2 diabetics with nonalcoholic fatty liver disease. J Am Soc Nephrol 2008; 19: 1564–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Musso G, Gambino R, Tabibian JH, et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med 2014; 11: e1001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Asghar MS, Hassan M, Rasheed U, et al. Impact of fasting lipid profile on chronic kidney disease patients having fatty liver disease. Cureus 2020; 12: e11146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kiapidou S, Liava C, Kalogirou M, et al. Chronic kidney disease in patients with non-alcoholic fatty liver disease: what the hepatologist should know? Ann Hepatol 2020; 19: 134–144. [DOI] [PubMed] [Google Scholar]
- 37. Sharma N, Sircar A, Anders HJ, et al. Crosstalk between kidney and liver in non-alcoholic fatty liver disease: mechanisms and therapeutic approaches. Arch Physiol Biochem. Epub ahead of print 30 March 2020. DOI: 10.1080/13813455.2020.1745851. [DOI] [PubMed] [Google Scholar]
- 38. Abdallah LR, de Matos RC, E Souza YPDM, et al. Non-alcoholic fatty liver disease and its links with inflammation and atherosclerosis. Curr Atheroscler Rep 2020; 22: 7. [DOI] [PubMed] [Google Scholar]
- 39. Gehrke N, Schattenberg JM. Metabolic inflammation – a role for hepatic inflammatory pathways as driver of comorbidities in non-alcoholic fatty liver disease (NAFLD)? Gastroenterology 2020; 158: 1929–1947.e6. [DOI] [PubMed] [Google Scholar]
- 40. Mihai S, Codrici E, Popescu ID, et al. Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. J Immunol Res 2018; 2018: 2180373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Raj D, Tomar B, Lahiri A, et al. The gut-liver-kidney axis: novel regulator of fatty liver associated chronic kidney disease. Pharmacol Res 2020; 152: 104617. [DOI] [PubMed] [Google Scholar]
- 42. Nagy J, Kovacs T. A brief review on the rising incidence of chronic kidney diseases and non-alcoholic fatty liver disease. Physiol Int 2019; 106: 305–310. [PubMed] [Google Scholar]
- 43. Budd J, Cusi K. Non-alcoholic fatty liver disease: what does the primary care physician need to know? Am J Med 2020; 133: 536–543. [DOI] [PubMed] [Google Scholar]
- 44. Vilar-Gomez E, Calzadilla-Bertot L, Friedman SL, et al. Improvement in liver histology due to lifestyle modification is independently associated with improved kidney function in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2017; 45: 332–344. [DOI] [PubMed] [Google Scholar]
- 45. Sun DQ, Zheng KI, Xu G, et al. PNPLA3 rs738409 is associated with renal glomerular and tubular injury in NAFLD patients with persistently normal ALT levels. Liver Int 2020; 40: 107–119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-taj-10.1177_20406223211024361 for Non-alcoholic fatty liver disease is associated with increased risk of chronic kidney disease by Xiaoyan Cai, Lichang Sun, Xiong Liu, Hailan Zhu, Yang Zhang, Sulin Zheng and Yuli Huang in Therapeutic Advances in Chronic Disease